Methionine Restriction Differentially Modulates Expression of Genes in the Base Excision Repair Pathway in Rat Brain and Liver
Abstract
1. Introduction
1.1. Aging and DNA Instability
1.2. Anti-Aging Dietary Interventions
2. Materials and Methods
2.1. Animals and Diets
2.2. RNA Isolation and RT-PCR
2.3. Statistical Analysis
3. Results
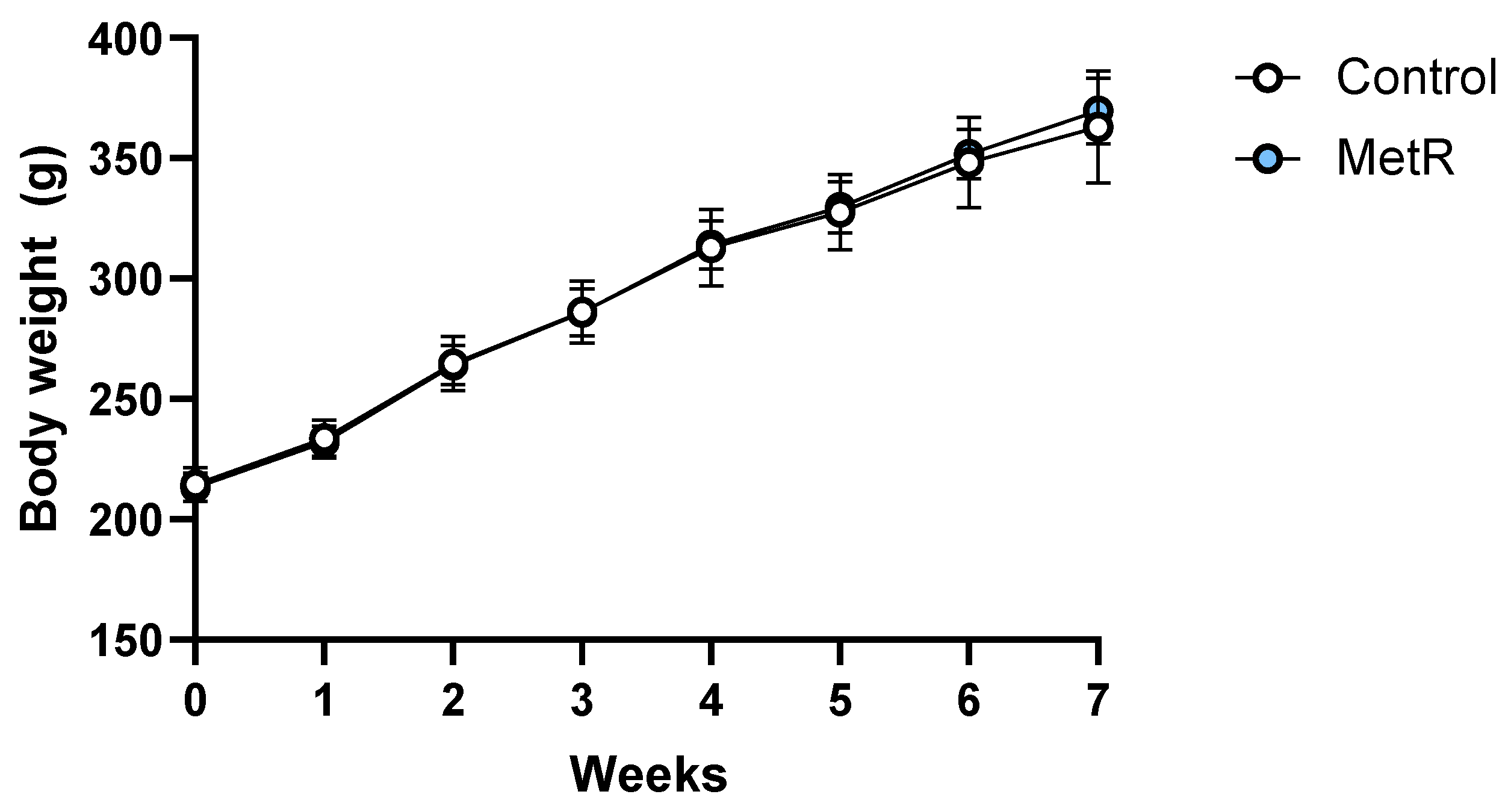
3.1. Body Weight of Rats and Food Intake Were Not Affected by Methionine Restriction
3.2. Rats Subjected to Methionine Restriction Show Similar Transcription Levels of Mnsod to Control Counterparts Both in Liver and Brain
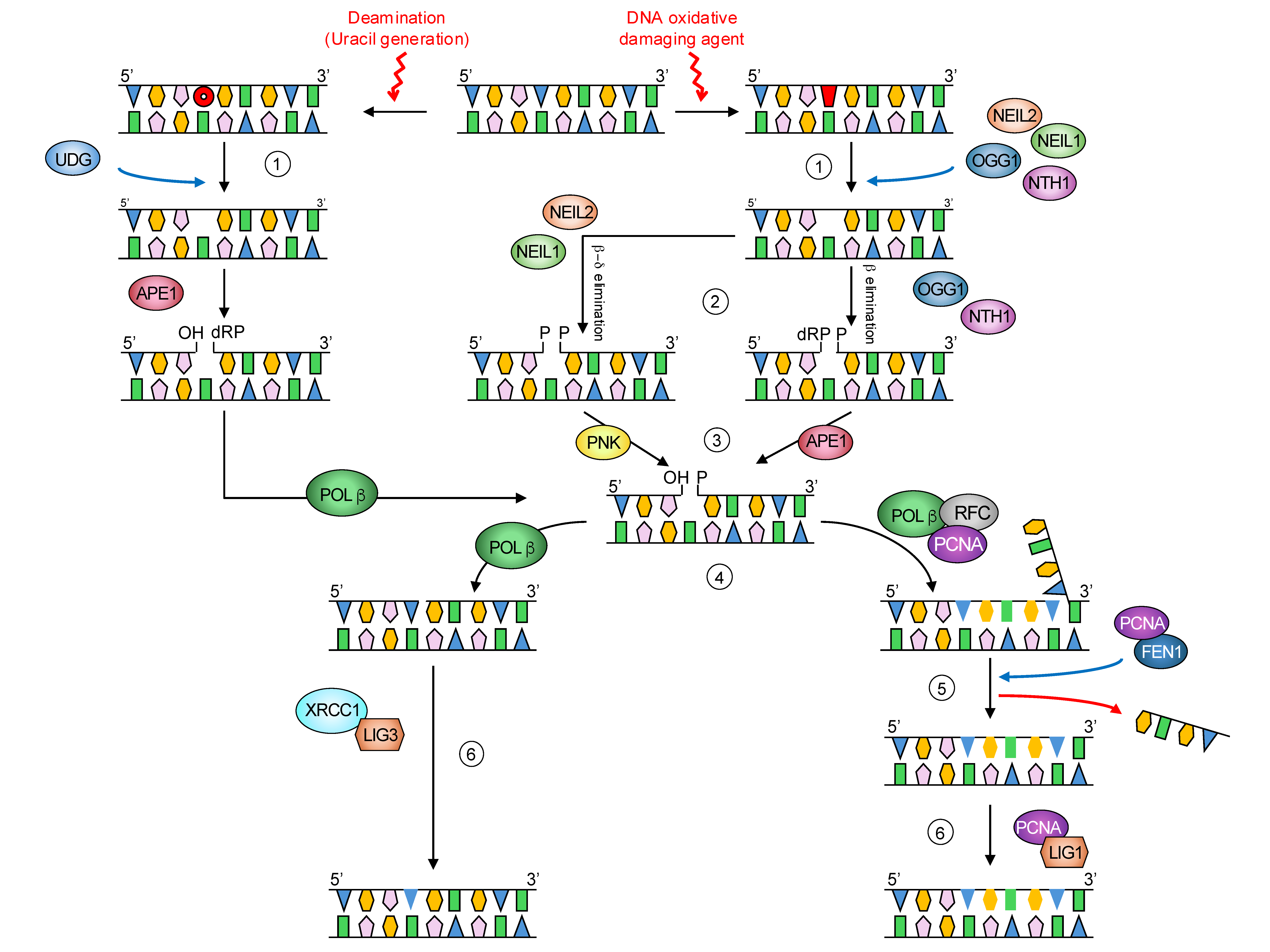
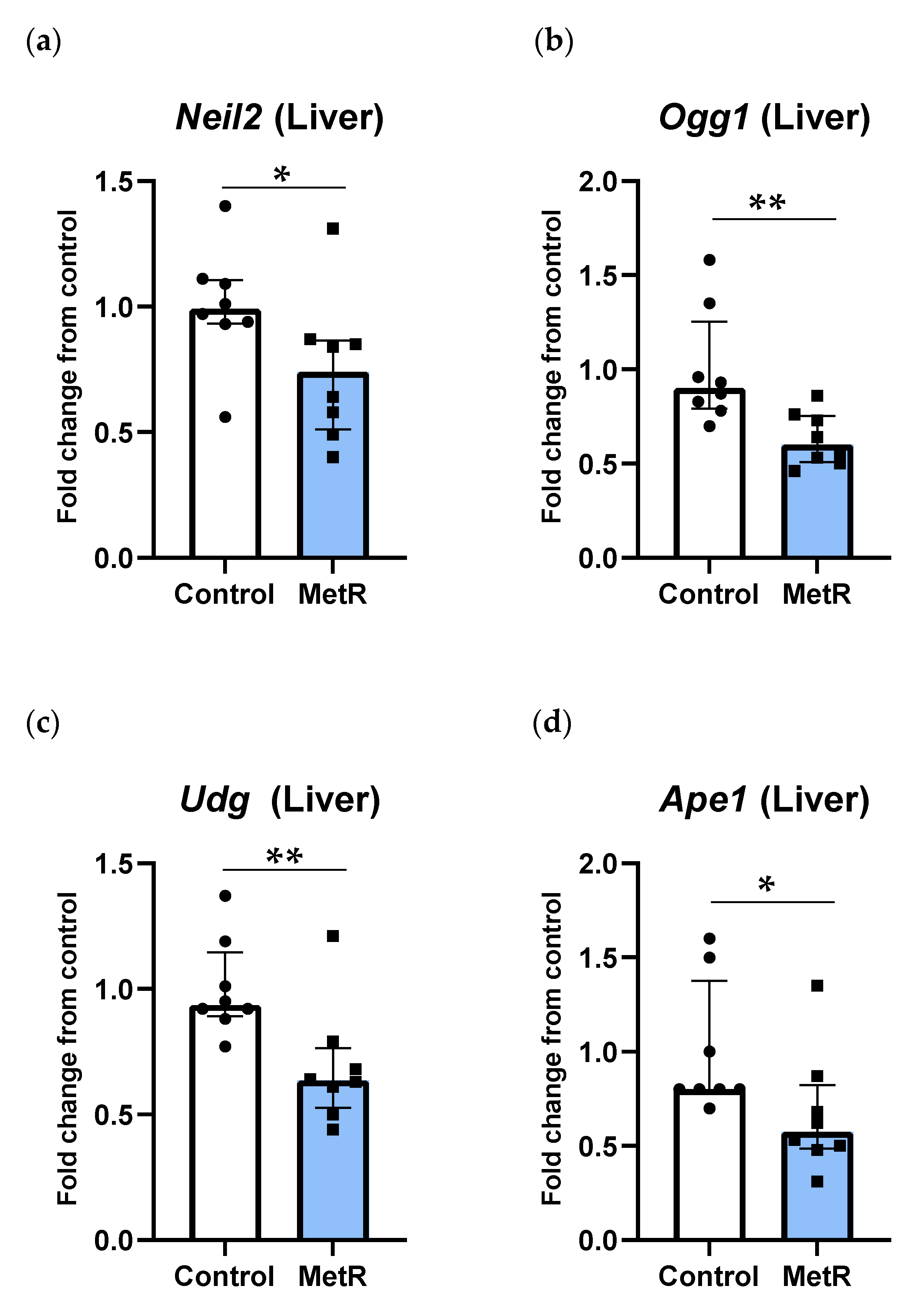
3.3. DNA Glycosylases and Ape1 Genes Were Downregulated by Methionine Restriction in Rat Liver
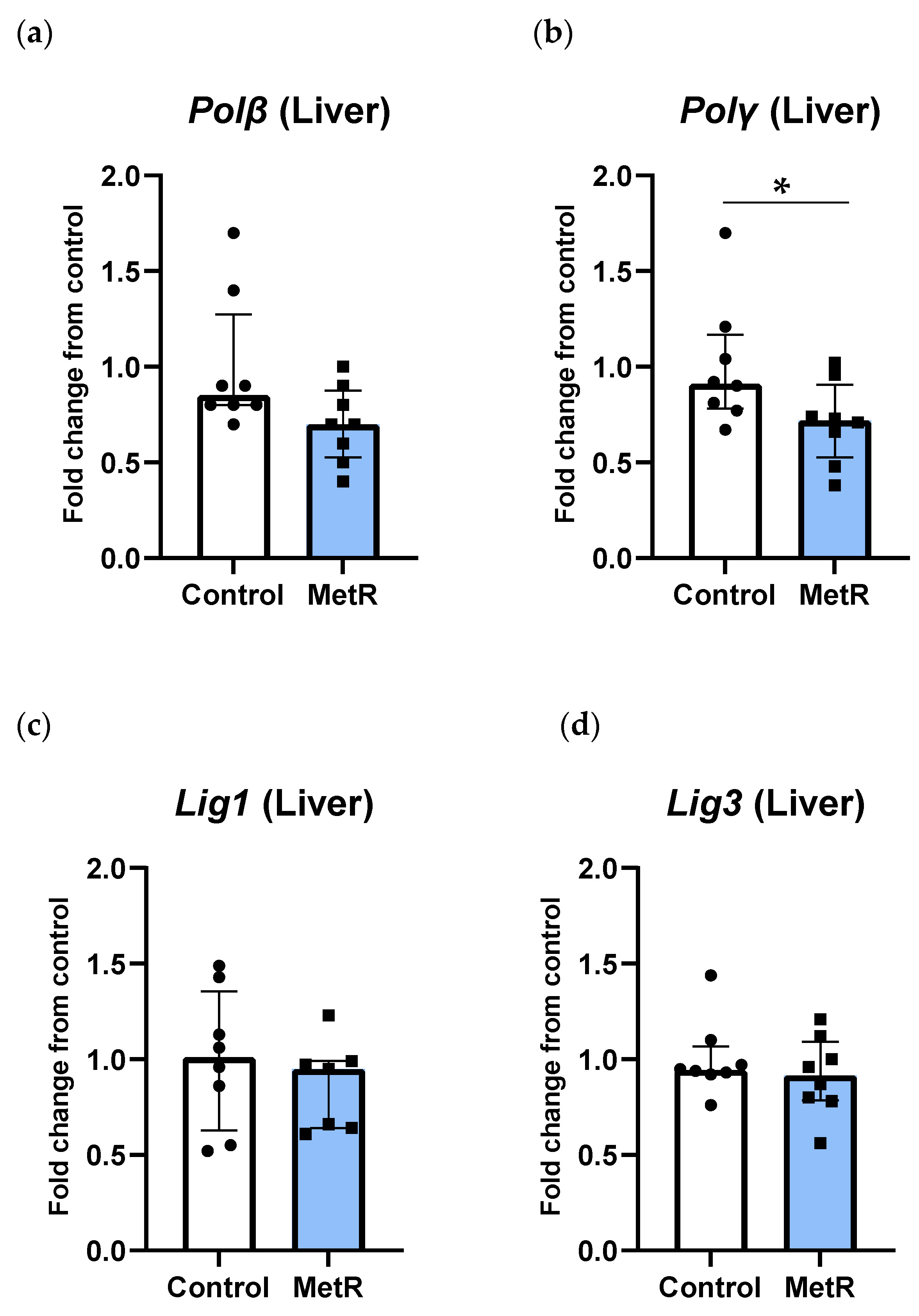
3.4. Transcription of Genes Related to DNA Polymerases but Not DNA Ligases Was Reduced After Methionine Restriction in Rat Liver
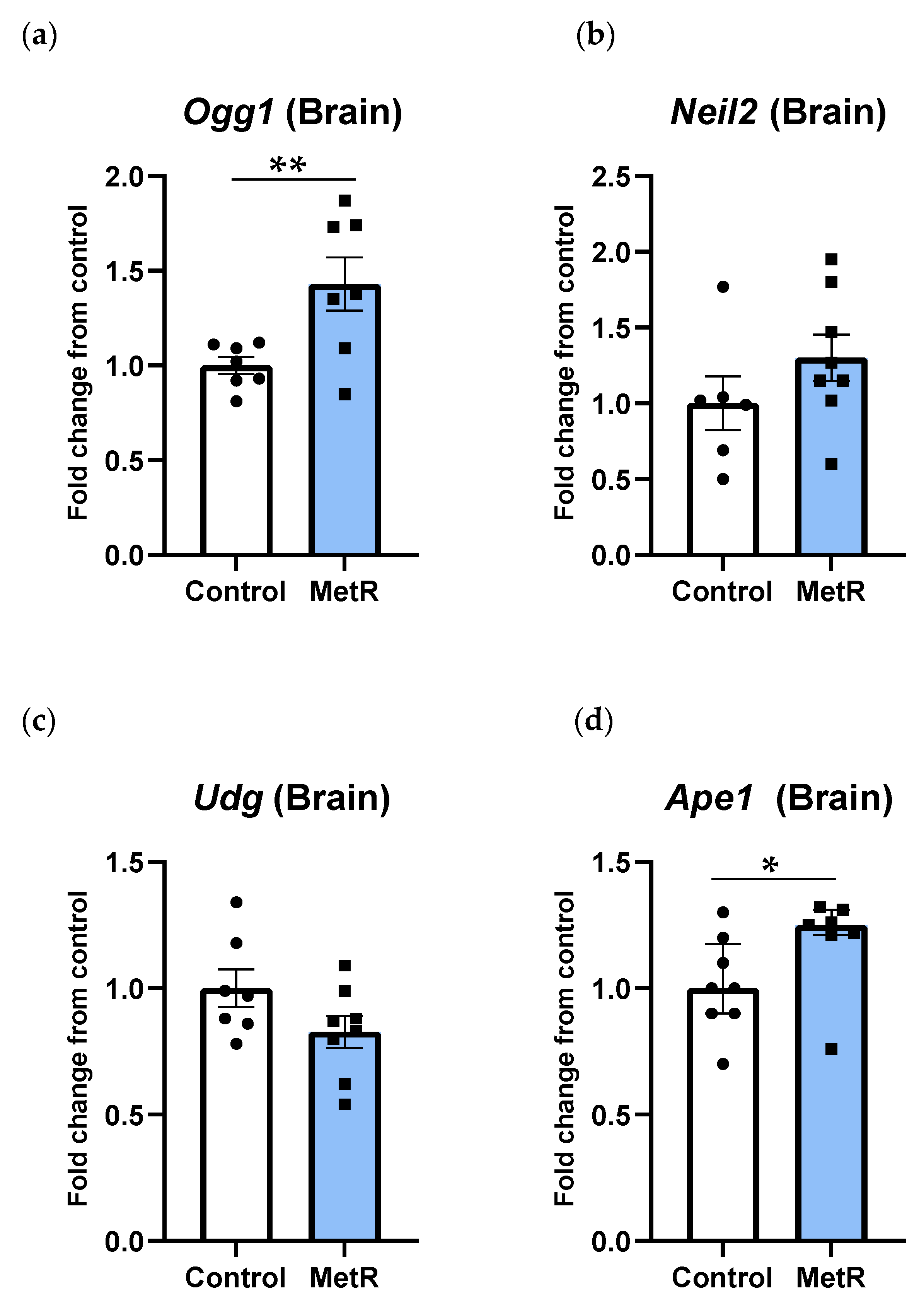
3.5. Ogg1 and Ape1 Genes Were Upregulated in Rat Brain, While Neil2 and Udg Genes Were Not Affected After Methionine Restriction in Rat Brain
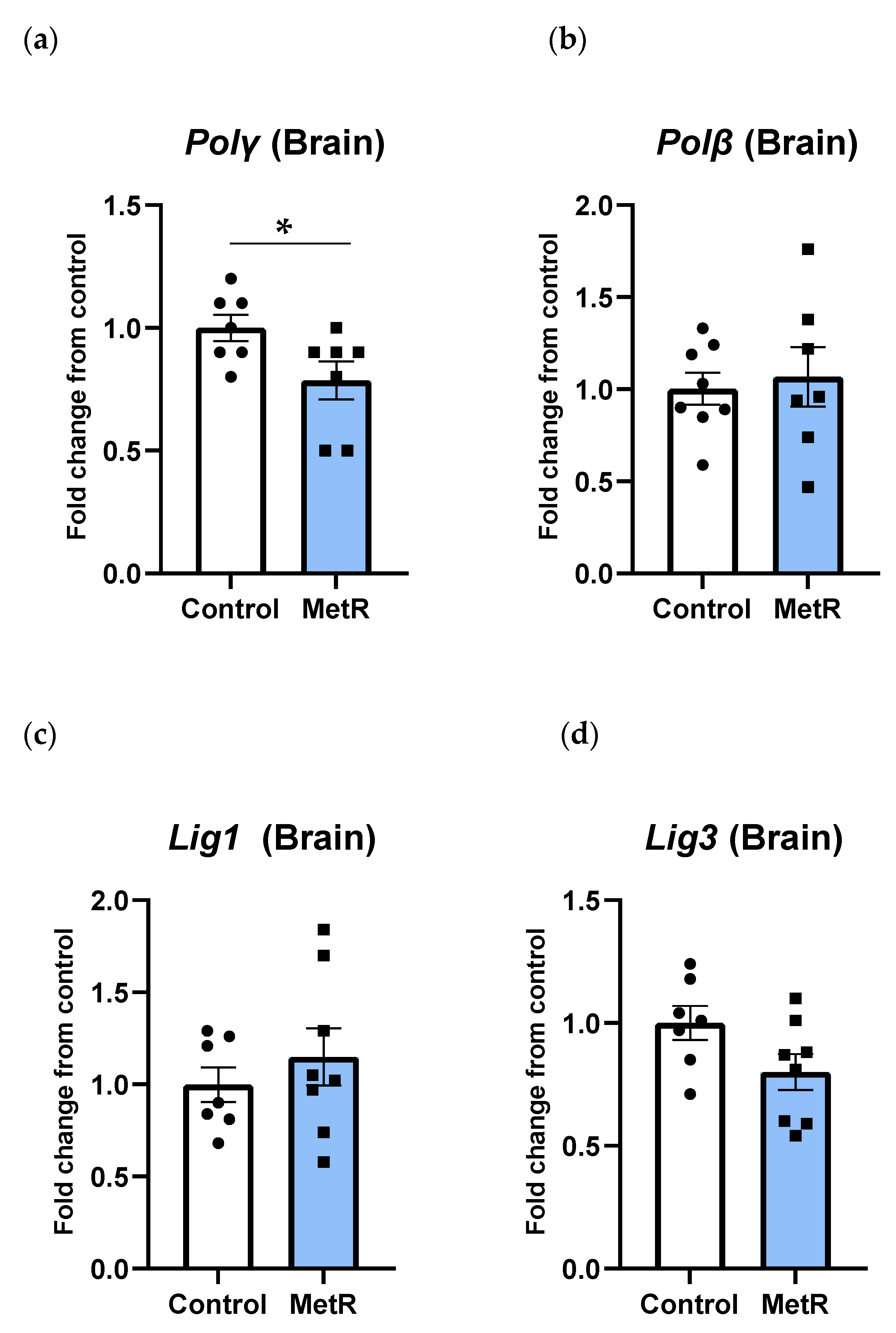
3.6. Gene Transcription of DNA Polymerases and DNA Ligases Was Differently Modulated by Methionine Restriction in Rat Brain
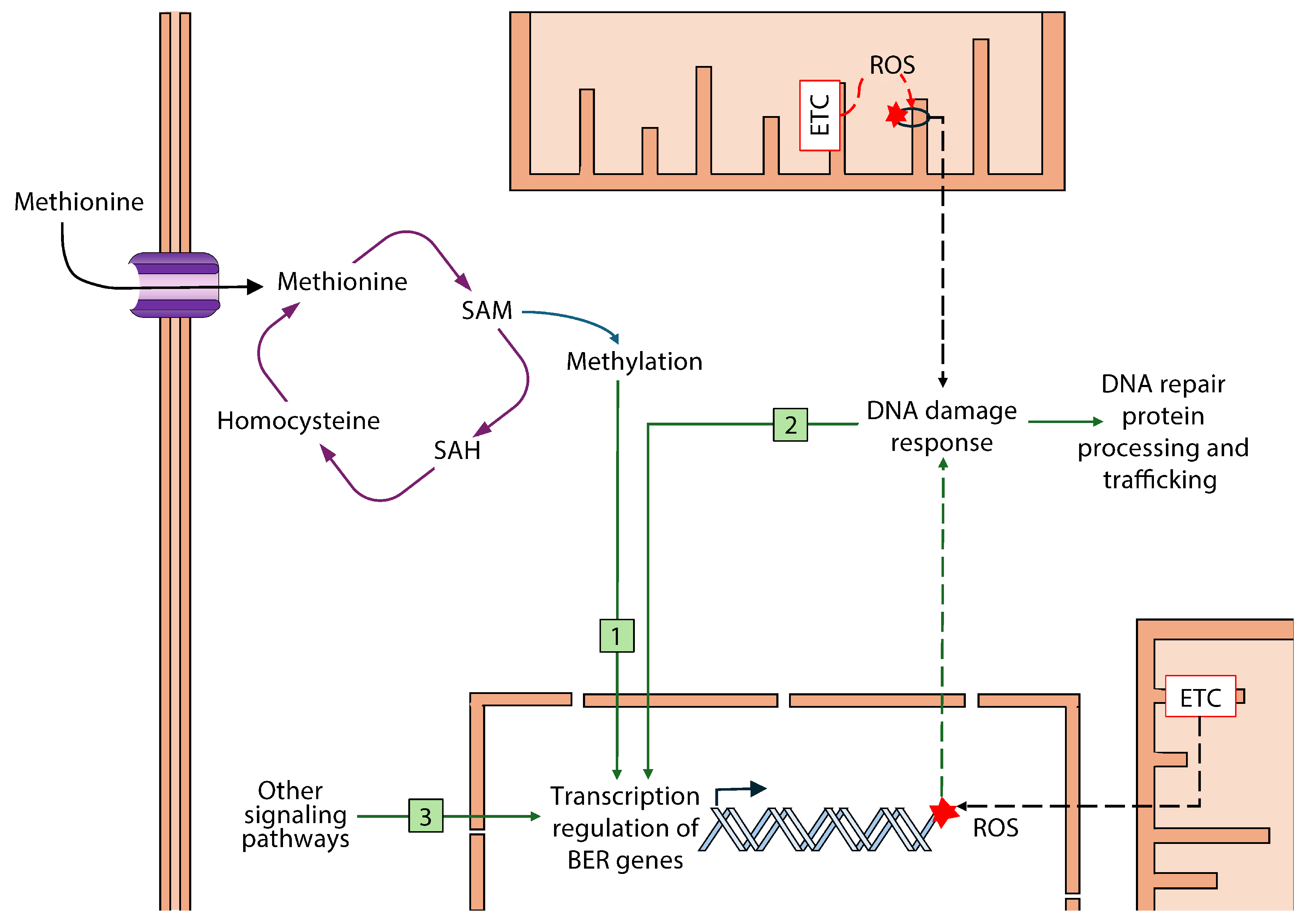
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136–137, 148–162. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The Effect of Retarded Growth Upon the Length of Life Span and Upon the Ultimate Body Size: One Figure. J. Nutr. 1935, 10, 63–79. [Google Scholar] [CrossRef]
- Weindruch, R. The retardation of aging by caloric restriction: Studies in rodents and primates. Toxicol. Pathol. 1996, 24, 742–745. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, J.; Liu, G.-H. Roles of chromatin and genome instability in cellular senescence and their relevance to ageing and related diseases. Nat. Rev. Mol. Cell Biol. 2024, 25, 979–1000. [Google Scholar] [CrossRef]
- Ropert, B.; Gallrein, C.; Schumacher, B. DNA repair deficiencies and neurodegeneration. DNA Repair 2024, 138, 103679. [Google Scholar] [CrossRef]
- Akbari, M.; Kirkwood, T.B.; Bohr, V.A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef]
- Gredilla, R.; Bohr, V.A.; Stevnsner, T. Mitochondrial DNA repair and association with aging—An update. Exp. Gerontol. 2010, 45, 478–488. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Bohr, V.A.; Stevnsner, T. DNA repair deficiency in neurodegeneration. Prog. Neurobiol. 2011, 94, 166–200. [Google Scholar] [CrossRef]
- Christmann, M.; Tomicic, M.T.; Roos, W.P.; Kaina, B. Mechanisms of human DNA repair: An update. Toxicology 2003, 193, 3–34. [Google Scholar] [CrossRef] [PubMed]
- de Souza-Pinto, N.C.; Mason, P.A.; Hashiguchi, K.; Weissman, L.; Tian, J.; Guay, D.; Lebel, M.; Stevnsner, T.V.; Rasmussen, L.J.; Bohr, V.A. Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA Repair 2009, 8, 704–719. [Google Scholar] [CrossRef]
- Aamann, M.D.; Sorensen, M.M.; Hvitby, C.; Berquist, B.R.; Muftuoglu, M.; Tian, J.; de Souza-Pinto, N.C.; Scheibye-Knudsen, M.; Wilson, D.M., 3rd; Stevnsner, T.; et al. Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane. FASEB J. 2010, 24, 2334–2346. [Google Scholar] [CrossRef] [PubMed]
- Krokan, E.H.; Nilsen, H.; Skorpen, F.; Otterlei, M.; Slupphaug, G. Base excision repair of DNA in mammalian cells. FEBS Lett. 2000, 476, 73–77. [Google Scholar] [CrossRef]
- Chattopadhyay, R.; Wiederhold, L.; Szczesny, B.; Boldogh, I.; Hazra, T.K.; Izumi, T.; Mitra, S. Identification and characterization of mitochondrial abasic (AP)-endonuclease in mammalian cells. Nucleic Acids Res. 2006, 34, 2067–2076. [Google Scholar] [CrossRef]
- Dianov, G.L.; Souza-Pinto, N.; Nyaga, S.G.; Thybo, T.; Stevnsner, T.; Bohr, V.A. Base excision repair in nuclear and mitochondrial DNA. Prog. Nucleic Acid Res. Mol. Biol. 2001, 68, 285–297. [Google Scholar] [PubMed]
- Bohr, V.A. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free. Radic. Biol. Med. 2002, 32, 804–812. [Google Scholar] [CrossRef]
- Behrouzi, A.; Kelley, M.R.; Fehrenbacher, J.C. Oxidative DNA Damage: A Role in Altering Neuronal Function. J. Cell. Signal. 2022, 3, 160–166. [Google Scholar] [CrossRef]
- Krasich, R.; Copeland, W.C. DNA polymerases in the mitochondria: A critical review of the evidence. Front. Biosci. 2017, 22, 692–709. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.S.; Seo, Y.R.; Maluf, S.W. Genomic Approach to Understand the Association of DNA Repair with Longevity and Healthy Aging Using Genomic Databases of Oldest-Old Population. Oxidat. Med. Cell. Longev. 2018, 2018, 2984730. [Google Scholar] [CrossRef]
- Garagnani, P.; Marquis, J.; Delledonne, M.; Pirazzini, C.; Marasco, E.; Kwiatkowska, K.M.; Iannuzzi, V.; Bacalini, M.G.; Valsesia, A.; Carayol, J.; et al. Whole-genome sequencing analysis of semi-supercentenarians. eLife 2021, 10, e57849. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Hou, Y.; Fang, E.F.; Bohr, V.A. Roles of NAD+ in Health and Aging. Cold Spring Harb. Perspect. Med. 2024, 14, a041193. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Hou, Y.; Lautrup, S.; Cordonnier, S.; Wang, Y.; Croteau, D.L.; Zavala, E.; Zhang, Y.; Moritoh, K.; O’connell, J.F.; Baptiste, B.A.; et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. USA 2018, 115, E1876–E1885. [Google Scholar] [CrossRef]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef]
- Weindruch, R.; Walford, R.L.; Fligiel, S.; Guthrie, D. The retardation of aging in mice by dietary restriction: Longevity, cancer, immunity and lifetime energy intake. J. Nutr. 1986, 116, 641–654. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- Dhahbi, J.M.; Kim, H.-J.; Mote, P.L.; Beaver, R.J.; Spindler, S.R. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 5524–5529. [Google Scholar] [CrossRef]
- Lee, C.-K.; Allison, D.B.; Brand, J.; Weindruch, R.; Prolla, T.A. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc. Natl. Acad. Sci. USA 2002, 99, 14988–14993. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P.; Masoro, E.J.; McMahan, C.A. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J. Gerontol. 1985, 40, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef]
- Mattson, M.P.; Chan, S.L.; Duan, W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol. Rev. 2002, 82, 637–672. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R. Caloric restriction, gene expression, and aging. Alzheimer Dis. Assoc. Disord. 2003, 17 (Suppl. 2), S58–S59. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014, 5, 3557. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S.; Holloszy, J.O. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. AGE 2010, 32, 97–108. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Gredilla, R.; Barja, G.; López-Torres, M. Effect of short-term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J. Bioenerg. Biomembr. 2001, 33, 279–287. [Google Scholar] [CrossRef]
- Gredilla, R.; Sanz, A.; Lopez-Torres, M.; Barja, G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001, 15, 1589–1591. [Google Scholar] [CrossRef]
- López-Torres, M.; Gredilla, R.; Sanz, A.; Barja, G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free. Radic. Biol. Med. 2002, 32, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, L.; Ramsey, J.J.; Hagopian, K.; Weindruch, R.; Harper, M.-E. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E852–E861. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.; Caro, P.; Ibañez, J.; Gómez, J.; Gredilla, R.; Barja, G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J. Bioenerg. Biomembr. 2005, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Agarwal, S.; Candas, M.; Forster, M.J.; Lal, H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech. Ageing Dev. 1994, 76, 215–224. [Google Scholar] [CrossRef]
- Hagopian, K.; Chen, Y.; Domer, K.S.; Hoo, R.S.; Bentley, T.; McDonald, R.B.; Ramsey, J.J. Caloric restriction influences hydrogen peroxide generation in mitochondrial sub-populations from mouse liver. J. Bioenerg. Biomembr. 2011, 43, 227–236. [Google Scholar] [CrossRef]
- Mair, W.; Piper, M.D.W.; Partridge, L.; Kirkwood, T. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005, 3, e223. [Google Scholar] [CrossRef]
- Austad, S.N.; Hoffman, J.M. Beyond calorie restriction: Aging as a biological target for nutrient therapies. Curr. Opin. Biotechnol. 2021, 70, 56–60. [Google Scholar] [CrossRef]
- López-Torres, M.; Barja, G. Lowered methionine ingestion as responsible for the decrease in rodent mitochondrial oxidative stress in protein and dietary restriction: Possible implications for humans. Biochim. Biophys. Acta 2008, 1780, 1337–1347. [Google Scholar] [CrossRef]
- Orentreich, N.; Matias, J.R.; DeFelice, A.; Zimmerman, J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [CrossRef]
- Richie, J.P., Jr.; Leutzinger, Y.; Parthasarathy, S.; Maixoy, V.; Orentreich, N.; Zimmerman, J.A. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994, 8, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Buehner, G.; Chang, Y.; Harper, J.M.; Sigler, R.; Smith-Wheelock, M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 2005, 4, 119–125. [Google Scholar] [CrossRef]
- Sun, L.; Akha, A.A.S.; Miller, R.A.; Harper, J.M. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Petti, A.A.; Crutchfield, C.A.; Rabinowitz, J.D.; Botstein, D. Survival of starving yeast is correlated with oxidative stress response and nonrespiratory mitochondrial function. Proc. Natl. Acad. Sci. USA 2011, 108, E1089–E1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Kaya, A.; Ma, S.; Kim, G.; Gerashchenko, M.V.; Yim, S.H.; Hu, Z.; Harshman, L.G.; Gladyshev, V.N. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014, 5, 359. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef]
- Malloy, V.L.; Krajcik, R.A.; Bailey, S.J.; Hristopoulos, G.; Plummer, J.D.; Orentreich, N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 2006, 5, 305–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Jelleschitz, J.; Grune, T.; Chen, W.; Zhao, Y.; Jia, M.; Wang, Y.; Liu, Z.; Höhn, A. Methionine restriction—Association with redox homeostasis and implications on aging and diseases. Redox Biol. 2022, 57, 102464. [Google Scholar] [CrossRef]
- Wang, C.; Hei, Y.; Liu, Y.; Bajpai, A.K.; Li, Y.; Guan, Y.; Xu, F.; Yao, C. Systems genetics identifies methionine as a high risk factor for Alzheimer’s disease. Front. Neurosci. 2024, 18, 1381889. [Google Scholar] [CrossRef]
- Sanz, A.; Caro, P.; Ayala, V.; Portero-Otin, M.; Pamplona, R.; Barja, G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006, 20, 1064–1073. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Gomez, A.; Gomez, J.; Suarez, H.; Sanchez, C.; Naudi, A.; Ayala, V.; Portero-Otin, M.; Lopez-Torres, M.; Pamplona, R.; et al. Forty percent methionine restriction lowers DNA methylation, complex I ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J. Bioenerg. Biomembr. 2011, 43, 699–708. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Racette, S.B.; The Washington University School of Medicine CALERIE Group; Weiss, E.P.; Villareal, D.T.; Arif, H.; Steger-May, K.; Schechtman, K.B.; Fontana, L.; Klein, S.; Holloszy, J.O. One year of caloric restriction in humans: Feasibility and effects on body composition and abdominal adipose tissue. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Cabelof, D. Caloric restriction promotes genomic stability by induction of base excision repair and reversal of its age-related decline. DNA Repair 2003, 2, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.A.; Karahalil, B.; Hogue, B.A.; Souza-Pinto, N.C.; Bohr, V.A. Mitochondrial and nuclear DNA base excision repair are affected differently by caloric restriction. FASEB J. 2004, 18, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Kisby, G.E.; Kohama, S.G.; Olivas, A.; Churchwell, M.; Doerge, D.; Spangler, E.; de Cabo, R.; Ingram, D.K.; Imhof, B.; Bao, G.; et al. Effect of caloric restriction on base-excision repair (BER) in the aging rat brain. Exp. Gerontol. 2009, 45, 208–216. [Google Scholar] [CrossRef]
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, N.; Zhang, H.; Liang, G.; Lin, Y.; Qian, Z.; Yang, X.; Yang, J.; Fu, Y.; Zhang, C.; et al. Systemic aging and aging-related diseases. FASEB J. 2025, 39, e70430. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Barja, G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013, 48, 1030–1042. [Google Scholar] [CrossRef]
- Mladenović, D.; Radosavljević, T.; Hrnčić, D.; Rasic-Markovic, A.; Stanojlović, O. The effects of dietary methionine restriction on the function and metabolic reprogramming in the liver and brain—Implications for longevity. Prog. Neurobiol. 2019, 30, 581–593. [Google Scholar] [CrossRef]
- Gredilla, R.; Garm, C.; Holm, R.; Bohr, V.A.; Stevnsner, T. Differential age-related changes in mitochondrial DNA repair activities in mouse brain regions. Neurobiol. Aging 2010, 31, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Mentch, S.J.; Gao, X.; Nichenametla, S.N.; Locasale, J.W. Methionine metabolism influences genomic architecture and gene expression through H3K4me3 peak width. Nat. Commun. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Cameron, K.M.; Ficz, G.; Oxley, D.; Tomaszewski, B.; Gorniak, J.P.; Maas, L.M.; Godschalk, R.W.L.; Van Schooten, F.J.; Reik, W.; et al. The Ageing Brain: Effects on DNA Repair and DNA Methylation in Mice. Genes 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Parkhitko, A.A.; Jouandin, P.; Mohr, S.E.; Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 2019, 18, e13034. [Google Scholar] [CrossRef]
- Gomez, J.; Caro, P.; Sanchez, I.; Naudi, A.; Jove, M.; Portero-Otin, M.; Lopez-Torres, M.; Pamplona, R.; Barja, G. Effect of methionine dietary supplementation on mitochondrial oxygen radical generation and oxidative DNA damage in rat liver and heart. J. Bioenerg. Biomembr. 2009, 41, 309–321. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Gómez, A.; Pérez, I.; Sanchez, C.; Suarez, H.; Naudí, A.; Jové, M.; Lopez-Torres, M.; Pamplona, R.; Barja, G. Effects of aging and methionine restriction applied at old age on ROS generation and oxidative damage in rat liver mitochondria. Biogerontology 2012, 13, 399–411. [Google Scholar] [CrossRef]
- Mattocks, D.A.; Mentch, S.J.; Shneyder, J.; Ables, G.P.; Sun, D.; Richie, J.P.; Locasale, J.W.; Nichenametla, S.N. Short term methionine restriction increases hepatic global DNA methylation in adult but not young male C57BL/6J mice. Exp. Gerontol. 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Lail, H.; Mabb, A.M.; Parent, M.B.; Pinheiro, F.; Wanders, D. Effects of Dietary Methionine Restriction on Cognition in Mice. Nutrients 2023, 15, 4950. [Google Scholar] [CrossRef]
- Dyrkheeva, N.S.; Lebedeva, N.A.; Lavrik, O.I. AP Endonuclease 1 as a Key Enzyme in Repair of Apurinic/Apyrimidinic Sites. Biochemistry 2016, 81, 951–967. [Google Scholar] [CrossRef]
- Kaguni, L.S. DNA polymerase gamma, the mitochondrial replicase. Annu. Rev. Biochem. 2004, 73, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; Vasko, M.R.; Kelley, M.R. DNA repair in neurons: So if they don’t divide what’s to repair? Mutat. Res. 2007, 614, 24–36. [Google Scholar] [CrossRef]
- Hollensworth, S.B.; Shen, C.-C.; Sim, J.E.; Spitz, D.R.; Wilson, G.L.; LeDoux, S.P. Glial cell type-specific responses to menadione-induced oxidative stress. Free. Radic. Biol. Med. 2000, 28, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Ables, G.P.; Perrone, C.E.; Orentreich, D.; Orentreich, N.; Borras, C. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS ONE 2012, 7, e51357. [Google Scholar] [CrossRef]
- Perrone, C.E.; Malloy, V.L.; Orentreich, D.S.; Orentreich, N. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp. Gerontol. 2013, 48, 654–660. [Google Scholar] [CrossRef]
- Caro, P.; Gómez, J.; López-Torres, M.; Sánchez, I.; Naudí, A.; Jove, M.; Pamplona, R.; Barja, G. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology 2008, 9, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Caro, P.; Gomez, J.; Sanchez, I.; Naudi, A.; Ayala, V.; López-Torres, M.; Pamplona, R.; Barja, G. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res. 2009, 12, 421–434. [Google Scholar] [CrossRef]
- Bordin, D.L.; Lirussi, L.; Nilsen, H. Cellular response to endogenous DNA damage: DNA base modifications in gene expression regulation. DNA Repair 2021, 99, 103051. [Google Scholar] [CrossRef]
- Akbari, M.; Otterlei, M.; Peña-Diaz, J.; Krokan, H. Different organization of base excision repair of uracil in DNA in nuclei and mitochondria and selective upregulation of mitochondrial uracil-DNA glycosylase after oxidative stress. Neuroscience 2007, 145, 1201–1212. [Google Scholar] [CrossRef]
- Holst, C.M.; Andersen, N.B.; Thinggaard, V.; Tilken, M.; Lautrup, S.; Tesauro, C.; Stevnsner, T. Phosphorylation of the Human DNA Glycosylase NEIL2 Is Affected by Oxidative Stress and Modulates Its Activity. Antioxidants 2023, 12, 355. [Google Scholar] [CrossRef]
- Maddineni, S.; Nichenametla, S.; Sinha, R.; Wilson, R.P.; Richie, J.P. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med. 2013, 238, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P.; Komninou, D.; Leutzinger, Y.; Kleinman, W.; Orentreich, N.; Malloy, V.; Zimmerman, J.A. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition 2004, 20, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yip, L.Y.; Lee, J.H.J.; Wu, Z.; Chew, H.Y.; Chong, P.K.W.; Teo, C.C.; Ang, H.Y.-K.; Peh, K.L.E.; Yuan, J.; et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat. Med. 2019, 25, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, M.; Boguszewska, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The role of AMPK in metabolism and its influence on DNA damage repair. Mol. Biol. Rep. 2020, 47, 9075–9086. [Google Scholar] [CrossRef]
- Geng, A.; Sun, J.; Tang, H.; Yu, Y.; Wang, X.; Zhang, J.; Wang, X.; Sun, X.; Zhou, X.; Gao, N.; et al. SIRT2 promotes base excision repair by transcriptionally activating OGG1 in an ATM/ATR-dependent manner. Nucleic Acids Res. 2024, 52, 5107–5120. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Pang, J.; Höhn, A.; Dong, W.; Gao, R.; Liu, Y.; Wang, D.; She, Y.; et al. Methionine restriction alleviates diabetes-associated cognitive impairment via activation of FGF21. Redox Biol. 2024, 77, 103390. [Google Scholar] [CrossRef]

| Component | Control (g/100 g) | 40% MetR (g/100 g) |
|---|---|---|
| L-Arginine | 1.12 | 1.124 |
| L-Lysine | 1.44 | 1.445 |
| L-Histidine | 0.33 | 0.331 |
| L-Leucine | 1.11 | 1.114 |
| L-Isoleucine | 0.82 | 0.823 |
| L-Valine | 0.82 | 0.823 |
| L-Threonine | 0.82 | 0.823 |
| L-Tryptophan | 0.18 | 0.181 |
| L-Methionine | 0.86 | 0.516 |
| L-Glutamic acid | 2.70 | 2.709 |
| L-Phenylalanine | 1.16 | 1.164 |
| L-Glycine | 2.33 | 2.338 |
| Dextrine | 5 | 5.017 |
| Corn starch | 31.80 | 31.92 |
| Sucrose | 31.79 | 31.92 |
| Cellulose | 5 | 5.017 |
| Choline bitartrate | 0.2 | 0.201 |
| MP Vitamin diet fortification mixture | 1.02 | 1.023 |
| Mineral mix (AIN) | 3.5 | 3.512 |
| Corn oil | 8 | 8.028 |
| Total (% weight) | 100 | 100 |
| Ogg1 | Forward | TATCATGGCTTCCCAAACCT |
| Reverse | CAACTTCTGAGGTGGGTCT | |
| Udg | Forward | GATGTGCGACATCCGTGA |
| Reverse | TGAGCTTGATTAGGTCCGTGA | |
| Neil2 | Forward | CAAAAGAGGTGACTGGATAGACC |
| Reverse | GGAAGCCACCACCACTAAAA | |
| Ape1 | Forward | GCATCTGCCACACTCAAG |
| Reverse | TGGTGCTTCTTCCTTTACCC | |
| Polß | Forward | CAGCGAGAATGATGAAAACG |
| Reverse | CTGATCTTTGGGGATCAACC | |
| Polγ | Forward | GCGAATGGTCCAGCGATTTC |
| Reverse | TTGACGAACAGTTCCCGAGG | |
| Lig1 | Forward | TGGTGTCCCGCAAAGTGATT |
| Reverse | CCTTTGGAGGGGGTTCCTTC | |
| Lig3 | Forward | TTGCAGAAAGGCTCCACAG |
| Reverse | CAAGTTGTACACACTCTTGATGACAC | |
| Mnsod | Forward | GCCTCAGCAATGTTGTGTCG |
| Reverse | ATTGTTCACGTAGGTCGCGT | |
| Ppargc1a | Forward | TTCAGGAGCTGGATGGCTTG |
| Reverse | GGGCAGCACACTCTATGTCA | |
| Bdnf | Forward | TGTTGGGGAGACGAGATTTT |
| Reverse | TCACCTGGTGGAACATTGTG | |
| 18S | Forward | GGTGCATGGCCGTTCTTA |
| Reverse | TCGTTCGTTATCGGAATTAACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gredilla, R.; Lopez-Torres, M.; Sanchez-Roman, I. Methionine Restriction Differentially Modulates Expression of Genes in the Base Excision Repair Pathway in Rat Brain and Liver. Biomolecules 2025, 15, 969. https://doi.org/10.3390/biom15070969
Gredilla R, Lopez-Torres M, Sanchez-Roman I. Methionine Restriction Differentially Modulates Expression of Genes in the Base Excision Repair Pathway in Rat Brain and Liver. Biomolecules. 2025; 15(7):969. https://doi.org/10.3390/biom15070969
Chicago/Turabian StyleGredilla, Ricardo, Monica Lopez-Torres, and Ines Sanchez-Roman. 2025. "Methionine Restriction Differentially Modulates Expression of Genes in the Base Excision Repair Pathway in Rat Brain and Liver" Biomolecules 15, no. 7: 969. https://doi.org/10.3390/biom15070969
APA StyleGredilla, R., Lopez-Torres, M., & Sanchez-Roman, I. (2025). Methionine Restriction Differentially Modulates Expression of Genes in the Base Excision Repair Pathway in Rat Brain and Liver. Biomolecules, 15(7), 969. https://doi.org/10.3390/biom15070969






