3D-Printed Poly (l-lactic acid) Scaffolds for Bone Repair with Oriented Hierarchical Microcellular Foam Structure and Biocompatibility
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Samples
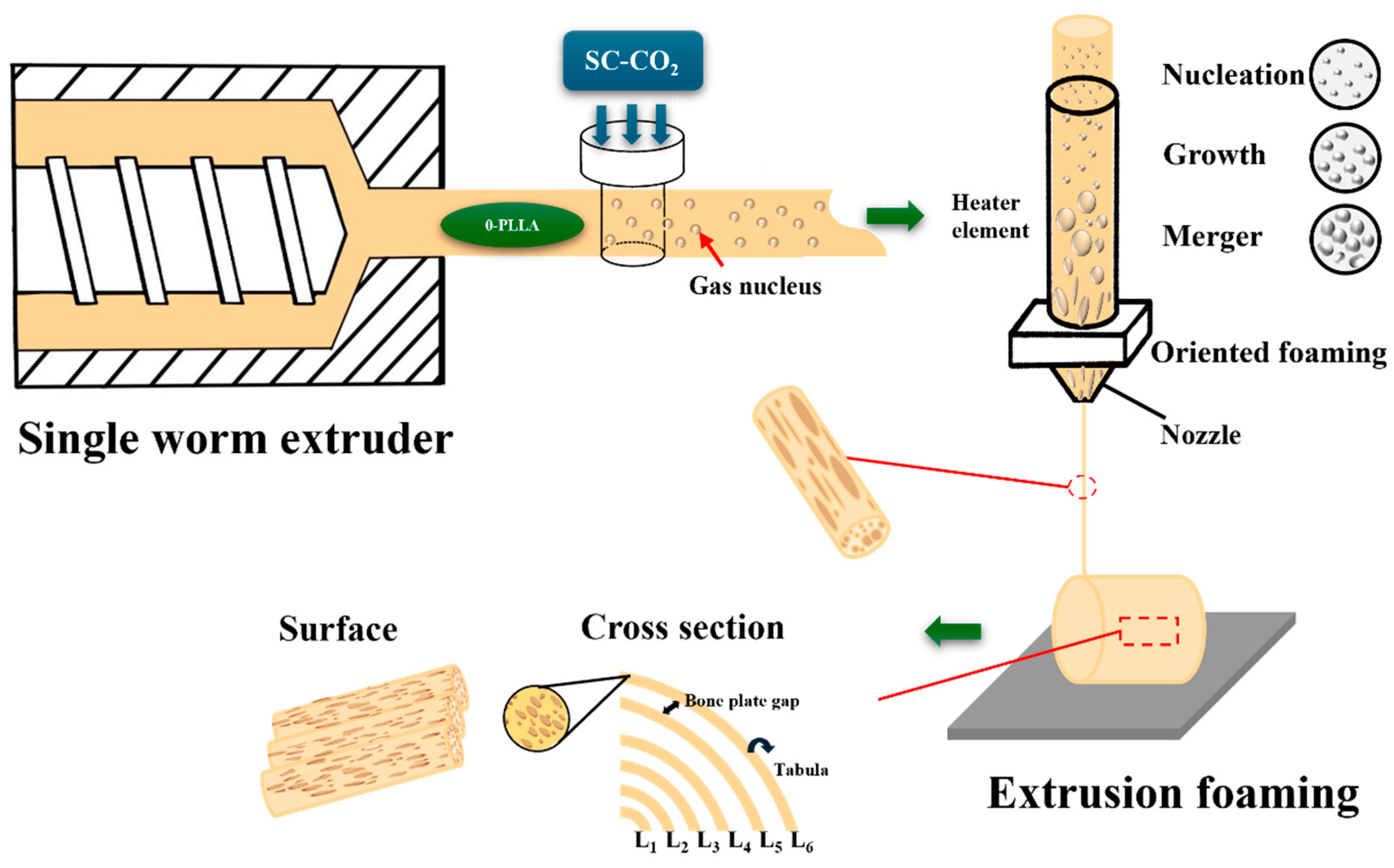
2.2.1. Preparation of FDM Filaments
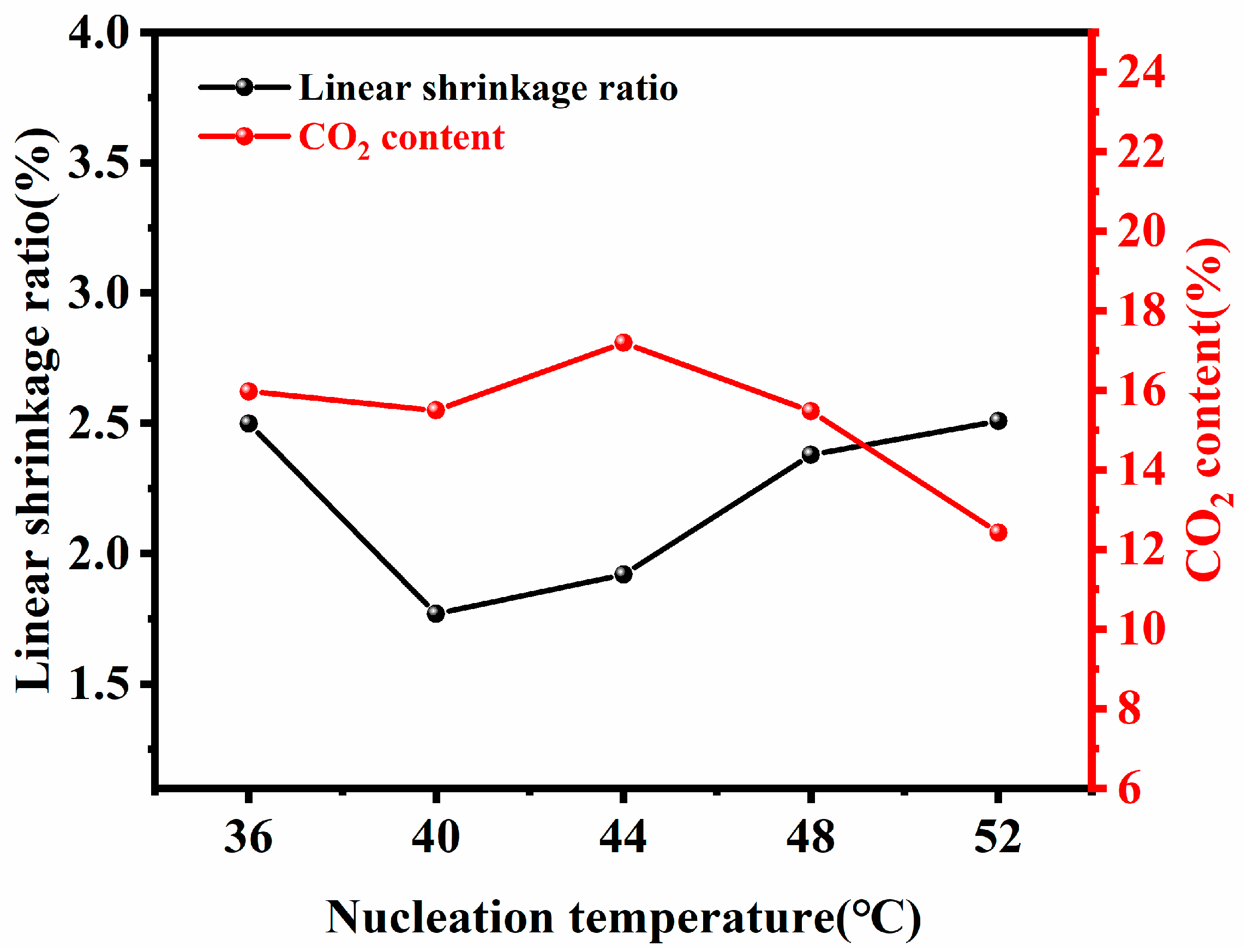
2.2.2. Preparation of Solid Phase Nucleation Filaments
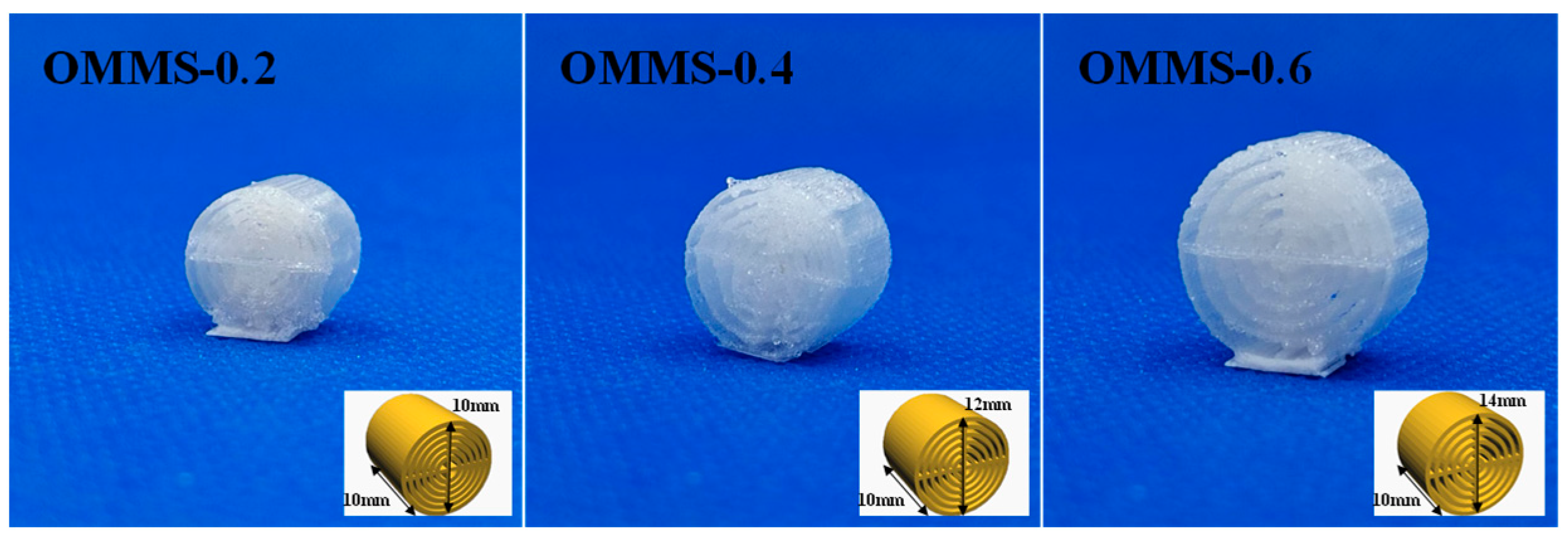
2.2.3. Preparation of Oriented Multi-Stage Microporous Scaffold by Extrusion Foaming Method
2.3. Measurements
2.3.1. Differential Scanning Calorimetry (DSC)
Non-Isothermal Crystallization and Melting Behavior
2.3.2. X-Ray Diffractometer (XRD)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Mechanical Property Measurement
2.3.5. Biological Evaluation
Cell Viability and Proliferation
Cell Morphology
Gene Expression Analysis
2.3.6. Surface Tension Measurement
3. Results and Discussion
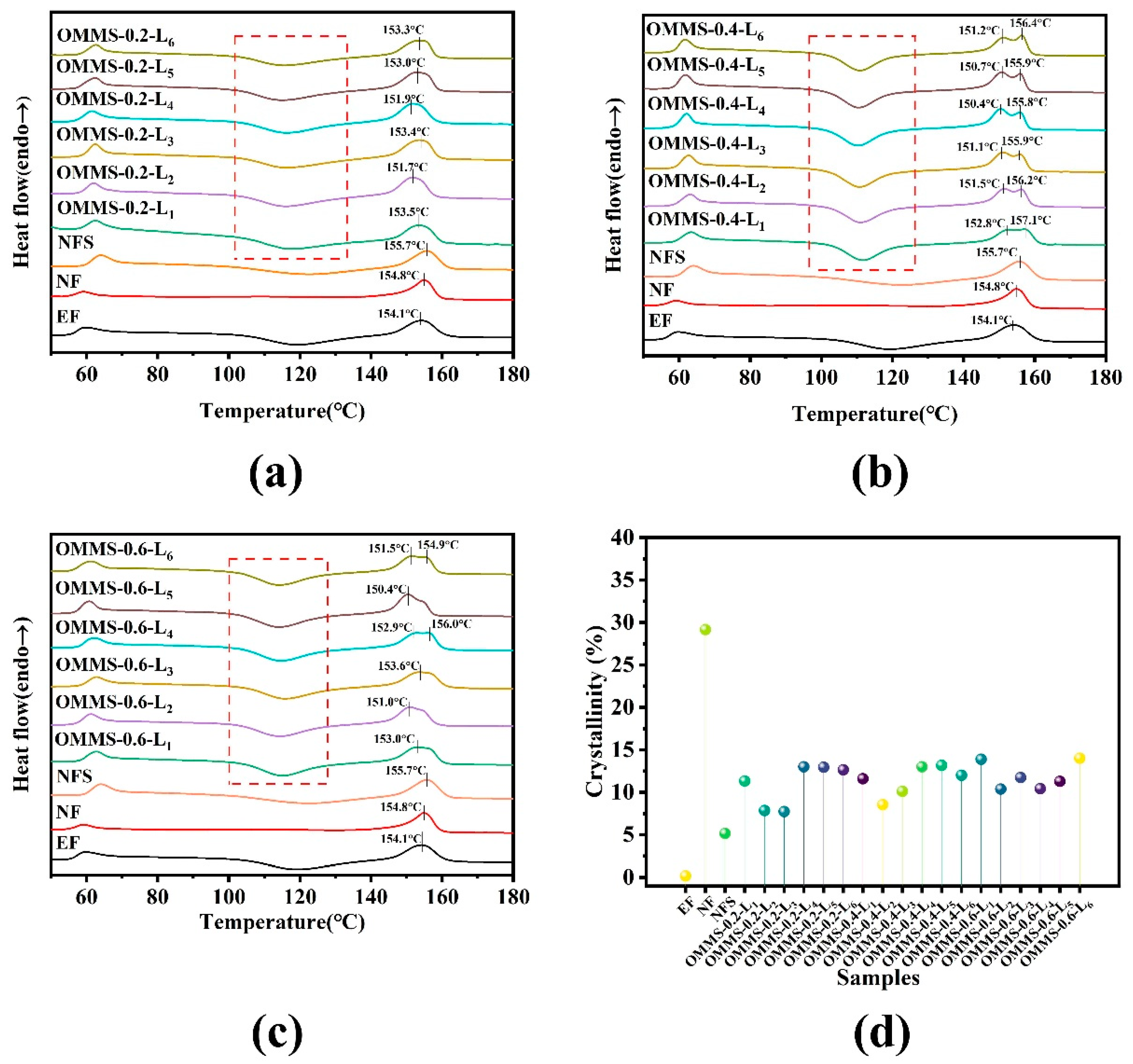
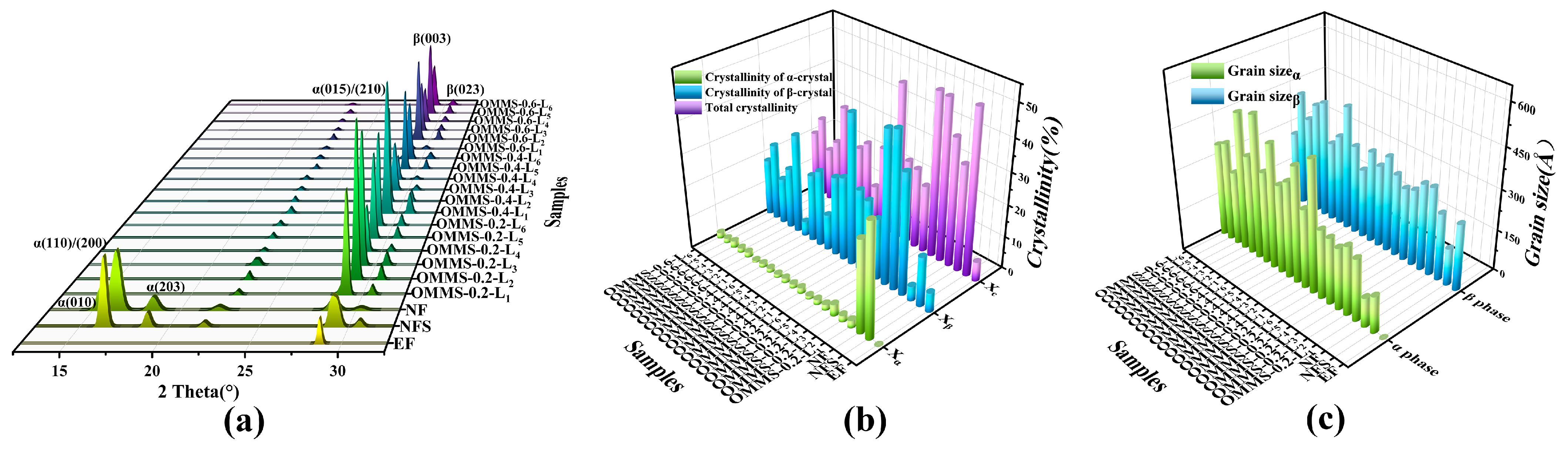
3.1. Thermal Properties and Crystal Structure
3.1.1. DSC
3.1.2. XRD
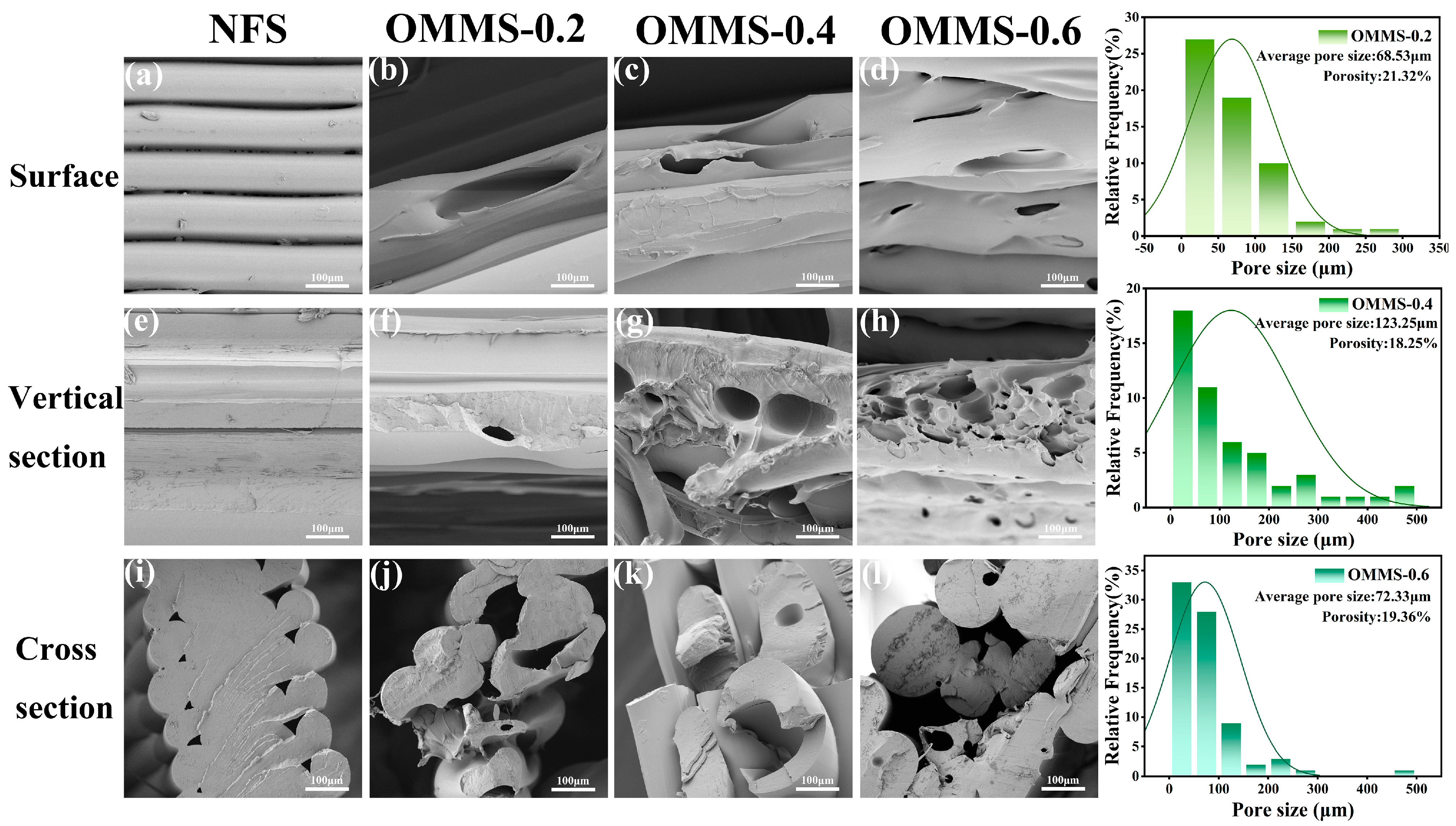
3.2. Morphology and Mechanical Properties
3.2.1. Morphology and Structure
3.2.2. Mechanical Property
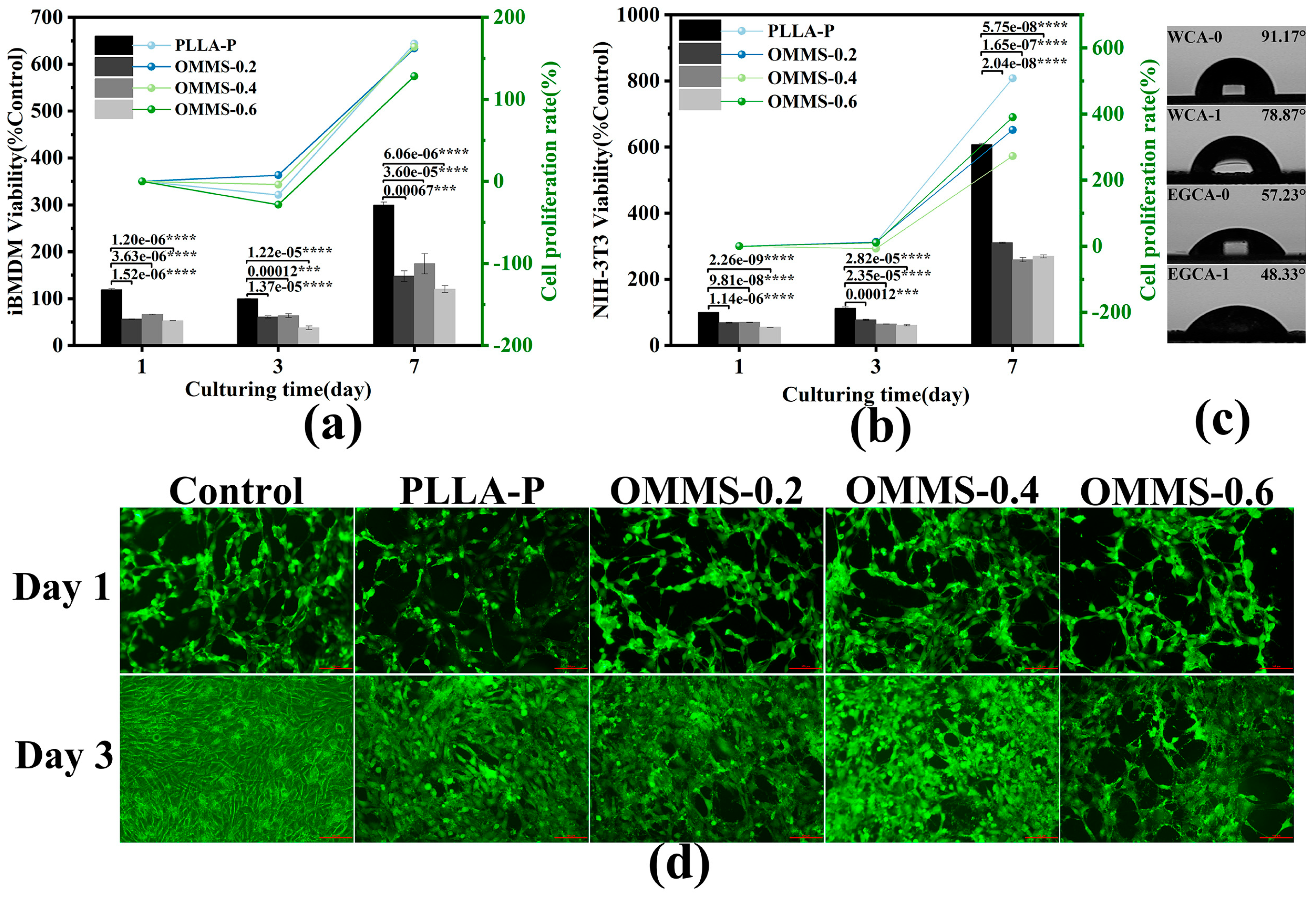
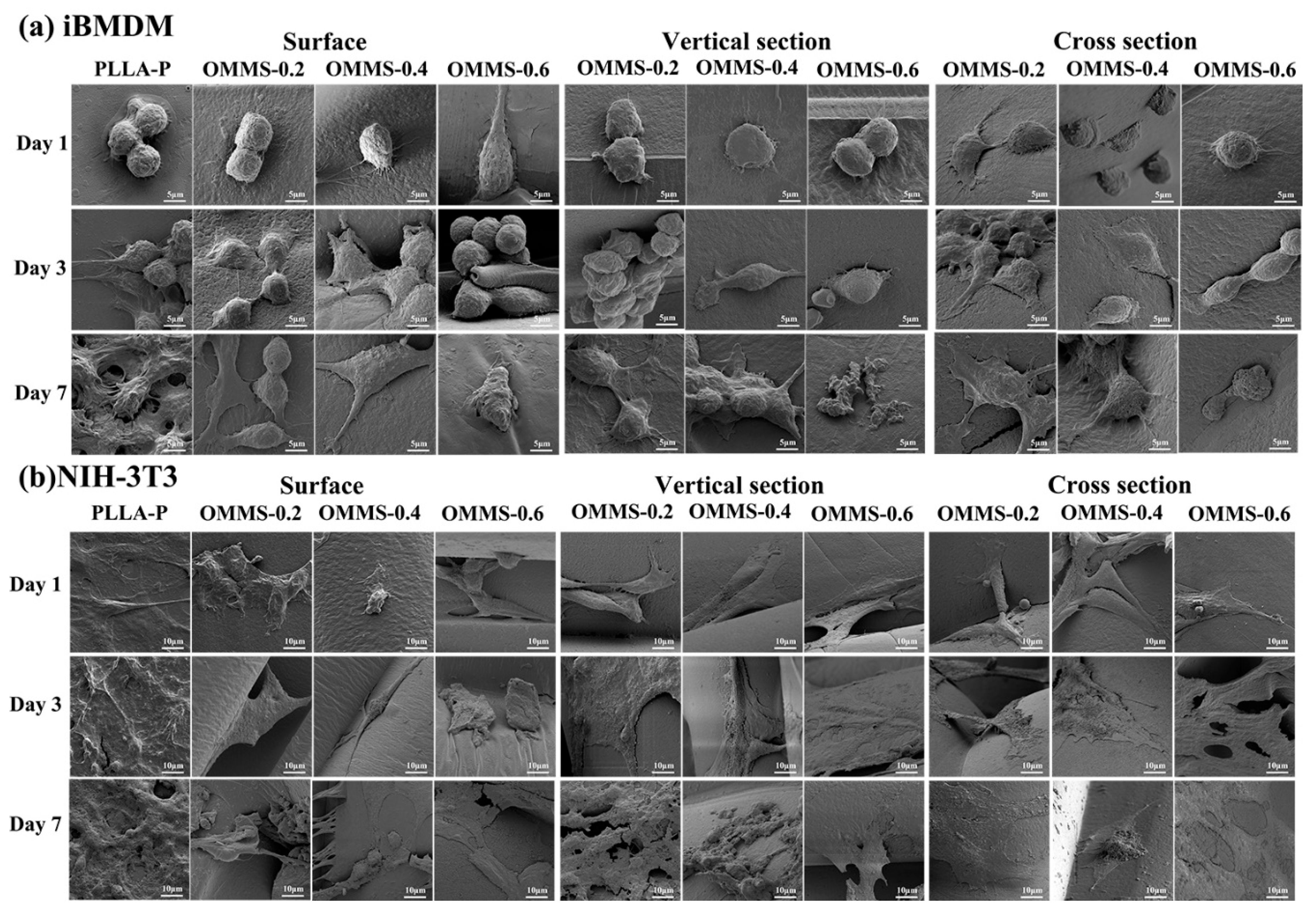
3.3. Biological Properties
3.3.1. Biocompatibility
3.3.2. Cell Morphology
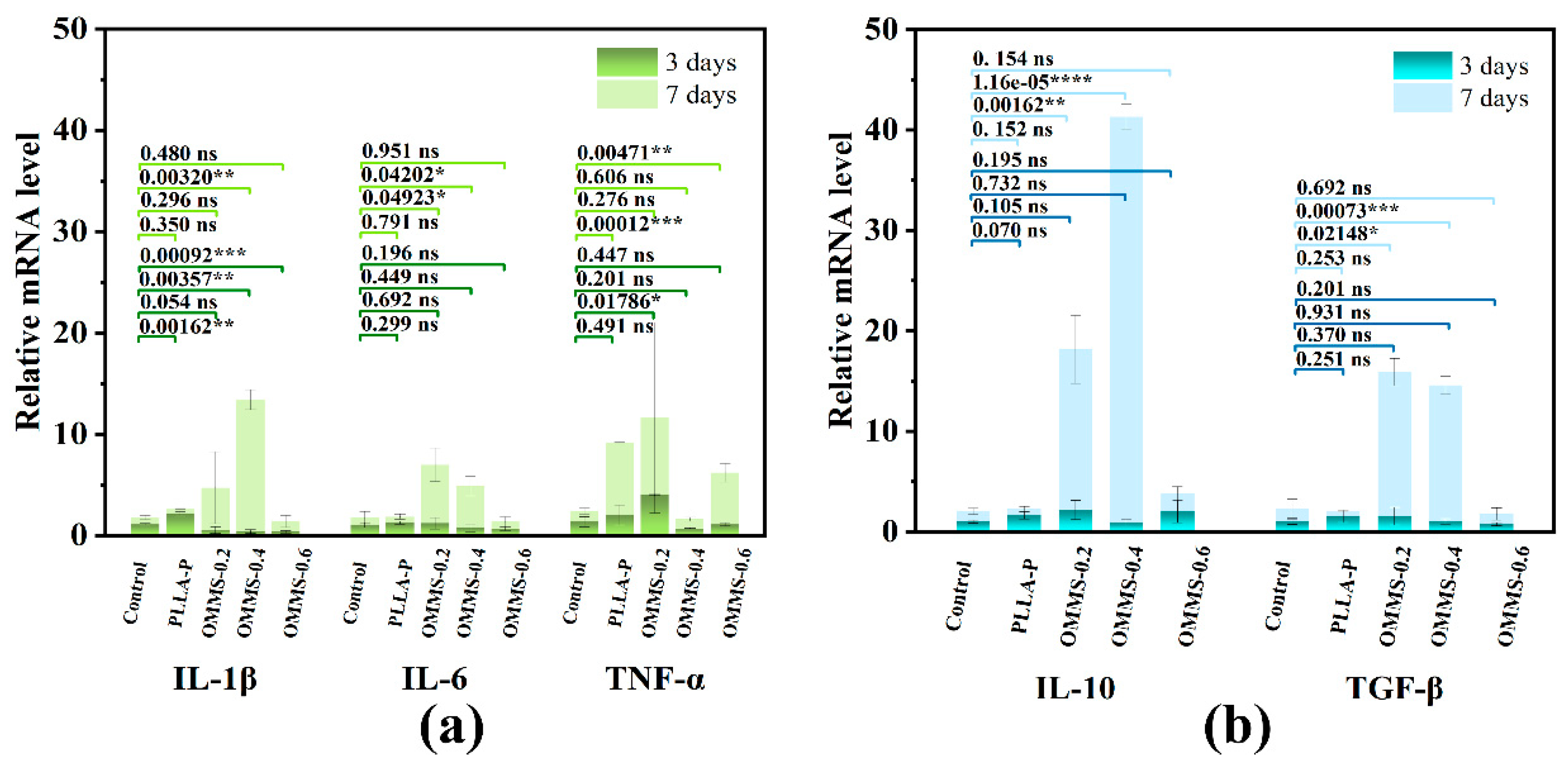
3.3.3. Gene Expression Analysis
3.4. Technology Optimization and Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Shi, X.; Miao, Z.; Zhang, H.; Zhao, X.; Wang, K.; Jian, Q.; Guang, Z. 3D-Printed Polyurethane Tissue-Engineering Scaffold with Hierarchical Microcellular Foam Structure and Antibacterial Properties. Adv. Eng. Mater. 2022, 24, 2101134. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Cao, Y.; Su, J.; Xiao, Y.; Ren, J.; Algadi, H.; Yeszhanov, E.; Sartayeva, A.; Huang, J.; Zhan, G.; Tynybekov, B.; et al. Functional biomass/biological macromolecular phase change composites and their applications in different scenarios: A review. Int. J. Biol. Macromol. 2025, 306, 141377. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical characteristics of poly-L-lactic acid (PLLA). Aesthetic Surg. J. 2018, 38 (Suppl. S1), S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Park, H.K. Fabrication of macroporous chitosan/poly (l-lactide) hybrid scaffolds by sodium acetate particulate-leaching method. J. Porous Mater. 2012, 19, 383–387. [Google Scholar] [CrossRef]
- Ezzati, P.; Ghasemi, I.; Karrabi, M.; Azizi, H.; Fortelny, I. Preparation of porous PLLA/PCL blend by a combination of PEO phase and NaCl particulate leaching in PLLA/PCL/PEO/NaCl blend. Iran. Polym. J. 2014, 23, 757–766. [Google Scholar] [CrossRef]
- Dong, Y.S.; Guo, C.; Lin, P.H.; Yin, L.H.; Pu, Y.P. Preparation of porous poly (L-lactic acid) (PLLA) scaffold by porogen leaching and freeze drying. Key Eng. Mater. 2005, 288, 381–384. [Google Scholar] [CrossRef]
- Dong, S.; Wang, L.; Li, Q.; Chen, X.; Liu, S.; Zhou, Y. Poly (L-lactide)-grafted bioglass/poly (lactide-co-glycolide) scaffolds with supercritical CO2 foaming reprocessing for bone tissue engineering. Chem. Res. Chin. Univ. 2017, 33, 499–506. [Google Scholar] [CrossRef]
- Athanasoulia, I.G.; Louli, V.; Schinas, P.; Rinotas, V.; Douni, E.; Tarantili, P.; Magoulas, K. The effect of foaming process with supercritical CO2 on the morphology and properties of 3D porous polylactic acid scaffolds. Polym. Eng. Sci. 2022, 62, 2459–2475. [Google Scholar] [CrossRef]
- Xu, L.Q.; Huang, H.X. Foaming of poly (lactic acid) using supercritical carbon dioxide as foaming agent: Influence of crystallinity and spherulite size on cell structure and expansion ratio. Ind. Eng. Chem. Res. 2014, 53, 2277–2286. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Chen, D.; Wang, M.; Wu, L. Facile fabrication of highly interconnected poly (lactic acid) -based scaffolds with good hydrophilicity using supercritical carbon dioxide. J. Appl. Polym. Sci. 2023, 140, e54047. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Jatteau, S.; Śliwka, M.; Ziąbka, M.; Menaszek, E. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing-for nasal cartilages and subchondral bone reconstruction. Mater. Des. 2018, 155, 297–306. [Google Scholar] [CrossRef]
- Yen, H.J.; Tseng, C.S.; Hsu, S.; Tsai, C.S. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed. Microdevices 2009, 11, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Bhagia, S.; Bornani, K.; Agrawal, R.; Satlewal, A.; Ďurkovič, J.; Lagaňa, R.; Bhagia, M.; Yoo, C.; Zhao, X.; Kunc, V. Critical review of FDM 3D printing of PLA biocomposites filled with biomass resources, characterization, biodegradability, upcycling and opportunities for biorefineries. Appl. Mater. Today 2021, 24, 101078. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Xiang, C.; Li, L. Electrospun porous PLLA and poly (LLA-co-CL) fibers by phase separation. New J. Chem. 2018, 42, 5102–5108. [Google Scholar] [CrossRef]
- Qi, Z.; Yu, H.; Chen, Y.; Zhu, M. Highly porous fibers prepared by electrospinning a ternary system of nonsolvent/solvent/poly (l-lactic acid). Mater. Lett. 2009, 63, 415–418. [Google Scholar] [CrossRef]
- Yu, Q.Z.; Qin, Y.M. Fabrication and formation mechanism of poly (L-lactic acid) ultrafine multi-porous hollow fiber by electrospinning. Express Polym. Lett. 2013, 7, 55–62. [Google Scholar] [CrossRef]
- Muniyandi, P.; Palaninathan, V.; Veeranarayanan, S.; Ukai, T.; Maekawa, T.; Hanajiri, T.; Mohamed, M.S. ECM mimetic electrospun porous poly (l-lactic acid) (PLLA) scaffolds as potential substrates for cardiac tissue engineering. Polymers 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Frochot, C.; Rahouadj, R.; Wang, X. An innovative method to obtain porous PLLA scaffolds with highly spherical and interconnected pores. J. Biomed. Mater. Res. Part B: Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 86, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Chang, W.V.; Nakamura, T.; Samant, D.M.; Thomas, P.B.; Trousdale, M.D.; Mircheff, A.K.; Schechter, J.E.; Yiu, S.C. Microporous poly (L-lactic acid) membranes fabricated by polyethylene glycol solvent-cast/particulate leaching technique. Tissue Eng. Part C Methods 2009, 15, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Viana, J.C.; Reis, R.L.; Mano, J.F. Development of porous lamellar poly (l-lactic acid) scaffolds by conventional injection molding process. Acta Biomater. 2008, 4, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Kuo, P.Y.; Hsieh, H.J.; Hsien, T.Y.; Hou, L.T.; Lai, J.Y.; Wang, D.M. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Tanaka, K.; Morino, D.; Sakurai, K. Morphology and Release Kinetics of Protein-Loaded Porous Poly (L-Lactic Acid) Spheres Prepared by Freeze-Drying Technique. Int. Sch. Res. Not. 2011, 2011, 490567. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Taki, K.; Nagamine, S.; Ohshima, M. Preparation of poly (L-lactic acid) honeycomb monolith structure by unidirectional freezing and freeze-drying. Chem. Eng. Sci. 2008, 63, 3858–3863. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Bagheri, A.; Petit-Rojo, O. 3D printing of porous scaffolds with controlled porosity and pore size values. Materials 2018, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Winarso, R.; Anggoro, P.W.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Application of fused deposition modeling (FDM) on bone scaffold manufacturing process: A review. Heliyon 2022, 8, e11701. [Google Scholar] [CrossRef] [PubMed]
- Prananingrum, W.; Naito, Y.; Galli, S.; Bae, J.; Sekine, K.; Hamada, K.; Tomotake, Y.; Wennerberg, A.; Jimbo, R.; Ichikawa, T. Bone ingrowth of various porous titanium scaffolds produced by a moldless and space holder technique: An in vivo study in rabbits. Biomed. Mater. 2016, 11, 015012. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Chao, L.; Xie, D.; Yang, Y.; Shi, J.; Zhang, Y.; Xue, B.; Shen, L.; Tian, Z.; Li, L.; et al. Trabecular-like Ti–6Al–4V scaffold for bone repair: A diversified mechanical stimulation environment for bone regeneration. Compos. Part B Eng. 2022, 241, 110057. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, X.; Yu, W.; Zhang, H.; Min, Y. Polyimide-based porous carbon/Ni nano-particle composites prepared by phase separation method with broadband absorption characteristics. Diam. Relat. Mater. 2025, 152, 111968. [Google Scholar] [CrossRef]
- Takahashi, K.; Sawai, D.; Yokoyama, T.; Kanamoto, T.; Hyon, S.H. Crystal transformation from the α-to the β-form upon tensile drawing of poly (l-lactic acid). Polymer 2004, 45, 4969–4976. [Google Scholar] [CrossRef]
- Sawai, D.; Takahashi, K.; Imamura, T.; Nakamura, K.; Kanamoto, T.; Hyon, S.H. Preparation of oriented β-form poly (l-lactic acid) by solid-state extrusion. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 95–104. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Huang, Q.; Luo, C.; Chen, W.; Gao, X.; Wang, K.; Li, Z.; Liu, L. Preparation and properties of polydimethylsiloxane-regulated oriented microporous poly (L-lactic acid) biomimetic bone repair materials. Int. J. Biol. Macromol. 2024, 280, 136189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal modifications and thermal behavior of poly (L-lactic acid) revealed by infrared spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Cocca, M.; Malinconico, M. Crystal polymorphism of poly (l-lactic acid) and its influence on thermal properties. Thermochim. Acta 2011, 522, 110–117. [Google Scholar] [CrossRef]
- Sarasua, J.R.; Prud’Homme, R.E.; Wisniewski, M.; Borgne, A.L.; Spassky, N. Crystallization and melting behavior of polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly (L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly (l-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, X.; Zhu, D.; Zheng, Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog. Mater. Sci. 2023, 134, 101072. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation mechanisms and acceleration strategies of poly (lactic acid) scaffold for bone regeneration. Mater. Des. 2021, 210, 110066. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, F. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Y.; Li, H.; Wang, E.; Mai, H.; Zhang, W.; Wen, J.; You, H.; Long, Y.; Guo, W.; et al. 3D-Printed Thermally Activated Shape Memory PLA/TBC Composite Scaffold with Body-Compatible Temperature for Minimally Invasive Bone Repair. ACS Appl. Polym. Mater. 2025, 7, 4572–4583. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Natarajan, E.; Mohan, S.; Krishnamurithy, C.; Murali, M.R.; Parasuraman, S.; Singh, S.; Kamarul, T. The functionalization of the electrospun PLLA fibrous scaffolds reduces the hydrogen peroxide induced cytokines secretion in vitro. Mater. Today Commun. 2021, 26, 101812. [Google Scholar] [CrossRef]
- Soares, D.G.; Zhang, Z.; Mohamed, F.; Eyster, T.W.; Costa, C.A.S.; Ma, P.X. Simvastatin and nanofibrous poly (l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater. 2018, 68, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, J.; You, X.; Huo, S.; Li, X.; Zhang, Y.; Zhang, W. Fabrication of calcium sulfate/PLLA composite for bone repair. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 73, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.; Zhao, Z.; Liu, L.; Li, Z.; Li, J. Biomimetic 2D-oriented microporous PLLA scaffolds: Fabrication and evaluation for bone repair. Int. J. Biol. Macromol. 2025, 311, 143918. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, J.; Deng, Y.; Li, Z.; Wei, J. Osteogenic and antibacterial PLLA membrane for bone tissue engineering. Int. J. Biol. Macromol. 2023, 247, 125671. [Google Scholar] [CrossRef] [PubMed]
| Materials | Melt Index (g/10 min) | Tg (°C) | Tm (°C) | Density (g/cm3) |
|---|---|---|---|---|
| PLLA | 14 | 50~66 | 145~160 | 1.24 |
| Temperature (°C) | Speed (mm/s) | Adhesion | |
|---|---|---|---|
| Nozzle | Platform | ||
| 190 | 50 | 60 | Brim |
| Abbreviation | Samples | Remark |
|---|---|---|
| EF | Extruded filament | -\- |
| NF | SC-CO2 solid-phase nucleation filament | -\- |
| NFS | Non-foamed PLLA scaffold | -\- |
| OMMS | Oriented multi-stage microporous scaffold | L1–6 represents the number of bone plate layers. |
| OMMS-0.2 | Oriented multi-stage microporous scaffold with a bone plate gap of 0.2 mm | L1–6 represents the number of bone plate layers. |
| OMMS-0.4 | Oriented multi-stage microporous scaffold with a bone plate gap of 0.4 mm | L1–6 represents the number of bone plate layers. |
| OMMS-0.6 | Oriented multi-stage microporous scaffold with a bone plate gap of 0.6 mm | L1–6 represents the number of bone plate layers. |
| PLLA-P | PLLA plate | -\- |
| WCA | Contact angles of water | 0 refers to PLLA plate, 1 refers to scaffold monolayer bone plate |
| EGCA | Contact angles of EG | 0 refers to PLLA plate, 1 refers to scaffold monolayer bone plate |
| Samples | Contact Angle (°) | γsd | γsp | γs | |
|---|---|---|---|---|---|
| Water | EG | (mN/m) | (mN/m) | (mN/m) | |
| PLLA-P | 91.17 | 57.23 | 35.79 | 1.17 | 36.96 |
| OMMS | 78.87 | 48.33 | 28.14 | 6.82 | 34.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Xue, J.; Huang, Q.; Deng, Y.; Zhao, Z.; Li, J.; Gao, X.; Li, Z. 3D-Printed Poly (l-lactic acid) Scaffolds for Bone Repair with Oriented Hierarchical Microcellular Foam Structure and Biocompatibility. Biomolecules 2025, 15, 1075. https://doi.org/10.3390/biom15081075
Luo C, Xue J, Huang Q, Deng Y, Zhao Z, Li J, Gao X, Li Z. 3D-Printed Poly (l-lactic acid) Scaffolds for Bone Repair with Oriented Hierarchical Microcellular Foam Structure and Biocompatibility. Biomolecules. 2025; 15(8):1075. https://doi.org/10.3390/biom15081075
Chicago/Turabian StyleLuo, Cenyi, Juan Xue, Qingyi Huang, Yuxiang Deng, Zhixin Zhao, Jiafeng Li, Xiaoyan Gao, and Zhengqiu Li. 2025. "3D-Printed Poly (l-lactic acid) Scaffolds for Bone Repair with Oriented Hierarchical Microcellular Foam Structure and Biocompatibility" Biomolecules 15, no. 8: 1075. https://doi.org/10.3390/biom15081075
APA StyleLuo, C., Xue, J., Huang, Q., Deng, Y., Zhao, Z., Li, J., Gao, X., & Li, Z. (2025). 3D-Printed Poly (l-lactic acid) Scaffolds for Bone Repair with Oriented Hierarchical Microcellular Foam Structure and Biocompatibility. Biomolecules, 15(8), 1075. https://doi.org/10.3390/biom15081075





