Adenosine as an Active Ingredient in Topical Preparations Against Hair Loss: A Systematic Review and Meta-Analysis of Published Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Meta-Analysis of Eligible Data
3. Results
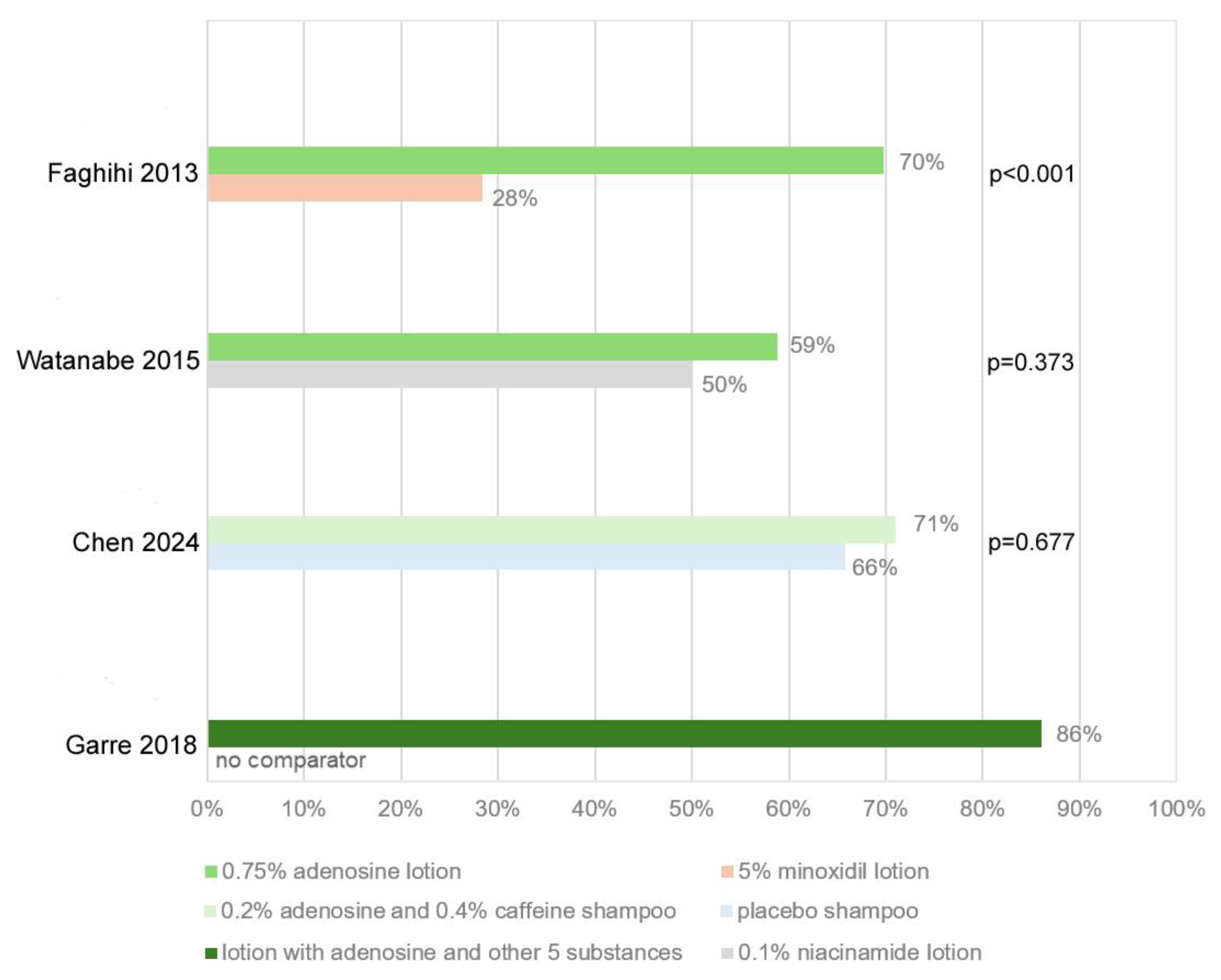
3.1. Systematic Review of Clinical Trials
3.2. Meta-Analysis
3.3. Patient Satisfaction and Safety Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | Androgenetic alopecia |
| FPHL | Female pattern hair loss |
| MNX | Minoxidil |
| TE | Telogen effluvium |
References
- Hasko, G.; Cronstein, B.N. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Do, C.W.; Avila, M.Y.; Peterson-Yatorno, K.; Stone, R.A.; Gao, Z.G.; Joshi, B.V.; Besada, P.; Jeong, L.S.; Jacobson, K.A.; et al. Nucleoside-derived antagonists to A3 adenosine receptors lower mouse intraocular pressure and act across species. Exp. Eye Res. 2010, 90, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Annabi, N.; Tamayol, A.; Oklu, R.; Ghanem, A.; Khademhosseini, A. Adenosine-associated delivery systems. J. Drug Target. 2015, 23, 580–596. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 60961, Adenosine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Adenosine (accessed on 16 March 2025).
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 3527–3552. [Google Scholar] [CrossRef]
- Zhang, C.; Du, S.; Guo, L.; Xu, Q.; Xie, X.; Chen, N. Enhancement of adenosine production by over expression of purA in Bacillis subtilis XGL. J. Chem. Pharm. Res. 2014, 6, 549–555. [Google Scholar]
- Marucci, G.; Buccioni, M.; Varlaro, V.; Volpini, R.; Amenta, F. The possible role of the nucleoside adenosine in countering skin aging: A review. Biofactors 2022, 48, 1027–1035. [Google Scholar] [CrossRef]
- Dakkak, M.; Forde, K.M.; Lanney, H. Hair Loss: Diagnosis and Treatment. Am. Fam. Physician. 2024, 110, 243–250. [Google Scholar]
- Braun, N.; Heinrich, U. What Can Complex Dietary Supplements Do for Hair Loss and How Can It Be Validly Measured—A Review. Appl. Sci. 2020, 10, 4996. [Google Scholar] [CrossRef]
- Rajput, R.J. Influence of Nutrition, Food Supplements and Lifestyle in Hair Disorders. Indian Dermatol. Online J. 2022, 13, 721–724. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, W.; Wang, C.; Yang, W. Mechanism and prevention of hair loss after metabolic and bariatric surgery. On behalf of Chinese Obesity Metabolic Surgery Collaborative. Precis. Nutr. 2022, 1, e00010. [Google Scholar] [CrossRef]
- Kwon, T.R.; Oh, C.T.; Park, H.M.; Han, H.J.; Ji, H.J.; Kim, B.J. Potential synergistic effects of human placental extract and minoxidil on hair growth-promoting activity in C57BL/6J mice. Clin. Exp. Dermatol. 2015, 40, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, E.; Dündar, C.; Türkoğlu, M. A proprietary herbal extract against hair loss in androgenetic alopecia and telogen effluvium: A placebo-controlled, single-blind, clinical-instrumental study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.L.; Lee, M.H.; Kim, N.R.; Roh, S.S.; Lee, Y.; Kim, C.D.; Lee, J.H.; Choi, K.C. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int. J. Mol. Med. 2012, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- El-Arabey, A.A.; Abdalla, M. Formononetin: Novel Deal for Androgenetic Alopecia. Arch. Med. Res. 2020, 51, 30–31. [Google Scholar] [CrossRef]
- Alsalhi, W.; Alalola, A.; Randolph, M.; Gwillim, E.; Tosti, A. Novel drug delivery approaches for the management of hair loss. Expert Opin. Drug Deliv. 2020, 17, 287–295. [Google Scholar] [CrossRef]
- Kadir, R.; Stempler, D.; Liron, Z.; Cohen, S. Penetration of adenosine into excised human skin from binary vehicles: The enhancement factor. J. Pharm. Sci. 1988, 77, 409–413. [Google Scholar] [CrossRef]
- Pathak, Y.; Thassu, D. Drug Delivery Nanoparticles Formulation and Characterization; Informa Healthcare; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Meidan, V.C.; Bonner, M.; Michniak, B. Transfollicular drug delivery—Is it a reality? Int. J. Pharm. 2005, 306, 1–14. [Google Scholar] [CrossRef]
- Lademann, J.; Knorr, F.; Richter, H.; Blume-Peytavi, U.; Vogt, A.; Antoniou, C.; Sterry, W.; Patzelt, A. Hair follicles—An efficient storage and penetration pathway for topically applied substances. Skin Pharmacol. Physiol. 2008, 21, 150–155. [Google Scholar] [CrossRef]
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schaefer, U.F.; Blume-Peytavi, U.; Teichmann, A.; Otberg, N.; Sterry, W. Hair follicles—A long-term reservoir for drug delivery. Skin Pharmacol. Physiol. 2006, 1, 232–236. [Google Scholar] [CrossRef]
- Linden, J. Adenosine in tissue protection and tissue regeneration. Mol. Pharmacol. 2005, 67, 1385–1387. [Google Scholar] [CrossRef]
- Iino, M.; Ehama, R.; Nakazawa, Y.; Iwabuchi, T.; Ogo, M.; Tajima, M.; Arase, S. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J. Investig. Dermatol. 2007, 127, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.H.; Kang, N.G.; Lee, S. Anti-Hair Loss Effect of Adenosine Is Exerted by cAMP Mediated Wnt/β-Catenin Pathway Stimulation via Modulation of Gsk3β Activity in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 2184. [Google Scholar] [CrossRef] [PubMed]
- Lisztes, E.; Tóth, B.T.; Bertolini, M.; Szabó, I.L.; Zákány, N.; Oláh, A.; Szöllősi, A.G.; Paus, R.; Bíró, T. Adenosine Promotes Human Hair Growth and Inhibits Catagen Transition In Vitro: Role of the Outer Root Sheath Keratinocytes. J. Investig. Dermatol. 2020, 140, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Marubayashi, A.; Nakaya, Y.; Fukui, K.; Li, M.; Arase, S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: Possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J. Investig. Dermatol. 2001, 6, 1594–1600. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Simmonds, M.S.J. Topical and nutricosmetic products for healthy hair and dermal antiaging using “dual-acting” (2 for 1) plant-based peptides, hormones, and cannabinoids. FASEB Bioadv. 2021, 3, 601–610. [Google Scholar] [CrossRef]
- Stenn, K.S.; Combates, N.J.; Eilertsen, K.J.; Gordon, J.S.; Pardinas, J.R.; Parimoo, S.; Prouty, S.M. Hair follicle growth controls. Dermatol. Clin. 1996, 14, 543–558. [Google Scholar] [CrossRef]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Investig. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef]
- Asanad, K.; Sholklapper, T.; Samplaski, M.K.; Cacciamani, G.E. Global online interest in finasteride sexual side effects. Int. J. Impot. Res. 2024, 36, 408–413. [Google Scholar] [CrossRef]
- Ciulkiewicz, Ł.; Pełka, M.; Fijałkowska, J.; Kania, A. An overview of incidence and mechanisms promoting weight gain as an adverse effect of oral minoxidil therapy for androgenetic alopecia. Eur. J. Clin. Exp. Med. 2024, 22, 424–431. [Google Scholar] [CrossRef]
- Jimenez-Cauhe, J.; Lo Sicco, K.I.; Shapiro, J.; Hermosa-Gelbard, A.; Burgos-Blasco, P.; Melian-Olivera, A.; Ortega-Quijano, D.; Pindado-Ortega, C.; Buendia-Castano, D.; Asz-Sigall, D.; et al. Characterization and Management of Adverse Events of Low-Dose Oral Minoxidil Treatment for Alopecia: A Narrative Review. J. Clin. Med. 2025, 14, 1805. [Google Scholar] [CrossRef]
- Kruk, A.; Dorosz, A.; Skoczeń, A.; Kulesza, M.; Wawrzynów, W.; Jakubowska, M.M.; Rutecka, N.; Kaczmarek, B.; Miłoś, M.; Kuśnierz-Gibała, A. Adverse effects of finasteride in men treated for benign prostatic hyperplasia and androgenetic alopecia—A literature review. J. Educ. Health Sport 2025, 79, 58442. [Google Scholar] [CrossRef]
- Ajao, A.A.; Sadgrove, N.J. Cosmetopoeia of African Plants in Hair Treatment and Care: Topical Nutrition and the Antidiabetic Connection? Diversity 2024, 16, 96. [Google Scholar] [CrossRef]
- Pyzik, M.; Plichta, D.; Spiewak, R. An occurrence analysis of ingredients declared as active in anti-hair loss products and systematic review of studies on their effectiveness. Estetol. Med. Kosmetol. 2020, 10. [Google Scholar] [CrossRef]
- Szendzielorz, E.; Spiewak, R. An analysis of the presence of ingredients that were declared by the producers as “active” in trichological shampoos for hair loss. Estetol. Med. Kosmetol. 2024, 14. [Google Scholar] [CrossRef]
- Chen, D.; Yu, F.; Wang, C.; Chen, H.; Tan, J.; Shi, Q.; He, X.; Liu, X.; Wang, F.; Zhao, H. Anti-hair loss effect of a shampoo containing caffeine and adenosine. J. Cosmet. Dermatol. 2024, 23, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, G.; Iraji, F.; Rajaee Harandi, M.; Nilforoushzadeh, M.A.; Askari, G. Comparison of the efficacy of topical minoxidil 5% and adenosine 0.75% solutions on male androgenetic alopecia and measuring patient satisfaction rate. Acta Dermatovenerol. Croat. 2013, 21, 155–159. [Google Scholar]
- Garre, A.; Piquero, J.; Trullas, C.; Martinez, G. Efficacy and Safety of a New Topical Hair Loss-Lotion Containing Oleanolic Acid, Apigenin, Biotinyl Tripeptide-1, Diaminopyrimidine Oxide, Adenosine, Biotin and Ginkgo biloba in Patients with Androgenetic Alopecia and Telogen effluvium: A Six-month Open-Label Prospective Clinical Study. J. Cosmo. Trichol. 2018, 4, 1000132. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Ideta, R.; Ehama, R.; Yamanishi, H.; Iino, M.; Nakazawa, Y.; Kobayashi, T.; Ohyama, M.; Kishimoto, J. Topical adenosine increases the proportion of thick hair in Caucasian men with androgenetic alopecia. J. Dermatol. 2016, 43, 567–570. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.y.; Choi, Y.-H.; Joo, J.H.; Kwack, M.H.; Sung, Y.K.; Kang, N.G. Hair Thickness Growth Effect of Adenosine Complex in Male/Female-Patterned Hair Loss via Inhibition of Androgen Receptor Signaling. Int. J. Mol. Sci. 2024, 25, 6534. [Google Scholar] [CrossRef]
- Kinoshita-Ise, M.; Fukuyama, M.; Ohyama, M. Recent Advances in Understanding of the Etiopathogenesis, Diagnosis, and Management of Hair Loss Diseases. J. Clin. Med. 2023, 12, 3259. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagashima, T.; Hanzawa, N.; Ishino, A.; Nakazawa, Y.; Ogo, M.; Iwabuchi, T.; Tajima, M. Topical adenosine increases thick hair ratio in Japanese men with androgenetic alopecia. Int. J. Cosmet. Sci. 2015, 37, 579–587. [Google Scholar] [CrossRef]
- Gupta, M.; Mysore, V. Classifications of Patterned Hair Loss: A Review. J. Cutan. Aesthetic Surg. 2016, 9, 3–12. [Google Scholar] [CrossRef]
- Oura, H.; Iino, M.; Nakazawa, Y.; Tajima, M.; Ideta, R.; Nakaya, Y.; Arase, S.; Kishimoto, J. Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: A pilot, double-blind, randomized, placebo-controlled trial. J. Dermatol. 2008, 35, 763–767. [Google Scholar] [CrossRef]
- Rebora, A. Telogen effluvium: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2019, 12, 583–590. [Google Scholar] [CrossRef]
- Oblong, J.E.; Peplow, A.W.; Hartman, S.M.; Davis, M.G. Topical niacinamide does not stimulate hair growth based on the existing body of evidence. Int. J. Cosmet. Sci. 2020, 42, 217–219. [Google Scholar] [CrossRef]
- Merja, A.; Patel, N.; Patel, M.; Patnaik, S.; Ahmed, A.; Maulekhi, S. Safety and efficacy of REGENDIL™ infused hair growth promoting product in adult human subject having hair fall complaints (alopecia). J. Cosmet. Dermatol. 2024, 23, 938–948. [Google Scholar] [CrossRef]
- Szendzielorz, E.; Spiewak, R. Placental extracts, proteins, and hydrolyzed proteins as active ingredients in cosmetic preparations for hair loss: A systematic review of available clinical evidence. Appl. Sci. 2024, 14, 10301. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.-E.; Ha, J.; Kim, Y.; Lee, C.; Hong, K.-W. Genetic Differences between Male and Female Pattern Hair Loss in a Korean Population. Life 2024, 14, 939. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef]
- Rathnayake, D.; Sinclair, R. Male androgenetic alopecia. Expert Opin. Pharmacother. 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss—Clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef]
- Aleissa, M. The Efficacy and Safety of Oral Spironolactone in the Treatment of Female Pattern Hair Loss: A Systematic Review and Meta-Analysis. Cureus 2023, 15, 43559. [Google Scholar] [CrossRef]
- Sawant, N.; Chikhalkar, S.; Mehta, V.; Ravi, M.; Madke, B.; Khopkar, U. Androgenetic alopecia: Quality-of-life and associated lifestyle patterns. Int. J. Trichology 2010, 2, 81–85. [Google Scholar] [CrossRef]
- Imhof, R.L.; Davis, D.M.R.; Tollefson, M.M. Hair Loss. Pediatr. Rev. 2020, 41, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Sinclair, R. Telogen effluvium. Clin. Exp. Dermatol. 2002, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tamashunas, N.L.; Bergfeld, W.F. Male and female pattern hair loss: Treatable and worth treating. Cleve. Clin. J. Med. 2021, 88, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Szendzielorz, E.; Spiewak, R. Caffeine as an Active Molecule in Cosmetic Products for Hair Loss: Its Mechanisms of Action in the Context of Hair Physiology and Pathology. Molecules 2025, 30, 167. [Google Scholar] [CrossRef]
- Szendzielorz, E.; Spiewak, R. Caffeine as an Active Ingredient in Cosmetic Preparations Against Hair Loss: A Systematic Review of Available Clinical Evidence. Healthcare 2025, 13, 395. [Google Scholar] [CrossRef]
- Heesen, R.; Bright, L.K. Publication bias is bad for science if not necessarily scientists. R. Soc. Open Sci. 2025, 12, 240688. [Google Scholar] [CrossRef]
- Hopewell, S.; Loudon, K.; Clarke, M.J.; Oxman, A.D.; Dickersin, K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst. Rev. 2009, 2009, MR000006. [Google Scholar] [CrossRef] [PubMed]
- van Lent, M.; Overbeke, J.; Out, H.J. Role of editorial and peer review processes in publication bias: Analysis of drug trials submitted to eight medical journals. PLoS ONE 2014, 9, e104846. [Google Scholar] [CrossRef]
- Guyatt, G.; Wang, Y.; Eachempati, P.; Iorio, A.; Murad, M.H.; Hultcrantz, M.; Chu, D.K.; Florez, I.D.; Hemkens, L.G.; Agoritsas, T.; et al. Core GRADE 4: Rating certainty of evidence-risk of bias, publication bias, and reasons for rating up certainty. BMJ 2025, 389, e083864. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Spuls, P.I.; Kottner, J.; Thomas, K.S.; Apfelbacher, C.; Chalmers, J.R.; Deckert, S.; Furue, M.; Gerbens, L.; Kirkham, J.; et al. Navigating the landscape of core outcome set development in dermatology. J. Am. Acad. Dermatol. 2019, 81, 297–305. [Google Scholar] [CrossRef]
- Ronsch, H.; Drewitz, K.P.; Atwater, A.R.; Becker, D.; Bentz, P.; Brans, R.; Chong, T.; Dickel, H.; Elsner, P.; Gimenez-Arnau, A.M.; et al. Consensus on core domains for hand eczema trials: Signs, symptoms, control and quality of life. J. Eur. Acad. Dermatol. Venereol. 2025. online version record. [Google Scholar] [CrossRef]
- Schmitt, J.; Lange, T.; Kottner, J.; Prinsen, C.A.C.; Weberschock, T.; Hahnel, E.; Apfelbacher, C.; Brandstetter, S.; Dreher, A.; Stevens, G.; et al. Cochrane Reviews and Dermatological Trials Outcome Concordance: Why Core Outcome Sets Could Make Trial Results More Usable. J. Investig. Dermatol. 2019, 139, 1045–1053. [Google Scholar] [CrossRef]



| PICO Criterion | Description |
|---|---|
| Patients | People suffering from baldness, hair loss, effluvium, or alopecia |
| Intervention | Adenosine in topical anti-hair loss preparations |
| Comparator/Control | Placebo or other topical anti-hair loss preparations, lack of controls |
| Outcomes | Phototrichogram, trichoscopy, investigator assessment, participant assessment |
| Types of Hair Loss | Clinical Trials | Classification and Severity Grading Systems |
|---|---|---|
| Androgenetic alopecia (AGA) | [38,39,40,41,42] | The Hamilton–Norwood classification is the most widely used classification system for AGA in men, which has seven stages of disease severity observed from the forehead line to the vertex [43] |
| [44] | Ogata created a scale to assess androgenic alopecia dedicated to Japanese men, suggesting that their balding patterns differ from white men. This scale is characterized by the occurrence of six subtypes of balding, each with 2–4 advancement stages [45] | |
| Female Pattern Hair Loss (FPHL) | [38,40,42,46] | The Ludwig classification assesses the degree of baldness in women and categorizes FPHL cases into three grades based on the extent of hair thinning on the top of the head [43] |
| Telogen effluvium (TE) | [40] | Headington made an attempt at TE classification, but it has not found a broad use, possibly due to its complexity. TE is classified according to the time and cause of the hair transitioning to the telogen phase and is divided into five subtypes, depending on whether the anagen or telogen phase is altered [47] |
| Year | Study Design | Study Group | Adenosine Formulation | Comparator | Main Outcome | Evidence Strength (GRADE) | Ref. |
|---|---|---|---|---|---|---|---|
| 2008 | Randomized double-blind, placebo-controlled trial | 30 F with FPHL | 0.75% adenosine lotion | Placebo lotion | Objective and subjective improvement | Moderate | [46] |
| 2013 | Prospective-randomized study | 110 M with AGA | 0.75% adenosine lotion | 5% MNX lotion | Effects of 0.75% adenosine comparable to 5% MNX | Low | [39] |
| 2015 | Randomized double-blind study | 102 M with AGA | 0.75% adenosine lotion | 0.1% niacinamide lotion | Objective and subjective improvement | Moderate | [44] |
| 2016 | Prospective randomized study | 38 M with AGA | 0.75% adenosine lotion | Placebo lotion | Improvement of hair density | Low | [41] |
| 2018 | Open-label prospective clinical study | 56 M and F with AGA and TE | Lotion with adenosine and oleanolic acid, apigenin, biotinyl tripeptide-1,2-4-diamino pyrimidine-3-oxide, Ginkgo biloba, and biotin | None | Improvement of hair parameters in whole scalp | Very low | [40] |
| 2024 | Randomized, controlled, single-blind study | 84 M and F with AGA | Shampoo with 0.2% adenosine and 0.4% caffeine | Placebo shampoo | Improvement in hair growth, decrease in hair loss | Low | [38] |
| 2024 | Randomized, controlled, double-blind study | 46 M and F with AGA | Complex lotion with 0.75% adenosine, 1% panthenol, and 2% niacinamide | 5% MNX lotion | Improvement of hair growth, condition, and hair thickness | Low | [42] |
| Author, Year | Adenosine Group Before Treatment | Adenosine Group After 6 Months | Placebo * Group Before Treatment | Placebo * Group After 6 Months |
|---|---|---|---|---|
| Iwabuchi 2016 [41] | 243.1 | 255.0 | 256.6 | 246.6 |
| Oura 2008 [46] | 210 | 205 | 195 | 195 |
| Watanabe * 2015 [44] | 220.2 | 226.1 | 230.1 | 232.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szendzielorz, E.; Spiewak, R. Adenosine as an Active Ingredient in Topical Preparations Against Hair Loss: A Systematic Review and Meta-Analysis of Published Clinical Trials. Biomolecules 2025, 15, 1093. https://doi.org/10.3390/biom15081093
Szendzielorz E, Spiewak R. Adenosine as an Active Ingredient in Topical Preparations Against Hair Loss: A Systematic Review and Meta-Analysis of Published Clinical Trials. Biomolecules. 2025; 15(8):1093. https://doi.org/10.3390/biom15081093
Chicago/Turabian StyleSzendzielorz, Ewelina, and Radoslaw Spiewak. 2025. "Adenosine as an Active Ingredient in Topical Preparations Against Hair Loss: A Systematic Review and Meta-Analysis of Published Clinical Trials" Biomolecules 15, no. 8: 1093. https://doi.org/10.3390/biom15081093
APA StyleSzendzielorz, E., & Spiewak, R. (2025). Adenosine as an Active Ingredient in Topical Preparations Against Hair Loss: A Systematic Review and Meta-Analysis of Published Clinical Trials. Biomolecules, 15(8), 1093. https://doi.org/10.3390/biom15081093







