Retinal Pigment Epithelium Transplantation in Retinal Disease: Clinical Trial Development, Challenges, and Future Directions
Abstract
1. Introduction
2. History and Development of RPE Transplantation
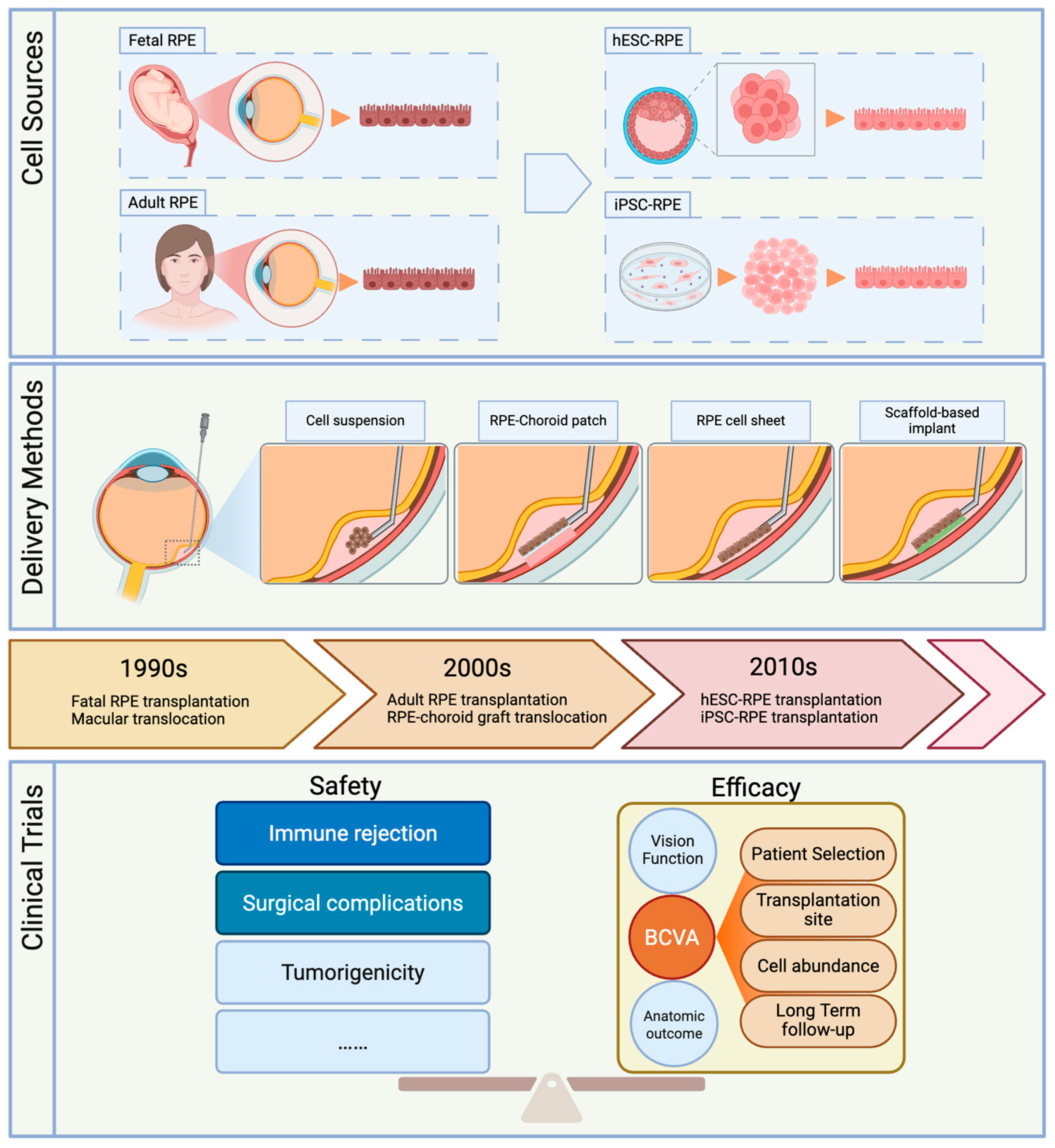
2.1. Cell Source Development
2.2. Comparison of Delivery Methods
2.2.1. Delivery of Cell Suspensions
2.2.2. RPE–choroid Patch Transplantation
2.2.3. RPE Cell Sheet Implants
2.2.4. Scaffold-Based RPE Implants
3. Overview and Analysis of Clinical Trials
| Clinical Trial ID | Target Disease | Phase | Actual Enrollment/Study Eyes | Cell Source | Autologous or Allogeneic | Delivery Method | Cell Amount | Delivery Position | Follow-Up Duration | Vision Outcome | Vision Function | Ocular Adverse Effect | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Dry and Wet AMD | NA | 84/88 | RPE–choroid patch | Autologous | RPE–choroid patch | N/A | Over the fovea | 2–10 years | 38% gained > 15 letters; 10% lost > 15 letters; rest stable | MP showed central fixation | RD, recurrent CNV, ERM, MH, hemorrhage, macular atrophy | [20] |
| NCT00401713 | Wet AMD | I | 7/7 | RPE–choroid sheet | Autologous | RPE–choroid patch | >1500 | Over the fovea | 2 years | 5/7 patients with BCVA improved or stable | MP showed more retinal fixation and stable | PVR, RD, ERM | [56] |
| 7/7 | RPE cells | Autologous | Cell suspension | >1500 | Over the fovea | 2 years | 6/7 patients with BCVA improved or stable | MP showed less retinal fixation and stable | ERM | [56] | |||
| NCT01344993 | Dry AMD | I/II | 9/9 | hESC-derived RPE (MA09-hRPE) | Allogeneic | Cell suspension | 50,000 100,000 150,000 (0.15 mL) | Between atrophic and healthy retina | 4 years | BCVA stable or improved | Not mentioned | Cataract, vitreous inflammation | [22,24] |
| NCT01345006 | STGD | I/II | 9/9 | hESC-derived RPE (MA09-hRPE) | Allogeneic | Cell suspension | 50,000 100,000 150,000 (0.15 mL) | Between atrophic and healthy retina | 4 years | BCVA stable or improved; one eye worsened | Not mentioned | Cataract, vitreous inflammation, endophthalmitis | [22,24] |
| NCT01469832 | STGD type 1 | I/II | 12/12 | hESC-derived RPE (MA09-hRPE) | Allogeneic | Cell suspension | 50,000 100,000 150,000 200,000 (0.15 mL) | Normal areas near vascular arch | 1 year | No significant BCVA improvement | No significant change in MP, mfERG, color vision | Subretinal/vitreous hemorrhage, ERM, preretinal/vitreous pigmentation | [57] |
| NCT01625559 | STGD | I | 3/3 | hESC-derived RPE (MA09-hRPE) | Allogeneic | Cell suspension | 50,000 (0.15 mL) | Relatively normal areas near macula | 3 years | BCVA improved >3 letters | No significant change in VF, ffERG, mfERG | Retinal hemorrhage, ERM, CNV, RRD, mild immune suppression effects | [58,59] |
| NCT01674829 | Dry AMD | I | 2/2 | hESC-derived RPE (MA09-hRPE) | Allogeneic | Cell suspension | 50,000 (0.15 mL) | Relatively normal areas near macula | 1 year | BCVA stable or improved | No significant change in VF, ERG, mfERG | Retinal hemorrhage, ELM, CNV, IOP elevation | [58] |
| NCT01691261 NCT03102138 | Wet AMD | I | 2/2 | hESC derived RPE (PF-05206388) | Allogeneic | Scaffold-based implant | 100,000 (3 × 6 mm) | Over the fovea | 5 years | BCVA improved 21–29 letters in 12 months, maintained for 2 years | MP improved short-term but declined; ERG, EOG showed photoreceptor reduction; reading speed improved | PVR-associated traction RD, epiretinal bands, macular traction, retinal thinning | [48,60] |
| NCT02286089 | Dry AMD and GA | I/IIa | 24/24 | hESC-derived RPE (OpRegen) | Allogeneic | Cell suspension | 50,000–200,000 | Not mentioned | 1–5 years | 75% vision improvement or maintenance in Cohort 4 | Not mentioned | ERM, RD, CNV (suprachoroidal route) | [61,62] |
| NCT02590692 | Dry AMD and GA | I/IIa | 16/16 | hESC-derived RPE (CPCB-RPE1) | Allogeneic | Scaffold-based implant | 100,000 (3.5 × 6.25 mm) | Over the GA and the fovea | 3 years | Treated eyes improved >5 letters and worsened <5 letters in BCVA | MP and mfERG unreliable | Retinal hemorrhage, edema, RD (surgery-related) | [42,47,63] |
| NCT02749734 | STGD type 1 | I | 7/7 | hESC-derived RPE (CTS-hESC-RPE) | Allogeneic | Cell suspension | 100,000 (0.1 mL) | Relatively normal areas near macula | 5 years | No significant BCVA improvement | No significant change in VF, fVEP, ffERG, mfERG | No severe ocular AE | [64] |
| Wet AMD | I | 3/3 | hESC-derived RPE (CTS-hESC-RPE) | Allogeneic | Cell suspension | 1,000,000 (0.1 mL) | Over the fovea | 1 year | BCVA improved > 10 letters (untreated eye improved less) | mfERG showed the response density was slightly increased; fVEP kept stable | No ocular severe AE. | [65] | |
| NCT02903576 | STGD type 3 | I | 12/12 | hESC-derived RPE (WA-099) | Allogeneic | Cell suspension | 1,000,000 (0.1 mL) | Over the fovea | 1 year | No significant BCVA improvement | No difference in VF, mfERG, ffERG | Cataract, epiretinal pigmented clumps, retinal atrophy | [66] |
| NCT03969154 | RP | I | 7/7 | hESC-derived RPE | Allogeneic | Scaffold-based implant | 14.5 mm2, 4800–15,000 cells/mm2 | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | [67] |
| UMIN000011929 | Wet AMD | I | 2/1 | iPSC-derived RPE | Autologous | RPE cell sheet | Around 50,000 (1.3 × 3.0 mm) | Over the fovea | 4 years | No significant improvement | MP unchanged | Temporary IOP elevation | [29,38] |
| UMIN000026003 | Wet AMD | I | 5/5 | iPSC-derived RPE | Allogeneic (HLA matched) | Cell suspension and anti-VEGF | 250,000 (0.05 mL) | Relatively normal areas near macula | 1 year | No significant improvement | ERG and mfERG mildly decreased | ERM, cystoid retinal edema, IOP elevation, recurrent CNV | [68] |
4. Safety and Efficacy of RPE Transplantation
4.1. Immune Safety
4.2. Surgical Complications
4.3. Improvement and Stability of Vision
4.4. Factors Influencing Vision Outcome
4.4.1. Patient Selection
4.4.2. Transplantation Site
4.4.3. The Number of Transplanted Cells
4.4.4. Long-Term Follow-Up
5. Challenge and Future Directions of RPE Transplantation
5.1. Cell Survival and Integration: Histological Evidence
5.2. Clinical Assessment of RPE Cell Survival
5.3. Factors That Influence Cell Survival
5.4. Enhancing RPE Survival and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPE | Retinal pigment epithelium |
| AMD | Age-related macular degeneration |
| STGD | Stargardt disease |
| hESC-RPE | Human embryonic stem cell-derived RPE |
| iPSC-RPE | Induced pluripotent stem cell-derived RPE |
| RCS | Royal College of Surgeons |
| OCT | Optical coherence tomography |
| PLGA | Poly lactic-co-glycolic acid |
| hAM | Human amniotic membrane |
| HLA | Human leukocyte antigen |
| IOP | Intraocular pressure |
| CNV | Choroidal neovascularization |
| BCVA | Best-corrected visual acuity |
| GA | Geographic atrophy |
| VA | Visual acuity |
| EOG | Electro-oculography |
| ERG | Electroretinography |
| ROCK | Rho-associated kinase |
References
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The Cell Biology of the Retinal Pigment Epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The Impact of Oxidative Stress and Inflammation on RPE Degeneration in Non-Neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Feher, J.; Kovacs, I.; Artico, M.; Cavallotti, C.; Papale, A.; Gabrieli, C.B. Mitochondrial Alterations of Retinal Pigment Epithelium in Age-Related Macular Degeneration. Neurobiol. Aging 2006, 27, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Valverde, D.; Riveiro-Alvarez, R.; Aguirre-Lamban, J.; Baiget, M.; Carballo, M.; Antiñolo, G.; Millán, J.M.; Sandoval, B.G.; Ayuso, C. Spectrum of the ABCA4 Gene Mutations Implicated in Severe Retinopathies in Spanish Patients. Investig. Ophthalmol. Vis. Sci. 2007, 48, 985–990. [Google Scholar] [CrossRef][Green Version]
- Hall, J.C.; Paull, D.; Pébay, A.; Lidgerwood, G.E. Human Pluripotent Stem Cells for the Modelling of Retinal Pigment Epithelium Homeostasis and Disease: A Review. Clin. Exp. Ophthalmol. 2022, 50, 667–677. [Google Scholar] [CrossRef]
- Nash, B.M.; Loi, T.H.; Fernando, M.; Sabri, A.; Robinson, J.; Cheng, A.; Eamegdool, S.S.; Farnsworth, E.; Bennetts, B.; Grigg, J.R.; et al. Evaluation for Retinal Therapy for RPE65 Variation Assessed in hiPSC Retinal Pigment Epithelial Cells. Stem Cells Int. 2021, 2021, 4536382. [Google Scholar] [CrossRef]
- Corazza, P.; D’Alterio, F.M.; Kabbani, J.; Alam, M.M.R.; Mercuri, S.; Orlans, H.O.; Younis, S. Long-Term Outcomes of Intravitreal Anti-VEGF Therapies in Patients Affected by Neovascular Age-Related Macular Degeneration: A Real-Life Study. BMC Ophthalmol. 2021, 21, 300. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Peyman, G.A.; Blinder, K.J.; Paris, C.L.; Alturki, W.; Nelson, N.C.; Desai, U. A Technique for Retinal Pigment Epithelium Transplantation for Age-Related Macular Degeneration Secondary to Extensive Subfoveal Scarring. Ophthalmic Surg. Lasers Imaging Retina 1991, 22, 102–108. [Google Scholar] [CrossRef]
- Algvere, P.V.; Berglin, L.; Gouras, P.; Sheng, Y. Transplantation of Fetal Retinal Pigment Epithelium in Age-Related Macular Degeneration with Subfoveal Neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y.; Lewis, J.M.; Hasegawa, T.; Tano, Y. Retinotomy and Foveal Translocation for Surgical Management of Subfoveal Choroidal Neovascular Membranes. Am. J. Ophthalmol. 1996, 122, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Akduman, L.; Karavellas, M.P.; MacDonald, J.C.; Olk, R.J.; Freeman, W.R. Macular Translocation with Retinotomy and Retinal Rotation for Exudative Age-Related Macular Degeneration. Retina 1999, 19, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Stanga, P.E.; Kychenthal, A.; Fitzke, F.W.; Halfyard, A.S.; Chan, R.; Bird, A.C.; Aylward, G.W. Retinal Pigment Epithelium Translocation and Central Visual Function in Age Related Macular Degeneration: Preliminary Results. Int. Ophthalmol. 2001, 23, 297–307. [Google Scholar] [CrossRef]
- Full Macular Translocation versus Photodynamic Therapy with Verteporfin in the Treatment of Neovascular Age-Related Macular Degeneration: 1-Year Results of a Prospective, Controlled, Randomised Pilot Trial (FMT-PDT)|Graefe’s Archive for Clinical and Experimental Ophthalmology. Available online: https://link.springer.com/article/10.1007/s00417-006-0524-y (accessed on 13 July 2025).
- Binder, S.; Stolba, U.; Krebs, I.; Kellner, L.; Jahn, C.; Feichtinger, H.; Povelka, M.; Frohner, U.; Kruger, A.; Hilgers, R.-D.; et al. Transplantation of Autologous Retinal Pigment Epithelium in Eyes with Foveal Neovascularization Resulting from Age-Related Macular Degeneration: A Pilot Study. Am. J. Ophthalmol. 2002, 133, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Krebs, I.; Hilgers, R.-D.; Abri, A.; Stolba, U.; Assadoulina, A.; Kellner, L.; Stanzel, B.V.; Jahn, C.; Feichtinger, H. Outcome of Transplantation of Autologous Retinal Pigment Epithelium in Age-Related Macular Degeneration: A Prospective Trial. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4151–4160. [Google Scholar] [CrossRef]
- Binder, S.; Stanzel, B.V.; Krebs, I.; Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007, 26, 516–554. [Google Scholar] [CrossRef]
- Joussen, A.M.; Joeres, S.; Fawzy, N.; Heussen, F.M.A.; Llacer, H.; van Meurs, J.C.; Kirchhof, B. Autologous Translocation of the Choroid and Retinal Pigment Epithelium in Patients with Geographic Atrophy. Ophthalmology 2007, 114, 551–560. [Google Scholar] [CrossRef]
- van Meurs, J.C.; Van Den Biesen, P.R. Autologous Retinal Pigment Epithelium and Choroid Translocation in Patients with Exudative Age-Related Macular Degeneration: Short-Term Follow-Up. Am. J. Ophthalmol. 2003, 136, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Parolini, B.; Di Salvatore, A.; Pinackatt, S.J.; Baldi, A.; Besozzi, G.; Finzi, A.; Cardillo, D.; Sallam, A.B.; Frisina, R. Long-term results of autologous retinal pigment epithelium and choroid transplantation for the treatment of exudative and atrophic maculopathies. Retina 2020, 40, 507–520. [Google Scholar] [CrossRef]
- Liao, J.-L.; Yu, J.; Huang, K.; Hu, J.; Diemer, T.; Ma, Z.; Dvash, T.; Yang, X.-J.; Travis, G.H.; Williams, D.S.; et al. Molecular Signature of Primary Retinal Pigment Epithelium and Stem-Cell-Derived RPE Cells. Hum. Mol. Genet. 2010, 19, 4229–4238. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Malcuit, C.; Wang, S.; Girman, S.; Francis, P.; Lemieux, L.; Lanza, R.; Lund, R. Long-Term Safety and Function of RPE from Human Embryonic Stem Cells in Preclinical Models of Macular Degeneration. Stem Cells 2009, 27, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9. [Google Scholar] [CrossRef]
- Subretinal Implantation of a Monolayer of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium: A Feasibility and Safety Study in Yucatán Minipigs|Graefe’s Archive for Clinical and Experimental Ophthalmology. Available online: https://link.springer.com/article/10.1007/s00417-016-3386-y?utm_source=chatgpt.com (accessed on 19 July 2025).
- Koster, C.; Wever, K.E.; Wagstaff, E.L.; van den Hurk, K.T.; Hooijmans, C.R.; Bergen, A.A. A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models. Int. J. Mol. Sci. 2020, 21, 2719. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Gu, Q.; Liu, Z.; Li, Q.; Li, Z.; Fang, J.; Liu, W.; Wu, J.; Zhang, Y.; et al. The Effect of Clinical-Grade Retinal Pigment Epithelium Derived from Human Embryonic Stem Cells Using Different Transplantation Strategies. Protein Cell 2019, 10, 455–460. [Google Scholar] [CrossRef]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Sheets Aiming for Clinical Application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retina 2019, 3, 850–859. [Google Scholar] [CrossRef]
- Maeda, T.; Takahashi, M. iPSC-RPE in Retinal Degeneration: Recent Advancements and Future Perspectives. Cold Spring Harb. Perspect. Med. 2023, 13, a041308. [Google Scholar] [CrossRef]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E.; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Oner, A.; Gonen, Z.B.; Sevim, D.G.; Smim Kahraman, N.; Unlu, M. Suprachoroidal Adipose Tissue-Derived Mesenchymal Stem Cell Implantation in Patients with Dry-Type Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: 6-Month Follow-Up Results of a Phase 2 Study. Cell. Reprogram. 2018, 20, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zeng, Y.; Li, Z.; Li, Q.; Xu, H.; Yin, Z.Q. Features Specific to Retinal Pigment Epithelium Cells Derived from Three-Dimensional Human Embryonic Stem Cell Cultures—A New Donor for Cell Therapy. Oncotarget 2016, 7, 22819–22833. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, G.; Lee, D.R.; Lee, J.E.; Kwon, H.J.; Song, W.K. Survival of Transplanted Human Embryonic Stem Cell–Derived Retinal Pigment Epithelial Cells in a Human Recipient for 22 Months. JAMA Ophthalmol. 2017, 135, 287–289. [Google Scholar] [CrossRef]
- van Zeeburg, E.J.T.; Maaijwee, K.J.M.; Missotten, T.O.A.R.; Heimann, H.; van Meurs, J.C. A Free Retinal Pigment Epithelium-Choroid Graft in Patients with Exudative Age-Related Macular Degeneration: Results up to 7 Years. Am. J. Ophthalmol. 2012, 153, 120–127.e2. [Google Scholar] [CrossRef]
- Han, L.; Ma, Z.; Wang, C.; Dou, H.; Hu, Y.; Feng, X.; Xu, Y.; Yin, Z.; Wang, X. Autologous Transplantation of Simple Retinal Pigment Epithelium Sheet for Massive Submacular Hemorrhage Associated with Pigment Epithelium Detachment. Investig. Opthalmol. Vis. Sci. 2013, 54, 4956–4963. [Google Scholar] [CrossRef]
- Gullapalli, V.K.; Sugino, I.K.; Van Patten, Y.; Shah, S.; Zarbin, M.A. Impaired RPE Survival on Aged Submacular Human Bruch’s Membrane. Exp. Eye Res. 2005, 80, 235–248. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Kajita, K.; Nishida, M.; Kurimoto, Y.; Yokota, S.; Sugita, S.; Semba, T.; Shirae, S.; Hayashi, N.; Ozaki, A.; Miura, Y.; et al. Graft Cell Expansion from hiPSC-RPE Strip after Transplantation in Primate Eyes with or without RPE Damage. Sci. Rep. 2024, 14, 10044. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ebeling, M.C.; Geng, Z.; Kapphahn, R.J.; Roehrich, H.; Montezuma, S.R.; Dutton, J.R.; Ferrington, D.A. Human iPSC- and Primary-Retinal Pigment Epithelial Cells for Modeling Age-Related Macular Degeneration. Antioxidants 2022, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wong, D.S.L.; Liu, Z.; Su, X. Quantitative Assessment of Choriocapillaris Following Subretinal Xenotransplantation of Retinal Pigment Epithelium Monolayers on Polyethylene Terephthalate Scaffolds. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3291. [Google Scholar]
- Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; Kashani, A.H.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; et al. Long-Term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy. Ophthalmology 2024, 131, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Tichotová, L.; Studenovska, H.; Petrovski, G.; Popelka, Š.; Nemesh, Y.; Sedláčková, M.; Drutovič, S.; Rohiwal, S.; Jendelová, P.; Erceg, S.; et al. Advantages of Nanofibrous Membranes for Culturing of Primary RPE Cells Compared to Commercial Scaffolds. Acta Ophthalmol. 2022, 100, e1172–e1185. [Google Scholar] [CrossRef] [PubMed]
- Macečková Brymová, A.; Rodriguez-Jimenez, F.J.; Konrad, A.; Nemesh, Y.; Thottappali, M.A.; Artero-Castro, A.; Nyshchuk, R.; Kolesnikova, A.; Müller, B.; Studenovska, H.; et al. Delivery of Human iPSC-Derived RPE Cells in Healthy Minipig Retina Results in Interaction Between Photoreceptors and Transplanted Cells. Adv. Sci. 2025, 12, e2412301. [Google Scholar] [CrossRef]
- Rajendran Nair, D.S.; Zhu, D.; Sharma, R.; Martinez Camarillo, J.C.; Bharti, K.; Hinton, D.R.; Humayun, M.S.; Thomas, B.B. Long-Term Transplant Effects of iPSC-RPE Monolayer in Immunodeficient RCS Rats. Cells 2021, 10, 2951. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ilmarinen, T.; Tan, G.S.W.; Hongisto, H.; Wong, E.Y.M.; Tsai, A.S.H.; Al-Nawaiseh, S.; Holder, G.E.; Su, X.; Barathi, V.A.; et al. Submacular Integration of hESC-RPE Monolayer Xenografts in a Surgical Non-Human Primate Model. Stem Cell Res. Ther. 2021, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W.; et al. One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.; Soomro, T.; Georgiadis, O.; Nommiste, B.; Sagoo, M.S.; Coffey, P. The Fate of RPE Cells Following hESC-RPE Patch Transplantation in Haemorrhagic Wet AMD: Pigmentation, Extension of Pigmentation, Thickness of Transplant, Assessment for Proliferation and Visual Function—A 5 Year-Follow Up. Diagnostics 2024, 14, 1005. [Google Scholar] [CrossRef]
- Liu, H.; Huang, S.S.; Lingam, G.; Kai, D.; Su, X.; Liu, Z. Advances in Retinal Pigment Epithelial Cell Transplantation for Retinal Degenerative Diseases. Stem Cell Res. Ther. 2024, 15, 390. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-Grade Stem Cell–Derived Retinal Pigment Epithelium Patch Rescues Retinal Degeneration in Rodents and Pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Tao, S.L.; Saint-Geniez, M. Porous Poly(ε-Caprolactone) Scaffolds for Retinal Pigment Epithelium Transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1754–1762. [Google Scholar] [CrossRef]
- Zadeh, M.A.; Khoder, M.; Al-Kinani, A.A.; Younes, H.M.; Alany, R.G. Retinal Cell Regeneration Using Tissue Engineered Polymeric Scaffolds. Drug Discov. Today 2019, 24, 1669–1678. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, K.; Gao, G.; Song, X.; Xu, P.; Zeng, J.; Xie, B.; Zheng, D.; He, L.; Ji, J.; et al. Amniotic Membrane Enhances the Characteristics and Function of Stem Cell-Derived Retinal Pigment Epithelium Sheets by Inhibiting the Epithelial–Mesenchymal Transition. Acta Biomater. 2022, 151, 183–196. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, C.; An, C.; Zhang, Y.; Zhang, H.; Li, Q.; Kong, L.; Wang, H.; Ma, X. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells via Injectable Microfluidic-Templated Microgels for Retinal Regeneration. Mater. Today Bio 2025, 32, 101880. [Google Scholar] [CrossRef]
- Yamashita, K.; Ostrovidov, S.; Raut, B.; Hori, T.; Nashimoto, Y.; Nagai, N.; Abe, T.; Kaji, H. Minimally Invasive Sub-Retinal Transplantation of RPE-J Cells on a Biodegradable Composite PCL/Collagen Nanosheet. Cell Transplant. 2023, 32, 9636897231165117. [Google Scholar] [CrossRef]
- Falkner-Radler, C.I.; Krebs, I.; Glittenberg, C.; Povazay, B.; Drexler, W.; Graf, A.; Binder, S. Human Retinal Pigment Epithelium (RPE) Transplantation: Outcome after Autologous RPE-Choroid Sheet and RPE Cell-Suspension in a Randomised Clinical Study. Br. J. Ophthalmol. 2011, 95, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kwon, O.W.; Song, W.K. Semiautomated Subretinal Fluid Injection Method Using Viscous Fluid Injection Mode. Retina 2019, 39, S174–S176. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell-Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Riemann, C.D.; Banin, E.; Barak, A.; Boyer, D.S.; Ehrlich, R.; Ho, A.; Jaouni, T.; McDonald, R.; Telander, D.; Mones, J.; et al. Phase I/IIa Clinical Trial of Transplanted Allogeneic Retinal Pigmented Epithelium (RPE, OpRegen) Cells in Advanced Dry Age-Related Macular Degeneration (AMD): Interim Results. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3316. [Google Scholar]
- Ho, A.C.; Banin, E.; Barak, A.; Boyer, D.S.; Ehrlich, R.; Jaouni, T.; MacDonald, H.R.; Riemann, C.D.; Telander, D.; Mones, J.; et al. Safety and Efficacy of a Phase 1/2a Clinical Trial of Transplanted Allogeneic Retinal Pigmented Epithelium (RPE, OpRegen) Cells in Advanced Dry Age-Related Macular Degeneration (AMD). Investig. Ophthalmol. Vis. Sci. 2022, 63, 1862. [Google Scholar]
- Kashani, A.H.; Uang, J.; Mert, M.; Rahhal, F.; Chan, C.; Avery, R.L.; Dugel, P.; Chen, S.; Lebkowski, J.; Clegg, D.O.; et al. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol. Retina 2020, 4, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.-T.; Li, Q.-Y.; Xu, H.-W.; Meng, X.-H.; Hao, J.; Zhou, Q.; et al. A Phase I Clinical Trial of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells for Early-Stage Stargardt Macular Degeneration: 5-Years’ Follow-Up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.W.; Wang, L.; Li, S.Y.; Zhao, C.J.; Hao, J.; Li, Q.Y.; Zhao, T.T.; Wu, W.; Wang, Y.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium Transplants as a Potential Treatment for Wet Age-Related Macular Degeneration. Cell Discov. 2018, 4, 50. [Google Scholar] [CrossRef]
- Brant Fernandes, R.A.; Lojudice, F.H.; Zago Ribeiro, L.; Santos da Cruz, N.F.; Polizelli, M.U.; Cristovam, P.C.; Innocenti, F.; Morimoto, L.; Magalhães, O.; Ferraz Sallum, J.M.; et al. Transplantation of subretinal stem cell-derived retinal pigment epithelium for stargardt disease: A Phase I Clinical Trial. Retina 2023, 43, 263–274. [Google Scholar] [CrossRef]
- Monville, C.; Bertin, S.; Devisme, C.; Brazhnikova, E.; Jaillard, C.; Walter, H.; Plancheron, A.; Jarraya, M.; Bejanariu, A.; Abbas, S.; et al. Phase I/II Open-Label Study of Implantation into One Eye of hESC-Derived RPE in Patients with Retinitis Pigmentosa Due to Monogenic Mutation: First Safety Results. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3829. [Google Scholar]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef]
- Masli, S.; Vega, J.L. Ocular Immune Privilege Sites. Methods Mol. Biol. Clifton NJ 2011, 677, 449–458. [Google Scholar] [CrossRef]
- Wenkel, H.; Streilein, J.W. Evidence That Retinal Pigment Epithelium Functions as an Immune-Privileged Tissue. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3467–3473. [Google Scholar]
- Sugita, S.; Kamao, H.; Iwasaki, Y.; Okamoto, S.; Hashiguchi, T.; Iseki, K.; Hayashi, N.; Mandai, M.; Takahashi, M. Inhibition of T-Cell Activation by Retinal Pigment Epithelial Cells Derived From Induced Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Kamao, H.; Takahashi, M. Immunological Aspects of RPE Cell Transplantation. Prog. Retin. Eye Res. 2021, 84, 100950. [Google Scholar] [CrossRef]
- Sung, Y.; Lee, M.J.; Choi, J.; Jung, S.Y.; Chong, S.Y.; Sung, J.H.; Shim, S.H.; Song, W.K. Long-Term Safety and Tolerability of Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Asian Stargardt Disease Patients. Br. J. Ophthalmol. 2021, 105, 829–837. [Google Scholar] [CrossRef]
- Petrash, C.C.; Palestine, A.G.; Canto-Soler, M.V. Immunologic Rejection of Transplanted Retinal Pigmented Epithelium: Mechanisms and Strategies for Prevention. Front. Immunol. 2021, 12, 621007. [Google Scholar] [CrossRef]
- Fragiotta, S.; Abdolrahimzadeh, S.; Dolz-Marco, R.; Sakurada, Y.; Gal-Or, O.; Scuderi, G. Significance of Hyperreflective Foci as an Optical Coherence Tomography Biomarker in Retinal Diseases: Characterization and Clinical Implications. J. Ophthalmol. 2021, 2021, 6096017. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Hinton, D.R.; Zhu, D.; Faynus, M.A.; Chen, S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chan, C.; et al. Survival of an HLA-Mismatched, Bioengineered RPE Implant in Dry Age-Related Macular Degeneration. Stem Cell Rep. 2022, 17, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Simakurthy, S.; Tripathy, K. Endophthalmitis. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2023. [Google Scholar]
- Pujari, A.; Agarwal, D.; Chawla, R.; Kumar, A.; Sharma, N. Intraoperative Optical Coherence Tomography Guided Ocular Surgeries: Critical Analysis of Clinical Role and Future Perspectives. Clin. Ophthalmol. 2020, 14, 2427–2440. [Google Scholar] [CrossRef]
- Holekamp, N.; Wykoff, C.C.; Schmitz-Valckenberg, S.; Monés, J.; Souied, E.H.; Lin, H.; Rabena, M.D.; Cantrell, R.A.; Henry, E.C.; Tang, F.; et al. Natural History of Geographic Atrophy Secondary to Age-Related Macular Degeneration: Results from the Prospective Proxima A and B Clinical Trials. Ophthalmology 2020, 127, 769–783. [Google Scholar] [CrossRef]
- Seiler, M.J.; Aramant, R.B. Cell Replacement and Visual Restoration by Retinal Sheet Transplants. Prog. Retin. Eye Res. 2012, 31, 661–687. [Google Scholar] [CrossRef]
- Ahluwalia, K.; Martinez-Camarillo, J.-C.; Thomas, B.B.; Naik, A.; Gonzalez-Calle, A.; Pollalis, D.; Lebkowski, J.; Lee, S.Y.; Mitra, D.; Louie, S.G.; et al. Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats. Cells 2023, 12, 1689. [Google Scholar] [CrossRef]
- Hurley, J.B. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci. 2021, 7, 665–692. [Google Scholar] [CrossRef]
- Falkner, C.I.; Leitich, H.; Frommlet, F.; Bauer, P.; Binder, S. The End of Submacular Surgery for Age-Related Macular Degeneration? A Meta-Analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 490–501. [Google Scholar] [CrossRef] [PubMed]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of Age-Related Macular Degeneration and Therapeutic Opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Salabati, M.; Huang, C.; Kamalipour, A.; Yu, H.J.; Mahmoudzadeh, R.; Jeng-Miller, K.; Chen, E.; Shah, C.P.; Wykoff, C.C.; Hsu, J. Magnitude of Visual Acuity Change with ETDRS versus Snellen Testing in Clinical Trials: Implications for Clinic-Based Outcomes. Ophthalmol. Sci. 2024, 4, 100372. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.C.; Phillips, R.L.; Hageman, G.S. Geographic Atrophy: A Histopathological Assessment. JAMA Ophthalmol. 2014, 132, 338–345. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Bohana-Kashtan, O.; Stoddard, J.W.; Andrews, M.D.; Pandit, N.; Rosenberg-Belmaker, L.R.; Wiser, O.; Matzrafi, L.; Banin, E.; Reubinoff, B.; et al. Long-Term Efficacy of GMP Grade Xeno-Free hESC-Derived RPE Cells Following Transplantation. Transl. Vis. Sci. Technol. 2017, 6, 17. [Google Scholar] [CrossRef]
- Khristov, V.; Maminishkis, A.; Amaral, J.; Rising, A.; Bharti, K.; Miller, S. Validation of iPS Cell-Derived RPE Tissue in Animal Models. Adv. Exp. Med. Biol. 2018, 1074, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.B.; Zhu, D.; Zhang, L.; Thomas, P.B.; Hu, Y.; Nazari, H.; Stefanini, F.; Falabella, P.; Clegg, D.O.; Hinton, D.R.; et al. Survival and Functionality of hESC-Derived Retinal Pigment Epithelium Cells Cultured as a Monolayer on Polymer Substrates Transplanted in RCS Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Klufas, M.A.; Sarraf, D. Clinical Applications of Fundus Autofluorescence in Retinal Disease. Int. J. Retina Vitr. 2016, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Cornish, E.E.; Vaze, A.; Jamieson, R.V.; Grigg, J.R. The Electroretinogram in the Genomics Era: Outer Retinal Disorders. Eye 2021, 35, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Fontrodona, L.; Albert, S.; Ramirez, D.M.; Seriola, A.; Salas, A.; Muoz, Y.; Ramos, D.; Villegas-Perez, M.P.; Zapata, M.A.; et al. Comparative Study of Human Embryonic Stem Cells (hESC) and Human Induced Pluripotent Stem Cells (hiPSC) as a Treatment for Retinal Dystrophies. Mol. Ther. Methods Clin. Dev. 2016, 3, 16010. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.; Fu, X.; Xu, Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front. Immunol. 2017, 8, 645. [Google Scholar] [CrossRef]
- Diniz, B.; Thomas, P.; Thomas, B.; Ribeiro, R.; Hu, Y.; Brant, R.; Ahuja, A.; Zhu, D.; Liu, L.; Koss, M.; et al. Subretinal Implantation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells: Improved Survival When Implanted as a Monolayer. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5087–5096. [Google Scholar] [CrossRef]
- Hsiung, J.; Zhu, D.; Hinton, D.R. Polarized Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cell Monolayers Have Higher Resistance to Oxidative Stress-Induced Cell Death than Nonpolarized Cultures. Stem Cells Transl. Med. 2015, 4, 10–20. [Google Scholar] [CrossRef]
- Klymenko, V.; González Martínez, O.G.; Zarbin, M. Recent Progress in Retinal Pigment Epithelium Cell-Based Therapy for Retinal Disease. Stem Cells Transl. Med. 2024, 13, 317–331. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A Bioengineered Retinal Pigment Epithelial Monolayer for Advanced, Dry Age-Related Macular Degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- Petrus-Reurer, S.; Bartuma, H.; Aronsson, M.; Westman, S.; Lanner, F.; André, H.; Kvanta, A. Integration of Subretinal Suspension Transplants of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in a Large-Eyed Model of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Wang, X.; Wang, S.; Huang, Z.; Li, C.; Liu, Y.; Cheng, Y.; Liu, C.; Wang, Z. Embryonic Stem Cell Microenvironment Enhances Proliferation of Human Retinal Pigment Epithelium Cells by Activating the PI3K Signaling Pathway. Stem Cell Res. Ther. 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Croze, R.H.; Thi, W.J.; Clegg, D.O. ROCK Inhibition Promotes Attachment, Proliferation, and Wound Closure in Human Embryonic Stem Cell–Derived Retinal Pigmented Epithelium. Transl. Vis. Sci. Technol. 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Sugita, S.; Makabe, K.; Fujii, S.; Futatsugi, Y.; Kamao, H.; Yamasaki, S.; Sakai, N.; Maeda, A.; Mandai, M.; et al. A ROCK Inhibitor Promotes Graft Survival during Transplantation of iPS-Cell-Derived Retinal Cells. Int. J. Mol. Sci. 2021, 22, 3237. [Google Scholar] [CrossRef]
- Vernardis, S.I.; Terzoudis, K.; Panoskaltsis, N.; Mantalaris, A. Human Embryonic and Induced Pluripotent Stem Cells Maintain Phenotype but Alter Their Metabolism after Exposure to ROCK Inhibitor. Sci. Rep. 2017, 7, 42138. [Google Scholar] [CrossRef]
- Caporossi, T.; Tartaro, R.; Giansanti, F.; Rizzo, S. The Amniotic Membrane for Retinal Pathologies. Insights on the Surgical Techniques. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2020, 258, 1347–1349. [Google Scholar] [CrossRef]
- Lee, I.K.; Xie, R.; Luz-Madrigal, A.; Min, S.; Zhu, J.; Jin, J.; Edwards, K.L.; Phillips, M.J.; Ludwig, A.L.; Gamm, D.M.; et al. Micromolded Honeycomb Scaffold Design to Support the Generation of a Bilayered RPE and Photoreceptor Cell Construct. Bioact. Mater. 2023, 30, 142–153. [Google Scholar] [CrossRef]
- Farjood, F.; Manos, J.D.; Wang, Y.; Williams, A.L.; Zhao, C.; Borden, S.; Alam, N.; Prusky, G.; Temple, S.; Stern, J.H.; et al. Identifying Biomarkers of Heterogeneity and Transplantation Efficacy in Retinal Pigment Epithelial Cells. J. Exp. Med. 2023, 220, e20230913. [Google Scholar] [CrossRef]
- Kole, C.; Klipfel, L.; Yang, Y.; Ferracane, V.; Blond, F.; Reichman, S.; Millet-Puel, G.; Clérin, E.; Aït-Ali, N.; Pagan, D.; et al. Otx2-Genetically Modified Retinal Pigment Epithelial Cells Rescue Photoreceptors after Transplantation. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 219–237. [Google Scholar] [CrossRef] [PubMed]
| RPE Cell Source | Strengths | Weaknesses | |
|---|---|---|---|
| Native RPE | Fetal RPE | A certain level of plasticity and regeneration; more closely mimics developmental stage-specific functions | Limited availability; ethical concerns over fetal tissue use; immune rejection risk |
| Adult RPE | Native morphology and gene expression; lower tumorigenicity risk; ethical acceptability | Scarce availability; limited proliferative capacity; variant quality of cells; high risk of immune rejection in non-autologous transplants | |
| Stem cell-derived RPE | hESC-RPE | Unlimited supply; high regenerative and proliferative potential; uniformity and scalability for standardizing therapeutic applications | Ethical concerns regarding use of embryonic cells; risk of tumorigenicity; mild to moderate immune rejection dependent on immunosuppressants manufacturing complexity |
| iPSC-RPE | Unlimited supply; high regenerative and proliferative potential; ethical acceptability; Patient-specific applications; genetic correction potential | complex manufacturing process; potential tumorigenicity; potential genomic instability; high cost of patient-specific derived cells; immune rejection risk in allogeneic transplantation | |
| RPE Transplant Delivery | Cell Amount and Implant Area | Advantages | Disadvantages |
|---|---|---|---|
| Cell suspension | Cells from native 0.5–10 × 105 cells in 0.1–0.15 mL | Simple, minimally invasive, adaptable | Cell survival issue, migration risk, low integration efficiency |
| RPE–choroid patch | Depends on lesion area, approximately three optic disc diameters | Natural structure, stable placement, native-like function | Complex surgery, donor limitations, retina damage at periphery |
| RPE cell sheet | 1 × 105 cells in 1.3–2 × 2–3 mm | Mimics native RPE, functional integration, reduce foreign material | Fragility, difficult handling, cell detachment risk |
| Scaffold-based implant | 1–10 × 105 cells in 3–3.5 × 6–6.25 mm | Stability, customizability, controlled placement | Foreign material immune reaction, complex production, potential barriers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhang, T.; Chen, Z.; Zeng, J.; O’Connor, A.; Zhu, M.; Gillies, M.C.; Lu, F.; Zhu, L. Retinal Pigment Epithelium Transplantation in Retinal Disease: Clinical Trial Development, Challenges, and Future Directions. Biomolecules 2025, 15, 1167. https://doi.org/10.3390/biom15081167
Chen Q, Zhang T, Chen Z, Zeng J, O’Connor A, Zhu M, Gillies MC, Lu F, Zhu L. Retinal Pigment Epithelium Transplantation in Retinal Disease: Clinical Trial Development, Challenges, and Future Directions. Biomolecules. 2025; 15(8):1167. https://doi.org/10.3390/biom15081167
Chicago/Turabian StyleChen, Qin, Ting Zhang, Zhi Chen, Jingwen Zeng, Aine O’Connor, Meidong Zhu, Mark C. Gillies, Fang Lu, and Ling Zhu. 2025. "Retinal Pigment Epithelium Transplantation in Retinal Disease: Clinical Trial Development, Challenges, and Future Directions" Biomolecules 15, no. 8: 1167. https://doi.org/10.3390/biom15081167
APA StyleChen, Q., Zhang, T., Chen, Z., Zeng, J., O’Connor, A., Zhu, M., Gillies, M. C., Lu, F., & Zhu, L. (2025). Retinal Pigment Epithelium Transplantation in Retinal Disease: Clinical Trial Development, Challenges, and Future Directions. Biomolecules, 15(8), 1167. https://doi.org/10.3390/biom15081167




_Kwok.png)




