CSF-Exosomal miRNAs and Delayed Cerebral Ischemia: Insights into Pathophysiology but No Definitive Biomarkers
Abstract
1. Introduction
2. Methods
2.1. Patient Enrollment
2.2. Sample Details
2.3. Exosomal RNA Isolation and Quantification
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Samples
3.2. Differential miRNA Expression Pattern Between Control and aSAH
3.3. Differential miRNA Expression Pattern for DCI vs. No-DCI Groups
3.4. Temporal Changes in CSF Exosome miRNA Expression in Cohorts with and Without DCI
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Souza, S. Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. Anesthesiol. 2015, 27, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, J.; Kerr, R.S.; Rinkel, G.J.E. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Sabri, M.; Lass, E.; Macdonald, R.L. Early brain injury: A common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res. Treat. 2013, 2013, 394036. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Kolls, B.J. Neuroprotection in subarachnoid hemorrhage. Stroke 2010, 41 (Suppl. S10), S79–S84. [Google Scholar] [CrossRef]
- Østergaard, L.; Aamand, R.; Karabegovic, S.; Tietze, A.; Blicher, J.U.; Mikkelsen, I.K.; Iversen, N.K.; Secher, N.; Engedal, T.S.; Anzabi, M.; et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2013, 33, 1825. [Google Scholar] [CrossRef]
- Abdulazim, A.; Heilig, M.; Rinkel, G.; Etminan, N. Diagnosis of Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage and Triggers for Intervention. Neurocritical Care 2023, 39, 311. [Google Scholar] [CrossRef]
- Etminan, N.; DI Vergouwen, M.; Ilodigwe, D.; Macdonald, R.L. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2011, 31, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, J.R.; Testai, F.D. Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Beyond Vasospasm and Towards a Multifactorial Pathophysiology. Curr. Atheroscler. Rep. 2017, 19, 50. [Google Scholar] [CrossRef]
- Megjhani, M.; Terilli, K.; Weiss, M.; Savarraj, J.; Chen, L.H.; Alkhachroum, A.; Roh, D.J.; Agarwal, S.; Connolly, E.S.; Velazquez, A.; et al. Dynamic Detection of Delayed Cerebral Ischemia: A Study in 3 Centers. Stroke 2021, 52, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-H.; Burkett, A.; Paz, A.; Savarraj, J.P.; Hinds, S.; Hergenroeder, G.; Gusdon, A.M.; Ren, X.; Hong, J.-H.; Choi, H.A. Systemic inflammatory markers of persistent cerebral edema after aneurysmal subarachnoid hemorrhage. J. Neuroinflammation 2022, 19, 199. [Google Scholar] [CrossRef]
- Chou, S.H.-Y.; Feske, S.K.; Atherton, J.; Konigsberg, R.G.; De Jager, P.L.; Du, R.; Ogilvy, C.S.; Lo, E.H.; Ning, M. Early Elevation of Serum Tumor Necrosis Factor-α is Associated with Poor Outcome in Subarachnoid Hemorrhage. J. Investig. Med. 2012, 60, 1054–1058. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakamura, S.; Endo, S.; Kuwayama, N.; Naruse, Y.; Takaku, A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem. Res. 1997, 22, 1249–1255. [Google Scholar] [CrossRef]
- Perez, P.; Lukaszewicz, A.-C.; Lenck, S.; Nizard, R.; Drouet, L.; Payen, D. Platelet activation and aggregation after aneurysmal subarachnoid hemorrhage. BMC Neurol. 2018, 18, 57. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Attanasio, L.; Creteur, J.; Grimaldi, D.; Schuind, S.; Taccone, F.S. The Impact of Extracerebral Infection After Subarachnoid Hemorrhage: A Single-Center Cohort Study. World Neurosurg. 2020, 144, e883–e897. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.; Schlenk, F.; Meisel, A.; Dreier, J.; Vajkoczy, P.; Meisel, C. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke 2011, 42, 53–58. [Google Scholar] [CrossRef]
- Burzyńska, M.; Uryga, A.; Woźniak, J.; Załuski, R.; Robba, C.; Goździk, W. The Role of Early Serum Biomarkers and Clinical Rating Scales in the Prediction of Delayed Cerebral Ischaemia and Short-Term Outcome after Aneurysmal Subarachnoid Haemorrhage: Single Centre Experience. J. Clin. Med. 2023, 12, 5614. [Google Scholar] [CrossRef]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS ONE 2014, 9, e94839. [Google Scholar] [CrossRef] [PubMed]
- Di Ieva, A.; Butz, H.; Niamah, M.; Rotondo, F.; De Rosa, S.; Sav, A.; Yousef, G.M.; Kovacs, K.; Cusimano, M.D. MicroRNAs as biomarkers in pituitary tumors. Neurosurgery 2014, 75, 181–189; discussion 188–189. [Google Scholar] [CrossRef] [PubMed]
- Sheinerman, K.S.; Umansky, S.R. Circulating cell-free microRNA as biomarkers for screening, diagnosis and monitoring of neurodegenerative diseases and other neurologic pathologies. Front. Cell. Neurosci. 2013, 7, 150. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Niu, W.; Guo, W.; Song, H.; Li, H.; Fan, H.; Zhao, L.; Zhong, A.; Dai, Y.; et al. A preliminary analysis of microRNA as potential clinical biomarker for schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2015, 168, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-J.; Tang, Z.-Y.; Tu, K.; Zhu, L.; Li, Y.-X.; Xie, L.; Xiao, H.-S. Identification and target prediction of miRNAs specifically expressed in rat neural tissue. BMC Genom. 2009, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Maki, M.; Ding, R.; Yang, Y.; Zhang, B.; Xiong, L. Genome-wide survey of tissue-specific microRNA and transcription factor regulatory networks in 12 tissues. Sci. Rep. 2014, 4, 5150. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Lee, E.J.; Baek, M.; Gusev, Y.; Brackett, D.J.; Nuovo, G.J.; Schmittgen, T.D. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA 2008, 14, 35–42. [Google Scholar] [CrossRef]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of microRNA expression profiles in normal human tissues. BMC Genom. 2007, 8, 166. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Bicker, S.; Lackinger, M.; Weiß, K.; Schratt, G. MicroRNA-132, -134, and -138: A microRNA troika rules in neuronal dendrites. Cell. Mol. Life Sci. CMLS 2014, 71, 3987–4005. [Google Scholar] [CrossRef]
- Cougot, N.; Bhattacharyya, S.N.; Tapia-Arancibia, L.; Bordonné, R.; Filipowicz, W.; Bertrand, E.; Rage, F. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 13793–13804. [Google Scholar] [CrossRef]
- Kye, M.-J.; Liu, T.; Levy, S.F.; Xu, N.L.; Groves, B.B.; Bonneau, R.; Lao, K.; Kosik, K.S. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. RNA 2007, 13, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.; Torvik, V.I.; Larson, J.; Smalheiser, N.R. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J. Neurochem. 2008, 106, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Schratt, G. microRNAs at the synapse. Nat. Rev. Neurosci. 2009, 10, 842–849. [Google Scholar] [CrossRef]
- Schratt, G.M.; Tuebing, F.; Nigh, E.A.; Kane, C.G.; Sabatini, M.E.; Kiebler, M.; Greenberg, M.E. A brain-specific microRNA regulates dendritic spine development. Nature 2006, 439, 283–289. [Google Scholar] [CrossRef]
- Smalheiser, N.R. The RNA-centred view of the synapse: Non-coding RNAs and synaptic plasticity. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2014, 369, 20130504. [Google Scholar] [CrossRef]
- Nail, H.M.; Chiu, C.-C.; Leung, C.-H.; Ahmed, M.M.M.; Wang, H.-M.D. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J. Biomed. Sci. 2023, 30, 69. [Google Scholar] [CrossRef]
- López-Pérez, Ó.; Sanz-Rubio, D.; Hernaiz, A.; Betancor, M.; Otero, A.; Castilla, J.; Andréoletti, O.; Badiola, J.J.; Zaragoza, P.; Bolea, R.; et al. Cerebrospinal Fluid and Plasma Small Extracellular Vesicles and miRNAs as Biomarkers for Prion Diseases. Int. J. Mol. Sci. 2021, 22, 6822. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Ogura, T.; Nakajima, H.; Ikeda, T.; Takeda, R.; Neki, H.; Kohyama, S.; Yamane, F.; Kurogi, R.; Amano, T.; et al. Altered Expression of MicroRNA-15a and Kruppel-Like Factor 4 in Cerebrospinal Fluid and Plasma After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2017, 108, 909–916.e3. [Google Scholar] [CrossRef]
- Pedrosa, L.; Hoyos, J.; Reyes, L.; Llull, L.; Santana, D.; de Riva, N.; Mellado, R.; Sala, X.; Rodríguez-Hernández, A.; Enseñat, J.; et al. MicroRNA cerebrospinal fluid profile during the early brain injury period as a biomarker in subarachnoid hemorrhage patients. Front. Cell. Neurosci. 2022, 16, 1016814. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Springer, J.E.; Xie, K.; Fardo, D.W.; Hatton, K.W. A Highly Predictive MicroRNA Panel for Determining Delayed Cerebral Vasospasm Risk Following Aneurysmal Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021, 8, 657258. [Google Scholar] [CrossRef]
- Simon, R.; Lam, A.; Li, C.; Ngan, M.; Menenzes, S.; Zhao, Y. Analysis of Gene Expression Data Using BRB-Array Tools. Cancer Inform. 2007, 3, 117693510700300022. [Google Scholar] [CrossRef]
- Gareev, I.; Beylerli, O.; Yang, G.; Izmailov, A.; Shi, H.; Sun, J.; Zhao, B.; Liu, B.; Zhao, S. Diagnostic and prognostic potential of circulating miRNAs for intracranial aneurysms. Neurosurg. Rev. 2021, 44, 2025–2039. [Google Scholar] [CrossRef]
- Liao, L.; Wang, H.; Wei, D.; Yi, M.; Gu, Y.; Zhang, M.; Wang, L. Exosomal microRNAs: Implications in the pathogenesis and clinical applications of subarachnoid hemorrhage. Front. Mol. Neurosci. 2023, 16, 1300864. [Google Scholar] [CrossRef]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- Saha, S. Role of microRNA in Oxidative Stress. Stresses 2024, 4, 269–281. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Zhu, X.; Li, X.; Li, Y.; Li, J. Targeting microRNAs to Regulate the Integrity of the Blood-Brain Barrier. Front. Bioeng. Biotechnol. 2021, 9, 673415. [Google Scholar] [CrossRef]
- Kiel, K.; Król, S.K.; Bronisz, A.; Godlewski, J. MiR-128-3p—A gray eminence of the human central nervous system. Mol. Ther. Nucleic Acids 2024, 35, 102141. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, F.; Fang, B.; Zhang, Z.; Dong, Y.; Tong, X.; Ma, H. MiR-128-3p Alleviates Spinal Cord Ischemia/Reperfusion Injury Associated Neuroinflammation and Cellular Apoptosis via SP1 Suppression in Rat. Front. Neurosci. 2020, 14, 609613. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, B.; Tang, Y.; Zhou, X.; Ji, Z.; Xu, F. Baicalein ameliorates oxidative stress and brain injury after intracerebral hemorrhage by activating the Nrf2/ARE pathway via miR-106a-5p/PHLPP2 axis. Int. J. Neurosci. 2023, 133, 1380–1393. [Google Scholar] [CrossRef]
- Hou, J.; Deng, Q.; Deng, X.; Zhong, W.; Liu, S.; Zhong, Z. MicroRNA-146a-5p alleviates lipopolysaccharide-induced NLRP3 inflammasome injury and pro-inflammatory cytokine production via the regulation of TRAF6 and IRAK1 in human umbilical vein endothelial cells (HUVECs). Ann. Transl. Med. 2021, 9, 1433. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.E.; Kim, S.W.; Jeong, S.; Moon, H.; Choi, W.S.; Lim, S.; Lee, S.; Hwang, K.-C.; Choi, J.-W. MicroRNA-26a/b-5p promotes myocardial infarction-induced cell death by downregulating cytochrome c oxidase 5a. Exp. Mol. Med. 2021, 53, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, C.A.; Fernández, A.; Ramírez, J.P.; Corvalán, K.; Carrasco, M.Á.; Iturriaga, A.; Bátiz, L.F.; Luarte, A.; Wyneken, U. A Role for mir-26a in Stress: A Potential sEV Biomarker and Modulator of Excitatory Neurotransmission. Cells 2020, 9, 1364. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Ebrahimi, V.; Rastegar-Moghaddam, S.H.; Mohammadipour, A. Therapeutic Potentials of MicroRNA-126 in Cerebral Ischemia. Mol. Neurobiol. 2023, 60, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.; Pérez-Serra, A.; Mangas, A.; Campuzano, O.; Sarquella-Brugada, G.; Quezada-Feijoo, M.; Ramos, M.; Alcalá, M.; Carrera, E.; García-Padilla, C.; et al. miR-16-5p Suppression Protects Human Cardiomyocytes against Endoplasmic Reticulum and Oxidative Stress-Induced Injury. Int. J. Mol. Sci. 2022, 23, 1036. [Google Scholar] [CrossRef] [PubMed]
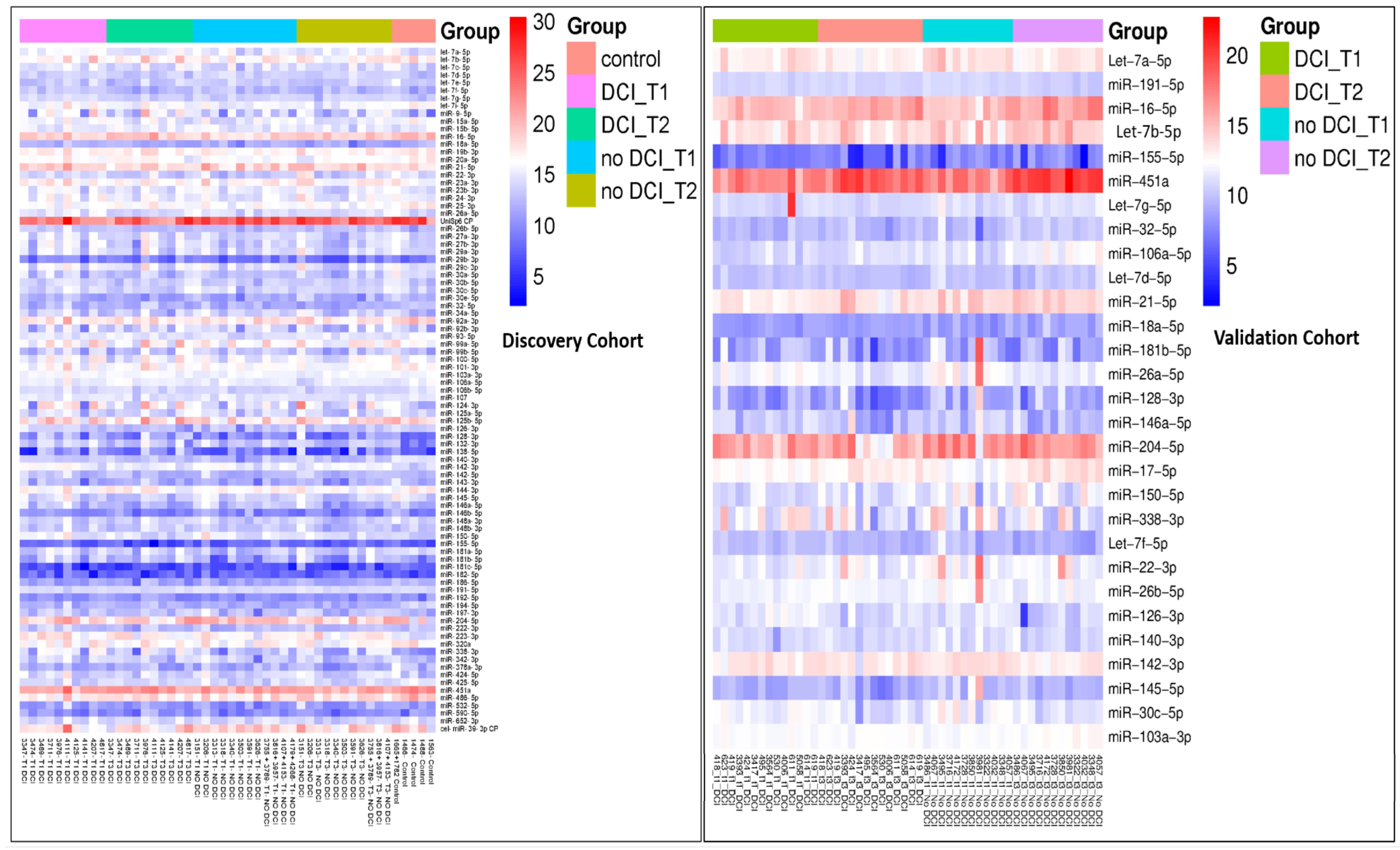


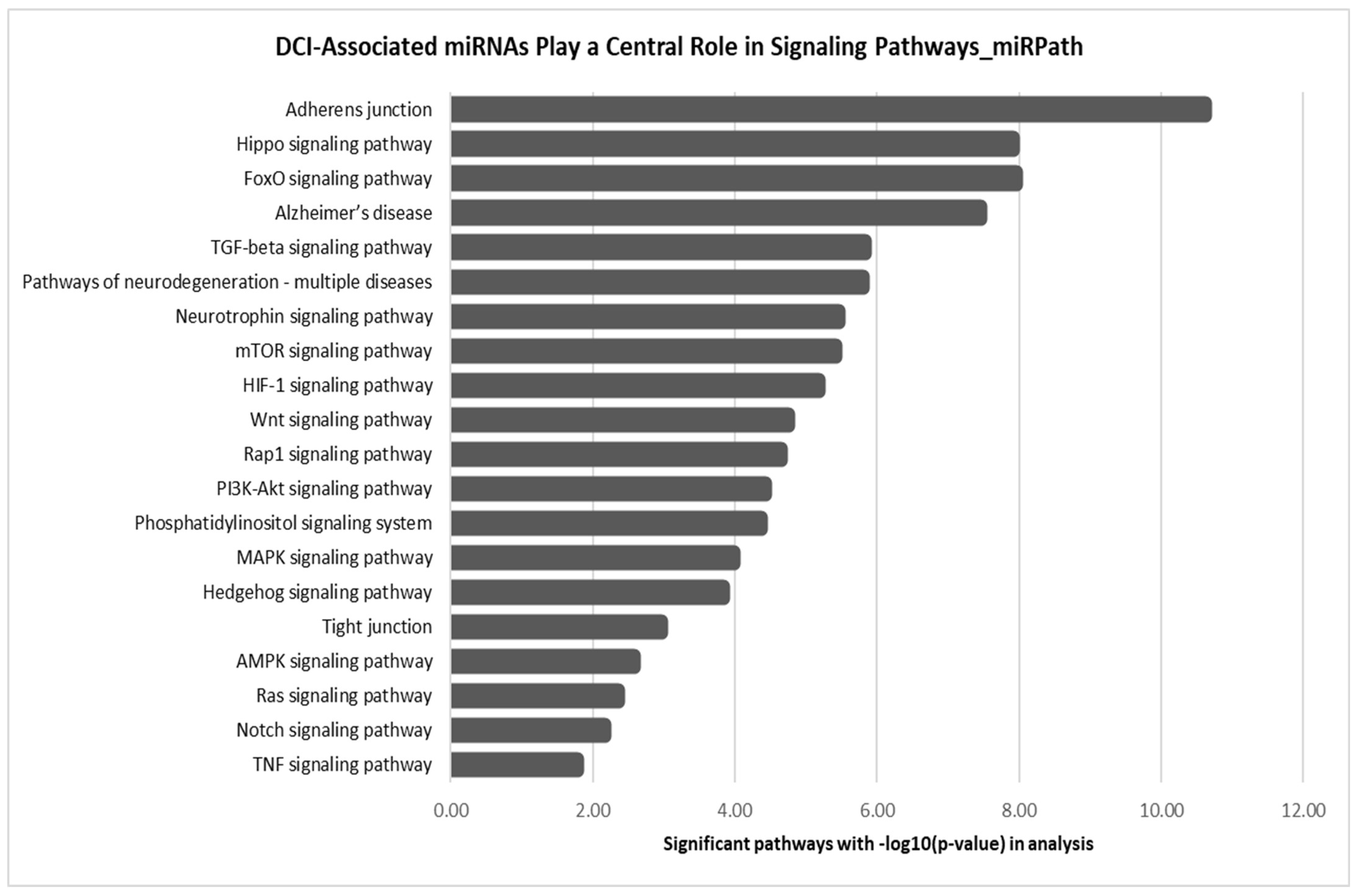
| Demographics | Discovery Cohort SAH: DCI vs. No DCI | Validation Cohort SAH: DCI vs. No DCI | Discovery SAH vs. Validation SAH | Discovery SAH_No DCI vs. Validation SAH_No DCI | Discovery SAH_DCI vs. Validation SAH_DCI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No DCI (N = 16) | DCI (N = 10) | p-Value | No DCI (N = 12) | DCI (N = 14) | p-Value | Discovery (N = 26) | Validation (N = 26) | p-Value | Discovery (N = 16) | Validation (N = 12) | p-Value | Discovery (N = 10) | Validation (N = 14) | p-Value | |
| Age | 0.786 | 0.229 | 0.237 | 0.584 | |||||||||||
| Mean (SD) | 51.6 | 50.6 | 58.3 | 53.3 | 0.433 | 51.2 | 55.6 | 51.6 | 58.3 | 50.6 | 53.3 | ||||
| Gender | 0.692 | 0.170 | 1.000 | ||||||||||||
| Male | 5 | 4 | 1 | 5 | 9 | 6 | 0.541 | 5 | 1 | 0.196 | 4 | 5 | |||
| Female | 11 | 6 | 11 | 9 | 17 | 20 | 11 | 11 | 6 | 9 | |||||
| Race | 0.538 | 0.836 | 0.276 | 0.568 | 0.948 | ||||||||||
| American Indian or Alaska native | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Black or African-American | 1 | 2 | 3 | 4 | 3 | 7 | 1 | 3 | 2 | 4 | |||||
| White | 15 | 8 | 8 | 9 | 23 | 17 | 15 | 8 | 8 | 9 | |||||
| Asian | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | |||||
| Hypertension (HTN) | 0.109 | 0.665 | 0.382 | 0.276 | 1.000 | ||||||||||
| No | 9 | 2 | 4 | 3 | 11 | 7 | 9 | 4 | 2 | 3 | |||||
| Yes | 7 | 8 | 8 | 11 | 15 | 19 | 7 | 8 | 8 | 11 | |||||
| Hyperlipidemia (HLD) | 1.000 | 0.090 | 0.044 | 1.000 | |||||||||||
| No | 14 | 9 | 6 | 12 | 23 | 18 | 0.173 | 14 | 6 | 9 | 12 | ||||
| Yes | 2 | 1 | 6 | 2 | 3 | 8 | 2 | 6 | 1 | 2 | |||||
| Diabetes Mellitus (DM) | 1.000 | 0.598 | 0.668 | 1.000 | 0.615 | ||||||||||
| No | 15 | 9 | 11 | 11 | 24 | 22 | 15 | 11 | 9 | 11 | |||||
| Yes | 1 | 1 | 1 | 3 | 2 | 4 | 1 | 1 | 1 | 3 | |||||
| Tobacco Use | 0.425 | 1.000 | 0.095 | 0.125 | 0.678 | ||||||||||
| No | 5 | 5 | 8 | 9 | 10 | 17 | 5 | 8 | 5 | 9 | |||||
| Yes | 11 | 5 | 4 | 5 | 16 | 9 | 11 | 4 | 5 | 5 | |||||
| Alcohol Use | 1.000 | 1.000 | 0.165 | 0.445 | 0.408 | ||||||||||
| No | 6 | 4 | 7 | 9 | 10 | 16 | 6 | 7 | 4 | 9 | |||||
| Yes | 10 | 6 | 5 | 5 | 16 | 10 | 10 | 5 | 6 | 5 | |||||
| Aspirin | 1.000 | 1.000 | 0.116 | 0.231 | 0.341 | ||||||||||
| No | 13 | 9 | 7 | 5 | 22 | 16 | 13 | 7 | 9 | 9 | |||||
| Yes | 3 | 1 | 9 | 5 | 4 | 10 | 3 | 5 | 1 | 5 | |||||
| Plavix | 1.000 | 0.462 | 1.000 | 0.429 | 1.000 | ||||||||||
| No | 16 | 10 | 11 | 14 | 26 | 25 | 16 | 11 | 10 | 14 | |||||
| Yes | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |||||
| GCS (3–15) | 0.141 | 0.038 | 0.023 | 0.428 | 0.053 | ||||||||||
| Mean (SD) | 13.1 | 11.5 | 12 | 8.6 | 12.5 | 10.2 | 13.1 | 12 | 11.5 | 8.6 | |||||
| Hunt Hess on arrival (1–5) | 0.440 | 0.516 | 0.248 | 0.516 | 0.459 | ||||||||||
| Mean (SD) | 2.8 | 3 | 3 | 3.2 | 2.9 | 3.1 | 2.8 | 3 | 3 | 3.2 | |||||
| Fischer Scale | 0.385 | 0.462 | 0.186 | 0.429 | 0.417 | ||||||||||
| 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |||||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 3 | 16 | 9 | 11 | 14 | 25 | 25 | 16 | 11 | 9 | 14 | |||||
| 4 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |||||
| Discovery Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|
| DCI vs. No-DCI | p-Value | FC | DCI vs. No-DCI | p-Value | FC |
| hsa-miR-194-5p | 0.045 | 1.50 | hsa-miR-21-5p | 0.001 | 0.59 |
| hsa-let-7i-5p | 0.051 | 1.50 | hsa-miR-106a-5p | 0.001 | 0.60 |
| hsa-miR-128-3p | 0.066 | 2.70 | hsa-miR-204-5p | 0.007 | 0.41 |
| hsa-miR-338-3p | 0.078 | 2.69 | hsa-miR-145-5p | 0.016 | 0.54 |
| hsa-miR-20a-5p | 0.079 | 1.35 | hsa-miR-22-3p | 0.028 | 0.54 |
| hsa-miR-138-5p | 0.084 | 3.14 | hsa-miR-128-3p | 0.030 | 0.48 |
| hsa-miR-18a-5p | 0.088 | 1.49 | hsa-miR-150-5p | 0.032 | 0.60 |
| hsa-miR-106b-5p | 0.098 | 1.28 | has-let7b-5p | 0.033 | 0.64 |
| hsa-miR-181b-5p | 0.098 | 2.38 | has-let7a-5p | 0.040 | 0.76 |
| hsa-miR-106a-5p | 0.100 | 1.30 | hsa-miR-451a | 0.064 | 0.56 |
| hsa-let-7d-5p | 0.103 | 0.78 | hsa-miR-126-3p | 0.073 | 1.53 |
| hsa-miR-9-5p | 0.106 | 2.60 | hsa-miR-26a-5p | 0.098 | 0.69 |
| hsa-miR-140-3p | 0.113 | 1.48 | hsa-miR-26b-5p | 0.118 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafeeque, C.M.; McBride, D.W.; Yan, Y.; Zeineddine, H.A.; Hagen, J.P.; Choi, H.A.; Savarraj, J.P.; Dienel, A.; Blackburn, S.L.; Thankamani, P.K. CSF-Exosomal miRNAs and Delayed Cerebral Ischemia: Insights into Pathophysiology but No Definitive Biomarkers. Biomolecules 2025, 15, 1161. https://doi.org/10.3390/biom15081161
Shafeeque CM, McBride DW, Yan Y, Zeineddine HA, Hagen JP, Choi HA, Savarraj JP, Dienel A, Blackburn SL, Thankamani PK. CSF-Exosomal miRNAs and Delayed Cerebral Ischemia: Insights into Pathophysiology but No Definitive Biomarkers. Biomolecules. 2025; 15(8):1161. https://doi.org/10.3390/biom15081161
Chicago/Turabian StyleShafeeque, Chathathayil M., Devin W. McBride, Yuanqing Yan, Hussein A. Zeineddine, John P. Hagen, H. Alex Choi, Jude P. Savarraj, Ari Dienel, Spiros L. Blackburn, and Peeyush Kumar Thankamani. 2025. "CSF-Exosomal miRNAs and Delayed Cerebral Ischemia: Insights into Pathophysiology but No Definitive Biomarkers" Biomolecules 15, no. 8: 1161. https://doi.org/10.3390/biom15081161
APA StyleShafeeque, C. M., McBride, D. W., Yan, Y., Zeineddine, H. A., Hagen, J. P., Choi, H. A., Savarraj, J. P., Dienel, A., Blackburn, S. L., & Thankamani, P. K. (2025). CSF-Exosomal miRNAs and Delayed Cerebral Ischemia: Insights into Pathophysiology but No Definitive Biomarkers. Biomolecules, 15(8), 1161. https://doi.org/10.3390/biom15081161








