Gosha-Jinki-Gan Reduces Inflammation in Chronic Ischemic Stroke Mouse Models by Suppressing the Infiltration of Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation for Gosha-Jinki-Gan (TJ107) Diet
2.3. Experimental Design
2.4. Histopathological Examination
2.5. Macrophage Cell Culture
2.6. Analysis of Cytokine mRNA Using Real-Time RT-PCR
2.7. Statistical Analysis
3. Results
3.1. TJ107 Recovered Body Weight in MCAO Mice
3.2. TJ107 Significantly Reduced Infarct Volume at Day 60 After MCAO
3.3. TJ107 Suppressed Brain-Infiltrating Macrophages and Reduced Expression of Immune Mediators in MCAO Mice
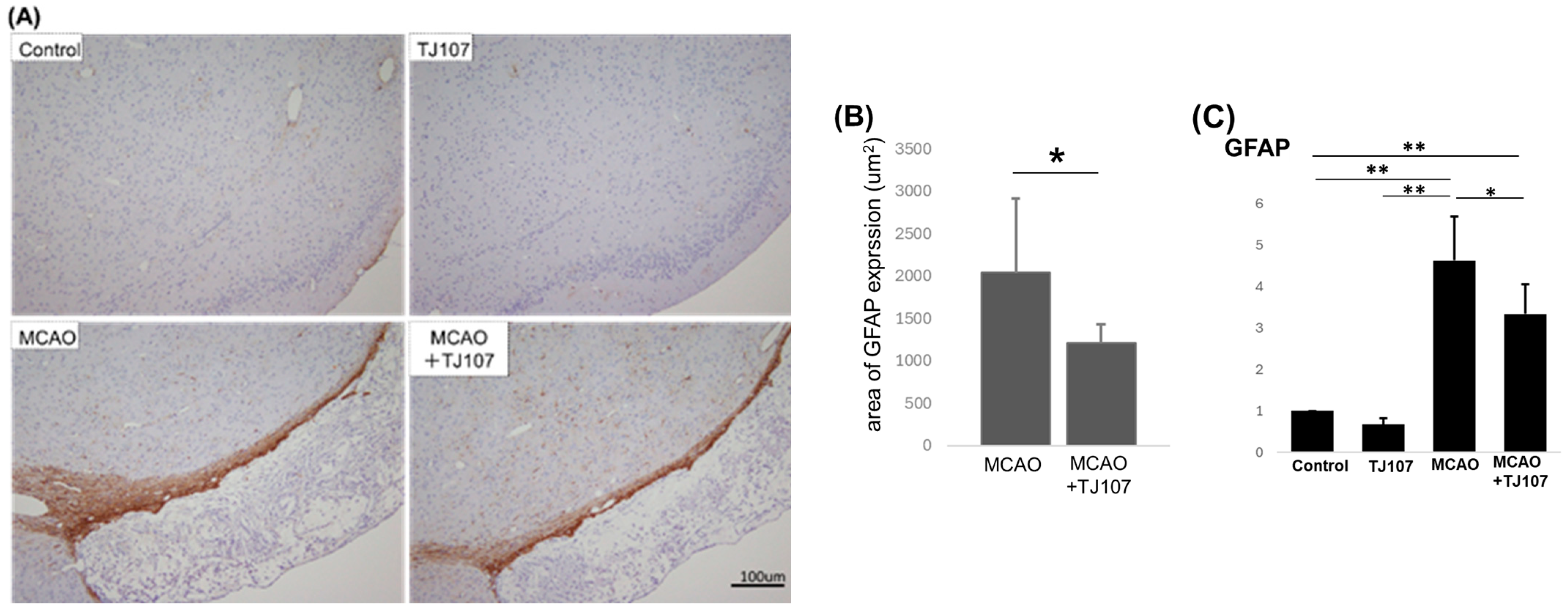
3.4. TJ107 Suppresses GFAP Expression in MCAO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCAO | middle cerebral artery occlusion |
| TJ107 | Gosha-jinki-gan |
| BBB | blood–brain barrier |
| MSCs | mesenchymal stem cells |
| DAMPs | danger-associated molecular patterns |
| TLR | tool-like receptor |
| GFAP | glial fibrillary acidic protein |
| AEVs | astrocytic extracellular vesicles |
References
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.B.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Fagan, S.C.; Hess, D.C.; Hohnadel, E.J.; Pollock, D.M.; Ergul, A. Targets for vascular protection after acute ischemic stroke. Stroke 2004, 35, 2220–2225. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Macrez, R.; Ali, C.; Toutirais, O.; Le Mauff, B.; Defer, G.; Dirnagl, U.; Vivien, D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011, 10, 471–480. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Hasegawa, E.; Kimura, A.; Morita, R.; Sakaguchi, R.; Takada, I.; Sekiya, T.; Ooboshi, H.; Kitazono, T.; Yanagawa, T.; et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012, 18, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Serrenho, I.; Ferreira, S.A.; Baltazar, G. Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact—A Systematic Review. Cells 2024, 13, 845. [Google Scholar] [CrossRef]
- Wei, L.; Fraser, J.L.; Lu, Z.Y.; Hu, X.; Yu, S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 2012, 46, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.H.; Wu, C.J.; Xu, X.Q.; Lu, S.S.; Zu, Q.Q.; Zhao, L.B.; Wang, J.; Liu, S.; Shi, H.B. Hypoxic conditioned medium derived from bone marrow mesenchymal stromal cells protects against ischemic stroke in rats. J. Cell. Physiol. 2019, 234, 1354–1368. [Google Scholar] [CrossRef]
- Kelly-Hayes, M.; Beiser, A.; Kase, C.S.; Scaramucci, A.; D’Agostino, R.B.; Wolf, P.A. The influence of gender and age on disability following ischemic stroke: The Framingham study. J. Stroke Cerebrovasc. Dis. 2003, 12, 119–126. [Google Scholar] [CrossRef]
- Bu, Y.; Lee, K.; Jung, H.S.; Moon, S.K. Therapeutic effects of traditional herbal medicine on cerebral ischemia: A perspective of vascular protection. Chin. J. Integr. Med. 2013, 19, 804–814. [Google Scholar] [CrossRef]
- Qu, N.; Kuramasu, M.; Hirayanagi, Y.; Nagahori, K.; Hayashi, S.; Ogawa, Y.; Terayama, H.; Suyama, K.; Naito, M.; Sakabe, K.; et al. Gosha-Jinki-Gan recovers spermatogenesis in mice with busulfan-induced aspermatogenesis. Int. J. Mol. Sci. 2018, 19, 2606. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Nagahori, K.; Kuramasu, M.; Ogawa, Y.; Suyama, K.; Hayashi, S.; Sakabe, K.; Itoh, M. Effect of Gosha-Jinki-Gan on levels of specific mRNA transcripts in mouse testes after busulfan treatment. Biomedicines 2020, 8, 432. [Google Scholar] [CrossRef]
- Nakano-Doi, A.; Nakagomi, T.; Fujikawa, M.; Nakagomi, N.; Kubo, S.; Lu, S.; Yoshikawa, H.; Soma, T.; Taguchi, A.; Matsuyama, T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells 2010, 28, 1292–1302. [Google Scholar] [CrossRef]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differntiate into neural and vascular lineage cells. Stem Cells 2015, 33, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Nakagomi, T.; Maeda, M.; Nakano-Doi, A.; Momota, Y.; Matsuyama, T. Induction of Perivascular Neural Stem Cells and Possible Contribution to Neurogenesis Following Transient Brain Ischemia/Reperfusion Injury. Transl. Stroke Res. 2017, 8, 131–143. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.B.; Chen, Z.; Wang, H.-Q.; Zhang, L.; Jiang, Y.; Li, T.; Yang, C.-F.; Wang, X.-Y.; Li, X.; et al. Inhibiting HMGB1-RAGE Axis Prevents Pro-Inflammatory Macrophages/Microglia Polarization and Affords Neuroprotection After Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 295. [Google Scholar] [CrossRef]
- Gelderblom, M.; Sobey, C.G.; Kleinschnitz, C.; Magnus, T. Danger signals in stroke. Ageing Res. Rev. 2015, 24 Pt A, 77–82. [Google Scholar] [CrossRef]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418774254. [Google Scholar] [CrossRef]
- Clark, W.M.; Lessov, N.; Lauten, J.D.; Hazel, K. Doxycycline treatment reduces ischemic brain damage in transient middle cerebral artery occlusion in the rat. J. Mol. Neurosci. 1997, 9, 103–108. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.-F.; Mao, L.; Xia, Y.-P.; Jin, H.-J.; Li, Y.-N.; You, M.-F.; et al. Microglia-derived TNF-αmediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef]
- Liberale, L.; Diaz-Cañestro, C.; Bonetti, N.R.; Paneni, F.; Akhmedov, A.; Beer, J.H.; Montecucco, F.; Lüscher, T.F.; Camici, G.G. Post-ischaemic administration of the murine Canakinumab-surrogate antibody improves outcome in experimental stroke. Eur. Heart J. 2018, 39, 3511–3517. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Fathali, N.; Doyle, K.P.; Williams, A.M.; Han, J.; Buckwalter, M.S. Astrocytic transforming growth factor beta signaling reduces subacute neuroinflammation after stroke in mice. Glia 2014, 62, 1227–1240. [Google Scholar] [CrossRef]
- Li, L.; Lundkvist, A.; Andersson, D.; Wilhelmsson, U.; Nagai, N.; Pardo, A.C.; Nodin, C.; Stahlberg, A.; Aprico, K.; Larsson, K.; et al. Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow. Metab. 2008, 28, 468–481. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and Is Neuroprotective Following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Choudhury, G.R.; Ding, S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol. Dis. 2016, 85, 234–244. [Google Scholar] [CrossRef]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef]
- Kaneko, S.; Iwanami, A.; Nakamura, M.; Kishino, A.; Kikuchi, K.; Shibata, S.; Okano, H.J.; Ikegami, T.; Moriya, A.; Konishi, O.; et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 2006, 12, 1380–1389. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regen eration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, N.; Yenari, M.A. Mechanisms and potential therapeutic applications of microglial activation after brain injury. CNS Neurosci. Ther. 2015, 21, 309–319. [Google Scholar] [CrossRef]
- Jin, X.; Yamashita, T. Microglia in central nervous system repair after injury. J. Biochem. 2016, 159, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kijima, C.; Inaba, T.; Hira, K.; Miyamoto, N.; Yamashiro, K.; Urabe, T.; Hattori, N.; Ueno, Y. Astrocytic Extracellular Vesicles Regulated by Microglial Inflammatory Responses Improve Stroke Recovery. Mol. Neurobiol. 2024, 61, 1002–1021. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Ryu, J.M.; Kim, Y.J.; Park, S.U.; Jahng, G.H.; Park, J.M.; Moon, S.; Ko, C.; Cho, K. Uhwang Chungsim won decreases blood oxygen level-dependent fMRI signal response to a motor stimulation task. Chin. J. Integr. Med. 2012, 21, 493–499. [Google Scholar] [CrossRef]
- Cho, K.; Noh, K.; Jung, W.; Park, S.; Moon, S.; Park, J.; Ko, C.; Kim, Y.S.; Bae, H.S. A preliminary study on the inhibitory effect of Chunghyul-dan on stroke recurrence in patients with small vessel disease. Neurol. Res. 2008, 30, 655–658. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Matsubara, M.; Kobayashi, S. Event-related brain potential changes after Choto-san administration in stroke patients with mild cognitive impairments. Psychopharmacology 2004, 171, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Yang, Q.; Kita, T.; Hikiami, H.; Shimada, Y.; Terasawa, K. Effects of Choto-san on microcirculation, serum nitric oxide and lipid peroxides in patients with asymptomatic cerebral infarction. Am. J. Chin. Med. 2001, 29, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, S.K.; Choi, I.Y.; Ju, C.; Nam, K.W.; Hwang, S.; Kim, B.W.; Yoon, M.J.; Won, M.H.; Park, Y.K.; et al. Amelioration of cerebral infarction and improvement of neurological deficit by a Korean herbal medicine, modified Bo-Yang-Hwan-O-Tang. J. Pharm. Pharmacol. 2011, 63, 695–706. [Google Scholar] [CrossRef]
- Wu, P.F.; Zhang, Z.; Wang, F.; Chen, J.G. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010, 31, 1523–1531. [Google Scholar] [CrossRef]
- Dohare, P.; Garg, P.; Sharma, U.; Jagannathan, N.R.; Ray, M. Neuroprotective efficacy and therapeutic window of curcuma oil: In rat embolic stroke model. BMC Complement. Altern. Med. 2008, 8, 55. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Sharma, S.S. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004, 74, 969–985. [Google Scholar] [CrossRef]
- Son, H.Y.; Han, H.S.; Jung, H.W.; Park, Y.K. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J. Pharmacol. Sci. 2009, 109, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, C.Q.; Chen, B.Y.; Zhang, S.P.; Liang, Y.; Luo, X.G. Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J. Ethnopharmacol. 2009, 121, 412–418. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Zheng, W.; Lu, Y.; Feng, G.; Yu, S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008, 1229, 224–232. [Google Scholar] [CrossRef]
- Al-Omar, F.A.; Nagi, M.N.; Abdulgadir, M.M.; Al Joni, K.S.; Al-Majed, A.A. Immediate and delayed treatments with curcumin prevents forebrain ischemia-induced neuronal damage and oxidative insult in the rat hippocampus. Neurochem. Res. 2006, 31, 611–618. [Google Scholar] [CrossRef]
- Ghoneim, A.I.; Abdel-Naim, A.B.; Khalifa, A.E.; El-Denshary, E.S. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol. Res. 2002, 46, 273–279. [Google Scholar] [CrossRef] [PubMed]



| Forward Primer (5′-3′) | Reverse Primer (5′-3′) | |
|---|---|---|
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| TLR2 | TGCAAGTACGAACTGGACTTCT | CCAGGTAGGTCTTGGTCATT |
| TLR4 | GTGCCATCATTATGAGTGCC | CAAGCCAAGAAATATACCATCGAAG |
| IL-1β | GAAGCTGGATGCTCTCATCTG | TTGACGGACCCCAAAAGAT |
| IL-17 | CAACAGTAGCAAAGACTTGACCA | CCCAGGAAGACATACTTAGAAGAAA |
| TNFα | TCTTCTCATTCCTGCTTGTGG | TCTGGGCCATAGAACTGATGA |
| F4/80 | CTTTGGCTATGGGCTTCCAGTC | GCAAGGAGGACAGAGTTTATCGTG |
| CD163 | GGGTCATTCAGAGGCACAGTG | CTGGCTGTCCTGTCAAGGCT |
| GFAP | TGGCCACTGTGAGGCAGAAG | ACCTCCTCCTCGTGGATCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Suyama, K.; Nagahori, K.; Kiyoshima, D.; Miyakawa, S.; Deguchi, H.; Katahira, Y.; Mizoguchi, I.; Terayama, H.; Hayashi, S.; et al. Gosha-Jinki-Gan Reduces Inflammation in Chronic Ischemic Stroke Mouse Models by Suppressing the Infiltration of Macrophages. Biomolecules 2025, 15, 1136. https://doi.org/10.3390/biom15081136
Xu M, Suyama K, Nagahori K, Kiyoshima D, Miyakawa S, Deguchi H, Katahira Y, Mizoguchi I, Terayama H, Hayashi S, et al. Gosha-Jinki-Gan Reduces Inflammation in Chronic Ischemic Stroke Mouse Models by Suppressing the Infiltration of Macrophages. Biomolecules. 2025; 15(8):1136. https://doi.org/10.3390/biom15081136
Chicago/Turabian StyleXu, Mingli, Kaori Suyama, Kenta Nagahori, Daisuke Kiyoshima, Satomi Miyakawa, Hiroshi Deguchi, Yasuhiro Katahira, Izuru Mizoguchi, Hayato Terayama, Shogo Hayashi, and et al. 2025. "Gosha-Jinki-Gan Reduces Inflammation in Chronic Ischemic Stroke Mouse Models by Suppressing the Infiltration of Macrophages" Biomolecules 15, no. 8: 1136. https://doi.org/10.3390/biom15081136
APA StyleXu, M., Suyama, K., Nagahori, K., Kiyoshima, D., Miyakawa, S., Deguchi, H., Katahira, Y., Mizoguchi, I., Terayama, H., Hayashi, S., Yoshimoto, T., & Qu, N. (2025). Gosha-Jinki-Gan Reduces Inflammation in Chronic Ischemic Stroke Mouse Models by Suppressing the Infiltration of Macrophages. Biomolecules, 15(8), 1136. https://doi.org/10.3390/biom15081136







