Glycosylated SARS-CoV-2 RBD Antigens Expressed in Glycoengineered Yeast Induce Strong Immune Responses Through High Antigen–Alum Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cloning and Expression of SARS-CoV-2 RBD Genes in Yeast Cells
2.3. Cloning and Expression of SARS-CoV-2 RBD Genes in Mammalian Cells
2.4. Cultivation and Purification of the Recombinant RBD Protein in Shake Flasks
2.5. Glycoform Identification
2.6. Mass Spectrometry for Measuring the Relative Molecular Masses (RMMs) of RBD Proteins
2.7. Measurement of the RBD Proteins’ Zeta Potential
2.8. Determination of the Level of Adsorption of the Recombinant RBD Antigen to Alum
2.9. Grayscale Analysis
2.10. Animal Experiments
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Preparation of the SARS-CoV-2 Pseudovirus
2.13. Pseudovirus Neutralization Assay
2.14. Statistical Analysis
3. Results
3.1. RBD Antigens Expressed by Wild-Type Pichia Pastoris Induced Higher Antibody Titers than Those Expressed in Mammalian Cells
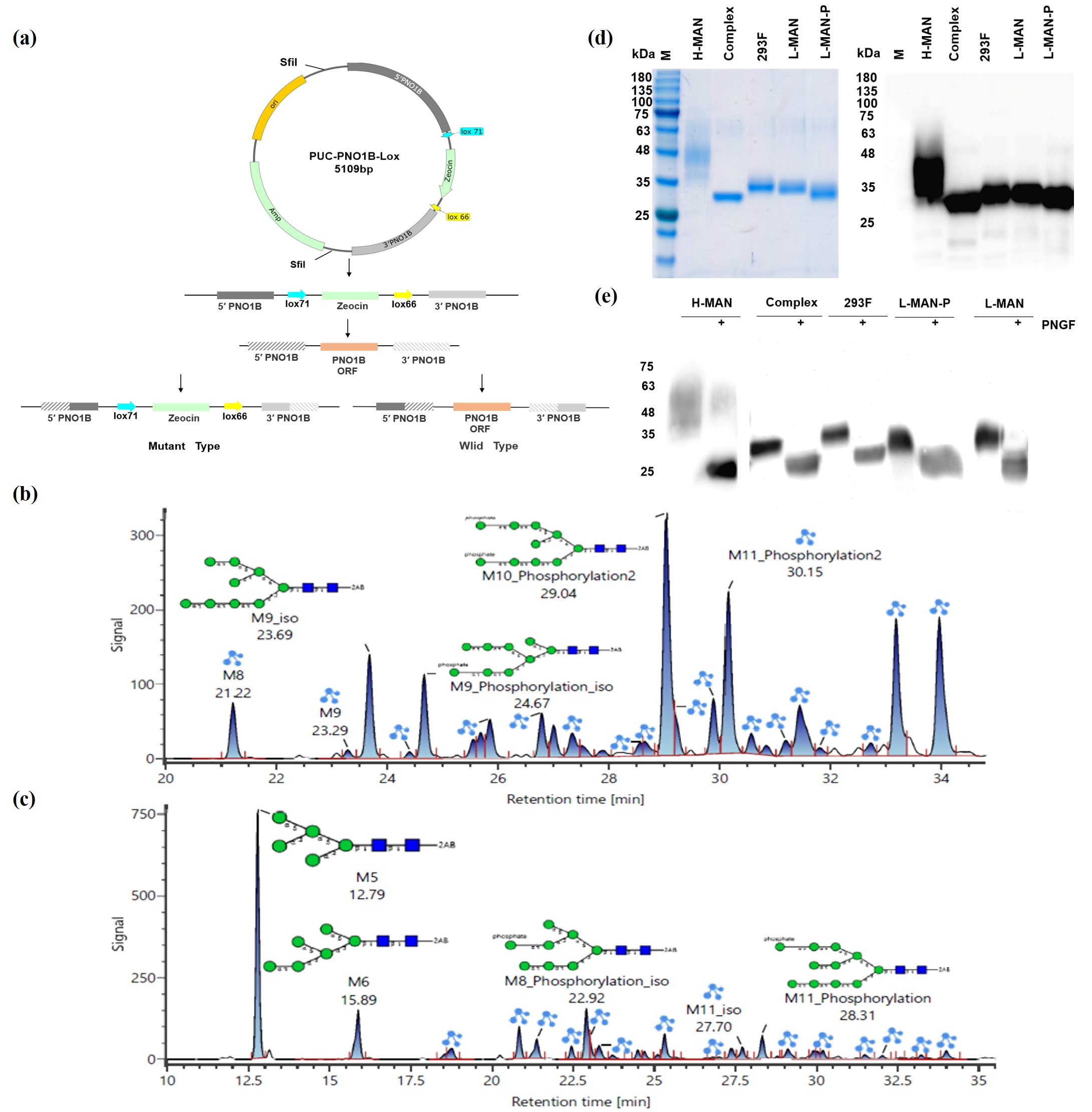
3.2. Reduced Construction of the Phosphate Mannose RBD Antigen by PNOIB and the MNN4B Knockout Yeast Strain, and Verification of Its Glycosylation
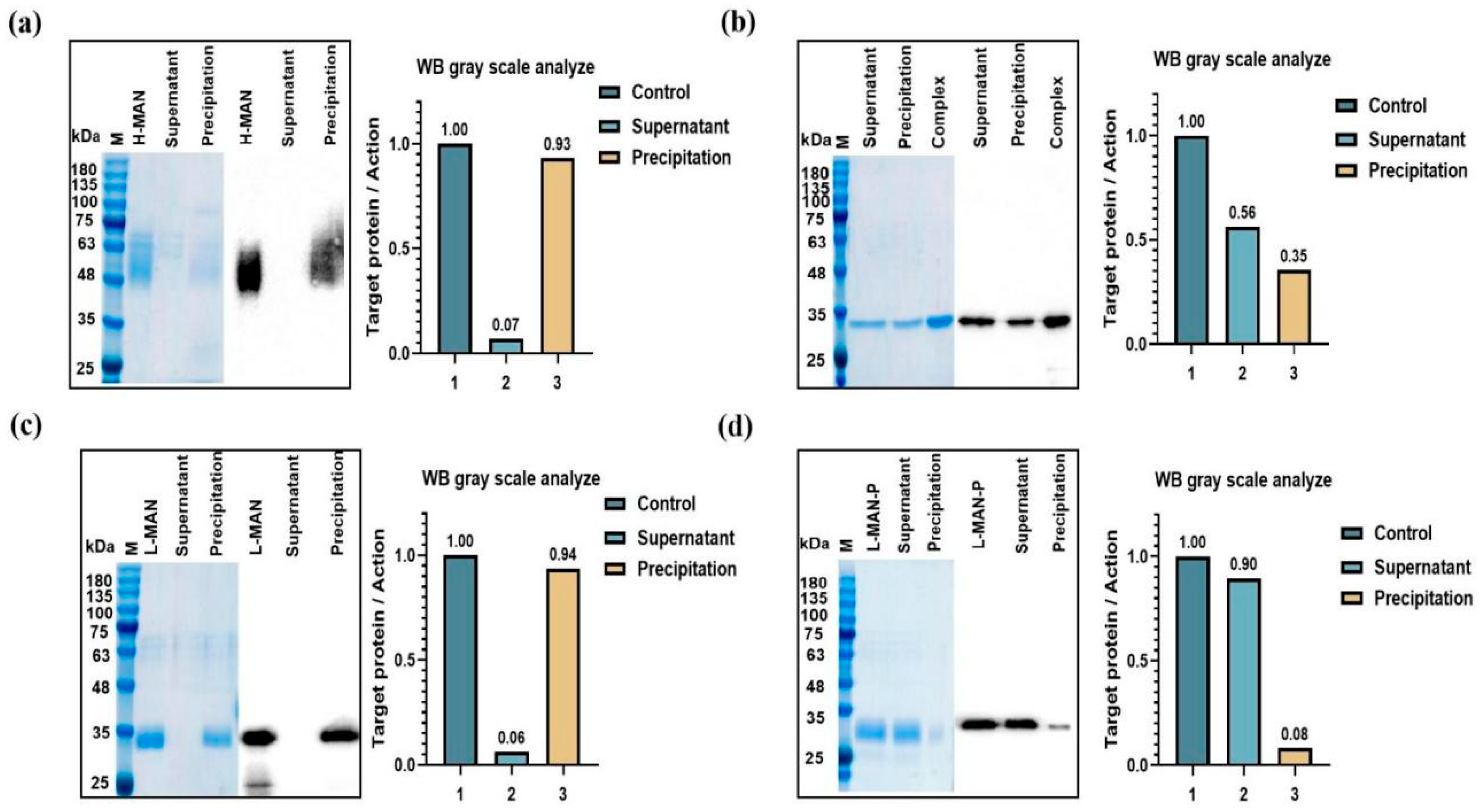
3.3. Phosphomannose Modification Enhanced the Adsorption of RBD Antigens onto Alum
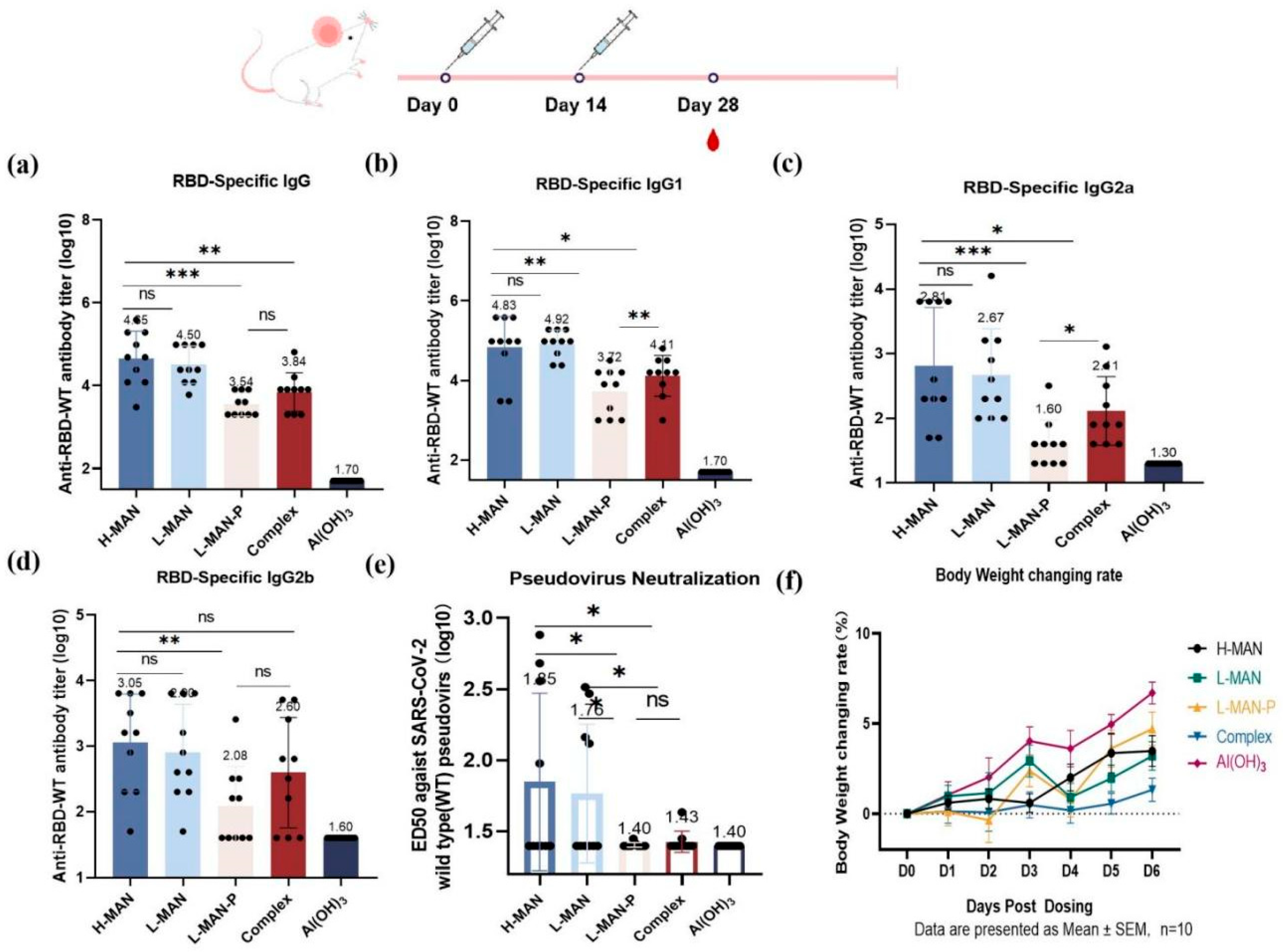
3.4. Higher Antibody Titers Elicited by RBD Antigens Expressed by Wild-Type Pichia Pastoris Were Correlated with Phosphomannose Modification
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Külper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance 2021, 26, 2100563. [Google Scholar] [CrossRef] [PubMed]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef]
- Ding, D.; Wen, Y.; Liao, C.-M.; Yin, X.-G.; Zhang, R.-Y.; Wang, J.; Zhou, S.-H.; Zhang, Z.-M.; Zou, Y.-K.; Gao, X.-F.; et al. Self-Adjuvanting Protein Vaccine Conjugated with a Novel Synthetic TLR4 Agonist on Virus-Like Liposome Induces Potent Immunity against SARS-CoV-2. J. Med. Chem. 2023, 66, 1467–1483. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, R.-Y.; Wang, J.; Zhou, S.-H.; Peng, X.-Q.; Ding, D.; Zhang, Z.-M.; Wei, H.-W.; Guo, J. Novel sialoglycan linkage for constructing adjuvant-protein conjugate as potent vaccine for COVID-19. J. Control. Release 2023, 355, 238–247. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, L.; Gao, S.; Zhou, Y.; Su, L.; Xin, W.; Su, Y.; Wang, J. Expression and purification of functional Clostridium perfringens alpha and epsilon toxins in Escherichia coli. Protein Expr. Purif. 2011, 77, 207–213. [Google Scholar] [CrossRef]
- Zhou, C.; Zai, X.; Zhou, Z.; Li, R.; Zhang, Y.; Li, Y.; Yin, Y.; Zhang, J.; Xu, J.; Chen, W. RBD(206)-sc-dimer induced robust cross-neutralization against SARS-CoV-2 and variants of concern. Signal Transduct. Target. Ther. 2021, 6, 390. [Google Scholar] [CrossRef]
- Wang, W.; Huang, B.; Zhu, Y.; Tan, W.; Zhu, M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell. Mol. Immunol. 2021, 18, 749–751. [Google Scholar] [CrossRef]
- Goldsmith, C.S.; Miller, S.E.; Martines, R.B.; Bullock, H.A.; Zaki, S.R. Electron microscopy of SARS-CoV-2: A challenging task. Lancet 2020, 395, e99. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Mao, Y.; Chen, Y.; Wang, S.; Zhong, Y.; Su, T.; Gong, M.; Du, D.; Lu, X.; et al. Site-specific N-glycosylation Characterization of Recombinant SARS-CoV-2 Spike Proteins. Mol. Cell. Proteom. 2021, 20, 100058. [Google Scholar] [CrossRef]
- Sanda, M.; Morrison, L.; Goldman, R. N- and O-Glycosylation of the SARS-CoV-2 Spike Protein. Anal. Chem. 2021, 93, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Sztain, T.; Ahn, S.-H.; Bogetti, A.T.; Casalino, L.; Goldsmith, J.A.; Seitz, E.; McCool, R.S.; Kearns, F.L.; Acosta-Reyes, F.; Maji, S.; et al. A glycan gate controls opening of the SARS-CoV-2 spike protein. Nat. Chem. 2021, 13, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Bowden, T.A.; Wilson, I.A.; Crispin, M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1480–1497. [Google Scholar] [CrossRef]
- Rowland, R.; Brandariz-Nuñez, A.; Neogi, U. Analysis of the Role of N-Linked Glycosylation in Cell Surface Expression, Function, and Binding Properties of SARS-CoV-2 Receptor ACE2. Microbiol. Spectr. 2021, 9, e0119921. [Google Scholar] [CrossRef]
- Crispin, M.; Doores, K.J. Targeting host-derived glycans on enveloped viruses for antibody-based vaccine design. Curr. Opin. Virol. 2015, 11, 63–69. [Google Scholar] [CrossRef]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Li, Y.; Ren, C.; Shen, Y.; Zhang, Y.; Chen, J.; Zheng, J.; Tian, R.; Cao, L.; Yan, R. Cryo-EM structures of SARS-CoV-2 BA.2-derived subvariants spike in complex with ACE2 receptor. Cell Discov. 2023, 9, 108. [Google Scholar] [CrossRef]
- Behrens, A.-J.; Harvey, D.J.; Milne, E.; Cupo, A.; Kumar, A.; Zitzmann, N.; Struwe, W.B.; Moore, J.P.; Crispin, M.; Ross, S.R. Molecular Architecture of the Cleavage-Dependent Mannose Patch on a Soluble HIV-1 Envelope Glycoprotein Trimer. J. Virol. 2017, 91, e01894-16. [Google Scholar] [CrossRef]
- Liu, B.; Yin, Y.; Liu, Y.; Wang, T.; Sun, P.; Ou, Y.; Gong, X.; Hou, X.; Zhang, J.; Ren, H.; et al. A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses. Engineering 2022, 13, 107–115. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X.; Chang, S.H.; Liu, B.; Song, M.; Huang, H.H.; Wu, J. A Pichia pastoris with alpha-1, 6-mannosyltransferases deletion and its use in expression of HSA/GM-CSF chimera. Chin. J. Biotechnol. 2007, 23, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Bobrowicz, P.; Davidson, R.C.; Hamilton, S.R.; Kung, D.H.; Li, H.; Miele, R.G.; Nett, J.H.; Wildt, S.; Gerngross, T.U. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc. Natl. Acad. Sci. USA 2003, 100, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, J.; Xu, W.; Deng, W.; Wang, Y.; Wang, M.; Wang, Q.; Hsieh, M.; Dong, J.; Wang, X.; et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022, 32, 269–287. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef]
- Rodrigues, K.A.; Rodriguez-Aponte, S.A.; Dalvie, N.C.; Lee, J.H.; Abraham, W.; Carnathan, D.G.; Jimenez, L.E.; Ngo, J.T.; Chang, J.Y.H.; Zhang, Z.; et al. Phosphate-mediated coanchoring of RBD immunogens and molecular adjuvants to alum potentiates humoral immunity against SARS-CoV-2. Sci. Adv. 2021, 7, eabj6538. [Google Scholar] [CrossRef]
- Zai, X.; Zhang, Z.; Zhou, C.; Zhao, F.; Zhang, Y.; Wang, X.; Li, R.; Li, Y.; Zhao, X.; Wang, S.; et al. Precise modification of the surface charge of antigen enhances vaccine immunogenicity. Innovation 2023, 4, 100451. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wen, Y.; He, C.B.; Zhou, S.H.; Wu, Y.H.; Wang, E.Y.; Feng, R.R.; Ding, D.; Du, J.J.; Gao, X.F.; et al. Conjugation of TLR7 and TLR7/8 agonists onto weak protein antigen via versatile oxime ligation for enhanced vaccine efficacy. Int. J. Biol. Macromol. 2024, 278 Pt 1, 134620. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zheng, T.; Xu, K.; Han, Y.; Xu, L.; Huang, E.; An, Y.; Cheng, Y.; Li, S.; Liu, M.; et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722–733.e11. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.-Q.; Liu, L.; Li, X.; Xu, M.; Lin, H.; Liu, S.; Hu, Y.; Li, B.; Liu, B.; et al. A triple-RBD-based mucosal vaccine provides broad protection against SARS-CoV-2 variants of concern. Cell. Mol. Immunol. 2022, 19, 1279–1289. [Google Scholar] [CrossRef]
- Hansen, B.; Sokolovska, A.; HogenEsch, H.; Hem, S.L. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine 2007, 25, 6618–6624. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Wang, T.; Zhang, B.; Hou, X.; Sun, P.; Wang, H.; Xu, H.; Tan, M.; Gong, X.; Wu, J.; et al. Glycosylated SARS-CoV-2 RBD Antigens Expressed in Glycoengineered Yeast Induce Strong Immune Responses Through High Antigen–Alum Adsorption. Biomolecules 2025, 15, 1172. https://doi.org/10.3390/biom15081172
Li A, Wang T, Zhang B, Hou X, Sun P, Wang H, Xu H, Tan M, Gong X, Wu J, et al. Glycosylated SARS-CoV-2 RBD Antigens Expressed in Glycoengineered Yeast Induce Strong Immune Responses Through High Antigen–Alum Adsorption. Biomolecules. 2025; 15(8):1172. https://doi.org/10.3390/biom15081172
Chicago/Turabian StyleLi, Ai, Tiantian Wang, Bin Zhang, Xuchen Hou, Peng Sun, Hao Wang, Huifang Xu, Min Tan, Xin Gong, Jun Wu, and et al. 2025. "Glycosylated SARS-CoV-2 RBD Antigens Expressed in Glycoengineered Yeast Induce Strong Immune Responses Through High Antigen–Alum Adsorption" Biomolecules 15, no. 8: 1172. https://doi.org/10.3390/biom15081172
APA StyleLi, A., Wang, T., Zhang, B., Hou, X., Sun, P., Wang, H., Xu, H., Tan, M., Gong, X., Wu, J., & Liu, B. (2025). Glycosylated SARS-CoV-2 RBD Antigens Expressed in Glycoengineered Yeast Induce Strong Immune Responses Through High Antigen–Alum Adsorption. Biomolecules, 15(8), 1172. https://doi.org/10.3390/biom15081172





