The Role of Mitochondrial DNA in Modulating Chemoresistance in Esophageal Cancer: Mechanistic Insights and Therapeutic Potential
Abstract
1. Introduction
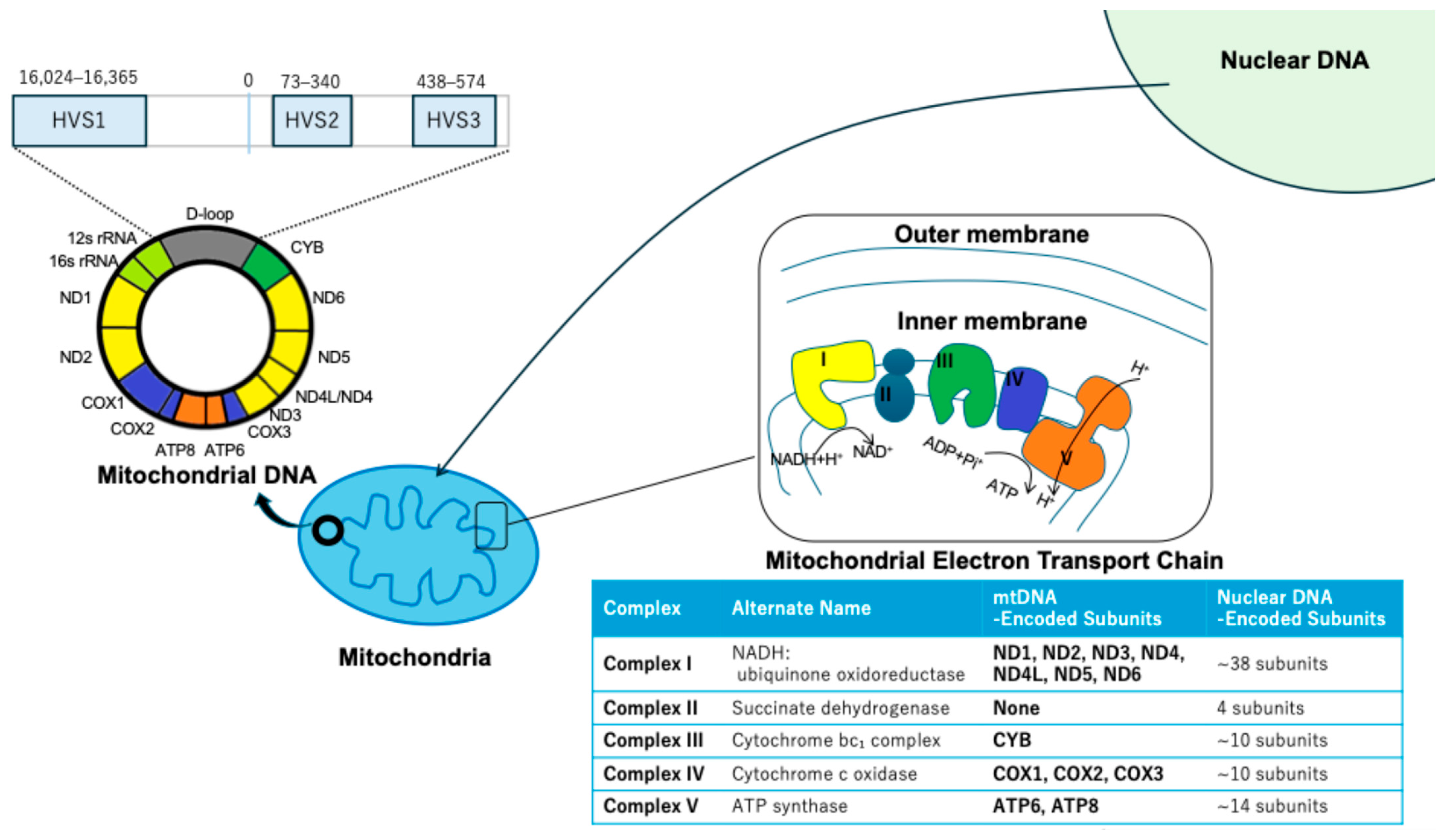
2. Clinical Correlations of mtDNA Alterations in EC
2.1. Prognostic Impact of mtDNA Copy Number Variations
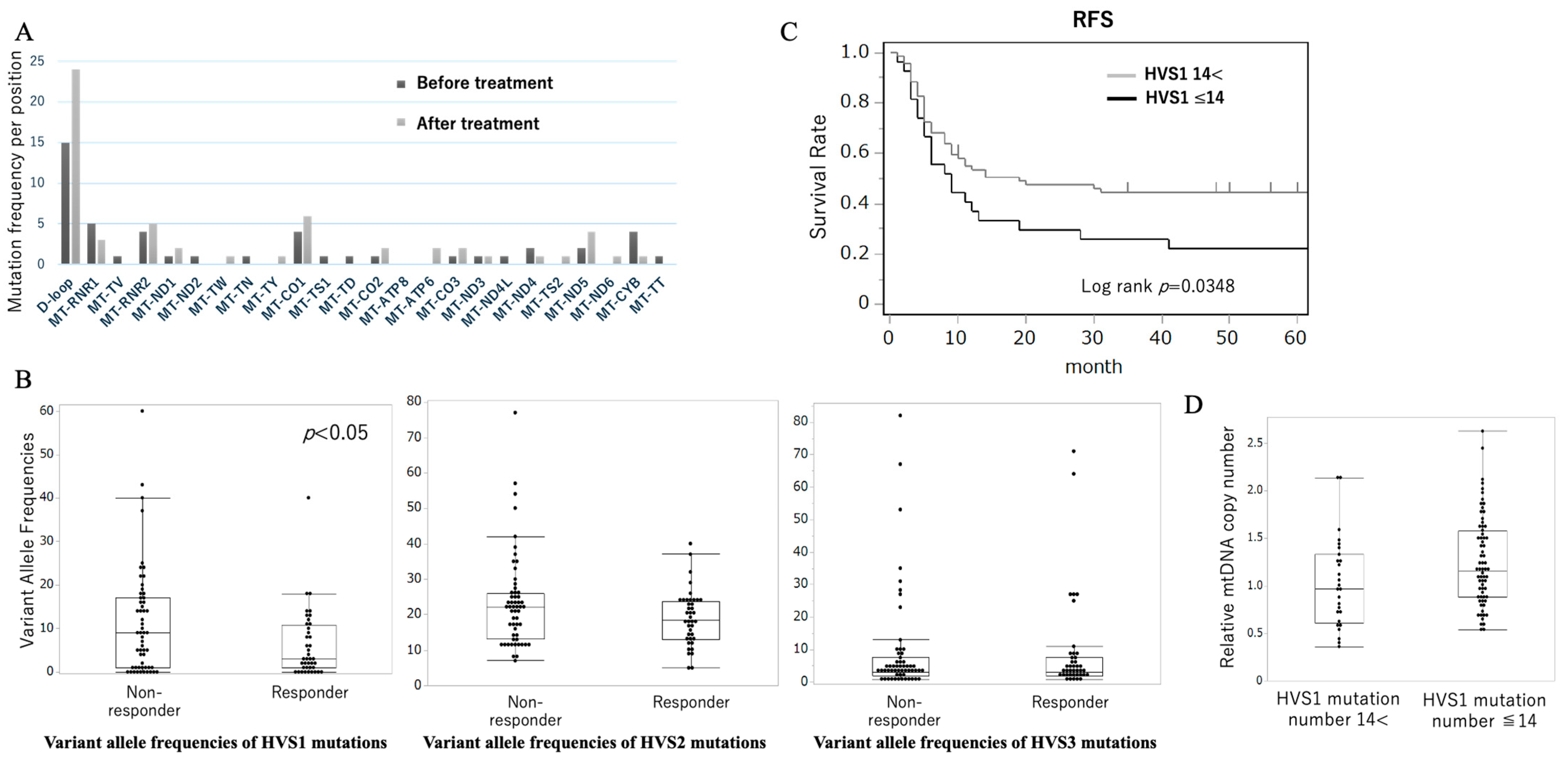
2.2. D-Loop HVS1 Mutations Correlate with Chemotherapy Response
3. Mechanistic Pathways Linking mtDNA Dysfunction to Chemoresistance
3.1. Bioenergetic Reprogramming and Metabolic Adaptation
3.2. ROS Signaling and Redox Homeostasis
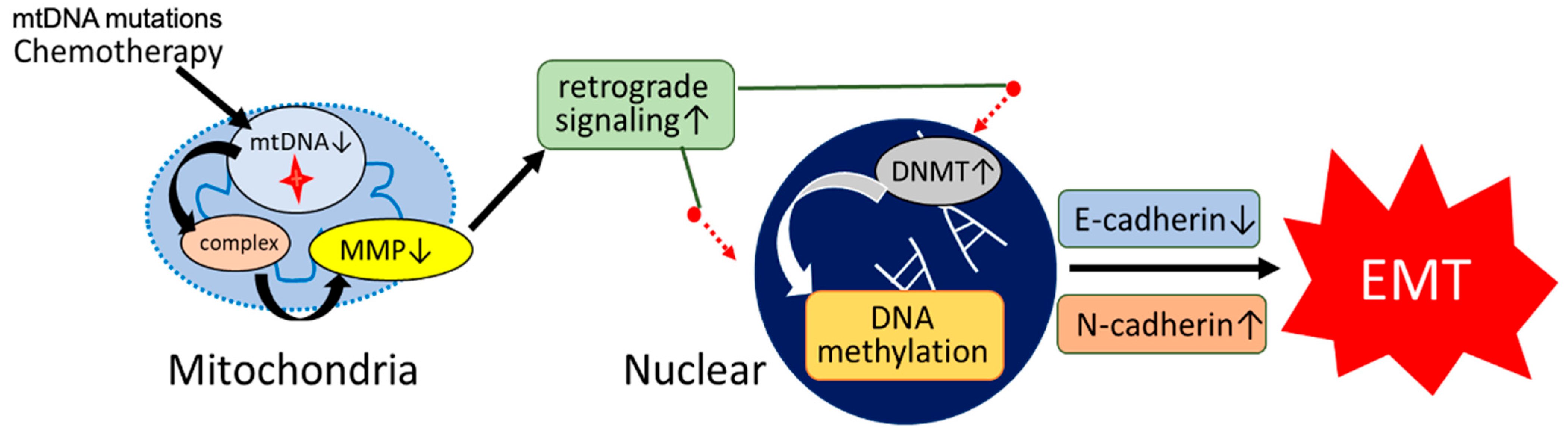
3.3. Epithelial–Mesenchymal Transition and Cancer Stem Cell Phenotypes
3.4. Epigenetic Remodeling via DNMT Upregulation
4. Therapeutic Strategies Targeting Mitochondrial Vulnerabilities
4.1. Targeting Epigenetic Dysregulation with DNMT Inhibitors
4.2. Exploiting Metabolic Vulnerabilities with Bioenergetic Modulators
4.3. Mitochondrial DNA Editing Technologies
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Ielpo, B.; Pernaute, A.S.; Elia, S.; Buonomo, O.C.; Valladares, L.D.; Aguirre, E.P.; Petrella, G.; Garcia, A.T. Impact of number and site of lymph node invasion on survival of adenocarcinoma of esophagogastric junction. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 704–708. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Yoshioka, A.; Yamasaki, M.; Nushijima, Y.; Takiguchi, S.; Fujiwara, Y.; Nishida, T.; Mano, M.; Mori, M.; Doki, Y. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer 2009, 115, 3324–3334. [Google Scholar] [CrossRef]
- Tachimori, Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: Efficacy of lymph node dissection according to tumor location. J. Thorac. Dis. 2017, 9, S724–S730. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshida, K.; Suetsugu, T.; Imai, T.; Matsuhashi, N.; Yamaguchi, K. Recent advancements in esophageal cancer treatment in Japan. Ann. Gastroenterol. Surg. 2018, 2, 253–265. [Google Scholar] [CrossRef]
- Yang, H.; Jia, X.; Chen, X.; Yang, C.S.; Li, N. Time-selective chemoprevention of vitamin E and selenium on esophageal carcinogenesis in rats: The possible role of nuclear factor kappaB signaling pathway. Int. J. Cancer 2012, 131, 1517–1527. [Google Scholar] [CrossRef]
- Chandramouleeswaran, P.M.; Guha, M.; Shimonosono, M.; Whelan, K.A.; Maekawa, H.; Sachdeva, U.M.; Ruthel, G.; Mukherjee, S.; Engel, N.; Gonzalez, M.V.; et al. Autophagy mitigates ethanol-induced mitochondrial dysfunction and oxidative stress in esophageal keratinocytes. PLoS ONE 2020, 15, e0239625. [Google Scholar] [CrossRef]
- Kondo, Y.; Ohashi, S.; Katada, C.; Nakai, Y.; Yamamoto, Y.; Tamaoki, M.; Kikuchi, O.; Yamada, A.; Hirohashi, K.; Mitani, Y.; et al. Aldh2 and the tumor suppressor Trp53 play important roles in alcohol-induced squamous field cancerization. J. Gastroenterol. 2025, 60, 546–560. [Google Scholar] [CrossRef]
- Tanaka, K.; Whelan, K.A.; Chandramouleeswaran, P.M.; Kagawa, S.; Rustgi, S.L.; Noguchi, C.; Guha, M.; Srinivasan, S.; Amanuma, Y.; Ohashi, S.; et al. ALDH2 modulates autophagy flux to regulate acetaldehyde-mediated toxicity thresholds. Am. J. Cancer Res. 2016, 6, 781–796. [Google Scholar]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Tuppen, H.A.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta 2010, 1797, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Shoffner, J.M.; Trounce, I.; Brown, M.D.; Ballinger, S.W.; Corral-Debrinski, M.; Horton, T.; Jun, A.S.; Lott, M.T. Mitochondrial DNA mutations in human degenerative diseases and aging. Biochim. Biophys. Acta 1995, 1271, 141–151. [Google Scholar] [CrossRef]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Boesch, P.; Weber-Lotfi, F.; Ibrahim, N.; Tarasenko, V.; Cosset, A.; Paulus, F.; Lightowlers, R.N.; Dietrich, A. DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim. Biophys. Acta 2011, 1813, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The maintenance of mitochondrial DNA integrity--critical analysis and update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Tann, A.W.; Longley, M.J.; Copeland, W.C.; Mitra, S. Long patch base excision repair in mammalian mitochondrial genomes. J. Biol. Chem. 2008, 283, 26349–26356. [Google Scholar] [CrossRef]
- de Souza-Pinto, N.C.; Mason, P.A.; Hashiguchi, K.; Weissman, L.; Tian, J.; Guay, D.; Lebel, M.; Stevnsner, T.V.; Rasmussen, L.J.; Bohr, V.A. Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA Repair 2009, 8, 704–719. [Google Scholar] [CrossRef]
- Gammage, P.A.; Frezza, C. Mitochondrial DNA: The overlooked oncogenome? BMC Biol. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Sabouny, R.; Shutt, T.E. The role of mitochondrial dynamics in mtDNA maintenance. J. Cell Sci. 2021, 134, jcs258944. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Alaylioglu, M.; Genc, G.; Sengul, B.; Keskin, E.; Sordu, P.; Gulec, Z.E.K.; Apaydin, H.; Bayram-Gurel, C.; Ulutin, T.; et al. Altered Transcriptional Profile of Mitochondrial DNA-Encoded OXPHOS Subunits, Mitochondria Quality Control Genes, and Intracellular ATP Levels in Blood Samples of Patients with Parkinson’s Disease. J. Alzheimers Dis. 2020, 74, 287–307. [Google Scholar] [CrossRef]
- Masuike, Y.; Tanaka, K.; Makino, T.; Yamasaki, M.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Nakajima, K.; Mori, M.; Doki, Y. Esophageal squamous cell carcinoma with low mitochondrial copy number has mesenchymal and stem-like characteristics, and contributes to poor prognosis. PLoS ONE 2018, 13, e0193159. [Google Scholar] [CrossRef] [PubMed]
- Challen, C.; Brown, H.; Cai, C.; Betts, G.; Paterson, I.; Sloan, P.; West, C.; Birch-Machin, M.; Robinson, M. Mitochondrial DNA mutations in head and neck cancer are infrequent and lack prognostic utility. Br. J. Cancer 2011, 104, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Harino, T.; Tanaka, K.; Motooka, D.; Masuike, Y.; Takahashi, T.; Yamashita, K.; Saito, T.; Yamamoto, K.; Makino, T.; Kurokawa, Y.; et al. D-loop mutations in mitochondrial DNA are a risk factor for chemotherapy resistance in esophageal cancer. Sci. Rep. 2024, 14, 31653. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA mutations in human cancer. Oncogene 2006, 25, 4663–4674. [Google Scholar] [CrossRef]
- Fukuda, S.; Miyata, H.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; Mori, M.; et al. Pyruvate Kinase M2 Modulates Esophageal Squamous Cell Carcinoma Chemotherapy Response by Regulating the Pentose Phosphate Pathway. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1461–S1468. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Kinugasa, H.; Whelan, K.A.; Tanaka, K.; Natsuizaka, M.; Long, A.; Guo, A.; Chang, S.; Kagawa, S.; Srinivasan, S.; Guha, M.; et al. Mitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene 2015, 34, 5229–5239. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Hirose, W.; Oshikiri, H.; Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System and Esophageal Cancer. Cancers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T.; et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer: A dance with the devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef]
- Miyauchi, W.; Shishido, Y.; Matsumi, Y.; Matsunaga, T.; Makinoya, M.; Shimizu, S.; Miyatani, K.; Sakamoto, T.; Umekita, Y.; Hasegawa, T.; et al. Simultaneous regulation of ferroptosis suppressor protein 1 and glutathione peroxidase 4 as a new therapeutic strategy of ferroptosis for esophageal squamous cell carcinoma. Esophagus 2023, 20, 492–501. [Google Scholar] [CrossRef]
- Feng, S.; Jia, J.; Wang, K.; Zhao, H.; Lv, G. FBXO10 inhibits ferroptosis and promotes the progression of esophageal squamous cell carcinoma by post-translational mediation of ACSL4 degradation. J. Mol. Histol. 2025, 56, 214. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Kubo, Y.; Tanaka, K.; Masuike, Y.; Takahashi, T.; Yamashita, K.; Makino, T.; Saito, T.; Yamamoto, K.; Tsujimoto, T.; Harino, T.; et al. Low mitochondrial DNA copy number induces chemotherapy resistance via epithelial-mesenchymal transition by DNA methylation in esophageal squamous cancer cells. J. Transl. Med. 2022, 20, 383. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Butow, R.A.; Avadhani, N.G. Mitochondrial signaling: The retrograde response. Mol. Cell 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Smiraglia, D.J.; Kulawiec, M.; Bistulfi, G.L.; Gupta, S.G.; Singh, K.K. A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol. Ther. 2008, 7, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.; Mackay, H.J.; Judson, I.; Plumb, J.A.; McCormick, C.; Strathdee, G.; Lee, C.; Barrett, S.; Reade, S.; Jadayel, D.; et al. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J. Clin. Oncol. 2007, 25, 4603–4609. [Google Scholar] [CrossRef]
- Santidrian, A.F.; Matsuno-Yagi, A.; Ritland, M.; Seo, B.B.; LeBoeuf, S.E.; Gay, L.J.; Yagi, T.; Felding-Habermann, B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J. Clin. Investig. 2013, 123, 1068–1081. [Google Scholar] [CrossRef]
- Masoud, R.; Reyes-Castellanos, G.; Lac, S.; Garcia, J.; Dou, S.; Shintu, L.; Abdel Hadi, N.; Gicquel, T.; El Kaoutari, A.; Dieme, B.; et al. Targeting Mitochondrial Complex I Overcomes Chemoresistance in High OXPHOS Pancreatic Cancer. Cell Rep. Med. 2020, 1, 100143. [Google Scholar] [CrossRef]
- Punetha, M.; Saini, S.; Chaudhary, S.; Bala, R.; Sharma, M.; Kumar, P.; Kumar, D.; Yadav, P.S. Mitochondria-targeted antioxidant MitoQ ameliorates ROS production and improves cell viability in cryopreserved buffalo fibroblasts. Tissue Cell 2023, 82, 102067. [Google Scholar] [CrossRef]
- Cheng, G.; Zielonka, J.; Dranka, B.P.; McAllister, D.; Mackinnon, A.C., Jr.; Joseph, J.; Kalyanaraman, B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012, 72, 2634–2644. [Google Scholar] [CrossRef]
- Gammage, P.A.; Gaude, E.; Van Haute, L.; Rebelo-Guiomar, P.; Jackson, C.B.; Rorbach, J.; Pekalski, M.L.; Robinson, A.J.; Charpentier, M.; Concordet, J.P.; et al. Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res. 2016, 44, 7804–7816. [Google Scholar] [CrossRef]
- Mok, B.Y.; de Moraes, M.H.; Zeng, J.; Bosch, D.E.; Kotrys, A.V.; Raguram, A.; Hsu, F.; Radey, M.C.; Peterson, S.B.; Mootha, V.K.; et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 2020, 583, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Baek, G.; Kim, J.S. Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat. Biotechnol. 2023, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Ham, S.; Lee, G.H.; Lee, Y.I.; Kim, S.; Lee, Y.S.; Shin, J.H.; Lee, Y. Efficient Mitochondrial Genome Editing by CRISPR/Cas9. Biomed. Res. Int. 2015, 2015, 305716. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Masuike, Y.; Kubo, Y.; Harino, T.; Kurokawa, Y.; Eguchi, H.; Doki, Y. The Role of Mitochondrial DNA in Modulating Chemoresistance in Esophageal Cancer: Mechanistic Insights and Therapeutic Potential. Biomolecules 2025, 15, 1128. https://doi.org/10.3390/biom15081128
Tanaka K, Masuike Y, Kubo Y, Harino T, Kurokawa Y, Eguchi H, Doki Y. The Role of Mitochondrial DNA in Modulating Chemoresistance in Esophageal Cancer: Mechanistic Insights and Therapeutic Potential. Biomolecules. 2025; 15(8):1128. https://doi.org/10.3390/biom15081128
Chicago/Turabian StyleTanaka, Koji, Yasunori Masuike, Yuto Kubo, Takashi Harino, Yukinori Kurokawa, Hidetoshi Eguchi, and Yuichiro Doki. 2025. "The Role of Mitochondrial DNA in Modulating Chemoresistance in Esophageal Cancer: Mechanistic Insights and Therapeutic Potential" Biomolecules 15, no. 8: 1128. https://doi.org/10.3390/biom15081128
APA StyleTanaka, K., Masuike, Y., Kubo, Y., Harino, T., Kurokawa, Y., Eguchi, H., & Doki, Y. (2025). The Role of Mitochondrial DNA in Modulating Chemoresistance in Esophageal Cancer: Mechanistic Insights and Therapeutic Potential. Biomolecules, 15(8), 1128. https://doi.org/10.3390/biom15081128





