Dormancy in Colorectal Carcinoma: Detection and Therapeutic Potential
Abstract
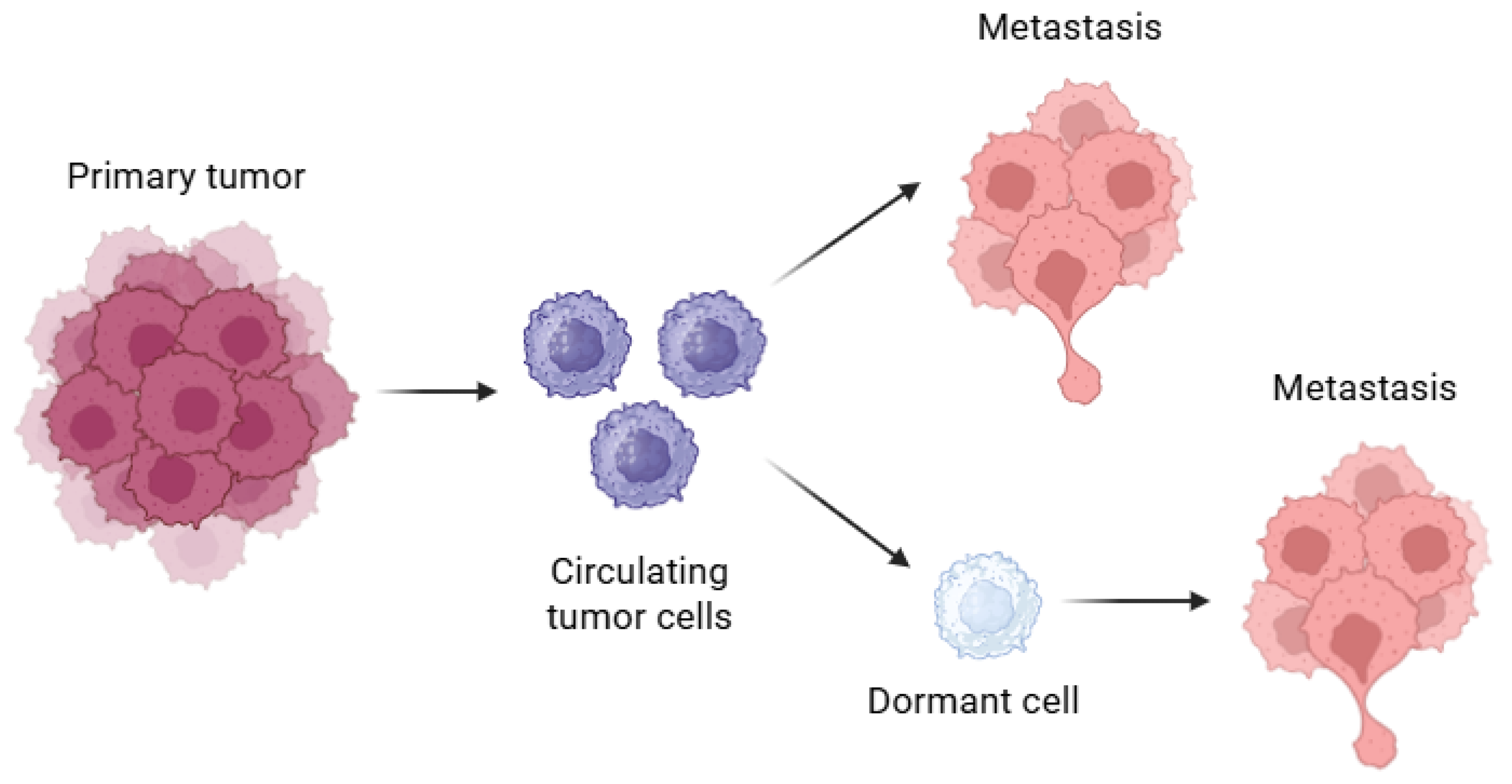
1. Introduction
2. Theoretical Foundation
2.1. Current Status: Cellular Dormancy
2.2. Current Status: Liquid Biopsy
3. Characterization of Dormant Cells
| Mechanism | Description | Key Molecules/Markers | Role in Dormancy | References |
|---|---|---|---|---|
| Epigenetic regulation. | Non-genetic program controlling dormancy via DNA modification and gene expression regulation. | TET2 protein, 5-hydroxymethylcytosine (5 hmC). | Maintains slow cell cycle state; modulates TNF-α signaling; restricts pro-apoptotic signals; biomarker of dormancy and relapse risk. | [27,31] |
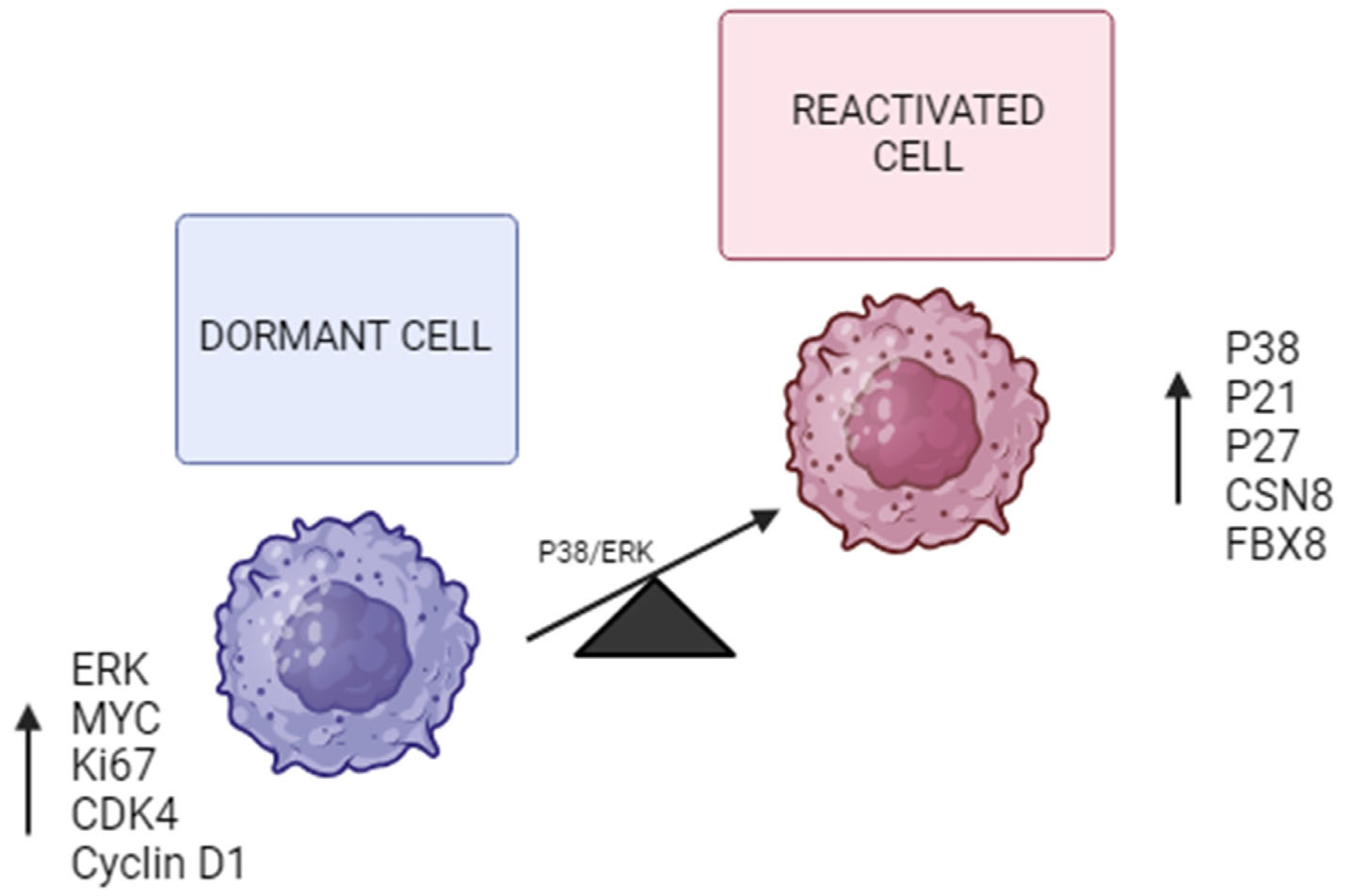
| MAPK signaling pathway. | Balance between P38 and ERK signaling influences dormancy induction. | P38, ERK 1/2, Cyclin D1, P53, P21, P16. | High P38/ERK ratio promotes growth arrest and survival; represses Cyclin D1; activates cell cycle checkpoints. | [20,21,32,33] |
| Stress response and chemoresistance. | Activation of survival pathways under stress conditions, including chemotherapy, hypoxia, serum deprivation. | PERK, BiP, BAX, CSN8, HIF-1α, NF-κB. | CSN8 promotes migration, invasion, dormancy markers (NR2F1, DEC2, P27) and hypoxia response; protects cells from apoptosis. | [19,32] |
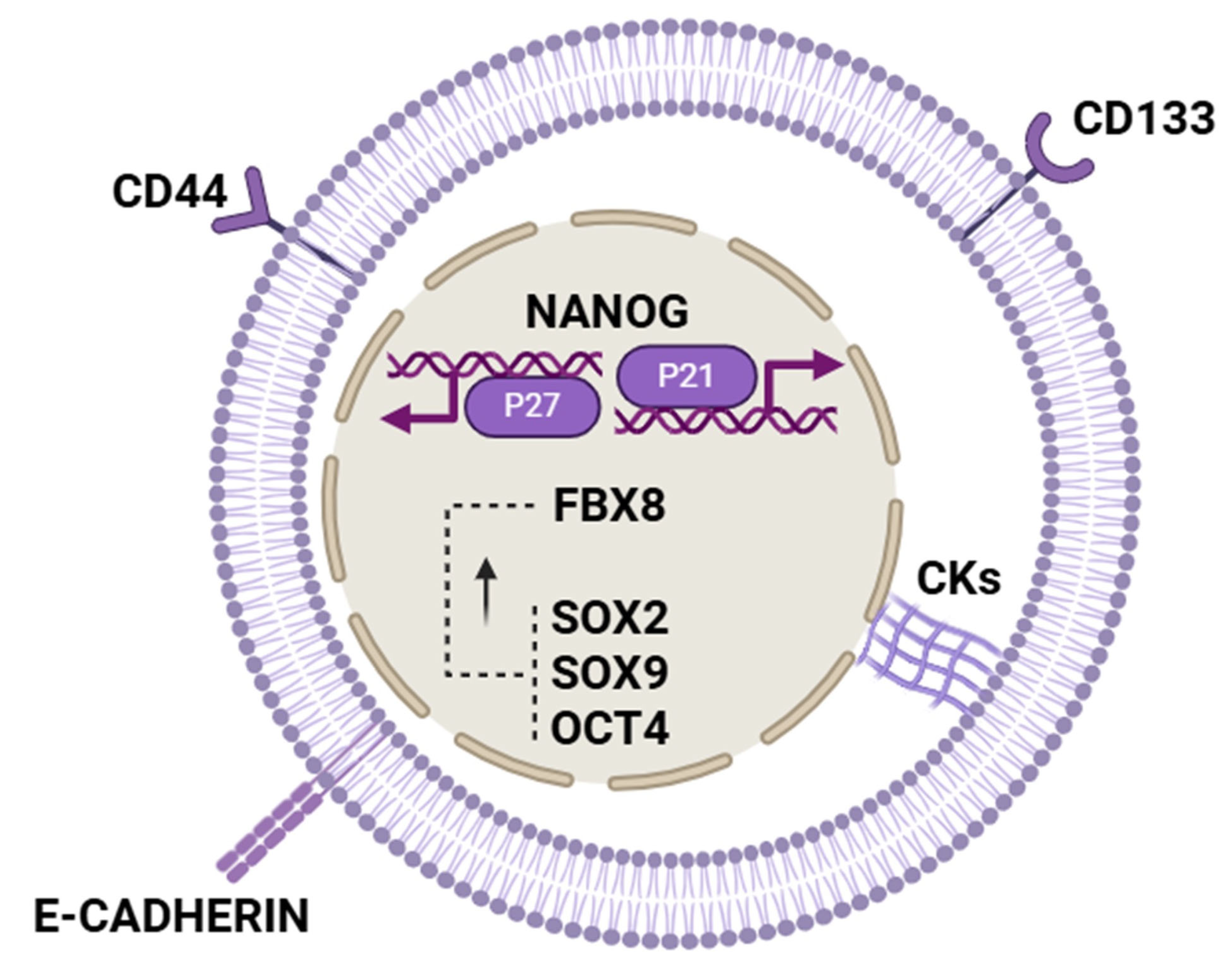
| Hypoxia-related pathways. | Hypoxia induces dormancy through the activation of HIF-1α and related signaling cascades. | HIF-1α, FBX8, NF-κB. | FBX8 regulates dormancy by modulating HIF-1α; promotes G0/G1 arrest and dormancy markers expression. | [5,12,19] |
| Stemness and dormancy overlap. | Dormant tumor cells share features and markers with cancer stem cells, suggesting overlapping mechanisms. | NANOG, SOX2, CD44, CD133, KLF4, AXIN2, LGR5, BMI1. | NANOG promotes dormancy via fatty acid oxidation and P21/P27 induction; other stemness factors mark dormant CSC-like. | [11,22,34,35,36,37] |
| Redox homeostasis. | Dormant cells maintain low ROS levels to evade oxidative damage and chemotherapy-induced apoptosis. | Antioxidant enzymes. | Reduces ROS below normal levels, avoiding ROS-mediated cell death and sustaining dormancy. | [30] |
| Dormant signature genes. | Shared gene expression patterns in dormant tumor cells across cancer types related to adhesion, TEM, TGF-b. | ZEB2, NANOG, factors related to cell adhesion and chemotaxis. | Regulate cell plasticity, migration, and dormancy across tumor types. | [10,11] |
| Cell cycle arrest regulation. | Induction and maintenance of cell cycle arrest at G0/G1 to maintain quiescence. | P21, P27, Cyclin D1, CDK4, c-MYC. | Cell cycle inhibitors P21 and P27 increase, Cyclin D1 and proliferative signals decrease, enforcing quiescence. | [5,11,32] |
4. Potential Detection via Liquid Biopsy
4.1. Analysis of CTDNA and Circulating Tumor Cells (CTCs)
4.2. Serum Analysis of Extracellular Vesicles (EVs)
5. Possible Therapeutic Approaches
5.1. Awakening Quiescent Cells
5.2. Maintenance of Quiescence
5.3. Eliminate Quiescent Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuñiga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.A.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019, 30, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- de Miguel Pérez, D.; Rodriguez Martínez, A.; Ortigosa Palomo, A.; Delgado Ureña, M.; Garcia Puche, J.L.; Robles Remacho, A.; Exposito Hernandez, J.; Lorente Acosta, J.A.; Ortega Sánchez, F.G.; Serrano, M.J. Extracellular vesicle-miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Sci. Rep. 2020, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Tamamouna, V.; Pavlou, E.; Neophytou, C.M.; Papageorgis, P.; Costeas, P. Regulation of Metastatic Tumor Dormancy and Emerging Opportunities for Therapeutic Intervention. Int. J. Mol. Sci. 2022, 23, 13931. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, F.; Wu, X.; Li, Z.; Wang, Z.; Ren, X.; Zhou, Y.; Song, F.; Liang, Y.; Zeng, Z.; et al. FBX8 promotes metastatic dormancy of colorectal cancer in liver. Cell Death Dis. 2020, 11, 622. [Google Scholar] [CrossRef]
- Patelli, G.; Vaghi, C.; Tosi, F.; Mauri, G.; Amatu, A.; Massihnia, D.; Ghezzi, S.; Bonazzina, E.; Bencardino, K.; Cerea, G.; et al. Liquid Biopsy for Prognosis and Treatment in Metastatic Colorectal Cancer: Circulating Tumor Cells vs Circulating Tumor DNA. Target. Oncol. 2021, 16, 309–324. [Google Scholar] [CrossRef]
- Wang, R.; Su, Q.; Yan, Z.-P. Reconsideration of recurrence and metastasis in colorectal cancer. World J. Clin. Cases 2021, 9, 6964–6968. [Google Scholar] [CrossRef]
- Summers, M.A.; McDonald, M.M.; Croucher, P.I. Cancer Cell Dormancy in Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037556. [Google Scholar] [CrossRef]
- Sistigu, A.; Musella, M.; Galassi, C.; Vitale, I.; De Maria, R. Tuning Cancer Fate: Tumor Microenvironment’s Role in Cancer Stem Cell Quiescence and Reawakening. Front. Immunol. 2020, 11, 2166. [Google Scholar] [CrossRef]
- Cuccu, A.; Francescangeli, F.; De Angelis, M.L.; Bruselles, A.; Giuliani, A.; Zeuner, A. Analysis of Dormancy-Associated Transcriptional Networks Reveals a Shared Quiescence Signature in Lung and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 9869. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, R.; Wang, H.; Yang, Z.; Zhang, H.; Zhang, Y.; Wang, M.; Wang, H.; Lin, J.; Zhao, Q.; et al. Nanog mediated by FAO/ACLY signaling induces cellular dormancy in colorectal cancer cells. Cell Death Dis. 2022, 13, 159. [Google Scholar] [CrossRef]
- Santos-de-Frutos, K.; Djouder, N. When dormancy fuels tumour relapse. Commun. Biol. 2021, 4, 747. [Google Scholar] [CrossRef]
- Khaleel Rehman, S.; Haynes, J.; Collignon, E.; Brown, K.R.; Wang, Y.; Nixon, A.M.L.; Bruce, J.P.; Wintersinger, J.A.; Mer, A.S.; Lo, E.B.L.; et al. Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell 2021, 184, 226–242.e21. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, J.S.; Akhter, T.; Bravo-Cordero, J.J. Remodeling the ECM: Implications for Metastasis and Tumor Dormancy. Cancers 2021, 13, 4916. [Google Scholar] [CrossRef] [PubMed]
- Senchukova, M.A. Colorectal cancer and dormant metastases: Put to sleep or destroy? World J. Gastrointest. Oncol. 2024, 16, 2304–2317. [Google Scholar] [CrossRef]
- Damen, M.P.F.; van Rheenen, J.; Scheele, C.L.G.J. Targeting dormant tumor cells to prevent cancer recurrence. FEBS J. 2021, 288, 6286–6303. [Google Scholar] [CrossRef]
- Ohsawa, I.; Murakami, T.; Uemoto, S.; Kobayashi, E. In vivo luminescent imaging of cyclosporin A-mediated cancer progression in rats. Transplantation 2006, 81, 1558–1567. [Google Scholar] [CrossRef]
- Ju, S.; Wang, F.; Wang, Y.; Ju, S. CSN8 is a key regulator in hypoxia-induced epithelial–mesenchymal transition and dormancy of colorectal cancer cells. Mol. Cancer 2020, 19, 168. [Google Scholar] [CrossRef]
- Sosa, M.S.; Avivar-Valderas, A.; Bragado, P.; Wen, H.-C.; Aguirre-Ghiso, J.A. ERK1/2 and p38α/β Signaling in Tumor Cell Quiescence: Opportunities to Control Dormant Residual Disease. Clin. Cancer Res. 2011, 17, 5850–5857. [Google Scholar] [CrossRef]
- Park, S.-Y.; Nam, J.-S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef]
- Xu, F.; Dai, C.; Zhang, R.; Zhao, Y.; Peng, S.; Jia, C. Nanog: A Potential Biomarker for Liver Metastasis of Colorectal Cancer. Dig. Dis. Sci. 2012, 57, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.C.; Tzeng, C.-W.D.; Cao, H.S.T.; Aloia, T.A.; Newhook, T.E.; Overman, M.J.; Kopetz, S.E.; Vauthey, J.-N.; Chun, Y.S. Preliminary Analysis of Liquid Biopsy after Hepatectomy for Colorectal Liver Metastases. J. Am. Coll. Surg. 2021, 233, 82–89.e1. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, D.; Narayan, S.; Thiele, J.-A.; McKinley, D.; Gerdtsson, A.S.; Welter, L.; Hošek, P.; Ostašov, P.; Vyčítal, O.; Brůha, J.; et al. Circulating Tumor Cell Kinetics and Morphology from the Liquid Biopsy Predict Disease Progression in Patients with Metastatic Colorectal Cancer Following Resection. Cancers 2022, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B.; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal–Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef]
- Barták, B.K.; Fodor, T.; Kalmár, A.; Nagy, Z.B.; Zsigrai, S.; Szigeti, K.A.; Valcz, G.; Igaz, P.; Dank, M.; Takács, I.; et al. A Liquid Biopsy-Based Approach for Monitoring Treatment Response in Post-Operative Colorectal Cancer Patients. Int. J. Mol. Sci. 2022, 23, 3774. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.Q.; Inchakalody, V.P.; Mestiri, S.; Yoosuf, Z.S.K.M.; Bedhiafi, T.; El-Ella, D.M.A.; Taib, N.; Hydrose, S.; Akbar, S.; et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J. Exp. Clin. Cancer Res. CR 2022, 41, 99. [Google Scholar] [CrossRef]
- Roviello, G.; Lavacchi, D.; Antonuzzo, L.; Catalano, M.; Mini, E. Liquid biopsy in colorectal cancer: No longer young, but not yet old. World J. Gastroenterol. 2022, 28, 1503–1507. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Zou, J.; Li, L.; Cui, N.; Hao, T.; Yi, K.; Yang, J.; Wu, Y. A meta-analysis of the value of circulating tumor cells in monitoring postoperative recurrence and metastasis of colorectal cancer. PLoS ONE 2022, 17, e0274282. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Ming, H.; Nice, E.C.; Xuan, R.; Huang, C. Redox Control of the Dormant Cancer Cell Life Cycle. Cells 2021, 10, 2707. [Google Scholar] [CrossRef]
- Puig, I.; Tenbaum, S.P.; Chicote, I.; Arqués, O.; Martínez-Quintanilla, J.; Cuesta-Borrás, E.; Ramírez, L.; Gonzalo, P.; Soto, A.; Aguilar, S.; et al. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J. Clin. Investig. 2018, 128, 3887–3905. [Google Scholar] [CrossRef]
- Kudaravalli, S.; den Hollander, P.; Mani, S.A. Role of p38 MAP kinase in cancer stem cells and metastasis. Oncogene 2022, 41, 3177–3185. [Google Scholar] [CrossRef]
- Paillas, S.; Boissière, F.; Bibeau, F.; Denouel, A.; Mollevi, C.; Causse, A.; Denis, V.; Vezzio-Vié, N.; Marzi, L.; Cortijo, C.; et al. Targeting the p38 MAPK pathway inhibits irinotecan resistance in colon adenocarcinoma. Cancer Res. 2011, 71, 1041–1049. [Google Scholar] [CrossRef]
- Khosravi, N.; Shahgoli, V.K.; Amini, M.; Safaei, S.; Mokhtarzadeh, A.; Mansoori, B.; Derakhshani, A.; Baghbanzadeh, A.; Baradaran, B. Suppression of Nanog inhibited cell migration and increased the sensitivity of colorectal cancer cells to 5-fluorouracil. Eur. J. Pharmacol. 2021, 894, 173871. [Google Scholar] [CrossRef]
- Noroozi Karimabad, M.; Roostaei, F.; Mahmoodi, M.; Hajizadeh, M.R. Evaluation of the Effect of Orlistatorlistat on Expression of OCT4, Nanog, SOX2, and KLF4 Genes in Colorectal Cancer SW40 Cell Line. Asian Pac. J. Cancer Prev. 2021, 22, 2335–2341. [Google Scholar] [CrossRef]
- Schölch, S.; García, S.A.; Iwata, N.; Niemietz, T.; Betzler, A.M.; Nanduri, L.K.; Bork, U.; Kahlert, C.; Thepkaysone, M.-L.; Swiersy, A.; et al. Circulating tumor cells exhibit stem cell characteristics in an orthotopic mouse model of colorectal cancer. Oncotarget 2016, 7, 27232–27242. [Google Scholar] [CrossRef] [PubMed]
- Alemohammad, H.; Asadzadeh, Z.; Motafakker Azad, R.; Hemmat, N.; Najafzadeh, B.; Vasefifar, P.; Najafi, S.; Baradaran, B. Signaling pathways and microRNAs, the orchestrators of NANOG activity during cancer induction. Life Sci. 2020, 260, 118337. [Google Scholar] [CrossRef]
- Henriksen, T.V.; Tarazona, N.; Frydendahl, A.; Reinert, T.; Gimeno-Valiente, F.; Carbonell-Asins, J.A.; Sharma, S.; Renner, D.; Hafez, D.; Roda, D.; et al. Circulating Tumor DNA in Stage III Colorectal Cancer, beyond Minimal Residual Disease Detection, toward Assessment of Adjuvant Therapy Efficacy and Clinical Behavior of Recurrences. Clin. Cancer Res. 2022, 28, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Lin, S.; Cai, M.; Zhu, Y.; Song, Y.; Sui, Y.; Lin, J.; Liu, J.; Lu, X.; Zhong, Y.; et al. 5-Hydroxymethylcytosine profiling from genomic and cell-free DNA for colorectal cancers patients. J. Cell. Mol. Med. 2019, 23, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Lu, X.; You, L.; Song, Y.; Luo, Z.; Zhang, J.; Nie, J.; Zheng, W.; Xu, D.; et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017, 27, 1243–1257. [Google Scholar] [CrossRef]
- Peng, H.; Park, J.K.; Katsnelson, J.; Kaplan, N.; Yang, W.; Getsios, S.; Lavker, R.M. microRNA-103/107 family regulates multiple epithelial stem cell characteristics. Stem Cells 2015, 33, 1642–1656. [Google Scholar] [CrossRef]
- Bhatia, R.; Chang, J.; Munoz, J.L.; Walker, N.D. Forging New Therapeutic Targets: Efforts of Tumor Derived Exosomes to Prepare the Pre-Metastatic Niche for Cancer Cell Dissemination and Dormancy. Biomedicines 2023, 11, 1614. [Google Scholar] [CrossRef]
- Khalil, B.D.; Sanchez, R.; Rahman, T.; Rodriguez-Tirado, C.; Moritsch, S.; Martinez, A.R.; Miles, B.; Farias, E.; Mezei, M.; Nobre, A.R.; et al. An NR2F1-specific agonist suppresses metastasis by inducing cancer cell dormancy. J. Exp. Med. 2021, 219, e20210836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Hernández, S.; Hidalgo-León, M.Á.; Lacalle-González, C.; Olivera-Salazar, R.; Ochieng’ Otieno, M.; García-Foncillas, J.; Martinez-Useros, J. Dormancy in Colorectal Carcinoma: Detection and Therapeutic Potential. Biomolecules 2025, 15, 1119. https://doi.org/10.3390/biom15081119
Fernández-Hernández S, Hidalgo-León MÁ, Lacalle-González C, Olivera-Salazar R, Ochieng’ Otieno M, García-Foncillas J, Martinez-Useros J. Dormancy in Colorectal Carcinoma: Detection and Therapeutic Potential. Biomolecules. 2025; 15(8):1119. https://doi.org/10.3390/biom15081119
Chicago/Turabian StyleFernández-Hernández, Sofía, Miguel Ángel Hidalgo-León, Carlos Lacalle-González, Rocío Olivera-Salazar, Michael Ochieng’ Otieno, Jesús García-Foncillas, and Javier Martinez-Useros. 2025. "Dormancy in Colorectal Carcinoma: Detection and Therapeutic Potential" Biomolecules 15, no. 8: 1119. https://doi.org/10.3390/biom15081119
APA StyleFernández-Hernández, S., Hidalgo-León, M. Á., Lacalle-González, C., Olivera-Salazar, R., Ochieng’ Otieno, M., García-Foncillas, J., & Martinez-Useros, J. (2025). Dormancy in Colorectal Carcinoma: Detection and Therapeutic Potential. Biomolecules, 15(8), 1119. https://doi.org/10.3390/biom15081119







