Mitochondrial Extracellular Vesicles: A Novel Approach to Mitochondrial Quality Control
Abstract
1. Introduction
2. Classical MQC
2.1. Mitochondrial Biogenesis
2.2. Mitochondrial Fusion and Fission
2.3. Mitochondrial Protein Homeostasis
2.4. Mitophagy
3. MitoEVs Participate in MQC
3.1. Role of MitoEVs Biogenesis and Sorting in MQC
3.1.1. Exophers
3.1.2. Migrasomes
3.1.3. Ectosomes
3.1.4. Exosomes
3.1.5. Mitopher
3.2. MitoEVs Involved in the Transfer of Mitochondria
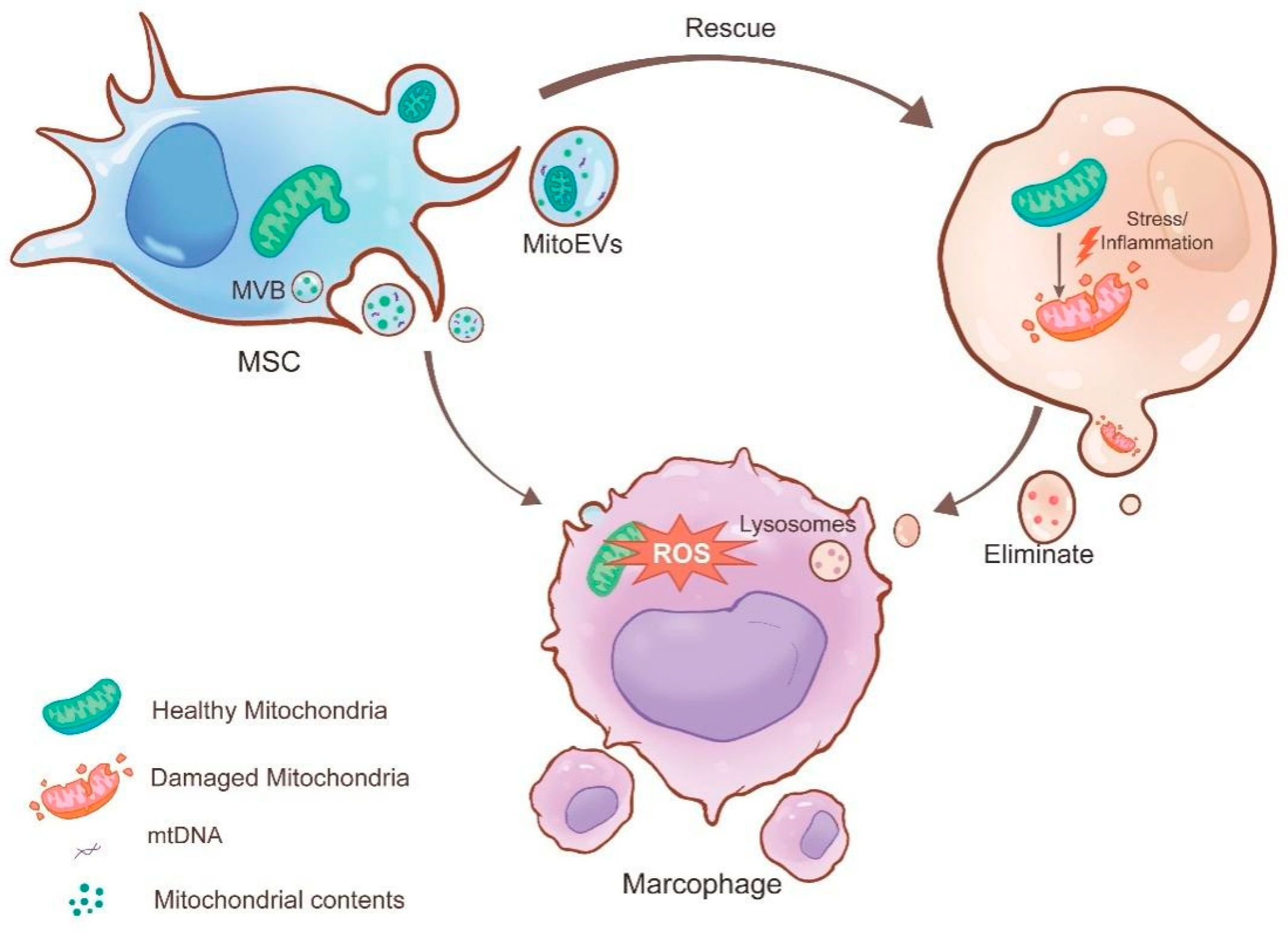
3.2.1. MitoEVs Act as Rescuers by Transferring Healthy Mitochondria to Damaged Cells
3.2.2. MitoEVs as Cleaners by Excluding Damaged Mitochondria
3.3. MitoEVs Transfer Mitochondrial Components
3.4. Cross-Talk Between Different Mechanisms of MQC
3.5. Mitochondrial Transfer Through Other Pathways
4. Clinical Translation of MitoEVs
4.1. Cardiovascular System
4.2. Nervous System
4.3. Respiratory System
4.4. Immune System
4.5. Digestive System
4.6. Urinary System
4.7. Other Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MitoEVs | mitochondrial extracellular vesicles |
| OXPHOS | oxidative phosphorylation |
| MQC | mitochondrial quality control |
| EVs | extracellular vesicles |
| PGC-1α | peroxisome proliferator-activated receptor-γ coactivator |
| Nrf2 | nuclear factor E2-related factor 2 |
| TFAM | mitochondrial transcription factor A |
| mtDNA | mitochondrial DNA |
| OMM | outer mitochondrial membrane |
| MP | mitochondrial protein |
| ROS | reactive oxygen species |
| TNT | tunneling nanotube |
| Cx43 | connexin 43 |
| MDVs | mitochondria-derived vesicles |
| MSCs | mesenchymal stem cells |
| MVs | microvesicles |
| BS | Behçet’s syndrome |
| MVB | multivesicular body |
| NETs | neutrophil extracellular traps |
| hUC-MSCs | human umbilical cord MSCs |
| IRI | ischemia–reperfusion injury |
| BAT | brown adipose tissue |
| cMacs | cardiac-resident macrophages |
| iCMs | cardiomyocytes |
| NSCs | neural stem cells |
References
- Cartalas, J.; Coudray, L.; Gobert, A. How RNases Shape Mitochondrial Transcriptomes. Int. J. Mol. Sci. 2022, 23, 6141. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in Aging and Age-Related Diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Liu, B.-H.; Xu, C.-Z.; Liu, Y.; Lu, Z.-L.; Fu, T.-L.; Li, G.-R.; Deng, Y.; Luo, G.-Q.; Ding, S.; Li, N.; et al. Mitochondrial Quality Control in Human Health and Disease. Mil. Med. Res. 2024, 11, 32. [Google Scholar] [CrossRef]
- Hao, T.; Yu, J.; Wu, Z.; Jiang, J.; Gong, L.; Wang, B.; Guo, H.; Zhao, H.; Lu, B.; Engelender, S.; et al. Hypoxia-Reprogramed Megamitochondrion Contacts and Engulfs Lysosome to Mediate Mitochondrial Self-Digestion. Nat. Commun. 2023, 14, 4105. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial Control of Inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.Á.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a Protein Corona on the Surface of Extracellular Vesicles in Blood Plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef] [PubMed]
- Sjoqvist, S.; Otake, K. Saliva and Saliva Extracellular Vesicles for Biomarker Candidate Identification-Assay Development and Pilot Study in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 5237. [Google Scholar] [CrossRef]
- Rahat, S.T.; Mäkelä, M.; Nasserinejad, M.; Ikäheimo, T.M.; Hyrkäs-Palmu, H.; Valtonen, R.I.P.; Röning, J.; Sebert, S.; Nieminen, A.I.; Ali, N.; et al. Clinical-Grade Patches as a Medium for Enrichment of Sweat-Extracellular Vesicles and Facilitating Their Metabolic Analysis. Int. J. Mol. Sci. 2023, 24, 7507. [Google Scholar] [CrossRef]
- Liu, B.; Jin, Y.; Yang, J.; Han, Y.; Shan, H.; Qiu, M.; Zhao, X.; Liu, A.; Jin, Y.; Yin, Y. Extracellular Vesicles from Lung Tissue Drive Bone Marrow Neutrophil Recruitment in Inflammation. J. Extracell. Vesicles 2022, 11, e12223. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- Tang, T.-T.; Wang, B.; Wu, M.; Li, Z.-L.; Feng, Y.; Cao, J.-Y.; Yin, D.; Liu, H.; Tang, R.-N.; Crowley, S.D.; et al. Extracellular Vesicle-Encapsulated IL-10 as Novel Nanotherapeutics against Ischemic AKI. Sci. Adv. 2020, 6, eaaz0748. [Google Scholar] [CrossRef]
- Lai, H.; Li, J.; Kou, X.; Mao, X.; Zhao, W.; Ma, L. Extracellular Vesicles for Dental Pulp and Periodontal Regeneration. Pharmaceutics 2023, 15, 282. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Chandra, S.; Lyon, C.; Ning, B.; Jiang, L.; Fan, J.; Hu, T.Y. Extracellular Vesicles: Emerging Tools as Therapeutic Agent Carriers. Acta Pharm. Sin. B 2022, 12, 3822–3842. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Xu, H.; Liu, S.; Li, Z.; Zhou, J.; Ding, F.; Zhang, X.; Wang, Y.; Jin, Y.; Wang, Q. Apoptotic Extracellular Vesicles Alleviate Pg-LPS Induced Inflammatory Responses of Macrophages via AMPK/SIRT1/NF-κB Pathway and Inhibit Osteoclast Formation. J. Periodontol. 2022, 93, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, J.; Cai, J.; Xiao, J.; Sui, X.; Yuan, X.; Li, R.; Li, Y.; Yao, J.; Lv, G.; et al. Extracellular Vesicles Derived from Mesenchymal Stromal Cells as Nanotherapeutics for Liver Ischaemia-Reperfusion Injury by Transferring Mitochondria to Modulate the Formation of Neutrophil Extracellular Traps. Biomaterials 2022, 284, 121486. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of Mitochondrial Biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α Signaling as a Potential Therapeutic Target for Mitochondrial Biogenesis in Neurodegenerative Disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef]
- Gao, B.; Doan, A.; Hybertson, B.M. The Clinical Potential of Influencing Nrf2 Signaling in Degenerative and Immunological Disorders. Clin. Pharmacol. 2014, 6, 19–34. [Google Scholar] [CrossRef]
- Hu, S.; Feng, J.; Wang, M.; Wufuer, R.; Liu, K.; Zhang, Z.; Zhang, Y. Nrf1 Is an Indispensable Redox-Determining Factor for Mitochondrial Homeostasis by Integrating Multi-Hierarchical Regulatory Networks. Redox Biol. 2022, 57, 102470. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ping, Y.; Li, X.; Mao, Y.; Chen, Y.; Shi, L.; Hong, X.; Chen, L.; Chen, S.; Cao, Z.; et al. Activation of PGC-1α-Dependent Mitochondrial Biogenesis Supports Therapeutic Effects of Silibinin against Type I Diabetic Periodontitis. J. Clin. Periodontol. 2023, 50, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Taddei, M.L.; Sacco, E.; Navari, N.; Correnti, M.; Piombanti, B.; Pastore, M.; Campani, C.; Pranzini, E.; Iorio, J.; et al. Mitochondrial Oxidative Metabolism Contributes to a Cancer Stem Cell Phenotype in Cholangiocarcinoma. J. Hepatol. 2021, 74, 1373–1385. [Google Scholar] [CrossRef]
- Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M.; Cordero, M.D. Lipophilic Antioxidants Prevent Lipopolysaccharide-Induced Mitochondrial Dysfunction through Mitochondrial Biogenesis Improvement. Pharmacol. Res. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Zheng, Y.; Zhang, Y.; Zhang, X.J.; Wang, H.; Du, Y.; Guan, J.; Wang, X.; Fu, J. NAD+ Improves Cognitive Function and Reduces Neuroinflammation by Ameliorating Mitochondrial Damage and Decreasing ROS Production in Chronic Cerebral Hypoperfusion Models through Sirt1/PGC-1α Pathway. J. Neuroinflamm. 2021, 18, 207. [Google Scholar] [CrossRef]
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021, 31, 62–74. [Google Scholar] [CrossRef]
- Tábara, L.C.; Burr, S.P.; Frison, M.; Chowdhury, S.R.; Paupe, V.; Nie, Y.; Johnson, M.; Villar-Azpillaga, J.; Viegas, F.; Segawa, M.; et al. MTFP1 Controls Mitochondrial Fusion to Regulate Inner Membrane Quality Control and Maintain mtDNA Levels. Cell 2024, 187, 3619–3637.e27. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Archer, S.L. Mitochondrial Dynamics--Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Perreten Lambert, H.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct Fission Signatures Predict Mitochondrial Degradation or Biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, L.; Song, Y.; Zhao, Z.; Li, X.; Wong, C.-Y.; Chen, R.; Feng, J.; Gou, Y.; Qi, Y.; et al. Force-Induced Tail-Autotomy Mitochondrial Fission and Biogenesis of Matrix-Excluded Mitochondrial-Derived Vesicles for Quality Control. Proc. Natl. Acad. Sci. USA 2024, 121, e2217019121. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.M.; Langer, T.; López-Otín, C. New Roles for Mitochondrial Proteases in Health, Ageing and Disease. Nat. Rev. Mol. Cell Biol. 2015, 16, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Sun, W.; Xue, J.; Zhou, Z.; Wang, W.; Guo, Q.; Chen, X.; Zhou, D.; Xu, Z.; Liu, L.; et al. Proteolytic Rewiring of Mitochondria by LONP1 Directs Cell Identity Switching of Adipocytes. Nat. Cell Biol. 2023, 25, 848–864. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, J.; Ran, Z. Emerging Views of Mitophagy in Immunity and Autoimmune Diseases. Autophagy 2020, 16, 3–17. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular Mitophagy: Mechanism, Roles in Diseases and Small Molecule Pharmacological Regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef]
- Ordureau, A.; Heo, J.-M.; Duda, D.M.; Paulo, J.A.; Olszewski, J.L.; Yanishevski, D.; Rinehart, J.; Schulman, B.A.; Harper, J.W. Defining Roles of PARKIN and Ubiquitin Phosphorylation by PINK1 in Mitochondrial Quality Control Using a Ubiquitin Replacement Strategy. Proc. Natl. Acad. Sci. USA 2015, 112, 6637–6642. [Google Scholar] [CrossRef]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality Control of the Mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Terešak, P.; Lapao, A.; Subic, N.; Boya, P.; Elazar, Z.; Simonsen, A. Regulation of PRKN-Independent Mitophagy. Autophagy 2022, 18, 24–39. [Google Scholar] [CrossRef]
- Lemasters, J.J. Variants of Mitochondrial Autophagy: Types 1 and 2 Mitophagy and Micromitophagy (Type 3). Redox Biol. 2014, 2, 749–754. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.-L.; Fon, E.A.; McBride, H.M. A New Pathway for Mitochondrial Quality Control: Mitochondrial-Derived Vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Xie, Y.; Sheng, H.; Wang, C.; Lian, Y.; Xie, N. Mitochondrial-Derived Vesicles: Gatekeepers of Mitochondrial Response to Oxidative Stress. Free Radic. Biol. Med. 2022, 188, 185–193. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, S.; Lu, Y.; Wan, M.; Cheng, J.; Liu, J. MitoEVs: A New Player in Multiple Disease Pathology and Treatment. J. Extracell. Vesicles 2023, 12, e12320. [Google Scholar] [CrossRef]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large Extracellular Vesicles: Size Matters in Tumor Progression. Cytokine Growth Factor Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef]
- Ciardiello, C.; Leone, A.; Lanuti, P.; Roca, M.S.; Moccia, T.; Minciacchi, V.R.; Minopoli, M.; Gigantino, V.; De Cecio, R.; Rippa, M.; et al. Large Oncosomes Overexpressing Integrin Alpha-V Promote Prostate Cancer Adhesion and Invasion via AKT Activation. J. Exp. Clin. Cancer Res. 2019, 38, 317. [Google Scholar] [CrossRef] [PubMed]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. C. elegans Neurons Jettison Protein Aggregates and Mitochondria under Neurotoxic Stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Gregory, C.D.; Rimmer, M.P. Extracellular Vesicles Arising from Apoptosis: Forms, Functions, and Applications. J. Pathol. 2023, 260, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, X.; Ye, J.; Ma, Y.; Mao, J.; Feng, D.; Wang, X. Migrasomes, a New Mode of Intercellular Communication. Cell Commun. Signal. 2023, 21, 105. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical Applications of Stem Cell-Derived Exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Liu, P.; Shi, J.; Sheng, D.; Lu, W.; Guo, J.; Gao, L.; Wang, X.; Wu, S.; Feng, Y.; Dong, D.; et al. Mitopherogenesis, a Form of Mitochondria-Specific Ectocytosis, Regulates Sperm Mitochondrial Quantity and Fertility. Nat. Cell Biol. 2023, 25, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, B.; Ou, Q.; Zhang, X.; He, Y.; Mao, X.; Wei, X.; Kou, X. ASC-Expressing Pyroptotic Extracellular Vesicles Alleviate Sepsis by Protecting B Cells. Mol. Ther. 2024, 32, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Sanchez, Z.C.; Kelley, N.M.; Hayes, J.B.; Ambroise, J.; Koory, E.N.; Krystofiak, E.; Taneja, N.; Zhang, Q.; Dungan, M.M.; et al. Blebbisomes Are Large, Organelle-Rich Extracellular Vesicles with Cell-like Properties. Nat. Cell Biol. 2025, 27, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Rizalar, F.S.; Lucht, M.T.; Petzoldt, A.; Kong, S.; Sun, J.; Vines, J.H.; Telugu, N.S.; Diecke, S.; Kaas, T.; Bullmann, T.; et al. Phosphatidylinositol 3,5-Bisphosphate Facilitates Axonal Vesicle Transport and Presynapse Assembly. Science 2023, 382, 223–230. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the Migrasome, an Organelle Mediating Release of Cytoplasmic Contents during Cell Migration. Cell Res. 2015, 25, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zou, Q.; Huang, R.; Li, Y.; Xing, X.; Fang, J.; Ma, L.; Li, L.; Yang, X.; Yu, L. Lateral Transfer of mRNA and Protein by Migrasomes Modifies the Recipient Cells. Cell Res. 2021, 31, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Shi, X.; Zhao, K.; Wang, L.; Shi, K.; Liu, Y.-J.; Li, H.; Ji, B.; Jiu, Y. Cell Migration Orchestrates Migrasome Formation by Shaping Retraction Fibers. J. Cell Biol. 2022, 221, e202109168. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Bi, M.; Liu, W.; Zhou, L.; Liu, H.; Yan, F.; Guan, L.; Zhang, J.; Xu, J. Migrasomes: From Biogenesis, Release, Uptake, Rupture to Homeostasis and Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 4525778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Ding, Y.; Zhang, J.; Xu, Y.; Xu, J.; Zheng, S.; Yang, H. Migrasome and Tetraspanins in Vascular Homeostasis: Concept, Present, and Future. Front. Cell Dev. Biol. 2020, 8, 438. [Google Scholar] [CrossRef]
- Jiao, H.; Jiang, D.; Hu, X.; Du, W.; Ji, L.; Yang, Y.; Li, X.; Sho, T.; Wang, X.; Li, Y.; et al. Mitocytosis, a Migrasome-Mediated Mitochondrial Quality-Control Process. Cell 2021, 184, 2896–2910.e13. [Google Scholar] [CrossRef]
- Kuang, L.; Wu, Y.; Shu, J.; Yang, J.; Zhou, H.; Huang, X. Pyroptotic Macrophage-Derived Microvesicles Accelerate Formation of Neutrophil Extracellular Traps via GSDMD-N-Expressing Mitochondrial Transfer during Sepsis. Int. J. Biol. Sci. 2024, 20, 733–750. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Wilen, C.B.; Moley, J.R.; Li, Y.; Zou, W.; Malvin, N.P.; Rowen, M.N.; Saunders, B.T.; Ma, H.; Mack, M.R.; et al. Intercellular Mitochondria Transfer to Macrophages Regulates White Adipose Tissue Homeostasis and Is Impaired in Obesity. Cell Metab. 2021, 33, 270–282.e8. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal Stem Cells Use Extracellular Vesicles to Outsource Mitophagy and Shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef]
- Wang, X.; Weidling, I.; Koppel, S.; Menta, B.; Perez Ortiz, J.; Kalani, A.; Wilkins, H.M.; Swerdlow, R.H. Detection of Mitochondria-Pertinent Components in Exosomes. Mitochondrion 2020, 55, 100–110. [Google Scholar] [CrossRef]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef]
- Konaka, H.; Kato, Y.; Hirano, T.; Tsujimoto, K.; Park, J.; Koba, T.; Aoki, W.; Matsuzaki, Y.; Taki, M.; Koyama, S.; et al. Secretion of Mitochondrial DNA via Exosomes Promotes Inflammation in Behçet’s Syndrome. EMBO J. 2023, 42, e112573. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, C.; Lv, N.; Liang, Z.; Ma, T.; Cheng, H.; Xia, Y.; Shi, L. AdMSC-Derived Exosomes Alleviate Acute Lung Injury via Transferring Mitochondrial Component to Improve Homeostasis of Alveolar Macrophages. Theranostics 2022, 12, 2928–2947. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Nakhle, J.; Griessinger, E.; Vignais, M.-L. Intercellular Mitochondria Trafficking Highlighting the Dual Role of Mesenchymal Stem Cells as Both Sensors and Rescuers of Tissue Injury. Cell Cycle 2018, 17, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Peng, J.; Zhang, X.; Zhang, F.; Wu, Y.; Huang, A.; Du, F.; Liao, Y.; He, Y.; et al. Astrocytic LRP1 Enables Mitochondria Transfer to Neurons and Mitigates Brain Ischemic Stroke by Suppressing ARF1 Lactylation. Cell Metab. 2024, 36, 2054–2068.e14. [Google Scholar] [CrossRef]
- Iorio, R.; Petricca, S.; Mattei, V.; Delle Monache, S. Horizontal Mitochondrial Transfer as a Novel Bioenergetic Tool for Mesenchymal Stromal/Stem Cells: Molecular Mechanisms and Therapeutic Potential in a Variety of Diseases. J. Transl. Med. 2024, 22, 491. [Google Scholar] [CrossRef]
- McLelland, G.-L.; Soubannier, V.; Chen, C.X.; McBride, H.M.; Fon, E.A. Parkin and PINK1 Function in a Vesicular Trafficking Pathway Regulating Mitochondrial Quality Control. EMBO J. 2014, 33, 282–295. [Google Scholar] [CrossRef]
- Ham, S.J.; Lee, D.; Yoo, H.; Jun, K.; Shin, H.; Chung, J. Decision between Mitophagy and Apoptosis by Parkin via VDAC1 Ubiquitination. Proc. Natl. Acad. Sci. USA 2020, 117, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.; Park, E. Cryo-EM Structure of the Mitochondrial Protein-Import Channel TOM Complex at near-Atomic Resolution. Nat. Struct. Mol. Biol. 2019, 26, 1158–1166. [Google Scholar] [CrossRef]
- Xiang, H.; Bao, C.; Chen, Q.; Gao, Q.; Wang, N.; Gao, Q.; Mao, L. Extracellular Vesicles (EVs)’ Journey in Recipient Cells: From Recognition to Cargo Release. J. Zhejiang Univ. Sci. B 2024, 25, 633–655. [Google Scholar] [CrossRef]
- Rosina, M.; Ceci, V.; Turchi, R.; Chuan, L.; Borcherding, N.; Sciarretta, F.; Sánchez-Díaz, M.; Tortolici, F.; Karlinsey, K.; Chiurchiù, V.; et al. Ejection of Damaged Mitochondria and Their Removal by Macrophages Ensure Efficient Thermogenesis in Brown Adipose Tissue. Cell Metab. 2022, 34, 533–548.e12. [Google Scholar] [CrossRef]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Levoux, J.; Prola, A.; Lafuste, P.; Gervais, M.; Chevallier, N.; Koumaiha, Z.; Kefi, K.; Braud, L.; Schmitt, A.; Yacia, A.; et al. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021, 33, 283–299.e9. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qi, Z.; Cao, L.; Ding, S. Mitochondrial Transfer/Transplantation: An Emerging Therapeutic Approach for Multiple Diseases. Cell Biosci. 2022, 12, 66. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem Cell-Based Therapy for Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhang, C.; Gao, J. Macrophages as Emerging Key Players in Mitochondrial Transfers. Front. Cell Dev. Biol. 2021, 9, 747377. [Google Scholar] [CrossRef]
- Turek, M.; Banasiak, K.; Piechota, M.; Shanmugam, N.; Macias, M.; Śliwińska, M.A.; Niklewicz, M.; Kowalski, K.; Nowak, N.; Chacinska, A.; et al. Muscle-derived Exophers Promote Reproductive Fitness. EMBO Rep. 2021, 22, e52071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, R.; Su, Y.; Sun, H.; Zheng, X.; Zhang, J.; Lu, X.; Zhao, B.; Jiang, X.; Huang, L.; et al. Efficient Intervention for Pulmonary Fibrosis via Mitochondrial Transfer Promoted by Mitochondrial Biogenesis. Nat. Commun. 2023, 14, 5781. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Im, G.-B.; Luo, A.C.; Zhu, Y.; Hong, X.; Neumeyer, J.; Tang, H.-W.; Perrimon, N.; Melero-Martin, J.M. Mitochondrial Transfer Mediates Endothelial Cell Engraftment through Mitophagy. Nature 2024, 629, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Dash, C.; Jayabalan, R.; Khiste, S.; Kulkarni, A.; Kurmi, K.; Mondal, J.; Majumder, P.K.; Bardia, A.; Jang, H.L.; et al. Intercellular Nanotubes Mediate Mitochondrial Trafficking between Cancer and Immune Cells. Nat. Nanotechnol. 2022, 17, 98–106. [Google Scholar] [CrossRef]
- Delvaeye, T.; Vandenabeele, P.; Bultynck, G.; Leybaert, L.; Krysko, D.V. Therapeutic Targeting of Connexin Channels: New Views and Challenges. Trends Mol. Med. 2018, 24, 1036–1053. [Google Scholar] [CrossRef]
- Wang, X.; Bukoreshtliev, N.V.; Gerdes, H.-H. Developing Neurons Form Transient Nanotubes Facilitating Electrical Coupling and Calcium Signaling with Distant Astrocytes. PLoS ONE 2012, 7, e47429. [Google Scholar] [CrossRef]
- Ariazi, J.; Benowitz, A.; De Biasi, V.; Den Boer, M.L.; Cherqui, S.; Cui, H.; Douillet, N.; Eugenin, E.A.; Favre, D.; Goodman, S.; et al. Tunneling Nanotubes and Gap Junctions-Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions. Front. Mol. Neurosci. 2017, 10, 333. [Google Scholar] [CrossRef]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles Transfer Mitochondria and Increase Mitochondrial Function in Brain Endothelial Cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Funcke, J.-B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular Vesicle-Based Interorgan Transport of Mitochondria from Energetically Stressed Adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.-H.; O’Brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-Rich Extracellular Vesicles From Autologous Stem Cell-Derived Cardiomyocytes Restore Energetics of Ischemic Myocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef]
- van der Vlist, M.; Raoof, R.; Willemen, H.L.D.M.; Prado, J.; Versteeg, S.; Martin Gil, C.; Vos, M.; Lokhorst, R.E.; Pasterkamp, R.J.; Kojima, T.; et al. Macrophages Transfer Mitochondria to Sensory Neurons to Resolve Inflammatory Pain. Neuron 2022, 110, 613–626.e9. [Google Scholar] [CrossRef]
- Berridge, M.V.; Schneider, R.T.; McConnell, M.J. Mitochondrial Transfer from Astrocytes to Neurons Following Ischemic Insult: Guilt by Association? Cell Metab. 2016, 24, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Willis, C.M.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.C.; Braga, A.; van den Bosch, A.; Leonardi, T.; et al. Neural Stem Cells Traffic Functional Mitochondria via Extracellular Vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef] [PubMed]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal Stromal Cell Extracellular Vesicles Rescue Mitochondrial Dysfunction and Improve Barrier Integrity in Clinically Relevant Models of ARDS. Eur. Respir. J. 2021, 58, 2002978. [Google Scholar] [CrossRef]
- Hough, K.P.; Trevor, J.L.; Strenkowski, J.G.; Wang, Y.; Chacko, B.K.; Tousif, S.; Chanda, D.; Steele, C.; Antony, V.B.; Dokland, T.; et al. Exosomal Transfer of Mitochondria from Airway Myeloid-Derived Regulatory Cells to T Cells. Redox Biol. 2018, 18, 54–64. [Google Scholar] [CrossRef]
- Maeda, A.; Fadeel, B. Mitochondria Released by Cells Undergoing TNF-α-Induced Necroptosis Act as Danger Signals. Cell Death Dis. 2014, 5, e1312. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.N.; Cheng, L.-C.; Kuo, C.-L.; Lo, Y.K.; Chou, H.-Y.; Chen, C.-H.; Wang, Y.-H.; Chuang, T.-H.; Cheng, S.-J.; Lee, A.Y.-L. Mitochondrial Lon-Induced mtDNA Leakage Contributes to PD-L1-Mediated Immunoescape via STING-IFN Signaling and Extracellular Vesicles. J. Immunother. Cancer 2020, 8, e001372. [Google Scholar] [CrossRef]
- Guan, B.; Liu, Y.; Xie, B.; Zhao, S.; Yalikun, A.; Chen, W.; Zhou, M.; Gu, Q.; Yan, D. Mitochondrial Genome Transfer Drives Metabolic Reprogramming in Adjacent Colonic Epithelial Cells Promoting TGFβ1-Mediated Tumor Progression. Nat. Commun. 2024, 15, 3653. [Google Scholar] [CrossRef]
- Thomas, M.A.; Fahey, M.J.; Pugliese, B.R.; Irwin, R.M.; Antonyak, M.A.; Delco, M.L. Human Mesenchymal Stromal Cells Release Functional Mitochondria in Extracellular Vesicles. Front. Bioeng. Biotechnol. 2022, 10, 870193. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Ma, K.; Zheng, Z.; Liu, X.; Hu, B.; Liu, H.; Zhao, Q.; Han, Y.; Xiao, Z.; et al. Circulating B Cell-Derived Small RNA Delivered by Extracellular Vesicles: A Dialogue Mechanism for Long-Range Targeted Renal Mitochondrial Injury in Obesity. Small 2024, 20, e2402526. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Wang, Y.; Qian, H.; Zhu, C.; Dong, H.; Hao, C.; Zhang, Y.; Ji, Z.; Li, X.; et al. Cardiac Fibroblast-Derived Mitochondria-Enriched sEVs Regulate Tissue Inflammation and Ventricular Remodeling Post-Myocardial Infarction through NLRP3 Pathway. Pharmacol. Res. 2025, 214, 107676. [Google Scholar] [CrossRef]
- Wang, J.; Moosavizadeh, S.; Jammes, M.; Tabasi, A.; Bach, T.; Ryan, A.E.; Ritter, T. In-Vitro Immunomodulatory Efficacy of Extracellular Vesicles Derived from TGF-Β1/IFN-γ Dual Licensed Human Bone Marrow Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2025, 16, 357. [Google Scholar] [CrossRef]
- Seo, W.; Gao, Y.; He, Y.; Sun, J.; Xu, H.; Feng, D.; Park, S.H.; Cho, Y.-E.; Guillot, A.; Ren, T.; et al. ALDH2 Deficiency Promotes Alcohol-Associated Liver Cancer by Activating Oncogenic Pathways via Oxidized DNA-Enriched Extracellular Vesicles. J. Hepatol. 2019, 71, 1000–1011. [Google Scholar] [CrossRef]
- Gao, Y.; Mi, N.; Wu, W.; Zhao, Y.; Fan, F.; Liao, W.; Ming, Y.; Guan, W.; Bai, C. Transfer of Inflammatory Mitochondria via Extracellular Vesicles from M1 Macrophages Induces Ferroptosis of Pancreatic Beta Cells in Acute Pancreatitis. J. Extracell. Vesicles 2024, 13, e12410. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Yan, E.; Do, M.; Kim, Y.; Lee, Y.; Cho, S.-G.; Kim, D.-H. Engineering Extracellular Vesicles for ROS Scavenging and Tissue Regeneration. Nano Converg. 2024, 11, 24. [Google Scholar] [CrossRef]
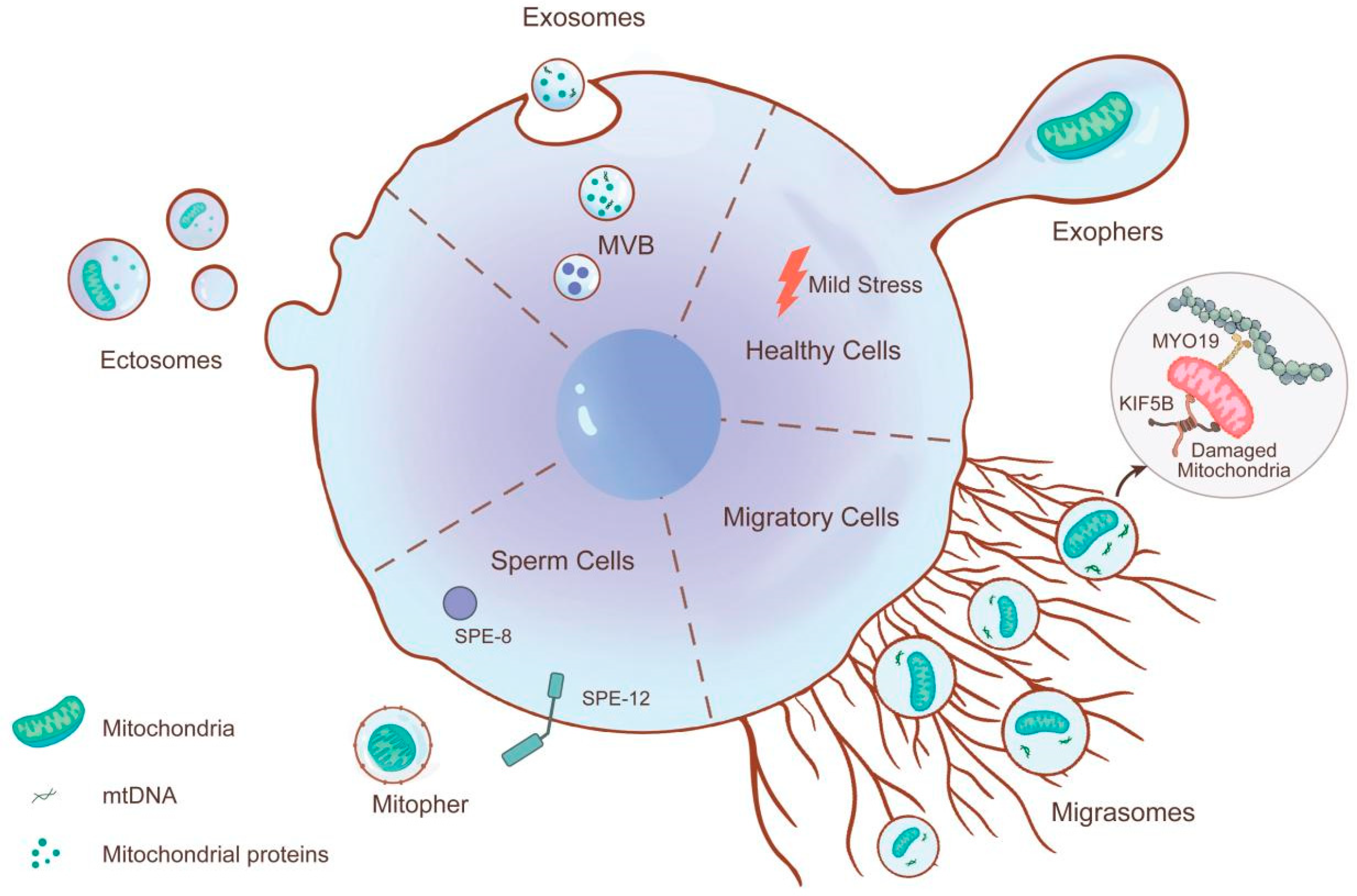
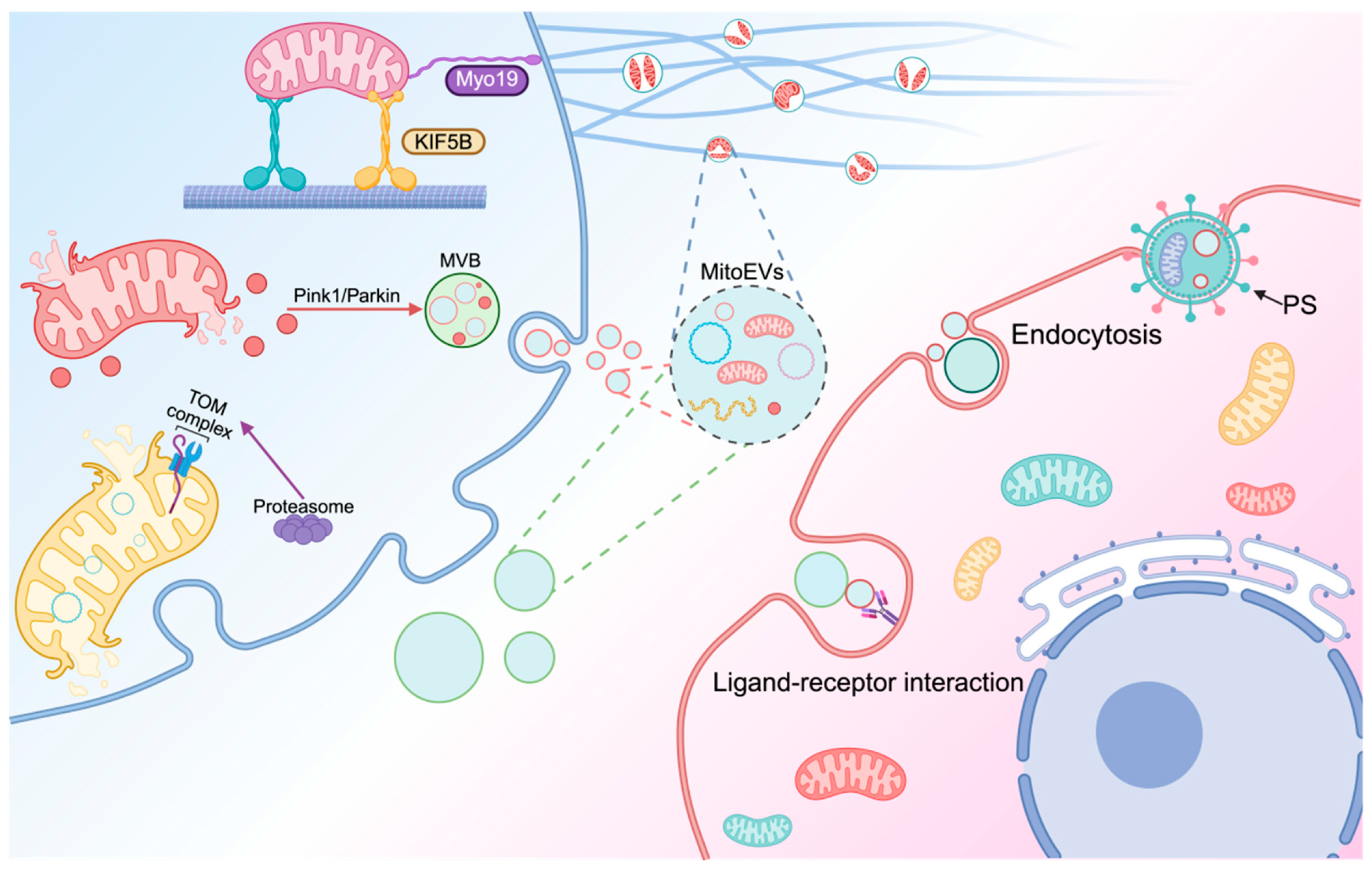

| Subtype | Size (nm) | Markers | Biogenesis/Release | Cargo | Refs. |
|---|---|---|---|---|---|
| Large oncosomes | 1000–10,000 | Caveolin 1, CK18, and GAPDH | Cancer cells | Proteins and nucleic acids | [46,47] |
| Exophers | 1000–7800 | Phosphatidyl-serine | Jettisoned from cell body | Mitochondria, lysosomes, and protein aggregates | [48] |
| Apoptotic bodies | 1000–5000 | CD9, CD63, CD81, C3b, and TSP | Budding from plasma membrane during apoptosis | Mitochondria, ribosomes, and proteins | [49] |
| Migrasomes | 500–3000 | TSPAN4, CPQ, EOGT, NDST1, and PIGK | Cell migration | Mitochondria, mtDNA, and proteins | [50] |
| Ectosomes (microparticles, microvesicles, and shedding vesicles) | 100–1000 | Annexin A1 and A2, and ARF6 | Outward budding of the plasma membrane | Mitochondria, mitochondrial proteins, proteins, lipids, and carbohydrates | [14] |
| Exosomes | 30–150 | CD63, CD9, CD81, TSG101, Alix, and HSP70 | Originating in the endosomal pathway in the MVB and released when MVB fused with plasma membrane | mtDNA, mitochondria-pertinent components, proteins, glycoconjugates, lipids, nucleic acids, and metabolites | [51,52] |
| Mitopher | 490–1100 | Unknown | Outward budding off | One single mitochondrion | [53] |
| Pyroptotic extracellular vesicles | 60–200 | ASC and Annexin V | Pyroptotic cells | Unknown | [54] |
| Blebbisomes | up to 20,000 | VDAC2, VDAC1, and TGN protein 2 | A single retraction event where a cell fragment remains attached to the substrate via a membrane nanotube and is released upon severing of the nanotube | Mitochondria and cellular organelles | [55] |
| Organ/System/Disease | Donor | Types of MitoEVs | Recipient | Cargos | Mechanism/Effect | Refs. |
|---|---|---|---|---|---|---|
| Cardiovascular System/Brain/Ischemic Stroke | Brain endothelial cell | Microvesicles | Endothelial cells and neurons | Polarized mitochondria | ATP production ↑, endothelial cell survival ↑ | [94] |
| Cardiovascular System/Cardiac/Ischemia–Reperfusion Injury | Adipocyte | Exosomes | Cardiomyocyte | Oxidatively-damaged mitochondrial particles | Induce adaptation in recipient cells, protect the heart from damage caused by obesity | [95] |
| Cardiovascular System/Cardiac/Ischemic Myocardium | Autologous-stem-cell-derived cardiomyocytes | Microvesicles | Cardiomyocyte | Mitochondria | Mitochondrial biogenesis ↑, cardiac function ↑ | [96] |
| Nervous System/Neuronal/Pain | Macrophages | Microvesicles | Sensory neurons | Mitochondria | CD200R/iSec1 receptor–ligand complex, inflammatory pain ↓ | [97] |
| Nervous System/Neuronal/Cerebral Ischemia | Astrocytes | Microvesicles | Neurons | Mitochondria | Protect neurons from hypoxia and glucose deprivation | [98] |
| Nervous System/Ventricular/ Degenerative Neurological Diseases | Neural stem cell | Microvesicles | Monocytes | Mitochondria | Restore mitochondrial dynamics and cellular metabolism | [99] |
| Respiratory System/Lung/Acute Respiratory Distress Syndrome | Mesenchymal stem cells | Microvesicles | Human pulmonary microvascular endothelial cells and human small airway epithelial cells | Functional mitochondria | Barrier integrity of human primary lung epithelial and endothelial cells ↑, symptoms of ARDS ↓ | [100] |
| Respiratory System/Lung/Acute Respiratory Distress Syndrome | Mesenchymal stem cells | Microvesicles | Monocyte-derived macrophages | Functional mitochondria | CD206 expression ↑, associate with ARDS | [83] |
| Respiratory System/Lung/Acute Lung Injury | Mesenchymal stem cells | Exosomes | Alveolar macrophages | mtDNA | Macrophage metabolism and immune homeostasis ↑, associate with acute lung injury | [69] |
| Respiratory System/Bronchial/Asthmatic | Airway myeloid-derived regulatory cells | Exosomes | Peripheral T cells | mtDNA | Alter the function of T cells, associate with asthma | [101] |
| Immune System/Multi-organ/Leukemia | Necroptotic cells | Microvesicles | Macrophage | Healthy mitochondria | Immune activation, inflammation ↓ | [102] |
| Immune System /Oral/Tumor | Oral squamous cell carcinoma cells | Exosomes | Macrophage | mtDNA | T-cell activation ↓, anti-tumor immunity ↓ | [103] |
| Immune System/Multi-organ/Behçet’s disease | Pyroptotic cells | Exosomes | Adjacent cells | mtDNA | Inflammatory response ↑, associate with BS | [68] |
| Digestive System/Hepatic/Ischemia–Reperfusion Injury | Mesenchymal stem cells | Microvesicles | Neutrophil | Healthy mitochondria | NET formation ↓, associate with liver IRI | [17] |
| Digestive System/Colon/Colon Cancer | Colon cancer cell | Exosomes | Adjacent colonic epithelial cells | mtDNA | ROS ↑, associate with colon cancer | [104] |
| Locomotor System/Bone/Regenerative Orthobiologic | Mesenchymal stem cells | Microvesicles | Chondrocytes | Healthy mitochondria | OA symptoms and pain ↓, preserve articular cartilage | [105] |
| Endocrine System/Adipose Tissue/Thermogenesis | Brown adipocytes | Microvesicles | Macrophages | Damaged mitochondria | Releases damaged mitochondria, restore thermogenic function | [77] |
| Urinary System/Kidney/Acute Kidney Injury | Mesenchymal stem cells | Exosomes | Renal proximal tubular cell lines | Mitochondrial proteins, mtDNA | TFAM expression ↑, treat kidney injury | [87] |
| Urinary System/Kidney/ Obesity-related Kidney Injury | B Lymphocyte | Exosomes | Proximal tubule epithelial cells | miR-3960 | Mitochondrial damage ↑, associate with obesity-related kidney damage | [106] |
| Reproductive System | Sperm cells | Mitopher | Unknown | Mitochondrion | Regulates sperm mitochondrial quantity and fertility | [53] |
| Multiple Systems | Migratingcell | Migrasome | Unknown | Damaged mitochondria | Remove damaged mitochondria and maintain cell viability | [62] |
| Cardiovascular System | Cardiomyocytes | Exophers | Macrophages | Dysfunctional mitochondria | Maintain cardiomyocyte health and cardiac function | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, J.; Sun, R.; Du, C.; Tang, Y.; Xie, C.; Li, Q.; Lin, L.; Wang, H. Mitochondrial Extracellular Vesicles: A Novel Approach to Mitochondrial Quality Control. Biomolecules 2025, 15, 1145. https://doi.org/10.3390/biom15081145
Kong J, Sun R, Du C, Tang Y, Xie C, Li Q, Lin L, Wang H. Mitochondrial Extracellular Vesicles: A Novel Approach to Mitochondrial Quality Control. Biomolecules. 2025; 15(8):1145. https://doi.org/10.3390/biom15081145
Chicago/Turabian StyleKong, Jie, Rui Sun, Chengying Du, Yiyang Tang, Chengzhi Xie, Qian Li, Li Lin, and Hongyan Wang. 2025. "Mitochondrial Extracellular Vesicles: A Novel Approach to Mitochondrial Quality Control" Biomolecules 15, no. 8: 1145. https://doi.org/10.3390/biom15081145
APA StyleKong, J., Sun, R., Du, C., Tang, Y., Xie, C., Li, Q., Lin, L., & Wang, H. (2025). Mitochondrial Extracellular Vesicles: A Novel Approach to Mitochondrial Quality Control. Biomolecules, 15(8), 1145. https://doi.org/10.3390/biom15081145






