Viscum coloratum (Komar.) Nakai: A Review of Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology
Abstract
1. Introduction
2. Botany
3. Traditional Uses
4. Phytochemistry
4.1. Flavonoids
4.2. Phenylpropanoids
4.3. Diphenylheptanes
4.4. Terpenoids
4.5. Alkaloids
4.6. Polysaccharides
4.7. Lectins
4.8. Other Compounds
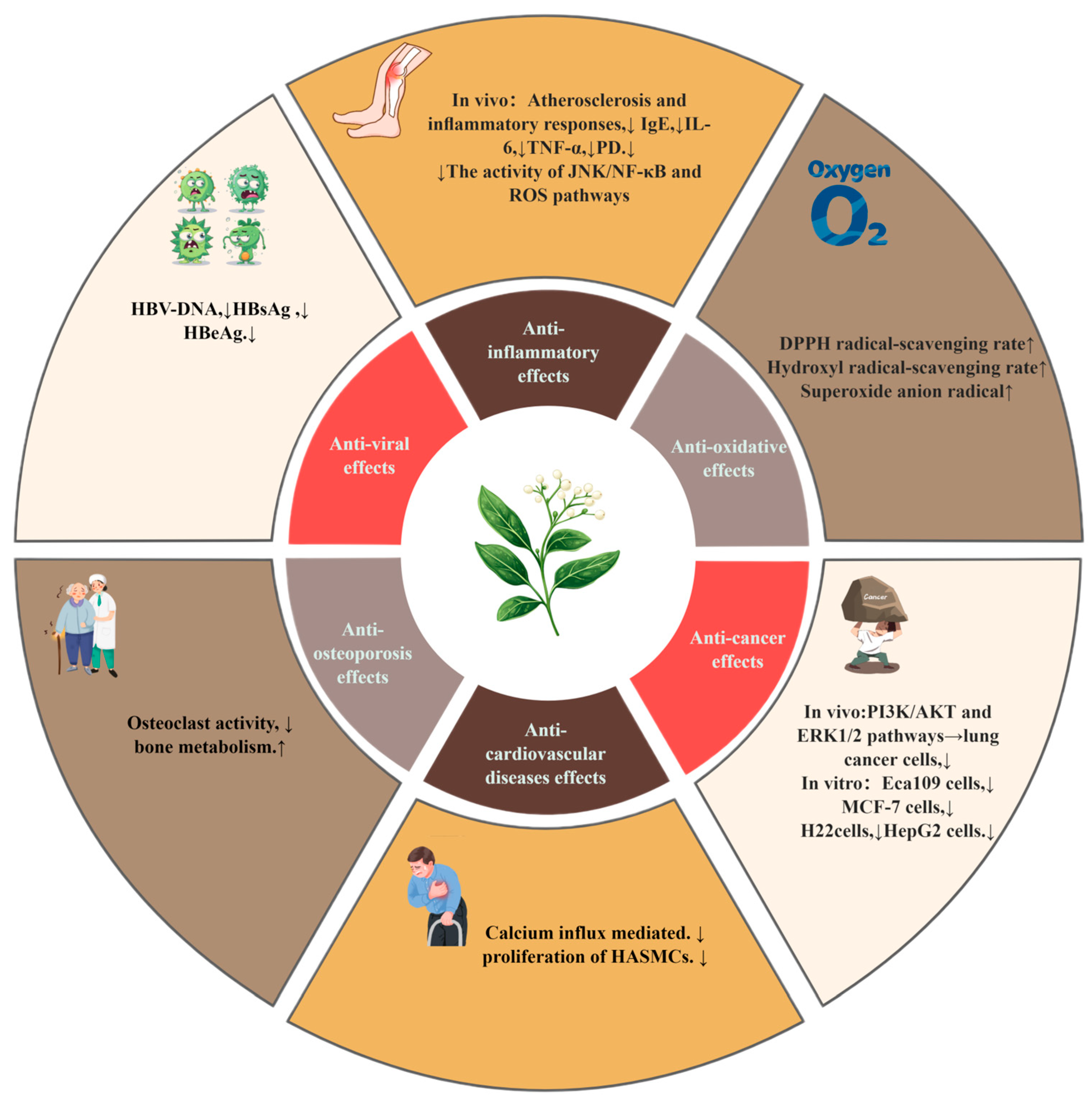
5. Pharmacology
5.1. Anti-Inflammatory Effect
5.2. Anticancer Effect
5.3. Antioxidant Effect
5.4. Anti-Cardiovascular Disease Effect
5.5. Other Effects
6. Pharmacokinetics
7. Toxicology
8. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Airway hyper-responsiveness |
| APA | Action potential amplitude |
| APD | Action potential |
| AQs | Aqueous extracts |
| BALF | Bronchoalveolar lavage fluid |
| CCK-8 | Cell Counting Kit-8 |
| CDDP | Cisplatin |
| CD | Crohn’s disease |
| CEEs | Crude ethanol extracts |
| CIA | Collagen-induced arthritis |
| CP | Chloroplast |
| EB30 | 1,7-Bis-(4-hydroxyphenyl)-1,4-heptadien-3-one |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| ER | Estrogen receptor |
| ESR | Electron spin resonance |
| EVC | Extract of Viscum coloratum (Komar.) Nakai |
| FMLP | Formyl-L-methionyl-L-leucyl-L-phenylalanine |
| HBV | Hepatitis B virus |
| Hedt-I | Homoeriodictyol-7-O-β-D-apiosiyl-(1→5)-β-D-apiosyl-(1→2)-β-D-glycoside |
| Hedt-II | Homoeriodictyol-7-O-β-D-apiose (1→2)-β-D-glycoside |
| Hedt-III | Homoeriodictyol-7-O-β-D-glycoside |
| Hedt-IV | Homoeriodictyol |
| Httf | 5-hydroxy-3,7,3′-trimethoxyflavone-4′-O-β-D-glucoside |
| IBD | Inflammatory bowel disease |
| Isor | Isornetin-3-O-β-D-glucoside |
| ITRAQ | Isobaric tags for relative and absolute quantitation |
| MDP | Maximum diastolic potential |
| ML | Maximum likelihood |
| MMP-2 | Matrix metalloprotease-2 |
| MMP-9 | Matrix metalloprotease-9 |
| MONO | Monomer |
| MIX | Mixture |
| MI | Ischemia |
| MTX | Methotrexate |
| MTT | Methylthiazolyldiphenyl tetrazolium bromide |
| OVA | Ovalbumin |
| PAF | Fast response action potential |
| Pes | Petroleum ether extracts |
| PPE-SVC | Partially purified extract of Viscum coloratum (Komar.) Nakai |
| PKA | Protein kinase A |
| PTH | Parathyroid hormone |
| P1A | 1-Phase amplitude |
| RA | Rheumatoid arthritis |
| Rham-I | Rhamnazin-3-O-β-D-glucoside |
| Rham-II | Rhamnazin-3-O-β-D-(6”-β-hydroxy-β-methyglutaryl)-glucoside |
| Rham-III | Rhamnazin-3-O-β-D-(6”-β-hydroxy-β-methyglutaryl)-β-D-glucoside-4′-O-β-D-glucoside |
| Syri | Syringin |
| U2OS | Human osteosarcoma cells |
| UC | Ulcerative colitis |
| VCE | Ethanol extract of Viscum coloratum (Komar.) Nakai |
| VCF | Viscum coloratum (Komar.) Nakai flavonoids |
| VCP | Viscum coloratum (Komar.) Nakai polysaccharides |
| V. coloratum | Viscum coloratum (Komar.) Nakai |
| V. album L. | Viscum album L. |
| ZO-1 | Zonula occludens-1 |
| 5-FU | 5-Fluorouracil |
References
- Zhang, R.Z.; Zhao, J.T.; Wang, W.Q.; Fan, R.H.; Rong, R.; Yu, Z.G.; Zhao, Y.L. Metabolomics-based comparative analysis of the effects of host and environment on Viscum coloratum metabolites and antioxidative activities. J. Pharm. Anal. 2022, 12, 243–252. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Noh, P.; Kim, W.J.; Yang, S.; Choi, G.; Moon, B.C. PCR-based rapid diagnostic tools for the authentication of medicinal mistletoe species. Phytomedicine 2021, 91, 153667. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, R.; Wang, W.; Yu, Z.; Li, Y.; Zhao, Y. Study on the dynamic variation of the secondary metabolites in Viscum coloratum using targeted metabolomics. Chin. J. Nat. Med. 2023, 21, 308–320. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, Z.; Liao, T.; Wang, K.; Wan, J.; Wei, Y.; Ouyang, Z. The herbal research on Viscum coloratum (Komar.) Nakai. Chin. J. Exp. Formula Sci. 2021, 27, 124–131. [Google Scholar] [CrossRef]
- Kim, B.Y.; Park, H.S.; Kim, S.; Kim, Y.D. Development of microsatellite markers for Viscum coloratum (Santalaceae) and their application to wild populations. Appl. Plant Sci. 2017, 5, 1600102. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, S.J.; Choi, Y.N.; Kim, S.D.; Kwag, E.B.; Song, S.Y.; Park, J.H.; Kim, J.K.; Seo, C.; Choi, J.J.; et al. Selective Immune Modulating Activities of Viscum album and Its Components; A Possibility of Therapeutics on Skin Rash Induced by EGFR Inhibitors. Integr. Cancer Ther. 2022, 21, 15347354221118332. [Google Scholar] [CrossRef]
- Jeong, J.; Park, C.H.; Kim, I.; Kim, Y.H.; Yoon, J.M.; Kim, K.S.; Kim, J.B. Korean mistletoe (Viscum album coloratum) extract regulates gene expression related to muscle atrophy and muscle hypertrophy. BMC Complement. Altern. Med. 2017, 17, 68. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, L.; Yang, Y.; Zhao, J.; Zhang, Y. Geographic variation of fruit color dimorphism in Viscum coloratum (Kom.) Nakai in Northeast China. Flora 2021, 280, 151846. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Park, S.M.; Choung, B.Y.; Park, W.B. Comparative study of Korean (Viscum album var. coloratum) and European mistletoes (Viscum album). Arch. Pharm. Res. 2000, 23, 592–598. [Google Scholar] [CrossRef]
- Bishayee, A.; Penn, A.; Bhandari, N.; Petrovich, R.; DeLiberto, L.K.; Burcher, J.T.; Barbalho, S.M.; Nagini, S. Dietary plants for oral cancer prevention and therapy: A review of preclinical and clinical studies. Phytother. Res. 2024, 38, 5225–5263. [Google Scholar] [CrossRef]
- Chaudhary, D.; Patel, S.; Gururani, R.; Chak, P.; Jain, S.; Dwivedi, J.; Sharma, S. A comprehensive review on anti-inflammatory plants: A mechanistic insight through preclinical and clinical studies. Inflammopharmacology 2025, 33, 2447–2476. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Li, Y.; Xue, X.; Huang, Y.; Luo, H.; Zhang, Y.; Lu, Z. Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes. Molecules 2018, 23, 359. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, M.; Gao, X.; Liu, T.; Yu, Z.; Bi, K. Isolation and determination of homoeriodictyol-7-O-beta-D-glycoside in Viscum coloratum. Se Pu 2006, 24, 479–481. [Google Scholar]
- Yin, J.; Han, N.; Xu, X.; Liu, Z.; Zhang, B.; Kadota, S. Inhibitory activity of the ethyl acetate fraction from Viscum coloratum on bone resorption. Planta Med. 2008, 74, 120–125. [Google Scholar] [CrossRef]
- Long, C.; Fan, R.; Zhang, Q.; Zhang, Z.; Wang, D.; Xia, Y.; Ma, Y.; Yu, Z.; Zhao, Y. Simultaneous identification and quantification of the common compounds of Viscum coloratum and its corresponding host plants by ultra-high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry and triple quadrupole mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 176–184. [Google Scholar] [CrossRef]
- Yao, H.; Zhou, G.X.; Wu, Q.; Lei, G.Q.; Chen, D.F.; Chen, J.K.; Zhou, T.S. Mistletonone, a novel antioxidative diarylheptanoid from the branches and leaves of Viscum coloratum. Molecules 2007, 12, 312–317. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Li, Y.-Y.; Wang, X.-C.; An, J.-D.; Wang, Y.-J.; Wang, X.-J. Mistletoe alkaloid fractions alleviates carbon tetrachloride-induced liver fibrosis through inhibition of hepatic stellate cell activation via TGF-β/Smad interference. J. Ethnopharmacol. 2014, 158, 230–238. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.F. [Extraction and content determination of polysaccharides in Viscum coloratum]. Zhongguo Zhong Yao Za Zhi 2007, 32, 2387–2390. [Google Scholar]
- Li, J.Q.; Yang, B. Lectins in Viscum: A progress review. Zhongguo Zhong Yao Za Zhi 2021, 46, 3551–3559. [Google Scholar] [CrossRef]
- Chai, Y.; Zhao, M. iTRAQ-Based Quantitative Proteomic Analysis of the Inhibitory Effects of Polysaccharides from Viscum coloratum (Kom.) Nakai on HepG2 Cells. Sci. Rep. 2017, 7, 4596. [Google Scholar] [CrossRef]
- Song, C.; Wei, X.-Y.; Qiu, Z.-D.; Gong, L.; Chen, Z.-Y.; Ma, Y.; Shen, Y.; Zhao, Y.-J.; Wang, W.-h.; Lai, C.-J.-S. Exploring the resources of the genus Viscum for potential therapeutic applications. J. Ethnopharmacol. 2021, 277, 114233. [Google Scholar] [CrossRef]
- Wei, X.; Guo, H.; Che, P.; Zhang, B.; Liu, H.; Qi, Y. The complete chloroplast genome sequence of Viscum coloratum (Viscaceae), a semiparasitic medicinal plant. Mitochondrial DNA B Resour. 2019, 4, 2904–2905. [Google Scholar] [CrossRef]
- Stirpe, F.; Legg, R.F.; Onyon, L.J.; Ziska, P.; Franz, H. Inhibition of protein synthesis by a toxic lectin from Viscum album L. (mistletoe). Biochem. J. 1980, 190, 843–845. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Fan, R.H.; Yuan, H.X.; Yu, M.; Bi, K.S.; Yu, Z.G. Development of the fingerprints for the quality evaluation of Viscum coloratum by high Performance liquid chromatography. J. Pharm. Anal. 2011, 1, 113–118. [Google Scholar] [CrossRef]
- Changhu, L. Biology of mistletoe (Viscum coloratum) and its seed dispersal by frugivoro us birds. Acta Ecol. Sin. 2003, 23, 834–839. [Google Scholar]
- Maul, K.; Krug, M.; Nickrent, D.L.; Müller, K.F.; Quandt, D.; Wicke, S. Morphology, geographic distribution, and host preferences are poor predictors of phylogenetic relatedness in the mistletoe genus Viscum L. Mol. Phylogenetics Evol. 2019, 131, 106–115. [Google Scholar] [CrossRef]
- Gorovoy, P.; Balyshev, M.; Krylov, A.; Schekina, V.; Nizkiy, S. Mistletoe coloring (Viscum coloratum (Kom.) Nakai) in the East Asia (taxonomy, area, possibilities applications). Acta Biol. Sib. 2018, 4, 103. [Google Scholar]
- Zengwei, G.; Xuehui, L.; Haixin, L.; Yuxin, C. A novel platelet-activating factor antagonist isolated from a Chinese herbal drug Viscum coloratum. J. Chin. Pharm. Sci. 2000, 9, 73. [Google Scholar]
- Li, S. Compendium of Materia Medica; Shanghai University of Traditional Chinese Medicine: Shanghai, China, 2011. [Google Scholar]
- Ye, F.; Du, G.Z.; Cui, A.Q.; Lu, X.T. Study on the mechanism of compound mistletoe fluidextract in relieving hypertension. J. Tradit. Chin. Med. 2009, 29, 291–295. [Google Scholar] [CrossRef]
- CB, W.; HS, Y.; Xu, Q. The ornamental value and cultivation characteristics of the excellent wild ornamental plant mistletoe. Abstr. Chin. Hortic. 2013, 29, 74–75. [Google Scholar]
- Wang, Y.; Hao, Z.; Lu, D.; Naseem, A.; Sun, Y.; Sun, Y.; Li, J.; Kuang, H.; Liu, Y.; Yang, B. Effects of Viscum coloratum (Kom.) Nakai on collagen-induced rheumatoid arthritis. J. Ethnopharmacol. 2024, 327, 118026. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.; Fan, R.; Gao, X.; Yu, M.; Li, H.; Wei, H.; Bi, K. Simultaneous determination of ten flavonoids from Viscum coloratum grown on different host species and different sources by LC-MS. Chem Pharm Bull 2011, 59, 1322–1328. [Google Scholar] [CrossRef]
- Yao, H.; Liao, Z.X.; Wu, Q.; Lei, G.Q.; Liu, Z.J.; Chen, D.F.; Chen, J.K.; Zhou, T.S. Antioxidative flavanone glycosides from the branches and leaves of Viscum coloratum. Chem. Pharm. Bull. 2006, 54, 133–135. [Google Scholar] [CrossRef]
- Chen, B. Research on the Chemical Composition of Viscum coloratum. Master’s Thesis, Shanxi Medical University, Shanxi, China, 2007. [Google Scholar]
- Zhao, Y.L.; Wang, X.Y.; Sun, L.X.; Fan, R.H.; Bi, K.S.; Yu, Z.G. Cytotoxic constituents of Viscum coloratum. Z. Naturforsch C J. Biosci. 2012, 67, 129–134. [Google Scholar] [CrossRef]
- Cao, D.; Han, C.; Gao, W.; Cheng, L.; Yang, P. Research on the Chemical Composition of Viscum coloratum. Chin. Herb. Med. 2016, 47, 4313–4317. [Google Scholar]
- Kong, D.; Luo, S.; Li, H.; Lei, X. Studies on chemical components of Viscum coloratum: I△. Pharm. Ind. 1987, 3, 123–127. [Google Scholar] [CrossRef]
- Kong, D.; Luo, S.; Li, H.; Lei, X. Studies on chemical components of Viscum coloratum: IV. Structure of viscumneoside IV. Yao Xue Xue Bao Acta Pharm. Sin. 1988, 23, 707–710. [Google Scholar]
- Cao, D.; Li, J.; He, Q.; Cheng, L. Research on the Chemical Composition of Viscum coloratum. China Pharm. Ind. J. 2016, 47, 861–864. [Google Scholar] [CrossRef]
- Hwang, T.L.; Leu, Y.L.; Kao, S.H.; Tang, M.C.; Chang, H.L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef]
- Kong, D.; Luo, S.; Li, H.; Lei, X. Studies on chemical components of Viscum coloratum: V. China Pharm. Ind. J. 1989, 3, 108–110. [Google Scholar] [CrossRef]
- Wen, B.; Pan, J.; Wang, Y.Q.; Hao, Z.C.; Guan, W.; Chen, Q.S.; Zhang, L.L.; Kuang, H.X.; Liu, Y.; Yang, B.Y. New cytotoxic compounds from Viscum coloratum (Kom.) Nakai. Nat. Prod. Res. 2025, 5, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hou, J.P.; Huang, L.; Khan, A.; Xing, F.F.; Zhang, X.H.; Han, D.F.; Yan, S.L.; Cao, G.D.; Jiao, Q.Y.; et al. Chemical constituents of Viscum coloratum (Kom.) Nakai and their cytotoxic activities. Nat. Prod. Res. 2022, 36, 1927–1933. [Google Scholar] [CrossRef]
- Sun, Y.; Ling, Y.; Ren, M.; Zhou, X.; Wang, J.; Ma, Y. Chemical constituents of Viscum coloratum f. rubroaurantiacum. Chin. Herb. Med. 2010, 41, 1418–1420. [Google Scholar]
- Sun, Y.; Liu, K.; Wang, S.; Xu, S. Research Progress of Viscum coloratum. Chin. Herb. Med. 2000, 23, 73–76. [Google Scholar]
- Lang, Y. Extraction, Separation and Pharmacokinetic Study of Alkaloids from Viscum coloratum. Master’s Thesis, Heilongjiang University of Chinese, Harbin, China, 2015. [Google Scholar]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Kim, M.K.; Yun, K.J.; Lim, D.H.; Kim, J.; Jang, Y.P. Anti-Inflammatory Properties of Flavone di-C-Glycosides as Active Principles of Camellia Mistletoe, Korthalsella japonica. Biomol. Ther. 2016, 24, 630–637. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Vikhorev, A.V.; Shmakov, N.A.; Morozov, S.V.; Chernyak, E.I.; Vasiliev, G.V.; Shatskaya, N.V.; Khlestkina, E.K.; Shoeva, O.Y. Features of Activity of the Phenylpropanoid Biosynthesis Pathway in Melanin-Accumulating Barley Grains. Front. Plant Sci. 2022, 13, 923717. [Google Scholar] [CrossRef]
- Wu, D.; Ge, D.; Dai, Y.; Chen, Y.; Fu, Q.; Jin, Y. Extraction and isolation of diphenylheptanes and flavonoids from Alpinia officinarum Hance using supercritical fluid extraction followed by supercritical fluid chromatography. J. Sep. Sci. 2023, 46, e2300156. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef]
- Cogo, E.; Elsayed, M.; Bhardwaj, S.; Cooley, K.; Aycho, C.; Liang, V.; Papadogianis, P.; Psihogios, A.; Seely, D. Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials. Curr. Oncol. 2023, 30, 8196–8219. [Google Scholar] [CrossRef]
- Song, Y.; Liu, X.; Feng, Y.; Liu, G.; Duan, Y. Recent insights into Hericium erinaceus polysaccharides: Gastrointestinal, gut microbiota, microbial metabolites, overall health and structure-function correlation. Int. J. Biol. Macromol. 2025, 311 Pt 3, 144013. [Google Scholar] [CrossRef]
- Chai, Y.; Zhao, M. Purification, characterization and anti-proliferation activities of polysaccharides extracted from Viscum coloratum (Kom.) Nakai. Carbohydr. Polym. 2016, 149, 121–130. [Google Scholar] [CrossRef]
- Rashidbaghan, A.; Mostafaie, A.; Yazdani, Y.; Mansouri, K. The Agglutinin of Common Nettle (Urtica dioica L.) Plant Effects on Gene Expression Related to Apoptosis of Human Acute Myeloid Leukemia Cell Line. Biochem. Genet. 2021, 59, 1049–1064. [Google Scholar] [CrossRef]
- Ma, Y.H.; Cheng, W.Z.; Gong, F.; Ma, A.L.; Yu, Q.W.; Zhang, J.Y.; Hu, C.Y.; Chen, X.H.; Zhang, D.Q. Active Chinese mistletoe lectin-55 enhances colon cancer surveillance through regulating innate and adaptive immune responses. World J. Gastroenterol. 2008, 14, 5274–5281. [Google Scholar] [CrossRef]
- Gong, F.; Ma, Y.; Ma, A.; Yu, Q.; Zhang, J.; Nie, H.; Chen, X.; Shen, B.; Li, N.; Zhang, D. A lectin from Chinese mistletoe increases gammadelta T cell-mediated cytotoxicity through induction of caspase-dependent apoptosis. Acta Biochim. Biophys. Sin. 2007, 39, 445–452. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Guan, W.; Li, Y.Y.; Wen, B.; Chen, Z.J.; Liu, S.; Wang, Y.F.; Hao, Z.C.; Chen, Q.S.; Zhang, L.L.; et al. A Comparative Research of the Flavonoid Metabolites From Viscum coloratum in Normal and RA Rats by an Integrated Analytical Strategy. Biomed. Chromatogr. 2025, 39, e70105. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef]
- Bruner, L.P.; White, A.M.; Proksell, S. Inflammatory Bowel Disease. Prim. Care 2023, 50, 411–427. [Google Scholar] [CrossRef]
- Yoo, J.M.; Park, K.I.; Ma, J.Y. Anticolitic Effect of Viscum coloratum through Suppression of Mast Cell Activation. Am. J. Chin. Med. 2019, 47, 203–221. [Google Scholar] [CrossRef]
- Liang, C.J.; Wang, S.H.; Chen, Y.H.; Chang, S.S.; Hwang, T.L.; Leu, Y.L.; Tseng, Y.C.; Li, C.Y.; Chen, Y.L. Viscolin reduces VCAM-1 expression in TNF-α-treated endothelial cells via the JNK/NF-κB and ROS pathway. Free Radic. Biol. Med. 2011, 51, 1337–1346. [Google Scholar] [CrossRef]
- Leskien, M.; Thiering, E.; Yu, Z.; Huels, A.; Yao, Y.; Merid, S.K.; Gruzieva, O.; Weidinger, S.; Waldenberger, M.; Peters, A.; et al. Childhood Asthma and Allergy Are Related to Accelerated Epigenetic Aging. Allergy 2025, 16583. [Google Scholar] [CrossRef]
- Shen, J.J.; Chiang, M.S.; Kuo, M.L.; Leu, Y.L.; Hwang, T.L.; Liou, C.J.; Huang, W.C. Partially purified extract and viscolin from Viscum coloratum attenuate airway inflammation and eosinophil infiltration in ovalbumin-sensitized mice. J. Ethnopharmacol. 2011, 135, 646–653. [Google Scholar] [CrossRef]
- Mullard, A. Addressing cancer’s grand challenges. Nat. Rev. Drug Discov. 2020, 19, 825–826. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Fan, J.; Wu, M.; Wang, J.; Ren, D.; Zhao, J.; Yang, G. 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one induces lung cancer cell apoptosis via the PI3K/Akt and ERK1/2 pathways. J. Cell Physiol. 2019, 234, 6336–6349. [Google Scholar] [CrossRef]
- Hong, J.; Meng, L.; Yu, P.; Zhou, C.; Zhang, Z.; Yu, Z.; Qin, F.; Zhao, Y. Novel drug isolated from mistletoe (1E,4E)-1,7-bis(4-hydroxyphenyl)hepta-1,4-dien-3-one for potential treatment of various cancers: Synthesis, pharmacokinetics and pharmacodynamics. RSC Adv. 2020, 10, 27794–27804. [Google Scholar] [CrossRef]
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Y.; Pang, L.; Zhang, L.; Zhai, Y.; Zhou, H. Proliferation, Apoptosis and Invasion effects of mistletoe alkali on human osteosarcoma U2OS in vitro. Int. Surg. 2016, 101, 282–290. [Google Scholar] [CrossRef]
- Peng, H.Y.; Zhang, Y.H.; Han, Y.; Wang, M. [Studies on the anticancer effects of total alkaloid from Viscum coloratum]. Zhongguo Zhong Yao Za Zhi 2005, 30, 381–382. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Chai, Y.; Kan, L.; Zhao, M. Enzymatic extraction optimization, anti-HBV and antioxidant activities of polysaccharides from Viscum coloratum (Kom.) Nakai. Int. J. Biol. Macromol. 2019, 134, 588–594. [Google Scholar] [CrossRef]
- Blumenthal, R.S.; Alfaddagh, A. THE ABCDE’S OF PRIMARY PREVENTION OF CARDIOVASCULAR DISEASE. Trans. Am. Clin. Clim. Assoc. 2022, 132, 135–154. [Google Scholar]
- Chu, W.; Qiao, G.; Bai, Y.; Pan, Z.; Li, G.; Piao, X.; Wu, L.; Lu, Y.; Yang, B. Flavonoids from Chinese Viscum coloratum produce cytoprotective effects against ischemic myocardial injuries: Inhibitory effect of flavonoids on PAF-induced Ca2+ overload. Phytother. Res. 2008, 22, 134–137. [Google Scholar] [CrossRef]
- Colman, M.A. Arrhythmia mechanisms and spontaneous calcium release: Bi-directional coupling between re-entrant and focal excitation. PLoS Comput. Biol. 2019, 15, e1007260. [Google Scholar] [CrossRef]
- Wu, J.X.; Yu, G.R.; Wang, B.Y.; Zhong, D.S.; Huang, D.J. [Effects of Viscum coloratum flavonoids on fast response action potentials of hearts]. Zhongguo Yao Li Xue Bao 1994, 15, 169–172. [Google Scholar]
- Chen, C.C.; Liang, C.J.; Leu, Y.L.; Chen, Y.L.; Wang, S.H. Viscolin Inhibits In Vitro Smooth Muscle Cell Proliferation and Migration and Neointimal Hyperplasia In Vivo. PLoS ONE 2016, 11, e0168092. [Google Scholar] [CrossRef]
- Trépo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- Jose-Abrego, A.; Roman, S.; Laguna-Meraz, S.; Panduro, A. Host and HBV Interactions and Their Potential Impact on Clinical Outcomes. Pathogens 2023, 12, 1146. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, R.; Duan, M.; Yu, Z.; Zhao, Y. A study of pharmacokinetic interactions among co-existing ingredients in Viscum coloratum after intravenous administration of three different preparations to rats. Pharmacogn. Mag. 2015, 11, 455–462. [Google Scholar] [CrossRef]
- Fan, R.H.; Ding, W.; Ma, Y.Y.; Lin, H.L.; Men, L.; Duan, M.M.; Zhao, Y.L.; Yu, Z.G. Development of a sensitive ultra high performance liquid chromatography with tandem mass spectrometry method for the simultaneous quantification of nine active compounds in rat plasma and its application to a pharmacokinetic study after administration of Viscum coloratum extracts. J. Sep. Sci. 2015, 38, 530–540. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Zhao, Y.; Gao, X.; Bi, K.; Yu, Z. HPLC determination and pharmacokinetic study of homoeriodictyol-7-O-beta-D-glucopyranoside in rat plasma and tissues. Biol. Pharm. Bull. 2007, 30, 617–620. [Google Scholar] [CrossRef][Green Version]
- Evens, Z.N.; Stellpflug, S.J. Holiday plants with toxic misconceptions. West. J. Emerg. Med. 2012, 13, 538–542. [Google Scholar] [CrossRef]
- Yang, G.E.; Chen, B.; Zhang, Z.; Gong, J.; Bai, H.; Li, J.; Wang, Y.; Li, B. Cytotoxic activities of extracts and compounds from Viscum coloratum and its transformation products by Rhodobacter sphaeroides. Appl. Biochem. Biotechnol. 2009, 152, 353–365. [Google Scholar] [CrossRef]
- Hall, A.H.; Spoerke, D.G.; Rumack, B.H. Assessing mistletoe toxicity. Ann. Emerg. Med. 1986, 15, 1320–1323. [Google Scholar] [CrossRef]
- Krenzelok, E.P.; Jacobsen, T.D.; Aronis, J. American mistletoe exposures. Am. J. Emerg. Med. 1997, 15, 516–520. [Google Scholar] [CrossRef]

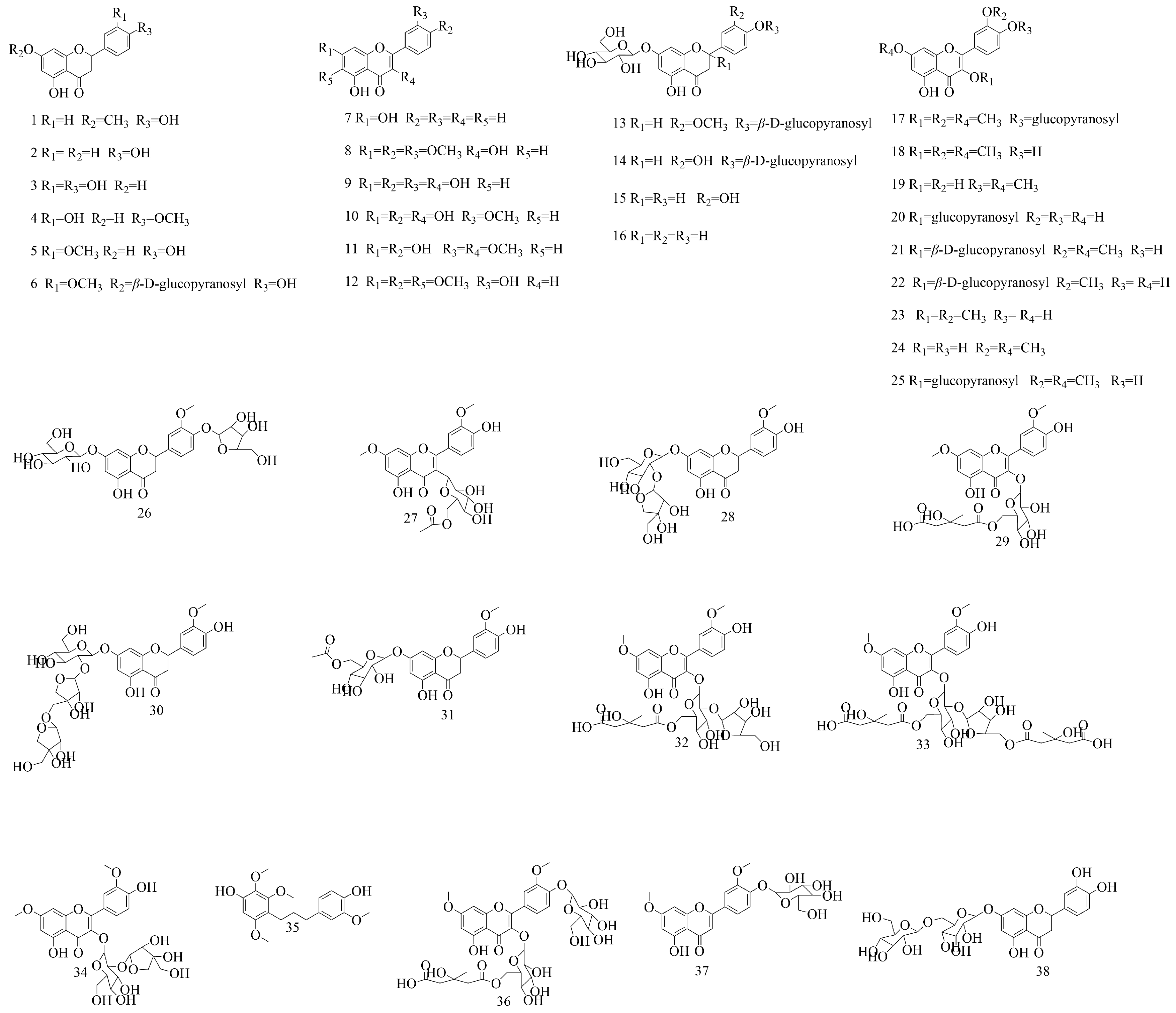
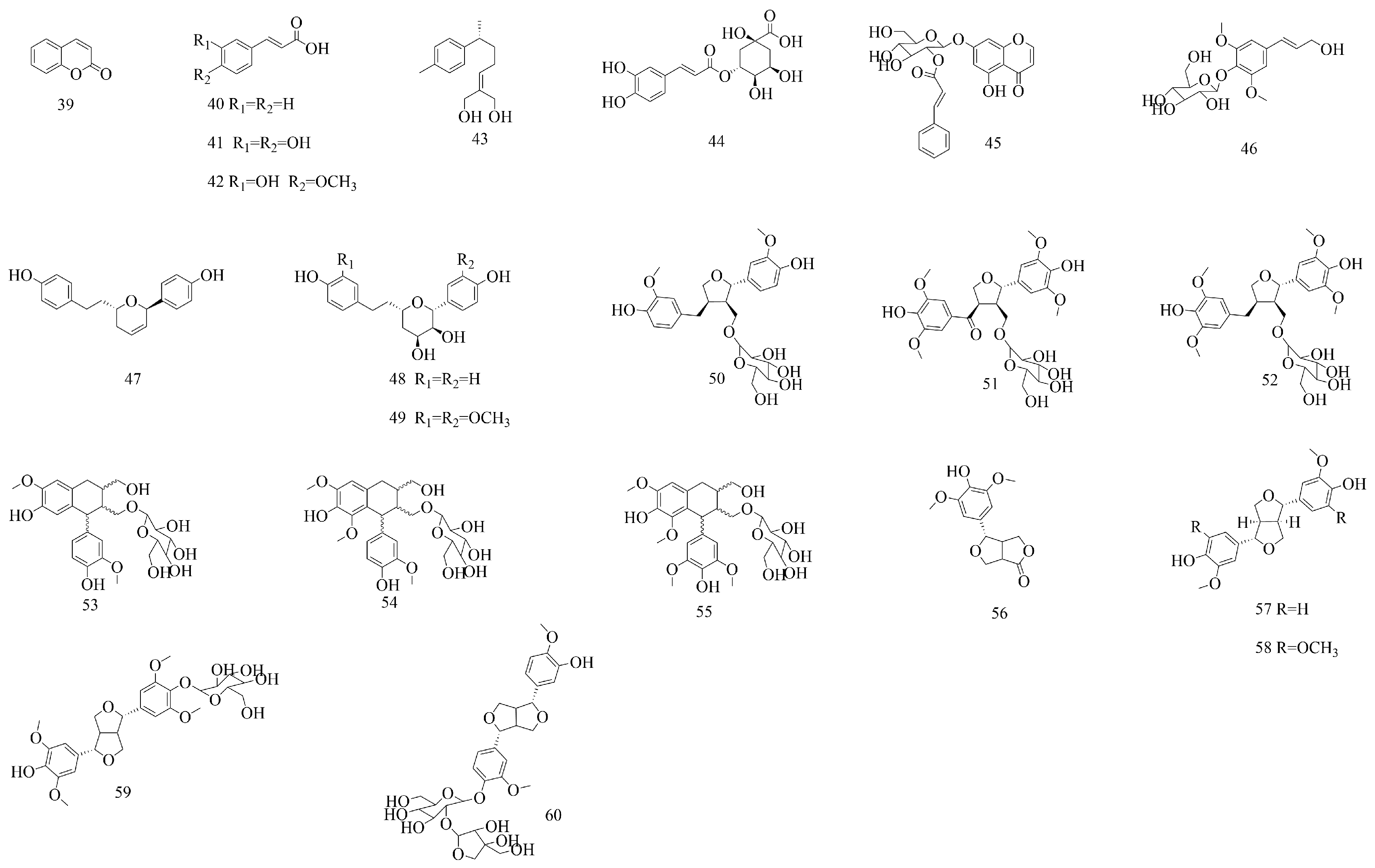
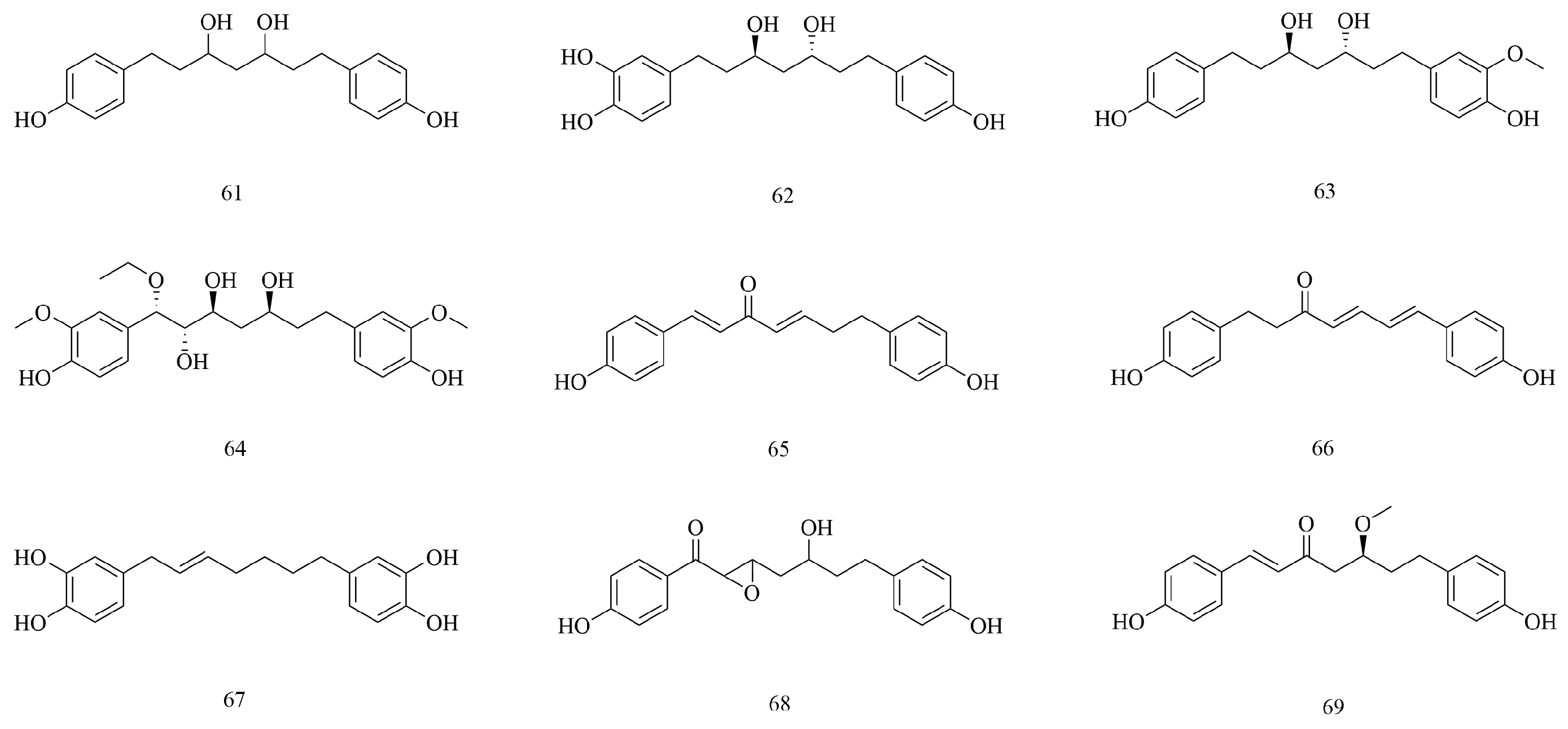
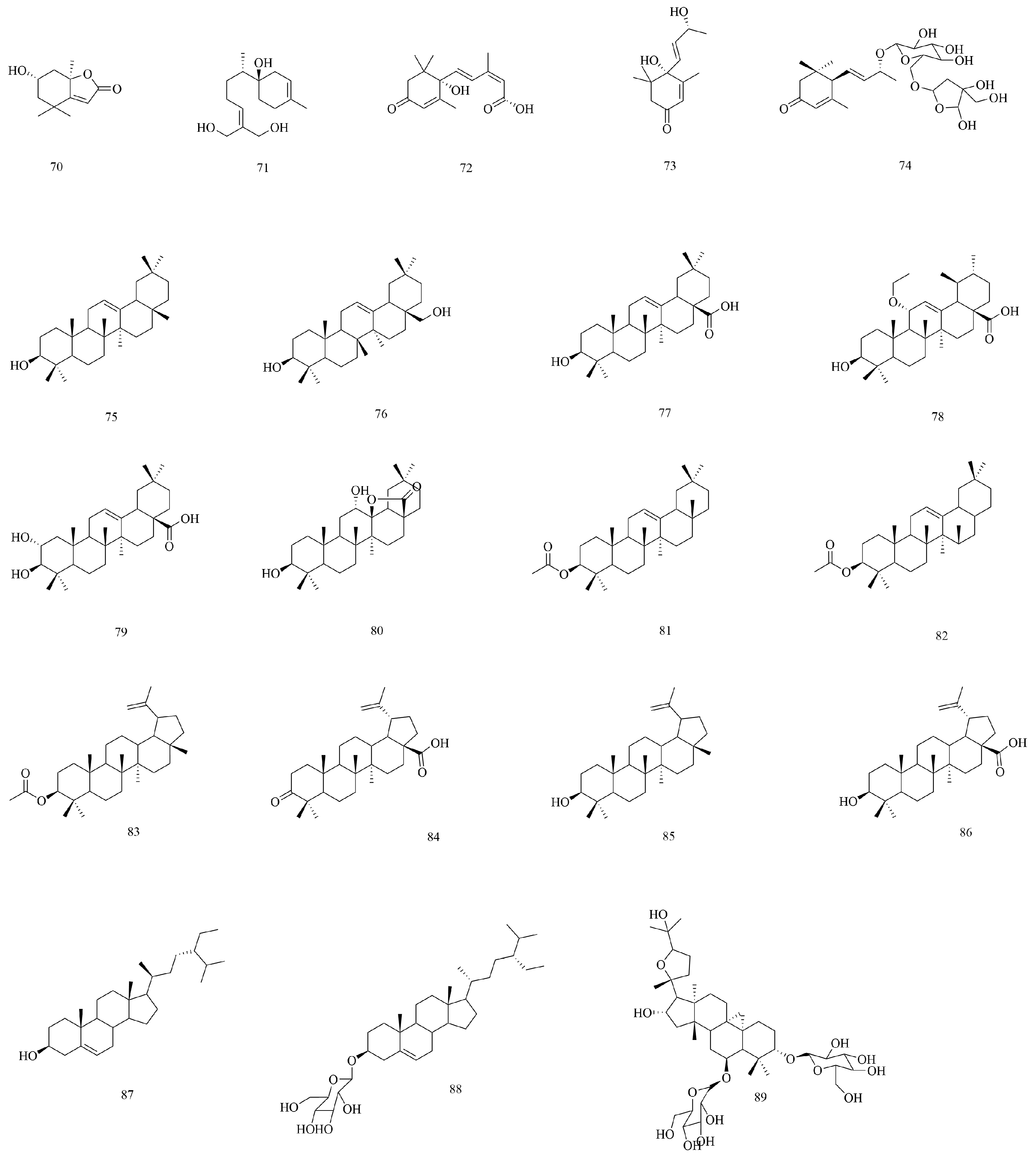
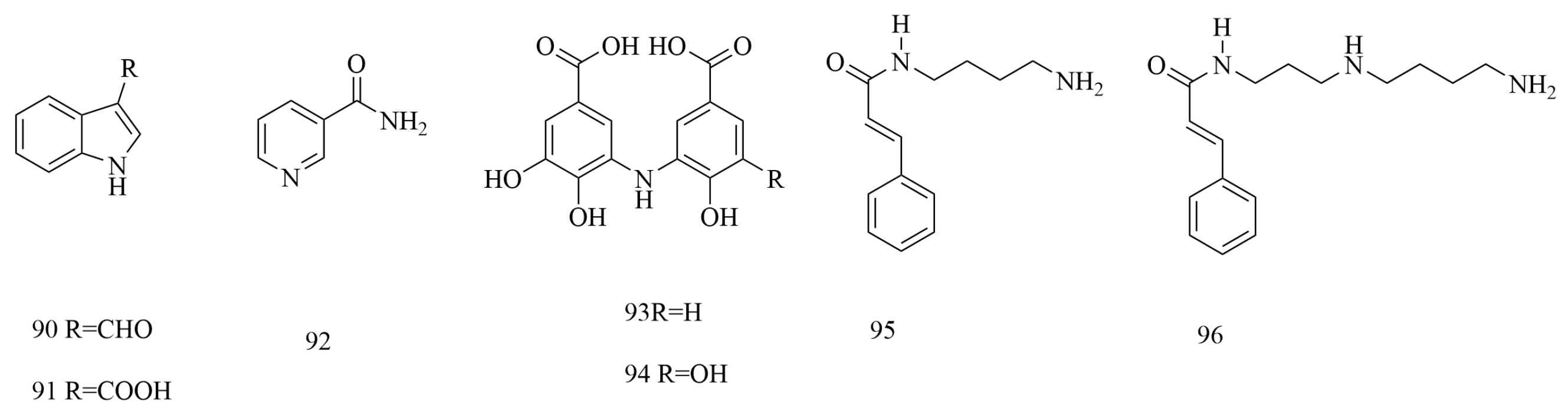
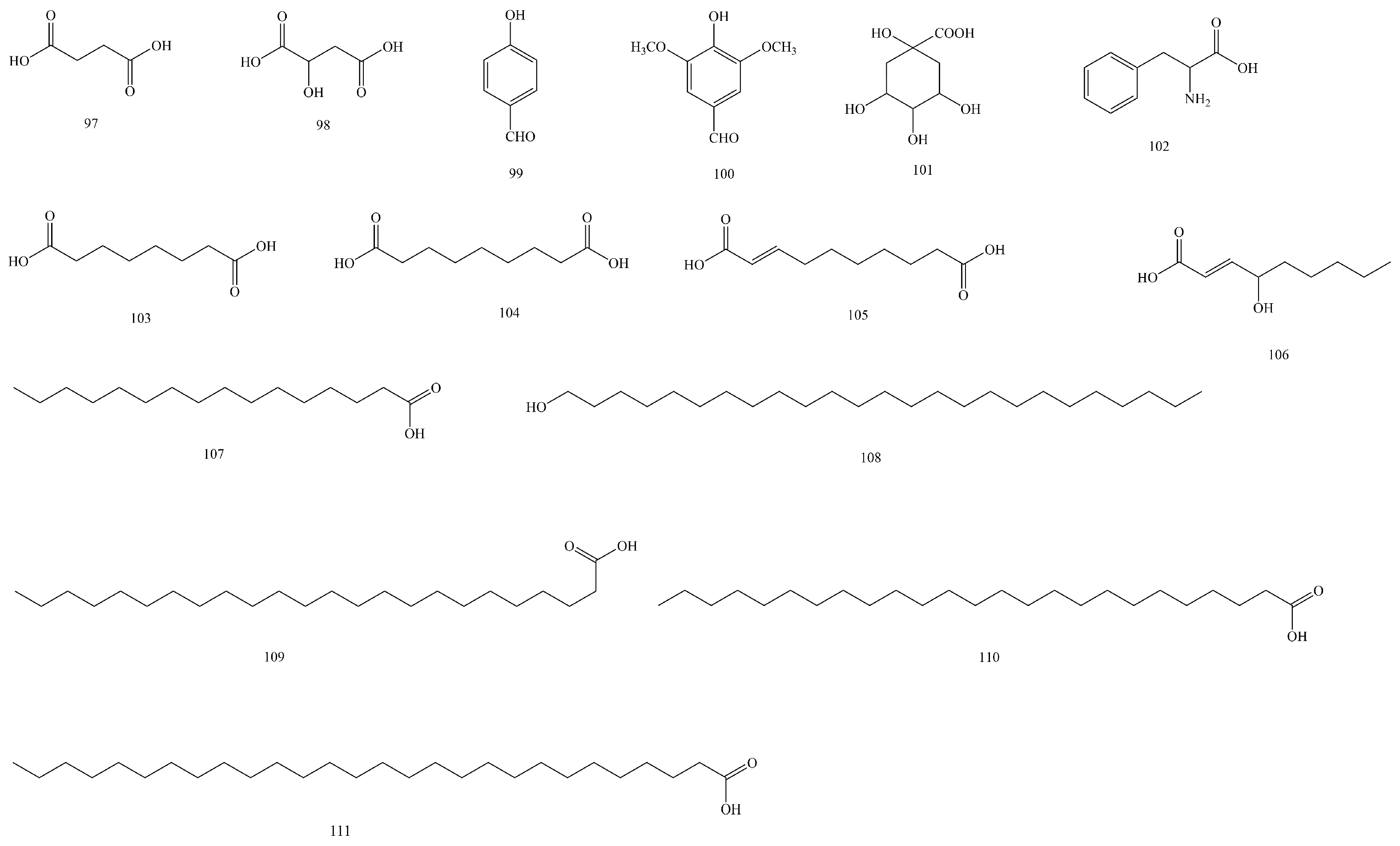
| No | Compounds | Molecular Formula | Extraction Solvent | Parts of the Plant | References |
|---|---|---|---|---|---|
| Flavonoids | |||||
| 1 | Sakuranetin | C16H14O5 | Water | Branches and leaves | [33] |
| 2 | Naringenin | C15H12O5 | Water | Branches and leaves | [33] |
| 3 | Eriodictyol | C15H12O6 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 4 | Hesperetin | C16H14O6 | Water | Branches and leaves | [33] |
| 5 | Homoeriodictyol | C16H14O6 | Water | Branches and leaves | [34] |
| 6 | Homoeriodictyol-7-O-β-D-glucoside | C22H24O11 | 95% ethanol | Stems and leaves | [34,35] |
| 7 | Chrysin | C15H10O4 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 8 | 7,3′,4′-Trimthylquercetin | C18H16O7 | 75% ethanol | Branches and leaves | [36] |
| 9 | Quercetin | C15H10O7 | Water | Branches and leaves | [33] |
| 10 | Isorhamnetin | C16H12O7 | Water | Branches and leaves | [33] |
| 11 | Quercetin-3,3′-dimethyl ether | C17H14O7 | 95% ethanol | Stems and leaves | [37] |
| 12 | Eupatorin | C18H16O7 | Water | Branches and leaves | [33] |
| 13 | (2S)-homoeriodictyol-7,4′-di-O-β-D-glucopyranoside | C28H34O16 | 90% ethanol | Branches and leaves | [35] |
| 14 | (2R)-eriodictyol 7,4′-di-O-β-D-glucopyranoside | C27H32O16 | 90% ethanol | Branches and leaves | [35] |
| 15 | (2S)-eriodictyol-7-O-β-D-glucopyranoside | C21H22O11 | 90% ethanol | Branches and leaves | [35] |
| 16 | (2S)-naringenin-7-O-β-D-glucopyranoside | C21H22O10 | 90% ethanol | Branches and leaves | [35] |
| 17 | 5-Hydroxy-3,7,3′-trimethoxyflavone-4′-O-β-D-glucoside | C24H26O12 | 50% methanol | Aboveground parts | [16,34,37] |
| 18 | Pachypodol | C18H16O7 | 50% methanol | Aboveground parts | [37] |
| 19 | Ombuine | C17H14O7 | 95% ethanol | Stems and leaves | [37] |
| 20 | Hyperoside | C21H20O12 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 21 | Rham-I | C23H24O12 | 50% methanol | Aboveground parts | [34] |
| 22 | Isorhamnetin-3-O-β-D-glucoside | C22H22O12 | 50% methanol | Aboveground parts | [34] |
| 23 | 5,7,4′-Trihydroxy-3,3′-dimethoxyflavone | C17H14O7 | 50% methanol | Aboveground parts | [34] |
| 24 | Rhamnazine | C17H14O7 | 95% ethanol | Stems and leaves | [38] |
| 25 | Rhamnazin-3-O-β-D-glucoside | C23H24O12 | 95% ethanol | Stems and leaves | [37] |
| 26 | Viscumneoside I | C27H32O15 | Water | Stems and leaves | [15] |
| 27 | Viscumneoside II | C25H26O12 | 95% ethanol | Stems and leaves | [39] |
| 28 | Viscumneoside III | C27H32O15 | 50% methanol | Aboveground parts | [37] |
| 29 | Viscumneoside IV | C29H32O16 | 95% ethanol | Stems and leaves | [40] |
| 30 | Viscumneoside V | C32H40O19 | Water | Branches and leaves | [33] |
| 31 | Viscumneoside VI | C24H26O12 | 95% ethanol | Stems and leaves | [37] |
| 32 | Viscumneoside VII | C34H40O20 | Water | Branches and leaves | [33] |
| 33 | Viscumneoside VIII | C40H48O24 | 95% ethanol | Stems and leaves | [41] |
| 34 | viscumneoside IX | C28H32O16 | 95% ethanol | Stems and leaves | [41] |
| 35 | Viscolin | C19H24O6 | Methanol | Stems | [42] |
| 36 | Rham-III | C35H42O21 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 37 | Flavoyadorinin-B | C23H24O11 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 38 | Eriocitrin | C27H32O15 | Water | Branches and leaves | [33] |
| Phenylpropanoids | |||||
| 39 | Coumarin | C9H6O2 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 40 | Cinnamic acid | C9H8O2 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 41 | Caffeic acid | C9H8O4 | 95% ethanol | Aboveground parts | [43] |
| 42 | Ferulic acid | C10H10O4 | 95% ethanol | Aboveground parts | [43] |
| 43 | Curcumene A | C15H22O2 | 95% ethanol | Stems and leaves | [44] |
| 44 | Chlorogenic acid | C16H18O9 | Water | Branches and leaves | [33] |
| 45 | Liquidamboside | C24H22O10 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 46 | Syringin | C17H24O9 | Water | Branches and leaves | [16,33,37] |
| 47 | 5,6-Dehydro-4″-de-O-methylcentrolobin | C19H20O3 | 95% ethanol | Stems and leaves | [44] |
| 48 | (2R,3S,4S,6S)-6-(4-hydroxyphenethyl)-2-(4-hydroxyphenyl)-tetrahydro-2H-pyran-3,4-diol | C19H22O5 | 95% ethanol | Stems and branches | [45] |
| 49 | (1R,2S,3S,5S)-2,3-dihydroxy-3′,3″-dimethoxy-4′de-O-methylcentrolobine. | C21H26O7 | 95% ethanol | Stems and branches | [45] |
| 50 | (+)-Lariciresinol-9-O-β-D-glucopyranoside | C26H34O11 | 95% ethanol | Stems and leaves | [44] |
| 51 | Aketrilignoside B | C28H36O14 | 95% ethanol | Stems and leaves | [44] |
| 52 | Alangilignoside C | C28H38O13 | 95% ethanol | Stems and leaves | [44] |
| 53 | (+)-Isolariciresinol-9′-O-β-glucopyranoside | C26H34O11 | 95% ethanol | Stems and leaves | [44] |
| 54 | (6R,7S,8S)-7α-[(β-D-glucopyranosyl)-oxy]-1-methoxyisolariciresinol | C27H36O12 | 95% ethanol | Stems and leaves | [44] |
| 55 | (8R,7′S,8′S)-7α-[(β-D-glucopyranosyl)-oxy]- lyoniresinol | C28H38O13 | 95% ethanol | Stems and leaves | [44] |
| 56 | Zhebeiresinol | C14H16O6 | 75% ethanol | Stems and leaves | [46] |
| 57 | (+)-Epipinoresinol | C20H22O6 | 75% ethanol | Branches and leaves | [45] |
| 58 | Syringaresinol | C22H26O8 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 59 | Syringaresinol-O-β-D-glucopyranoside | C29H40O13 | 95% ethanol | Stems and leaves | [15] |
| 60 | pinoresinol-4-O-β-D-apiosly-(1→2)-β-D-glucoside | C31H40O15 | 95% ethanol | Aboveground parts | [44] |
| Diphenylheptanes | |||||
| 61 | 1,7-Bis(4-hydroxyphenyl)-heptane-3,5-diol | C19H24O4 | 95% ethanol | Stems and branches | [45] |
| 62 | (3R,5R)-3,5-dihydroxy-1-(3,4-dihydroxyphe-nyl)-7-(4-hydroxyphenyl)-heptane | C19H24O5 | 95% ethanol | Stems and leaves | [44] |
| 63 | (3S,5S)-1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-heptane-3,5-diol | C20H26O5 | 95% ethanol | Stems and leaves | [44] |
| 64 | Diphenylheptane C. | C23H32O8 | 95% ethanol | Stems and leaves | [44] |
| 65 | 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one | C19H18O3 | 95% ethanol | Stems and leaves | [37] |
| 66 | 1,7-Di-(4-hydroxyphenyl)-4E,6E-heptadiene-3-ketone | C19H18O3 | 95% ethanol | Stems and leaves | [44] |
| 67 | Diphenylheptane B | C19H22O4 | 95% ethanol | Stems and leaves | [44] |
| 68 | Mistletonone | C19H20O5 | 90% ethanol | Branches and leaves | [17] |
| 69 | 1,7-Bis(4-hydroxyphenyl)-5-methoxyhept-1-en-3-one | C20H22O4 | 95% ethanol | Stems and branches | [45] |
| Terpenoids | |||||
| 70 | Loliolide | C11H16O3 | 75% ethanol | Branches and leaves | [36] |
| 71 | (1R,7S)-1,12,13-trihydroxybisabola-3,10-diene. | C15H26O3 | 95% ethanol | Stems and branches | [45] |
| 72 | (2Z,4E)-5-((S)-1-hydroxy-2,6,6-trimethyl-4-oxocyclohex-2-en-1-yl)-3-methylpenta-2,4-dienoic acid | C15H20O4 | 95% ethanol | Stems and branches | [45] |
| 73 | Vomifoliol | C13H20O3 | 95% ethanol | Stems and leaves | [44] |
| 74 | Eriobotroside II | C24H38O11 | 95% ethanol | Stems and leaves | [44] |
| 75 | β-Amyrin | C30H50O | 75% ethanol | Branches and leaves | [36] |
| 76 | Erythordiol | C30H50O2 | 95% ethanol | Aboveground parts | [46] |
| 77 | Oleanolic acid | C30H48O3 | Water | Branches and leaves | [16,37] |
| 78 | Alstolarnoid D | C32H52O4 | 95% ethanol | Stems and leaves | [44] |
| 79 | Maslinic acid | C30H48O4 | 70% ethanol | Aboveground parts | [33] |
| 80 | Oleanane-type triterpene | C30H48O4 | 95% ethanol | Stems and leaves | [44] |
| 81 | β-Acetylamyrin | C32H52O2 | 95% ethanol | Stems and leaves | [47] |
| 82 | β-Amyrin acetate | C32H52O2 | 75% ethanol | Branches and leaves | [36] |
| 83 | Lupeol acetate | C32H52O2 | 95% ethanol | Stems and leaves | [37] |
| 84 | Betulonic acid | C30H46O3 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 85 | Lupeol | C30H50O | 75% ethanol | Branches and leaves | [36] |
| 86 | 3-Epi-betulinic acid | C30H48O3 | 75% ethanol | Branches and leaves | [36] |
| 87 | β-Sitosterol | C29H50O | 50% (v/v) methanol–water | Aboveground parts | [37] |
| 88 | Daucosterol | C35H60O6 | 75% ethanol | Stems and leaves | [46] |
| 89 | Astragaloside IV | C41H68O14 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| Alkaloids | |||||
| 90 | Indole-3-carboxaldehyde | C9H7NO | 95% ethanol | Stems and branches | [45] |
| 91 | Indole-3-carboxylic acid | C9H7NO2 | 95% ethanol | Stems and branches | [45] |
| 92 | Nicotinamide | C6H6N2O | 75% ethanol | Branches and leaves | [36] |
| 93 | 4,5,4′-Trihydroxy-3,3′-iminodibenzoic acid | C14H11NO7 | Methanol | Aboveground parts | [22] |
| 94 | 4,5,4′,5′-Tetrahydroxy-3,3′-iminodibenzoic acid | C14H11NO8 | Methanol | Aboveground parts | [22] |
| 95 | N-cinnamoylbutanediamine | C13H18N2O | Hydrochloric acid | Aboveground parts | [48] |
| 96 | N-cinnamidylspermidine | C16H25N3O | Hydrochloric acid | Aboveground parts | [33,48] |
| Other compounds | |||||
| 97 | Succinic acid | C4H6O4 | 95% ethanol | Aboveground parts | [43] |
| 98 | Malic acid | C4H6O5 | Water | Branches and leaves | [33] |
| 99 | 4-Hydroxybenzaldehyde | C7H6O2 | 95% ethanol | Stems and branches | [45] |
| 100 | 4-Hydroxy-3,5-dimethoxybenzaldehyde | C9H10O4 | 95% ethanol | Stems and branches | [45] |
| 101 | Quinic acid | C7H12O6 | Water | Branches and leaves | [33] |
| 102 | Phenylalanine | C9H11NO2 | 50% (v/v) methanol–water | Aboveground parts | [16] |
| 103 | Octanedioic acid | C8H14O4 | 95% ethanol | Stems and branches | [45] |
| 104 | Nonanedioic acid | C9H16O4 | 95% ethanol | Stems and branches | [45] |
| 105 | (E)-Dec-2-enedioic acid | C10H16O4 | 95% ethanol | Stems and branches | [45] |
| 106 | (E)-4-hydroxynon-2-enoic acid | C9H16O3 | 95% ethanol | Stems and branches | [45] |
| 107 | Palmitic acid | C16H32O2 | 95% ethanol | Aboveground parts | [43] |
| 108 | Pentacosanol | C25H52O | 75% ethanol | Branches and leaves | [36] |
| 109 | Lignoceric acid | C24H48O2 | 95% ethanol | Aboveground parts | [43] |
| 110 | Cerotic acid | C25H50O2 | 95% ethanol | Aboveground parts | [43] |
| 111 | Octacosanioc acid | C28H56O2 | 95% ethanol | Aboveground parts | [43] |
| No | Name | Extraction Solvent | Composition | Molar Ratio | Mw (kDa) | Total Yield (%) | References |
|---|---|---|---|---|---|---|---|
| 1 | VCP1 | Water | Glc, Gal, Ara, Rha, Man | 30.6:34.3:14.9:1.7:18.5 | 32 | 15 | [57] |
| 2 | VCP2 | Water | Glc, Gal, Ara, GluA, GalA, Rha, Man | 8.4:14.5:43.2:1.8:18.8:6.3:7.0 | 280 | 10 | [57] |
| 3 | VCP3 | Water | Glc, Gal, Ara, GluA, GalA, Rha, Man | 5.6:10.5:33.3:1.3:31.1:13.8:4.4 | 21 | 5 | [57] |
| No | Name | Relative Molecular Weight of Subunits/k Da | Sugar Specificity | References |
|---|---|---|---|---|
| 1 | CM-1 | 27, 31 | D-Galactose | [20] |
| 2 | CM-2 | 29, 32 | D-Galactose | [20] |
| 3 | ACML-55 | 29, 35 | D-Galactose | [59] |
| 4 | VCL | 29, 35 | D-Galactose | [20] |
| 5 | ML | 30, 34 | D-Galactose | [60] |
| 6 | CM-0 | Not detected | D-Galactose | [20] |
| Activity | Study design | Models | Dosages | Results | References |
|---|---|---|---|---|---|
| Anti-inflammatory effect | In vivo | Collagen-induced arthritis (CIA) mode | 2 g/kg | ↓ Inflammation and bone erosion, ↑ cartilage protection | [33] |
| In vivo | DSS-induced colitis mode | 0–200 mg/kg | ↓ In vivo: DSS-induced colitis | [65] | |
| In vitro | Human neutrophil model | 1–30 μM 1–100 μg/mL | ↓ Human neutrophil proinflammatory responses | [42] | |
| In vivo | TNF-α-treated mouse model | 10 mg/kg/day | ↓ Atherosclerosis and inflammatory responses | [66] | |
| In vivo | VA-sensitized mouse model | 5 mg/kg | ↓ Airway inflammation and eosinophil infiltration | [68] | |
| Anticancer effect | In vitro | HepG 2 cells | 0.2, 0.4, 0.6, 0.8, 1.0 mg/mL | ↑ VCP concentration, ↑ inhibition rate | [78] |
| In vitro | A549 cells, NCI-H292 cells | 0, 2.5, 5, 10, 20, 30, 40 μM | ↑ Dose and inhibition rate | [71] | |
| In vitro | Twelve types of cancer cells | 1~100 μmol/L | Significant therapeutic effects on lung cancer and breast cancer | [72] | |
| In vitro | Human osteosarcoma cells | 1.25, 2.5, 5, 10, 20, 40, and 80 μg/mL | IC50 of V. coloratum >5-FU | [74] | |
| In vitro in vivo | Eca109 cells, MCF-7 cells, H22cells | 60, 90,120 mg/kg | ↑ The dose, ↑ Inhibition rate of cancer cells | [75] | |
| Antioxidant effect | In vitro | Hydroxyl radicals, superoxide anion radicals | 0.18, 0.36, 0.54, 0.72,0.90 mM. 0.06, 0.12, 0.18, 0.24, 0.30 mM. | IC50 values are 0.485 mM and 0.273 mM | [1] |
| In vitro | DPPH and hydroxyl radical | 2–10 mg/mL | ↑ 2–6 mg/mL of VCP, DPPH Radical scavenging rate ↑ 2–10 mg/mL of VCP, hydroxyl radical scavenging rate | [78] | |
| In vitro | Hydroxyl radicals, superoxide anion radicals | 100 μL | The antioxidant property of (2S)-naringenin 7-O-β-D-glucopyranoside is the strongest | [35] | |
| Anti-cardiovascular disease effect | In vivo | Myocardial infarction model | 15 mg/kg, 75 mg/kg | ↓ Calcium influx mediated | [80] |
| In vivo | Dog heart Purkinje cells, guinea pig ventricular myocytes | 100 μg/mL | VCF is effective for rapid arrhythmias | [82] | |
| In vivo | Intracavitary mechanical injury model | 100 μg/kg | ↓ Proliferation of HASMCs | [83] | |
| Other effects | In vitro | HepG2.2.15 cells | 10 mg/mL | ↑ VCP concentration and inhibition rate | [21] |
| in vivo | Ovariectomized rat model | 50 mg/kg 100 mg/kg | ↓ Osteoclast activity | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, H.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Viscum coloratum (Komar.) Nakai: A Review of Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology. Biomolecules 2025, 15, 974. https://doi.org/10.3390/biom15070974
Di H, Shen C, Zhang S, Wang Y, Guan F. Viscum coloratum (Komar.) Nakai: A Review of Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology. Biomolecules. 2025; 15(7):974. https://doi.org/10.3390/biom15070974
Chicago/Turabian StyleDi, Han, Congcong Shen, Shengyu Zhang, Yanhong Wang, and Feng Guan. 2025. "Viscum coloratum (Komar.) Nakai: A Review of Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology" Biomolecules 15, no. 7: 974. https://doi.org/10.3390/biom15070974
APA StyleDi, H., Shen, C., Zhang, S., Wang, Y., & Guan, F. (2025). Viscum coloratum (Komar.) Nakai: A Review of Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology. Biomolecules, 15(7), 974. https://doi.org/10.3390/biom15070974






