Non-Canonical, Strongly Selective Protein Disulfide Isomerases as Anticancer Therapeutic Targets
Abstract
1. Introduction
2. Protein Disulfide Isomerases as a Druggable Vulnerability in a Subset of Human Cancers
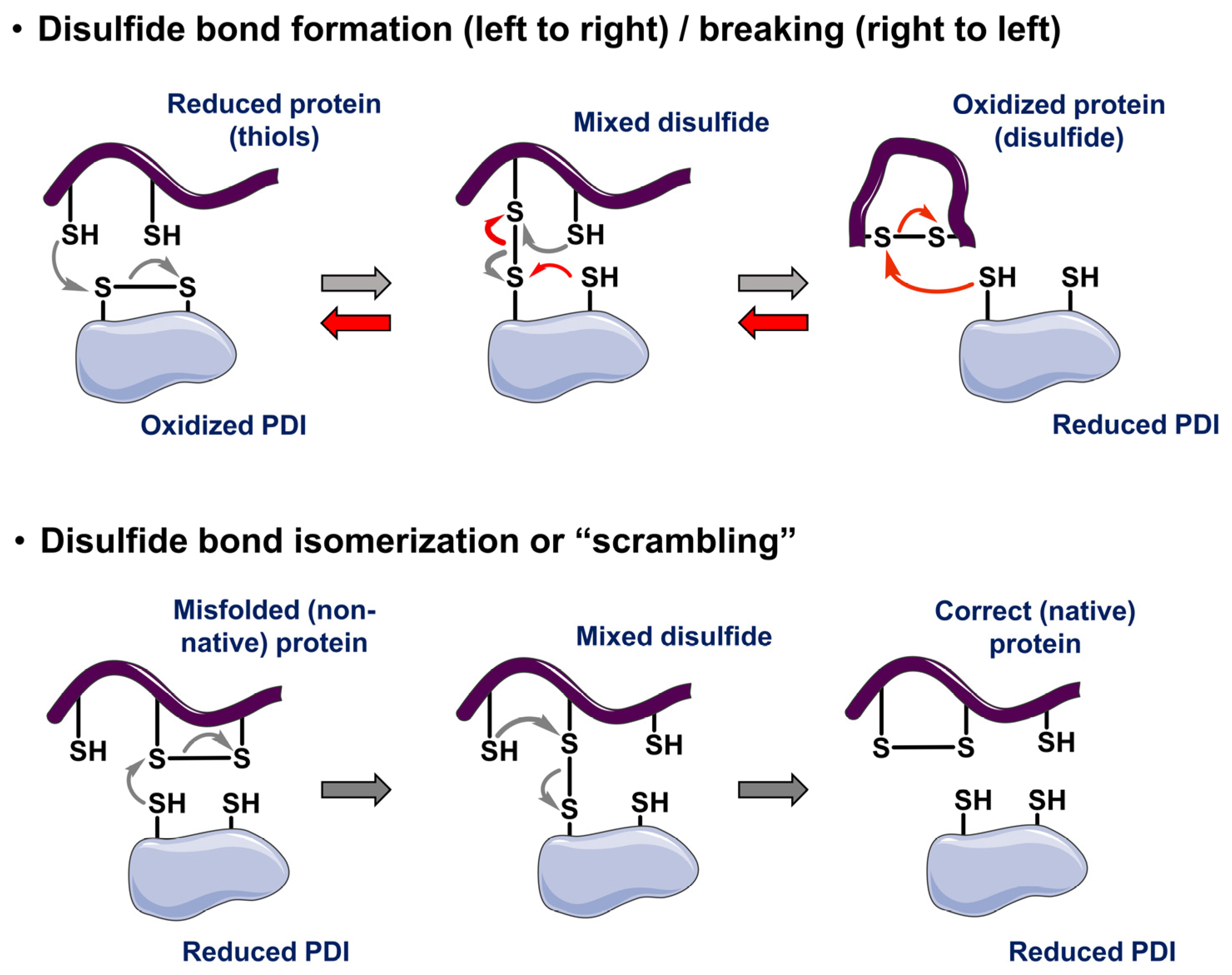
3. Roles of Strongly Selective Disulfide Isomerases in Protein Folding and Cellular Signaling
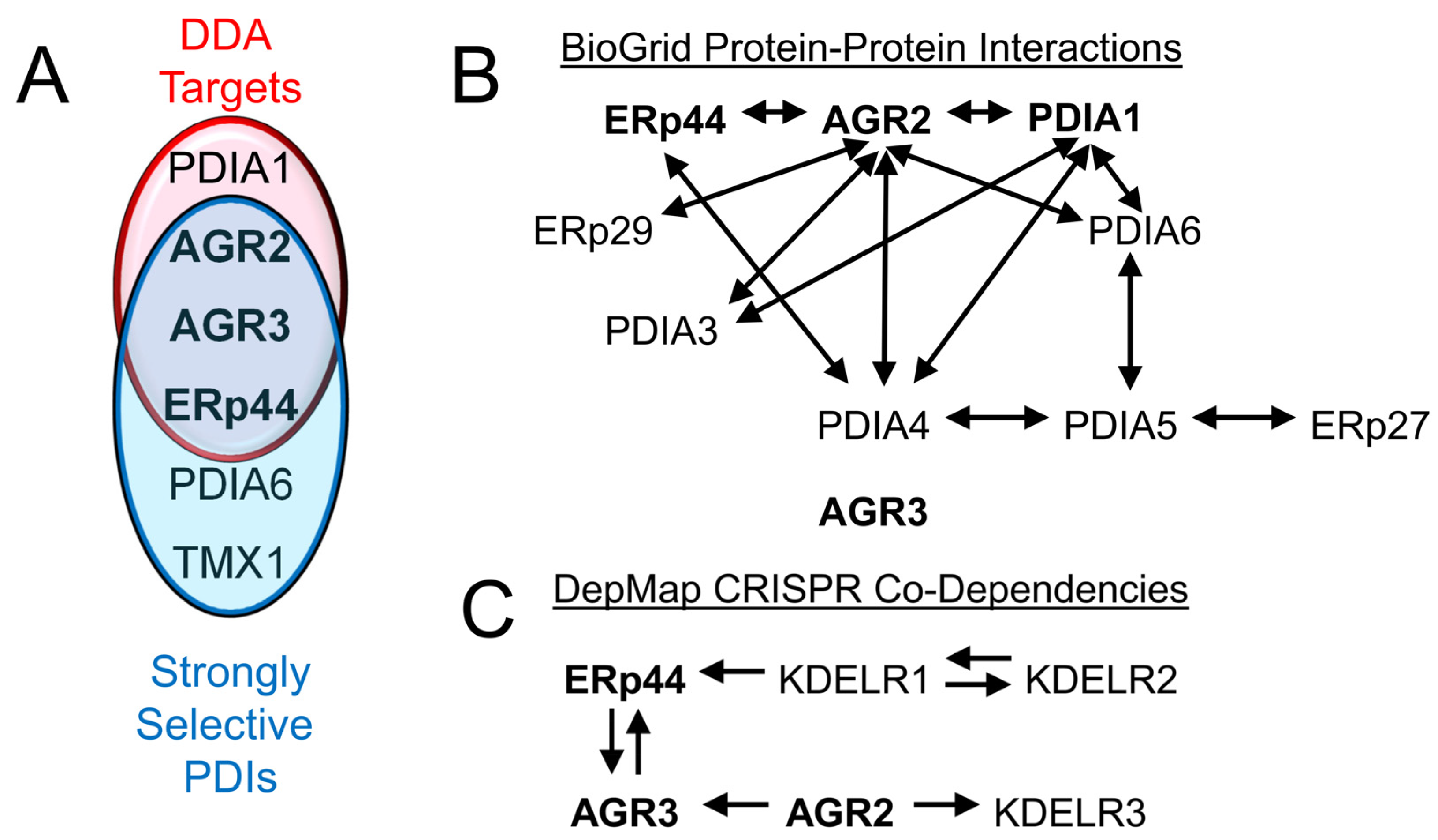
4. Small Molecule Inhibitors of Strongly Selective and Non-Canonical Disulfide Isomerases
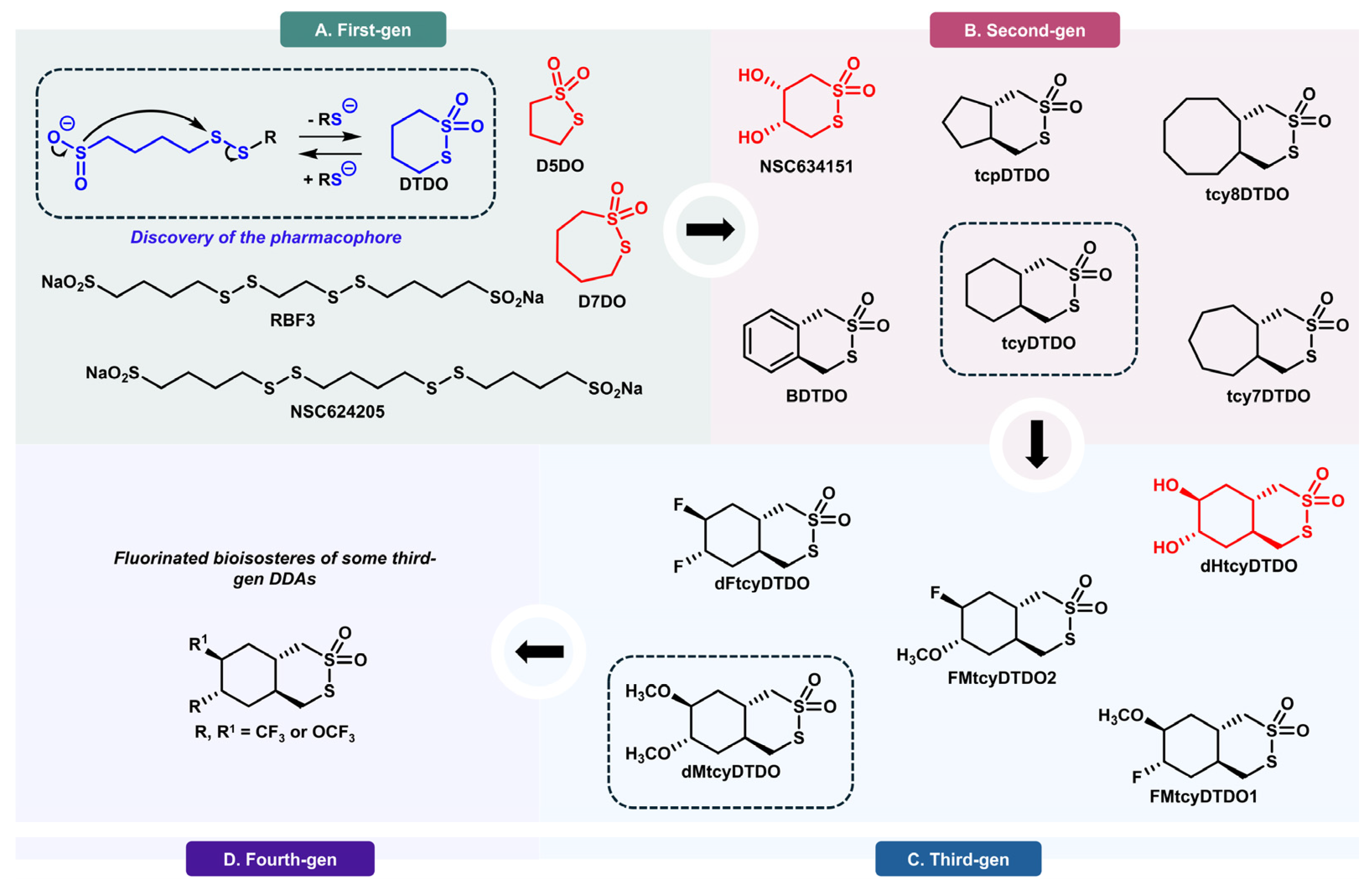
5. DDAs: Disulfide Isomerase Inhibitors with a Unique Selectivity Profile
6. Unanswered Questions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Pourdehnad, M.; Truitt, M.L.; Siddiqi, I.N.; Ducker, G.S.; Shokat, K.M.; Ruggero, D. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proc. Natl. Acad. Sci. USA 2013, 110, 11988–11993. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, D. The role of Myc-induced protein synthesis in cancer. Cancer Res. 2009, 69, 8839–8843. [Google Scholar] [CrossRef]
- Smit, W.L.; Spaan, C.N.; Johannes de Boer, R.; Ramesh, P.; Martins Garcia, T.; Meijer, B.J.; Vermeulen, J.L.M.; Lezzerini, M.; MacInnes, A.W.; Koster, J.; et al. Driver mutations of the adenoma-carcinoma sequence govern the intestinal epithelial global translational capacity. Proc. Natl. Acad. Sci. USA 2020, 117, 25560–25570. [Google Scholar] [CrossRef]
- Stams, W.A.; den Boer, M.L.; Holleman, A.; Appel, I.M.; Beverloo, H.B.; van Wering, E.R.; Janka-Schaub, G.E.; Evans, W.E.; Pieters, R. Asparagine synthetase expression is linked with L-asparaginase resistance in TEL-AML1-negative but not TEL-AML1-positive pediatric acute lymphoblastic leukemia. Blood 2005, 105, 4223–4225. [Google Scholar] [CrossRef]
- Wells, R.J.; Woods, W.G.; Lampkin, B.C.; Nesbit, M.E.; Lee, J.W.; Buckley, J.D.; Versteeg, C.; Hammond, G.D. Impact of high-dose cytarabine and asparaginase intensification on childhood acute myeloid leukemia: A report from the Childrens Cancer Group. J. Clin. Oncol. 1993, 11, 538–545. [Google Scholar] [CrossRef]
- Chow, A.K.; Ng, L.; Sing Li, H.; Cheng, C.W.; Lam, C.S.; Yau, T.C.; Cheng, P.N.; Fan, S.T.; Poon, R.T.; Pang, R.W. Anti-tumor efficacy of a recombinant human arginase in human hepatocellular carcinoma. Curr. Cancer Drug Targets 2012, 12, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Kobayashi, S.; Inanami, O.; Fujii, J.; Nakajima, O.; Ito, T.; Yasui, H. LAT1 inhibitor JPH203 sensitizes cancer cells to radiation by enhancing radiation-induced cellular senescence. Transl. Oncol. 2021, 14, 101212. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Coles, G.; Broer, A.; McLeod, M.D.; George, A.J.; Hannan, R.D.; Broer, S. Identification and characterization of a novel SNAT2 (SLC38A2) inhibitor reveals synergy with glucose transport inhibition in cancer cells. Front. Pharmacol. 2022, 13, 963066. [Google Scholar] [CrossRef]
- Nishikubo, K.; Ohgaki, R.; Liu, X.; Okanishi, H.; Xu, M.; Endou, H.; Kanai, Y. Combination effects of amino acid transporter LAT1 inhibitor nanvuranlat and cytotoxic anticancer drug gemcitabine on pancreatic and biliary tract cancer cells. Cancer Cell Int. 2023, 23, 116. [Google Scholar] [CrossRef]
- Jakobsen, S.; Nielsen, C.U. Exploring Amino Acid Transporters as Therapeutic Targets for Cancer: An Examination of Inhibitor Structures, Selectivity Issues, and Discovery Approaches. Pharmaceutics 2024, 16, 197. [Google Scholar] [CrossRef]
- Rii, J.; Sakamoto, S.; Mizokami, A.; Xu, M.; Fujimoto, A.; Saito, S.; Koike, H.; Tamura, T.; Arai, T.; Yamada, Y.; et al. L-type amino acid transporter 1 inhibitor JPH203 prevents the growth of cabazitaxel-resistant prostate cancer by inhibiting cyclin-dependent kinase activity. Cancer Sci. 2024, 115, 937–953. [Google Scholar] [CrossRef]
- Thomas, S.E.; Dalton, L.; Malzer, E.; Marciniak, S.J. Unravelling the story of protein misfolding in diabetes mellitus. World J. Diabetes 2011, 2, 114–118. [Google Scholar] [CrossRef]
- Ashraf, G.M.; Greig, N.H.; Khan, T.A.; Hassan, I.; Tabrez, S.; Shakil, S.; Sheikh, I.A.; Zaidi, S.K.; Akram, M.; Jabir, N.R.; et al. Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Morales-Scheihing, D.; Butler, P.C.; Soto, C. Type 2 diabetes as a protein misfolding disease. Trends Mol. Med. 2015, 21, 439–449. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Edwards Iii, G.; Salvadores, N.; Shahnawaz, M.; Diaz-Espinoza, R.; Soto, C. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol. Psychiatry 2017, 22, 1327–1334. [Google Scholar] [CrossRef]
- Costes, S. Targeting protein misfolding to protect pancreatic beta-cells in type 2 diabetes. Curr. Opin. Pharmacol. 2018, 43, 104–110. [Google Scholar] [CrossRef]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef]
- Agorogiannis, E.I.; Agorogiannis, G.I.; Papadimitriou, A.; Hadjigeorgiou, G.M. Protein misfolding in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2004, 30, 215–224. [Google Scholar] [CrossRef]
- Kwong, L.K.; Uryu, K.; Trojanowski, J.Q.; Lee, V.M. TDP-43 proteinopathies: Neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals 2008, 16, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Lipton, S.A. Cell death: Protein misfolding and neurodegenerative diseases. Apoptosis 2009, 14, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Rzymski, T.; Mellor, H.R.; Pike, L.; Bottini, A.; Generali, D.; Harris, A.L. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009, 69, 4415–4423. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Sharma, N.; Golden, E.B.; Cho, H.; Agarwal, P.; Gaffney, K.J.; Petasis, N.A.; Chen, T.C.; Hofman, F.M.; Louie, S.G.; et al. Preferential killing of triple-negative breast cancer cells in vitro and in vivo when pharmacological aggravators of endoplasmic reticulum stress are combined with autophagy inhibitors. Cancer Lett. 2012, 325, 63–71. [Google Scholar] [CrossRef]
- Arora, S.; Golemis, E.A. A new strategy to ERADicate HER2-positive breast tumors? Sci. Signal. 2015, 8, fs11. [Google Scholar] [CrossRef]
- Singh, N.; Joshi, R.; Komurov, K. HER2-mTOR signaling-driven breast cancer cells require ER-associated degradation to survive. Sci. Signal. 2015, 8, ra52. [Google Scholar] [CrossRef] [PubMed]
- Calvo, V.; Zheng, W.; Adam-Artigues, A.; Staschke, K.A.; Huang, X.; Cheung, J.F.; Nobre, A.R.; Fujisawa, S.; Liu, D.; Fumagalli, M.; et al. A PERK-Specific Inhibitor Blocks Metastatic Progression by Limiting Integrated Stress Response-Dependent Survival of Quiescent Cancer Cells. Clin. Cancer Res. 2023, 29, 5155–5172. [Google Scholar] [CrossRef] [PubMed]
- Urano, F.; Bertolotti, A.; Ron, D. IRE1 and efferent signaling from the endoplasmic reticulum. J. Cell Sci. 2000, 113 Pt 21, 3697–3702. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef]
- Brewer, J.W.; Diehl, J.A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA 2000, 97, 12625–12630. [Google Scholar] [CrossRef]
- Harding, H.P.; Novoa, I.; Zhang, Y.H.; Zeng, H.Q.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Iwawaki, T.; Hosoda, A.; Okuda, T.; Kamigori, Y.; Nomura-Furuwatari, C.; Kimata, Y.; Tsuru, A.; Kohno, K. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat. Cell Biol. 2001, 3, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K.; Murakami, S. Role of the unfolded protein response in cell death. Apoptosis 2006, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hiss, D.C.; Gabriels, G.A. Implications of endoplasmic reticulum stress, the unfolded protein response and apoptosis for molecular cancer therapy. Part I: Targeting p53, Mdm2, GADD153/CHOP, GRP78/BiP and heat shock proteins. Expert. Opin. Drug Discov. 2009, 4, 799–821. [Google Scholar] [CrossRef]
- Hazari, Y.M.; Bashir, A.; Haq, E.U.; Fazili, K.M. Emerging tale of UPR and cancer: An essentiality for malignancy. Tumour Biol. 2016, 37, 14381–14390. [Google Scholar] [CrossRef]
- Wang, M.; Law, M.E.; Castellano, R.K.; Law, B.K. The unfolded protein response as a target for anticancer therapeutics. Crit. Rev. Oncol./Hematol. 2018, 127, 66–79. [Google Scholar] [CrossRef]
- DuRose, J.B.; Scheuner, D.; Kaufman, R.J.; Rothblum, L.I.; Niwa, M. Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol. Cell. Biol. 2009, 29, 4295–4307. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Scheraga, H.A. Catalysis of the oxidative folding of bovine pancreatic ribonuclease A by protein disulfide isomerase. J. Mol. Biol. 2000, 300, 995–1003. [Google Scholar] [CrossRef]
- Scheraga, H.A.; Konishi, Y.; Ooi, T. Multiple pathways for regenerating ribonuclease A. Adv. Biophys. 1984, 18, 21–41. [Google Scholar] [CrossRef]
- Konishi, Y.; Ooi, T.; Scheraga, H.A. Regeneration of RNase A from the reduced protein: Models of regeneration pathways. Proc. Natl. Acad. Sci. USA 1982, 79, 5734–5738. [Google Scholar] [CrossRef]
- Isenman, D.E.; Lancet, D.; Pecht, I. Folding pathways of immunoglobulin domains. The folding kinetics of the Cgamma3 domain of human IgG1. Biochemistry 1979, 18, 3327–3336. [Google Scholar] [CrossRef]
- Schmid, F.X.; Baldwin, R.L. Acid catalysis of the formation of the slow-folding species of RNase A: Evidence that the reaction is proline isomerization. Proc. Natl. Acad. Sci. USA 1978, 75, 4764–4768. [Google Scholar] [CrossRef]
- McPhie, P. Kinetic studies on the unfolding and refolding of pepsinogen in urea. The nature of the rate-limiting step. J. Biol. Chem. 1980, 255, 4048–4052. [Google Scholar] [CrossRef]
- Fisher, K.E.; Ruan, B.; Alexander, P.A.; Wang, L.; Bryan, P.N. Mechanism of the kinetically-controlled folding reaction of subtilisin. Biochemistry 2007, 46, 640–651. [Google Scholar] [CrossRef]
- Nagradova, N. Enzymes catalyzing protein folding and their cellular functions. Curr. Protein Pept. Sci. 2007, 8, 273–282. [Google Scholar] [CrossRef]
- Page, A.P. Cyclophilin and protein disulfide isomerase genes are co-transcribed in a functionally related manner in Caenorhabditis elegans. DNA Cell Biol. 1997, 16, 1335–1343. [Google Scholar] [CrossRef]
- Okumura, M.; Kadokura, H.; Inaba, K. Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum. Free Radic. Biol. Med. 2015, 83, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.S.A.; Zahari, S.; Syafruddin, S.E.; Firdaus-Raih, M.; Low, T.Y.; Mohtar, M.A. Functions and mechanisms of protein disulfide isomerase family in cancer emergence. Cell Biosci. 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jackson, C.; Karapetyan, E.; Dutta, P.; Kermah, D.; Wu, Y.; Wu, Y.; Schloss, J.; Vadgama, J.V. Roles of Protein Disulfide Isomerase in Breast Cancer. Cancers 2022, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Zhang, J.; Aslam, M.; Blumental-Perry, A.; Tew, K.D.; Townsend, D.M. Protein disulfide isomerase family mediated redox regulation in cancer. Adv. Cancer Res. 2023, 160, 83–106. [Google Scholar] [CrossRef]
- Pierre, A.S.; Gavriel, N.; Guilbard, M.; Ogier-Denis, E.; Chevet, E.; Delom, F.; Igbaria, A. Modulation of Protein Disulfide Isomerase Functions by Localization: The Example of the Anterior Gradient Family. Antioxid. Redox Signal. 2024, 41, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Yang, J.; Zhou, X.; Li, N.; Zhang, J. The Role of Protein Disulfide Isomerase Inhibitors in Cancer Therapy. ChemMedChem 2025, 20, e202400590. [Google Scholar] [CrossRef]
- Laboissiere, M.C.; Sturley, S.L.; Raines, R.T. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J. Biol. Chem. 1995, 270, 28006–28009. [Google Scholar] [CrossRef] [PubMed]
- Bulleid, N.J. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 4, a013219. [Google Scholar] [CrossRef]
- Walker, K.W.; Gilbert, H.F. Scanning and escape during protein-disulfide isomerase-assisted protein folding. J. Biol. Chem. 1997, 272, 8845–8848. [Google Scholar] [CrossRef]
- Furie, B.; Flaumenhaft, R. Thiol isomerases in thrombus formation. Circ. Res. 2014, 114, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Johannes, L.; Mallard, F.; Girod, A.; Grill, S.; Reinsch, S.; Keller, P.; Tzschaschel, B.; Echard, A.; Goud, B.; et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 1999, 147, 743–760. [Google Scholar] [CrossRef]
- Murshid, A.; Presley, J.F. ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell. Mol. Life Sci. 2004, 61, 133–145. [Google Scholar] [CrossRef]
- Vavassori, S.; Cortini, M.; Masui, S.; Sannino, S.; Anelli, T.; Caserta, I.R.; Fagioli, C.; Mossuto, M.F.; Fornili, A.; van Anken, E.; et al. A pH-regulated quality control cycle for surveillance of secretory protein assembly. Mol. Cell 2013, 50, 783–792. [Google Scholar] [CrossRef]
- Watanabe, S.; Harayama, M.; Kanemura, S.; Sitia, R.; Inaba, K. Structural basis of pH-dependent client binding by ERp44, a key regulator of protein secretion at the ER-Golgi interface. Proc. Natl. Acad. Sci. USA 2017, 114, E3224–E3232. [Google Scholar] [CrossRef]
- Watanabe, S.; Amagai, Y.; Sannino, S.; Tempio, T.; Anelli, T.; Harayama, M.; Masui, S.; Sorrentino, I.; Yamada, M.; Sitia, R.; et al. Zinc regulates ERp44-dependent protein quality control in the early secretory pathway. Nat. Commun. 2019, 10, 603. [Google Scholar] [CrossRef]
- Raghu, R.; Kurlak, L.O.; Lee, E.D.; Mistry, H.D. The differential placental expression of ERp44 and pre-eclampsia; association with placental zinc, the ERAP1 and the renin-angiotensin-system. Placenta 2023, 134, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.W.; Verhaeghe, C.; Park, S.W.; Nguyenvu, L.T.; Huang, X.; Zhen, G.; Erle, D.J. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 178–185. [Google Scholar] [CrossRef]
- Cloots, E.; Guilbert, P.; Provost, M.; Neidhardt, L.; Van de Velde, E.; Fayazpour, F.; De Sutter, D.; Savvides, S.N.; Eyckerman, S.; Janssens, S. Activation of goblet-cell stress sensor IRE1β is controlled by the mucin chaperone AGR2. EMBO J. 2024, 43, 695–718. [Google Scholar] [CrossRef]
- Neidhardt, L.; Cloots, E.; Friemel, N.; Weiss, C.A.M.; Harding, H.P.; McLaughlin, S.H.; Janssens, S.; Ron, D. The IRE1beta-mediated unfolded protein response is repressed by the chaperone AGR2 in mucin producing cells. EMBO J. 2024, 43, 719–753. [Google Scholar] [CrossRef]
- Al-Shaibi, A.A.; Abdel-Motal, U.M.; Hubrack, S.Z.; Bullock, A.N.; Al-Marri, A.A.; Agrebi, N.; Al-Subaiey, A.A.; Ibrahim, N.A.; Charles, A.K.; Group, C.i.I.-Q.S.; et al. Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1809–1830. [Google Scholar] [CrossRef]
- Anelli, T.; Alessio, M.; Mezghrani, A.; Simmen, T.; Talamo, F.; Bachi, A.; Sitia, R. ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J. 2002, 21, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Otsu, M.; Bertoli, G.; Fagioli, C.; Guerini-Rocco, E.; Nerini-Molteni, S.; Ruffato, E.; Sitia, R. Dynamic retention of Ero1alpha and Ero1beta in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid. Redox Signal. 2006, 8, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Parekh, V.V.; Mendez-Fernandez, Y.; Olivares-Villagomez, D.; Dragovic, S.; Hill, T.; Roopenian, D.C.; Joyce, S.; Van Kaer, L. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 2006, 203, 647–659. [Google Scholar] [CrossRef]
- Kakihana, T.; Araki, K.; Vavassori, S.; Iemura, S.; Cortini, M.; Fagioli, C.; Natsume, T.; Sitia, R.; Nagata, K. Dynamic regulation of Ero1alpha and peroxiredoxin 4 localization in the secretory pathway. J. Biol. Chem. 2013, 288, 29586–29594. [Google Scholar] [CrossRef]
- Hisatsune, C.; Ebisui, E.; Usui, M.; Ogawa, N.; Suzuki, A.; Mataga, N.; Takahashi-Iwanaga, H.; Mikoshiba, K. ERp44 Exerts Redox-Dependent Control of Blood Pressure at the ER. Mol. Cell 2015, 58, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Firat, E.; Saveanu, L.; Aichele, P.; Staeheli, P.; Huai, J.; Gaedicke, S.; Nil, A.; Besin, G.; Kanzler, B.; van Endert, P.; et al. The role of endoplasmic reticulum-associated aminopeptidase 1 in immunity to infection and in cross-presentation. J. Immunol. 2007, 178, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Batliwala, M.; Bouvier, M. ERAP1 enzyme-mediated trimming and structural analyses of MHC I-bound precursor peptides yield novel insights into antigen processing and presentation. J. Biol. Chem. 2019, 294, 18534–18544. [Google Scholar] [CrossRef] [PubMed]
- Rastall, D.P.; Aldhamen, Y.A.; Seregin, S.S.; Godbehere, S.; Amalfitano, A. ERAP1 functions override the intrinsic selection of specific antigens as immunodominant peptides, thereby altering the potency of antigen-specific cytolytic and effector memory T-cell responses. Int. Immunol. 2014, 26, 685–695. [Google Scholar] [CrossRef]
- York, I.A.; Brehm, M.A.; Zendzian, S.; Towne, C.F.; Rock, K.L. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc. Natl. Acad. Sci. USA 2006, 103, 9202–9207. [Google Scholar] [CrossRef]
- Chang, S.C.; Momburg, F.; Bhutani, N.; Goldberg, A.L. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 17107–17112. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, Y.; Shi, X.; Li, D.; Han, J. ERp44 depletion exacerbates ER stress and aggravates diabetic nephropathy in db/db mice. Biochem. Biophys. Res. Commun. 2018, 504, 921–926. [Google Scholar] [CrossRef]
- Nyirimigabo, E.; Jin, M.; Yang, Z.; Wang, J.; Zhai, K.; Mao, Y.; Wang, H.; Ji, G. The role of ERp44 in glucose and lipid metabolism. Arch. Biochem. Biophys. 2019, 671, 175–184. [Google Scholar] [CrossRef]
- Bi, Y.; Chang, Y.; Liu, Q.; Mao, Y.; Zhai, K.; Zhou, Y.; Jiao, R.; Ji, G. ERp44/CG9911 promotes fat storage in Drosophila adipocytes by regulating ER Ca(2+) homeostasis. Aging 2021, 13, 15013–15031. [Google Scholar] [CrossRef]
- Anelli, T.; Ceppi, S.; Bergamelli, L.; Cortini, M.; Masciarelli, S.; Valetti, C.; Sitia, R. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J. 2007, 26, 4177–4188. [Google Scholar] [CrossRef]
- Cortini, M.; Sitia, R. ERp44 and ERGIC-53 synergize in coupling efficiency and fidelity of IgM polymerization and secretion. Traffic 2010, 11, 651–659. [Google Scholar] [CrossRef]
- Wang, Z.V.; Schraw, T.D.; Kim, J.Y.; Khan, T.; Rajala, M.W.; Follenzi, A.; Scherer, P.E. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol. Cell. Biol. 2007, 27, 3716–3731. [Google Scholar] [CrossRef]
- Wolf, G. New insights into thiol-mediated regulation of adiponectin secretion. Nutr. Rev. 2008, 66, 642–645. [Google Scholar] [CrossRef]
- Mahameed, M.; Boukeileh, S.; Obiedat, A.; Darawshi, O.; Dipta, P.; Rimon, A.; McLennan, G.; Fassler, R.; Reichmann, D.; Karni, R.; et al. Pharmacological induction of selective endoplasmic reticulum retention as a strategy for cancer therapy. Nat. Commun. 2020, 11, 1304. [Google Scholar] [CrossRef]
- Anelli, T.; Alessio, M.; Bachi, A.; Bergamelli, L.; Bertoli, G.; Camerini, S.; Mezghrani, A.; Ruffato, E.; Simmen, T.; Sitia, R. Thiol-mediated protein retention in the endoplasmic reticulum: The role of ERp44. EMBO J. 2003, 22, 5015–5022. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Vavassori, S.; Li, S.; Ke, H.; Anelli, T.; Degano, M.; Ronzoni, R.; Sitia, R.; Sun, F.; et al. Crystal structure of human ERp44 shows a dynamic functional modulation by its carboxy-terminal tail. EMBO Rep. 2008, 9, 642–647. [Google Scholar] [CrossRef]
- Hampe, L.; Xu, C.; Harris, P.W.R.; Chen, J.; Liu, M.; Middleditch, M.; Radjainia, M.; Wang, Y.; Mitra, A.K. Synthetic peptides designed to modulate adiponectin assembly improve obesity-related metabolic disorders. Br. J. Pharmacol. 2017, 174, 4478–4492. [Google Scholar] [CrossRef]
- Hampe, L.; Harris, P.W.R.; Rushton, B.; Radjainia, M.; Brimble, M.A.; Mitra, A.K. Engineering a stable complex of ERp44 with a designed peptide ligand for analyzing the mode of interaction of ERp44 with its clients. Pept. Sci. 2021, 113, e24230. [Google Scholar] [CrossRef]
- Tian, H.; Shi, S.; You, B.; Zhang, Q.; Gu, M.; You, Y. ER resident protein 44 promotes malignant phenotype in nasopharyngeal carcinoma through the interaction with ATP citrate lyase. J. Transl. Med. 2021, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, X.; Wang, F. Protein tyrosine phosphatase receptor type O (PTPRO) knockdown enhances the proliferative, invasive and angiogenic activities of trophoblast cells by suppressing ER resident protein 44 (ERp44) expression in preeclampsia. Bioengineered 2021, 12, 9561–9574. [Google Scholar] [CrossRef] [PubMed]
- Solda, T.; Galli, C.; Guerra, C.; Hoefner, C.; Molinari, M. TMX5/TXNDC15, a natural trapping mutant of the PDI family is a client of the proteostatic factor ERp44. Life Sci. Alliance 2024, 7, e202403047. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Yang, Z.; Jin, M.; Zhai, K.; Wang, J.; Mao, Y.; Liu, Y.; Ding, M.; Wang, H.; Wang, F.; et al. ERp44 is required for endocardial cushion development by regulating VEGFA secretion in myocardium. Cell Prolif. 2022, 55, e13179. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jiang, Z.; Yu, X.; Zhang, Y.; Sun, M.; Wang, W.; Ge, Z.; De, W.; Sun, L. MiR-101 regulates apoptosis of trophoblast HTR-8/SVneo cells by targeting endoplasmic reticulum (ER) protein 44 during preeclampsia. J. Hum. Hypertens. 2014, 28, 610–616. [Google Scholar] [CrossRef]
- Cho, J.H.; Jeon, Y.J.; Park, S.M.; Shin, J.C.; Lee, T.H.; Jung, S.; Park, H.; Ryu, J.; Chen, H.; Dong, Z.; et al. Multifunctional effects of honokiol as an anti-inflammatory and anti-cancer drug in human oral squamous cancer cells and xenograft. Biomaterials 2015, 53, 274–284. [Google Scholar] [CrossRef]
- Thompson, D.A.; Weigel, R.J. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem. Biophys. Res. Commun. 1998, 251, 111–116. [Google Scholar] [CrossRef]
- Aberger, F.; Weidinger, G.; Grunz, H.; Richter, K. Anterior specification of embryonic ectoderm: The role of the Xenopus cement gland-specific gene XAG-2. Mech. Dev. 1998, 72, 115–130. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.G.; Lee, P.Y.; Cho, S.; Lee, D.H.; Kim, G.H.; Kim, J.H.; Park, B.C. Dimerization of pro-oncogenic protein Anterior Gradient 2 is required for the interaction with BiP/GRP78. Biochem. Biophys. Res. Commun. 2013, 430, 610–615. [Google Scholar] [CrossRef]
- Clarke, D.J.; Murray, E.; Faktor, J.; Mohtar, A.; Vojtesek, B.; MacKay, C.L.; Smith, P.L.; Hupp, T.R. Mass spectrometry analysis of the oxidation states of the pro-oncogenic protein anterior gradient-2 reveals covalent dimerization via an intermolecular disulphide bond. Biochim. Biophys. Acta 2016, 1864, 551–561. [Google Scholar] [CrossRef]
- Patel, P.; Clarke, C.; Barraclough, D.L.; Jowitt, T.A.; Rudland, P.S.; Barraclough, R.; Lian, L.Y. Metastasis-promoting anterior gradient 2 protein has a dimeric thioredoxin fold structure and a role in cell adhesion. J. Mol. Biol. 2013, 425, 929–943. [Google Scholar] [CrossRef]
- Shih, L.J.; Lu, Y.F.; Chen, Y.H.; Lin, C.C.; Chen, J.A.; Hwang, S.P. Characterization of the agr2 gene, a homologue of X. laevis anterior gradient 2, from the zebrafish, Danio rerio. Gene Expr. Patterns 2007, 7, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.S.; Shandarin, I.N.; Ermakova, G.V.; Minin, A.A.; Tereshina, M.B.; Zaraisky, A.G. The secreted factor Ag1 missing in higher vertebrates regulates fins regeneration in Danio rerio. Sci. Rep. 2015, 5, 8123. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Tereshina, M.B.; Ermakova, G.V.; Belousov, V.V.; Zaraisky, A.G. Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci. Rep. 2013, 3, 1279. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Zhang, Y.; Lu, M.M.; DeMayo, F.J.; Dekker, J.D.; Tucker, P.W.; Morrisey, E.E. Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development 2012, 139, 2500–2509. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, M.; Liu, X.; Ji, X.; Li, S.; Jin, E. New insights into the unfolded protein response (UPR)-anterior gradient 2 (AGR2) pathway in the regulation of intestinal barrier function in weaned piglets. Anim. Nutr. 2023, 15, 225–232. [Google Scholar] [CrossRef]
- Park, S.W.; Zhen, G.; Verhaeghe, C.; Nakagami, Y.; Nguyenvu, L.T.; Barczak, A.J.; Killeen, N.; Erle, D.J. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. USA 2009, 106, 6950–6955. [Google Scholar] [CrossRef]
- Bertoli-Avella, A.; Hotakainen, R.; Al Shehhi, M.; Urzi, A.; Pareira, C.; Marais, A.; Al Shidhani, K.; Aloraimi, S.; Morales-Torres, G.; Fisher, S.; et al. A disorder clinically resembling cystic fibrosis caused by biallelic variants in the AGR2 gene. J. Med. Genet. 2022, 59, 993–1001. [Google Scholar] [CrossRef]
- Dong, A.; Wodziak, D.; Lowe, A.W. Epidermal growth factor receptor (EGFR) signaling requires a specific endoplasmic reticulum thioredoxin for the post-translational control of receptor presentation to the cell surface. J. Biol. Chem. 2015, 290, 8016–8027. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Q.; Chen, H.; Hu, L.; Negi, H.; Zheng, Y.; Ahmed, Y.; Wu, Z.; Li, D. Binding of anterior gradient 2 and estrogen receptor-alpha: Dual critical roles in enhancing fulvestrant resistance and IGF-1-induced tumorigenesis of breast cancer. Cancer Lett. 2016, 377, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Salmans, M.L.; Zhao, F.; Andersen, B. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: A potential drug target and biomarker. Breast Cancer Res. 2013, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Rudland, P.S.; Sibson, D.R.; Platt-Higgins, A.; Barraclough, R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005, 65, 3796–3805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hao, Y.; Lowe, A.W. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008, 68, 492–497. [Google Scholar] [CrossRef]
- Gupta, A.; Dong, A.; Lowe, A.W. AGR2 gene function requires a unique endoplasmic reticulum localization motif. J. Biol. Chem. 2012, 287, 4773–4782. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Jia, M.; Guo, Y.; Zhu, D.; Zhang, N.; Li, L.; Jiang, J.; Dong, Y.; Xu, Q.; Zhang, X.; Wang, M.; et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-kappaB pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1622–1633. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Q.; Yu, X.; Merugu, S.B.; Mangukiya, H.B.; Smith, N.; Li, Z.; Zhang, B.; Negi, H.; Rong, R.; et al. Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene 2017, 36, 5098–5109. [Google Scholar] [CrossRef]
- Hong, X.Y.; Wang, J.; Li, Z. AGR2 expression is regulated by HIF-1 and contributes to growth and angiogenesis of glioblastoma. Cell Biochem. Biophys. 2013, 67, 1487–1495. [Google Scholar] [CrossRef]
- Arumugam, T.; Deng, D.; Bover, L.; Wang, H.; Logsdon, C.D.; Ramachandran, V. New Blocking Antibodies against Novel AGR2-C4.4A Pathway Reduce Growth and Metastasis of Pancreatic Tumors and Increase Survival in Mice. Mol. Cancer Ther. 2015, 14, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.D.; Wu, K.; Liu, Y.J.; Zhang, Q.; Shen, H.; Ji, J.; Fang, D.; Xi, S.Y. LY6/PLAUR domain containing 3 (LYPD3) maintains melanoma cell stemness and mediates an immunosuppressive microenvironment. Biol. Direct 2023, 18, 72. [Google Scholar] [CrossRef]
- Cocce, K.J.; Jasper, J.S.; Desautels, T.K.; Everett, L.; Wardell, S.; Westerling, T.; Baldi, R.; Wright, T.M.; Tavares, K.; Yllanes, A.; et al. The Lineage Determining Factor GRHL2 Collaborates with FOXA1 to Establish a Targetable Pathway in Endocrine Therapy-Resistant Breast Cancer. Cell Rep. 2019, 29, 889–903 e810. [Google Scholar] [CrossRef]
- Fessart, D.; Domblides, C.; Avril, T.; Eriksson, L.A.; Begueret, H.; Pineau, R.; Malrieux, C.; Dugot-Senant, N.; Lucchesi, C.; Chevet, E.; et al. Secretion of protein disulphide isomerase AGR2 confers tumorigenic properties. Elife 2016, 5, e13887. [Google Scholar] [CrossRef] [PubMed]
- Maurel, M.; Obacz, J.; Avril, T.; Ding, Y.P.; Papadodima, O.; Treton, X.; Daniel, F.; Pilalis, E.; Horberg, J.; Hou, W.; et al. Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol. Med. 2019, 11, e10120. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Yordanov, G.N.; Hao, Y.; Deschenes, A.; Hinds, J.; Belleau, P.; Klingbeil, O.; Brosnan, E.; Doshi, A.; Park, Y.; et al. A mucus production programme promotes classical pancreatic ductal adenocarcinoma. Gut 2024, 73, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Bonser, L.R.; Schroeder, B.W.; Ostrin, L.A.; Baumlin, N.; Olson, J.L.; Salathe, M.; Erle, D.J. The Endoplasmic Reticulum Resident Protein AGR3. Required for Regulation of Ciliary Beat Frequency in the Airway. Am. J. Respir. Cell Mol. Biol. 2015, 53, 536–543. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, H.; Hu, J.; Song, Y.; Li, J.; Wang, L.; Wang, Z. AGR3 promotes the stemness of colorectal cancer via modulating Wnt/β-catenin signalling. Cell. Signal. 2020, 65, 109419. [Google Scholar] [CrossRef]
- Adam, P.J.; Boyd, R.; Tyson, K.L.; Fletcher, G.C.; Stamps, A.; Hudson, L.; Poyser, H.R.; Redpath, N.; Griffiths, M.; Steers, G.; et al. Comprehensive Proteomic Analysis of Breast Cancer Cell Membranes Reveals Unique Proteins with Potential Roles in Clinical Cancer*. J. Biol. Chem. 2003, 278, 6482–6489. [Google Scholar] [CrossRef] [PubMed]
- Maresh, E.L.; Mah, V.; Alavi, M.; Horvath, S.; Bagryanova, L.; Liebeskind, E.S.; Knutzen, L.A.; Zhou, Y.; Chia, D.; Liu, A.Y.; et al. Differential expression of anterior gradient gene AGR2 in prostate cancer. BMC Cancer 2010, 10, 680. [Google Scholar] [CrossRef]
- Armes, J.E.; Davies, C.M.; Wallace, S.; Taheri, T.; Perrin, L.C.; Autelitano, D.J. AGR2 expression in ovarian tumours: A potential biomarker for endometrioid and mucinous differentiation. Pathology 2013, 45, 49–54. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Law, M.E.; Jahn, S.C.; Davis, B.J.; Heldermon, C.D.; Reinhard, M.; Castellano, R.K.; Law, B.K. Novel agents that downregulate EGFR, HER2, and HER3 in parallel. Oncotarget 2015, 6, 10445–10459. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Wang, M.; Law, M.E.; Davis, B.J.; Bartley, A.N.; Higgins, P.J.; Kilberg, M.S.; Santostefano, K.E.; Terada, N.; Heldermon, C.D.; et al. Disulfide bond disrupting agents activate the unfolded protein response in EGFR- and HER2-positive breast tumor cells. Oncotarget 2017, 8, 28971–28989. [Google Scholar] [CrossRef]
- Wang, M.; Ferreira, R.B.; Law, M.E.; Davis, B.J.; Yaaghubi, E.; Ghilardi, A.F.; Sharma, A.; Avery, B.A.; Rodriguez, E.; Chiang, C.W.; et al. A novel proteotoxic combination therapy for EGFR+ and HER2+ cancers. Oncogene 2019, 38, 4264–4282. [Google Scholar] [CrossRef]
- Wang, M.; Law, M.E.; Davis, B.J.; Yaaghubi, E.; Ghilardi, A.F.; Ferreira, R.B.; Chiang, C.W.; Guryanova, O.A.; Kopinke, D.; Heldermon, C.D.; et al. Disulfide bond-disrupting agents activate the tumor necrosis family-related apoptosis-inducing ligand/death receptor 5 pathway. Cell Death Discov. 2019, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Law, M.E.; Yaaghubi, E.; Ghilardi, A.F.; Davis, B.J.; Ferreira, R.B.; Koh, J.; Chen, S.; DePeter, S.F.; Schilson, C.M.; Chiang, C.W.; et al. Inhibitors of ERp44, PDIA1, and AGR2 induce disulfide-mediated oligomerization of Death Receptors 4 and 5 and cancer cell death. Cancer Lett. 2022, 534, 215604. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, A.F.; Yaaghubi, E.; Ferreira, R.B.; Law, M.E.; Yang, Y.; Davis, B.J.; Schilson, C.M.; Ghiviriga, I.; Roitberg, A.E.; Law, B.K.; et al. Anticancer Agents Derived from Cyclic Thiosulfonates: Structure-Reactivity and Structure-Activity Relationships. ChemMedChem 2022, 17, e202200165. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhu, Q.; Gao, G.W.; Zhou, C.C.; Li, D.W. [Preparation, characterization and potential application of monoclonal antibody 18A4 against AGR2]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2010, 26, 49–51. [Google Scholar]
- Negi, H.; Merugu, S.B.; Mangukiya, H.B.; Li, Z.; Zhou, B.; Sehar, Q.; Kamle, S.; Yunus, F.-u.-N.; Mashausi, D.S.; Wu, Z.; et al. Anterior Gradient-2 monoclonal antibody inhibits lung cancer growth and metastasis by upregulating p53 pathway and without exerting any toxicological effects: A preclinical study. Cancer Lett. 2019, 449, 125–134. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Zhu, Q.; Yu, X.; Rong, R.; Merugu, S.B.; Mangukiya, H.B.; Li, D. A humanized monoclonal antibody targeting secreted anterior gradient 2 effectively inhibits the xenograft tumor growth. Biochem. Biophys. Res. Commun. 2016, 475, 57–63. [Google Scholar] [CrossRef]
- Roy, D.; Liu, G.-S.; Zeling Wang, A.; Zhou, B.; Yunus, F.-U.-N.; Raza, G.; Bharath Merugu, S.; Saidi Mashausi, D.; Li, D.; Zhao, B. Construction and stable gene expression of AGR2xPD1 bi-specific antibody that enhances attachment between T-Cells and lung tumor cells, suppress tumor cell migration and promoting CD8 expression in cytotoxic T-cells. Saudi Pharm. J. 2023, 31, 85–95. [Google Scholar] [CrossRef]
- Garri, C.; Howell, S.; Tiemann, K.; Tiffany, A.; Jalali-Yazdi, F.; Alba, M.M.; Katz, J.E.; Takahashi, T.T.; Landgraf, R.; Gross, M.E.; et al. Identification, characterization and application of a new peptide against anterior gradient homolog 2 (AGR2). Oncotarget 2018, 9, 27363–27379. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khan, S.U.; Khan, A.; Junaid, M.; Rafiq, H.; Htar, T.T.; Zhao, Y.; Shah, S.A.A.; Wadood, A. Prospect of Anterior Gradient 2 homodimer inhibition via repurposing FDA-approved drugs using structure-based virtual screening. Mol. Divers. 2022, 26, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Deng, Y.; Schuck, K.; Raulefs, S.; Maeritz, N.; Yu, Y.; Hechler, T.; Pahl, A.; Fernández-Sáiz, V.; et al. AGR2-Dependent Nuclear Import of RNA Polymerase II Constitutes a Specific Target of Pancreatic Ductal Adenocarcinoma in the Context of Wild-Type p53. Gastroenterology 2021, 161, 1601–1614.e1623. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, M.A.; Hernychova, L.; O’Neill, J.R.; Lawrence, M.L.; Murray, E.; Vojtesek, B.; Hupp, T.R. The Sequence-specific Peptide-binding Activity of the Protein Sulfide Isomerase AGR2 Directs Its Stable Binding to the Oncogenic Receptor EpCAM. Mol. Cell. Proteom. 2018, 17, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Yang, J.; Wang, X.; Xu, K.; Ikezoe, T. miR-217 sensitizes chronic myelogenous leukemia cells to tyrosine kinase inhibitors by targeting pro-oncogenic anterior gradient 2. Exp. Hematol. 2018, 68, 80–88.e82. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Wang, Y.; Wu, D.; Zhang, H. miR-199a-3p plays an anti-tumorigenic role in lung adenocarcinoma by suppressing anterior gradient 2. Bioengineered 2021, 12, 7859–7871. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Dou, M.; Li, Z.; Liu, Z.; Li, J.; Tian, C.; Yao, Y.; Wang, C.; Li, Y.; et al. 125I seeds inhibit proliferation and promote apoptosis in cholangiocarcinoma cells by regulating the AGR2-mediated p38 MAPK pathway. Cancer Lett. 2022, 524, 29–41. [Google Scholar] [CrossRef]
- Ly, T.T.G.; Yun, J.; Ha, J.-S.; Kim, Y.-J.; Jang, W.-B.; Van Le, T.H.; Rethineswaran, V.K.; Choi, J.; Kim, J.-H.; Min, S.-H.; et al. Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 944. [Google Scholar] [CrossRef]
- Sheng, N.; Wang, Y.-q.; Wang, C.-f.; Jia, M.-q.; Niu, H.-m.; Lu, Q.-q.; Wang, Y.-n.; Feng, D.; Zheng, X.-x.; Yuan, H.-q. AGR2-induced cholesterol synthesis drives lovastatin resistance that is overcome by combination therapy with allicin. Acta Pharmacol. Sin. 2022, 43, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.A.; MacLaine, N.J.; Michie, C.O.; Bouchalova, P.; Murray, E.; Howie, J.; Hrstka, R.; Maslon, M.M.; Nenutil, R.; Vojtesek, B.; et al. Anterior Gradient-3: A novel biomarker for ovarian cancer that mediates cisplatin resistance in xenograft models. J. Immunol. Methods 2012, 378, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Obacz, J.; Sommerova, L.; Sicari, D.; Durech, M.; Avril, T.; Iuliano, F.; Pastorekova, S.; Hrstka, R.; Chevet, E.; Delom, F.; et al. Extracellular AGR3 regulates breast cancer cells migration via Src signaling. Oncol. Lett. 2019, 18, 4449–4456. [Google Scholar] [CrossRef]
- Jian, L.; Xie, J.; Guo, S.; Yu, H.; Chen, R.; Tao, K.; Yang, C.; Li, K.; Liu, S. AGR3 promotes estrogen receptor-positive breast cancer cell proliferation in an estrogen-dependent manner. Oncol. Lett. 2020, 20, 1441–1451. [Google Scholar] [CrossRef]
- Xu, Q.; Shao, Y.; Zhang, J.; Zhang, H.; Zhao, Y.; Liu, X.; Guo, Z.; Chong, W.; Gu, F.; Ma, Y. Anterior Gradient 3 Promotes Breast Cancer Development and Chemotherapy Response. Cancer Res. Treat. 2020, 52, 218–245. [Google Scholar] [CrossRef]
- Law, M.E.; Davis, B.J.; Ghilardi, A.F.; Yaaghubi, E.; Dulloo, Z.M.; Wang, M.; Guryanova, O.A.; Heldermon, C.D.; Jahn, S.C.; Castellano, R.K.; et al. Repurposing Tranexamic Acid as an Anticancer Agent. Front. Pharmacol. 2022, 12, 792600. [Google Scholar] [CrossRef] [PubMed]
- Besse, L.; Besse, A.; Mendez-Lopez, M.; Vasickova, K.; Sedlackova, M.; Vanhara, P.; Kraus, M.; Bader, J.; Ferreira, R.B.; Castellano, R.K.; et al. A metabolic switch in proteasome inhibitor-resistant multiple myeloma ensures higher mitochondrial metabolism, protein folding and sphingomyelin synthesis. Haematologica 2019, 104, e415–e419. [Google Scholar] [CrossRef]
- Law, M.E.; Dulloo, Z.M.; Eggleston, S.R.; Takacs, G.P.; Alexandrow, G.M.; Lee, Y.i.; Wang, M.; Hardy, B.; Su, H.; Forsyth, B.; et al. DR5 disulfide bonding functions as a sensor and effector of protein folding stress. Mol. Cancer Res. 2024, 23, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Koumantou, D.; Barnea, E.; Martin-Esteban, A.; Maben, Z.; Papakyriakou, A.; Mpakali, A.; Kokkala, P.; Pratsinis, H.; Georgiadis, D.; Stern, L.J.; et al. Editing the immunopeptidome of melanoma cells using a potent inhibitor of endoplasmic reticulum aminopeptidase 1 (ERAP1). Cancer Immunol. Immunother. 2019, 68, 1245–1261. [Google Scholar] [CrossRef]
- Field, L.; Khim, Y.H. Organic disulfides and related substances. 33. Sodium 4-(2-acetamidoethyldithio)butanesulfinate and related compounds as antiradiation drugs. J. Med. Chem. 1972, 15, 312–315. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Field, L. Organic Disulfides and Related Substances. 45. Synthesis and Properties of Some Disulfide Sulfinate Salts Containing No Nitrogen. J. Chem. Eng. Data 1986, 31, 252–254. [Google Scholar] [CrossRef]
- Bruice, T.C.; Pandit, U.K. Intramolecular Models Depicting the Kinetic Importance of “Fit” in Enzymatic Catalysis. Proc. Natl. Acad. Sci. USA 1960, 46, 402–404. [Google Scholar] [CrossRef]
- Houk, J.; Whitesides, G.M. Characterization and Stability of Cyclic Disulfides and Cyclic Dimeric Bis(Disulfides). Tetrahedron 1989, 45, 91–102. [Google Scholar] [CrossRef]
- Kavanagh, M.E.; Horning, B.D.; Khattri, R.; Roy, N.; Lu, J.P.; Whitby, L.R.; Ye, E.; Brannon, J.C.; Parker, A.; Chick, J.M.; et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. Nat. Chem. Biol. 2022, 18, 1388–1398. [Google Scholar] [CrossRef]
- Shi, L.; Zhong, Z.; Li, X.; Zhou, Y.; Pan, Z. Discovery of an Orally Available Janus Kinase 3 Selective Covalent Inhibitor. J. Med. Chem. 2019, 62, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Barragan, A.M.; Ghaby, K.; Pond, M.P.; Roux, B. Computational Investigation of the Covalent Inhibition Mechanism of Bruton’s Tyrosine Kinase by Ibrutinib. J. Chem. Inf. Model. 2024, 64, 3488–3502. [Google Scholar] [CrossRef] [PubMed]
- Awoonor-Williams, E.; Abu-Saleh, A.A.A. Molecular Insights into the Impact of Mutations on the Binding Affinity of Targeted Covalent Inhibitors of BTK. J. Phys. Chem. B 2024, 128, 2874–2884. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Du, Y.; Huang, L.; Cui, J.; Niu, J.; Xu, Y.; Zhu, Q. Discovery of novel potent covalent inhibitor-based EGFR degrader with excellent in vivo efficacy. Bioorg. Chem. 2022, 120, 105605. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, E.; Zaro, B.W.; Dix, M.M.; Cravatt, B.; Wong, C.H. Refinement of Covalent EGFR Inhibitor AZD9291 to Eliminate Off-target Activity. Tetrahedron Lett. 2021, 74, 153178. [Google Scholar] [CrossRef]
- Li, N.; Liu, C.F.; Zhang, W.; Rao, G.W. A New Dawn for Targeted Cancer Therapy: Small Molecule Covalent Binding Inhibitor Targeting K-Ras (G12C). Curr. Med. Chem. 2025, 32, 647–677. [Google Scholar] [CrossRef]
- Selles, B.; Zannini, F.; Couturier, J.; Jacquot, J.P.; Rouhier, N. Atypical protein disulfide isomerases (PDI): Comparison of the molecular and catalytic properties of poplar PDI-A and PDI-M with PDI-L1A. PLoS ONE 2017, 12, e0174753. [Google Scholar] [CrossRef]
- Lu, X.; Gilbert, H.F.; Harper, J.W. Conserved residues flanking the thiol/disulfide centers of protein disulfide isomerase are not essential for catalysis of thiol/disulfide exchange. Biochemistry 1992, 31, 4205–4210. [Google Scholar] [CrossRef]
- Wunderlich, M.; Otto, A.; Maskos, K.; Mucke, M.; Seckler, R.; Glockshuber, R. Efficient catalysis of disulfide formation during protein folding with a single active-site cysteine. J. Mol. Biol. 1995, 247, 28–33. [Google Scholar] [CrossRef]
- Coelho, F.; Saidjalolov, S.; Moreau, D.; Thorn-Seshold, O.; Matile, S. Inhibition of Cell Motility by Cell-Penetrating Dynamic Covalent Cascade Exchangers: Integrins Participate in Thiol-Mediated Uptake. JACS Au 2023, 3, 1010–1016. [Google Scholar] [CrossRef]
- Shybeka, I.; Maynard, J.R.J.; Saidjalolov, S.; Moreau, D.; Sakai, N.; Matile, S. Dynamic Covalent Michael Acceptors to Penetrate Cells: Thiol-Mediated Uptake with Tetrel-Centered Exchange Cascades, Assisted by Halogen-Bonding Switches. Angew. Chem. Int. Ed. Engl. 2022, 61, e202213433. [Google Scholar] [CrossRef]
- Kato, T.; Lim, B.; Cheng, Y.; Pham, A.T.; Maynard, J.; Moreau, D.; Poblador-Bahamonde, A.I.; Sakai, N.; Matile, S. Cyclic Thiosulfonates for Thiol-Mediated Uptake: Cascade Exchangers, Transporters, Inhibitors. JACS Au 2022, 2, 839–852. [Google Scholar] [CrossRef]
- Laurent, Q.; Martinent, R.; Lim, B.; Pham, A.T.; Kato, T.; Lopez-Andarias, J.; Sakai, N.; Matile, S. Thiol-Mediated Uptake. JACS Au 2021, 1, 710–728. [Google Scholar] [CrossRef]
- Zeisel, L.; Felber, J.G.; Scholzen, K.C.; Schmitt, C.; Wiegand, A.J.; Komissarov, L.; Arner, E.S.J.; Thorn-Seshold, O. Piperazine-Fused Cyclic Disulfides Unlock High-Performance Bioreductive Probes of Thioredoxins and Bifunctional Reagents for Thiol Redox Biology. J. Am. Chem. Soc. 2024, 146, 5204–5214. [Google Scholar] [CrossRef]
- Felber, J.G.; Kitowski, A.; Zeisel, L.; Maier, M.S.; Heise, C.; Thorn-Seshold, J.; Thorn-Seshold, O. Cyclic Dichalcogenides Extend the Reach of Bioreductive Prodrugs to Harness Thiol/Disulfide Oxidoreductases: Applications to seco-Duocarmycins Targeting the Thioredoxin System. ACS Cent. Sci. 2023, 9, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Obacz, J.; Brychtova, V.; Podhorec, J.; Fabian, P.; Dobes, P.; Vojtesek, B.; Hrstka, R. Anterior gradient protein 3 is associated with less aggressive tumors and better outcome of breast cancer patients. Onco Targets Ther. 2015, 8, 1523–1532. [Google Scholar] [CrossRef][Green Version]
- Garczyk, S.; von Stillfried, S.; Antonopoulos, W.; Hartmann, A.; Schrauder, M.G.; Fasching, P.A.; Anzeneder, T.; Tannapfel, A.; Ergonenc, Y.; Knuchel, R.; et al. AGR3 in breast cancer: Prognostic impact and suitable serum-based biomarker for early cancer detection. PLoS ONE 2015, 10, e0122106. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kang, H.; Lyu, L.; Xiong, W.; Hu, Y. A target map of clinical combination therapies in oncology: An analysis of clinicaltrials.gov. Discov. Oncol. 2023, 14, 151. [Google Scholar] [CrossRef]
- Bergstrom, J.H.; Berg, K.A.; Rodriguez-Pineiro, A.M.; Stecher, B.; Johansson, M.E.; Hansson, G.C. AGR2, an endoplasmic reticulum protein, is secreted into the gastrointestinal mucus. PLoS ONE 2014, 9, e104186. [Google Scholar] [CrossRef] [PubMed]
| PDIA# | Alternate Names | Catalytic Motif(s) | ER Retention Sequence | Strongly Selective in DepMap (6/16/25) | Confirmed as DDA Targets | Highly Expressed (The Human Protein Atlas; 6/16/25) |
|---|---|---|---|---|---|---|
| 1 | P4HB | CGHC, CGHC | –KDEL | – | + | Liver, Pancreas |
| 2 | PDA2, PDIp | CGHC, CTHC | –KEEL | – | – | Pancreas |
| 3 | ERp57 | CGHC, CGHC | –QEDL | – | – | Thyroid Gland |
| 4 | ERp72 | CGHC, CGHC, CGHC | –KEEL | – | – | (Broadly Expressed) |
| 5 | PDIR | CSMC, CGHC, CPHC | –KEEL | – | – | Liver |
| 6 | ERp5 | CGHC, CGHC | –KDEL | +CRISPR | – | (Broadly Expressed) |
| 7 | PDILT | – | –KEEL | +RNAi | – | Stomach |
| 8 | ERp27 | – | –KTEL | +RNAi | – | Pancreas |
| 9 | ERp29 | – | –KEEL | – | – | (Broadly Expressed) |
| 10 | ERp44, TXNDC4 | CRFS | –RDEL | +CRISPR +RNAi | + | (Broadly Expressed) |
| 11 | TMX1 | CPAC | Transmembrane | +CRISPR | – | (Broadly Expressed) |
| 12 | TMX2 | – | Transmembrane | – | – | (Broadly Expressed) |
| 13 | TMX3 | CGHC | Transmembrane | – | – | (Broadly Expressed) |
| 14 | TMX4 | CPSC | Transmembrane | – | – | (Broadly Expressed) |
| 15 | TXNDC5, ERp46 | CGHC, CGHC, CGHC | –KDEL | – | – | (Broadly Expressed) |
| 16 | TXNDC12, AGR1 | CGAC | –KTEL | – | – | (Broadly Expressed) |
| 17 | AGR2 | CPHS | –KTEL | +CRISPR +RNAi | + | Cervix, Intestine, Stomach |
| 18 | AGR3 | CQYS | –QSEL | +CRISPR | + | Fallopian Tube, Intestine, Lung |
| 19 | DnaJC10, DnaJ | CSHC, CPPC, CHPC, CGPC | –KDEL | – | – | Epididymis |
| PDIB1 | Calsequestrin 1 | – | – | +RNAi | – | Skeletal Muscle, Tongue |
| PDIB2 | Calsequestrin 2 | – | – | +RNAi | – | Heart Muscle |
| TMX5, TXNDC15 | CRFS | Transmembrane | – | – | (Broadly Expressed) |
| AGR2 Selectively Dependent Cancer Line | AGR2 Essentiality Rank (out of 17,347 Genes) | Tumor Type | Other Top 10 Essential Genes (Rank) |
|---|---|---|---|
| C80 | 1 | Colorectal | CDX2 (2) |
| COLO205 | 3 | Colorectal | FOXP4 (2), FOXA2 (9) |
| LS513 | 3 | Colorectal | CDX2 (2), CDX1 (5) |
| SNUC4 | 5 | Colorectal | Axin1 (8) |
| MAPACHS77 | 2 | Pancreatic | FZD5 (1), WNT7B (3), DVL1 (4), PORCN (6), WLS (7), LRP5 (8) |
| HSC39 | 7 | Esophagogastric | |
| 2313287 | 8 | Gastric | AXIN2 (9) |
| Rank | Gene |
|---|---|
| 1 | SOX9 |
| 2 | AGR3 |
| 3 | TTC7A |
| 4 | TCF7L2 (Wnt effector) |
| 5 | KDELR3 |
| Rank | Gene | Importance | Corr. Type |
|---|---|---|---|
| 1 | ERN2 (IRE1β) | 22.5% | – |
| 2 | MUC3A | 2.4% | – |
| 3 | LRRC19 | 1.6% | – |
| 4 | SH3BGRL2 | 1.3% | – |
| 5 | TCEA2 | 1.2% | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, M.E.; Dulloo, Z.M.; Hardy, B.; Kelegama, A.; Clark, R.; Rivas Montbrun, M.; Antmann, G.; Nooka, S.; Castellano, R.K.; Law, B.K. Non-Canonical, Strongly Selective Protein Disulfide Isomerases as Anticancer Therapeutic Targets. Biomolecules 2025, 15, 1146. https://doi.org/10.3390/biom15081146
Law ME, Dulloo ZM, Hardy B, Kelegama A, Clark R, Rivas Montbrun M, Antmann G, Nooka S, Castellano RK, Law BK. Non-Canonical, Strongly Selective Protein Disulfide Isomerases as Anticancer Therapeutic Targets. Biomolecules. 2025; 15(8):1146. https://doi.org/10.3390/biom15081146
Chicago/Turabian StyleLaw, Mary E., Zaafir M. Dulloo, Brian Hardy, Ania Kelegama, Reagan Clark, Mariana Rivas Montbrun, Gabriella Antmann, Srihith Nooka, Ronald K. Castellano, and Brian K. Law. 2025. "Non-Canonical, Strongly Selective Protein Disulfide Isomerases as Anticancer Therapeutic Targets" Biomolecules 15, no. 8: 1146. https://doi.org/10.3390/biom15081146
APA StyleLaw, M. E., Dulloo, Z. M., Hardy, B., Kelegama, A., Clark, R., Rivas Montbrun, M., Antmann, G., Nooka, S., Castellano, R. K., & Law, B. K. (2025). Non-Canonical, Strongly Selective Protein Disulfide Isomerases as Anticancer Therapeutic Targets. Biomolecules, 15(8), 1146. https://doi.org/10.3390/biom15081146






