Benefits of Maternal Choline Supplementation on Aged Basal Forebrain Cholinergic Neurons (BFCNs) in a Mouse Model of Down Syndrome and Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Cohort Generation
2.2. Tissue Preparation and Immunohistochemistry
2.3. Laser Capture Microdissection
2.4. RNA Processing
2.5. Library Preparation
2.6. Single Population RNA-Seq
2.7. Statistical Analysis
2.8. Bioinformatic Pathway Analyses
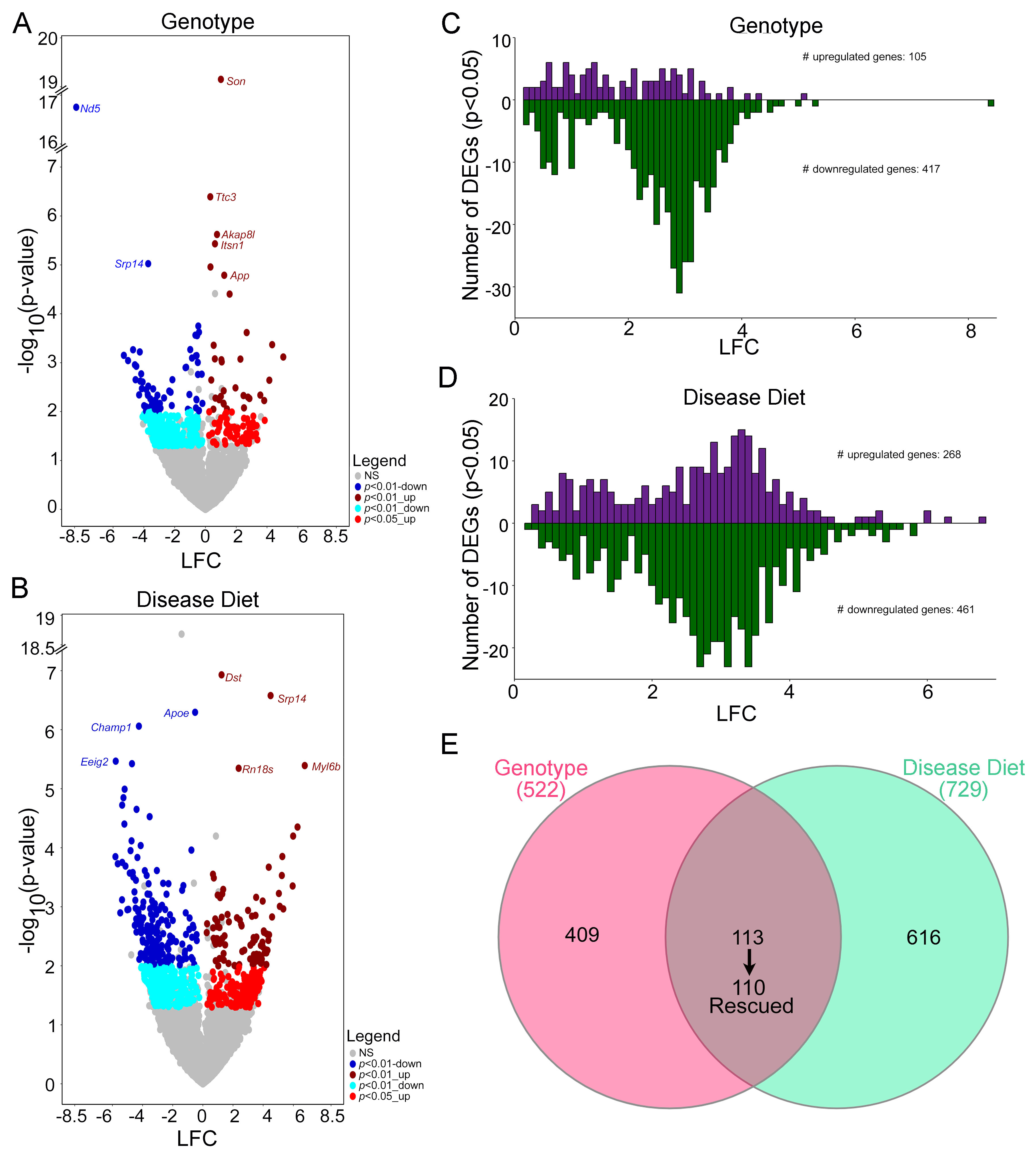
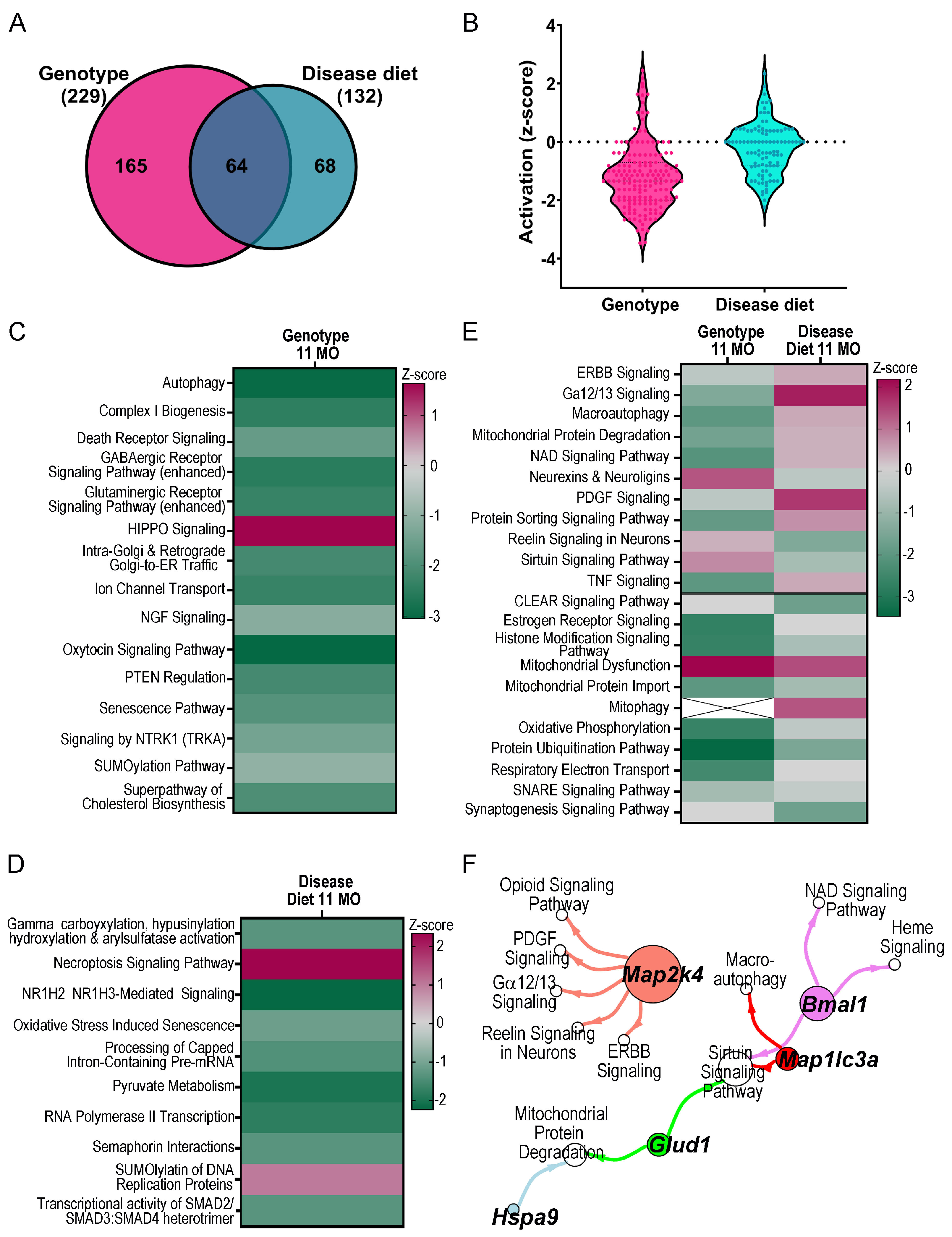
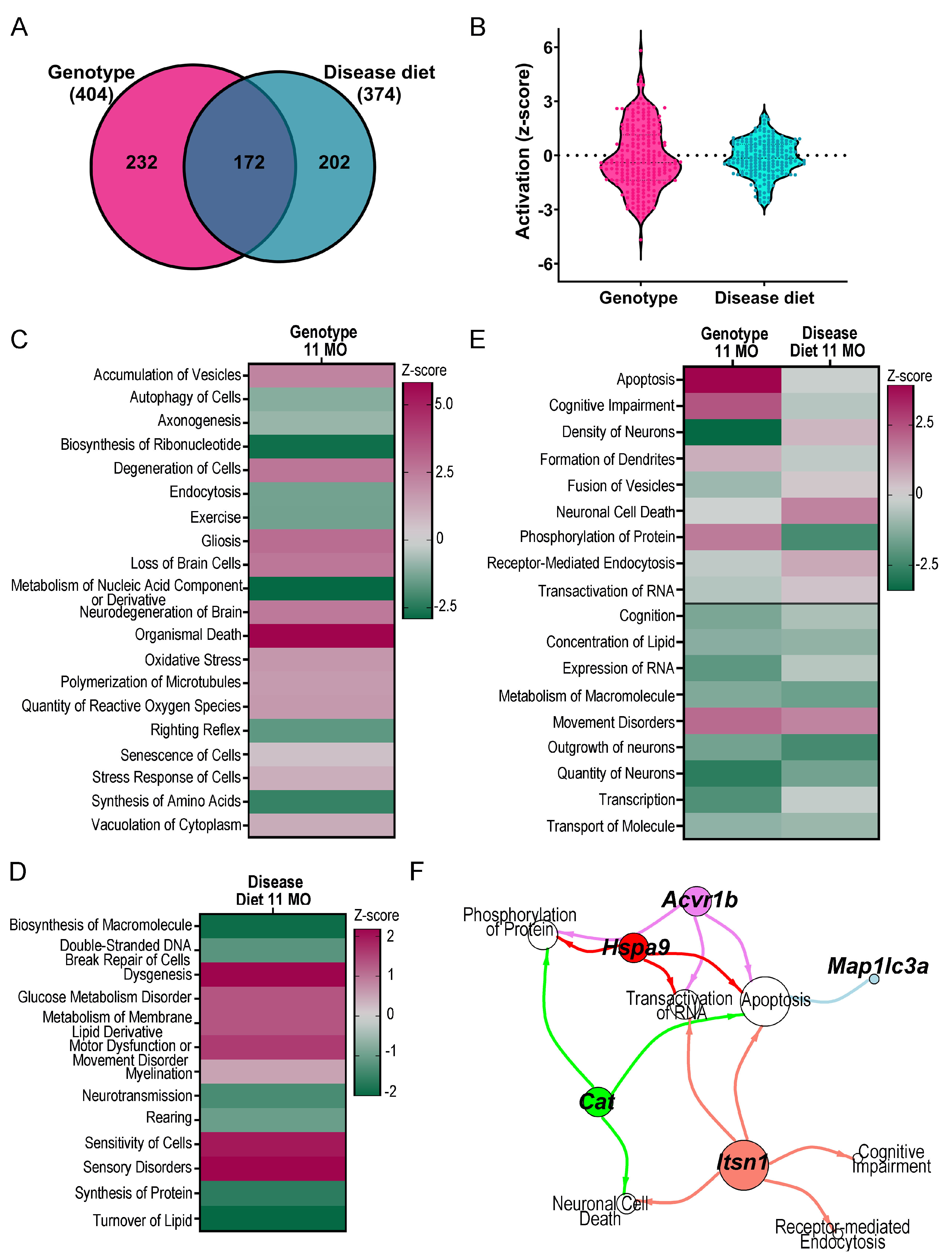
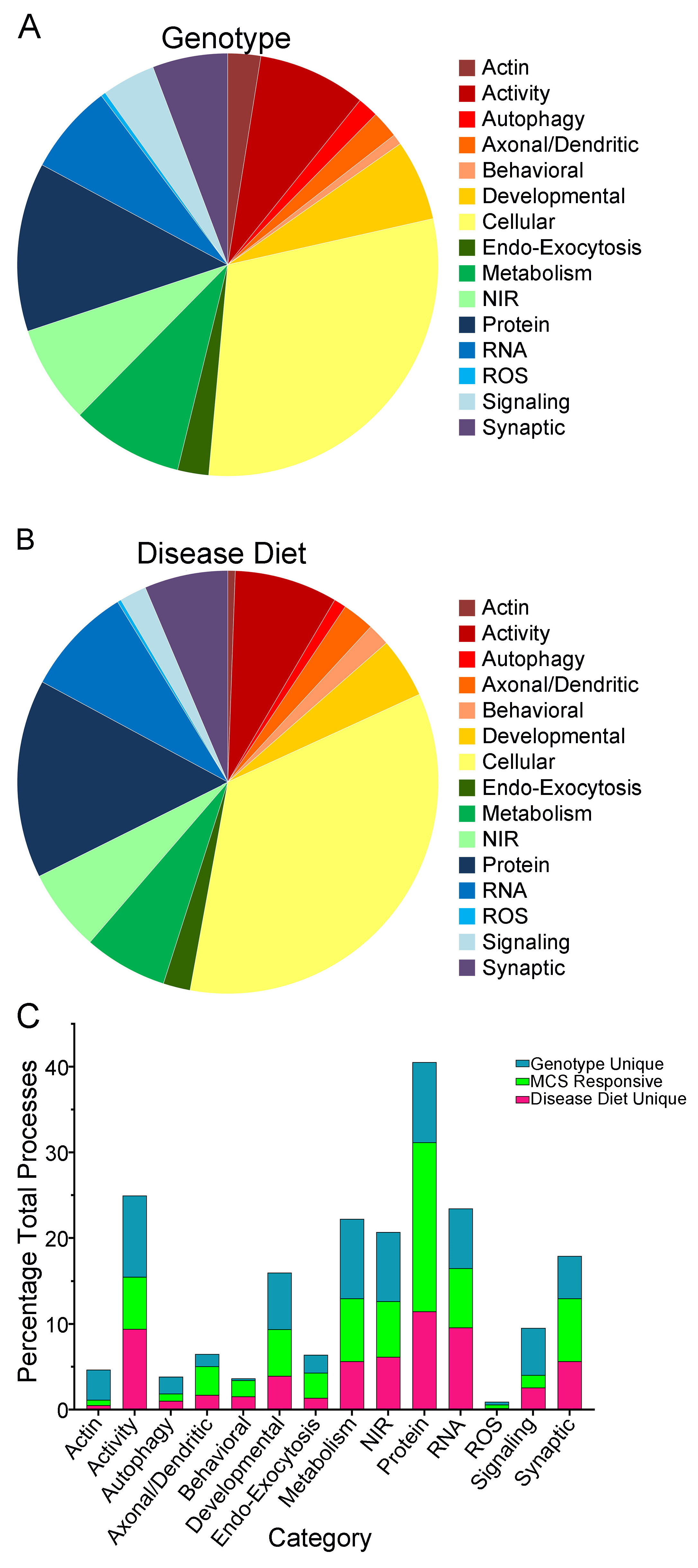
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2N | Disomic mouse |
| 2N+ | Disomic mouse plus choline supplementation |
| Aβ | Amyloid-beta peptide |
| Acvr1b | activin A receptor, type 1B |
| AD | Alzheimer’s disease |
| App | Amyloid-beta precursor protein |
| Bace1 | beta-site amyloid precursor protein cleaving enzyme 1 |
| BFCN | basal forebrain cholinergic neuron |
| Bmal1 | basic helix-loop-helix ARNT like 1 |
| Cat | Catalase |
| ChAT | choline acetyltransferase |
| ChAT-ir | ChAT-immunoreactivity |
| CS | Combat-seq |
| DEGs | Differentially expressed genes |
| Diet plus genotype | Ts+ vs. 2N |
| Disease diet compare | Ts+ vs. Ts |
| Disomic diet compare | 2N+ vs. 2N |
| D/Fs | Disease and Functions |
| DS | Down syndrome |
| DS + AD | Down syndrome + Alzheimer’s disease |
| E0 | Embryonic day 0 |
| Genotype compare | Ts vs. 2N |
| GO | Gene Ontology |
| Glud1 | glutamate dehydrogenase 1 |
| GTC | Genome Technology Center |
| HSA21 | Human chromosome 21 |
| Hspa9 | Heat-shock protein 9 |
| IPA | Ingenuity Pathway Analysis |
| Itsn1 | Intersectin 1 |
| LCM | Laser capture microdissection |
| LFC | Log fold change |
| LMM | Linear mixed model |
| Map1lc3a | microtubule-associated protein 1 light chain 3 alpha |
| Map2k4 | mitogen-activated protein kinase kinase 4 |
| MCS | Maternal choline supplementation |
| MDS | Multidimensional scale plots |
| MO | Months of age |
| MSN | Medial septal nucleus |
| NAD | nicotinamide adenine dinucleotide |
| ns | not significant |
| NTRK1 | Cognate NGF receptor TrkA |
| Ndufb1 | NADH:ubiquinone oxidoreductase subunit B1 |
| NYUGSOM | New York University Grossman School of Medicine |
| P21 | Postnatal day 21 |
| PEMT | phosphatidylethanolamine N-methyltransferase |
| PEN | polyethylene naphthalate |
| QC | Quality control |
| RE | Random effects |
| RNA-seq | RNA sequencing |
| RT | Room temperature |
| Sdhc | succinate dehydrogenase complex, subunit C, integral membrane protein |
| Supplemented | Ts+ vs. 2N+ |
| TMM | Trimmed means of M-values |
| Ts | Ts65Dn trisomic mouse |
| Ts+ | Ts65Dn trisomic mouse plus choline supplementation |
| VP | Variance partition |
| WGCNA | weighted gene co-expression analysis |
References
- Chapman, R.S.; Hesketh, L.J. Behavioral phenotype of individuals with Down syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.; Yates, P.O.; Marcyniuk, B. Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down’s syndrome in middle age form an age related continuum of pathological changes. Neuropathol. Appl. Neurobiol. 1984, 10, 185–207. [Google Scholar] [CrossRef]
- Videla, L.; Benejam, B.; Pegueroles, J.; Carmona-Iragui, M.; Padilla, C.; Fernández, S.; Barroeta, I.; Altuna, M.; Valldeneu, S.; Garzón, D.; et al. Longitudinal Clinical and Cognitive Changes Along the Alzheimer Disease Continuum in Down Syndrome. JAMA Netw. Open 2022, 5, e2225573. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Collaborators. The Burden of Dementia due to Down Syndrome, Parkinson’s Disease, Stroke, and Traumatic Brain Injury: A Systematic Analysis for the Global Burden of Disease Study 2019. Neuroepidemiology 2021, 55, 286–296. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Lott, I.T.; Head, E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019, 15, 135–147. [Google Scholar] [CrossRef]
- Wisniewski, K.E.; Dalton, A.J.; Crapper McLachlan, D.R.; Wen, G.Y.; Wisniewski, H.M. Alzheimer’s disease in Down’s syndrome: Clinicopathologic studies. Neurology 1985, 35, 957–961. [Google Scholar] [CrossRef]
- Yates, C.M.; Simpson, J.; Maloney, A.F.; Gordon, A.; Reid, A.H. Alzheimer-like cholinergic deficiency in Down syndrome. Lancet 1980, 2, 979. [Google Scholar] [CrossRef]
- Sendera, T.J.; Ma, S.Y.; Jaffar, S.; Kozlowski, P.B.; Kordower, J.H.; Mawal, Y.; Saragovi, H.U.; Mufson, E.J. Reduction in TrkA-immunoreactive neurons is not associated with an overexpression of galaninergic fibers within the nucleus basalis in Down’s syndrome. J. Neurochem. 2000, 74, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Remy, S. Septo-hippocampal interaction. Cell Tissue Res. 2018, 373, 565–575. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Mufson, E.J.; Wainer, B.H.; Levey, A.I. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 1983, 10, 1185–1201. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Mufson, E.J.; Levey, A.I.; Wainer, B.H. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 1983, 214, 170–197. [Google Scholar] [CrossRef] [PubMed]
- Rye, D.B.; Wainer, B.H.; Mesulam, M.M.; Mufson, E.J.; Saper, C.B. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 1984, 13, 627–643. [Google Scholar] [CrossRef]
- Fernández-Cabello, S.; Kronbichler, M.; Van Dijk, K.R.A.; Goodman, J.A.; Spreng, R.N.; Schmitz, T.W. Basal forebrain volume reliably predicts the cortical spread of Alzheimer’s degeneration. Brain 2020, 143, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.K.; Conley, A.C.; Wilson, J.E.; Newhouse, P.A. Cholinergic System Structure and Function Changes in Individuals with Down Syndrome During the Development of Alzheimer’s Disease. Curr. Top. Behav. Neurosci. 2024, 69, 49–78. [Google Scholar]
- Cooper, J.D.; Salehi, A.; Delcroix, J.D.; Howe, C.L.; Belichenko, P.V.; Chua-Couzens, J.; Kilbridge, J.F.; Carlson, E.J.; Epstein, C.J.; Mobley, W.C. Failed retrograde transport of NGF in a mouse model of Down’s syndrome: Reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc. Natl. Acad. Sci. USA 2001, 98, 10439–10444. [Google Scholar] [CrossRef]
- Hunter, C.L.; Bachman, D.; Granholm, A.C. Minocycline prevents cholinergic loss in a mouse model of Down’s syndrome. Ann. Neurol. 2004, 56, 675–688. [Google Scholar] [CrossRef]
- Salehi, A.; Delcroix, J.D.; Belichenko, P.V.; Zhan, K.; Wu, C.; Valletta, J.S.; Takimoto-Kimura, R.; Kleschevnikov, A.M.; Sambamurti, K.; Chung, P.P.; et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 2006, 51, 29–42. [Google Scholar] [CrossRef]
- Granholm, A.C.; Sanders, L.A.; Crnic, L.S. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp. Neurol. 2000, 161, 647–663. [Google Scholar] [CrossRef]
- Rueda, N.; Florez, J.; Martinez-Cue, C. Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural Plast. 2012, 2012, 584071. [Google Scholar] [CrossRef]
- Ash, J.A.; Velazquez, R.; Kelley, C.M.; Powers, B.E.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation improves spatial mapping and increases basal forebrain cholinergic neuron number and size in aged Ts65Dn mice. Neurobiol. Dis. 2014, 70, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.M.; Ginsberg, S.D.; Alldred, M.J.; Strupp, B.J.; Mufson, E.J. Maternal choline supplementation alters basal forebrain cholinergic neuron gene expression in the Ts65Dn mouse model of Down syndrome. Dev. Neurobiol. 2019, 79, 664–683. [Google Scholar] [CrossRef] [PubMed]
- Ebell, M.H.; Barry, H.C.; Baduni, K.; Grasso, G. Clinically Important Benefits and Harms of Monoclonal Antibodies Targeting Amyloid for the Treatment of Alzheimer Disease: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2024, 22, 50–62. [Google Scholar] [CrossRef]
- Thangwaritorn, S.; Lee, C.; Metchikoff, E.; Razdan, V.; Ghafary, S.; Rivera, D.; Pinto, A.; Pemminati, S. A Review of Recent Advances in the Management of Alzheimer’s Disease. Cureus 2024, 16, e58416. [Google Scholar] [CrossRef]
- Gautier, M.K.; Kelley, C.M.; Lee, S.H.; Alldred, M.J.; McDaid, J.; Mufson, E.J.; Stutzmann, G.E.; Ginsberg, S.D. Maternal choline supplementation protects against age-associated cholinergic and GABAergic basal forebrain neuron degeneration in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. Neurobiol. Dis. 2023, 188, 106332. [Google Scholar] [CrossRef]
- Meck, W.H.; Smith, R.A.; Williams, C.L. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 1989, 103, 1234–1241. [Google Scholar] [CrossRef]
- Meck, W.H.; Smith, R.A.; Williams, C.L. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 1988, 21, 339–353. [Google Scholar] [CrossRef]
- Strupp, B.J.; Powers, B.E.; Velazquez, R.; Ash, J.A.; Kelley, C.M.; Alldred, M.J.; Strawderman, M.; Caudill, M.A.; Mufson, E.J.; Ginsberg, S.D. Maternal Choline Supplementation: A Potential Prenatal Treatment for Down Syndrome and Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 97–106. [Google Scholar] [CrossRef]
- Zeisel, S.H. Nutrition in pregnancy: The argument for including a source of choline. Int. J. Womens Health 2013, 5, 193–199. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Albright, C.D.; Mar, M.H.; Craciunescu, C.N.; Song, J.; Zeisel, S.H. Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Res. Dev. Brain Res. 2005, 159, 149–154. [Google Scholar] [CrossRef]
- Albright, C.D.; Tsai, A.Y.; Friedrich, C.B.; Mar, M.H.; Zeisel, S.H. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res. 1999, 113, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.M.; Schambra, U.B. Morphogenetic roles of acetylcholine. Environ. Health Perspect. 1999, 107, 65–69. [Google Scholar] [PubMed]
- Yan, J.; Ginsberg, S.D.; Powers, B.; Alldred, M.J.; Saltzman, A.; Strupp, B.J.; Caudill, M.A. Maternal choline supplementation programs greater activity of the phosphatidylethanolamine N-methyltransferase (PEMT) pathway in adult Ts65Dn trisomic mice. FASEB J. 2014, 28, 4312–4323. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Mellott, T.J. Choline nutrition programs brain development via DNA and histone methylation. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Chen, M.; Gandhy, S.U.; Strawderman, M.; Levitsky, D.A.; Maclean, K.N.; Strupp, B.J. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav. Neurosci. 2010, 124, 346–361. [Google Scholar] [CrossRef]
- Powers, B.E.; Kelley, C.M.; Velazquez, R.; Ash, J.A.; Strawderman, M.S.; Alldred, M.J.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation in a mouse model of Down syndrome: Effects on attention and nucleus basalis/substantia innominata neuron morphology in adult offspring. Neuroscience 2017, 340, 501–514. [Google Scholar] [CrossRef]
- Powers, B.E.; Velazquez, R.; Kelley, C.M.; Ash, J.A.; Strawderman, M.S.; Alldred, M.J.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Attentional function and basal forebrain cholinergic neuron morphology during aging in the Ts65Dn mouse model of Down syndrome. Brain Struct. Funct. 2016, 221, 4337–4352. [Google Scholar] [CrossRef]
- Velazquez, R.; Ash, J.A.; Powers, B.E.; Kelley, C.M.; Strawderman, M.; Luscher, Z.I.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 2013, 58, 92–101. [Google Scholar] [CrossRef]
- Ward, B.C.; Kolodny, N.H.; Nag, N.; Berger-Sweeney, J.E. Neurochemical changes in a mouse model of Rett syndrome: Changes over time and in response to perinatal choline nutritional supplementation. J. Neurochem. 2009, 108, 361–371. [Google Scholar] [CrossRef]
- Chin, E.W.M.; Lim, W.M.; Ma, D.; Rosales, F.J.; Goh, E.L.K. Choline Rescues Behavioural Deficits in a Mouse Model of Rett Syndrome by Modulating Neuronal Plasticity. Mol. Neurobiol. 2019, 56, 3882–3896. [Google Scholar] [CrossRef]
- Scremin, O.U.; Roch, M.; Norman, K.M.; Djazayeri, S.; Liu, Y.Y. Brain acetylcholine and choline concentrations and dynamics in a murine model of the Fragile X syndrome: Age, sex and region-specific changes. Neuroscience 2015, 301, 520–528. [Google Scholar] [CrossRef]
- El Feil, N.S.; Elmahdy, H.S.; Elmahdy, R.A.; Aboelezz, A.A.; Dawoud, H.S.; Al-Beltagi, M. Brain metabolic profile assessed by magnetic resonance spectroscopy in children with Down syndrome: Relation to intelligence quotient. World J. Clin. Pediatr. 2023, 12, 310–318. [Google Scholar] [CrossRef]
- Bahnfleth, C.L.; Strupp, B.J.; Caudill, M.A.; Canfield, R.L. Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J. 2022, 36, e22054. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Fink, B.A.; Hoecker, H.L.; Boys, C.J.; Radke, J.P.; Kroupina, M.G.; Miller, N.C.; Brearley, A.M.; et al. Choline supplementation in children with fetal alcohol spectrum disorders: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2015, 102, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Kroupina, M.G.; Miller, N.C.; Boys, C.J.; Brearley, A.M.; Fink, B.A.; Hoecker, H.L.; Zeisel, S.H.; et al. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr. Res. 2013, 33, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Powers, B.E.; Velazquez, R.; Strawderman, M.S.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal Choline Supplementation as a Potential Therapy for Down Syndrome: Assessment of Effects Throughout the Lifespan. Front. Aging Neurosci. 2021, 13, 723046. [Google Scholar] [CrossRef]
- Kelley, C.M.; Ash, J.A.; Powers, B.E.; Velazquez, R.; Alldred, M.J.; Ikonomovic, M.D.; Ginsberg, S.D.; Strupp, B.J.; Mufson, E.J. Effects of maternal choline supplementation on the septohippocampal cholinergic system in the Ts65Dn mouse model of Down syndrome. Curr. Alzheimer Res. 2016, 13, 84–96. [Google Scholar] [CrossRef]
- Gautier, M.K.; Kelley, C.M.; Lee, S.H.; Mufson, E.J.; Ginsberg, S.D. Maternal choline supplementation rescues early endosome pathology in basal forebrain cholinergic neurons in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. Neurobiol. Aging 2024, 144, 30–42. [Google Scholar] [CrossRef]
- Alldred, M.J.; Pidikiti, H.; Heguy, A.; Roussos, P.; Ginsberg, S.D. Basal forebrain cholinergic neurons are vulnerable in a mouse model of Down syndrome and their molecular fingerprint is rescued by maternal choline supplementation. FASEB J. 2023, 37, e22944. [Google Scholar] [CrossRef]
- Gotti, S.; Caricati, E.; Panzica, G. Alterations of brain circuits in Down syndrome murine models. J. Chem. Neuroanat. 2011, 42, 317–326. [Google Scholar] [CrossRef]
- Alldred, M.J.; Ibrahim, K.W.; Pidikiti, H.; Lee, S.H.; Heguy, A.; Chiosis, G.; Mufson, E.J.; Stutzmann, G.E.; Ginsberg, S.D. Alldred, M.J.; et al. Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer’s disease (AD). Brain Struct. Funct. 2015, 220, 2983–2996. [Google Scholar] [CrossRef]
- Alldred, M.J.; Lee, S.H.; Petkova, E.; Ginsberg, S.D. Expression profile analysis of vulnerable CA1 pyramidal neurons in young-middle-aged Ts65Dn mice. J. Comp. Neurol. 2015, 523, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Panagiotakos, D.B.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2008, 87, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Duchon, A.; Raveau, M.; Chevalier, C.; Nalesso, V.; Sharp, A.J.; Herault, Y. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: Relevance for modeling Down syndrome. Mamm. Genome 2011, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Alldred, M.J.; Chao, H.M.; Lee, S.H.; Beilin, J.; Powers, B.E.; Petkova, E.; Strupp, B.J.; Ginsberg, S.D. CA1 pyramidal neuron gene expression mosaics in the Ts65Dn murine model of Down syndrome and Alzheimer’s disease following maternal choline supplementation. Hippocampus 2018, 28, 251–268. [Google Scholar] [CrossRef]
- Alldred, M.J.; Ginsberg, S.D. Microisolation of Spatially Characterized Single Populations of Neurons for RNA Sequencing from Mouse and Postmortem Human Brain Tissues. J. Clin. Med. 2023, 12, 3304. [Google Scholar] [CrossRef]
- Alldred, M.J.; Penikalapati, S.C.; Lee, S.H.; Heguy, A.; Roussos, P.; Ginsberg, S.D. Profiling Basal Forebrain Cholinergic Neurons Reveals a Molecular Basis for Vulnerability Within the Ts65Dn Model of Down Syndrome and Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 5141–5162. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk//projects/fastqc/ (accessed on 7 June 2024).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Picard Toolkit. 2019 8/24/22. Available online: https://broadinstitute.github.io/picard/ (accessed on 11 June 2024).
- Zehetmayer, S.; Posch, M.; Graf, A. Impact of adaptive filtering on power and false discovery rate in RNA-seq experiments. BMC Bioinform. 2022, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.; Gallopin, M.; Celeux, G.; Jaffrézic, F. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 2013, 29, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Parmigiani, G.; Johnson, W.E. ComBat-seq: Batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform. 2020, 2, lqaa078. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Fertig, E.J.; Jaffe, A.E.; Zhang, Y.; Storey, J.D.; Torres, L.C. SVA: Surrogate Variable Analysis. 2025. R package Version 3.56.0. Available online: https://bioconductor.org/packages/sva (accessed on 27 June 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Schadt, E.E. variancePartition: Interpreting drivers of variation in complex gene expression studies. BMC Bioinform. 2016, 17, 483. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Roussos, P. Dream: Powerful differential expression analysis for repeated measures designs. Bioinformatics 2021, 37, 192–201. [Google Scholar] [CrossRef]
- Qiagen. 2020. Available online: https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis (accessed on 4 April 2025).
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Alldred, M.J.; Pidikiti, H.; Ibrahim, K.W.; Lee, S.H.; Heguy, A.; Hoffman, G.E.; Roussos, P.; Wisniewski, T.; Wegiel, J.; Stutzmann, G.E.; et al. Analysis of microisolated frontal cortex excitatory layer III and V pyramidal neurons reveals a neurodegenerative phenotype in individuals with Down syndrome. Acta Neuropathol. 2024, 148, 16. [Google Scholar] [CrossRef] [PubMed]
- Alldred, M.J.; Pidikiti, H.; Ibrahim, K.W.; Lee, S.H.; Heguy, A.; Hoffman, G.E.; Mufson, E.J.; Stutzmann, G.E.; Ginsberg, S.D. Hippocampal CA1 Pyramidal Neurons Display Sublayer and Circuitry Dependent Degenerative Expression Profiles in Aged Female Down Syndrome Mice. J. Alzheimers Dis. 2024, 100, S341–S362. [Google Scholar] [CrossRef]
- Alldred, M.J.; Ibrahim, K.W.; Pidikiti, H.; Lee, S.H.; Heguy, A.; Chiosis, G.; Mufson, E.J.; Stutzmann, G.E.; Ginsberg, S.D. Profiling hippocampal neuronal populations reveals unique gene expression mosaics reflective of connectivity-based degeneration in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. Front. Mol. Neurosci. 2025, 18, 1546375. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Mathys, H.; Peng, Z.; Boix, C.A.; Victor, M.B.; Leary, N.; Babu, S.; Abdelhady, G.; Jiang, X.; Ng, A.P.; Ghafari, K.; et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell 2023, 186, 4365–4385.e27. [Google Scholar] [CrossRef]
- Sierra, C.; Sabariego-Navarro, M.; Fernández-Blanco, Á.; Cruciani, S.; Zamora-Moratalla, A.; Novoa, E.M.; Dierssen, M. The lncRNA Snhg11, a new candidate contributing to neurogenesis, plasticity, and memory deficits in Down syndrome. Mol. Psychiatry 2024, 29, 2117–2134. [Google Scholar] [CrossRef]
- Palmer, C.R.; Liu, C.S.; Romanow, W.J.; Lee, M.H.; Chun, J. Altered cell and RNA isoform diversity in aging Down syndrome brains. Proc. Natl. Acad. Sci. USA 2021, 118, e2114326118. [Google Scholar] [CrossRef]
- Alldred, M.J.; Ibrahim, K.W.; Pidikiti, H.; Chiosis, G.; Mufson, E.J.; Stutzmann, G.E.; Ginsberg, S.D. Down syndrome frontal cortex layer III and layer V pyramidal neurons exhibit lamina specific degeneration in aged individuals. Acta Neuropathol. Commun. 2024, 12, 182. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.A.; Cermak, J.M.; Blusztajn, J.K. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front. Integr. Neurosci. 2008, 2, 119. [Google Scholar] [CrossRef]
- Mellott, T.J.; Williams, C.L.; Meck, W.H.; Blusztajn, J.K. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004, 18, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H.; Williams, C.L. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003, 27, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Zheng, Y.; Zhang, Y.; Zhang, X.J.; Wang, H.; Du, Y.; Guan, J.; Wang, X.; Fu, J. NAD+ improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J. Neuroinflamm. 2021, 18, 207. [Google Scholar] [CrossRef]
- Xiong, X.; Hou, J.; Zheng, Y.; Jiang, T.; Zhao, X.; Cai, J.; Huang, J.; He, H.; Xu, J.; Qian, S.; et al. NAD+-boosting agent nicotinamide mononucleotide potently improves mitochondria stress response in Alzheimer’s disease via ATF4-dependent mitochondrial UPR. Cell Death Dis. 2024, 15, 744. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Hung, S.Y.; Huang, W.P.; Liou, H.C.; Fu, W.M. LC3 overexpression reduces Aβ neurotoxicity through increasing α7nAchR expression and autophagic activity in neurons and mice. Neuropharmacology 2015, 93, 243–251. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Function of Autophagy in Neurodegenerative Diseases. Int. J. Mol. Sci. 2015, 16, 26797–26812. [Google Scholar] [CrossRef]
- Marcus, D.L.; Thomas, C.; Rodriguez, C.; Simberkoff, K.; Tsai, J.S.; Strafaci, J.A.; Freedman, M.L. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp. Neurol. 1998, 150, 40–44. [Google Scholar] [CrossRef]
- Keating, D.J.; Chen, C.; Pritchard, M.A. Alzheimer’s disease and endocytic dysfunction: Clues from the Down syndrome-related proteins, DSCR1 and ITSN1. Ageing Res. Rev. 2006, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Shin, J.H.; Jeong, J.I.; Song, J.H.; Jo, Y.K.; Kim, E.S.; Lee, E.H.; Hwang, J.J.; Lee, E.K.; Chung, S.J.; et al. Down-regulation of mortalin exacerbates Aβ-mediated mitochondrial fragmentation and dysfunction. J. Biol. Chem. 2014, 289, 2195–2204. [Google Scholar] [CrossRef]
- Alldred, M.J.; Chao, H.M.; Lee, S.H.; Beilin, J.; Powers, B.E.; Petkova, E.; Strupp, B.J.; Ginsberg, S.D. Long-term effects of maternal choline supplementation on CA1 pyramidal neuron gene expression in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. FASEB J. 2019, 33, 9871–9884. [Google Scholar] [CrossRef]
- Kelley, C.M.; Powers, B.E.; Velazquez, R.; Ash, J.A.; Ginsberg, S.D.; Strupp, B.J.; Mufson, E.J. Sex differences in the cholinergic basal forebrain in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. Brain Pathol. 2014, 24, 33–44. [Google Scholar] [CrossRef]
- Mesulam, M.; Shaw, P.; Mash, D.; Weintraub, S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann. Neurol. 2004, 55, 815–828. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- Mufson, E.J.; Ikonomovic, M.D.; Counts, S.E.; Perez, S.E.; Malek-Ahmadi, M.; Scheff, S.W.; Ginsberg, S.D. Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav. Brain Res. 2016, 311, 54–69. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Che, S.; Counts, S.E.; Mufson, E.J. Shift in the ratio of three-repeat tau and four-repeat tau mRNAs in individual cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J. Neurochem. 2006, 96, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Kazuki, Y.; Gao, F.J.; Li, Y.; Moyer, A.J.; Devenney, B.; Hiramatsu, K.; Miyagawa-Tomita, S.; Abe, S.; Kazuki, K.; Kajitani, N.; et al. A non-mosaic transchromosomic mouse model of Down syndrome carrying the long arm of human chromosome 21. Elife 2020, 9, e56223. [Google Scholar] [CrossRef] [PubMed]
- Mustaly-Kalimi, S.; Gallegos, W.; Marr, R.A.; Gilman-Sachs, A.; Peterson, D.A.; Sekler, I.; Stutzmann, G.E. Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2211999119. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alldred, M.J.; Pidikiti, H.; Ibrahim, K.W.; Lee, S.H.; Heguy, A.; Chiosis, G.; Mufson, E.J.; Stutzmann, G.E.; Ginsberg, S.D. Benefits of Maternal Choline Supplementation on Aged Basal Forebrain Cholinergic Neurons (BFCNs) in a Mouse Model of Down Syndrome and Alzheimer’s Disease. Biomolecules 2025, 15, 1131. https://doi.org/10.3390/biom15081131
Alldred MJ, Pidikiti H, Ibrahim KW, Lee SH, Heguy A, Chiosis G, Mufson EJ, Stutzmann GE, Ginsberg SD. Benefits of Maternal Choline Supplementation on Aged Basal Forebrain Cholinergic Neurons (BFCNs) in a Mouse Model of Down Syndrome and Alzheimer’s Disease. Biomolecules. 2025; 15(8):1131. https://doi.org/10.3390/biom15081131
Chicago/Turabian StyleAlldred, Melissa J., Harshitha Pidikiti, Kyrillos W. Ibrahim, Sang Han Lee, Adriana Heguy, Gabriela Chiosis, Elliott J. Mufson, Grace E. Stutzmann, and Stephen D. Ginsberg. 2025. "Benefits of Maternal Choline Supplementation on Aged Basal Forebrain Cholinergic Neurons (BFCNs) in a Mouse Model of Down Syndrome and Alzheimer’s Disease" Biomolecules 15, no. 8: 1131. https://doi.org/10.3390/biom15081131
APA StyleAlldred, M. J., Pidikiti, H., Ibrahim, K. W., Lee, S. H., Heguy, A., Chiosis, G., Mufson, E. J., Stutzmann, G. E., & Ginsberg, S. D. (2025). Benefits of Maternal Choline Supplementation on Aged Basal Forebrain Cholinergic Neurons (BFCNs) in a Mouse Model of Down Syndrome and Alzheimer’s Disease. Biomolecules, 15(8), 1131. https://doi.org/10.3390/biom15081131






