Tauopathies: Calmodulin Regulates Tau Hyperphosphorylation and Its Transformation into Disease-Specific Aggregates
Abstract
1. Introduction
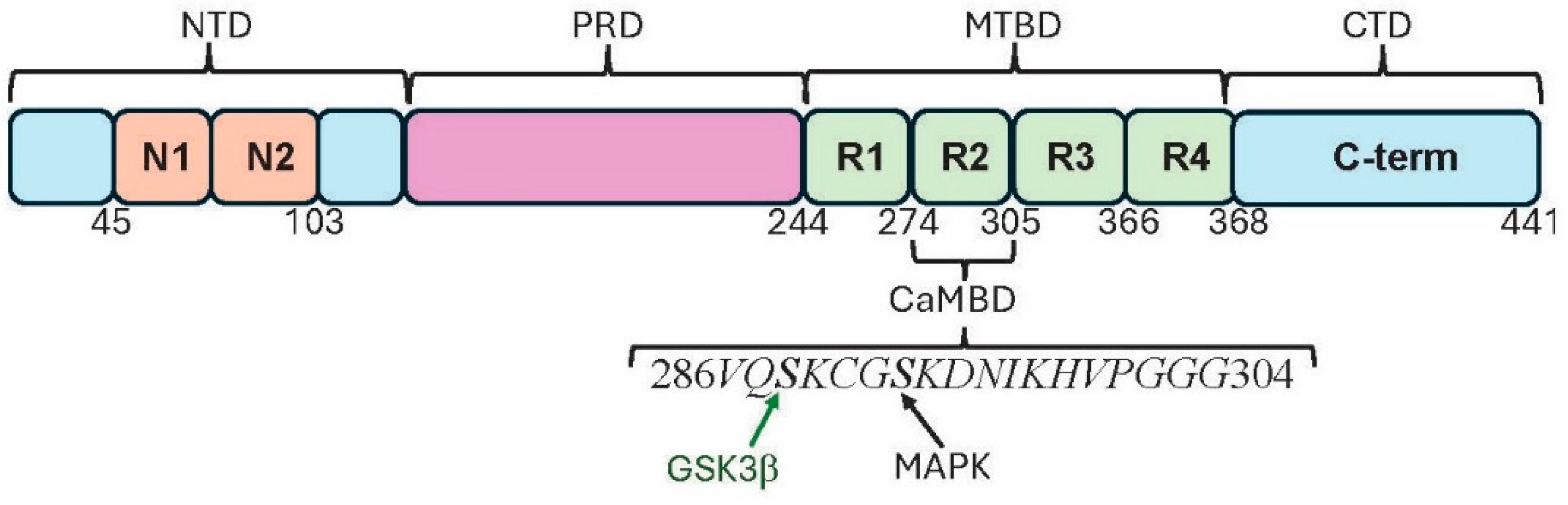
2. Tau Structure
3. Calcium Dysregulation in Tauopathies
4. Calmodulin: The Basics
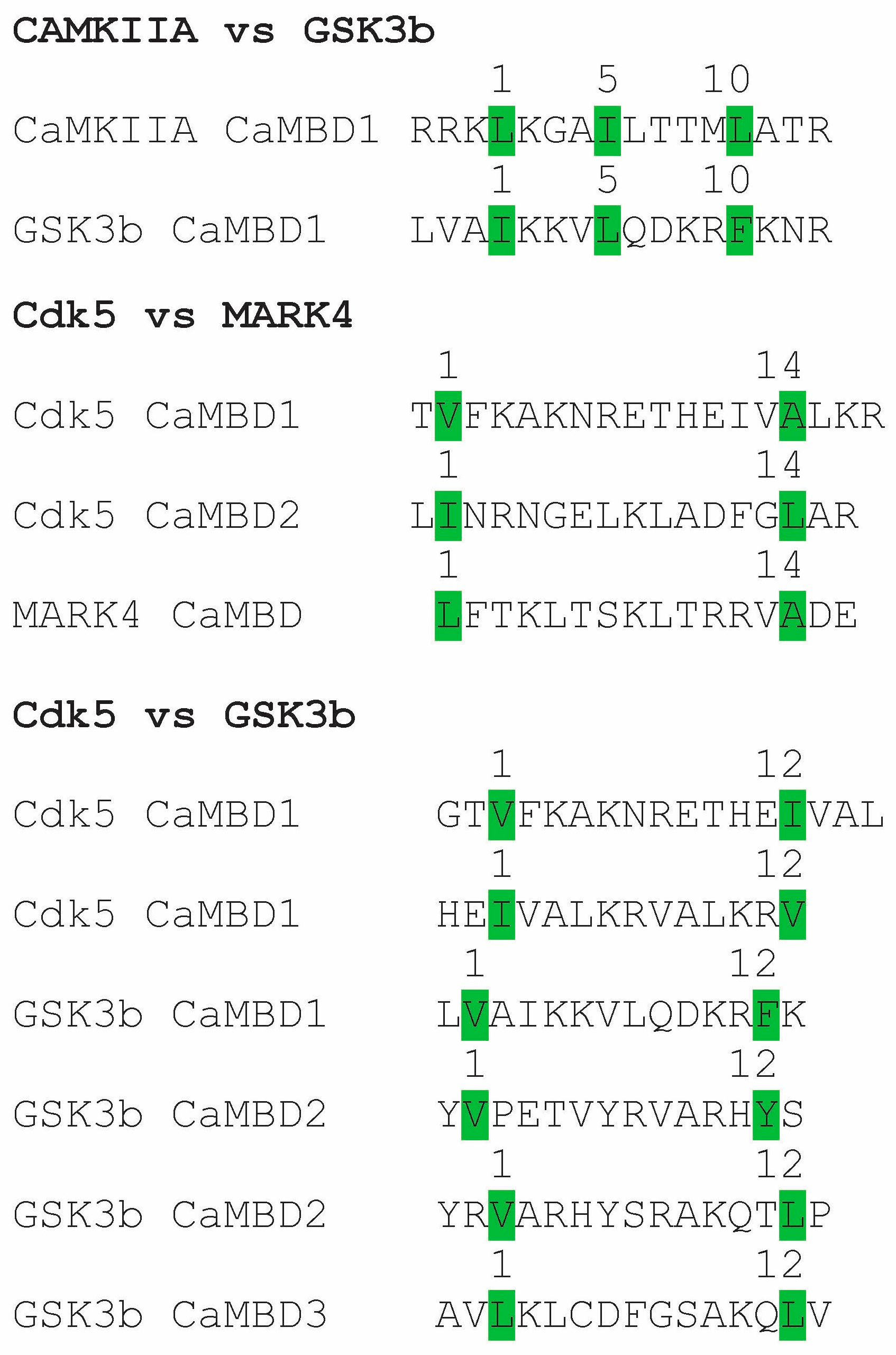
5. Tau Binds to Calmodulin
6. Tau Phosphorylation
6.1. CaMKII
6.2. GSK3
6.3. Cdk5
6.4. MARK4
6.5. ROCK and Rho
6.6. Calcineurin
6.7. Bottom of Form
7. From Prephosphorylation to Hyperphosphorylation
8. Tau Aggregation
9. Discussion
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovacs, G.G. Tauopathies. Handb. Clin. Neurol. 2017, 145, 355–368. [Google Scholar]
- Chu, Y.; Hirst, W.D.; Kardover, J.H. Mixed pathology as a rule, not exception: Time to reconsider disease nosology. Handb. Clin. Neurol. 2023, 192, 57–71. [Google Scholar]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An english translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Delacourte, A.; David, J.P.; Sergeant, N.; Buée, L.; Wattez, A.; Vermersch, P.; Ghozali, F.; Fallet-Bianco, C.; Pasquier, F.; Lebert, F.; et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 1999, 52, 1158–1165. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Marcatti, M.; Tumurbaatar, B.; Borghi, M.; Guptarak, J.; Zhang, W.R.; Krishnan, B.; Kayed, R.; Fracassi, A.; Taglialatela, G. Inhibition of calcineurin with FK506 reduces Tau levels and attenuates synaptic impairment driven by Tau oligomers in the Hippocampus of male mouse models. Int. J. Mol. Sci. 2024, 25, 9092. [Google Scholar] [CrossRef]
- LaCroix, M.S.; Mirbaha, H.; Shang, P.; Zandee, S.; Foong, C.; Prat, A.; White, C.L., 3rd; Stuve, O.; Diamond, M.I. Tau seeding in cases of multiple sclerosis. Acta Neuropathol. Commun. 2022, 10, 146. [Google Scholar] [CrossRef]
- Mees, I.; Nisbet, R.M.; Hannan, A.J.; Renoir, T. Implications of Tau dysregulation in Huntington’s disease and potential for new therapeutics. J. Huntingt.’s Disease. 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Walker, L.; Attems, J. Prevalence of concomitant pathologies in Parkinson’s disease: Implications for prognosis, diagnosis, and Insights into common pathogenic mechanisms. J. Park. Dis. 2024, 14, 35–52. [Google Scholar] [CrossRef]
- Creekmore, B.C.; Watanabe, R.; Lee, E.B. Neurodegenerative disease tauopathies. Annu. Rev. Pathol. 2024, 19, 345–370. [Google Scholar] [CrossRef]
- Josephs, K.A. Current understanding of neurodegenerative diseases associated with the protein Tau. Mayo Clin. Proc. 2017, 92, 1291–1303. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, K.M.; Yang, L.; Dong, Q.; Yu, J.T. Tauopathies: New perspectives and challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef]
- Saito, Y.; Ruberu, N.N.; Sawabe, M.; Arai, T.; Tanaka, N.; Kakuta, Y.; Yamanouchi, H.; Murayama, S. Staging of argyrophilic grains: An age-associated tauopathy. J. Neuropathol. Exp. Neurol. 2004, 63, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, F.; Park, S.; Sengupta, U.; Puangmalai, N.; Bhatt, N.; Shchankin, N.; Jerez, C.; Moreno, N.; Bittar, A.; Xavier, R.; et al. Brain-derived tau oligomer polymorphs: Distinct aggregations, stability profiles, and biological activities. Commun. Biol. 2025, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, H.; Cai, Q.; Tai, H.C. The enigma of Tau protein aggregation: Mechanistic insights and future challenges. Int. J. Mol. Sci. 2024, 25, 4969. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.; Zhang, W.; Murzin, A.G.; Mushudov, G.; Garringer, H.J.; Vidal, R.; Crowther, R.A.; Ghetti, B.; Scheres, S.H.W.; Goedert, M. Structures of filaments from Pick’s disease reveal a novel tau protein 481-fold. Nature 2018, 561, 137–140. [Google Scholar] [CrossRef]
- Han, Y.; He, Z. Concomitant protein pathogenesis in Parkinson’s disease and perspective mechanisms. Front. Aging Neurosci. 2023, 15, 1189809. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Shafiei, S.S.; Guerrero-Muñoz, M.J.; Castillo-Carranza, D.L. Tau oligomers: Cytotoxicity, propagation, and mitochondrial damage. Front. Aging Neurosci. 2017, 9, 83. [Google Scholar] [CrossRef]
- Zwang, T.J.; Sastre, E.D.; Wolf, N.; Ruiz-Uribe, N.; Woost, B.; Hoglund, Z.; Fan, Z.; Bailey, J.; Nfor, L.; Buée, L.; et al. Neurofibrillary tangle-bearing neurons have reduced risk of cell death in mice with Alzheimer’s pathology. Cell Rep. 2024, 43, 114574. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, N.M. Tau here, tau there, tau almost everywhere: Clarifying the distribution of tau in the adult CNS. Cytoskeleton 2024, 81, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Trabzuni, D.; Wray, S.; Vandrovcova, J.; Ramasamy, A.; Walker, R.; Smith, C.; Trabzuni, D.; Wray, S.; Vandrovcova, J.; Ramasamy, A.; et al. MAPT expression and splicing is differentially regulated by brain region: Relation to genotype and implication for tauopathies. Hum. Mol. Genet. 2012, 21, 4094–4103. [Google Scholar] [CrossRef]
- Reddy, P.H. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res. 2011, 1415, 136–148. [Google Scholar] [CrossRef]
- Baas, P.W.; Qiang, L. Tau: It’s not what you think. Trends Cell Biol. 2019, 29, 452–461. [Google Scholar] [CrossRef]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau associates with changes in its function beyond microtubule stability. Front. Cell. Neurosci. 2018, 12, 338. [Google Scholar] [CrossRef]
- Nishida, K.; Matsumura, K.; Tamura, M.; Nakamichi, T.; Shimamori, K.; Kuragano, M.; Kabir, A.M.R.; Kakugo, A.; Kotani, S.; Nishishita, N.; et al. Effects of three microtubule-associated proteins (MAP2, MAP4, and Tau) on microtubules’ physical properties and neurite morphology. Sci. Rep. 2023, 13, 8870. [Google Scholar] [CrossRef]
- Paterno, G.; Bell, B.M.; Gorion, K.M.M.; Prokop, S.; Giasson, B.I. Reassessment of neuronal Tau distribution in adult human brain and implications for Tau pathobiology. Acta Neuropathol. Commun. 2022, 10, 94. [Google Scholar] [CrossRef]
- Götz, J.; Halliday, G.; Nisbet, R.M. Molecular pathogenesis of the tauopathies. Annu. Rev. Pathol. 2019, 14, 239–261. [Google Scholar] [CrossRef]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau post-translational modifications: Dynamic transformers of Tau function, degradation, and aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Potier, M.C.; Ulrich, J.; Crowther, R.A. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: Differential expression of tau protein mRNAs in human brain. EMBO J. 1989, 8, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Prokop, S.; Giasson, B.I. “Don’t Phos Over Tau”: Recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol. Neurodegener. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, N.; Malina, L.; Malohlava, J.; Hajdúch, M.; Das, V. Tau R2 and R3 are essential regions for tau aggregation, seeding and propagation. Biochimie 2022, 200, 79–86. [Google Scholar] [CrossRef]
- Fyfe, I. Tau folds differently between diseases. Nat. Rev. Neurol. 2018, 14, 633. [Google Scholar] [CrossRef]
- Webber, E.K.; Fivaz, M.; Stutzmann, G.E.; Griffoen, G. Cytosolic calcium: Judge jury and executioner of neurodegeneration in Alzheimer’s disease and beyond. Alzheimer’s Dement. 2023, 19, 3701–3717. [Google Scholar] [CrossRef]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The role of Ca2+ signaling in Parkinson’s disease. Dis. Models Mech. 2017, 10, 519–535. [Google Scholar] [CrossRef]
- Alzheimer’s Association Calcium Hypothesis Workgroup; Khachaturian, Z.S. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer’s Dement. 2017, 13, 178–182. [Google Scholar]
- O’Day, D.H. Alzheimer’s disease beyond calcium dysregulation: The complex interplay between calmodulin, calmodulin binding proteins and amyloid beta from disease onset through progression. Curr. Issues Mol. Biol. 2023, 45, 6246–6261. [Google Scholar] [CrossRef]
- Datta, D.; Leslie, S.N.; Wang, M.; Morozov, Y.M.; Yang, S.; Mentone, S.; Zeiss, C.; Duque, A.; Rakic, P.; Horvath, T.L.; et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimer’s Dement. 2021, 17, 920–932. [Google Scholar] [CrossRef]
- Guan, P.-P.; Cao, L.-L.; Wang, P. Elevating the levels of calcium Ions exacerbate Alzheimer’s disease via inducing the production and aggregation of b-amyloid protein and phosphorylated tau. Int. J. Mol. Sci. 2021, 22, 5900. [Google Scholar] [CrossRef]
- Diepenbroek, M.; Casadei, N.; Esmer, H.; Saido, T.C.; Takano, J.; Kahle, P.J.; Nixon, R.A.; Rao, M.V.; Melki, R.; Pieri, L.; et al. Over expression of the calpain-specific inhibitor calpastatin reduces human alpha-Synuclein processing, aggregation and synaptic impairment in [A30P] aSyn transgenic mice. Hum. Mol. Genet. 2014, 23, 3975–3989. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. Intracellular calcium homeostasis. Ann. Rev. Biochem. 1987, 56, 395–433. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Neuronal calcium signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Schrank, S.; Barrington, N.; Stutzmann, G.E. Calcium-handling defects and neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a035212. [Google Scholar] [CrossRef]
- Deb, B.K.; Hasan, G. Regulation of store-operated Ca2+ entry by septins. Front. Cell. Dev. Biol. 2016, 4, 142. [Google Scholar] [CrossRef]
- Princen, K.; Van Dooren, T.; van Gorsel, M.; Louros, N.; Yang, X.; Dumbacher, M.; Bastiaens, I.; Coupet, K.; Dupont, S.; Cuveliers, E.; et al. Pharmacological modulation of septins restores calcium homeostasis and is neuroprotective in models of Alzheimer’s disease. Science 2024, 384, eadd6260. [Google Scholar] [CrossRef]
- O’Day, D.H. The complex interplay between toxic hallmark proteins, calmodulin, calmodulin binding proteins and ion channels linked to calcium dyshomeostasis in Alzheimer’s, Huntington’s and Parkinson’s diseases. Biomolecules 2024, 14, 173. [Google Scholar] [CrossRef]
- Chung, D.C.; Roemer, S.; Petrucelli, L.; Strong, M.G. Cellular and pathological heterogeneity of primary tauopathies. Mol. Neurodegener. 2021, 16, 57. [Google Scholar] [CrossRef]
- Devi, G. The tauopathies. Handb. Clin. Neurol. 2023, 196, 251–265. [Google Scholar]
- Khachaturian, Z.S. Calcium hypothesis of Alzheimer’s disease and brain aging. Ann. N. Y. Acad. Sci. 1994, 747, 1–11. [Google Scholar] [CrossRef]
- O’Day, D.H.; Myre, M.A. Calmodulin Binding Domains in Alzheimer’s Disease Proteins: Extending the Calcium Hypothesis. Biochem. Biophys. Res. Commun. 2004, 320, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H.; Eshak, K.; Myre, M.A. Calmodulin Binding Proteins and Alzheimer’s Disease: A Review. J. Alzheimer’s Dis. 2015, 46, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; García, M.A.; González, I.L.; Lucena, D.D.; Villalonga, A.R.; Tech, M.C.; Llorens, F.; Garcia-Esparcia, P.; Martinez-Maldonado, A.; Mendez, M.F.; et al. Aging-related tau astrogliopathy (ARTAG): Not only tau phosphorylation in astrocytes. Brain Pathol. 2018, 28, 965–985. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. Calcium and Non-Penetrating Traumatic Brain Injury: A Proposal for the Implementation of an Early Therapeutic Treatment for Initial Head Insults. Biomolecules 2024, 14, 853. [Google Scholar] [CrossRef]
- Stein-O’Brien, G.L.; Palaganas, R.; Meyer, E.M.; Redding-Ochoa, J.; Pletnikova, O.; Guo, H.; Bell, W.R.; Troncoso, J.C.; Huganir, R.L.; Morris, M. Transcriptional signatures of hippocampal tau pathology in primary age-related tauopathy and Alzheimer’s disease. Cell Rep. 2025, 44, 115422. [Google Scholar] [CrossRef]
- Imamura, K.; Sahara, N.; Kanaan, N.; Tsukita, K.; Kondo, T.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Kawakami, K.; Hotta, A.; et al. Calcium dysregulation contributes to neurodegeneration in FTLD patient iPSC-derived neurons. Sci. Rep. 2016, 6, 34904. [Google Scholar] [CrossRef]
- Berrocal, M.; Corbacho, I.; Vázquez-Hernández, M.; Ávila, J.; Sepúlveda, M.R.; Mata, A.M. Inhibition of PMCA activity by tau as a function of aging and Alzheimer’s neuropathology. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1465–1476. [Google Scholar] [CrossRef]
- Atlante, A.; Valenti, D.; Latina, V.; Amadoro, G. Dysfunction of Mitochondria in Alzheimer’s Disease: ANT and VDAC Interact with Toxic Proteins and Aid to Determine the Fate of Brain Cells. Int. J. Mol. Sci. 2022, 23, 7722. [Google Scholar] [CrossRef] [PubMed]
- Koren, T.D.K.; Shrivastava, R.; Siddiqui, S.I.; Ghosh, S. Calmodulin modulates the gating properties of voltage-dependent anion channel from rat brain mitochondria. J. Phys. Chem. B 2022, 126, 4857–4871. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, A.R.; Friedberg, F. Sequence motifs for calmodulin recognition. FASEB J. 1997, 11, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tidow, H.; Nissen, P. Structural diversity of calmodulin binding to its target sites. FEBS J. 2013, 280, 5551–5565. [Google Scholar] [CrossRef]
- Grant, B.M.M.; Enomoto, M.; Ikura, M.; Marshall, C.B. A non-canonical calmodulin target motif comprising a polybasic region and lapidated terminal residue regulates localization. Int. J. Mol. Sci. 2020, 21, 2751. [Google Scholar] [CrossRef]
- Kirberger, M.; Yang, J.J. Structural aspects and prediction of calmodulin-binding proteins. Int. J. Mol. Sci. 2021, 22, 308. [Google Scholar]
- Lee, Y.C.; Wolff, J. Calmodulin binds to both microtubule-associated protein 2 and tau proteins. J. Biol. Chem. 1984, 259, 1226–1230. [Google Scholar] [CrossRef]
- Padilla, R.; Maccioni, R.; Avila, J. Calmodulin binds to a tubulin binding site of the microtubule-associated protein tau. Mol. Cell. Biochem. 1990, 97, 35–41. [Google Scholar] [CrossRef]
- Yap, K.L.; Kim, J.; Truong, K.; Sherman, M.; Yuan, T.; Ikura, M. Calmodulin target database. J. Struct. Funct. Genom. 2000, 1, 8–14. [Google Scholar] [CrossRef]
- Mruk, K.; Farley, B.M.; Ritacco, A.W.; Kobertz, W.R. Calmodulation meta-analysis: Predicting calmodulin binding via canonical motif clustering. J. Gen. Physiol. 2014, 144, 105–114. [Google Scholar] [CrossRef]
- Myre, M.A.; Tesco, G.; Tanzi, R.E.; Wasco, W. Calmodulin binding to APP and the APLPs. In Molecular Mechanisms of Neurodegeneration: A Joint Biochemical Society/Neuroscience Ireland Focused Meeting; University College Dublin: Dublin, Ireland, 2005. [Google Scholar]
- Chavez, S.E.; O’Day, D.H. Calmodulin binds to and regulates the activity of beta-secretase (BACE1). Curr. Res. Alzheimers Dis. 2007, 1, 37–47. [Google Scholar]
- Canobbio, I.; Catricalà, S.; Balduini, C.; Torti, M. Calmodulin regulates the non-amyloidogenic metabolism of amyloid precursor protein in platelets. Biochem. Biophys. Acta 2011, 1813, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Man, V.H.; He, X.; Gao, J.; Wang, J. Phosphorylation of Tau R2 Repeat Destabilizes Its Binding to Microtubules: A Molecular Dynamics Simulation Study. ACS Chem. Neurosci. 2023, 14, 458–467. [Google Scholar] [CrossRef] [PubMed]
- von Bergen, M.; Barghorn, S.; Jeganathan, S.; Mandelkow, E.M.; Mandelkow, E. Spectroscopic approaches to the conformation of tau protein in solution and in paired helical filaments. Neurodegener. Dis. 2006, 3, 197–206. [Google Scholar] [CrossRef]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Huang, L.; Lei, F.; Li, T.; Luo, Y.; Zeng, M.; Wang, Z. The role and pathogenesis of Tau protein in Alzheimer’s disease. Biomolecules 2025, 15, 824. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.; Wang, J.Z.; Liu, R.; Wang, X. Tau Ubiquitination in Alzheimer’s Disease. Front. Neurol. 2022, 12, 786353. [Google Scholar] [CrossRef]
- Basheer, N.; Smolek, T.; Hassan, I.; Liu, F.; Iqbal, K.; Zilka, N.; Novak, P. Does modulation of tau hyperphosphorylation represent a reasonable therapeutic strategy for Alzheimer’s disease? From preclinical studies to the clinical trials. Mol. Psychiatry 2023, 28, 2197–2214. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Yang, Y.; Murzin, A.G.; Falcon, B.; Kotecha, A.; van Beers, M.; Tarutani, A.; Kametani, F.; Garringer, H.J.; et al. Structure-based classification of tauopathies. Nature 2021, 598, 359–363. [Google Scholar] [CrossRef]
- Kimura, T.; Sharma, G.; Ishiguro, K.; Hisanaga, S.I. Phospho-Tau Bar Code: Analysis of Phosphoisotypes of Tau and Its Application to Tauopathy. Front. Neurosci. 2018, 12, 44. [Google Scholar] [CrossRef]
- Branca, C.; Shaw, D.M.; Belfiore, R.; Gokhale, V.; Shaw, A.Y.; Foley, C.; Smith, B.; Hulme, C.; Dunckley, T.; Meechoovet, B.; et al. Dyrk1 inhibition improves Alzheimer’s disease-like pathology. Aging Cell 2017, 16, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Sander, A.; Oswald, M.S.; Gartner, F.; Knippschild, U.; Bischof, J. Comprehensive characterization of CK1δ-mediated tau phosphorylation in Alzheimer’s disease. Front. Mol. Biosci. 2022, 9, 872171. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.M.F.; dos Reis, R.; Franco, G.; Gontijo, V.S.; Viegas, C., Jr. Protein kinases as therapeutic targets for Alzheimer’s disease: A brief review. Explor. Neuroprot. Ther. 2024, 4, 411–441. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef]
- Yu, D.Y.; Tong, L.; Song, G.J.; Lin, W.L.; Zhang, L.Q.; Bai, W.; Gong, H.; Yin, Y.X.; Wei, Q. Tau binds both subunits of calcineurin, and binding is impaired by calmodulin. Biochim. Biophys. Acta 2008, 1783, 2255–2261. [Google Scholar] [CrossRef]
- Hanson, P.I.; Schulman, H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem. 1992, 61, 559–601. [Google Scholar] [CrossRef]
- Payne, M.E.; Fong, Y.L.; Ono, T.; Colbran, R.J.; Kemp, B.E.; Soderling, T.R.; Means, A.R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J. Biol. Chem. 1988, 263, 7190–7195. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, G.H.; Hu, X.Y.; Lu, Y.P.; Zhou, J.N.; Liu, R.Y. The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer’s disease and its links with AD-related pathology. Brain Res. 2005, 1031, 101–108. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ichinose, T.; Yamauchi, T. Phosphorylation of tau protein to sites found in Alzheimer’s disease brain is catalyzed by Ca2+/calmodulin-dependent protein kinase II as demonstrated tandem mass spectrometry. Neurosci. Lett. 2003, 353, 185–188. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hiragami, Y.; Murayama, M.; Ishizuka, K.; Kawahara, M.; Takashima, A. Phosphorylation of tau at serine 416 by Ca2+/calmodulin-dependent protein kinase II in neuronal soma in brain. J. Neurochem. 2005, 94, 1438–1447. [Google Scholar] [CrossRef]
- Sałaciak, K.; Koszałka, A.; Żmudzka, E.; Pytka, K. The Calcium/Calmodulin-Dependent Kinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders. Int. J. Mol. Sci. 2021, 22, 4307. [Google Scholar] [CrossRef]
- Augustinack, J.C.; Schneider, A.; Mandelkow, E.-M.; Hyman, B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002, 103, 26–35. [Google Scholar] [CrossRef]
- Islam, T.; Hill, E.; Abrahamson, E.E.; Servaes, S.; Smirnov, D.S.; Zeng, X.; Sehrawat, A.; Chen, Y.; Kac, P.R.; Kvartsberg, H.; et al. Phospho-tau serine-262 and serine-356 as biomarkers of pre-tangle soluble tau assemblies in Alzheimer’s disease. Nat. Med. 2025, 31, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Grundke-Iqbal, I.; Iqbal, K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 2007, 25, 59–68. [Google Scholar] [CrossRef]
- Payne, M.E.; Soderling, T.R. Calmodulin-dependent glycogen synthase kinase. J. Biol. Chem. 1980, 255, 8054–8056. [Google Scholar] [CrossRef]
- Woodgett, J.R.; Tonks, N.K.; Cohen, P. Identification of a calmodulin-dependent glycogen synthase kinase in rabbit skeletal muscle, distinct from phosphorylase kinase. FEBS Lett. 1982, 148, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Woodgett, J.R.; Davison, M.T.; Cohen, P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur. J. Biochem. 1983, 136, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Freeman, T.A.; Ahmad, F.; Shang, X.; Mangano, E.; Gao, E.; Farber, J.; Wang, Y.; Ma, X.L.; Woodgett, J.; et al. GSK-3α is a central regulator of age-related pathologies in mice. J. Clin. Investig. 2013, 123, 1821–1832. [Google Scholar] [CrossRef]
- Song, B.; Lai, B.; Zheng, Z.; Zhang, Y.; Luo, J.; Wang, C.; Chen, Y.; Woodgett, J.R.; Li, M. Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J. Biol. Chem. 2010, 285, 41122–41134. [Google Scholar] [CrossRef]
- Noble, W.; Olm, V.; Takata, K.; Casey, E.; Mary, O.; Meyerson, J.; Gaynor, K.; Lafrancois, J.; Wang, L.; Kondo, T.; et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 2003, 38, 555–565. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The role of Cdk5 in Alzheimer’s disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef]
- Cruz, J.C.; Tseng, H.C.; Goldman, J.A.; Shih, H.; Tsai, L.H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 2003, 40, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.T.; McKenna, R.; Evans, D.B.; Sharma, S.K.; Matthews, W.R. Characterization of the in vitro phosphorylation of human tau by tau protein kinase II (cdk5/p20) using mass spectrometry. J. Neurochem. 2001, 76, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Saito, T.; Hisanaga, S.; Hashiguchi, T. Truncation of CDK5 activator p35 induces intensive phos-phorylation of Ser202/Thr205 of human tau. J. Biol. Chem. 2002, 277, 44525–44530. [Google Scholar] [CrossRef] [PubMed]
- Neddens, J.; Temmel, M.; Flunkert, S.; Kerschbaumer, B.; Hoeller, C.; Loeffler, T.; Niederkofler, V.; Duam, G.; Attems, J.; Hutter-Paier, B. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 52. [Google Scholar] [CrossRef]
- Piedrahita, D.; Herna’ndez, I.; Lo’pez-Tobo’n, A.; Fedorov, D.; Obara, B.; Manjuath, B.S.; Boudreau, R.L.; Davidson, B.; LaFerla, F.; Gallego-Go’mez, J.C.; et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic Alzheimer’s mice. J. Neurosci. 2010, 30, 13966–13976. [Google Scholar] [CrossRef]
- Asada, A.; Saito, T.; Hisanaga, S. Phosphorylation of p35 and p39 by Cdk5 determines the subcellular location of the holokinase in a phosphorylation site-specific manner. J. Cell Sci. 2012, 125, 3421–3429. [Google Scholar] [CrossRef]
- Ao, C.; Li, C.; Chen, J.; Tan, J.; Zeng, L. The role of Cdk5 in neurological disorders. Front. Cell. Neurosci. 2022, 16, 951202. [Google Scholar] [CrossRef]
- Huber, R.J.; Catalano, A.; O’Day, D.H. Cyclin-dependent kinase 5 is a calmodulin-binding protein that associates with puromycin-sensitive aminopeptidase in the nucleus of Dictyostelium. Biochim. Biophys. Acta 2013, 1833, 11–20. [Google Scholar] [CrossRef][Green Version]
- Oba, T.; Saito, T.; Asada, A.; Shimizu, S.; Iijima, K.M.; Ando, K. Microtubule affinity-regulating kinase 4 with an Alzheimer’s disease-related mutation promotes tau accumulation and exacerbates neurodegeneration. J. Biol. Chem. 2020, 295, 17138–17147. [Google Scholar] [CrossRef]
- Saito, T.; Oba, T.; Shimizu, S.; Asada, A.; Iijima, K.M.; Ando, K. Cdk5 increases MARK4 activity and augments pathological tau accumulation and toxicity through tau phosphorylation at Ser262. Hum. Mol. Genet. 2019, 28, 3062–3071. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, N.; Agrawal, K.; Džubák, P.; Hajdúch, M.; Das, V. Microtubule affinity-regulating kinases are potential druggable targets for Alzheimer’s disease. Cell. Mol. Life Sci. 2017, 74, 4159–4169. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.E.; Rizzo, M.A. A novel single-color FRET sensor for rho-kinase reveals calcium-dependent activation of rhoA and ROCK. Sensors 2024, 24, 6869. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jia, Y.; Liu, H.; He, M.; Yang, Y.; Xiao, W.; Li, Y. RhoA/ROCK pathway: Implication in osteoarthritis and therapeutic targets. Am. J. Transl. Res. 2019, 11, 5324–5331. [Google Scholar]
- Rostas, J.A.P.; Skelding, K.A. Calcium/calmodulin-stimulated protein kinase II (CaMKII): Different functional outcomes from activation, depending on the cellular microenvironment. Cells 2023, 12, 401. [Google Scholar] [CrossRef]
- Klee, C.B.; Ren, H.; Wang, X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 1998, 273, 13367–13370. [Google Scholar] [CrossRef]
- Ulengin-Talkish, I.; Cyert, M.S. A cellular atlas of calcineurin signaling. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119366. [Google Scholar] [CrossRef]
- O’Neal, M.A.; Stallings, N.R.; Malter, J.S. Alzheimer’s disease, dendritic spines, and calcineurin inhibitors: A New Approach? ACS Chem. Neurosci. 2018, 9, 1233–1234. [Google Scholar] [CrossRef]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Oda, Y.; Tomizawa, K.; Gong, C.X. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J. Biol. Chem. 2005, 280, 37755–37762. [Google Scholar] [CrossRef]
- Rahman, A.; Grundke-Iqbal, I.; Iqbal, K. PP2B isolated from human brain preferentially dephosphorylates Ser-262 and Ser-396 of the Alzheimer disease abnormally hyper-phosphorylated tau. J. Neural Transm. 2006, 113, 219–230. [Google Scholar] [CrossRef]
- Sama, D.M.; Norris, C.M. Calcium dysregulation and neuroinflammation: Discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res. Rev. 2013, 12, 982–995. [Google Scholar] [CrossRef]
- Lian, Q.; Ladner, C.J.; Magnuson, D.; Lee, J.M. Selective changes of calcineurin (protein phosphatase 2B) activity in Alzheimer’s disease cerebral cortex. Exp. Neurol. 2001, 167, 158–165. [Google Scholar] [CrossRef]
- Hoekman, J.D.; Tokheim, A.M.; Spannaus-Martin, D.J.; Martin, B.L. Molecular modeling of the calmodulin binding region of calcineurin. Protein J. 2006, 25, 175–182. [Google Scholar] [CrossRef]
- Sontag, E.; Nunbhakdi-Craig, V.; Lee, G.; Bloom, G.S.; Mumby, M.C. Regulation of the phosphorylation state and microtubule binding activity of tau by protein phosphatase 2A. Neuron 1996, 17, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Grundke-Iqbal, I.; Wu, W.; Chauhan, V.; Novak, M.; Kontzekova, E.; Iqbal, K. Protein kinase C and calcium/calmodulin-dependent protein kinase II phosphorylate three-repeat and four-repeat tau isoforms at different rates. Mol. Cell. Biochem. 1997, 168, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the pathogenesis of Alzheimer’s disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Zaidi, T.; Novak, M.; Grundke-Iqbal, I.; Iqbal, K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA 2001, 98, 6923–6928. [Google Scholar] [CrossRef]
- Hernandez, P.; Lee, G.; Sjoberg, M.; Maccioni, R. Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Abeta (25-35): Involvement of lipid rafts. J. Alzheimer’s Dis. 2009, 16, 149–156. [Google Scholar] [CrossRef]
- Terwel, D.; Muyllaert, D.; Dewachter, I.; Borghgraef, P.; Croes, S.; Devijver, H.; Van Leuven, F. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am. J. Pathol. 2008, 172, 786–798. [Google Scholar] [CrossRef]
- Kim, G.E.; Park, H.H. Structures of human transglutaminase 2: Finding clues for interference in cross-linking mediated activity. Int. J. Mol. Sci. 2020, 21, 2225. [Google Scholar] [CrossRef]
- Min, B.; Chung, K.C. New insight into transglutaminase 2 and link to neurodegenerative diseases. BMB Rep. 2018, 51, 5–13. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. The search for a universal treatment for defined and mixed pathology neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25, 13424. [Google Scholar] [CrossRef] [PubMed]
- Norlund, M.A.; Lee, J.M.; Zainelli, G.M.; Muma, N.A. Elevated transglutaminase-induced bonds in PHF tau in Alzheimer’s disease. Brain Res. 1999, 851, 154–163. [Google Scholar] [CrossRef]
- Ishizawa, T.; Mattila, P.; Davies, P.; Wang, D.; Dickson, D.W. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J. Neuropathol. Exp. Neurol. 2003, 62, 389–397. [Google Scholar] [CrossRef]
- Wang, D.S.; Dickson, D.W.; Malter, J.S. Tissue transglutaminase, protein cross-linking and Alzheimer’s disease: Review and views. Int. J. Clin. Exp. Pathol. 2008, 1, 5–18. [Google Scholar]
- Miller, M.L.; Johnson, G.V. Transglutaminase cross-linking of the tau protein. J. Neurochem. 1995, 65, 1760–1770. [Google Scholar] [CrossRef]
- Zemaitaitis, M.O.; Lee, J.M.; Troncoso, J.C.; Muma, N.A. Transglutaminase-induced cross-linking of tau proteins in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2000, 59, 983–989. [Google Scholar] [CrossRef]
- Halverson, R.A.; Lewis, J.; Frausto, S.; Hutton, M.; Muma, N.A. Tau protein is cross-linked by transglutaminase in P301L tau transgenic mice. J. Neurosci. 2005, 25, 1226–1233. [Google Scholar] [CrossRef]
- Puszkin, E.G.; Raghuraman, V. Catalytic properties of a calmodulin-regulated transglutaminase from human platelet and chicken gizzard. J. Biol. Chem. 1985, 260, 16012–16020. [Google Scholar] [CrossRef]
- Zainelli, G.M.; Ross, C.A.; Troncoso, J.C.; Fitzgerald, J.K.; Mumna, N.A. Calmodulin regulates transglutaminase 2 cross-linking of huntingtin. J. Neurosci. 2004, 24, 1954–1961. [Google Scholar] [CrossRef]
- O’Day, D.H. Calmodulin binding domains in critical risk proteins involved in neurodegeneration. Curr. Issues Mol. Biol. 2022, 44, 5802–5814. [Google Scholar] [CrossRef]
- Patil, S.; Chan, C. Palmitic and stearic fatty acids induce Alzheimer-like hyperphosphorylation of Tau in primary rat cortical neurons. Neurosci. Lett. 2005, 384, 288–293. [Google Scholar] [CrossRef]
- García-Cruz, V.M.; Coria, R.; Arias, C. Role of saturated fatty acid metabolism in posttranslational modifications of the Tau protein. Mol. Cell. Biochem. 2025, 1–14. [Google Scholar] [CrossRef]
- Ma, X.-H.; Dai, H.; Liu, S.-Y.; Liu, X.-N.; Zhang, J.; Meng, X.-L. Protection of dauricine and daurisoline on PC12 cells damaged by glutamate or Aβ25-35. Brain Res. 2025, 1857, 149609. [Google Scholar] [CrossRef] [PubMed]
- Scaduto, P.; Marcatti, M.; Bhatt, N.; Kayed, R.; Taglialatela, G. Calcineurin inhibition prevents synaptic plasticity deficit induced by brain-derived tau oligomers. Brain Communic. 2024, 6, fcae277. [Google Scholar] [CrossRef]
- Stallings, N.R.; O’Neal, M.A.; Hu, J.; Shen, Z.J.; Malter, J.S. Long-term normalization of calcineurin activity in model mice rescues Pin1 and attenuates Alzheimer’s phenotypes without blocking peripheral T cell IL-2 response. Alzheimer’s Res. Ther. 2023, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela, G.; Rastellini, C.; Cicalese, L. Reduced incidence of dementia in solid organ transplant patients treated with calcineurin inhibitors. J. Alzheimer’s Dis. 2015, 47, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Garver, T.D.; Kincaid, R.L.; Conn, R.A.; Billingsley, M.L. Reduction of calcineurin activity in brain by antisense oligonucleotides leads to persistent phosphorylation of tau protein at Thr181 and Thr231. Mol. Pharmacol. 1999, 55, 632–641. [Google Scholar] [CrossRef]
- Luo, J.; Ma, J.; Yu, D.Y.; Bu, F.; Zhang, W.; Tu, L.H.; Wei, Q. Infusion of FK506, a specific inhibitor of calcineurin, induces potent tau hyperphosphorylation in mouse brain. Brain Res. Bull. 2008, 76, 464–468. [Google Scholar] [CrossRef]
- Martin, Z.S.; Neugebauer, V.; Dineley, K.T.; Kayed, R.; Zhang, W.; Reese, L.C.; Taglialatela, G. α-Synuclein oligomers oppose long-term potentiation and impair memory through a calcineurin-dependent mechanism: Relevance to human synucleopathic diseases. J. Neurochem. 2012, 120, 440–452. [Google Scholar] [CrossRef]
- Dineley, K.T.; Hogan, D.; Zhang, W.R.; Taglialatela, G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol. Learn. Mem. 2007, 88, 217–224. [Google Scholar] [CrossRef]
- Cavallucci, V.; Berretta, N.; Nobili, A.; Nistic, R.; Mercuri, N.B.; D’amelio, M. Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuromol. Med. 2013, 15, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Radiske, A.; de Castro, C.M.; Rossato, J.I.; Gonzalez, M.C.; Cammarota, M. Hippocampal CaMKII inhibition induces reactivation-dependent amnesia for extinction memory and causes fear relapse. Sci. Rep. 2023, 13, 21712. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.N.; Bayer, K.U. Studying CaMKII: Tools and standards. Cell Rep. 2024, 43, 113982. [Google Scholar] [CrossRef]
- Ali, A.S.; Al-Nasser, M.S.; Sattar, M.A.A.; Alkreathy, H.M.; Al-Amma, M.N.; Alsulaimani, R.A.; Abdulfattah, E.H. Major Adverse Effects Associated with Tacrolimus (Fk506) Based Regimen among Saudi Kidney Transplant Patients. J. Pharm. Res. Int. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Gunes, S.; Aizawa, Y.; Sugashi, T.; Sugimoto, M.; Rodrigues, P.P. Biomarkers for Alzheimer’s Disease in the Current State: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 4962. [Google Scholar] [CrossRef]
- Sexton, C.E.; Bitan, G.; Bowles, K.R.; Brys, M.; Buée, L.; Maina, M.B.; Clelland, C.D.; Cohen, A.D.; Crary, J.F.; Dage, J.L.; et al. Novel avenues of tau research. Alzheimer’s Dement. 2024, 20, 2240–2261. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Day, D.H. Tauopathies: Calmodulin Regulates Tau Hyperphosphorylation and Its Transformation into Disease-Specific Aggregates. Biomolecules 2025, 15, 1133. https://doi.org/10.3390/biom15081133
O’Day DH. Tauopathies: Calmodulin Regulates Tau Hyperphosphorylation and Its Transformation into Disease-Specific Aggregates. Biomolecules. 2025; 15(8):1133. https://doi.org/10.3390/biom15081133
Chicago/Turabian StyleO’Day, Danton H. 2025. "Tauopathies: Calmodulin Regulates Tau Hyperphosphorylation and Its Transformation into Disease-Specific Aggregates" Biomolecules 15, no. 8: 1133. https://doi.org/10.3390/biom15081133
APA StyleO’Day, D. H. (2025). Tauopathies: Calmodulin Regulates Tau Hyperphosphorylation and Its Transformation into Disease-Specific Aggregates. Biomolecules, 15(8), 1133. https://doi.org/10.3390/biom15081133





