Abstract
Tumor drug resistance, a major cause of treatment failure, involves complex multi-gene networks, remodeling of signaling pathways, and interactions with the tumor microenvironment. Yin Yang 1 (YY1) is a critical oncogene overexpressed in many tumors and mediates multiple tumor-related processes, such as cell proliferation, metabolic reprogramming, immune evasion, and drug resistance. Notably, YY1 drives resistance through multiple mechanisms, such as upregulation of drug efflux, maintenance of cancer stemness, enhancement of DNA repair capacity, modulation of the tumor microenvironment, and epithelial–mesenchymal transition, thereby positioning it as a pivotal regulator of drug resistance. This review examines the pivotal role of YY1 in resistance, elucidating its molecular mechanisms and clinical relevance. We demonstrate that YY1 inhibition could effectively reverse drug resistance and restore therapeutic sensitivity across various treatment modalities. Importantly, we highlight the promising potential of YY1-targeted strategies, particularly combined with anti-tumor agents, to overcome resistance barriers. Furthermore, we discuss critical translational considerations for advancing these combinatorial approaches into clinical practice.
1. Introduction
Yin Yang 1 (YY1) is a zinc-finger protein that belongs to the GLI-Krüppel family. In humans, YY1 encodes a protein with 414 amino acids and four C2H2 zinc fingers domain, possessing activation and repression domains. The regulatory effect of YY1 on its target genes can be either positive or negative, which is determined by the specific binding context [1,2,3]. YY1 is ubiquitously expressed and capable of regulating approximately 7% of the total mammalian genome [4]. YY1 regulates transcription by binding to DNA sequences that most frequently contain the consensus core motifs 5′-CCAT-3′ and 5′-ACAT-3′. In addition to its transcriptional role, YY1 mediates post-translational modifications [5,6]. YY1 is a multifaceted protein with crucial functions in various biological processes, such as cell proliferation, chromatin remodeling, DNA synthesis, and embryogenesis [7,8,9,10,11]. Due to its critical role in biological procedures, dysregulation of YY1 is tightly bound to diseases, including cardiovascular diseases, nervous system diseases, metabolic disorders, and tumor diseases. Numerous studies have shown increased YY1 expression in various tumor cells, including breast, gastrointestinal, hepatocellular, and pancreatic cancers [12,13,14]. Extensive studies have demonstrated that YY1, as an oncogene, regulates many pathways in tumor cell growth, metabolic reprogramming, invasion, and chemotherapy resistance [15,16]. Mechanistically, YY1 activates oncogenic pathways, such as c-Myc and human epidermal growth factor receptor 2 (HER2), while suppressing those of tumor suppressors, including p53 as well as phosphatase and tensin homolog (PTEN), thereby promoting the cancer process. Furthermore, YY1 can rewire tumor cell metabolism by upregulating glycolytic enzymes and glutamine transporters and enhance tumor cells’ metastatic potential by reshaping the tumor microenvironment. In addition, YY1 facilitates tumor progression by regulating tumor immune response. YY1 reshapes the tumor immune microenvironment, including DNA methylation patterns, histone post-translational modifications, non-coding RNA networks, and core oncogenic signaling pathways, thereby promoting immune escape [17,18,19,20]. These findings position YY1 as a central node in cancer signaling networks, where it coordinately regulates uncontrolled tumor cell proliferation, metastasis, immune escape, and survival pathways, demonstrating the multifaceted programs driving tumor progression.
Despite being the therapeutic backbone in oncology, conventional and targeted anticancer agents universally face the formidable challenge of acquired resistance [21]. Tumor cells’ remarkable capacity for evolutionary adaptation systematically undermines treatment efficacy, invariably leading to either explosive cancer recurrence or accelerated malignant progression, representing the principal challenge to achieving durable remissions and improved survival [21,22]. Drug resistance is a primary cause of treatment non-response in oncology. Resistance arises through multiple molecular mechanisms, which collectively enable tumor cells to evade therapeutic pressures and sustain malignant progression [23,24]. Primary resistance describes tumors’ innate insensitivity to therapies due to their genetic and molecular features, reducing treatment efficacy from the outset [24]. Acquired resistance arises through tumors’ evolution under therapy, mediated by mechanisms like somatic mutations, signaling pathway dysregulation, increased drug efflux, and defective cell death in tumor cells [23,25].
Notably, numerous studies have shown that YY1 drives drug resistance development in tumors [26]. Mechanistically, YY1 mediates resistance through various mechanisms, including dysregulated signaling pathways, such as DNA repair modulation, drug efflux activation, cancer stemness maintenance, and apoptosis regulation. Moreover, preclinical models have shown that genetic or pharmacological inhibition of YY1 sensitizes resistant tumor cells to conventional treatments, highlighting its potential as a biomarker for predicting therapy and a treatment target for overcoming drug resistance [27,28,29].
In the present review, we highlight the function of YY1 in driving drug resistance in tumor cells, encompassing its regulation of survival pathways. We also discuss YY1 as a potential biomarker of drug resistance and its therapeutic potential for overcoming therapy resistance, with a focus on recent advances in pharmacological inhibition and potential combination strategies with conventional anticancer therapies.
2. The Function of YY1 in Tumor Drug Resistance Mechanisms
Despite remarkable advances in cancer therapeutics, resistance still is a critical challenge in cancer therapy, acting as the primary cause of treatment failure in most poor prognosis and survival rates. Drug resistance is associated with molecular changes promoting adaptive mechanisms. Elucidating the mechanisms underlying cancer drug resistance is therefore of paramount importance for improving therapeutic outcomes [25,30]. YY1 drives drug resistance through various pathways, including DNA repair signaling, anti-apoptosis processes, and stemness maintenance progress. A number of studies have shown that YY1 plays crucial roles in cancer drug resistance.
2.1. YY1 Enhances Drug Resistance Through Upregulation of Drug Efflux
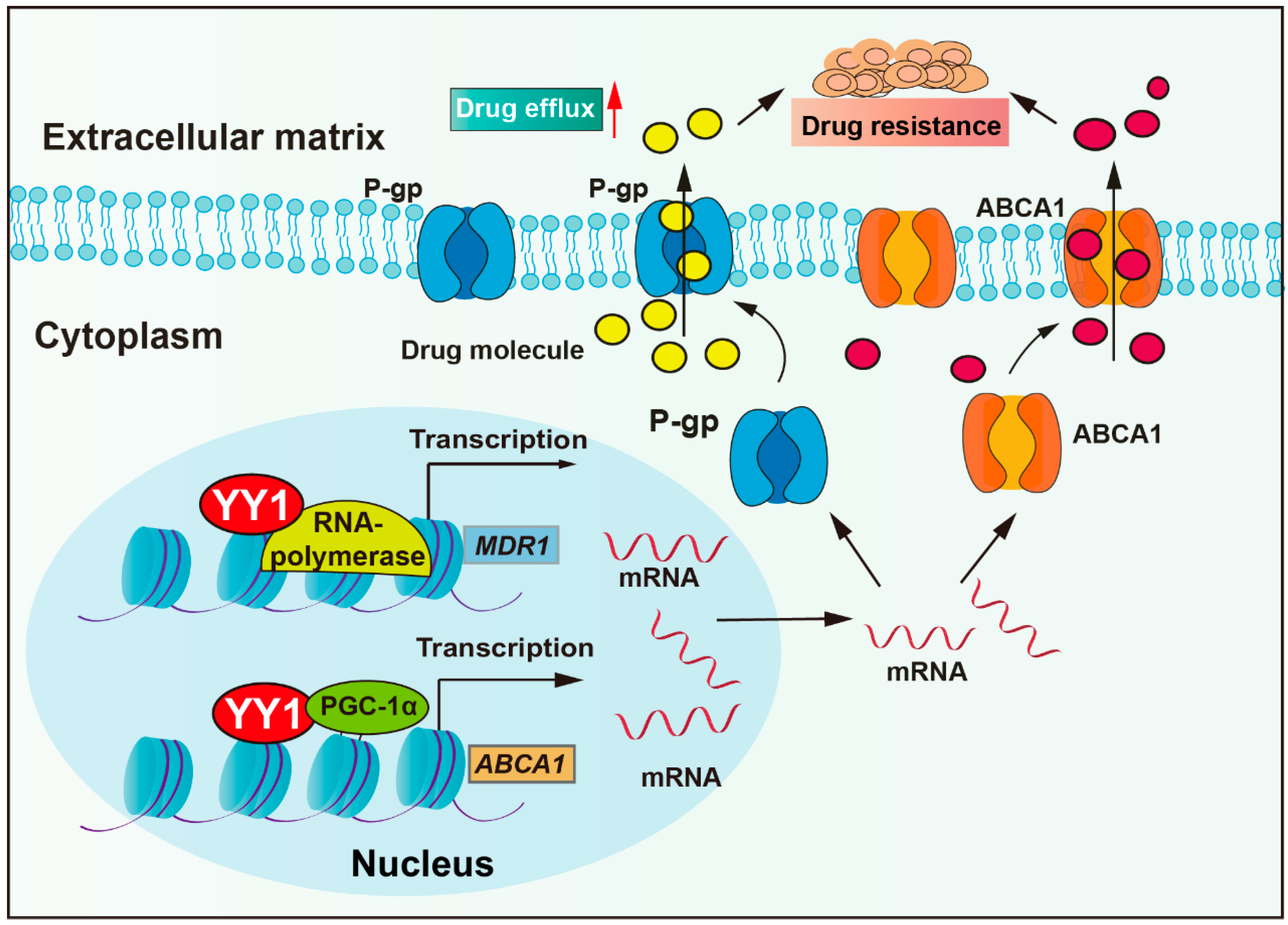
Anti-tumor drugs require sufficient cellular concentrations to achieve therapeutic efficacy. However, tumor cells could actively reduce intracellular drug concentration by overexpressing pump proteins, thereby enhancing drug efflux. Drug transporter mediated efflux, particularly by ATP-binding cassette (ABC) proteins, is the most clinically significant drug efflux mechanism. ABC transporters utilize ATP-derived energy to drive the active translocation of various substrates. Numerous studies have unequivocally identified ABC transporters as pivotal players in mediating cancer multidrug resistance (MDR) development, such as P-glycoprotein (P-gp, also named MDR1), multidrug resistance-associated protein 1 (MRP1), and breast cancer resistance protein (BCRP) [31]. P-gp, encoded by the multidrug resistance-1 (MDR1) gene, is a central member of the ABCB subfamily within the ABC transporter family. P-gp actively extrudes therapeutic agents and is overexpressed in most cancers (colorectal cancer, breast cancer, glioma, lung cancer, and others), and it has been confirmed to correlate with clinical drug resistance [32]. Studies have revealed that YY1 binds to the promoter of the MDR1 gene, enhancing transcriptional activity and upregulating P-gp protein expression, thereby reducing cellular sensitivity to multiple chemotherapeutic agents, including vincristine, cytarabine, and adriamycin (Figure 1) [33,34,35]. Moreover, clinical studies show that YY1 correlates positively with P-gp expression in acute lymphoblastic leukemia (ALL), with high YY1 expression patients exhibiting significantly lower 5-year overall survival rates [34,36]. In addition, a study demonstrated that the bendamustine hydrochloride (BH) resistant mantle cell lymphoma (MCL)-derived subline KPUM-YY1R showed significant upregulation of MDR1 mRNA and cross-resistance to vincristine and cytarabine. Moreover, the restoration of BH sensitivity in KPUM-YY1R cells through P-gp inhibition confirmed that YY1 drives chemoresistance in tumor cells by upregulating MDR1 gene expression and amplifying drug efflux capacity [33]. YY1 cooperates with transcriptional coactivator PGC-1α and then enhances transcriptional activity of the ATP-binding cassette transporter 1 (ABCA1) gene, resulting in elevated ABCA1 expression levels, leading to insensitivity of colorectal cancer cells to isoliquiritigenin and tumor metastasis [37].
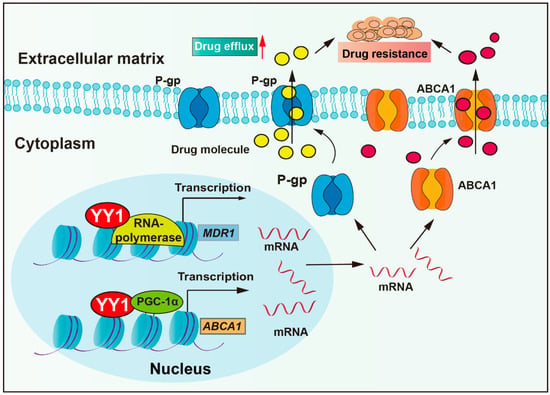
Figure 1.
YY1 enhances drug resistance through upregulation of drug efflux. YY1 promotes drug excretion through cells by increasing the expression of P-gp and ABCA, leading to drug resistance. Arrows are used to show activation; arrows terminated with a bar represent inhibition.
In addition to drug transporters, studies have reported that exosomes also actively contribute to enhanced drug efflux. Emerging evidence indicates that the phospholipid composition of exosome membranes and internal lumen facilitates the binding and sequestration of hydrophobic drugs, such as docetaxel, resulting in reduced intracellular drug accumulation [38,39]. YY1 promotes the secretion of exosomes to promote prostate cancer progression, and YY1 upregulates polycom, also named pentraxin domain containing 1 (SVEP1), in cancer-associated fibroblasts (CAFs), which secrete miR-146a-5p and increase urothelial bladder cancer resistance [7,40,41,42]. These studies demonstrate that YY1 enhances tumor drug efflux and promotes multidrug resistance by regulating ABC transporters and promoting exosome-mediated drug efflux.
2.2. YY1 as a Critical Regulator of Cell Death Pathways in Cancer Drug Resistance
Cell death acts as a crucial factor in regulating impaired cells during developmental stages and is controlled by multiple signaling pathways. Nonetheless, its dysregulation may lead to tumor progression and therapeutic resistance [43]. Tumor cells can evade apoptosis by inhibiting the pro-apoptotic signal or upregulating the anti-apoptotic signal. This enables them to resist chemotherapy-induced cell death and contributes to therapeutic failure. The key mechanisms involved in apoptotic inhibition include overexpression of anti-apoptotic proteins, BCL-2, BCL-XL, and MCL-1, or downregulation of pro-apoptotic proteins (such as p53 and BAX) [44,45,46].
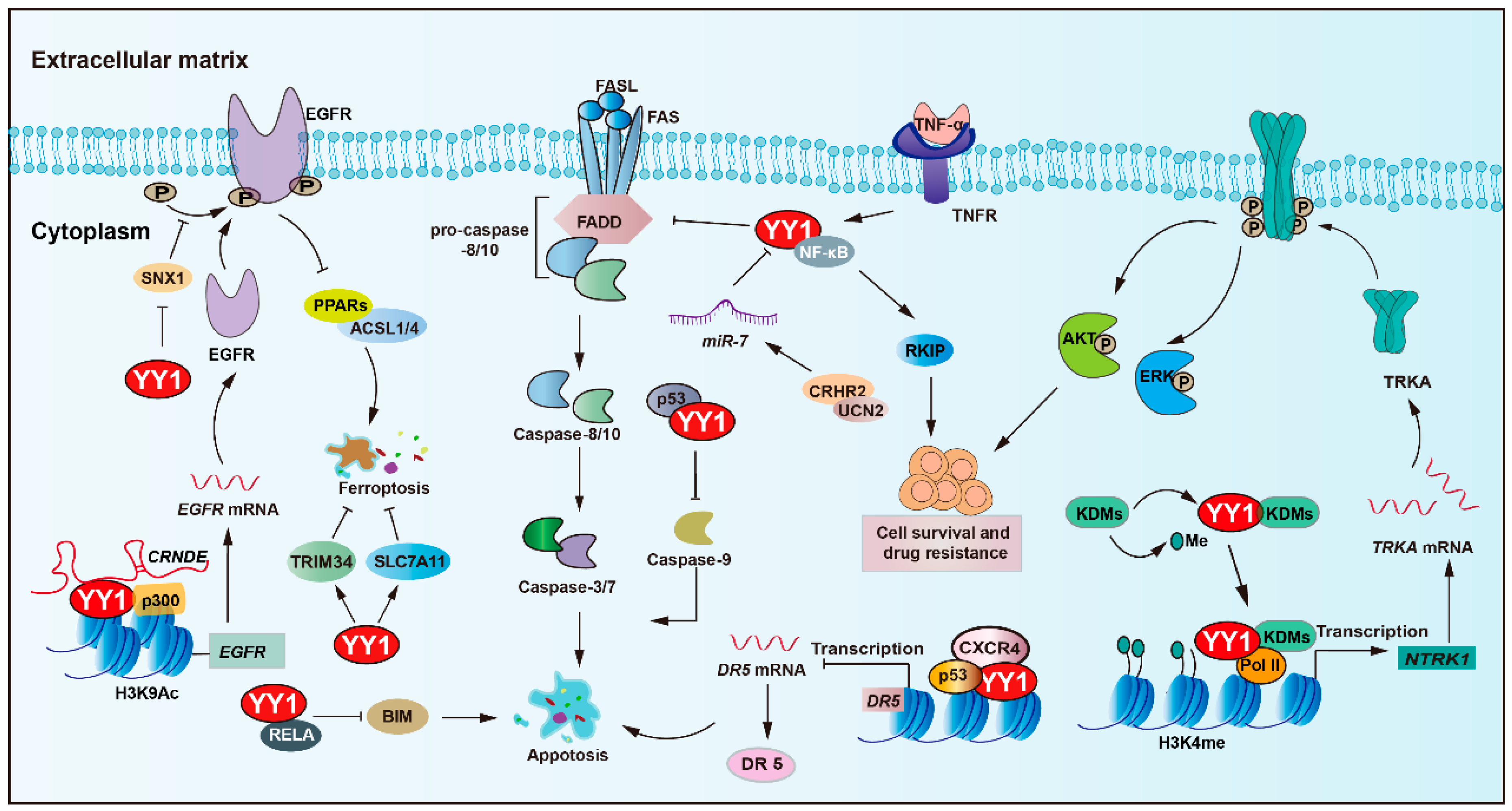
YY1 plays a central role in regulating apoptosis and mediating resistance to anticancer therapies (Figure 2 and Table 1). An important pathway involving YY1 is the NF-κB/YY1/ RKIP regulatory axis, which has been shown to significantly contribute to tumor cell resistance against both chemotherapy and immunotherapy-induced cytotoxicity [47,48,49]. For instance, YY1 upregulates the expression of MCL-1, while concurrently downregulating pro-apoptotic caspases-3, -7, and -9 [43,50]. Such a dual regulatory mechanism promotes cancer cell survival and contributes to therapy resistance, potentially in a p53-dependent manner. Simultaneously, obatoclax-induced death receptor 5 (DR5) downregulation via YY1 and NF-κB activation concurrently promotes MCL-1, further influencing sensitivity to TRAIL-based therapies [51,52,53]. Additionally, YY1 cooperates with p53 to transcriptionally suppress DR5 expression upon chemokine receptor (CXCR4) activation, thereby promoting chemoresistance and tumor progression in solid tumors [54].
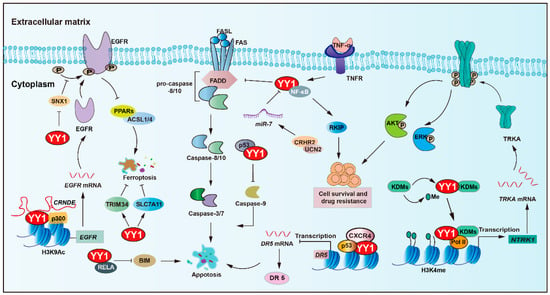
Figure 2.
YY1 as a key regulator of cell death pathways in tumor drug resistance. YY1 promotes tumor cell survival and drug resistance through multifaceted anti-apoptotic mechanisms, including engagement in the NF-κB/SNAIl/YY1/RAF regulatory loop, upregulation of MCL-1 alongside inhibition of caspases-3, -7, and -9, and suppression of the death receptor pathway via p53/YY1-mediated downregulation of DR5 and direct inhibition of FAS expression. Additionally, YY1 inhibits BIM by forming a complex with the NF-κB subunit RELA, confers resistance to ferroptosis by inhibiting the PPARs-ACSL1/4 signaling axis and upregulating TRIM34 and amplifies EGFR signaling through YY1-mediated inhibition of SNX1 under nutrient stress. Collectively, these mechanisms enable YY1 to drive therapeutic resistance by robustly promoting anti-apoptotic responses in tumor cells. Arrows are used to show activation; arrows terminated with a bar represent inhibition.

Table 1.
YY1 regulates cell death pathways in cancer drug resistance.
Furthermore, YY1 suppresses the expression of BIM, a pro-apoptotic gene, by forming a transcriptional repressor complex with the NF-κB subunit (RELA), further promoting resistance to programmed cell death [44]. Recent findings have also identified corticotropin-releasing hormone receptor 2 and its ligand urocortin-2 as modulators of colorectal cancer (CRC) cell sensitivity to FAS/FASL-induced apoptosis. Restoration of CRHR2/UCN2 signaling leads to miR-7 overexpression and decreased YY1 expression, thereby resensitizing tumor cells to FAS/FASL-mediated cell apoptosis [55].
The tropomyosin receptor kinase A (TRKA), encoded by the neurotrophic tropomyosin receptor kinase 1 (NTRK1) gene, has been implicated in imatinib resistance. Mechanistically, YY1 recruits histone lysine demethylase 6A (KDM6A) to the NTRK1 promoter, leading to upregulation of TRKA expression and activation of downstream survival pathways that confer resistance to imatinib [56]. Another histone demethylase, lysine demethylase 5C (KDM5C), which specifically demethylates H3K4, exhibits context-dependent oncogenic or tumor suppressor roles. Notably, YY1 and KDM5C form a synthetic lethal pair. KDM5C can recruit YY1 to the promoters of genes associated with cell cycle control and apoptotic processes, which promote drug resistance [57].
Additionally, non-coding RNA networks play a pivotal role in YY1-mediated anti-apoptotic regulation in tumors. The lncRNA CRNDE enhanced p300/YY1 binding to the EGFR promoter, leading to EGFR upregulation and resulting in enhanced tumor cell proliferation and sorafenib resistance [58]. In ovarian cancer, YY1 induces the transcription of PART1, enhances resistance to cisplatin, and inhibits apoptosis by modulating the miR-512-3p/chromatin accessibility complex subunit 1 (CHRAC1) axis [59]. Moreover, YY1 transcriptionally activates miR-135b, which directly suppresses the core circadian regulator BMAL1, to target and repress the circadian core gene BMAL1, thereby forming a YY1/miR-135b/BMAL1 axis, inhibiting tumor cell apoptosis, and promoting gemcitabine resistance in pancreatic cancer [60].
Ferroptosis is a type of regulated cell death that relies on iron and is marked by the accumulation of lipid peroxides. A study showed that YY1 activates TRIM34 transcription, contributing to TRIM34 overexpression, thereby suppressing ferroptosis and promoting cell proliferation and migration in HCC [61]. Moreover, YY1 acts as a pivotal regulator in oxymatrine-induced liver cancer ferroptosis by modulating the silent information regulator 1 (SIRT1)/YY1/GPX4 signaling axis [62]. In addition, YY1 could bind to the solute carrier family 7-member 11 (SLC7A11) promoter and upregulate its transcription, which sustains cystine uptake and glutathione synthesis, ultimately conferring insensitivity to ferroptosis-inducing agents erastin and RSL-3 [63].
Another key regulator linked to YY1-mediated survival is sorting nexin 1 (SNX1), a protein involved in EGFR trafficking and degradation. SNX1 deficiency enhances EGFR phosphorylation, thereby increasing resistance to gefitinib. Under a nutrient-deficient environment, YY1 becomes activated, represses SNX1 expression, and consequently activates EGFR signaling. This cascade also inhibits the PPARs-ACSL1/4-mediated ferroptosis pathway, facilitating colorectal cancer cell survival and resistance to 5-FU [64].
Collectively, these findings underscore the central role of YY1 as a pivotal transcriptional regulator that bridges apoptotic resistance, cancer progression, and treatment failure. Targeting YY1 and its regulatory network represents a promising strategy for enhancing the efficacy of anticancer therapies.
2.3. YY1 Drives Drug Resistance by Regulating Cancer Stemness
Cancer stem cells (CSCs) drive tumor initiation, progression, and recurrence [65]. CSCs are the triggers of tumorigenesis and the initiators of tumor treatment resistance [66,67]. Among the transcriptional regulators involved, YY1 has emerged as a modulator of CSC-associated phenotypes and drug resistance across multiple cancer types (Figure 3 and Table 2).
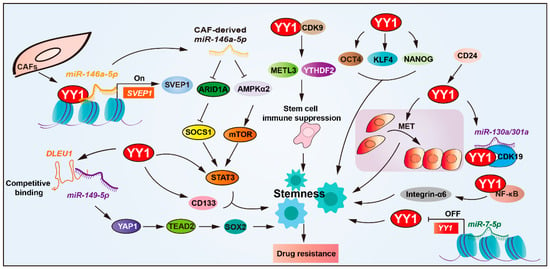
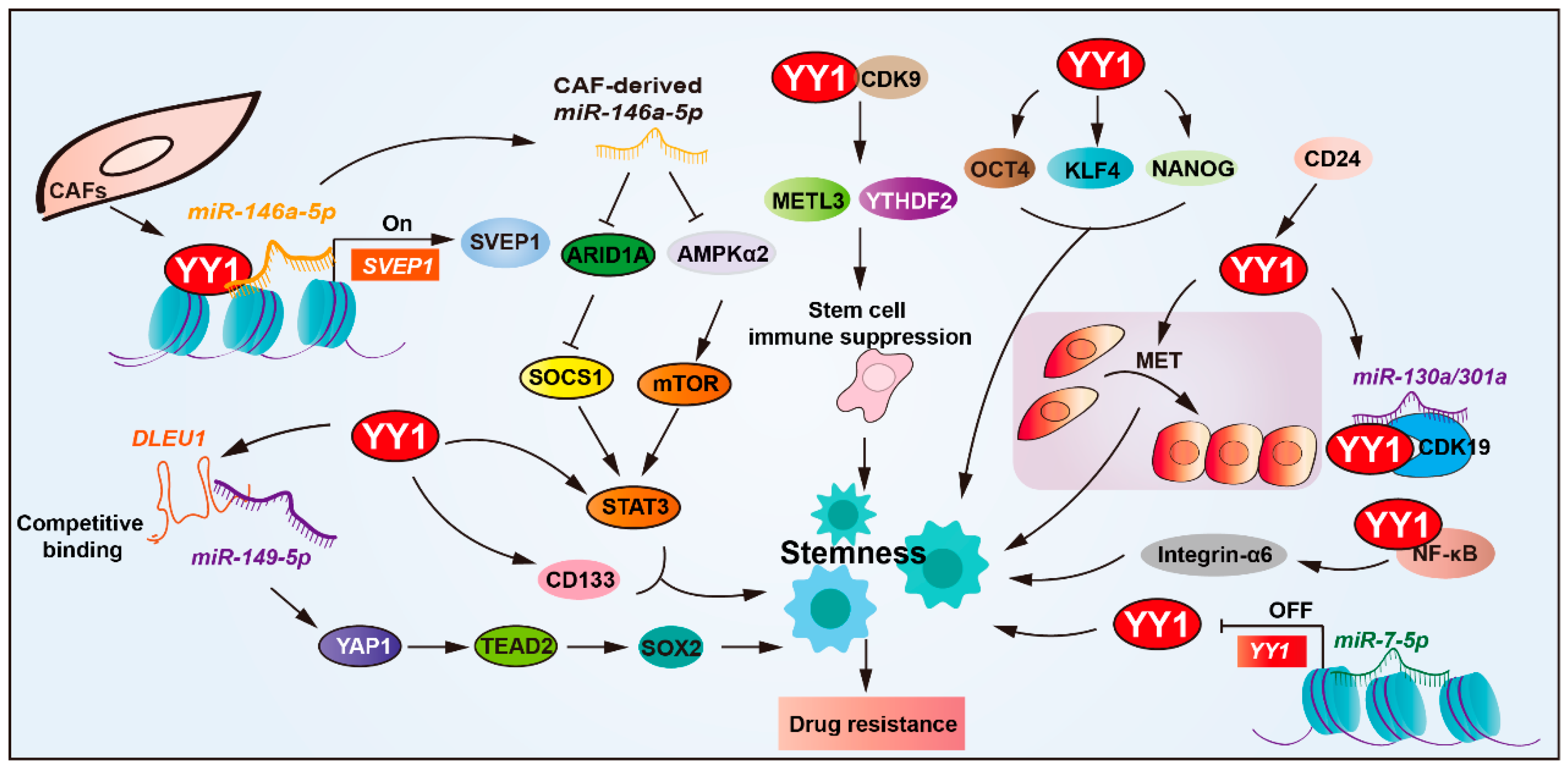
Figure 3.
YY1 drives drug resistance by regulating cancer stemness. YY1 promotes tumor drug resistance by enhancing cancer stem cell (CSC) properties, directly upregulating CSC markers (CD133, STAT3, and integrin-α6). Through NF-κB/PI3K/AKT signaling, it forms regulatory loops with SOX2, OCT4, and BMI1 to reinforce CSC traits. The microRNA network modulates this process; miR-146a-5p enhances YY1 recruitment to upregulate SVEP1 in CAFs for CSC amplification, while miR-7-5p suppresses YY1 to reduce stemness. CD24 activates the YY1-miR-130a/301a-CDK19 axis to maintain CSC quiescence, and the YY1–CDK9 complex diminishes tumor immunogenicity, collectively driving CSC-mediated drug resistance. Arrows are used to show activation; arrows terminated with a bar represent inhibition.
YY1 overexpression exerts a positive regulatory effect on CSC stemness and drug resistance [7,68,69,70]. Studies have revealed that YY1 upregulates the expression of CSC markers’ (such as CD133, STAT3, and integrin-α6) expression, thereby promoting the resistance of glioblastoma cells to cisplatin and temozolomide. Deletion in lymphocytic leukemia 1 (DLEU1) enhances cancer stemness by upgrading upregulating stem cell markers (SOX2, OCT4, NANOG, and KLF4) in cholangiocarcinoma. YY1-mediated DLEU1 activation promotes resistance to cisplatin and gemcitabine, establishing a YY1/DLEU1/stemness axis in chemoresistance [71].
YY1 is also involved in modulating TME to support CSC-mediated resistance. In bladder cancer, the extracellular matrix protein SVEP1, a matrix-type CAF marker, has been implicated in chemoresistance. Mechanistically, miR-146a-5p enhances YY1 recruitment to upregulate SVEP1 expression in CAFs and enhances CSCs’ properties, and depletion of either miR-146a-5p or SVEP1 significantly sensitized urothelial bladder carcinoma (UBC) cells to gemcitabine–cisplatin combination therapy both in vitro and in vivo [7,72].
Post-transcriptional regulation of YY1 also influences stemness and therapy resistance. YY1 downregulated by miR-7-5p via direct 3′UTR targeting reduces stemness and enhances temozolomide sensitivity in glioblastoma, positioning the miR-7-5p/YY1 axis as a key regulator of chemoresistance. In ovarian cancer, YY1 expression is upregulated by CD24, a surface marker associated with CSC phenotypes and malignant progression. CD24-induced YY1 promotes mesenchymal-to-epithelial transition (MET), a process linked to enhanced stemness and platinum-based chemoresistance [73,74]. Furthermore, CD24 facilitates aberrant activation of the YY1-miR-130a/301a-CDK19 regulatory axis, promoting ovarian CSCs’ quiescence and platinum-based drug resistance [75].
YY1 also interacts with core stemness transcription factors, such as SOX2, OCT4, and BMI1. Together, they form part of a regulatory circuit involving NF-κB/PI3K/AKT signaling that reinforces CSCs’ maintenance and cisplatin resistance [76]. YY1 also interacts with CDK9 in glioblastoma stem cells and maintains stemness. Moreover, YY1–CDK9 complexes downregulate the immune response of tumor cells via NF-κB signaling, thereby enhancing resistance to immunotherapy in glioblastoma [77].
In summary, YY1 is a central transcriptional regulator of CSC biology and therapy resistance. It operates through direct transcriptional activation of stemness genes, modulation of tumor–stromal interactions, engagement with non-coding RNAs, and integration into broader oncogenic signaling pathways. Elucidating YY1’s role in maintaining CSCs and mediating resistance mechanisms provides promising avenues for enhanced cancer therapy.


Table 2.
The stemness-maintaining function of YY1 contributes to drug resistance.
Table 2.
The stemness-maintaining function of YY1 contributes to drug resistance.
| Molecular Pathway | Target Gene or Pathway | Regulation | Resistance Type | Refs |
|---|---|---|---|---|
| YY1/CD133 | STAT3 and integrin-α6 | Increases the CSC markers | Resistance to cisplatin and temozolomide | [78] |
| YY1/DLEU1 | SOX2, OCT4, NANOG, KLF4 | Actives DLEU1 | Resistance to cisplatin and gemcitabine | [71] |
| MiR-146a-5p/YY1/SVEP1 | SVEP1 | MiR-146a-5p enhances the recruitment of YY1 and upregulates SVEP1 | Resistance to the combination therapy of gemcitabine and cisplatin | [72] |
| MiR-7-5p/YY1 | YY1’ 3′UTR | Downregulates YY1 by binding to 3′UTR of YY1 | Resistance to temozolomide | [79] |
| CD24/YY1 | YY1 | CD24 upregulates YY1 and promotes mesenchymal– epithelial transition | Resistance to platinum drugs | [73] |
| YY1-miR-130a/301a-CDK19 | YY1-miR-130a/301a-CDK19 | CD24 promotes abnormal activation of the YY1-miR-130a/301a-CDK19 regulatory axis | Resistance to platinum drugs | [75] |
| YY1 with SOX2, OCT4, BMI1 | NF-κB/PI3K/AKT | Strengthens the maintenance of CSCs | Resistant to cisplatin | [76] |
| YY1–CDK9 complexes | NF-κB | The complex downregulates the immune response of tumor cells | Resistant to immunotherapy | [77] |
2.4. YY1-Mediated DNA Repair Pathways in Drug Resistance
Multiple anti-tumor agents, including 5-FU, platinum drugs, and temozolomide, achieve anti-tumor efficacy by inducing DNA damage to trigger tumor cell death [80]. However, tumor cells respond to DNA damage through coordinated signaling pathways, such as homologous recombination, non-homologous end joining, mismatch repair, and base excision repair pathways, to ensure survival. Consequently, enhanced DNA repair capacity constitutes a critical determinant of chemotherapeutic resistance in tumor cells [81,82].
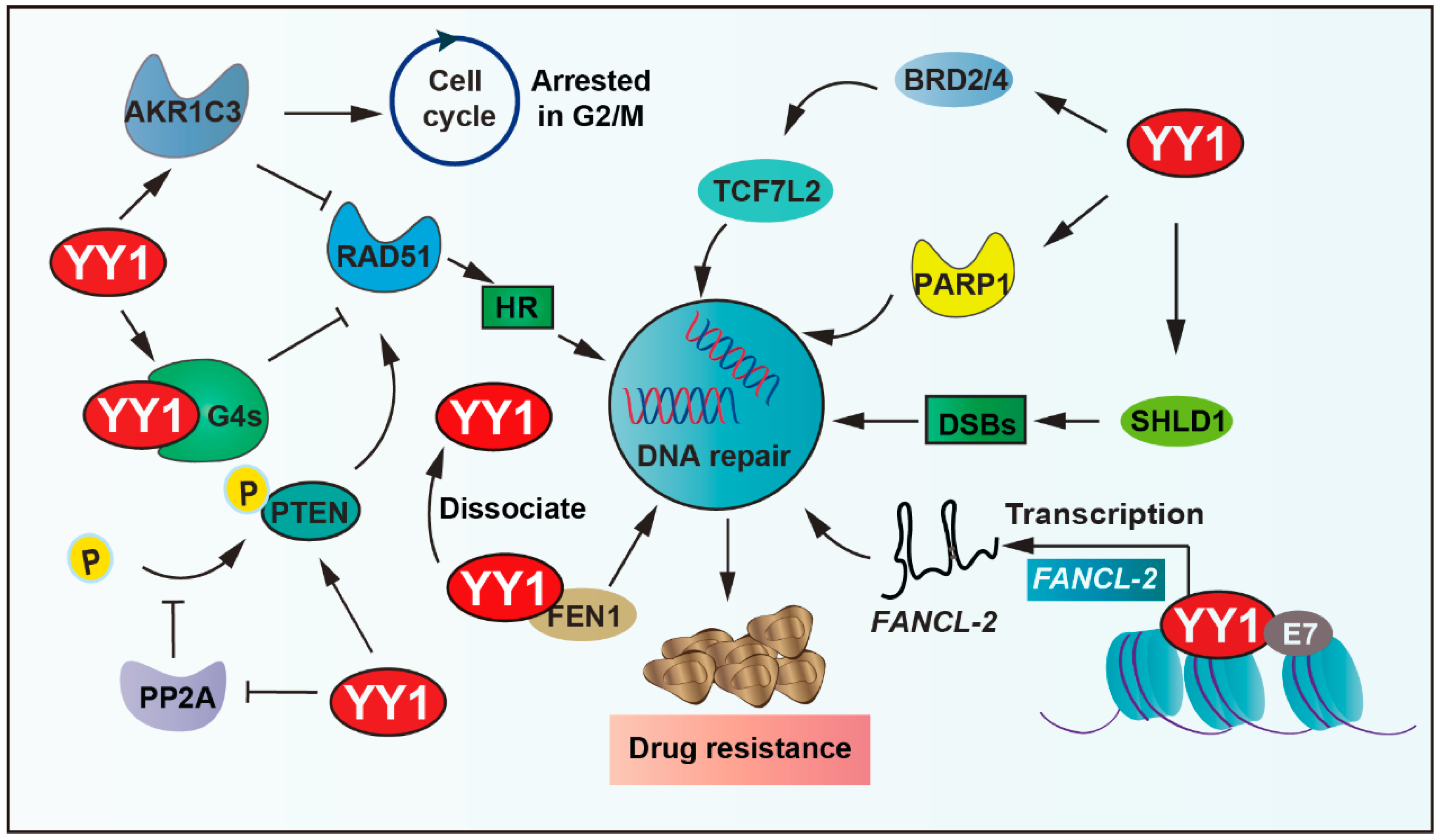
RAD51 recombinase, an ATPase, assembles into a nucleoprotein filament on single-stranded DNA, which is a crucial player in the HR process, including searching for homologous sequences and strand exchange. YY1 binds to guanine quadruplexes’ (G4s) structures, which restricts RAD51 access and thereby shields excessive HR activity, ultimately protecting genomic stability [83]. YY1 also upregulates various DNA-repair-related proteins, including PTEN and RAD51. YY1 reduces the phosphorylation of PTEN by inhibiting the activity of protein phosphatase 2A (PP2A), thus ensuring the fidelity of the DNA repair process [84]. Moreover, the shieldin complex shields double-strand DNA breaks (DSBs) from nucleolytic resection. YY1 inhibits SHLD1 transcription, thereby leading cross-resistance to poly ADP-ribose polymerase (PARP) inhibitor and cisplatin in breast cancer susceptibility gene 1 (BRCA1)-deficient ovarian cancer cells [85]. In addition, bromodomain and extraterritorial domain family factors modulate the expression of several genes related to carcinogenesis. It is overexpressed in many types of cancer and associated with the resistance of tumor cells to chemotherapeutic drugs [86,87]. YY1 recruits BETs (including BRD2, BRD4) to bind to cis-regulatory elements of transcription factor 7 like 2 (TCF7L2), thereby regulating the expression of TCF7L2, cooperatively activating tumor cell DNA repair capacity and driving therapeutic resistance [88,89].
Furthermore, YY1 can regulate DNA repair by affecting the expression of related enzymes. PARP1 plays an important role in DNA repair processes. YY1 binds to the PARP1 promoter and then drives its expression; more importantly, the YY1–PARP1 axis highlights the potential of DNA repair and promotes resistance [90,91,92]. In addition, the elevated expression of flap endonuclease 1 (FEN1) correlates with drug resistance and patient survival in breast cancer. Studies demonstrate that YY1 functions as a transcriptional repressor of FEN1, and YY1 dissociates from the FEN1 promoter to increase its expression under DNA-damaging agent (mitomycin and taxol) treatment, consequently promoting resistance to drugs in breast cancer cells [93,94,95]. Similarly, aldo-keto reductase family 1 member C3 (AKR1C3) regulates cell cycle G2 arrest and the expression of RAD51, which is involved in the mechanism of DNA damage repair ability. YY1 upregulates AKR1C3 and the hedgehog axis to enhance lenalidomide (LEN) resistance in multiple myeloma cells [96,97].
Additionally, YY1 upregulates the expression of lnc-FANCI-2 through interaction with a conserved region 3 (CR3) of E7 (human papillomaviruses oncoprotein) core motif and transactivates the promoter of lnc-FANCI-2, which is expressed from a genomic locus adjacent to the FANCI gene encoding an important DNA repair factor in in vivo and in vitro tumor cells [98]. YY1 further binds directly to the miR-140 promoter, which leads to decreased miR-140 expression, and then directly associates with its 3′UTR to inhibit flap endonuclease 1 (FEN1), which results in impaired DNA repair and reduced doxorubicin resistance [94]. In summary, YY1 drives tumor cell resistance by promoting DNA damage repair (Figure 4).
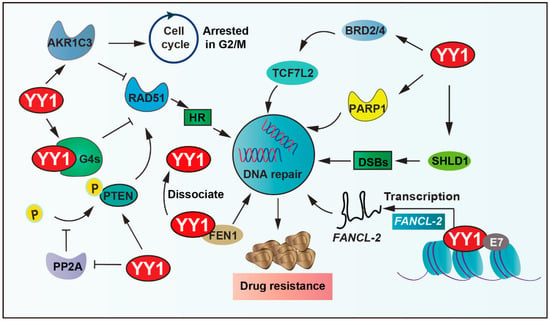
Figure 4.
YY1-mediated DNA repair pathways in drug resistance. YY1 promotes DNA repair via transcriptional activation of SHLD1/PARP1, inhibiting PP2A-mediated dephosphorylation of PTEN, binding guanine quadruplexes (G4s) to restrict RAD51-dependent homologous recombination, and activating AKR1C3 and Hedgehog signaling, and recruits BRD2/4 to cis-regulatory elements of TCF7L2 to modulate its expression. These mechanisms enhance DNA repair and enable evasion of DNA-damage-induced cell death, establishing YY1 as a key mediator of therapeutic resistance. Arrows are used to show activation; arrows terminated with a bar represent inhibition.
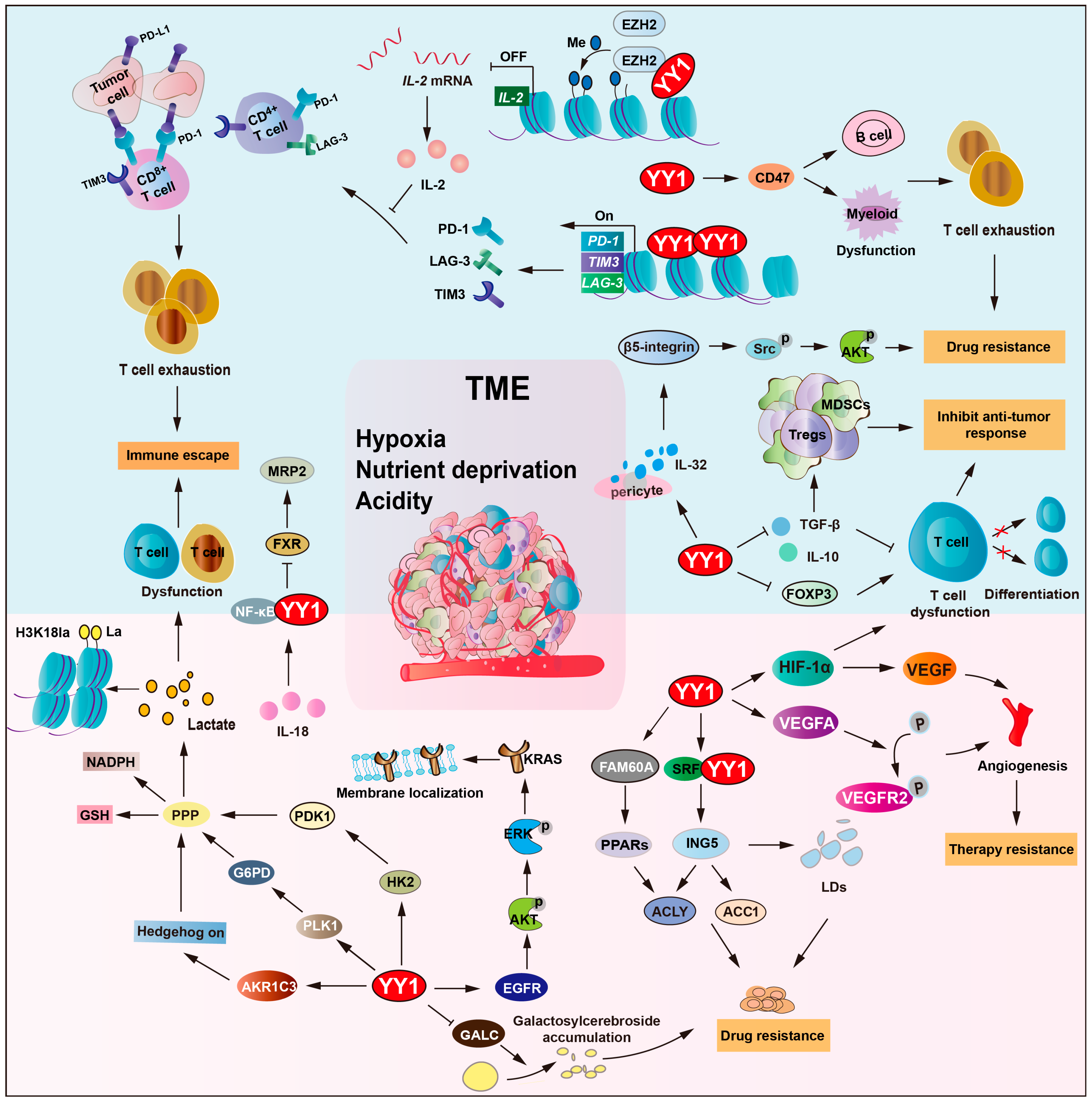
2.5. YY1 Orchestrates TME Remodeling to Drive Drug Resistance
The tumor microenvironment (TME) is a complicated system that includes the effects of the metabolic microenvironment, the immune microenvironment, the extracellular matrix, and angiogenesis [99,100]. The TME plays a crucial role in drug resistance development, which is a promising and strategic target for tumor therapies [101].
Due to the inadequacy of the vascular system, hypoxia and nutrient deprivation are hallmark features of the TME [102]. Under hypoxia, hypoxia-inducible factor (HIF) is induced, which plays a crucial role in the survival of tumor cells, as well as in tumor drug resistance [103]. YY1 directly binds to HIF-1a promoter and recruits RNA polymerase II and the histone acetyltransferase p300 to promote HIF-1a transcription under hypoxia. Moreover, YY1 binds to the oxygen-dependent degradation (ODD) domain of HIF-1a, blocks von Hippel–Lindau (VHL)-mediated ubiquitination, and promotes HIF-1a protein stabilization and nuclear translocation [6,104,105]. Consequently, YY1/HIF-1a upregulates VEGF expression and drives tumor vascularization to augment oxygen and nutrient perfusion. Also, YY1 upregulates the expression of VEGFA, resulting in the activation of the phosphorylation of VEGFR2, and triggers vascular endothelial morphogenesis and transmigration capacity. Subsequently, it promotes hepatocellular carcinoma (HCC) angiogenesis and resistance to bevacizumab. However, these newly formed blood vessels have abnormal structures and functions, leading to drug delivery failure [65,106,107]. Furthermore, YY1/HIF-1a promotes P-gp and medicine-related problems (MRPs) expression and increases drug efflux. Thereby, reduced intertumoral drug concentration contributes to the formation of multidrug resistance.
2.5.1. YY1-Driven Metabolic Remodeling of the TME Promotes Drug Resistance
The hypoxic conditions and nutrient deficiency within the TME prompt tumor cells to undergo metabolic reprogramming, including upregulating glycolysis and altering nutrient transport and metabolic pathways, to meet the demands of proliferation and adapt to the harsh conditions [108,109]. Conversely, tumor cells alter metabolism patterns, remodel the extracellular matrix, and reshape the TME, exacerbating hypoxia and acidity, promoting angiogenesis, and creating a more suitable microenvironment for tumor cell survival, invasion, and metastasis. This interaction establishes a pathological ecosystem driving tumor progression and therapy resistance [17,110,111].
In multiple myeloma, YY1 promotes AKR1C3 expression and activates hedgehog signaling to promote glycolytic activity and lenalidomide resistance [96]. KRAS mutations occur in approximately 30–50% of patients and are a key determinant of non-responsiveness to chemotherapy in colorectal cancer (CRC) [112,113]. YY1 triggers EGFR/AKT/ERK pathway activation, enhancing KRAS membrane localization and reinforcing cetuximab resistance in KRAS-mutant CRC cells [114]. Polo like kinase 1 (PLK1) is another downstream effector of YY1, regulating cell cycle progression and metabolism. YY1-induced PLK1 expression elevates G6PD levels, activating the pentose phosphate pathway and increasing NADPH and glutathione (GSH) production, thereby driving tumor cells’ resistance to paclitaxel and cisplatin [115]. Furthermore, YY1 promotes lactate production through the upregulation of glycolytic enzymes, hexokinase 2 (HK2) and pyruvate dehydrogenase kinase 1 (PDK1). This leads to increased histone H3K18 lactylation (H3K18la), establishing a YY1–lactate–H3K18la positive loop, ultimately leading to cancer cell resistance to cisplatin [116].
Moreover, YY1 regulates lipid metabolism and affects drug resistance in the TME. Inhibitor of growth 5 (ING5) upregulated the expression of acetyl-CoA carboxylase 1 (ACC1) and ATP-citrate lyase (ACLY) and mediated tumor lipogenesis, which is involved in the malignant progression of cancers. Serum response factor (SRF)–YY1 complexes can bind to the promoter of ING5 and upregulate its transcription, thereby promoting lipid droplets’ (LDs) formation and increasing sorafenib resistance of HCC cells [117]. Galactosylcerebroside is known to be overexpressed on the surface of tumor cells and plays an important role in the inhibition of cellular adhesion and apoptosis. Lysosomal enzyme galactocerebrosidase (GALC) is responsible for the hydrolysis of galactosyl cerebroside. YY1 suppressed GALC gene transcription, consequently increasing galactosyl cerebroside accumulation and enhancing tumor cells’ resistance to doxorubicin [118]. Family with sequence similarity 60 member A (FAM60A) increases essential metabolic enzymes acyl-CoA synthetase long chain family (ACSL1/4) and glutathione peroxidase 4 (GPX4) as a key regulator.
Additionally, the low amino acid signal activates the synergistic interaction between YY1 and yes-associated protein (YAP), activating the expression of the PH domain containing protein 6 (FGD6) gene and thereby forming the YY1–FGD6 axis. It can replenish the amino acid reserves within the cells, bypassing the dependence on free glutamine and maintaining metabolic balance. Studies have found that mTOR inhibitors can inhibit the growth of tumor regions rich in nutrients, but, in the central regions that rely on the YY1–FGD6 axis, the inhibitory function of mTOR inhibitors on cell growth is weakened [119].
2.5.2. YY1-Mediated Immunomodulation in TME Remodeling Contributes to Drug Resistance
Tumor immunity is another critical factor in reshaping the TME. Immune cells within the TME, such as T cells (Tregs), B cells, NK cells, and macrophages, modulate tumor behavior by secreting cytokines, altering angiogenesis, and reshaping metabolic profiles [120,121]. Compelling evidence indicates that YY1 modulates tumor immunity by regulating immune checkpoint molecules, influencing T cell differentiation and function, and modulating myeloid-derived suppressor cell (MDSC) polarization [16,48]. YY1 directly transcriptionally activates PD-L1, LAG-3, and TIM3 transcription, which induce T cell exhaustion across various cancer types while enhancing tumor resistance to cisplatin and other chemotherapeutic agents [122]. Additionally, YY1 upregulates CD47 expression, inhibiting macrophage phagocytosis, facilitating tumor cell immune escape, and contributing to treatment resistance [123]. Moreover, YY1 also regulates immunosuppressive molecules, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), promoting the immunosuppressive function of regulatory T cells and inducing a tolerogenic phenotype in myeloid cells [123]. Regarding drug resistance mechanisms, YY1 not only mediates immune escape through immune checkpoints but also coordinately regulates tumor metabolism. For example, YY1 activates Hexokinase 2 (HK2)/pyruvate dehydrogenase kinase-1 (PDK1) to promote glycolysis, so that tumor cells generate excessive lactic acid, and impairs T cell function, expands regulatory T cells, and fosters an immunosuppressive microenvironment, thereby enhancing tumor cell resistance to both chemotherapy and immunotherapy.
The TME harbors multiple immunosuppressive components that collectively impair immune cells’ (including NK cells and T cells) function. The cytokine TGF-β suppresses T/B cell function and promotes T cell differentiation, while interleukin (IL-2/6/10) impairs antigen presentation and inhibits T/NK cell activity [124]. These immunosuppressive factors suppress anti-tumor immune responses through direct or indirect mechanisms that lead to tumor drug resistance [123,125,126]. YY1 inhibits the role of effector T cells by regulating the expression of TGF-β and IL-10. The functional inhibition of effector T cells leads to the escape of tumor cells from immune killing, which leads to resistance to immunotherapy. Researchers found that YY1 negatively regulates IL-2 (in collaboration with enhancer of zeste homolog 2 (EZH2) histone methyltransferase). As a type I cytokine, IL-2 plays a pivotal role in clonal expansion and the persistence of tumor-reactive T cells. However, YY1 represses IL-2 gene activation, and repeat stimulation leads to CD8+ and CD4+ T cells showing markers of PD-1, LAG-3, and TIM3, which are all signs of T cell exhaustion [127,128].
EGFR-mutated patients notably secrete IL-32, which affects signaling pathways and biological processes linked to tyrosine kinase inhibitors’ (TKIs) sensitivity. YY1 upregulates the secretion of IL-32 in pericytes and subsequently activates the β5-integrin-Src-Akt (protein kinase B) pathway in EGFR-mutated lung cancer cells, thereby influencing sensitivity to TKIs [129]. Normally, the farnesoid X receptor (FXR) functions as a bile acid receptor that activates multidrug resistance-associated protein 2 (MRP2) expression. However, IL-18 (a pro-inflammatory cytokine) suppresses FXR activity while simultaneously activating the NF-κB/YY1 axis, contributing to the downregulation of MRP2 expression, therefore promoting cancer cells’ chemoresistance under the tumor microenvironment [130].
Interestingly, YY1 cooperates with HIF-1α to upregulate VEGF expression and facilitate the accumulation of immunosuppressive cells, including MDSCs and regulatory T cells, particularly in glioblastoma [41]. A study showed that YY1 could suppress anti-tumor immunity by enhancing HIF-1α stability in tumor-associated macrophages [131]. It also associates with small mother against decapentaplegic (SMAD)3/4 to inhibit forkhead box protein 3 (FOXP3) transcription, blocking the differentiation and function of T cells and further promoting immune suppression [132].
Overall, YY1 plays a key part in modulating tumor cell survival and chemoresistance within the TME by orchestrating important biological processes, such as metabolic reprogramming, immune evasion, and other cancer-associated pathways (Figure 5 and Table 3).
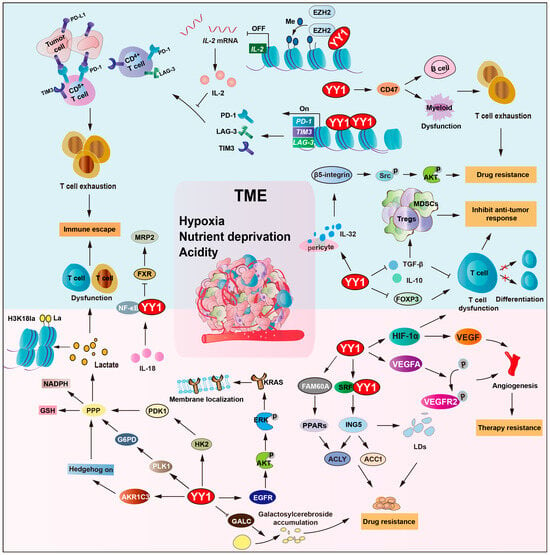
Figure 5.
YY1 orchestrates TME remodeling to drive drug resistance. YY1 regulates glycolysis via HIF-1a and G6PD and creates an acidic hypoxic niche. It also upregulates CD47 to block macrophage phagocytosis and induces T cell exhaustion by repressing IL-2, GALC, TGF-b, IL-10, and FOXP3. Additionally, YY1 mediates TKI resistance through the IL-32/b-5-integrin/AKT pathway and activates the IL-18/NF-κB axis to downregulate MRP2, forming an inflammatory feedback loop. This metabolic–immune crosstalk enables tumor cells to escape immune surveillance and resist therapy-induced cell death, thereby developing drug resistance. Arrows are used to show activation; arrows terminated with a bar represent inhibition.

Table 3.
YY1 reshapes the TME to drive drug resistance.
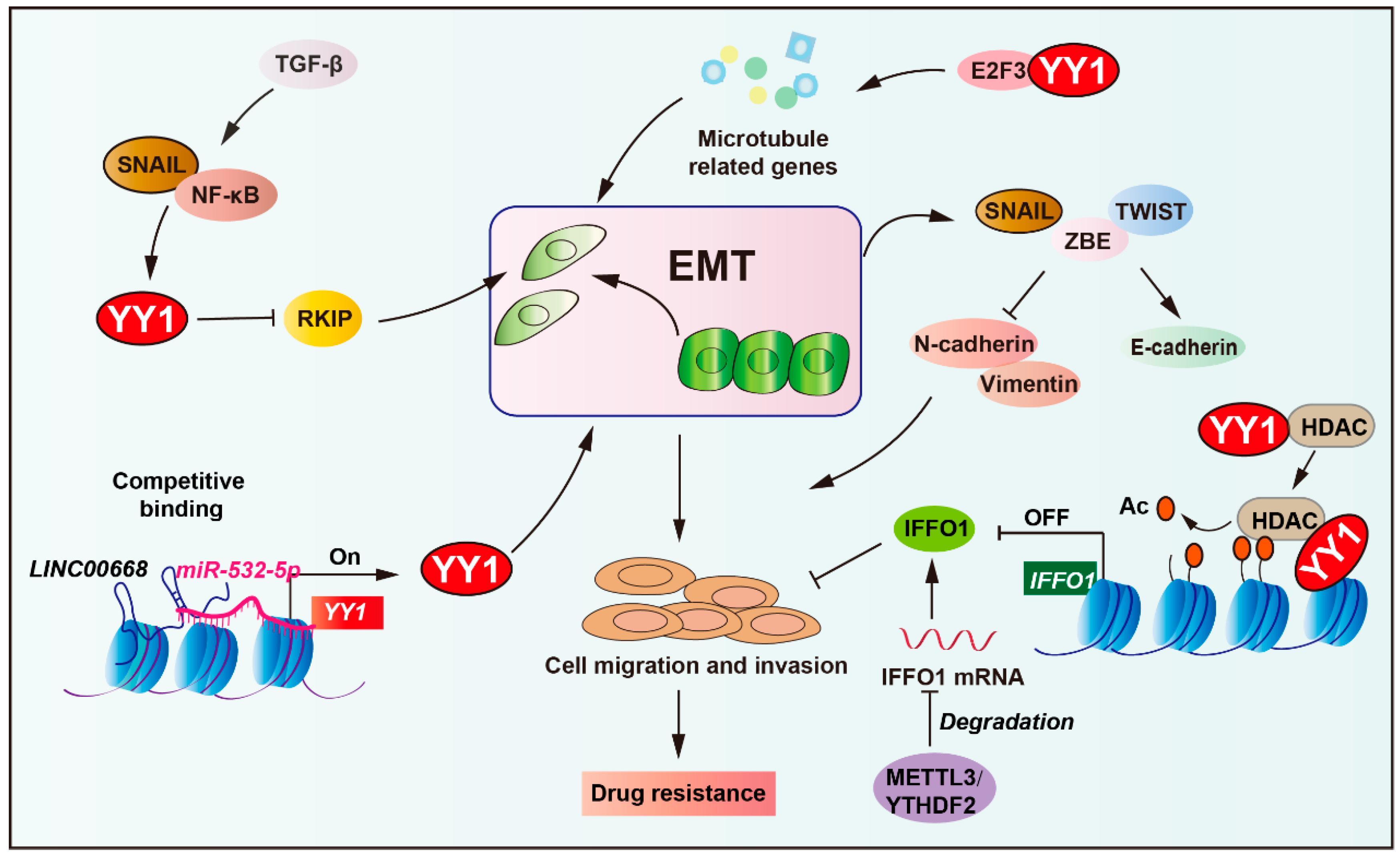
2.6. Role of YY1 in EMT-Mediated Tumor Drug Resistance
Epithelial–mesenchymal transition (EMT) enables epithelial cells to acquire invasive mesenchymal properties while fostering drug resistance through enhanced cellular plasticity and cancer stem cell traits [68,136,137]. More precisely, EMT core regulators (TWIST1, SNAIL, ZEB1) promote tumor cell migration by upregulating N-cadherin/vimentin [138,139,140]. These transcription factors concurrently activate stemness properties and drive therapy resistance (such as tamoxifen, platinum drugs, and paclitaxel) through exosomal signaling and RHOJ-mediated DNA damage repair [141,142,143].
Notably, EMT is regulated by multiple pathways (such as TGF-β, Wnt, RTK, and NF-κB), activating SNAIL/ZEB/TWIST to suppress E-cadherin and induce N-cadherin/vimentin expression, promoting cell migration and invasion. The tumor microenvironment (including hypoxia and inflammation) and epigenetic regulators (miRNAs and lncRNAs) critically modulate EMT dynamics, facilitating cancer dissemination and therapeutic resistance [68,144]. YY1 acts as a downstream effector in the NF-κB/SNAIL pathway, repressing RKIP expression to promote tumor EMT and treatment resistance, while NO donors downregulate YY1 to restore RKIP activity and reverse drug resistance (Figure 6) [145,146]. Additionally, YY1 is required for TGF-β-induced EMT in lung epithelial cells, acting through the NF-κB/SNAIL/RAF pathway dependent on chromatin remodeling [147].
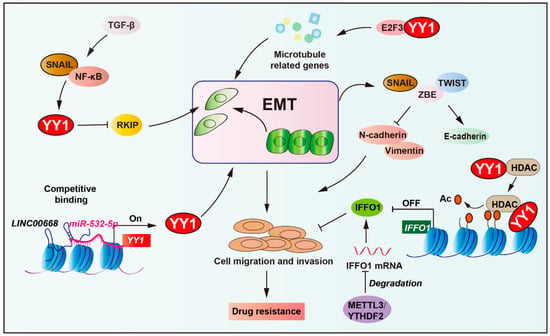
Figure 6.
Role of YY1 in EMT-mediated tumor drug resistance. YY1, acting as a downstream effector of the NF-κB/SNAIL pathway, represses RKIP expression to drive tumor epithelial–mesenchymal transition (EMT). Concurrently, the long non-coding RNA LINC00668 competitively binds to miR-532-5p to upregulate YY1, further enhancing EMT progression. Mechanistically, YY1 promotes tumor drug resistance via epigenetic regulation by recruiting HDAC5 to suppress IFFO1 for metastasis and cisplatin resistance. In addition, YY1 collaborates with E2F3 proteins to activate the expression of microtubule-related genes, thereby mediating drug resistance in tumors. This integrated network highlights YY1 as a key mediator linking EMT to therapeutic resistance. Arrows are used to show activation; arrows terminated with a bar represent inhibition.
Long non-coding RNAs, such as LINC00668, can promote EMT by regulating YY1 expression via competitive binding to miR-532-5p, thereby enhancing cell proliferation, migration, and invasion [148]. Moreover, YY1 cooperates with transcription factor 3 (E2F3) proteins to enhance the expression of microtubule-related genes, promote the ovarian cancer cell mesenchymal transition, and increase the resistance to paclitaxel and docetaxel [149]. In ovarian cancer, intermediate filament family orphan 1 (IFFO1) suppresses tumor metastasis and cisplatin resistance by inhibiting β-catenin nuclear translocation. Specifically, YY1 recruits histone deacetylase 5 (HDAC5) to the IFFO1 promoter, suppressing its expression, resulting in metastasis and cisplatin resistance [150]. Collectively, these findings identify YY1 as a critical nexus between EMT and treatment resistance, reinforcing its therapeutic potential in malignancies driven by EMT.
3. Targeting YY1 to Overcome Therapeutic Resistance in Cancer Treatment
3.1. Clinical Implications of Targeting YY1 in Overcoming Drug Resistance
As a multifunctional transcription factor, YY1 is abnormally highly expressed in most tumors and plays a key role in tumorigenesis and malignant progression. It could extensively regulate the core biological processes of tumors, such as cell growth, apoptosis, invasion, and metastasis, by directly binding to the promoter of target genes or interacting with other transcription factors [29,151]. Clinically, elevated YY1 expression is associated with advanced stage, metastasis, and treatment resistance, positioning it as both a valuable prognostic molecular marker and a therapeutic target [29].
Notably, YY1 is a central mediator of tumor drug resistance, driving multiple escape mechanisms, including ABC transporter upregulation, cancer stem cell maintenance, tumor microenvironment shaping, and pro-survival pathway activation. YY1 establishes a comprehensive defense system against anticancer agents. This central role in drug resistance pathways makes YY1 a promising therapeutic target, as its inhibition holds the potential to simultaneously dismantle multiple resistance mechanisms and resensitize refractory tumors to therapy [115,152,153]. Thus, YY1 represents a theranostic target capable of refining treatment strategies while improving clinical outcomes.
3.2. Combination Therapy Strategies Targeting YY1
Combination therapy has become a fundamental paradigm in modern oncology that overcomes the limitations of single-agent treatments. This multiple targeting strategy overcomes drug resistance, enhances efficacy, and reduces toxicity through synergistic effects. The rational combination of molecularly targeted agents with conventional therapies or immunotherapies provides comprehensive tumor control, overcoming heterogeneity while improving treatment outcomes. YY1’s central role in tumor progression and drug resistance makes it a promising target for combination therapy. Studies demonstrate that YY1 inhibitors promote the efficacy of therapeutic drugs, suggesting their potential in combination therapies, as shown in Table 4. Therefore, combining YY1 inhibitors with existing therapies (such as chemotherapy, immunotherapy, and targeted drugs) improves efficacy and overcomes resistance, offering a more effective strategy for cancer treatment [29,69].
3.2.1. Combination with Chemotherapy Drugs
Chemotherapy still plays an important role in cancer drug treatment, leveraging cytotoxic agents to eliminate tumor cells. However, its efficacy is frequently hindered by insufficient drug accumulation in tumors and the formation of resistance-related mechanisms, leading to failed therapy and cancer recurrence [154,155,156]. Emerging evidence establishes YY1 as a key regulator of chemoresistance, coordinating multiple resistance pathways in various malignancies [43,84,157]. Chemotherapeutic drugs (5-FU, cisplatin, and paclitaxel) are limited by enhanced DNA repair capacity and the anti-apoptotic pathway in tumor cells, with YY1 mediating these processes. For example, in prostate cancer cells that are resistant to cisplatin, YY1 directly binds to the promoter region of the DNA repair gene SHILD, thereby increasing its expression and enhancing nucleotide excision repair efficiency. YY1 inhibitors, such as the small-molecule compound diethylamine NONOate (DETA NONOate), induce the s-nitrosation of YY1, leading to downregulation of BCL-XL expression and activating the apoptotic pathway, simultaneously increasing the sensitization of prostate carcinoma cells to cisplatin [158,159]. Resistance to doxorubicin is driven by P-gp-mediated drug efflux, which is upregulated under intermittent hypoxia via HIF-1α activation. YY1 enhances P-gp expression by stabilizing HIF-1α. A promising therapeutic strategy involves the combination of YY1 inhibitors (lercanidipine and amlodipine, calcium channel blockers with identified YY1 inhibitory activity) with doxorubicin. This combination therapy mechanistically targets multiple resistance mechanisms by disrupting the HIF-1α/P-gp axis while suppressing ERK/MAPK survival pathway and restoring doxorubicin sensitivity. The observed inhibition of tumor spheroid formation and the reversal of chemoresistance support its potential for cancer therapy [160,161,162,163]. Additionally, galiximab enhances non-Hodgkin lymphoma (NHL) cells’ sensitivity to cisplatin and TRAIL-induced apoptosis by inhibiting the YY1/BCL-XL pathway, and combination therapy improves anti-tumor efficacy [164].
Furthermore, nucleic-acid-based YY1 inhibitors (such as siRNAs, miRNAs, and CRISPR/Cas9) demonstrate effects when combined with conventional anti-tumor agents, effectively overcoming chemoresistance through targeted YY1 pathway inhibition. This combinatorial strategy has shown promising results in both in vitro and in vivo models of drug-resistant cancers. In a gastric cancer model, YY1 activates the transcription of NLRC5 and leads to cell invasion, migration, and resistance to 5-FU chemotherapy. Combined treatment with siRNA-YY1 and 5-FU decreased NLRC5 expression and increased tumor cell sensitivity to 5-FU-induced apoptosis [165]. Recently, research found that T7 ligand-functionalized exosomes (T7-exo) could enhance the delivery of cholesterol-modified siYY1. When combined with temozolomide, targeted knockdown of YY1 through T7-siYY1-exo overcomes chemotherapy resistance and suppresses tumor cell growth. T7-exosome binds to glioblastoma cells and improves the delivery of siYY1. Combined with temozolomide, targeting the knockdown of YY1 via T7-siYY1-exo overcomes chemotherapy resistance and inhibits the growth of GBM [42]. In addition, knocking out YY1 decreases the expression of TP73-AS1 and results in sensitivity to chemotherapeutic temozolomide in GBM [166]. These findings indicate that using nucleic acid technologies to inhibit YY1 and in combination with drugs might be potential tactics to reverse chemoradiotherapy resistance in tumors.
Therefore, the increasing development of YY1 inhibitors is a hopeful strategy for overcoming drug resistance and increasing therapeutic efficacy in combination with conventional chemotherapeutic drugs.
3.2.2. Targeted Therapy Combination
Targeted therapies play a pivotal role in current cancer therapy, with high specificity to precisely engage with distinct molecular targets in tumor cells. By blocking oncogenic signaling pathways, inducing apoptosis, or inhibiting tumor angiogenesis, these agents significantly enhance tumor therapeutic effects. However, drug resistance still remains a critical obstacle limiting their clinical efficacy [29]. Typically, YY1 serves as a key regulator in activating bypass signaling pathways, thereby mediating resistance to targeted therapies. For example, DR5 is downregulated in TRAIL-resistant cells. Mechanistically, YY1 represses DR5 transcription by binding to its promoter, leading to resistance to TRAIL-induced apoptosis. DETA NONOate, as an inhibitor of YY1, restores the sensitivity of prostate cancer cells to TRAIL-induced apoptosis by inhibiting YY1 and increasing DR5 expression [52]. Additionally, DETA NONOate and rituximab inhibit YY1, resulting in the upregulation of FAS expression and sensitization to TRAIL-induced and FAS agonist antibody (CH-11) induced apoptosis in B-NHL cells [167].
Recent studies demonstrate that rituximab targeting YY1 reverses TRAIL resistance in B-NHL by suppressing YY1 expression and impairing its DNA-binding activity. This approach effectively sensitizes cells to TRAIL-induced apoptosis through robust activation of the type II mitochondrial pathway [168]. Furthermore, similar therapeutic effects were replicated using YY1-specific siRNA, validating YY1 as a critical molecular target for overcoming TRAIL resistance [51]. Notably, the combination therapy of rituximab and artesunate leads to significant downregulation of YY1 and activation of the FAS/CD95 pathway, accompanied by mitochondrial membrane potential disruption, which ultimately reduces drug resistance in NHL [169]. Recently, research revealed that ursolic acid (UA) exerts remarkable anti-tumor effects by reducing the binding of YY1 and serum response factor (SRF) to the promoter of ING5, thereby downregulating ING5 expression. This mechanism disrupts the ING5-mediated PI3K/Akt axis, reversing the chemoresistance of HCC cells to sorafenib [117].
Overall, understanding the regulation between YY1 and target therapy resistance is key to the development of innovative therapies. The combination of YY1 inhibitors to combat YY1-driven resistance may contribute to more effective cancer therapy.
3.2.3. Immunotherapy Combination
Tumor immunotherapy, which activates the host immune system to attack tumors, has demonstrated durable response advantages. For instance, PD-1/PD-L1 inhibitors can induce long-term remission in melanoma and NSCLC. However, clinical benefits remain limited, with most patients showing primary resistance due to immunosuppressive microenvironments or impaired antigen presentation [123,170]. Additionally, acquired resistance frequently occurs through mechanisms like the upregulation of immune checkpoint factors or the accumulation of regulatory T cells [171]. YY1 promotes tumor immune escape by directly regulating immune checkpoint molecules, influencing T cell differentiation and function, and modulating the immune microenvironment [132,172]. NADPH oxidase 4 (NOX4) drives NSCLC resistance to EGFR-TKIs (gefitinib and osimertinib) and tumor immune escape through a YY1-dependent pathway. Specifically, NOX4 upregulation enhances YY1-mediated transcriptional activation of IL-8, consequently promoting PD-L1 expression and forming an immunosuppressive tumor microenvironment. Furthermore, the combination of GKT137831, which is a specific NOX4 inhibitor and reduces YY1 expression, and gefitinib synergistically promoted apoptosis and reduced resistance to both TKIs and immunotherapy [173]. The deubiquitinase ubiquitin-specific protease 7 (USP7) stabilizes YY1 expression by preventing its proteasomal degradation, thereby promoting HCC cell proliferation, migration, and therapy resistance. Isorhamnetin reverses this process by targeting USP7 to promote YY1 degradation. Notably, HMSN-ISO@ProA-PD-L1 Ab nanoparticles, which are dual-functional mesoporous silica nanoparticles combining isorhamnetin (ISO) and anti-PD-L1 antibody, demonstrate therapeutic effects, including specific tumor targeting coupled with significant modulation of the tumor immune microenvironment through MDSC reduction and enhanced T cell infiltration [69]. YY1 has been implicated in the regulation of PD-L1 expression through several signaling pathways involving complex crosstalk [16,105,153]. Therefore, targeting YY1 and its regulatory networks represents a promising combinatorial strategy to enhance anti-tumor immunity and overcome resistance to immune checkpoint inhibitors.


Table 4.
Combination therapy with YY1 inhibitor.
Table 4.
Combination therapy with YY1 inhibitor.
| YY1 Inhibitor | Combination Drug/Therapy | Mechanism | Outcome | Cancer | Current Status | Refs |
|---|---|---|---|---|---|---|
| Galiximab | Fludarabine | Inhibit survival/anti-apoptotic NF-κB pathway SNAIL/YY1 | Inhibit tumor growth | DHBL | Preclinical research | [174] |
| Galiximab | Cisplatin | Inhibit NF-κB /SNAIL/YY1/BCL-XL circuit | Induce apoptosis | NHL | Preclinical research | [164] |
| MiR-302b | Cisplatin | Downregulate E2Fs and YY1 and then inhibit ITGA6 | Affect DNA Repair and stemness and increase sensitivity to cisplatin | TNBC | Preclinical research | [175] |
| Lercanidipine, Amlodipine | Doxorubicin | Inhibit the YY1/ ERK/ TGF-β pathway | Inhibit the potential of cellular proliferation and spheroid formation | GC | Preclinical research | [163] |
| siYY1 | 5-FU | Inhibit YY1 and decrease NLRC5 expression | Increase tumor cell sensitivity to 5-FU-induced apoptosis | GC | Preclinical research | [165] |
| CRISPR/Cas9 | Temozolomide | Knock out YY1 and decrease the expression of TP73-AS1 | Decrease tumor aggressiveness and drug resistance | GBM | Preclinical research | [166] |
| l-NAME, DETA NONOate | Photodynamic therapy | Inhibit the NF-κB/ SNAIL/YY1/RKIP loop | Inhibit cell growth and EMT | PCa | Preclinical research | [176,177] |
| CA3 | Osimertinib | Inhibit YAP1 and YY1 expression, activate the EGFR/MAPK axis | Induce autophagy | NSCLC | Preclinical research | [178] |
| siYY1 | Oxaliplatin | Inhibit the YY1/GLUT3 axis | Inhibit glucose metabolism and cell proliferation, sensitize tumor cells to treatment | CRC | Preclinical research | [179] |
| MiR-103a | Radiotherapy | Inhibit NF-κB and YY1 expression | Decrease DNA damage repair and radioresistance | UBC | Preclinical research | [180] |
| DETA NONOate | Cisplatin | Inhibit YY1, BCL-XL | Sensitize the resistant tumor cells to apoptosis | PCa | Preclinical research | [159] |
| DETA NONOate | TRAIL | Inhibit YY1 and increase DR5 expression | Enhance sensitivity to TRAIL apoptosis | PCa | Preclinical research | [52] |
| DETA NONOate | Rituximab | Inhibit YY1 and upregulate FAS expression | Increase apoptosis | B-NHL | Preclinical research | [167] |
| MiR-7-5p | Temozolomide | Target the 3′-UTR of YY1 and decrease YY1 expression | Suppress cancer stemness and increase sensitivity to drugs | GBM | Preclinical research | [79] |
| Obatoclax | TRAIL | Inhibit YY1, upregulate DR5, and decrease MCL-1 expression | Reverse resistance to TRAIL-induced apoptosis | B-NHL | Preclinical research | [53] |
| Rituximab | TRAIL | Suppress YY1 expression and impair its DNA-binding activity | Sensitive to TRAIL-induced apoptosis | B-NHL | Preclinical research | [168] |
| Rituximab | Artesunate | Downregulate YY1 and activate the FAS/CD95 pathway | Increase apoptosis and sensitize to therapy | NHL | Preclinical research | [169] |
| Ursolic acid | Sorafenib | Reduce YY1 and disrupt the ING5-mediated PI3K/AKT signaling pathway | Inhibit tumorigenesis and reverse sorafenib resistance | HCC | Preclinical research | [117] |
| T7-siYY1-Exo | Temozolomide, irradiation | Decrease YY1 expression | Enhance chemoradiotherapy sensitivity and improve survival | GBM | Preclinical research | [42] |
| siYY1 | Chemo radiotherapy | Downregulate YY1 and PLK1 | Increase cell death | ESCC | Preclinical research | [115] |
| MiR-411-3p | Methotrexate | Inhibit YY1 expression | Motivate MTX’s cellular uptake and cytotoxic | ALL | Preclinical research | [181] |
| MiR-7 | Cisplatin | Downregulate YY1 and KLF4 | Inhibit proliferation and cell viability, decrease resistance | NHL | Preclinical research | [182] |
| MiR-7 | CH11 | Inhibit YY1 expression and increase FAS activity | Increase apoptosis | CRC | Preclinical research | [55] |
| MiR-186 | Cisplatin | Degrade YY1 protein | Inhibit the formation of the tumor-initiating cell phenotype, reverse cisplatin resistance | GBM | Preclinical research | [78] |
| AMD3100 | Cytarabine | Increase let-7a and inhibit YY1 | Extend survival | AML | Phase I/II clinical trial stage | [46] |
| GKT137831 | Gefitinib | Inhibit NOX4 expression and downregulate YY1 | Increase cellular apoptosis | NSCLC | Preclinical research | [173] |
| Photodynamic | DR2 | Inhibit the NF-κB/SNAIl/YY1/RKIP loop | Induce cell death | PCa | Preclinical research | [183] |
| Isorhamnetin | Anti-PD-L1 antibody | Target USP7 and promote YY1 ubiquitin-dependent degradation | Improve the tumor immune microenvironment and inhibit progression | HCC | Preclinical research | [69] |
| Vitamin D | Anti-PD-1 drug | Promote VDR interaction with YY1 and activate the transcription of VDBP | Improve anti-tumor efficacy | HCC | Preclinical research | [184] |
| Kenpaullone | Doxorubicin | Inhibit YY1, downregulate BCL-2 | Increase apoptosis | NHL | Preclinical research | [185] |
4. Challenges and Future Perspectives
YY1 acts as a regulator in the network of tumor drug resistance, exerting multifaceted resistance-related pathways, ranging from enhancing drug efflux through upregulation of ABC transporters to facilitating DNA damage repair. Moreover, YY1 reinforces therapeutic resistance by maintaining cancer stemness, driving EMT, and reprogramming tumor metabolism, creating a multifaceted defense against chemotherapy, targeted therapy, and immunotherapy [41,43,114]. Simultaneously, YY1 drives tumor cells’ drug-resistant phenotypes by regulating tumor metabolism and reshaping the immune microenvironment. Thus, YY1’s critical role as a regulator coordinating multiple drug resistance mechanisms highlights its potential as a therapeutic target for overcoming drug resistance in tumors.
Despite the promising therapeutic value of targeting YY1, significant challenges and limitations persist. YY1 exhibits dual roles in tumor biology, functioning as either an oncogenic driver or a tumor suppressor depending on tumor type, molecular context, and the tumor microenvironment. In the majority of tumors, YY1 acts as a prototypical oncogene, driving multiple hallmarks of cancer progression, including cell proliferation, invasion, metastasis, angiogenesis, and resistance to therapeutic interventions [186]. Notably, in pancreatic cancer (PDAC), YY1 is mainly a tumor suppressor, inhibiting cell proliferation, metastasis, invasion, and pancreatic clock regulation. Its high expression is associated with better outcomes in PDAC patients, and YY1 may indirectly inhibit PDAC by reducing the incidence of diabetes, providing a theoretical basis for the diagnosis and targeted therapy of pancreatic cancer [187,188]. Furthermore, YY1 exhibits dual regulatory functions in lung cancer, modulating both tumor-promoting and tumor-suppressing activities through diverse epigenetic modifications and signaling pathways [189]. This functional duality presents a therapeutic dilemma, as inhibition of YY1 might suppress oncogenic roles while potentially disrupting tumor-suppressive functions. Thus, targeting YY1 is infeasible, as inhibition may accelerate tumor growth in YY1-protective cancers. This context dependence requires more precise molecular stratification to guide therapeutic decision making regarding YY1 inhibition, activation, or pathway-selective regulation.
Additionally, tumor heterogeneity also affects the treatment outcome. The molecular mechanisms of this heterogeneity include epigenetic regulation, post-translational modifications, and protein interaction networks, among others [29]. Studies show that YY1 demonstrates significant heterogeneity in diverse tumor types. The molecular mechanisms of this heterogeneity include epigenetic regulation, post-translational modifications, and protein interaction networks, among others [190]. In pancreatic cancer neuroendocrine tumors (PNETs), the occurrence of YY1 gene mutations is different in insulinomas and non-insulinomas, and its protein expression is related to clinic outcomes [191]. Within the same tumor, both the single-cell level and microenvironmental factors can lead to the heterogeneity of YY1. For instance, in glioblastoma, YY1 is highly expressed in the stem-cell-like cell subpopulation, while its expression is lower in differentiated cells. During the cisplatin-resistant epithelial cell transformation process in bladder cancer, YY1 expression gradually increases, and histone lactylation modification (H3K18la) can activate its expression by enriching in the YY1 promoter region, resulting in cisplatin resistance [116]. Therefore, given the heterogeneity of YY1 in tumors, it is rather difficult to select a treatment plan.
A mountain of evidence supports the therapeutic value of targeting YY1 in drug-resistant malignancies. Small-molecule inhibitors, nucleic-acid-based agents (such as siRNAs, antisense oligonucleotides), and epigenetic drugs that modulate YY1 expression or activity have demonstrated promising results in preclinical models. Importantly, when combined with conventional chemotherapies, targeted therapies, or immunotherapies, these agents synergize to reverse resistance phenotypes, enhancing tumor cell vulnerability and improving treatment responses. This combinatorial strategy is especially attractive in the era of immuno-oncology. However, their effectiveness is often blunted by resistance mechanisms, many of which are reinforced by YY1 activity. For example, YY1-mediated immune evasion and CSC maintenance contribute to immunotherapy failure. Integrating YY1 inhibition into immunotherapy regimens may thus unlock durable anti-tumor immunity and expand the pool of patients who benefit from these treatments [192].
5. Conclusions
Collectively, YY1 serves as a central hub coordinating drug resistance mechanisms, making its therapeutic targeting a compelling strategy against refractory cancers. Therefore, future work should bridge mechanistic insights to clinical translation through network mapping, rational drug combinations, and biomarker development to harness YY1 inhibition’s full potential in precision oncology.
Author Contributions
Writing—original draft preparation, visualization, Z.L. and X.J.; formal analysis, visualization, writing—review and editing, I.T.S.M. and Y.L.; funding acquisition, resources, supervision, writing—review and editing, Y.L. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Chongqing, grant number CSTB2023NSCQ-MSX0451.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We note that during the preparation of this manuscript AI tools (DeepSeek Chat) used to improve language. However, the review’s core viewpoints, content organization, tables, and figures are the authors’ independent work.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Abbreviations
| YY1 | Yin Yang 1 |
| MDR | mediating cancer multidrug resistance |
| CAFs | cancer-associated fibroblasts |
| SVEP1 | pentraxin domain containing 1 |
| TKIs | tyrosine kinase inhibitors |
| HDAC2 | histone deacetylase 2 |
| FEN1 | flap endonuclease 1 |
| LEN | lenalidomide |
| TME | tumor microenvironment |
| VEGF | vascular endothelial growth factor |
| PLK1 | polo like kinase 1 |
| HK2 | hexokinase 2 |
| ING5 | inhibitor of growth 5 |
| ACLY | ATP-citrate lyase |
| LDs | lipid droplets |
| FAM60A | family with sequence similarity 60 member A |
| GPX4 | glutathione peroxidase 4 |
| IL-32 | interleukin 32 |
| MRP2 | multidrug resistance-associated protein 2 |
| TRIM34 | tripartite motif-containing protein 34 |
| NO | nitric oxide |
| CRHR2 | corticotropin-releasing-hormone-receptor-2 |
| NTRK1 | neurotrophic tropomyosin receptor kinase 1 |
| IFFO1 | intermediate filament family orphan 1 |
| CHRAC1 | chromatin accessibility complex subunit 1 |
| DIOB | diosbulbin B |
| PARPi | polymerase inhibitors |
| ITGA6 | integrin subunit alpha 6 |
| IR | irradiation |
| PP2A | protein phosphatase 2A |
| SMAD | small mother against decapentaplegic |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| USP7 | ubiquitin-specific protease 7 |
| VDR | vitamin D receptor |
| NHL | non-Hodgkin’s lymphoma |
| GBM | glioblastoma |
| BC | breast cancer |
| DHBL | disseminated human B-lymphoma |
| BH | bendamustine hydrochloride |
| ABCB | ATP-binding cassette subfamily B |
| TRKA | tropomyosin receptor kinase A |
| NOX4 | NADPH oxidase 4 |
| HR | homologous recombination |
| BER | base excision repair |
| G4s | guanine quadruplexes |
| SHLD1 | shieldin component |
| AKR1C3 | aldo-keto reductase family 1 member C3 |
| BET | bromodomain and extraterminal |
| HIFs | hypoxia-inducible factor |
| GTPase | guanosine triphosphatase |
| PPP | pentose phosphate pathway |
| PDK1 | pyruvate dehydrogenase kinase 1 |
| ACC1 | acetyl-CoA carboxylase 1 |
| SRF | serum response factor |
| GALC | galactocerebrosidase |
| ACSL | acyl-CoA synthetase long chain family |
| IFN-γ | interferon-gamma |
| FXR | farnesoid X receptor |
| FOXP3 | forkhead box protein P3 |
| SIRT1 | silent information regulator 1 |
| FASL | fas ligand |
| UCN2 | urocortin-2 |
| KDM5C | lysine demethylase 5C |
| HDAC5 | histone deacetylase 5 |
| EGFR | epidermal growth factor receptor |
| CSCs | cancer stem cells |
| mCAFs | matrix-type cancer-associated fibroblasts |
| RKIP | raf kinase inhibitor protein |
| ISO | isorhamnetin |
| VDBP | vitamin D binding protein |
| MTX | methotrexate |
| PTEN | phosphatase and tensin homolog |
| BRCA1 | breast-cancer susceptibility gene 1 |
| VHL | von Hippel–Lindau |
| EZH2 | enhancer of zeste homolog 2 |
| MAPK | mitogen-activated protein kinases |
| UA | ursolic acid |
References
- Shi, Y.; Seto, E.; Chang, L.S.; Shenk, T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell 1991, 67, 377–388. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lossie, A.C.; Camper, S.A.; Gumucio, D.L. Chromosomal localization of the transcription factor YY1 in the mouse and human. Mamm. Genome 1994, 5, 234–236. [Google Scholar] [CrossRef]
- Schug, J.; Schuller, W.P.; Kappen, C.; Salbaum, J.M.; Bucan, M.; Stoeckert, C.J., Jr. Promoter features related to tissue specificity as measured by Shannon entropy. Genome Biol. 2005, 6, R33. [Google Scholar] [CrossRef]
- Khachigian, L.M. The Yin and Yang of YY1 in tumor growth and suppression. Int. J. Cancer 2018, 143, 460–465. [Google Scholar] [CrossRef]
- Wu, S.; Kasim, V.; Kano, M.R.; Tanaka, S.; Ohba, S.; Miura, Y.; Miyata, K.; Liu, X.; Matsuhashi, A.; Chung, U.I.; et al. Transcription factor YY1 contributes to tumor growth by stabilizing hypoxia factor HIF-1α in a p53-independent manner. Cancer Res. 2013, 73, 1787–1799. [Google Scholar] [CrossRef]
- Zhuang, J.; Shen, L.; Li, M.; Sun, J.; Hao, J.; Li, J.; Zhu, Z.; Ge, S.; Zhang, D.; Guo, H.; et al. Cancer-Associated Fibroblast-Derived miR-146a-5p Generates a Niche That Promotes Bladder Cancer Stemness and Chemoresistance. Cancer Res. 2023, 83, 1611–1627. [Google Scholar] [CrossRef]
- Thomas, M.J.; Seto, E. Unlocking the mechanisms of transcription factor YY1: Are chromatin modifying enzymes the key? Gene 1999, 236, 197–208. [Google Scholar] [CrossRef]
- Shi, Y.; Lee, J.S.; Galvin, K.M. Everything you have ever wanted to know about Yin Yang 1. Biochim. Biophys. Acta 1997, 1332, F49–F66. [Google Scholar] [CrossRef]
- Wilkinson, F.H.; Park, K.; Atchison, M.L. Polycomb recruitment to DNA in vivo by the YY1 REPO domain. Proc. Natl. Acad. Sci. USA 2006, 103, 19296–19301. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kong, G.; Lee, D.H.; Kim, M.; Tran, Q.; Cho, H.; Kim, C.; Park, S.; Kim, S.H.; et al. Yin Yang 1 is required for PHD finger protein 20-mediated myogenic differentiation in vitro and in vivo. Cell Death Differ. 2020, 27, 3321–3336. [Google Scholar] [CrossRef]
- Bao, Y.; Teng, S.; Zhai, H.; Zhang, Y.; Xu, Y.; Li, C.; Chen, Z.; Ren, F.; Wang, Y. SE-lncRNAs in Cancer: Classification, Subcellular Localisation, Function and Corresponding TFs. J. Cell. Mol. Med. 2024, 28, e70296. [Google Scholar] [CrossRef]
- Wang, H.; He, K.; Huo, R.; Li, W.; Zhang, S.; Jiang, L.J.; Wu, H.; Yu, M.; Jiang, S.H.; Xue, J. MAGEA6 Engages a YY1-Dependent Transcription to Dictate Perineural Invasion in Colorectal Cancer. Adv. Sci. 2025, 12, e2501119. [Google Scholar] [CrossRef]
- Shimizu, T.; Shindo, T.; Ogawa, H.; Teranaka, K.; Watanabe, A.; Takaori-Kondo, A. Upregulation of YY1/EZH2 and MLH1 as Therapeutic Targets for Adult T-Cell Leukemia/Lymphoma. Cancer Sci. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meliala, I.T.S.; Hosea, R.; Kasim, V.; Wu, S. The biological implications of Yin Yang 1 in the hallmarks of cancer. Theranostics 2020, 10, 4183–4200. [Google Scholar] [CrossRef] [PubMed]
- Hays, E.; Bonavida, B. YY1 regulates cancer cell immune resistance by modulating PD-L1 expression. Drug Resist. Updat. 2019, 43, 10–28. [Google Scholar] [CrossRef]
- Li, M.; Wei, J.; Xue, C.; Zhou, X.; Chen, S.; Zheng, L.; Duan, Y.; Deng, H.; Xiong, W.; Tang, F.; et al. Dissecting the roles and clinical potential of YY1 in the tumor microenvironment. Front. Oncol. 2023, 13, 1122110. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, J.; Cao, Y.H. Crosstalk between YY1 and lncRNAs in cancer: A review. Medicine 2022, 101, e31990. [Google Scholar] [CrossRef]
- Lin, J.; He, Y.; Wang, B.; Xun, Z.; Chen, S.; Zeng, Z.; Ou, Q. Blocking of YY1 reduce neutrophil infiltration by inhibiting IL-8 production via the PI3K-Akt-mTOR signaling pathway in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 195, 226–236. [Google Scholar] [CrossRef]
- Zhou, X.; Xian, W.; Zhang, J.; Zhu, Y.; Shao, X.; Han, Y.; Qi, Y.; Ding, X.; Wang, X. YY1 binds to the E3’ enhancer and inhibits the expression of the immunoglobulin κ gene via epigenetic modifications. Immunology 2018, 155, 491–498. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Crucitta, S.; Cucchiara, F.; Mathijssen, R.; Mateo, J.; Jager, A.; Joosse, A.; Passaro, A.; Attili, I.; Petrini, I.; van Schaik, R.; et al. Treatment-driven tumour heterogeneity and drug resistance: Lessons from solid tumours. Cancer Treat. Rev. 2022, 104, 102340. [Google Scholar] [CrossRef] [PubMed]
- Aldea, M.; Andre, F.; Marabelle, A.; Dogan, S.; Barlesi, F.; Soria, J.C. Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov. 2021, 11, 874–899. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J. Clin. Oncol. 2022, 40, 598–610. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef]
- Rizkallah, R.; Hurt, M.M. The Multilayered Regulation of the Oncogenic Protein YY1. Crit. Rev. Oncog. 2017, 22, 109–129. [Google Scholar] [CrossRef]
- Fu, X.; Ji, F.; He, Q.; Qiu, X. A Systematic Pan-Cancer Analysis of YY1 Aberrations and their Relationship with Clinical Outcome, Tumor Microenvironment, and Therapeutic Targets. J. Immunol. Res. 2022, 2022, 5826741. [Google Scholar] [CrossRef]
- Zhang, Q.; Stovall, D.B.; Inoue, K.; Sui, G. The oncogenic role of Yin Yang 1. Crit. Rev. Oncog. 2011, 16, 163–197. [Google Scholar] [CrossRef]
- Hosea, R.; Hillary, S.; Wu, S.; Kasim, V. Targeting Transcription Factor YY1 for Cancer Treatment: Current Strategies and Future Directions. Cancers 2023, 15, 3506. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Duan, C.; Yu, M.; Xu, J.; Li, B.Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-drug Resistance (MDR): Reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug-Drug Interaction Studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Takimoto-Shimomura, T.; Nagoshi, H.; Maegawa, S.; Fujibayashi, Y.; Tsukamoto, T.; Matsumura-Kimoto, Y.; Mizuno, Y.; Chinen, Y.; Mizutani, S.; Shimura, Y.; et al. Establishment and Characteristics of a Novel Mantle Cell Lymphoma-derived Cell Line and a Bendamustine-resistant Subline. Cancer Genom. Proteom. 2018, 15, 213–223. [Google Scholar] [CrossRef]
- Grubach, L.; Juhl-Christensen, C.; Rethmeier, A.; Olesen, L.H.; Aggerholm, A.; Hokland, P.; Ostergaard, M. Gene expression profiling of Polycomb, Hox and Meis genes in patients with acute myeloid leukaemia. Eur. J. Haematol. 2008, 81, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, X.; Wei, M.; Li, Z.; Li, Y.; Zhao, H.; Miyagishi, M.; Kasim, V.; Wu, S. YY2/PHGDH axis suppresses tumorigenesis by inhibiting tumor cell de novo serine biosynthesis. Biomed. Pharmacother. 2023, 165, 115006. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Andrés, G.; Rangel-Santiago, J.; Tirado-Rodríguez, B.; Martinez-Ruiz, G.U.; Klunder-Klunder, M.; Vega, M.I.; Lopez-Martinez, B.; Jiménez-Hernández, E.; Torres Nava, J.; Medina-Sanson, A.; et al. Role of Yin Yang-1 (YY1) in the transcription regulation of the multi-drug resistance (MDR1) gene. Leuk. Lymphoma 2018, 59, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Q.; Dai, X.; Chen, X.; Zhang, C.; Bai, R.; Chen, Y.; Zhang, K.; Duan, X.; Qiao, Y.; et al. PGC-1α promotes colorectal carcinoma metastasis through regulating ABCA1 transcription. Oncogene 2023, 42, 2456–2470. [Google Scholar] [CrossRef]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.F.; et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell 2016, 29, 653–668. [Google Scholar] [CrossRef]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef]
- Guan, H.; Tao, H.; Luo, J.; Wan, L.; Hu, H.; Chen, L.; Wen, Z.; Tao, Y.; Chen, S.; Gu, M. Upregulation of YY1 in M2 macrophages promotes secretion of exosomes containing hsa-circ-0000326 via super-enhancers to facilitate prostate cancer progression. Mol. Cell Biochem. 2025, 480, 3873–3888. [Google Scholar] [CrossRef]
- Navasardyan, I.; Zaravinos, A.; Bonavida, B. Therapeutic Implications of Targeting YY1 in Glioblastoma. Cancers 2024, 16, 2074. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Liu, N.; Gao, G.; Du, M.; Wang, Y.; Cheng, B.; Zhu, M.; Jia, B.; Pan, L.; et al. Kill two birds with one stone: Engineered exosome-mediated delivery of cholesterol modified YY1-siRNA enhances chemoradiotherapy sensitivity of glioblastoma. Front. Pharmacol. 2022, 13, 975291. [Google Scholar] [CrossRef]
- Jung, M.; Bui, I.; Bonavida, B. Role of YY1 in the Regulation of Anti-Apoptotic Gene Products in Drug-Resistant Cancer Cells. Cancers 2023, 15, 4267. [Google Scholar] [CrossRef]
- Potluri, V.; Noothi, S.K.; Vallabhapurapu, S.D.; Yoon, S.O.; Driscoll, J.J.; Lawrie, C.H.; Vallabhapurapu, S. Transcriptional repression of Bim by a novel YY1-RelA complex is essential for the survival and growth of Multiple Myeloma. PLoS ONE 2013, 8, e66121. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Yepez, S.; Liu, H.; Baritaki, S.; Del Lourdes Cebrera-Muñoz, M.; Rivera-Pazos, C.; Maldonado-Valenzuela, A.; Valencia-Hipolito, A.; Vega, M.I.; Chen, H.; Berenson, J.R.; et al. Overexpression of Yin Yang 1 in bone marrow-derived human multiple myeloma and its clinical significance. Int. J. Oncol. 2014, 45, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jacamo, R.; Konopleva, M.; Garzon, R.; Croce, C.; Andreeff, M. CXCR4 downregulation of let-7a drives chemoresistance in acute myeloid leukemia. J. Clin. Investig. 2013, 123, 2395–2407. [Google Scholar] [CrossRef]
- Bonavida, B. RKIP-mediated chemo-immunosensitization of resistant cancer cells via disruption of the NF-κB/Snail/YY1/RKIP resistance-driver loop. Crit. Rev. Oncog. 2014, 19, 431–445. [Google Scholar] [CrossRef]
- Ho, M.; Bonavida, B. Cross-Talks between Raf Kinase Inhibitor Protein and Programmed Cell Death Ligand 1 Expressions in Cancer: Role in Immune Evasion and Therapeutic Implications. Cells 2024, 13, 864. [Google Scholar] [CrossRef]
- Shvartsur, A.; Givechian, K.B.; Garban, H.; Bonavida, B. Overexpression of RKIP and its cross-talk with several regulatory gene products in multiple myeloma. J. Exp. Clin. Cancer Res. 2017, 36, 62. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, W.; Meng, X.; Li, M.; Hou, P.; Li, Z.; Chu, S.; Zheng, J.; Bai, J. The role of transcription factor Yin Yang-1 in colorectal cancer. Cancer Med. 2023, 12, 11177–11190. [Google Scholar] [CrossRef]
- Martínez-Paniagua, M.A.; Baritaki, S.; Huerta-Yepez, S.; Ortiz-Navarrete, V.F.; González-Bonilla, C.; Bonavida, B.; Vega, M.I. Mcl-1 and YY1 inhibition and induction of DR5 by the BH3-mimetic Obatoclax (GX15-070) contribute in the sensitization of B-NHL cells to TRAIL apoptosis. Cell Cycle 2011, 10, 2792–2805. [Google Scholar] [CrossRef]
- Huerta-Yepez, S.; Vega, M.; Escoto-Chavez, S.E.; Murdock, B.; Sakai, T.; Baritaki, S.; Bonavida, B. Nitric oxide sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of the DR5 transcription repressor Yin Yang 1. Nitric Oxide 2009, 20, 39–52. [Google Scholar] [CrossRef]
- Lee, J.Y.; Huerta-Yepez, S.; Vega, M.; Baritaki, S.; Spandidos, D.A.; Bonavida, B. The NO TRAIL to YES TRAIL in cancer therapy (review). Int. J. Oncol. 2007, 31, 685–691. [Google Scholar] [CrossRef]
- Nengroo, M.A.; Maheshwari, S.; Singh, A.; Verma, A.; Arya, R.K.; Chaturvedi, P.; Saini, K.K.; Singh, A.K.; Sinha, A.; Meena, S.; et al. CXCR4 intracellular protein promotes drug resistance and tumorigenic potential by inversely regulating the expression of Death Receptor 5. Cell Death Dis. 2021, 12, 464. [Google Scholar] [CrossRef]
- Pothoulakis, C.; Torre-Rojas, M.; Duran-Padilla, M.A.; Gevorkian, J.; Zoras, O.; Chrysos, E.; Chalkiadakis, G.; Baritaki, S. CRHR2/Ucn2 signaling is a novel regulator of miR-7/YY1/Fas circuitry contributing to reversal of colorectal cancer cell resistance to Fas-mediated apoptosis. Int. J. Cancer 2018, 142, 334–346. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Zhu, Y.; Xu, R.; Deng, Z.; Liu, X.; Ding, Y.; Wang, C.; Shi, Y.; Bei, L.; et al. KDM6A promotes imatinib resistance through YY1-mediated transcriptional upregulation of TRKA independently of its demethylase activity in chronic myelogenous leukemia. Theranostics 2021, 11, 2691–2705. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, P.; Qiang, Y.; Fan, J.; Xing, Y.; Zhang, Y.; Yang, F.; Li, F.; Xiong, J. Targeting the transcription factor YY1 is synthetic lethal with loss of the histone demethylase KDM5C. EMBO Rep. 2024, 25, 5408–5428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lin, Y.H.; Chi, H.C.; Huang, P.S.; Liao, C.J.; Liou, Y.S.; Lin, C.C.; Yu, C.J.; Yeh, C.T.; Huang, Y.H.; et al. CRNDE acts as an epigenetic modulator of the p300/YY1 complex to promote HCC progression and therapeutic resistance. Clin. Epigenetics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Zhu, L.; Yang, Y.; Yin, X. YY1-Induced lncRNA PART1 Enhanced Resistance of Ovarian Cancer Cells to Cisplatin by Regulating miR-512-3p/CHRAC1 Axis. DNA Cell Biol. 2021, 40, 821–832. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, S.; Shen, J.; Guo, L.; Sun, Y.; Zhu, Y.; Ma, Z.; Zhang, X.; Hu, Y.; Xiao, W.; et al. The MiR-135b-BMAL1-YY1 loop disturbs pancreatic clockwork to promote tumourigenesis and chemoresistance. Cell Death Dis. 2018, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhou, S.; Zhang, R.; Chen, Y.; Huang, W.; Yu, K.; Yang, N.; Qian, X.; Tie, X.; Xu, J.; et al. CRISPR/Cas9 screen reveals that targeting TRIM34 enhances ferroptosis sensitivity and augments immunotherapy efficacy in hepatocellular carcinoma. Cancer Lett. 2024, 593, 216935. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Zhang, F.; Qin, X.M.; Nong, X.; Shi, X.; Zhou, X.M.; Qin, Y.M. Oxymatrine Inhibits Liver Cancer Progression by Regulating SIRT1/YY1/GPX4 Axis-Mediated Ferroptosis. Chem. Res. Toxicol. 2025, 38, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Li, Z.; Wei, M.; Zhao, H.; Miyagishi, M.; Wu, S.; Kasim, V. Homeostasis Imbalance of YY2 and YY1 Promotes Tumor Growth by Manipulating Ferroptosis. Adv. Sci. 2022, 9, e2104836. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.H.; Wen, K.L.; Guo, Y.; Liao, Y.N.; Li, M.Y.; Li, Z.Q.; Li, S.X.; Nie, H.Z. Nutrient deficiency-induced downregulation of SNX1 inhibits ferroptosis through PPARs-ACSL1/4 axis in colorectal cancer. Apoptosis 2025, 30, 1391–1409. [Google Scholar] [CrossRef]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef]
- Malik, S.; Sikander, M.; Wahid, M.; Dhasmana, A.; Sarwat, M.; Khan, S.; Cobos, E.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Deciphering cellular and molecular mechanism of MUC13 mucin involved in cancer cell plasticity and drug resistance. Cancer Metastasis Rev. 2024, 43, 981–999. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Liu, H.; Han, J.; Lv, Y.; Zhao, Z.; Zheng, S.; Sun, Y.; Sun, T. Isorhamnetin and anti-PD-L1 antibody dual-functional mesoporous silica nanoparticles improve tumor immune microenvironment and inhibit YY1-mediated tumor progression. J. Nanobiotechnol. 2023, 21, 208. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes. Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Xu, Y.; Kang, P.; Huang, P.; Meng, N.; Wang, H.; Zheng, W.; Wang, H.; Wang, Z.; et al. YY1-induced DLEU1/miR-149-5p Promotes Malignant Biological Behavior of Cholangiocarcinoma through Upregulating YAP1/TEAD2/SOX2. Int. J. Biol. Sci. 2022, 18, 4301–4315. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Liu, Q.; Wong, P.P.; Song, E. Cancer stem cell mimicry for immune evasion and therapeutic resistance. Cell Stem Cell 2024, 31, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Jang, Y.; Yun, B.S.; Kang, S.; Kim, Y.H.; Kim, B.G.; Cho, N.H. MET overexpression in ovarian cancer via CD24-induced downregulation of miR-181a: A signalling for cellular quiescence-like state and chemoresistance in ovarian CSCs. Cell Prolif. 2024, 57, e13582. [Google Scholar] [CrossRef]
- Jang, Y.; Kang, S.; Han, H.H.; Kim, B.G.; Cho, N.H. CD24 induced cellular quiescence-like state and chemoresistance in ovarian cancer cells via miR-130a/301a-dependent CDK19 downregulation. Cell Death Discov. 2024, 10, 81. [Google Scholar] [CrossRef]
- Kaufhold, S.; Garbán, H.; Bonavida, B. Yin Yang 1 is associated with cancer stem cell transcription factors (SOX2, OCT4, BMI1) and clinical implication. J. Exp. Clin. Cancer Res. 2016, 35, 84. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, L.; Shen, J.Z.; Liang, Z.; Wu, Q.; Yang, K.; Min, L.; Gimple, R.C.; Yang, Q.; Bhargava, S.; et al. Transcription Elongation Machinery Is a Druggable Dependency and Potentiates Immunotherapy in Glioblastoma Stem Cells. Cancer Discov. 2022, 12, 502–521. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Guo, F. miR-186 reverses cisplatin resistance and inhibits the formation of the glioblastoma-initiating cell phenotype by degrading Yin Yang 1 in glioblastoma. Int. J. Mol. Med. 2019, 43, 517–524. [Google Scholar] [CrossRef]
- Jia, B.; Liu, W.; Gu, J.; Wang, J.; Lv, W.; Zhang, W.; Hao, Q.; Pang, Z.; Mu, N.; Zhang, W.; et al. MiR-7-5p suppresses stemness and enhances temozolomide sensitivity of drug-resistant glioblastoma cells by targeting Yin Yang 1. Exp. Cell Res. 2019, 375, 73–81. [Google Scholar] [CrossRef]
- Chang, J.; Zhao, X.; Wang, Y.; Liu, T.; Zhong, C.; Lao, Y.; Zhang, S.; Liao, H.; Bai, F.; Lin, D.; et al. Genomic alterations driving precancerous to cancerous lesions in esophageal cancer development. Cancer Cell 2023, 41, 2038–2050.e2035. [Google Scholar] [CrossRef]
- Tiek, D.; Cheng, S.Y. DNA damage and metabolic mechanisms of cancer drug resistance. Cancer Drug Resist. 2022, 5, 368–379. [Google Scholar] [CrossRef]
- Cordes, J.; Zhao, S.; Engel, C.M.; Stingele, J. Cellular responses to RNA damage. Cell 2025, 188, 885–900. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, C.; Fu, C.; Hu, J.; Li, T.; Li, L. YY1 is involved in homologous recombination inhibition at guanine quadruplex sites in human cells. Nucleic Acids Res. 2024, 52, 7401–7413. [Google Scholar] [CrossRef]
- Zhao, L.; Li, R.; Qiu, J.Z.; Yu, J.B.; Cao, Y.; Yuan, R.T. YY1-mediated PTEN dephosphorylation antagonizes IR-induced DNA repair contributing to tongue squamous cell carcinoma radiosensitization. Mol. Cell. Probes 2020, 53, 101577. [Google Scholar] [CrossRef]
- Shinoda, K.; Zong, D.; Callen, E.; Wu, W.; Dumitrache, L.C.; Belinky, F.; Chari, R.; Wong, N.; Ishikawa, M.; Stanlie, A.; et al. The dystonia gene THAP1 controls DNA double-strand break repair choice. Mol. Cell 2021, 81, 2611–2624.e2610. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, G.; Han, F.; Fu, S.; Cao, Y.; Zhang, F.; Zhang, Q.; Meslamani, J.; Xu, Y.; Ji, D.; et al. Spatially constrained tandem bromodomain inhibition bolsters sustained repression of BRD4 transcriptional activity for TNBC cell growth. Proc. Natl. Acad. Sci. USA 2018, 115, 7949–7954. [Google Scholar] [CrossRef] [PubMed]
- Sarnik, J.; Popławski, T.; Tokarz, P. BET Proteins as Attractive Targets for Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 11102. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Zhou, X.; Zhang, X.; Qiu, F.; Zhang, S.; Chen, Y.; Yang, J.; Wang, J.; Tan, W. BRD4-binding enhancer promotes CRC progression by interacting with YY1 to activate the Wnt pathway through upregulation of TCF7L2. Biochem. Pharmacol. 2023, 218, 115877. [Google Scholar] [CrossRef]
- Xu, C.; Tsai, Y.H.; Galbo, P.M.; Gong, W.; Storey, A.J.; Xu, Y.; Byrum, S.D.; Xu, L.; Whang, Y.E.; Parker, J.S.; et al. Cistrome analysis of YY1 uncovers a regulatory axis of YY1:BRD2/4-PFKP during tumorigenesis of advanced prostate cancer. Nucleic Acids Res. 2021, 49, 4971–4988. [Google Scholar] [CrossRef]
- Yang, C.; Qu, J.; Cheng, Y.; Tian, M.; Wang, Z.; Wang, X.; Li, X.; Zhou, S.; Zhao, B.; Guo, Y.; et al. YY1 drives PARP1 expression essential for PARylation of NONO in mRNA maturation during neuroblastoma progression. J. Transl. Med. 2024, 22, 1153. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Zhou, Q.Q.; Sun, Y.; Yu, T.; Jiang, Y.; Li, H.J. The anti-non-small cell lung cancer effect of Diosbulbin B: Targeting YY1 induced cell cycle arrest and apoptosis. Phytomedicine 2024, 130, 155734. [Google Scholar] [CrossRef]
- Myadelets, D.; Parfenyev, S.; Vasileva, J.; Shuvalov, O.; Petukhov, A.; Fedorova, O.; Barlev, N.; Daks, A. Methyltransferase Set7/9 controls PARP1 expression and regulates cisplatin response of breast cancer cells. Biochem. Biophys. Res. Commun. 2024, 691, 149328. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Li, Z.; Zhang, T.; Liu, W.; Liu, Z.; Yuan, Y.C.; Su, F.; Xu, L.; Wang, Y.; et al. YY1 suppresses FEN1 over-expression and drug resistance in breast cancer. BMC Cancer 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, R.; Wang, M.; Kumar, A.K.; Pan, F.; He, L.; Hu, Z.; Guo, Z. MicroRNA-140 impedes DNA repair by targeting FEN1 and enhances chemotherapeutic response in breast cancer. Oncogene 2020, 39, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jing, L.; Tan, S.; Zeng, E.M.; Lin, Y.; He, L.; Hu, Z.; Liu, J.; Guo, Z. Inhibition of miR-1193 leads to synthetic lethality in glioblastoma multiforme cells deficient of DNA-PKcs. Cell Death Dis. 2020, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, A.; Wang, Y.; Qi, D.; Peng, C.; Liang, Z.; Guo, J.; Gu, Y. YY1-induced transcription of AKR1C3 activates the Hedgehog signalling pathway to enhance lenalidomide resistance and glycolytic activity in multiple myeloma cells. Clin. Exp. Med. 2025, 25, 99. [Google Scholar] [CrossRef]
- Meng, F.; Qi, T.; Liu, X.; Wang, Y.; Yu, J.; Lu, Z.; Cai, X.; Li, A.; Jung, D.; Duan, J. Enhanced pharmacological activities of AKR1C3-activated prodrug AST-3424 in cancer cells with defective DNA repair. Int. J. Cancer 2025, 156, 417–430. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Yang, Y.; Wang, X.; Wu, E.; Majerciak, V.; Zhang, T.; Steenbergen, R.D.M.; Wang, H.K.; Banerjee, N.S.; et al. Oncogenic HPV promotes the expression of the long noncoding RNA lnc-FANCI-2 through E7 and YY1. Proc. Natl. Acad. Sci. USA 2021, 118, e2014195118. [Google Scholar] [CrossRef]
- Xiao, Y.; Hassani, M.; Moghaddam, M.B.; Fazilat, A.; Ojarudi, M.; Valilo, M. Contribution of tumor microenvironment (TME) to tumor apoptosis, angiogenesis, metastasis, and drug resistance. Med. Oncol. 2025, 42, 108. [Google Scholar] [CrossRef]
- Goenka, A.; Khan, F.; Verma, B.; Sinha, P.; Dmello, C.C.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B.C. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun. 2023, 43, 525–561. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Choi, K.S.; Bae, M.K.; Jeong, J.W.; Moon, H.E.; Kim, K.W. Hypoxia-induced angiogenesis during carcinogenesis. J. Biochem. Mol. Biol. 2003, 36, 120–127. [Google Scholar] [CrossRef]
- Mohamed, O.A.A.; Tesen, H.S.; Hany, M.; Sherif, A.; Abdelwahab, M.M.; Elnaggar, M.H. The role of hypoxia on prostate cancer progression and metastasis. Mol. Biol. Rep. 2023, 50, 3873–3884. [Google Scholar] [CrossRef] [PubMed]
- de Nigris, F.; Rossiello, R.; Schiano, C.; Arra, C.; Williams-Ignarro, S.; Barbieri, A.; Lanza, A.; Balestrieri, A.; Giuliano, M.T.; Ignarro, L.J.; et al. Deletion of Yin Yang 1 protein in osteosarcoma cells on cell invasion and CXCR4/angiogenesis and metastasis. Cancer Res. 2008, 68, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Meo, C.; de Nigris, F. Clinical Potential of YY1-Hypoxia Axis for Vascular Normalization and to Improve Immunotherapy. Cancers 2024, 16, 491. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Vijayappa, S.; Kozin, S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol. Cancer Ther. 2006, 5, 1275–1279. [Google Scholar] [CrossRef]
- Kachalaki, S.; Ebrahimi, M.; Mohamed Khosroshahi, L.; Mohammadinejad, S.; Baradaran, B. Cancer chemoresistance; biochemical and molecular aspects: A brief overview. Eur. J. Pharm. Sci. 2016, 89, 20–30. [Google Scholar] [CrossRef]
- Li, X.; Ma, T.K.; Wang, M.; Zhang, X.D.; Liu, T.Y.; Liu, Y.; Huang, Z.H.; Zhu, Y.H.; Zhang, S.; Yin, L.; et al. YY1-induced upregulation of LncRNA-ARAP1-AS2 and ARAP1 promotes diabetic kidney fibrosis via aberrant glycolysis associated with EGFR/PKM2/HIF-1α pathway. Front. Pharmacol. 2023, 14, 1069348. [Google Scholar] [CrossRef]
- Marcucci, F.; Rumio, C. Glycolysis-induced drug resistance in tumors-A response to danger signals? Neoplasia 2021, 23, 234–245. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.; Vaghi, C.; Baraibar, I.; Saoudi González, N.; Rodríguez-Castells, M.; García, A.; Alcaraz, A.; Salva, F.; Tabernero, J.; Elez, E. Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver. Int. J. Mol. Sci. 2024, 25, 3304. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lin, Y.; Wang, C.; Lv, Y.; Chen, W. YY1 as a mediator to enhance the resistance of KRAS mutant colorectal cancer cells to cetuximab. J. Genet. 2025, 104, 3. [Google Scholar] [CrossRef]
- Zhao, M.; Lu, T.; Bi, G.; Hu, Z.; Liang, J.; Bian, Y.; Feng, M.; Zhan, C. PLK1 regulating chemoradiotherapy sensitivity of esophageal squamous cell carcinoma through pentose phosphate pathway/ferroptosis. Biomed. Pharmacother. 2023, 168, 115711. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Huang, Y.; Li, D.; Zheng, Z.; Xie, K.; Cao, C.; Wang, Q.; Zhao, X.; Huang, Z.; et al. Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist. Updat. 2024, 73, 101059. [Google Scholar] [CrossRef]
- Fan, Y.J.; Pan, F.Z.; Cui, Z.G.; Zheng, H.C. The Antitumor and Sorafenib-resistant Reversal Effects of Ursolic Acid on Hepatocellular Carcinoma via Targeting ING5. Int. J. Biol. Sci. 2024, 20, 4190–4208. [Google Scholar] [CrossRef]
- Beier, U.H.; Görögh, T. Implications of galactocerebrosidase and galactosylcerebroside metabolism in cancer cells. Int. J. Cancer 2005, 115, 6–10. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Q.; Huang, P.Q.; Su, T.; Jiang, S.H.; Hu, L.P.; Zhang, X.L.; Sun, Y.; Pan, H.; Yang, X.M.; et al. A low amino acid environment promotes cell macropinocytosis through the YY1-FGD6 axis in Ras-mutant pancreatic ductal adenocarcinoma. Oncogene 2022, 41, 1203–1215. [Google Scholar] [CrossRef]
- Baharom, F.; Ramirez-Valdez, R.A.; Khalilnezhad, A.; Khalilnezhad, S.; Dillon, M.; Hermans, D.; Fussell, S.; Tobin, K.K.S.; Dutertre, C.A.; Lynn, G.M.; et al. Systemic vaccination induces CD8(+) T cells and remodels the tumor microenvironment. Cell 2022, 185, 4317–4332.e4315. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, A.; Obeidat, L.; Zaravinos, A.; Bonavida, B. The Role of YY1 in the Regulation of LAG-3 Expression in CD8 T Cells and Immune Evasion in Cancer: Therapeutic Implications. Cancers 2024, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, N.; Fan, L.; Wu, R.; Cao, L.; Ren, Y.; Lu, C.; Zhang, L.; Cai, Y.; Shi, Y.; et al. Single-cell transcriptomic and spatial analysis reveal the immunosuppressive microenvironment in relapsed/refractory angioimmunoblastic T-cell lymphoma. Blood Cancer J. 2024, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, D.Y.; Tang, K.T.; Chao, Y.H.; Shen, C.H.; Lui, P.W. Inhibitory effects of propofol on Th17 cell differentiation. Immunopharmacol. Immunotoxicol. 2017, 39, 211–218. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Baritaki, S.; Zaravinos, A. Cross-Talks between RKIP and YY1 through a Multilevel Bioinformatics Pan-Cancer Analysis. Cancers 2023, 15, 4932. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Kwiatkowska, D.; Mazur, E.; Reich, A. YY1 Is a Key Player in Melanoma Immunotherapy/Targeted Treatment Resistance. Front. Oncol. 2022, 12, 856963. [Google Scholar] [CrossRef]
- Huang, C.; Huang, X.; Qiu, X.; Kong, X.; Wu, C.; Jiang, X.; Yao, M.; Wang, M.; Su, L.; Lv, C.; et al. Pericytes Modulate Third-Generation Tyrosine Kinase Inhibitor Sensitivity in EGFR-Mutated Lung Cancer Cells Through IL32-β5-Integrin Paracrine Signaling. Adv. Sci. 2024, 11, e2405130. [Google Scholar] [CrossRef]
- Liu, X.C.; Lian, W.; Zhang, L.J.; Feng, X.C.; Gao, Y.; Li, S.X.; Liu, C.; Cheng, Y.; Yang, L.; Wang, X.J.; et al. Interleukin-18 Down-Regulates Multidrug Resistance-Associated Protein 2 Expression through Farnesoid X Receptor Associated with Nuclear Factor Kappa B and Yin Yang 1 in Human Hepatoma HepG2 Cells. PLoS ONE 2015, 10, e0136215. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Lu, J.; Mao, W.; Zheng, S.; Zhang, M.; Wu, T.; Chen, Y.; Lu, K.; Chu, C.; et al. YY1 enhances HIF-1α stability in tumor-associated macrophages to suppress anti-tumor immunity of prostate cancer in mice. Nat. Commun. 2025, 16, 6261. [Google Scholar] [CrossRef]
- Hwang, S.S.; Jang, S.W.; Kim, M.K.; Kim, L.K.; Kim, B.S.; Kim, H.S.; Kim, K.; Lee, W.; Flavell, R.A.; Lee, G.R. YY1 inhibits differentiation and function of regulatory T cells by blocking Foxp3 expression and activity. Nat. Commun. 2016, 7, 10789. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.; Qin, R.; Wang, X.; An, H.; Wang, Y.; Zhu, Y.; Liu, Y.; Cai, S.; Chen, S.; et al. Corrigendum: YY1 Promotes Endothelial Cell-Dependent Tumor Angiogenesis in Hepatocellular Carcinoma by Transcriptionally Activating VEGFA. Front. Oncol. 2021, 11, 828861. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, S.; Peng, L.; Li, H.; Zhao, Y.; Hu, Y. The hypoxia-mimetic agent CoCl2 induces chemotherapy resistance in LOVO colorectal cancer cells. Mol. Med. Rep. 2016, 13, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Kosasih, F.R.; Bonavida, B. Involvement of Yin Yang 1 (YY1) Expression in T-Cell Subsets Differentiation and Their Functions: Implications in T Cell-Mediated Diseases. Crit. Rev. Immunol. 2019, 39, 491–510. [Google Scholar] [CrossRef]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009, 69, 5820–5828. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, H.; Ren, G. Epithelial-mesenchymal transition and drug resistance in breast cancer (Review). Int. J. Oncol. 2015, 47, 840–848. [Google Scholar] [CrossRef]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Guan, T.; Li, M.; Song, Y.; Chen, J.; Tang, J.; Zhang, C.; Wen, Y.; Yang, X.; Huang, L.; Zhu, Y.; et al. Phosphorylation of USP29 by CDK1 Governs TWIST1 Stability and Oncogenic Functions. Adv. Sci. 2023, 10, e2205873. [Google Scholar] [CrossRef]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef]
- Debaugnies, M.; Rodríguez-Acebes, S.; Blondeau, J.; Parent, M.A.; Zocco, M.; Song, Y.; de Maertelaer, V.; Moers, V.; Latil, M.; Dubois, C.; et al. RHOJ controls EMT-associated resistance to chemotherapy. Nature 2023, 616, 168–175. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Bonavida, B.; Baritaki, S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide 2011, 24, 1–7. [Google Scholar] [CrossRef]
- Cho, A.A.; Bonavida, B. Targeting the Overexpressed YY1 in Cancer Inhibits EMT and Metastasis. Crit. Rev. Oncog. 2017, 22, 49–61. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Hua, Y.; Zhao, Q.; Wang, K.; Zhen, L.; Wang, G.; Lü, J.; Luo, A.; Cho, W.C.; et al. YY1 mediates TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial cells. Respir. Res. 2019, 20, 249. [Google Scholar] [CrossRef]
- Xuan, W.; Zhou, C.; You, G. LncRNA LINC00668 promotes cell proliferation, migration, invasion ability and EMT process in hepatocellular carcinoma by targeting miR-532-5p/YY1 axis. Biosci. Rep. 2020, 40, BSR20192697. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Huang, Z.; Baba, T.; Lee, P.S.; Barnett, J.C.; Mori, S.; Chang, J.T.; Kuo, W.L.; Gusberg, A.H.; Whitaker, R.S.; et al. Yin yang 1 modulates taxane response in epithelial ovarian cancer. Mol. Cancer Res. 2009, 7, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, J.G.; Jia, X.Y.; Ke, Y.; Zhang, M.K.; Stieg, D.; Liu, W.J.; Liu, L.Z.; Wang, L.; Jiang, B.H. METTL3-mediated N6-methyladenosine modification and HDAC5/YY1 promote IFFO1 downregulation in tumor development and chemo-resistance. Cancer Lett. 2023, 553, 215971. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bang, S.; Jee, S.; Park, S.; Kim, Y.; Park, H.; Jang, K.; Paik, S.S. Loss of YY1 expression predicts unfavorable prognosis in stage III colorectal cancer. Indian J. Pathol. Microbiol. 2021, 64, S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, Z.; Zhao, C.; Yin, X.; Feng, L.; Wang, Z.; Liu, C.; Li, B. YY1 modulates the radiosensitivity of esophageal squamous cell carcinoma through KIF3B-mediated Hippo signaling pathway. Cell Death Dis. 2023, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Dillen, A.; Bui, I.; Jung, M.; Agioti, S.; Zaravinos, A.; Bonavida, B. Regulation of PD-L1 Expression by YY1 in Cancer: Therapeutic Efficacy of Targeting YY1. Cancers 2024, 16, 1237. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Qin, S.Y.; Cheng, Y.J.; Lei, Q.; Zhang, A.Q.; Zhang, X.Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef]
- Lee, M.H.; Lahusen, T.; Wang, R.H.; Xiao, C.; Xu, X.; Hwang, Y.S.; He, W.W.; Shi, Y.; Deng, C.X. Yin Yang 1 positively regulates BRCA1 and inhibits mammary cancer formation. Oncogene 2012, 31, 116–127. [Google Scholar] [CrossRef]
- Hongo, F.; Garban, H.; Huerta-Yepez, S.; Vega, M.; Jazirehi, A.R.; Mizutani, Y.; Miki, T.; Bonavida, B. Inhibition of the transcription factor Yin Yang 1 activity by S-nitrosation. Biochem. Biophys. Res. Commun. 2005, 336, 692–701. [Google Scholar] [CrossRef]
- Huerta-Yepez, S.; Baritaki, S.; Baay-Guzman, G.; Hernandez-Luna, M.A.; Hernandez-Cueto, A.; Vega, M.I.; Bonavida, B. Contribution of either YY1 or BclXL-induced inhibition by the NO-donor DETANONOate in the reversal of drug resistance, both in vitro and in vivo. Nitric Oxide 2013, 29, 17–24. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef]
- Wang, G.; Xie, G.; Han, L.; Wang, D.; Du, F.; Kong, X.; Su, G. Involvement of hypoxia-inducible factor-1 alpha in the upregulation of P-glycoprotein in refractory epilepsy. Neuroreport 2019, 30, 1191–1196. [Google Scholar] [CrossRef]
- Wartenberg, M.; Ling, F.C.; Müschen, M.; Klein, F.; Acker, H.; Gassmann, M.; Petrat, K.; Pütz, V.; Hescheler, J.; Sauer, H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003, 17, 503–505. [Google Scholar] [CrossRef]
- Panneerpandian, P.; Rao, D.B.; Ganesan, K. Calcium channel blockers lercanidipine and amlodipine inhibit YY1/ERK/TGF-β mediated transcription and sensitize the gastric cancer cells to doxorubicin. Toxicol. Vitr. 2021, 74, 105152. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Paniagua, M.A.; Vega, M.I.; Huerta-Yepez, S.; Baritaki, S.; Vega, G.G.; Hariharan, K.; Bonavida, B. Galiximab signals B-NHL cells and inhibits the activities of NF-κB-induced YY1- and snail-resistant factors: Mechanism of sensitization to apoptosis by chemoimmunotherapeutic drugs. Mol. Cancer Ther. 2012, 11, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xiang, T.; Liu, S.; Xiang, W. Inhibition of NLRC5 attenuates the malignant growth and enhances the sensitivity of gastric cancer cells to 5-FU chemotherapy by blocking the carcinogenic effect of YY1. Exp. Ther. Med. 2022, 24, 601. [Google Scholar] [CrossRef]
- Mazor, G.; Smirnov, D.; Ben David, H.; Khrameeva, E.; Toiber, D.; Rotblat, B. TP73-AS1 is induced by YY1 during TMZ treatment and highly expressed in the aging brain. Aging 2021, 13, 14843–14861. [Google Scholar] [CrossRef]
- Vega, M.I.; Jazirehi, A.R.; Huerta-Yepez, S.; Bonavida, B. Rituximab-induced inhibition of YY1 and Bcl-xL expression in Ramos non-Hodgkin’s lymphoma cell line via inhibition of NF-kappa B activity: Role of YY1 and Bcl-xL in Fas resistance and chemoresistance, respectively. J. Immunol. 2005, 175, 2174–2183. [Google Scholar] [CrossRef]
- Vega, M.I.; Baritaki, S.; Huerta-Yepez, S.; Martinez-Paniagua, M.A.; Bonavida, B. A potential mechanism of rituximab-induced inhibition of tumor growth through its sensitization to tumor necrosis factor-related apoptosis-inducing ligand-expressing host cytotoxic cells. Leuk. Lymphoma 2011, 52, 108–121. [Google Scholar] [CrossRef]
- Sieber, S.; Gdynia, G.; Roth, W.; Bonavida, B.; Efferth, T. Combination treatment of malignant B cells using the anti-CD20 antibody rituximab and the anti-malarial artesunate. Int. J. Oncol. 2009, 35, 149–158. [Google Scholar] [CrossRef][Green Version]
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021, 121, 3297–3351. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Zhao, J.L.; Huang, F.; He, F.; Gao, C.C.; Liang, S.Q.; Ma, P.F.; Dong, G.Y.; Han, H.; Qin, H.Y. Forced Activation of Notch in Macrophages Represses Tumor Growth by Upregulating miR-125a and Disabling Tumor-Associated Macrophages. Cancer Res. 2016, 76, 1403–1415. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, L.; Zhou, F.M.; Liu, S.W.; Wang, W.; Zhao, E.J.; Yao, Q.J.; Li, W.; Zhao, Y.Q.; Shi, Z.; et al. Elevated NOX4 promotes tumorigenesis and acquired EGFR-TKIs resistance via enhancing IL-8/PD-L1 signaling in NSCLC. Drug Resist. Updat. 2023, 70, 100987. [Google Scholar] [CrossRef]
- Hariharan, K.; Chu, P.; Murphy, T.; Clanton, D.; Berquist, L.; Molina, A.; Ho, S.N.; Vega, M.I.; Bonavida, B. Galiximab (anti-CD80)-induced growth inhibition and prolongation of survival in vivo of B-NHL tumor xenografts and potentiation by the combination with fludarabine. Int. J. Oncol. 2013, 43, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.; Romero-Cordoba, S.; Plantamura, I.; Cosentino, G.; Hidalgo-Miranda, A.; Tagliabue, E.; Iorio, M.V. MiR-302b as a Combinatorial Therapeutic Approach to Improve Cisplatin Chemotherapy Efficacy in Human Triple-Negative Breast Cancer. Cancers 2020, 12, 2261. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Della Pietra, E.; Bonavida, B. Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol. 2015, 6, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Pietra, E.D.; Bonavida, B.; Xodo, L.E. The Role Of Nitric Oxide After Repeated Low Dose Photodynamic Treatments In Prostate Carcinoma Cells. Redox Biol. 2015, 5, 422–423. [Google Scholar] [CrossRef]
- Ning, Y.; Zheng, H.; Yang, Y.; Zang, H.; Wang, W.; Zhan, Y.; Wang, H.; Luo, J.; Wen, Q.; Peng, J.; et al. YAP1 synergize with YY1 transcriptional co-repress DUSP1 to induce osimertinib resistant by activating the EGFR/MAPK pathway and abrogating autophagy in non-small cell lung cancer. Int. J. Biol. Sci. 2023, 19, 2458–2474. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Huang, C.; Li, Y.; Zhao, H.; Kasim, V. Yin Yang 1 promotes the Warburg effect and tumorigenesis via glucose transporter GLUT3. Cancer Sci. 2018, 109, 2423–2434. [Google Scholar] [CrossRef]
- Gu, J.; Mu, N.; Jia, B.; Guo, Q.; Pan, L.; Zhu, M.; Zhang, W.; Zhang, K.; Li, W.; Li, M.; et al. Targeting radiation-tolerant persister cells as a strategy for inhibiting radioresistance and recurrence in glioblastoma. Neuro Oncol. 2022, 24, 1056–1070. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, S.; Yang, Z.; Meng, M.; Dai, Y.; Li, X.; Chen, X. MicroRNA-411-3p motivates methotrexate’s cellular uptake and cytotoxicity via targeting Yin-yang 1 in leukemia cells. Acta Biochim. Pol. 2023, 70, 721–727. [Google Scholar] [CrossRef]
- Morales-Martinez, M.; Vega, G.G.; Neri, N.; Nambo, M.J.; Alvarado, I.; Cuadra, I.; Duran-Padilla, M.A.; Huerta-Yepez, S.; Vega, M.I. MicroRNA-7 Regulates Migration and Chemoresistance in Non-Hodgkin Lymphoma Cells Through Regulation of KLF4 and YY1. Front. Oncol. 2020, 10, 588893. [Google Scholar] [CrossRef]
- Rapozzi, V.; Varchi, G.; Della Pietra, E.; Ferroni, C.; Xodo, L.E. A photodynamic bifunctional conjugate for prostate cancer: An in vitro mechanistic study. Investig. New Drugs 2017, 35, 115–123. [Google Scholar] [CrossRef]
- Qin, L.N.; Zhang, H.; Li, Q.Q.; Wu, T.; Cheng, S.B.; Wang, K.W.; Shi, Y.; Ren, H.R.; Xing, X.W.; Yang, C.; et al. Vitamin D binding protein (VDBP) hijacks twist1 to inhibit vasculogenic mimicry in hepatocellular carcinoma. Theranostics 2024, 14, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Montecillo-Aguado, M.; Morales-Martínez, M.; Huerta-Yepez, S.; Vega, M.I. KLF4 inhibition by Kenpaullone induces cytotoxicity and chemo sensitization in B-NHL cell lines via YY1 independent. Leuk. Lymphoma 2021, 62, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Sarvagalla, S.; Kolapalli, S.P.; Vallabhapurapu, S. The Two Sides of YY1 in Cancer: A Friend and a Foe. Front. Oncol. 2019, 9, 1230. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, W.J.; Jia, Y.X.; Yuan, H.; Wu, P.F.; Ge, W.L.; Meng, L.D.; Huang, X.M.; Shen, P.; Yang, T.Y.; et al. Effect of the transcription factor YY1 on the development of pancreatic endocrine and exocrine tumors: A narrative review. Cell Biosci. 2021, 11, 86. [Google Scholar] [CrossRef]
- Hackeng, W.M.; Brosens, L.A.A.; Dreijerink, K.M.A. Aggressive versus indolent insulinomas: New clinicopathological insights. Endocr. Relat. Cancer 2023, 30, e220321. [Google Scholar] [CrossRef]
- Kumari, S.; Ponamgi, S.P.D.; Chelikani, P.; Srilatha, M.; Nagaraju, G.P.; Peela, S. Role of Yin Yang 1 (YY1) on microenvironment, signaling pathways, and epigenetics in lung cancer. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189359. [Google Scholar] [CrossRef]
- Son, H.J.; Choi, E.J.; Yoo, N.J.; Lee, S.H. Somatic Mutations and Intratumoral Heterogeneity of Cancer-Related Genes NLK, YY1 and PA2G4 in Gastric and Colorectal Cancers. Pathol. Oncol. Res. 2020, 26, 2813–2815. [Google Scholar] [CrossRef]
- Song, Y.L.; Xu, J.; Zhao, D.C.; Zhang, T.P.; Jin, K.Z.; Zhu, L.M.; Yu, S.; Chen, Y.J. Mutation and Expression of Gene YY1 in Pancreatic Neuroendocrine Tumors and Its Clinical Significance. Endocr. Pract. 2021, 27, 874–880. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).