Kidney Stone Disease: Epigenetic Dysregulation in Homocystinuria and Mitochondrial Sulfur Trans-Sulfuration Ablation Driven by COVID-19 Pathophysiology
Abstract
1. Introduction
2. Epigenetics of COVID-19 Infection and KSD
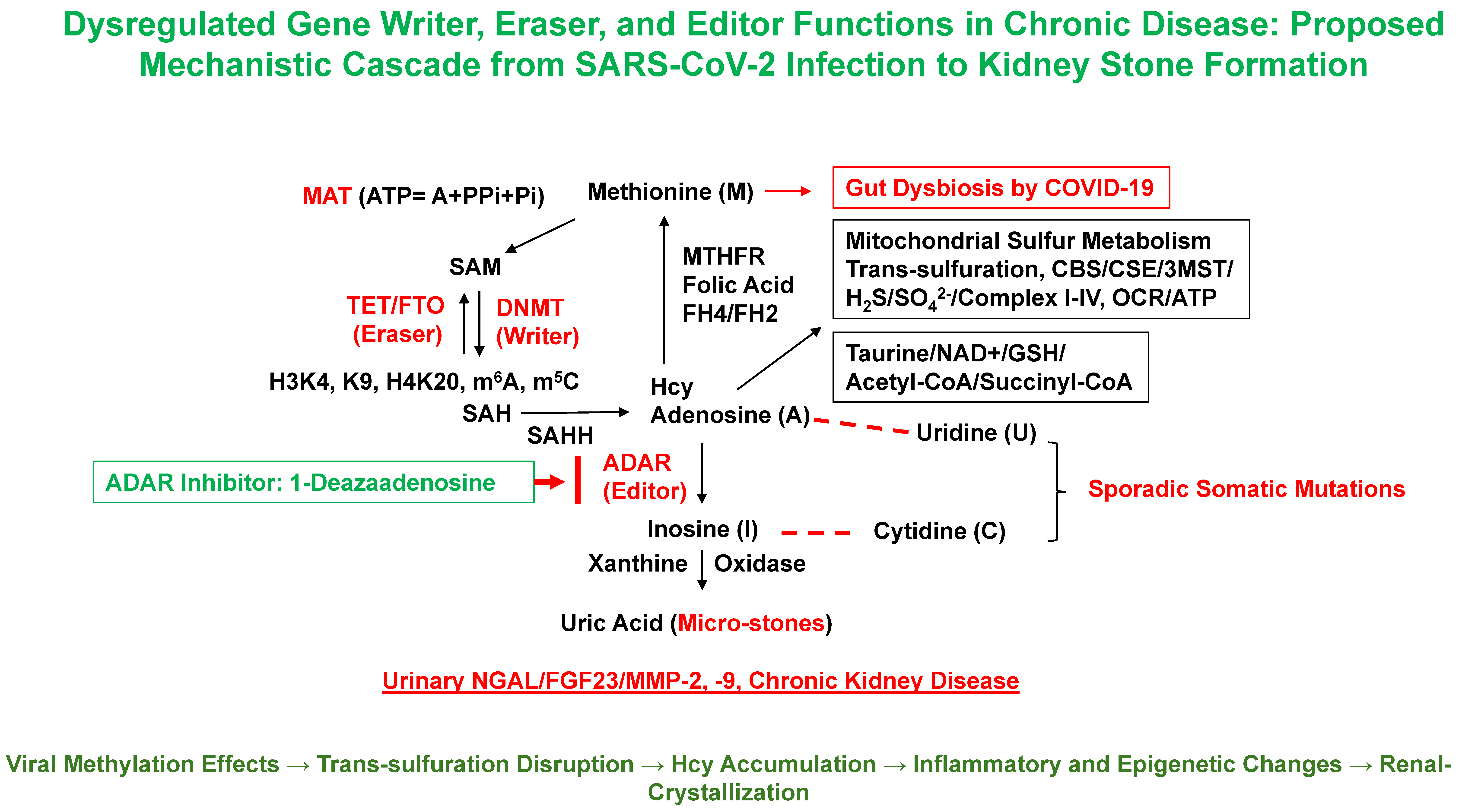
2.1. Epigenetic Methylation Dysregulation and SARS-CoV-2
2.2. Hcy and Sulfur Metabolism: Implications for KSD
2.3. Immune-Mediated Endothelial Injury and Fibrosis
2.4. Dysfunction and miRNA-Mediated Reprogramming
2.5. From Biomarkers to Therapeutics: Neopterin and iNOS
| Biomarker | Source | Relevance |
|---|---|---|
| Homocysteine (Hcy) | Plasma/Urine | Elevated in HHcy; pro-oxidant; stone-promoting |
| NGAL | Urine/Plasma | Early kidney injury marker; linked to COVID-19 |
| BH4 (Tetrahydrobiopterin) | Blood/Urine | Reflects redox imbalance and NOS uncoupling |
| Neopterin | Urine/Serum | Marker of macrophage activation, immune stress |
| NETs | Plasma | Linked to both COVID-19 and renal inflammation |
2.6. Epigenetics, Viral Persistence, and Long-Term Risk of KSD
3. Kidney Stones
4. Underlying Mechanisms Linking COVID-19 Infection to Kidney Stone Formation
4.1. Mitochondrial Sulfur Metabolism and Hcy Accumulation
4.2. Trans-Sulfuration Pathway Disruption and Epigenetic Consequences
4.3. COVID-19-Induced Mitochondrial Dysfunction
4.4. Oxidative and Nitrosative Stress
4.5. Immune Activation, Macrophages, and NETosis
4.6. Tubular Transport Dysfunction and Osmotic Stress
4.7. Role of Gut Microbiome and Uremic Toxins
4.8. Systemic Hypoxia and Dehydration
5. Potential Limitations and Future Directions
5.1. Animal Models
5.2. Epigenetic Analyses
5.3. Clinical Biomarker Studies
5.4. Gut Microbiome Sequencing
5.5. Therapeutic Trials
5.6. Sex-Differentiated Renal Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin de Francisco, Á.; Fernández Fresnedo, G. Long COVID-19 renal disease: A present medical need for nephrology. Nefrologia 2023, 43, 1–5. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M. Long-term interplay between COVID-19 and chronic kidney disease. Int. Urol. Nephrol. 2023, 55, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kissling, S.; Rotman, S.; Gerber, C.; Halfon, M.; Lamoth, F.; Comte, D.; Lhopitallier, L.; Sadallah, S.; Fakhouri, F. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020, 98, 228–231. [Google Scholar] [CrossRef]
- Caceres, P.S.; Savickas, G.; Murray, S.L.; Umanath, K.; Uduman, J.; Yee, J.; Liao, T.D.; Bolin, S.; Levin, A.M.; Khan, M.N.; et al. High SARS-CoV-2 Viral Load in Urine Sediment Correlates with Acute Kidney Injury and Poor COVID-19 Outcome. J. Am. Soc. Nephrol. 2021, 32, 2517–2528. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Singh, M. Multi-organ damage by COVID-19: Congestive (cardio-pulmonary) heart failure, and blood-heart barrier leakage. Mol. Cell Biochem. 2021, 476, 1891–1895. [Google Scholar] [CrossRef]
- Homme, R.P.; George, A.K.; Singh, M.; Smolenkova, I.; Zheng, Y.; Pushpakumar, S.; Tyagi, S.C. Mechanism of Blood-Heart-Barrier Leakage: Implications for COVID-19 Induced Cardiovascular Injury. Int. J. Mol. Sci. 2021, 22, 13546. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Kaynar, M.; Yildiz, M.; Batur, A.F.; Akand, M.; Kilic, O.; Goktas, S. The Increased Risk of Complicated Ureteral Stones in the Era of COVID-19 Pandemic. J. Endourol. 2020, 34, 882–886. [Google Scholar] [CrossRef]
- Spooner, J.; Masoumi-Ravandi, K.; MacNevin, W.; Ilie, G.; Skinner, T.; Powers, A.L. Septic and febrile kidney stone presentations during the COVID-19 pandemic What is the effect of reduced access to care during pandemic restrictions? Can. Urol. Assoc. J. 2024, 18, E19–E25. [Google Scholar]
- Kasiri, H.; Moradimajd, P.; Samaee, H.; Ghazaeian, M. The Increased Risk of Renal Stones in Patients With COVID-19 Infection. Pharm. Biomed. Res. 2022, 8, 333–340. [Google Scholar] [CrossRef]
- Carollo, C.; Benfante, A.; Sorce, A.; Montalbano, K.; Cirafici, E.; Calandra, L.; Geraci, G.; Mulè, G.; Scichilone, N. Predictive Biomarkers of Acute Kidney Injury in COVID-19: Distinct Inflammatory Pathways in Patients with and Without Pre-Existing Chronic Kidney Disease. Life 2025, 15, 720. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Xiao, X.; Zhou, H.; Peng, Z.; Wang, W.; Huang, L.; Xie, Y.; Xu, H.; Tao, L.; et al. Identification of common molecular signatures of SARS-CoV-2 infection and its influence on acute kidney injury and chronic kidney disease. Front. Immunol. 2023, 14, 961642. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Gameiro, J.; Oliveira, J.; Fonseca, J.A.; Duarte, I.; Bernardo, J.; Branco, C.; Costa, C.; Carreiro, C.; Braz, S.; et al. Acute Kidney Disease and Mortality in Acute Kidney Injury Patients with COVID-19. J. Clin. Med. 2021, 10, 4599. [Google Scholar] [CrossRef]
- Kemble, J.P.; Liaw, C.W.; Alamiri, J.M.; Ungerer, G.N.; Potretzke, A.M.; Koo, K. Public Interest in Vitamin C Supplementation During the COVID-19 Pandemic as a Potential Risk for Oxalate Nephrolithiasis. Cureus 2025, 17, e79452. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Benita, S.; Kumari, M.; Ganesan, D.; Paul, E.; Sasikumar, P.; Mahesh, A.; Yuvaraj, S.; Ramprasath, T.; Selvam, G.S. Molecular analysis of oxalate-induced endoplasmic reticulum stress mediated apoptosis in the pathogenesis of kidney stone disease. J. Physiol. Biochem. 2017, 73, 561–573. [Google Scholar] [CrossRef]
- Kaur, M.; Varanasi, R.; Nayak, D.; Tandon, S.; Agrawal, V.; Tandon, C. Molecular insights into cell signaling pathways in kidney stone formation. Urolithiasis 2025, 53, 30. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Zhu, J.; Berisa, M.; Schwörer, S.; Qin, W.; Cross, J.R.; Thompson, C.B. Transsulfuration Activity Can Support Cell Growth upon Extracellular Cysteine Limitation. Cell Metab. 2019, 30, 865–876.e5. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Thomas, M.; Ghorpade, A.; Gendelman, H.E.; Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006, 281, 35785–35793. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef]
- Perła-Kaján, J.; Jakubowski, H. COVID-19 and One-Carbon Metabolism. Int. J. Mol. Sci. 2022, 23, 4181. [Google Scholar] [CrossRef] [PubMed]
- Scammahorn, J.J.; Nguyen, I.T.N.; Bos, E.M.; Van Goor, H.; Joles, J.A. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants 2021, 10, 373. [Google Scholar] [CrossRef]
- Chen, C.J.; Cheng, M.C.; Hsu, C.N.; Tain, Y.L. Sulfur-Containing Amino Acids, Hydrogen Sulfide, and Sulfur Compounds on Kidney Health and Disease. Metabolites 2023, 13, 688. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal 2014, 20, 770–782. [Google Scholar] [CrossRef]
- Tavasoli, S.; Borumandnia, N.; Basiri, A.; Taheri, M. Effects of COVID-19 pandemics on urinary metabolites in kidney stone patients: Our kidney stone prevention clinic experience. Environ. Health Prev. Med. 2021, 26, 112. [Google Scholar] [CrossRef]
- Chen, I.W.; Chang, L.C.; Ho, C.N.; Wu, J.Y.; Tsai, Y.W.; Lin, C.M.; Chang, Y.J.; Hung, K.C. Association between COVID-19 and the development of chronic kidney disease in patients without initial acute kidney injury. Sci. Rep. 2025, 15, 10924. [Google Scholar] [CrossRef]
- Lang, S.M.; Schiffl, H. Long-term renal consequences of COVID-19. Emerging evidence and unanswered questions. Int. Urol. Nephrol. 2025. [Google Scholar] [CrossRef]
- du Preez, H.N.; Lin, J.; Maguire, G.E.M.; Aldous, C.; Kruger, H.G. COVID-19 vaccine adverse events: Evaluating the pathophysiology with an emphasis on sulfur metabolism and endotheliopathy. Eur. J. Clin. Invest. 2024, 54, e14296. [Google Scholar] [CrossRef] [PubMed]
- du Preez, H.N.; Aldous, C.; Hayden, M.R.; Kruger, H.G.; Lin, J. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. FASEB J. 2022, 36, e22052. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Zhang, H.; Sui, L.; Li, L.; Xu, W.; Du, S.; Hao, P.; Jiang, Y.; Chen, J.; et al. The global succinylation of SARS-CoV-2-infected host cells reveals drug targets. Proc. Natl. Acad. Sci. USA 2022, 119, e2123065119. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.T.; Bruce, A.W.; Anbalagan, M.; Srinivasan, H.; Chinnappan, S.; Rajagopal, M.; Khanna, K.; Chandramoorthy, H.C.; Mani, R.R. COVID-19 influenced gut dysbiosis, post-acute sequelae, immune regulation, and therapeutic regimens. Front. Cell Infect. Microbiol. 2024, 14, 1384939. [Google Scholar] [CrossRef]
- An, L.; Wu, W.; Li, S.; Lai, Y.; Chen, D.; He, Z.; Chang, Z.; Xu, P.; Huang, Y.; Lei, M.; et al. Escherichia coli Aggravates Calcium Oxalate Stone Formation via PPK1/Flagellin-Mediated Renal Oxidative Injury and Inflammation. Oxid. Med. Cell Longev. 2021, 2021, 9949697. [Google Scholar] [CrossRef]
- Li, H.; Xue, X.; Meng, G.; He, C.; Tong, L.; Lai, Y. The roles of bacteria on urolithiasis progression and associated compounds. Biochem. Pharmacol. 2025, 237, 116958. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Jeng, T.H.; Lee, M.Y.; Wang, H.C.; Tsai, K.F.; Chou, C.K. Viral mitochondriopathy in COVID-19. Redox Biol. 2025, 85, 103766. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 2021, 12, 2506. [Google Scholar] [CrossRef]
- Bhat, S.; Rishi, P.; Chadha, V.D. Understanding the epigenetic mechanisms in SARS CoV-2 infection and potential therapeutic approaches. Virus Res. 2022, 318, 198853. [Google Scholar] [CrossRef]
- Rath, S.; Perikala, V.; Jena, A.B.; Dandapat, J. Factors regulating dynamics of angiotensin-converting enzyme-2 (ACE2), the gateway of SARS-CoV-2: Epigenetic modifications and therapeutic interventions by epidrugs. Biomed. Pharmacother. 2021, 143, 112095. [Google Scholar] [CrossRef] [PubMed]
- Beacon, T.H.; Delcuve, G.P.; Davie, J.R. Epigenetic regulation of ACE2, the receptor of the SARS-CoV-2 virus(1). Genome 2021, 64, 386–399. [Google Scholar] [CrossRef]
- Singh, M.; Pushpakumar, S.; Bard, N.; Zheng, Y.; Homme, R.P.; Mokshagundam, S.P.L.; Tyagi, S.C. Simulation of COVID-19 symptoms in a genetically engineered mouse model: Implications for the long haulers. Mol. Cell Biochem. 2023, 478, 103–119. [Google Scholar] [CrossRef]
- Ponti, G.; Roli, L.; Oliva, G.; Manfredini, M.; Trenti, T.; Kaleci, S.; Iannella, R.; Balzano, B.; Coppola, A.; Fiorentino, G.; et al. Homocysteine (Hcy) assessment to predict outcomes of hospitalized Covid-19 patients: A multicenter study on 313 Covid-19 patients. Clin. Chem. Lab. Med. 2021, 59, e354–e357. [Google Scholar] [CrossRef]
- Eslamifar, Z.; Behzadifard, M.; Zare, E. Investigation of homocysteine, D-dimer and platelet count levels as potential predictors of thrombosis risk in COVID-19 patients. Mol. Cell Biochem. 2025, 480, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Ruini, C.; Tomasi, A. Homocysteine as a potential predictor of cardiovascular risk in patients with COVID-19. Med. Hypotheses 2020, 143, 109859. [Google Scholar] [CrossRef]
- Martins, M.C.; Meyers, A.A.; Whalley, N.A.; Rodgers, A.L. Cystine: A promoter of the growth and aggregation of calcium oxalate crystals in normal undiluted human urine. J. Urol. 2002, 167, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, B.; Lu, H.; Lai, Y.; Liu, J.; Luo, J.; Zhu, F.; Hu, Z.; Zhou, M.; Tian, J.; et al. Hyperhomocysteinemia Accelerates Acute Kidney Injury to Chronic Kidney Disease Progression by Downregulating Heme Oxygenase-1 Expression. Antioxid. Redox Signal 2019, 30, 1635–1650. [Google Scholar] [CrossRef]
- Khezri, M.R.; Ghasemnejad-Berenji, M. Neurological effects of elevated levels of angiotensin II in COVID-19 patients. Hum. Cell 2021, 34, 1941–1942. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gheblawi, M.; Nikhanj, A.; Munan, M.; MacIntyre, E.; O’Neil, C.; Poglitsch, M.; Colombo, D.; Del Nonno, F.; Kassiri, Z.; et al. Dysregulation of ACE (Angiotensin-Converting Enzyme)-2 and Renin-Angiotensin Peptides in SARS-CoV-2 Mediated Mortality and End-Organ Injuries. Hypertension 2022, 79, 365–378. [Google Scholar] [CrossRef]
- Caputo, I.; Caroccia, B.; Frasson, I.; Poggio, E.; Zamberlan, S.; Morpurgo, M.; Seccia, T.M.; Calì, T.; Brini, M.; Richter, S.N.; et al. Angiotensin II Promotes SARS-CoV-2 Infection via Upregulation of ACE2 in Human Bronchial Cells. Int. J. Mol. Sci. 2022, 23, 5125. [Google Scholar] [CrossRef]
- Lip, S.; Tran, T.Q.B.; Hanna, R.; Nichol, S.; Guzik, T.J.; Delles, C.; McClure, J.; McCallum, L.; Touyz, R.M.; Berry, C.; et al. Long-term effects of SARS-CoV-2 infection on blood vessels and blood pressure—LOCHINVAR. J. Hypertens. 2025, 43, 1057–1065. [Google Scholar] [CrossRef]
- Ravichandran, B.; Grimm, D.; Krüger, M.; Kopp, S.; Infanger, M.; Wehland, M. SARS-CoV-2 and hypertension. Physiol. Rep. 2021, 9, e14800. [Google Scholar] [CrossRef]
- Singh, M.; Pushpakumar, S.; Zheng, Y.; Smolenkova, I.; Akinterinwa, O.E.; Luulay, B.; Tyagi, S.C. Novel mechanism of the COVID-19 associated coagulopathy (CAC) and vascular thromboembolism. Npj viruses 2023, 1, 3. [Google Scholar] [CrossRef]
- Belen Apak, F.B.; Yuce, G.; Topcu, D.I.; Gultekingil, A.; Felek, Y.E.; Sencelikel, T. Coagulopathy is Initiated with Endothelial Dysfunction and Disrupted Fibrinolysis in Patients with COVID-19 Disease. Indian. J. Clin. Biochem. 2023, 38, 220–230. [Google Scholar] [CrossRef]
- Ward, S.E.; Fogarty, H.; Karampini, E.; Lavin, M.; Schneppenheim, S.; Dittmer, R.; Morrin, H.; Glavey, S.; Ni Cheallaigh, C.; Bergin, C.; et al. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J. Thromb. Haemost. 2021, 19, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Henry, B.M.; Lippi, G. Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro)Thrombosis. Semin. Thromb. Hemost. 2021, 47, 400–418. [Google Scholar] [CrossRef]
- Chau, C.W.; To, A.; Au-Yeung, R.K.H.; Tang, K.; Xiang, Y.; Ruan, D.; Zhang, L.; Wong, H.; Zhang, S.; Au, M.T.; et al. SARS-CoV-2 infection activates inflammatory macrophages in vascular immune organoids. Sci. Rep. 2024, 14, 8781. [Google Scholar] [CrossRef] [PubMed]
- Hönzke, K.; Obermayer, B.; Mache, C.; Fatykhova, D.; Kessler, M.; Dökel, S.; Wyler, E.; Baumgardt, M.; Löwa, A.; Hoffmann, K.; et al. Human lungs show limited permissiveness for SARS-CoV-2 due to scarce ACE2 levels but virus-induced expansion of inflammatory macrophages. Eur. Respir. J. 2022, 60, 2102725. [Google Scholar] [CrossRef] [PubMed]
- Pode Shakked, N.; de Oliveira, M.H.S.; Cheruiyot, I.; Benoit, J.L.; Plebani, M.; Lippi, G.; Benoit, S.W.; Henry, B.M. Early prediction of COVID-19-associated acute kidney injury: Are serum NGAL and serum Cystatin C levels better than serum creatinine? Clin. Biochem. 2022, 102, 1–8. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, D.H.; Lee, H.W.; Kim, S.G.; Kim, Y.K.; Kim, J.K. Role of increased neutrophil extracellular trap formation on acute kidney injury in COVID-19 patients. Front. Immunol. 2023, 14, 1122510. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Cheruiyot, I.; Benoit, J.; Rose, J.; Favaloro, E.J.; Lippi, G.; Benoit, S.; Pode Shakked, N. Cell-Free DNA, Neutrophil extracellular traps (NETs), and Endothelial Injury in Coronavirus Disease 2019- (COVID-19-) Associated Acute Kidney Injury. Mediat. Inflamm. 2022, 2022, 9339411. [Google Scholar] [CrossRef]
- Gemmati, D.; Bramanti, B.; Serino, M.L.; Secchiero, P.; Zauli, G.; Tisato, V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int. J. Mol. Sci. 2020, 21, 3474. [Google Scholar] [CrossRef] [PubMed]
- Chanana, N.; Palmo, T.; Sharma, K.; Kumar, R.; Graham, B.B.; Pasha, Q. Sex-derived attributes contributing to SARS-CoV-2 mortality. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E562–E567. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.K.; Thévenod, F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Salomão, R.; Assis, V.; de Sousa Neto, I.V.; Petriz, B.; Babault, N.; Durigan, J.L.Q.; de Cássia Marqueti, R. Involvement of Matrix Metalloproteinases in COVID-19: Molecular Targets, Mechanisms, and Insights for Therapeutic Interventions. Biology 2023, 12, 843. [Google Scholar] [CrossRef]
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.A. SARS-CoV-2 triggering autoimmune diseases. Cytokine 2022, 154, 155873. [Google Scholar] [CrossRef]
- Galipeau, Y.; Cooper, C.; Langlois, M.A. Autoantibodies in COVID-19: Implications for disease severity and clinical outcomes. Front. Immunol. 2024, 15, 1509289. [Google Scholar] [CrossRef]
- Brinkmann, M.; Traby, L.; Kussmann, M.; Weiss-Tessbach, M.; Buchtele, N.; Staudinger, T.; Gaidoschik, E.; Perkmann, T.; Haslacher, H.; Ratzinger, F.; et al. Autoantibody development is associated with clinical severity of COVID-19: A cohort study. Clin. Immunol. 2025, 274, 110471. [Google Scholar] [CrossRef]
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2022, 29, 217–231.e8. [Google Scholar] [CrossRef]
- Reiser, J.; Spear, R.; Luo, S. SARS-CoV-2 pirates the kidneys: A scar(y) story. Cell Metab. 2022, 34, 352–354. [Google Scholar] [CrossRef]
- Larrue, R.; Fellah, S.; Van der Hauwaert, C.; Hennino, M.F.; Perrais, M.; Lionet, A.; Glowacki, F.; Pottier, N.; Cauffiez, C. The Versatile Role of miR-21 in Renal Homeostasis and Diseases. Cells 2022, 11, 3525. [Google Scholar] [CrossRef]
- McDonald, J.T.; Enguita, F.J.; Taylor, D.; Griffin, R.J.; Priebe, W.; Emmett, M.R.; Sajadi, M.M.; Harris, A.D.; Clement, J.; Dybas, J.M.; et al. Role of miR-2392 in driving SARS-CoV-2 infection. Cell Rep. 2021, 37, 109839. [Google Scholar] [CrossRef]
- Valle, A.; Soto, Z.; Muhamadali, H.; Hollywood, K.A.; Xu, Y.; Lloyd, J.R.; Goodacre, R.; Cantero, D.; Cabrera, G.; Bolivar, J. Metabolomics for the design of new metabolic engineering strategies for improving aerobic succinic acid production in Escherichia coli. Metabolomics Off. J. Metabolomic Soc. 2022, 18, 56. [Google Scholar] [CrossRef]
- Tong, W.; Hannou, S.A.; Wang, Y.; Astapova, I.; Sargsyan, A.; Monn, R.; Thiriveedi, V.; Li, D.; McCann, J.R.; Rawls, J.F.; et al. The intestine is a major contributor to circulating succinate in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22546. [Google Scholar] [CrossRef]
- Kalan Sarı, I.; Keskin, O.; Seremet Keskin, A.; Elli Dağ, H.Y.; Harmandar, O. Is Homocysteine Associated with the Prognosis of Covid-19 Pneumonia. Int. J. Clin. Pract. 2023, 2023, 9697871. [Google Scholar] [CrossRef] [PubMed]
- Carpenè, G.; Negrini, D.; Henry, B.M.; Montagnana, M.; Lippi, G. Homocysteine in coronavirus disease (COVID-19): A systematic literature review. Diagnosis 2022, 9, 306–310. [Google Scholar] [CrossRef]
- Lee, S.R.; Roh, J.Y.; Ryu, J.; Shin, H.J.; Hong, E.J. Activation of TCA cycle restrains virus-metabolic hijacking and viral replication in mouse hepatitis virus-infected cells. Cell Biosci. 2022, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H(2)S(2), H(2)S(3) and H(2)S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.; Sathnur, P.B.; Kundu, S.; Givvimani, S.; Coley, D.M.; Mishra, P.K.; Qipshidze, N.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am. J. Physiol. Cell Physiol. 2012, 303, C41–C51. [Google Scholar] [CrossRef]
- Agrawal, R.; Pal, V.K.; KS, S.; Menon, G.J.; Singh, I.R.; Malhotra, N.; CS, N.; Ganesh, K.; Rajmani, R.S.; Narain Seshasayee, A.S.; et al. Hydrogen sulfide (H2S) coordinates redox balance, carbon metabolism, and mitochondrial bioenergetics to suppress SARS-CoV-2 infection. PLoS Pathog. 2025, 21, e1013164. [Google Scholar] [CrossRef]
- Han, S.J.; Noh, M.R.; Jung, J.M.; Ishii, I.; Yoo, J.; Kim, J.I.; Park, K.M. Hydrogen sulfide-producing cystathionine γ-lyase is critical in the progression of kidney fibrosis. Free Radic. Biol. Med. 2017, 112, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Jang, H.S.; Kim, J.I.; Han, S.J.; Park, J.W.; Park, K.M. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim. Biophys. Acta 2013, 1832, 1989–1997. [Google Scholar] [CrossRef]
- Fabbrizi, E.; Fiorentino, F.; Carafa, V.; Altucci, L.; Mai, A.; Rotili, D. Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection. Cells 2023, 12, 852. [Google Scholar] [CrossRef]
- Sanchez-Russo, L.; Billah, M.; Chancay, J.; Hindi, J.; Cravedi, P. COVID-19 and the Kidney: A Worrisome Scenario of Acute and Chronic Consequences. J. Clin. Med. 2021, 10, 900. [Google Scholar] [CrossRef]
- Madsen, H.B.; Durhuus, J.A.; Andersen, O.; Straten, P.T.; Rahbech, A.; Desler, C. Mitochondrial dysfunction in acute and post-acute phases of COVID-19 and risk of non-communicable diseases. NPJ Metab. Health Dis. 2024, 2, 36. [Google Scholar] [CrossRef]
- Fong, P.; Wusirika, R.; Rueda, J.; Raphael, K.L.; Rehman, S.; Stack, M.; de Mattos, A.; Gupta, R.; Michels, K.; Khoury, F.G.; et al. Increased Rates of Supplement-Associated Oxalate Nephropathy During COVID-19 Pandemic. Kidney Int. Rep. 2022, 7, 2608–2616. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Yantiss, R.K. The pathogenesis of coronavirus-19 disease. J. Biomed. Sci. 2022, 29, 87. [Google Scholar] [CrossRef]
- Karam, A.; Mjaess, G.; Younes, H.; Aoun, F. Increase in urolithiasis prevalence due to vitamins C and D supplementation during the COVID-19 pandemic. J. Public. Health 2022, 44, e625–e626. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Herbrechter, R.; Schlabs, C.; Pethe, A.; Lee, W.K.; Wolff, N.A.; Roussa, E. Role of the SLC22A17/lipocalin-2 receptor in renal endocytosis of proteins/metalloproteins: A focus on iron- and cadmium-binding proteins. Am. J. Physiol. Ren. Physiol. 2023, 325, F564–F577. [Google Scholar] [CrossRef] [PubMed]
- Engström, J.; Koozi, H.; Didriksson, I.; Larsson, A.; Friberg, H.; Frigyesi, A.; Spångfors, M. Plasma neutrophil gelatinase-associated lipocalin independently predicts dialysis need and mortality in critical COVID-19. Sci. Rep. 2024, 14, 6695. [Google Scholar] [CrossRef]
- Kaufman, D.W.; Kelly, J.P.; Curhan, G.C.; Anderson, T.E.; Dretler, S.P.; Preminger, G.M.; Cave, D.R. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol. 2008, 19, 1197–1203. [Google Scholar] [CrossRef]
- Troxel, S.A.; Sidhu, H.; Kaul, P.; Low, R.K. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J. Endourol. 2003, 17, 173–176. [Google Scholar] [CrossRef]
- Mehta, M.; Goldfarb, D.S.; Nazzal, L. The role of the microbiome in kidney stone formation. Int. J. Surg. 2016, 36, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Su, J.; Liu, S.; Li, Y.; Xu, G. Causal effects of gut microbiota on the risk of urinary tract stones: A bidirectional two-sample mendelian randomization study. Heliyon 2024, 10, e25704. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Corbella, X.; Rello, J. Management of hypoxemia in SARS-CoV-2 infection: Lessons learned from one year of experience, with a special focus on silent hypoxemia. J. Intensive Med. 2021, 1, 26–30. [Google Scholar] [CrossRef]
- George, C.E.; Scheuch, G.; Seifart, U.; Inbaraj, L.R.; Chandrasingh, S.; Nair, I.K.; Hickey, A.J.; Barer, M.R.; Fletcher, E.; Field, R.D.; et al. COVID-19 symptoms are reduced by targeted hydration of the nose, larynx and trachea. Sci. Rep. 2022, 12, 4599. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Kemp, J.A.; Cardozo, L.; Borges, N.A.; Nerbass, F.B.; Alvarenga, L.; Kalantar-Zadeh, K. COVID-19 and Nutrition: Focus on Chronic Kidney Disease. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2023, 33, S118–S127. [Google Scholar] [CrossRef]
- Vuorio, A.; Raal, F.; Kovanen, P.T. Familial hypercholesterolemia: The nexus of endothelial dysfunction and lipoprotein metabolism in COVID-19. Curr. Opin. Lipidol. 2023, 34, 119–125. [Google Scholar] [CrossRef]
- Camp, T.M.; Smiley, L.M.; Hayden, M.R.; Tyagi, S.C. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. J. Hypertens. 2003, 21, 1719–1727. [Google Scholar] [CrossRef]
- Grover, P.K.; Kim, D.S.; Ryall, R.L. The effect of seed crystals of hydroxyapatite and brushite on the crystallization of calcium oxalate in undiluted human urine in vitro: Implications for urinary stone pathogenesis. Mol. Med. 2002, 8, 200–209. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, S.; Guo, J.; Xu, N.; Wang, Q.; Zhang, G.; Zhao, L.; Zhou, Q.; Fu, X.; Li, L.; et al. ADAMTS13 protects mice against renal ischemia-reperfusion injury by reducing inflammation and improving endothelial function. Am. J. Physiol. Ren. Physiol. 2019, 316, F134–F145. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alzahrani, K.J.; Cruz-Martins, N.; Batiha, G.E. The potential role of neopterin in COVID-19: A new perspective. Mol. Cell. Biochem. 2021, 476, 4161–4166. [Google Scholar] [CrossRef]
- Anderson, S.; McNicholas, D.; Murphy, C.; Cheema, I.; McLornan, L.; Davis, N.; Quinlan, M. The impact of COVID-19 on acute urinary stone presentations: A single-centre experience. Ir. J. Med. Sci. 2022, 191, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Üntan, İ. How did COVID-19 affect acute urolithiasis? An inner Anatolian experience. Ulus. Travma Acil Cerrahi Derg. 2023, 29, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Turney, B.W.; Demaire, C.; Klöcker, S.; Woodward, E.; Sommerfeld, H.J.; Traxer, O. An analysis of stone management over the decade before the COVID-19 pandemic in Germany, France and England. BJU Int. 2023, 132, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, N.; Nantha Kumar, D.; Joshi, H. The Impact of Early COVID-19 Pandemic on the Presentation and Management of Urinary Calculi Across the Globe: A Systematic Review. J. Endourol. 2022, 36, 1255–1264. [Google Scholar] [CrossRef]
- The, L. Long COVID: 3 years in. Lancet 2023, 401, 795. [Google Scholar] [CrossRef]
- Makhluf, H.; Madany, H.; Kim, K. Long COVID: Long-Term Impact of SARS-CoV2. Diagnostics 2024, 14, 711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babbarwal, A.; Singh, M.; Sen, U.; Tyagi, M.; Tyagi, S.C. Kidney Stone Disease: Epigenetic Dysregulation in Homocystinuria and Mitochondrial Sulfur Trans-Sulfuration Ablation Driven by COVID-19 Pathophysiology. Biomolecules 2025, 15, 1163. https://doi.org/10.3390/biom15081163
Babbarwal A, Singh M, Sen U, Tyagi M, Tyagi SC. Kidney Stone Disease: Epigenetic Dysregulation in Homocystinuria and Mitochondrial Sulfur Trans-Sulfuration Ablation Driven by COVID-19 Pathophysiology. Biomolecules. 2025; 15(8):1163. https://doi.org/10.3390/biom15081163
Chicago/Turabian StyleBabbarwal, Anmol, Mahavir Singh, Utpal Sen, Mahima Tyagi, and Suresh C. Tyagi. 2025. "Kidney Stone Disease: Epigenetic Dysregulation in Homocystinuria and Mitochondrial Sulfur Trans-Sulfuration Ablation Driven by COVID-19 Pathophysiology" Biomolecules 15, no. 8: 1163. https://doi.org/10.3390/biom15081163
APA StyleBabbarwal, A., Singh, M., Sen, U., Tyagi, M., & Tyagi, S. C. (2025). Kidney Stone Disease: Epigenetic Dysregulation in Homocystinuria and Mitochondrial Sulfur Trans-Sulfuration Ablation Driven by COVID-19 Pathophysiology. Biomolecules, 15(8), 1163. https://doi.org/10.3390/biom15081163








