Changes in Epithelial Cell Polarity and Adhesion Guide Human Endometrial Receptivity: How In Vitro Systems Help to Untangle Mechanistic Details
Abstract
1. Introduction
2. Part I: In Vivo Observations
2.1 Dynamic Changes of the Endometrial Epithelium Occur During the Menstrual Cycle
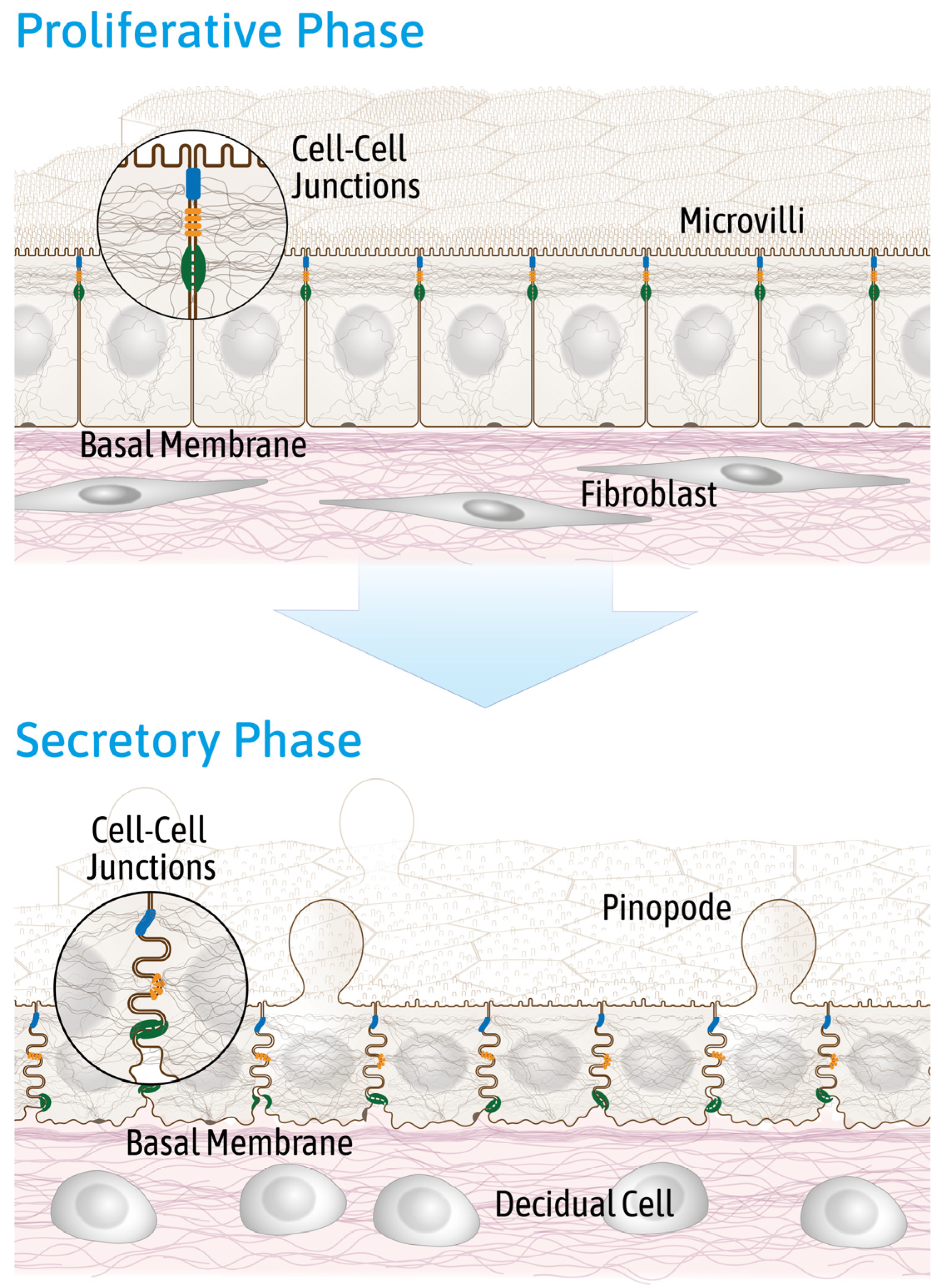
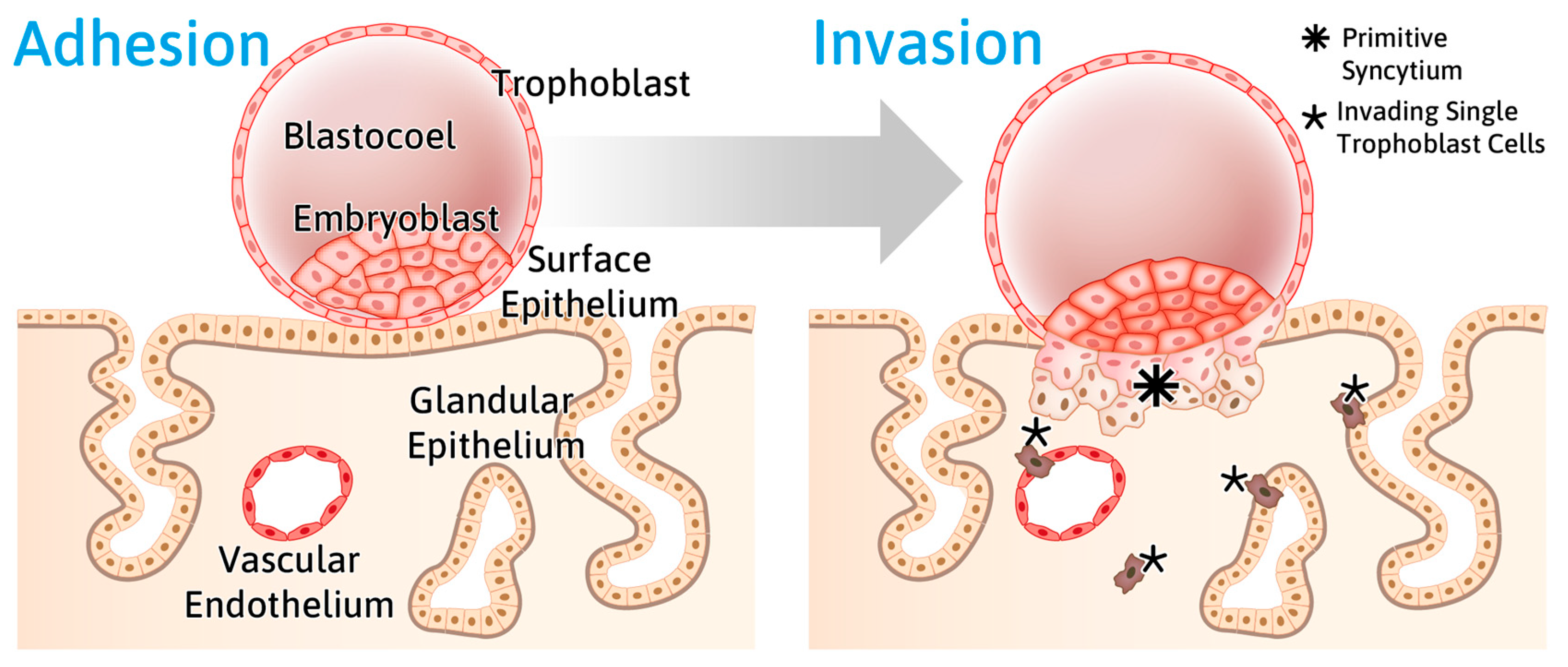
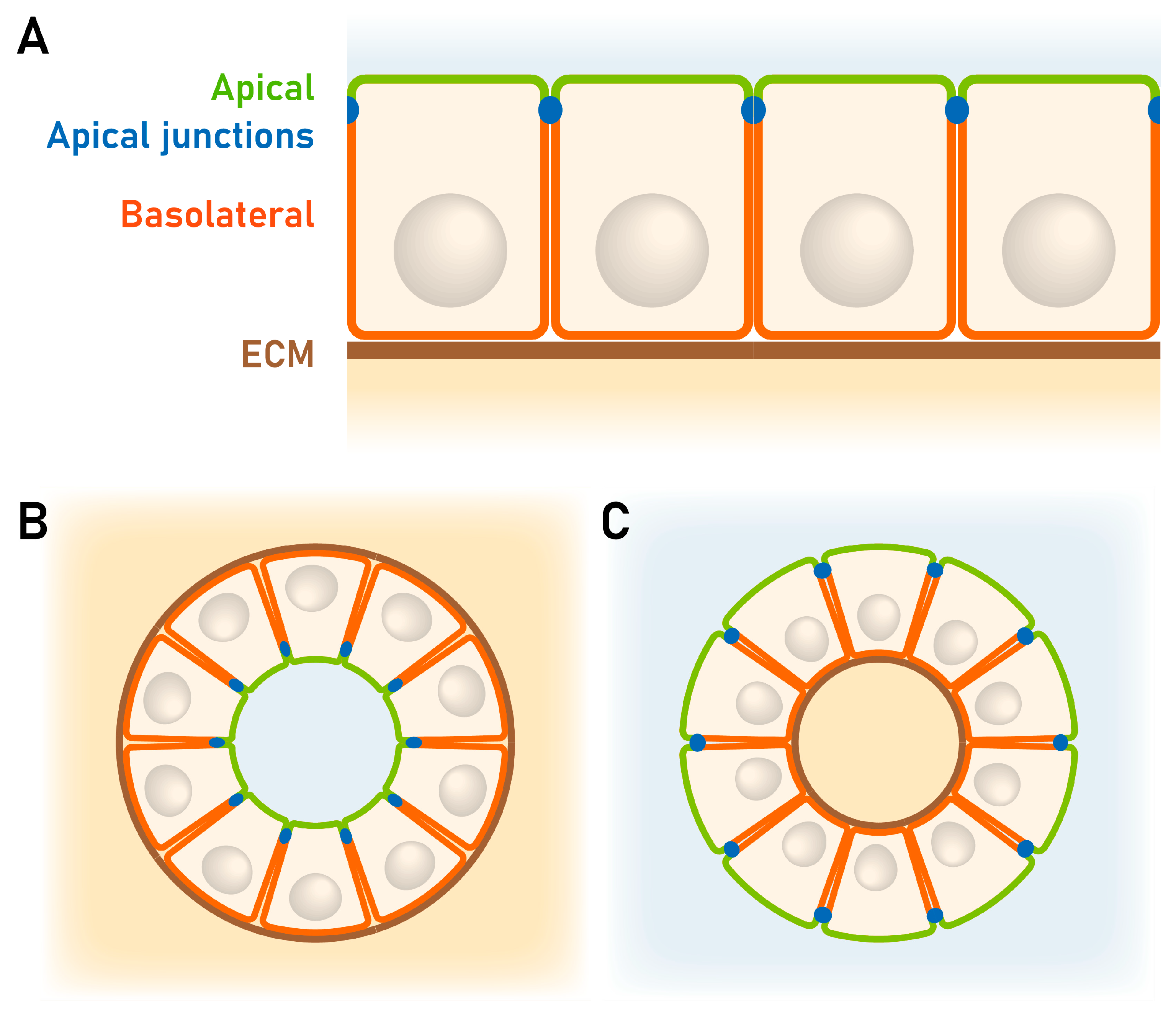
2.2. Reduced Endometrial Epithelial Cell Polarity Favors Receptivity
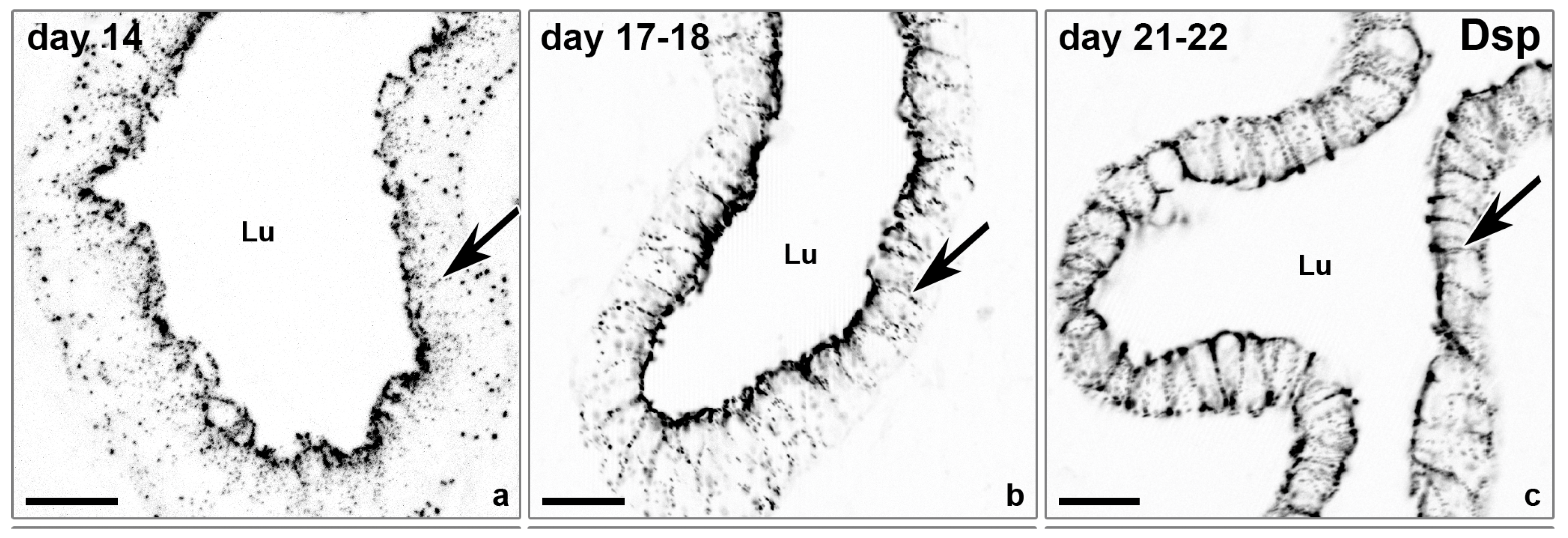
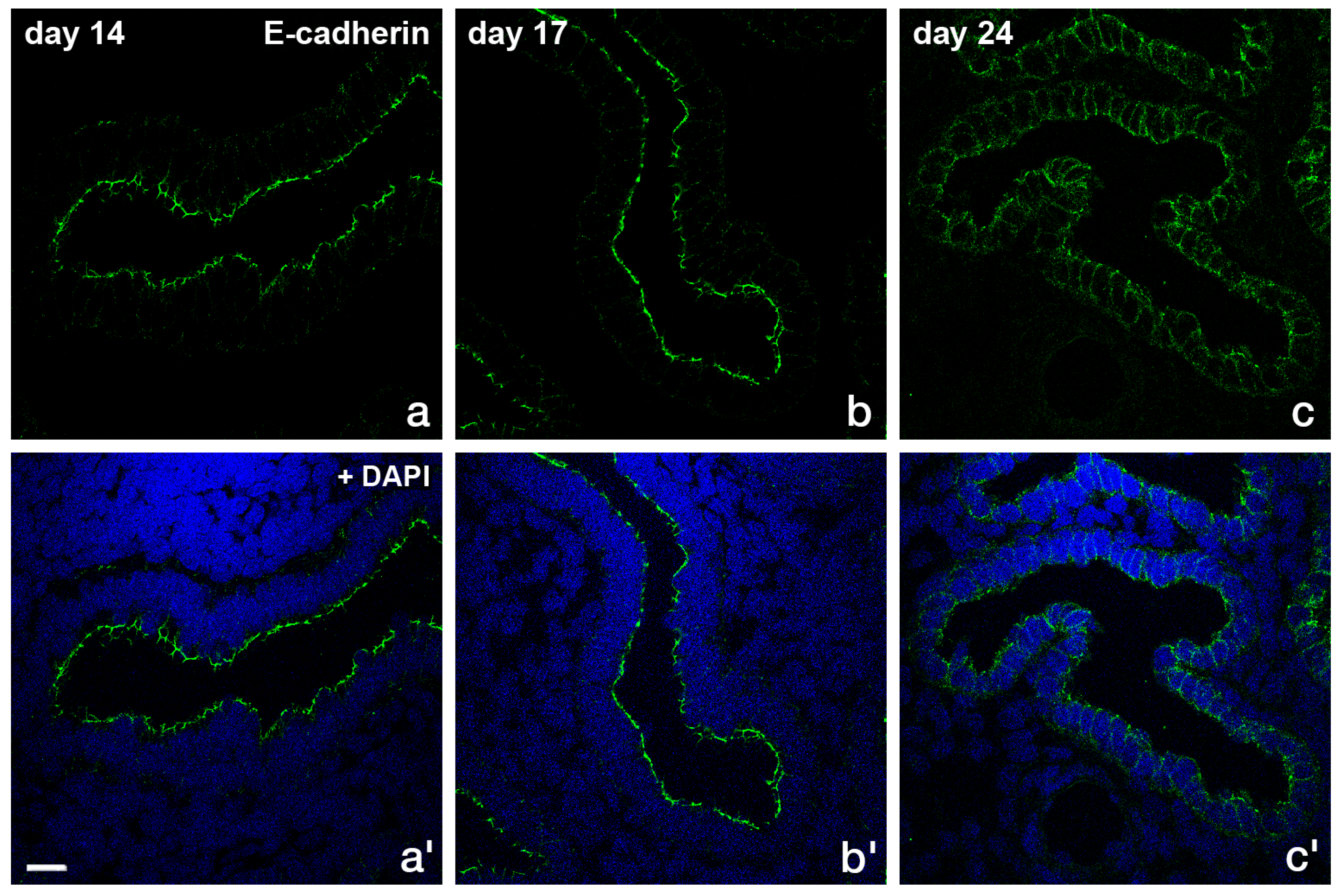
2.3 Rearrangement of Adhesion Complexes Occurs in the Endometrial Epithelium During Acquisition of the Receptive State
3. Part II: In Vitro Observations
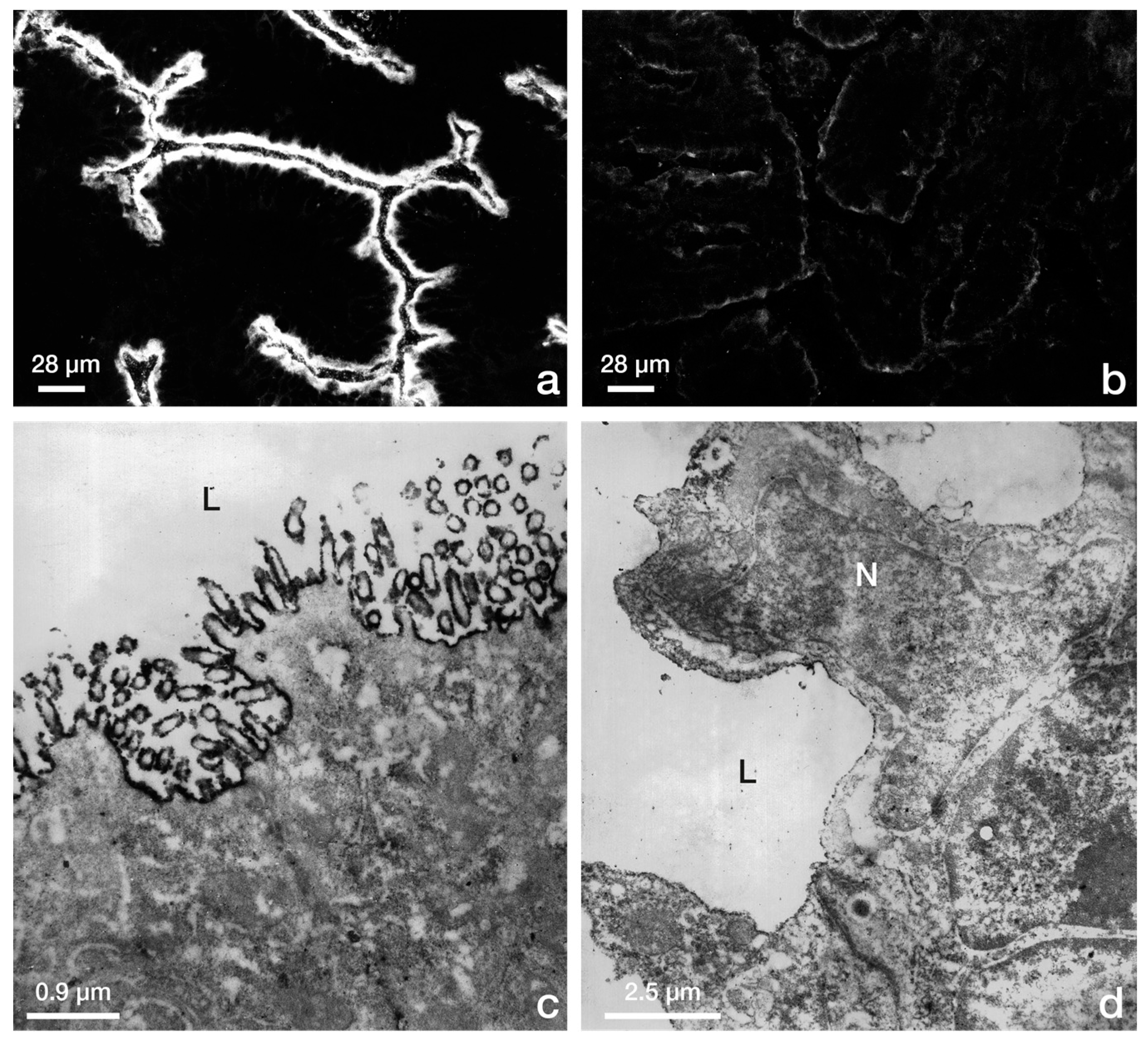
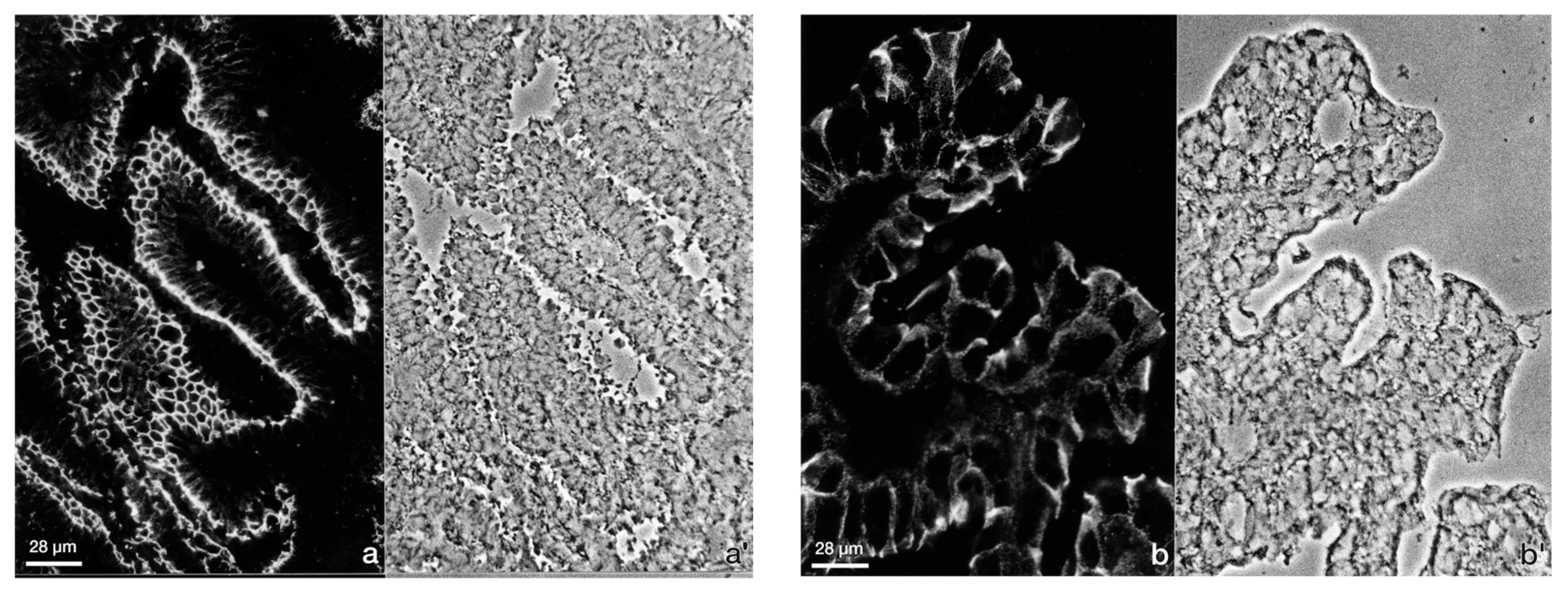
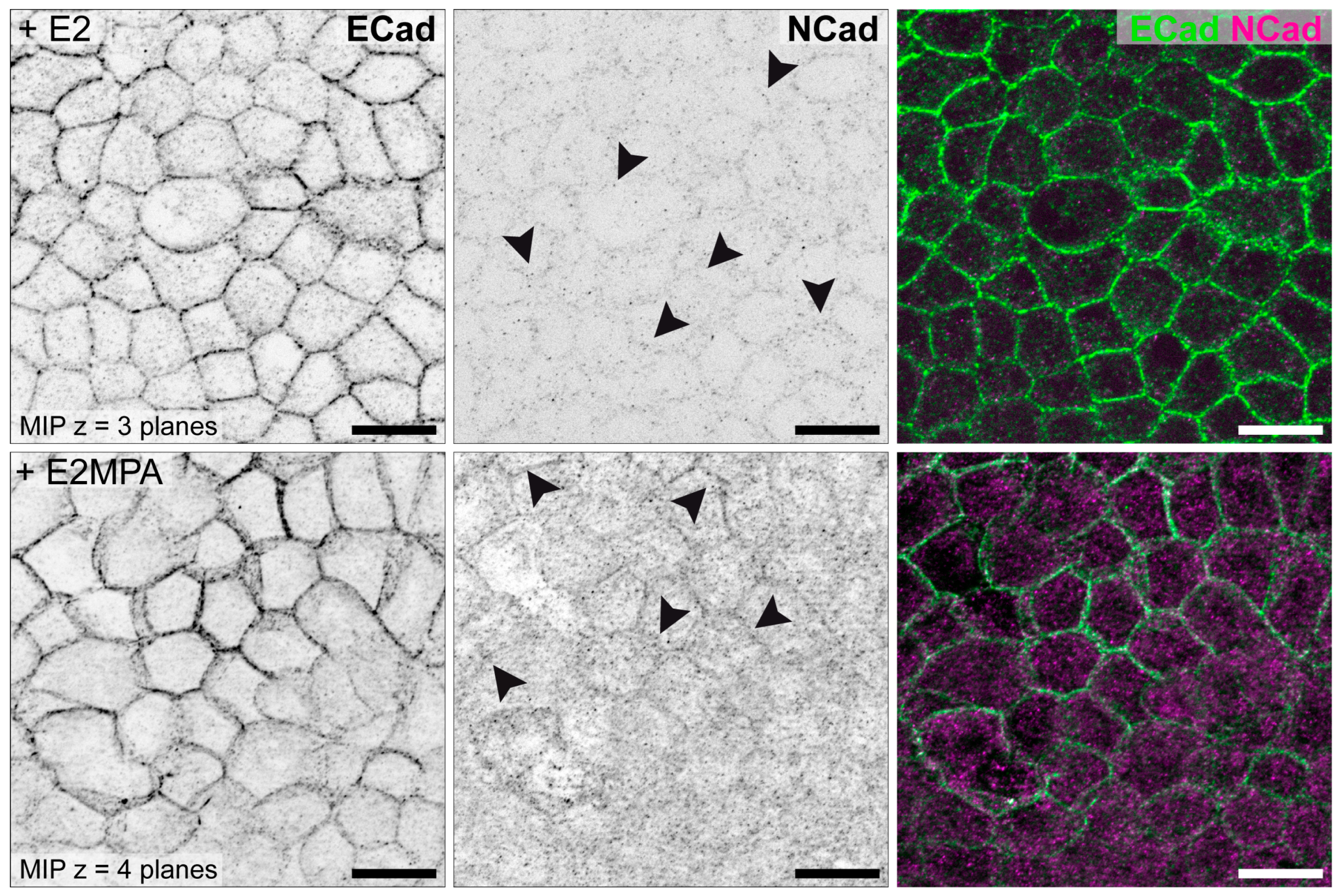
3.1. The Degree of Polarization and Adhesion Differs Between Human Endometrial Epithelial Cell Lines
3.1.1. Studies with Monolayers
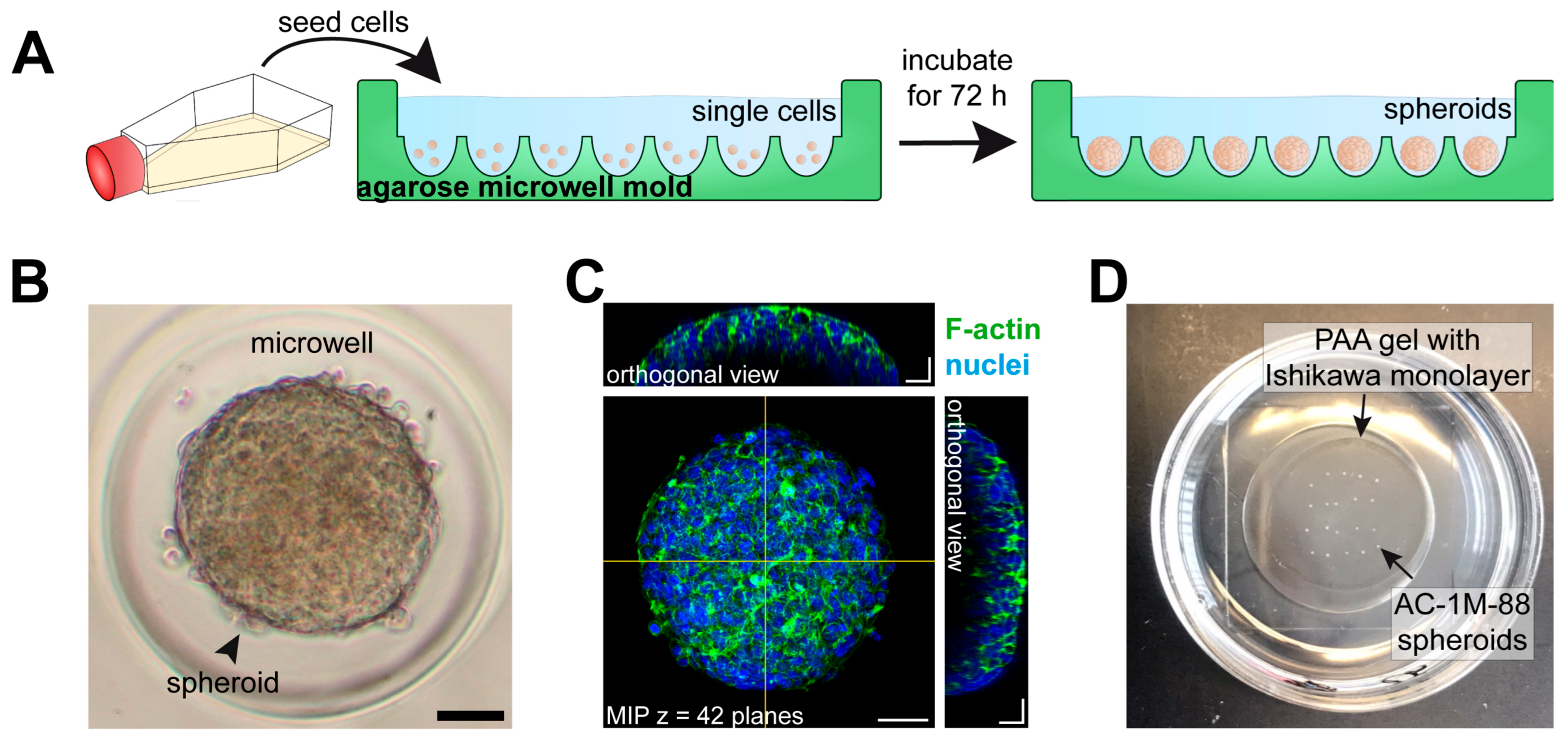
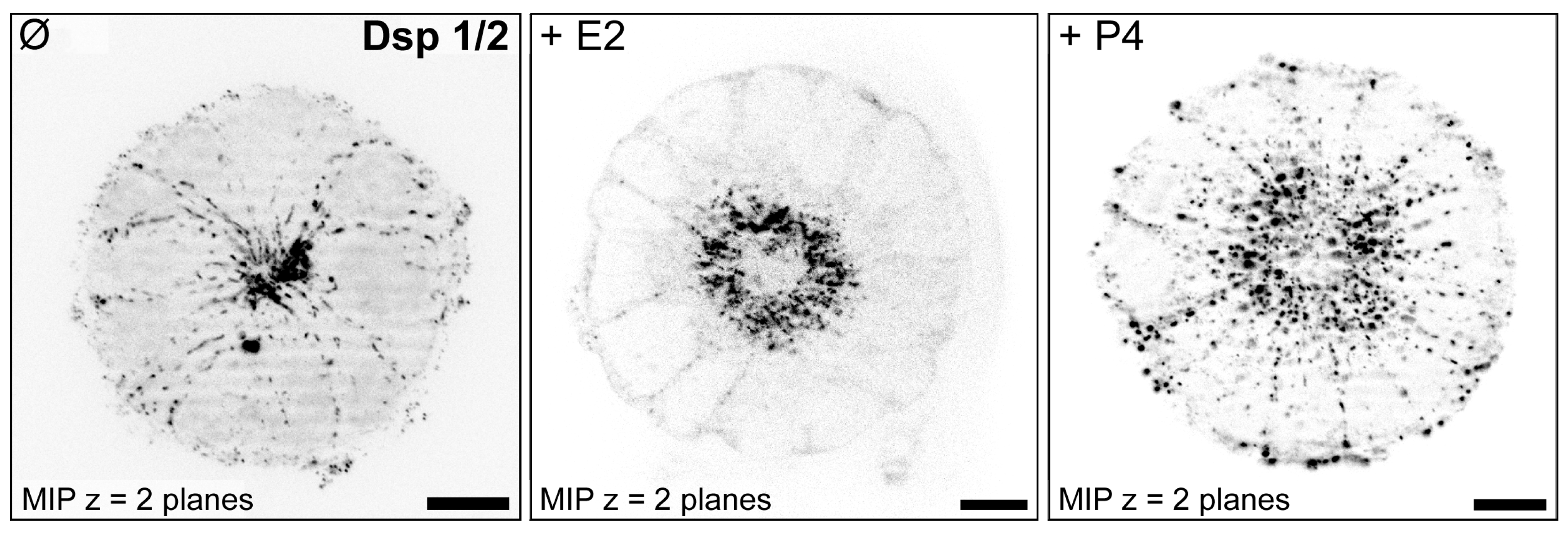
3.1.2. Studies with 3D Endometrial Epithelial Spheroids
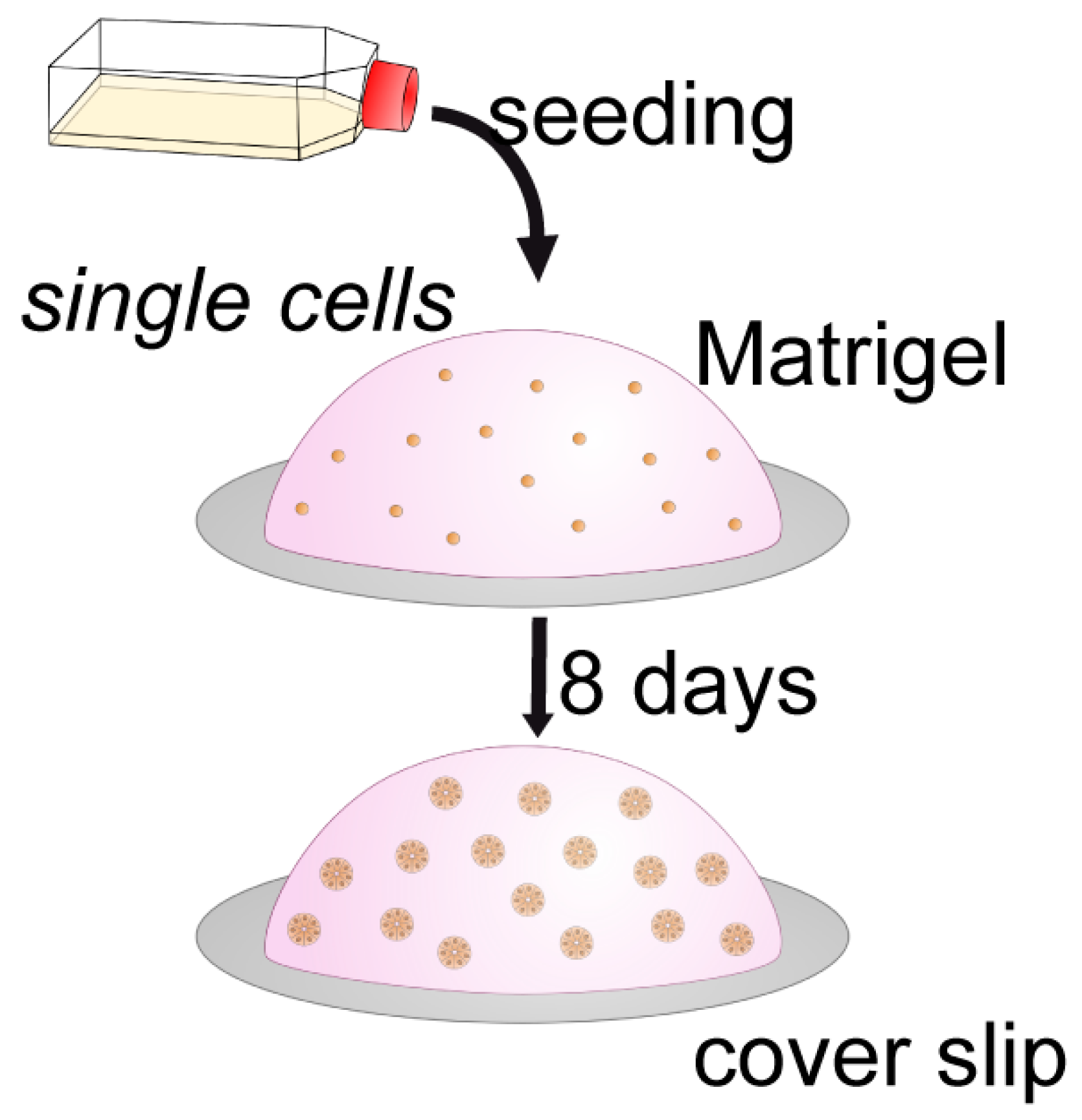
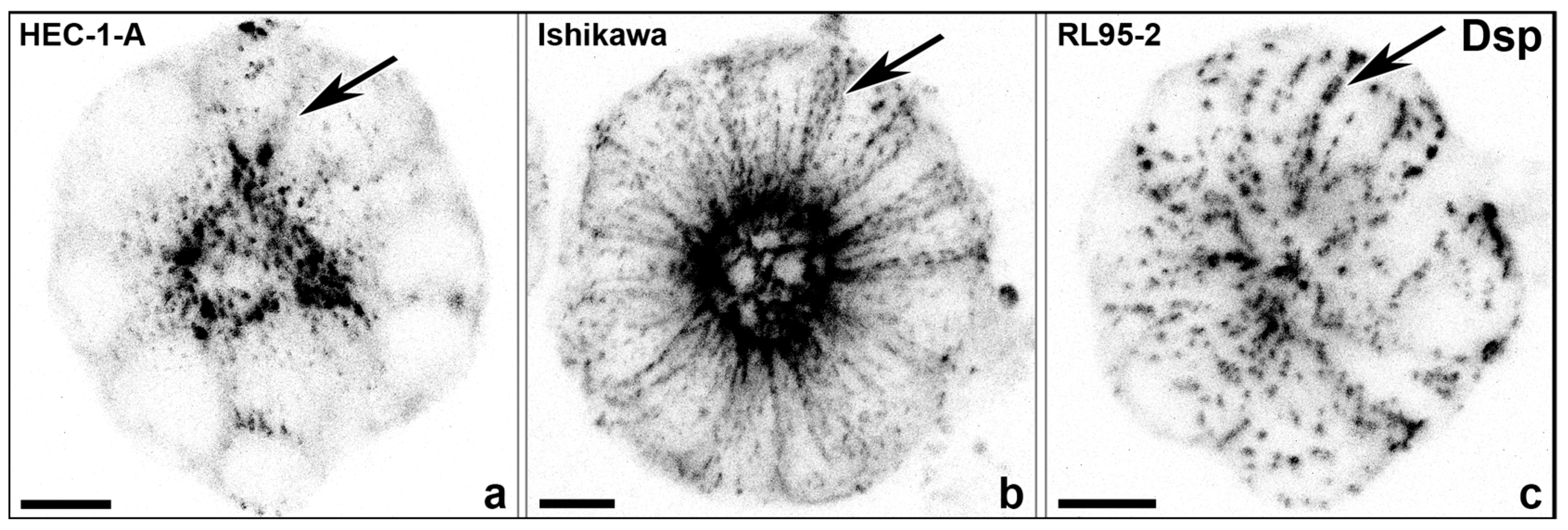

3.2. Hormone Responses Can Be Studied in 3D Endometrial Epithelial Spheroids
3.3. Endometrial Receptivity Can Be Examined in Confrontation Assays with Trophoblasts
3.3.1. Confrontation of Endometrial Epithelial Spheroids with Trophoblast Cells from the Basal Cell Pole
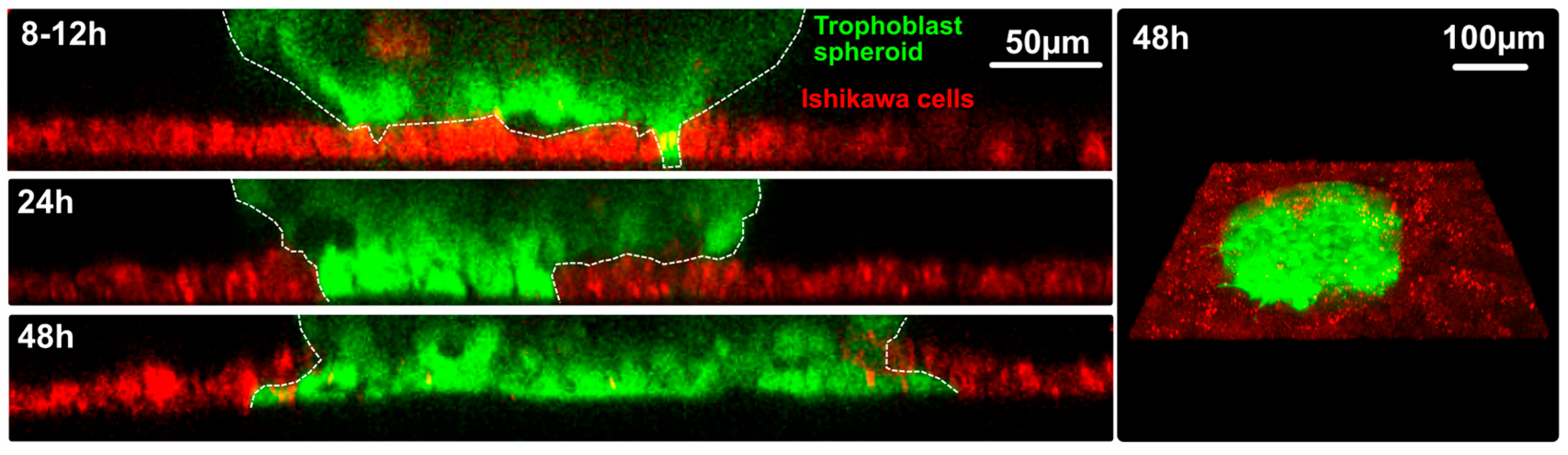
3.3.2 Confrontation of Trophoblast Spheroids with the Apical Cell Pole of Ishikawa Cell Monolayers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, W. Mother or nothing: The agony of infertility. Bull. World Health Organ. 2010, 88, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.K. Sir Robert Edwards: From the science of human conception to the reality of IVF birth. Reprod. Biomed. Online 2024, 49, 104478. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.J.; Giudice, L.C. Retrospective. Robert G. Edwards (1925–2013). Science 2013, 340, 825. [Google Scholar] [CrossRef]
- Macklon, N.S.; Brosens, J.J. The human endometrium as a sensor of embryo quality. Biol. Reprod. 2014, 91, 98. [Google Scholar] [CrossRef]
- Macklon, N.S.; Geraedts, J.P.; Fauser, B.C. Conception to ongoing pregnancy: The ‘black box’ of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef]
- Evans, J.; Hannan, N.J.; Hincks, C.; Rombauts, L.J.; Salamonsen, L.A. Defective soil for a fertile seed? Altered endometrial development is detrimental to pregnancy success. PLoS ONE 2012, 7, e53098. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Morrison, L.; Longfellow, M.; Ritson, A.; Pace, D. Granulated lymphocytes in human endometrium: Histochemical and immunohistochemical studies. Hum. Reprod. 1991, 6, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Classen-Linke, I.; Alfer, J.; Hey, S.; Krusche, C.A.; Kusche, M.; Beier, H.M. Marker molecules of human endometrial differentiation can be hormonally regulated under in-vitro conditions as in-vivo. Hum. Reprod. Update 1998, 4, 539–549. [Google Scholar] [CrossRef]
- Beier, H.M.; Beier-Hellwig, K. Molecular and cellular aspects of endometrial receptivity. Hum. Reprod. Update 1998, 4, 448–458. [Google Scholar] [CrossRef]
- Nikas, G. Pinopodes as markers of endometrial receptivity in clinical practice. Hum. Reprod. 1999, 14 (Suppl. 2), 99–106. [Google Scholar] [CrossRef]
- Develioglu, O.H.; Hsiu, J.G.; Nikas, G.; Toner, J.P.; Oehninger, S.; Jones, H.W. Endometrial estrogen and progesterone receptor and pinopode expression in stimulated cycles of oocyte donors. Fertil. Steril. 1999, 71, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.E.; Matson, B.C.; Wetendorf, M.; Caron, K.M. Pinopodes: Recent advancements, current perspectives, and future directions. Mol. Cell Endocrinol. 2020, 501, 110644. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Reprint of: Dating the Endometrial Biopsy. Fertil. Steril. 2019, 112, e93–e115. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, M.R.; Kempson, R.L. Normal histology of the uterus and fallopian tubes. In Histology for Pathologists, 2nd ed.; Sternberg, S.S., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 1997. [Google Scholar]
- Heller, D.S. Handbook of Endometrial Pathology, 1st ed.; JP Medical Limited: London, UK, 2012. [Google Scholar]
- Aplin, J.D.; Charlton, A.K.; Ayad, S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988, 253, 231–240. [Google Scholar] [CrossRef]
- Tanaka, T.; Wang, C.; Umesaki, N. Remodeling of the human endometrial epithelium is regulated by laminin and type IV collagen. Int. J. Mol. Med. 2009, 23, 173–180. [Google Scholar] [CrossRef]
- Sarani, S.A.; Ghaffari-Novin, M.; Warren, M.A.; Dockery, P.; Cooke, I.D. Morphological evidence for the ‘implantation window’ in human luminal endometrium. Hum. Reprod. 1999, 14, 3101–3106. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Psychoyos, A. Uterine receptivity for nidation. Ann. N. Y. Acad. Sci. 1986, 476, 36–42. [Google Scholar] [CrossRef]
- Aplin, J.D. The cell biology of human implantation. Placenta 1996, 17, 269–275. [Google Scholar] [CrossRef]
- Bergh, P.A.; Navot, D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil. Steril. 1992, 58, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J. Adaptation of core mechanisms to generate cell polarity. Nature 2003, 422, 766–774. [Google Scholar] [CrossRef]
- Knust, E.; Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 2002, 298, 1955–1959. [Google Scholar] [CrossRef]
- Margolis, B.; Borg, J.-P. Apicobasal polarity complexes. J. Cell Sci. 2005, 118, 5157–5159. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, C.G.; de la Serna, E.L.; Dunn, A.R. How cells tell up from down and stick together to construct multicellular tissues—interplay between apicobasal polarity and cell-cell adhesion. J. Cell Sci. 2021, 134, 248757. [Google Scholar] [CrossRef]
- Whitby, S.; Salamonsen, L.A.; Evans, J. The Endometrial Polarity Paradox: Differential Regulation of Polarity Within Secretory-Phase Human Endometrium. Endocrinology 2018, 159, 506–518. [Google Scholar] [CrossRef]
- Denker, H.W. Implantation: A cell biological paradox. J. Exp. Zool. 1993, 266, 541–558. [Google Scholar] [CrossRef]
- Denker, H.-W. Trophblast-Endometrial Interactions at Embryo Implantation: A Cell Biological Paradox. In Trophoblast Invasion and Endometrial Receptivity; Denker, H.-W., Aplin, J.D., Eds.; Trophoblast Research; Plenum Medical Book Company: New York, NY, USA; London, UK, 1990; Volume 4, pp. 3–29. [Google Scholar] [CrossRef]
- Denker, H.W. Endometrial receptivity: Cell biological aspects of an unusual epithelium. A review. Ann. Anat. 1994, 176, 53–60. [Google Scholar] [CrossRef]
- Mak, L.L. Ultrastructural studies of amphibian neural fold fusion. Dev. Biol. 1978, 65, 435–446. [Google Scholar] [CrossRef]
- Eom, D.S.; Amarnath, S.; Agarwala, S. Apicobasal polarity and neural tube closure. Dev. Growth Differ. 2013, 55, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Agarwala, S. Cell-cycle-dependent TGFbeta-BMP antagonism regulates neural tube closure by modulating tight junctions. J. Cell Sci. 2017, 130, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, N.; Jaulin, F.; Peglion, F. Inverted apicobasal polarity in health and disease. J. Cell Sci. 2024, 137, 261659. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R. Uterine receptivity and the plasma membrane transformation. Cell Res. 2004, 14, 259–267. [Google Scholar] [CrossRef]
- Hay, E.D. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995, 154, 8–20. [Google Scholar] [CrossRef]
- Whitby, S.; Zhou, W.; Dimitriadis, E. Alterations in Epithelial Cell Polarity During Endometrial Receptivity: A Systematic Review. Front. Endocrinol. 2020, 11, 596324. [Google Scholar] [CrossRef]
- Classen-Linke, I.; Denker, H.W.; Winterhager, E. Apical plasma membrane-bound enzymes of rabbit uterine epithelium. Pattern Changes During the Periimplantation phase. Histochemistry 1987, 87, 517–529. [Google Scholar] [CrossRef]
- Classen-Linke, I.; Denker, H.-W. Preparation of rabbit uterine epithelium for trophoblast attachment: Histochemical changes in the apical and lateral membrane compartment. In Trophoblast Invasion and Endometrial Receptivity; Denker, H.W., Aplin, J.D., Eds.; Trophoblast Research; Plenum Medical Book Company: New York, NY, USA, 1990; Volume 4, pp. 307–322. [Google Scholar] [CrossRef]
- Murphy, C.R.; Shaw, T.J. Plasma membrane transformation: A common response of uterine epithelial cells during the peri-implantation period. Cell Biol. Int. 1994, 18, 1115–1128. [Google Scholar] [CrossRef]
- Murphy, C.R. The cytoskeleton of uterine epithelial cells: A new player in uterine receptivity and the plasma membrane transformation. Hum. Reprod. Update 1995, 1, 567–580. [Google Scholar] [CrossRef]
- Yoshihara, M.; Mizutani, S.; Kato, Y.; Matsumoto, K.; Mizutani, E.; Mizutani, H.; Fujimoto, H.; Osuka, S.; Kajiyama, H. Recent Insights into Human Endometrial Peptidases in Blastocyst Implantation via Shedding of Microvesicles. Int. J. Mol. Sci. 2021, 22, 13479. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J. Remodeling epithelial cell organization: Transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a000513. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Holthofer, B.; Windoffer, R.; Troyanovsky, S.; Leube, R.E. Structure and function of desmosomes. Int. Rev. Cytol. 2007, 264, 65–163. [Google Scholar] [CrossRef]
- Lessey, L.R.; Robinson, S.C.; Chaudhary, R.; Daniel, J.M. Adherens junction proteins on the move-From the membrane to the nucleus in intestinal diseases. Front. Cell Dev. Biol. 2022, 10, 998373. [Google Scholar] [CrossRef]
- Illingworth, I.M.; Kiszka, I.; Bagley, S.; Ireland, G.W.; Garrod, D.R.; Kimber, S.J. Desmosomes are reduced in the mouse uterine luminal epithelium during the preimplantation period of pregnancy: A mechanism for facilitation of implantation. Biol. Reprod. 2000, 63, 1764–1773. [Google Scholar] [CrossRef]
- Preston, A.M.; Lindsay, L.A.; Murphy, C.R. Progesterone treatment and the progress of early pregnancy reduce desmoglein 1&2 staining along the lateral plasma membrane in rat uterine epithelial cells. Acta Histochem. 2004, 106, 345–351. [Google Scholar] [CrossRef]
- Preston, A.M.; Lindsay, L.A.; Murphy, C.R. Desmosomes in uterine epithelial cells decrease at the time of implantation: An ultrastructural and morphometric study. J. Morphol. 2006, 267, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Schlafke, S.; Enders, A.C. Cellular basis of interaction between trophoblast and uterus at implantation. Biol. Reprod. 1975, 12, 41–65. [Google Scholar] [CrossRef]
- Enders, A.C.; Schlafke, S. Implantation in the ferret: Epithelial penetration. Am. J. Anat. 1972, 133, 291–315. [Google Scholar] [CrossRef]
- Bentin-Ley, U.; Horn, T.; Sjogren, A.; Sorensen, S.; Falck Larsen, J.; Hamberger, L. Ultrastructure of human blastocyst-endometrial interactions in vitro. J. Reprod. Fertil. 2000, 120, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hertig, A.T.; Rock, J.; Adams, E.C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 1956, 98, 435–493. [Google Scholar] [CrossRef]
- O’Rahilly, R.; Müller, F. Developmental stages in human embryos: Revised and new measurements. Cells Tissues Organs 2010, 192, 73–84. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, R.; Müller, F. Developmental Stages in Human Embryos, Including a Revision of Streeter’s ‘Horizons’ and a Survey of the Carnegie Collection; Carnegie Institution of Washington: Washington, WA, USA, 1987; Available online: https://publicationsonline.carnegiescience.edu/publications_online/developmental_stages.pdf (accessed on 27 April 2025).
- Aplin, J.D.; Ruane, P.T. Embryo-epithelium interactions during implantation at a glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Buck, V.U.; Windoffer, R.; Leube, R.E.; Classen-Linke, I. Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem. Cell Biol. 2012, 137, 777–790. [Google Scholar] [CrossRef]
- Singh, H.; Aplin, J.D. Endometrial apical glycoproteomic analysis reveals roles for cadherin 6, desmoglein-2 and plexin b2 in epithelial integrity. Mol. Hum. Reprod. 2015, 21, 81–94. [Google Scholar] [CrossRef]
- Murphy, C.R.; Swift, J.G.; Need, J.A.; Mukherjee, T.M.; Rogers, A.W. A freeze-fracture electron microscopic study of tight junctions of epithelial cells in the human uterus. Anat. Embryol. 1982, 163, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Rogers, P.A.; Hosie, M.J.; Leeton, J.; Beaton, L. Tight junctions of human uterine epithelial cells change during the menstrual cycle: A morphometric study. Acta Anat. 1992, 144, 36–38. [Google Scholar] [CrossRef]
- Grund, S.; Grummer, R. Direct Cell(-)Cell Interactions in the Endometrium and in Endometrial Pathophysiology. Int. J. Mol. Sci. 2018, 19, 2227. [Google Scholar] [CrossRef]
- Grund, S.C.; Wu, X.X.; Muller, D.; Wennemuth, G.; Grummer, R. Impact of endometrial claudin-3 deletion on murine implantation, decidualization, and embryo development. Biol. Reprod. 2022, 107, 984–997. [Google Scholar] [CrossRef]
- Schumann, S.; Buck, V.U.; Classen-Linke, I.; Wennemuth, G.; Grummer, R. Claudin-3, claudin-7, and claudin-10 show different distribution patterns during decidualization and trophoblast invasion in mouse and human. Histochem. Cell Biol. 2015, 144, 571–585. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xia, X.; Gao, L.; Gao, C.; Zhou, J.; Yan, Z.; Cui, Y.; Ma, X.; Kwak-Kim, J.Y.H.; et al. Hyperlipidemia negatively impacts implantation by dysregulating tight junction and Claudin-3 and Claudin-4 expression in the endometrium. J. Reprod. Immunol. 2024, 166, 104326. [Google Scholar] [CrossRef]
- Matsuyama, S.; Whiteside, S.; Li, S.Y. Implantation and Decidualization in PCOS: Unraveling the Complexities of Pregnancy. Int. J. Mol. Sci. 2024, 25, 1203. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.H.; Giribabu, N.; Salleh, N. Testosterone Reduces Tight Junction Complexity and Down-regulates Expression of Claudin-4 and Occludin in the Endometrium in Ovariectomized, Sex-steroid Replacement Rats. In Vivo 2020, 34, 225–231. [Google Scholar] [CrossRef]
- Mokhtar, M.H.; Giribabu, N.; Salleh, N. Testosterone Decreases the Number of Implanting Embryos, Expression of Pinopode and L-selectin Ligand (MECA-79) in the Endometrium of Early Pregnant Rats. Int. J. Environ. Res. Public Health 2020, 17, 2293. [Google Scholar] [CrossRef] [PubMed]
- Thie, M.; Harrach-Ruprecht, B.; Sauer, H.; Fuchs, P.; Albers, A.; Denker, H.W. Cell adhesion to the apical pole of epithelium: A function of cell polarity. Eur. J. Cell Biol. 1995, 66, 180–191. [Google Scholar]
- Thie, M.; Denker, H.W. Endometrial Receptivity for Trophoblast Attachment: Model Studies Using Cell Lines. In Microscopy for Reproduction and Development: A Dynamic Approach; Motta, P.M., Editore, A.D., Eds.; Rome, Italy, 1997; pp. 241–249. [Google Scholar]
- John, N.J.; Linke, M.; Denker, H.-W. Quantitation of human choriocarcinoma spheroid attachment to uterine epithelial cell monolayers. Vitr. Cell. Dev. Biol.-Anim. 1993, 29, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for study of human embryo implantation: Choice of cell lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Kuramoto, H.; Tamura, S.; Notake, Y. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am. J. Obs. Gynecol. 1972, 114, 1012–1019. [Google Scholar] [CrossRef]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. [Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors]. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar]
- Nishida, M. The Ishikawa cells from birth to the present. Hum. Cell 2002, 15, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Way, D.L.; Grosso, D.S.; Davis, J.R.; Surwit, E.A.; Christian, C.D. Characterization of a new human endometrial carcinoma (RL95-2) established in tissue culture. Vitr. Cell. Dev. Biol.-Plant 1983, 19, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Thie, M.; Fuchs, P.; Denker, H.W. Epithelial cell polarity and embryo implantation in mammals. Int. J. Dev. Biol. 1996, 40, 389–393. [Google Scholar] [CrossRef]
- Thie, M.; Fuchs, P.; Butz, S.; Sieckmann, F.; Hoschutzky, H.; Kemler, R.; Denker, H.W. Adhesiveness of the apical surface of uterine epithelial cells: The role of junctional complex integrity. Eur. J. Cell Biol. 1996, 70, 221–232. [Google Scholar] [PubMed]
- Thie, M.; Rospel, R.; Dettmann, W.; Benoit, M.; Ludwig, M.; Gaub, H.E.; Denker, H.W. Interactions between trophoblast and uterine epithelium: Monitoring of adhesive forces. Hum. Reprod. 1998, 13, 3211–3219. [Google Scholar] [CrossRef]
- Thie, M.; Denker, H.W. In vitro studies on endometrial adhesiveness for trophoblast: Cellular dynamics in uterine epithelial cells. Cells Tissues Organs 2002, 172, 237–252. [Google Scholar] [CrossRef]
- Heneweer, C.; Kruse, L.H.; Kindhauser, F.; Schmidt, M.; Jakobs, K.H.; Denker, H.W.; Thie, M. Adhesiveness of human uterine epithelial RL95-2 cells to trophoblast: Rho protein regulation. Mol. Hum. Reprod. 2002, 8, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Heneweer, C.; Adelmann, H.G.; Kruse, L.H.; Denker, H.W.; Thie, M. Human uterine epithelial RL95-2 cells reorganize their cytoplasmic architecture with respect to Rho protein and F-actin in response to trophoblast binding. Cells Tissues Organs 2003, 175, 1–8. [Google Scholar] [CrossRef]
- Heneweer, C.; Schmidt, M.; Denker, H.W.; Thie, M. Molecular mechanisms in uterine epithelium during trophoblast binding: The role of small GTPase RhoA in human uterine Ishikawa cells. J. Exp. Clin. Assist. Reprod. 2005, 2, 4. [Google Scholar] [CrossRef]
- Sternberg, A.K. Establishment and Characterization of in Vitro Systems to Study Human Hormone-Dependent Endometrial Epithelial Plasticity and Mechanics. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2023. Available online: https://publications.rwth-aachen.de/record/968796/files/968796.pdf (accessed on 21 April 2025).
- Pan-Castillo, B.; Gazze, S.A.; Thomas, S.; Lucas, C.; Margarit, L.; Gonzalez, D.; Francis, L.W.; Conlan, R.S. Morphophysical dynamics of human endometrial cells during decidualization. Nanomedicine 2018, 14, 2235–2245. [Google Scholar] [CrossRef]
- Sternberg, A.K.; Izmaylova, L.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. An Assessment of the Mechanophysical and Hormonal Impact on Human Endometrial Epithelium Mechanics and Receptivity. Int. J. Mol. Sci. 2024, 25, 3726. [Google Scholar] [CrossRef] [PubMed]
- Buck, V.U. Studies of Endometrial Epithelial Cell Junctions and Trophoblast-Endometrial Interaction in a 3D Cell Culture System. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2013. Available online: https://publications.rwth-aachen.de/record/229374/files/4867.pdf (accessed on 20 April 2025).
- Buck, V.U.; Gellersen, B.; Leube, R.E.; Classen-Linke, I. Interaction of human trophoblast cells with gland-like endometrial spheroids: A model system for trophoblast invasion. Hum. Reprod. 2015, 30, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S. Differential sensitivity of epithelial cells to extracellular matrix in polarity establishment. PLoS ONE 2014, 9, e112922. [Google Scholar] [CrossRef]
- Buck, V.; Kohlen, M.; Sternberg, A.; Rösing, B.; Neulen, J.; Leube, R.; Classen-Linke, I. Steroid hormones and human choriogonadotropin influence the distribution of alpha6-integrin and desmoplakin 1 in gland-like endometrial epithelial spheroids. Histochem. Cell Biol. 2021, 155, 581–591. [Google Scholar] [CrossRef]
- Hohn, H.P.; Denker, H.W. Experimental modulation of cell-cell adhesion, invasiveness and differentiation in trophoblast cells. Cells Tissues Organs 2002, 172, 218–236. [Google Scholar] [CrossRef]
- Grummer, R.; Hohn, H.P.; Mareel, M.M.; Denker, H.W. Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three-dimensional organ culture system. Placenta 1994, 15, 411–429. [Google Scholar] [CrossRef]
- Albers, A.; Thie, M.; Hohn, H.P.; Denker, H.W. Differential expression and localization of integrins and CD44 in the membrane domains of human uterine epithelial cells during the menstrual cycle. Acta Anat. 1995, 153, 12–19. [Google Scholar] [CrossRef]
- Frank, H.G.; Gunawan, B.; Ebeling-Stark, I.; Schulten, H.J.; Funayama, H.; Cremer, U.; Huppertz, B.; Gaus, G.; Kaufmann, P.; Fuzesi, L. Cytogenetic and DNA-fingerprint characterization of choriocarcinoma cell lines and a trophoblast/choriocarcinoma cell hybrid. Cancer Genet. Cytogenet. 2000, 116, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Asbach, P.; Streitberger, K.J.; Thomas, A.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. In vivo high-resolution magnetic resonance elastography of the uterine corpus and cervix. Eur. Radiol. 2014, 24, 3025–3033. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early human trophoblast development: From morphology to function. Cell Mol. Life Sci. 2022, 79, 345. [Google Scholar] [CrossRef]
- Ruane, P.T.; Garner, T.; Parsons, L.; Babbington, P.A.; Wangsaputra, I.; Kimber, S.J.; Stevens, A.; Westwood, M.; Brison, D.R.; Aplin, J.D. Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation. Hum. Reprod. 2022, 37, 777–792. [Google Scholar] [CrossRef]
- Heng, S.; Samarajeewa, N.; Aberkane, A.; Essahib, W.; Van de Velde, H.; Scelwyn, M.; Hull, M.L.; Vollenhoven, B.; Rombauts, L.J.; Nie, G. Podocalyxin inhibits human embryo implantation in vitro and luminal podocalyxin in putative receptive endometrium is associated with implantation failure in fertility treatment. Fertil. Steril. 2021, 116, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Paule, S.G.; Heng, S.; Samarajeewa, N.; Li, Y.; Mansilla, M.; Webb, A.I.; Nebl, T.; Young, S.L.; Lessey, B.A.; Hull, M.L.; et al. Podocalyxin is a key negative regulator of human endometrial epithelial receptivity for embryo implantation. Hum. Reprod. 2021, 36, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Maruyama, T.; Nishikawa-Uchida, S.; Oda, H.; Miyazaki, K.; Yamasaki, A.; Yoshimura, Y. Studies using an in vitro model show evidence of involvement of epithelial-mesenchymal transition of human endometrial epithelial cells in human embryo implantation. J. Biol. Chem. 2012, 287, 4441–4450. [Google Scholar] [CrossRef]
- Uchida, H.; Maruyama, T.; Masuda, H.; Uchida, S.; Miki, F.; Hihara, H.; Katakura, S.; Yoshimasa, Y.; Tanaka, M. How to Create an Embryo Penetration Route. Am. J. Reprod. Immunol. 2016, 75, 326–332. [Google Scholar] [CrossRef]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horne, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef]
- Uchida, H. Epithelial mesenchymal transition in human menstruation and implantation. Endocr. J. 2024, 71, 745–751. [Google Scholar] [CrossRef]
- Shi, C.; Shen, H.; Fan, L.J.; Guan, J.; Zheng, X.B.; Chen, X.; Liang, R.; Zhang, X.W.; Cui, Q.H.; Sun, K.K.; et al. Endometrial MicroRNA Signature during the Window of Implantation Changed in Patients with Repeated Implantation Failure. Chin. Med. J. 2017, 130, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Riyanti, A.; Febri, R.R.; Zakirah, S.C.; Harzif, A.K.; Rajuddin, R.; Muharam, R.; Asmarinah, A.; Wiweko, B. Suppressing HOXA-10 Gene Expression by MicroRNA 135b During the Window of Implantation in Infertile Women. J. Reprod. Infertil. 2020, 21, 217–221. [Google Scholar]
- Zhou, W.; Van Sinderen, M.; Rainczuk, K.; Menkhorst, E.; Sorby, K.; Osianlis, T.; Pangestu, M.; Santos, L.; Rombauts, L.; Rosello-Diez, A.; et al. Dysregulated miR-124-3p in endometrial epithelial cells reduces endometrial receptivity by altering polarity and adhesion. Proc. Natl. Acad. Sci. USA 2024, 121, e2401071121. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Graves, M.L.; Chelliah, S.; Vogl, A.W.; Roskelley, C.D.; McNagny, K.M. The CD34-related molecule podocalyxin is a potent inducer of microvillus formation. PLoS ONE 2007, 2, e237. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, M.; Aplin, J.D.; Caballero-Campo, P.; O’Connor, J.E.; Martin, J.C.; Remohi, J.; Pellicer, A.; Simon, C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol. Reprod. 2001, 64, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D. Embryo implantation: The molecular mechanism remains elusive. Reprod. Biomed. Online 2006, 13, 833–839. [Google Scholar] [CrossRef]
- Ruane, P.T.; Paterson, I.; Reeves, B.; Adlam, D.; Berneau, S.C.; Renshall, L.; Brosens, J.J.; Kimber, S.J.; Brison, D.R.; Aplin, J.D.; et al. Glucose influences endometrial receptivity to embryo implantation through O-GlcNAcylation-mediated regulation of the cytoskeleton. Am. J. Physiol. Cell Physiol. 2024, 327, C634–C645. [Google Scholar] [CrossRef]
- Yu, L.; Wei, Y.; Duan, J.; Schmitz, D.A.; Sakurai, M.; Wang, L.; Wang, K.; Zhao, S.; Hon, G.C.; Wu, J. Blastocyst-like structures generated from human pluripotent stem cells. Nature 2021, 591, 620–626. [Google Scholar] [CrossRef]
- Shibata, S.; Endo, S.; Nagai, L.A.E.; Kobayashi, E.H.; Oike, A.; Kobayashi, N.; Kitamura, A.; Hori, T.; Nashimoto, Y.; Nakato, R.; et al. Modeling embryo-endometrial interface recapitulating human embryo implantation. Sci. Adv. 2024, 10, eadi4819. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kodithuwakku, S.P.; Chan, R.W.S.; Yeung, W.S.B.; Yao, Y.; Ng, E.H.Y.; Chiu, P.C.N.; Lee, C.L. Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation. Reprod. Biol. Endocrinol. 2022, 20, 120. [Google Scholar] [CrossRef]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef]
- Nallasamy, S.; Li, Q.; Bagchi, M.K.; Bagchi, I.C. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet. 2012, 8, e1002500. [Google Scholar] [CrossRef]
- Daikoku, T.; Cha, J.; Sun, X.; Tranguch, S.; Xie, H.; Fujita, T.; Hirota, Y.; Lydon, J.; DeMayo, F.; Maxson, R.; et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev. Cell 2011, 21, 1014–1025. [Google Scholar] [CrossRef]
- Bolnick, A.D.; Bolnick, J.M.; Kilburn, B.A.; Stewart, T.; Oakes, J.; Rodriguez-Kovacs, J.; Kohan-Ghadr, H.R.; Dai, J.; Diamond, M.P.; Hirota, Y.; et al. Reduced homeobox protein MSX1 in human endometrial tissue is linked to infertility. Hum. Reprod. 2016, 31, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ni, Z.L.; Kong, S.B.; Lu, J.H.; Wang, H.B. Homeobox genes for embryo implantation: From mouse to human. Anim. Model. Exp. Med. 2018, 1, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ashary, N.; Laheri, S.; Modi, D. Homeobox genes in endometrium: From development to decidualization. Int. J. Dev. Biol. 2020, 64, 227–237. [Google Scholar] [CrossRef] [PubMed]


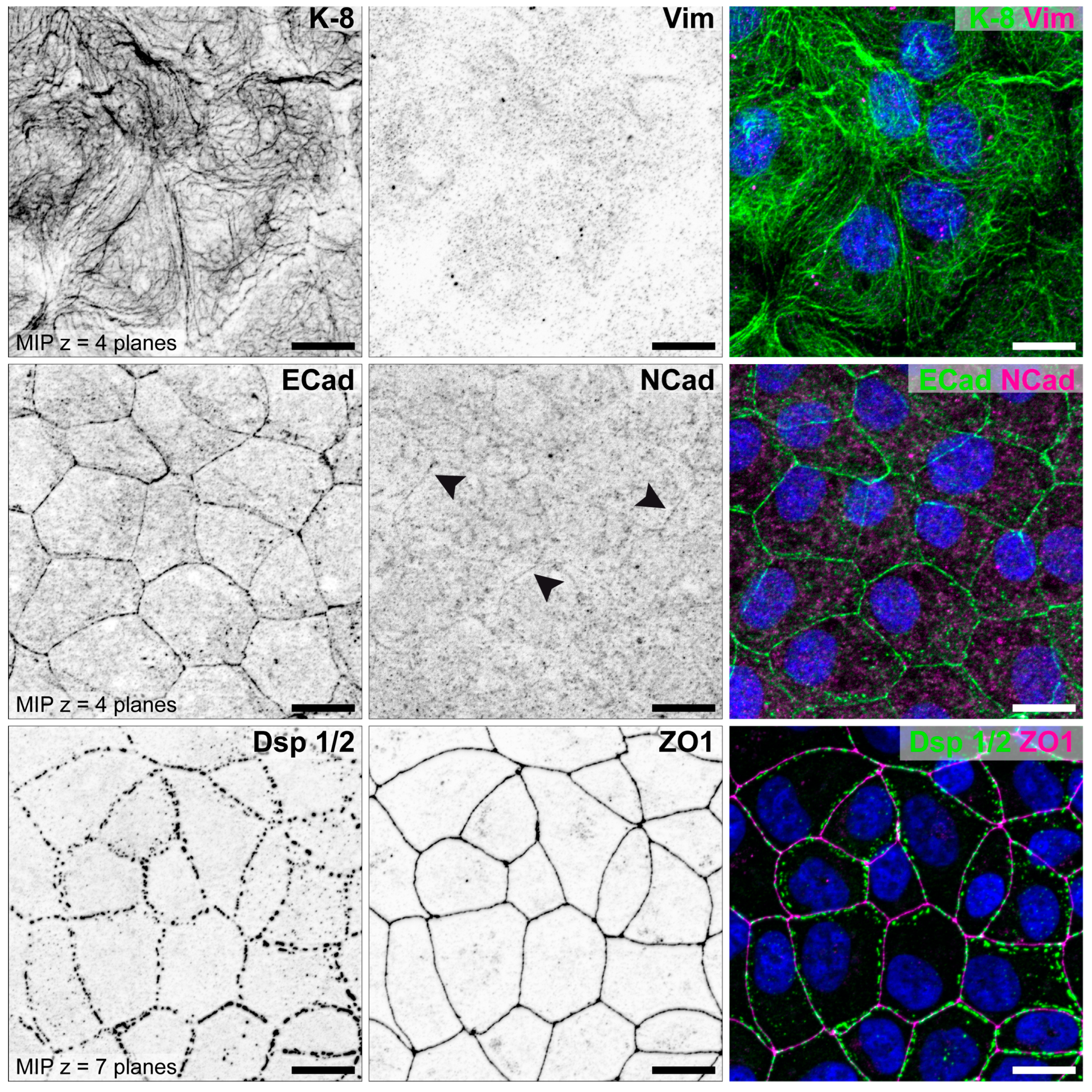
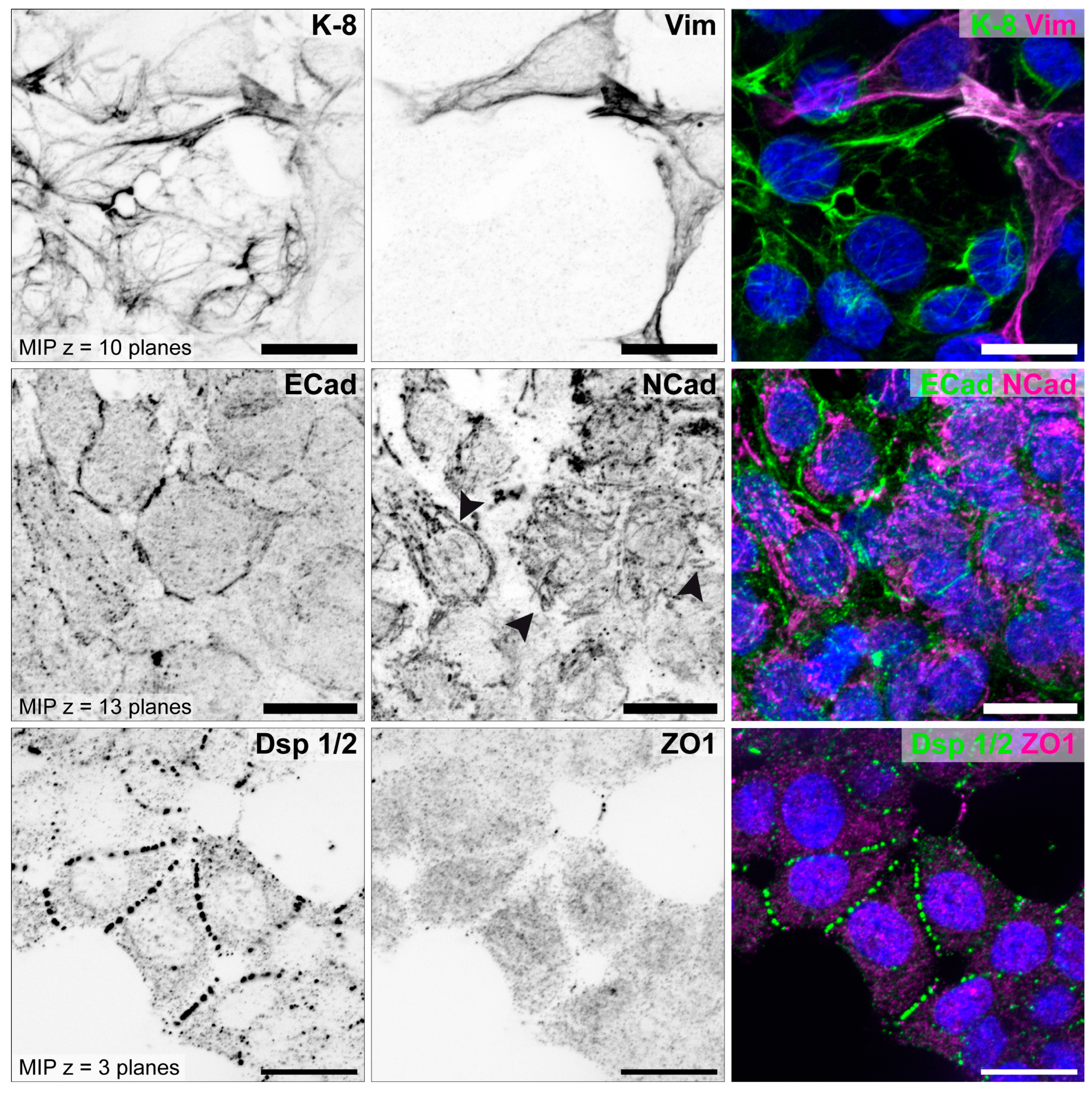
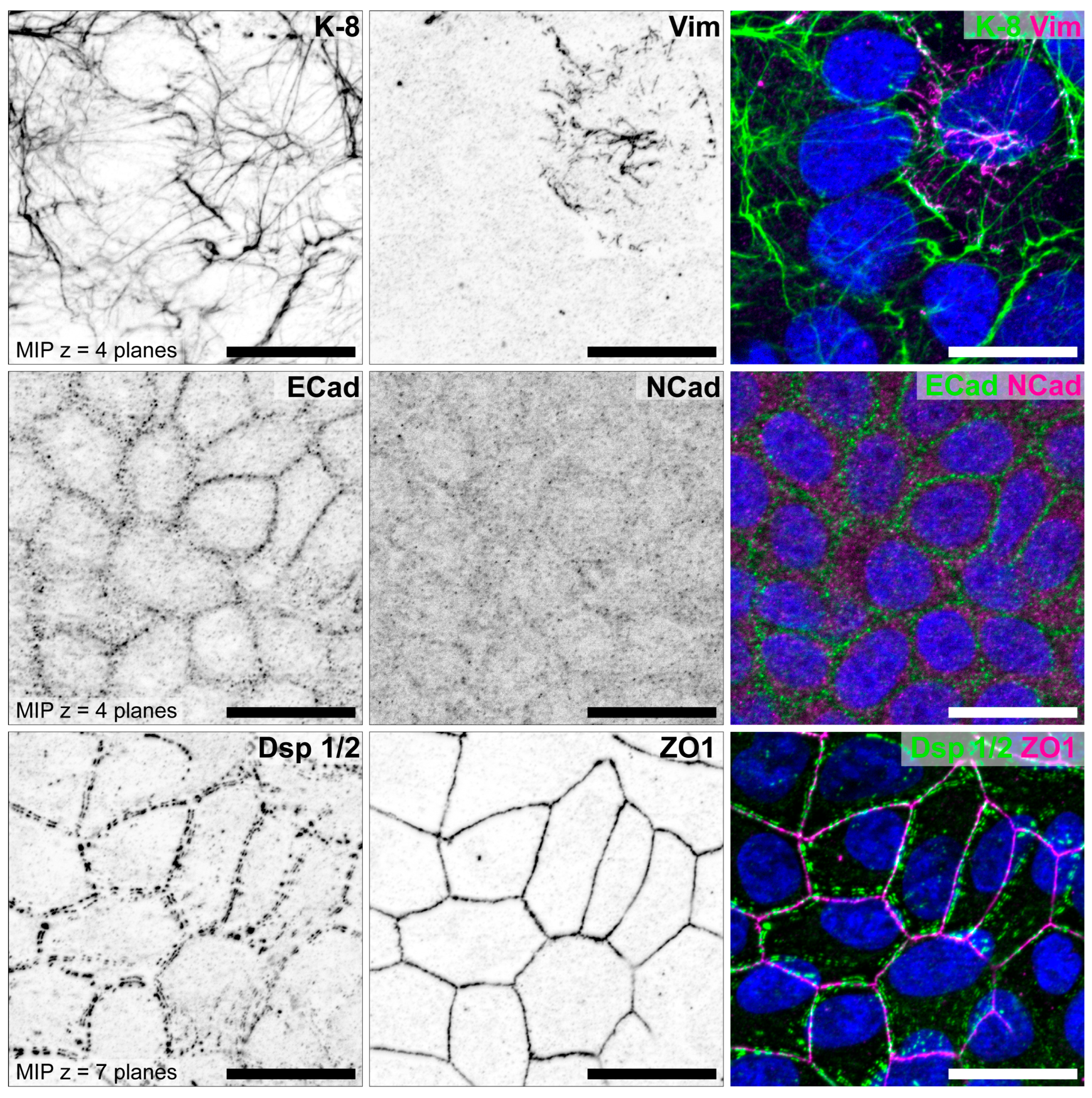




| HEC-1-A | Ishikawa | RL95-2 | References | |
|---|---|---|---|---|
| Epithelial cell polarity | high | moderate | low | [70,71,73,88,89] |
| Adhesiveness | non-adhesive | adhesive | highly adhesive | [70,73,89] |
| Keratin 8 | ++ | + | (+) | [70,85] |
| Vimentin | Ø | single cells (+) | some cells (+) | [70,85] |
| E-cadherin | ++ | + | (+) | [70,85] |
| N-cadherin | Ø | Ø | (+) | [85] |
| Desmoplakin 1/2 | ++ | + | + | [79,85,88,89] |
| ZO1 | ++ | + | Ø | [79,85,88,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Classen-Linke, I.; Buck, V.U.; Sternberg, A.K.; Kohlen, M.; Izmaylova, L.; Leube, R.E. Changes in Epithelial Cell Polarity and Adhesion Guide Human Endometrial Receptivity: How In Vitro Systems Help to Untangle Mechanistic Details. Biomolecules 2025, 15, 1057. https://doi.org/10.3390/biom15081057
Classen-Linke I, Buck VU, Sternberg AK, Kohlen M, Izmaylova L, Leube RE. Changes in Epithelial Cell Polarity and Adhesion Guide Human Endometrial Receptivity: How In Vitro Systems Help to Untangle Mechanistic Details. Biomolecules. 2025; 15(8):1057. https://doi.org/10.3390/biom15081057
Chicago/Turabian StyleClassen-Linke, Irmgard, Volker U. Buck, Anna K. Sternberg, Matthias Kohlen, Liubov Izmaylova, and Rudolf E. Leube. 2025. "Changes in Epithelial Cell Polarity and Adhesion Guide Human Endometrial Receptivity: How In Vitro Systems Help to Untangle Mechanistic Details" Biomolecules 15, no. 8: 1057. https://doi.org/10.3390/biom15081057
APA StyleClassen-Linke, I., Buck, V. U., Sternberg, A. K., Kohlen, M., Izmaylova, L., & Leube, R. E. (2025). Changes in Epithelial Cell Polarity and Adhesion Guide Human Endometrial Receptivity: How In Vitro Systems Help to Untangle Mechanistic Details. Biomolecules, 15(8), 1057. https://doi.org/10.3390/biom15081057







