Levels of Zinc, Iron, and Copper in the Aqueous Humor of Patients with Primary Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Clinical Variables
2.4. Sample Preparation and Quantitative Analysis of Trace Metals
2.5. Statistical and Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Changes in Trace Metal Concentrations in the AH
3.3. Relationship Between Trace Metal Concentrations in the AH
3.4. Relationship Between Clinical Variables and Trace Metal Concentrations in the AH
3.5. Analysis of Potential Biomarkers for the Discrimination of Primary Glaucoma
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGCs | Retinal ganglion cells |
| IOP | Intraocular pressure |
| TM | Trabecular meshwork |
| PACG | Primary angle-closure glaucoma |
| POAG | Primary open-angle glaucoma |
| AACC | Acute angle-closure crisis |
| Zn | Zinc |
| Fe | Iron |
| Cu | Copper |
| MMPs | Matrix metalloproteinases |
| ECM | Extracellular matrix |
| NMDA | N-methyl-D-aspartic acid |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AH | Aqueous humor |
| FI-ICP-MS | Flow injection inductively coupled plasma mass spectrometry |
| VA | Visual acuity |
| CDR | Cup-to-disc ratio |
| logMAR | Logarithm of minimum angle of resolution |
| AL | Axial length |
| RNFL | Retinal nerve fiber layer |
| OCT | Optical coherence tomography |
| ACA | Anterior chamber angle |
| UBM | Ultrasound biomicroscopy |
| ACD | Anterior chamber depth |
| MD | Mean deviation |
| PSD | Pattern standard deviation |
| VF | Visual field |
| HNO3 | Nitric acid |
| Ge | Germanium |
| Sc | Scandium |
| In | Indium |
| ICP-MS | Inductively coupled plasma mass spectrometer |
| ln | Natural logarithmic transformation |
| ROC | Receiver operating characteristic |
| AUC | Area under the ROC curve |
| ORs | Odds ratios |
| AAS | Atomic absorption spectrometry |
| RPE | Retinal pigment epithelial |
| TGF-β2 | Transforming growth factor-β2 |
| ROS | Reactive oxygen species |
| LOX | Lysyl oxidase |
| LOXL2 | Lysyl oxidase like-2 |
References
- Quigley, H.A. The Number of People with Glaucoma Worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, J.; Chen, Y.; Zhang, Q.; Lin, J.; Wu, S.; Han, J.; Liu, Z.; Wu, C.; Zhuo, Y.; et al. Intracellular Zn2+ Promotes Extracellular Matrix Remodeling in Dexamethasone-Treated Trabecular Meshwork. Am. J. Physiol. Cell Physiol. 2024, 326, C1293–C1307. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Calkins, D.J. The Neurovascular Unit in Glaucomatous Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 452. [Google Scholar] [CrossRef]
- Tezel, G. Molecular Regulation of Neuroinflammation in Glaucoma: Current Knowledge and the Ongoing Search for New Treatment Targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Hui, F.; Hercus, J.; Bui, B.; Jusuf, P.R. Restoring the Oxidative Balance in Age-Related Diseases—An Approach in Glaucoma. Ageing Res. Rev. 2022, 75, 101572. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, G.-L.; Cheng, S.; Wang, Z.; Yang, X.-L. Activation of Retinal Glial Cells Contributes to the Degeneration of Ganglion Cells in Experimental Glaucoma. Prog. Retin. Eye Res. 2023, 93, 101169. [Google Scholar] [CrossRef]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults—Screening, Diagnosis, and Management. JAMA 2021, 325, 164. [Google Scholar] [CrossRef]
- Wu, A.M.; Stein, J.D.; Shah, M. Potentially Missed Opportunities in Prevention of Acute Angle-Closure Crisis. JAMA Ophthalmol. 2022, 140, 598. [Google Scholar] [CrossRef]
- Allam, A.A.; Rudayni, H.A.; Ahmed, N.A.; Aba Alkhayl, F.F.; Lamsabhi, A.M.; Kamel, E.M. Comprehensive Insights into Carbonic Anhydrase Inhibition: A Triad of In Vitro, In Silico, and In Vivo Perspectives. Enzyme Microb. Technol. 2025, 189, 110657. [Google Scholar] [CrossRef]
- Kamińska, A.; Romano, G.L.; Rejdak, R.; Zweifel, S.; Fiedorowicz, M.; Rejdak, M.; Bajka, A.; Amato, R.; Bucolo, C.; Avitabile, T.; et al. Influence of Trace Elements on Neurodegenerative Diseases of The Eye—The Glaucoma Model. Int. J. Mol. Sci. 2021, 22, 4323. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhuo, Y.; Li, Y. Effects of Iron and Zinc on Mitochondria: Potential Mechanisms of Glaucomatous Injury. Front. Cell Dev. Biol. 2021, 9, 720288. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Drago, F. Carbon Monoxide and the Eye: Implications for Glaucoma Therapy. Pharmacol. Ther. 2011, 130, 191–201. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Wu, J.; Li, S.; Lin, W.; Wang, N.; Bai, L. Ferroptosis in Glaucoma: A Promising Avenue for Therapy. Adv. Biol. 2024, 8, 2300530. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Y.; Yu, C.; Huang, R.; Du, Q.; Zhao, J.; Sun, W.; Wang, W. Enhanced Fenton-like Catalytic Activity and Stability of g-C3N4 Nanosheet-Wrapped Copper Phosphide with Strong Anti-Interference Ability: Kinetics and Mechanistic Study. J. Colloid Interface Sci. 2021, 595, 129–141. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, Zinc, and Copper in Retinal Physiology and Disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef]
- Huang, K.; Schofield, C.; Nguy, T.; Dere, R.; Wolowski, V.; Siebourg-Polster, J.; Dieckmann, A.; Garweg, J.G.; Chang, M.; Honigberg, L.; et al. Proteomics Approach Identifies Aqueous Humor Biomarkers in Retinal Diseases. Commun. Med. 2025, 5, 134. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, Y.; Zhang, S.; Huo, Y.; Wu, Y.; Shen, L.; Mao, J. Metabolomic Characteristics of Aqueous Humor in Wet Age-Related Macular Degeneration and the Impact of Anti-VEGF Treatment. Invest. Ophthalmol. Vis. Sci. 2025, 66, 37. [Google Scholar] [CrossRef]
- Koyanagi, Y.; Sajiki, A.F.; Yuki, K.; Ushida, H.; Kawano, K.; Fujita, K.; Shimizu, H.; Okuda, D.; Kosaka, M.; Yamada, K.; et al. Application of Metagenomic Long-Read Sequencing for the Diagnosis of Herpetic Uveitis. Invest. Ophthalmol. Vis. Sci. 2025, 66, 50. [Google Scholar] [CrossRef]
- Hohberger, B.; Chaudhri, M.A.; Michalke, B.; Lucio, M.; Nowomiejska, K.; Schlötzer-Schrehardt, U.; Grieb, P.; Rejdak, R.; Jünemann, A.G.M. Levels of Aqueous Humor Trace Elements in Patients with Open-Angle Glaucoma. J. Trace Elem. Med. Biol. 2018, 45, 150–155. [Google Scholar] [CrossRef]
- Aranaz, M.; Costas-Rodríguez, M.; Lobo, L.; García, M.; González-Iglesias, H.; Pereiro, R.; Vanhaecke, F. Homeostatic Alterations Related to Total Antioxidant Capacity, Elemental Concentrations and Isotopic Compositions in Aqueous Humor of Glaucoma Patients. Anal. Bioanal. Chem. 2022, 414, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Forte, G.; Pisano, A.; Farace, C.; Giancipoli, E.; Pinna, A.; Dore, S.; Madeddu, R. A Pilot Study to Evaluate the Levels of Aqueous Humor Trace Elements in Open-Angle Glaucoma. J. Trace Elem. Med. Biol. 2020, 61, 126560. [Google Scholar] [CrossRef] [PubMed]
- NikhalaShree, S.; George, R.J.; Shantha, B.; Vijaya, L.; Sulochana, K.N.; Coral, K. Copper, Lysyl Oxidase Activity, and Collagen in Aqueous Humour of Primary Glaucoma: An Association with Clinical Parameters. Ophthalmic Res. 2023, 66, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Prum, B.E.; Herndon, L.W.; Moroi, S.E.; Mansberger, S.L.; Stein, J.D.; Lim, M.C.; Rosenberg, L.F.; Gedde, S.J.; Williams, R.D. Primary Angle Closure Preferred Practice Pattern® Guidelines. Ophthalmology 2016, 123, P1–P40. [Google Scholar] [CrossRef]
- Prum, B.E.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern® Guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef]
- Olson, R.J.; Braga-Mele, R.; Chen, S.H.; Miller, K.M.; Pineda, R.; Tweeten, J.P.; Musch, D.C. Cataract in the Adult Eye Preferred Practice Pattern®. Ophthalmology 2017, 124, P1–P119. [Google Scholar] [CrossRef]
- Miao, T.-Y.; Wang, G.-J.; Pomerantzeff, O.; Miao, T.-Y.; Wang, G.-J.; Pomerantzeff, O.; Miao, T.-Y.; Wang, G.-J.; Pomerantzeff, O. A Visual Acuity Chart with Geometric Gradation and Logarithmic Notation. Am. J. Oph-thalmol. 1983, 95, 835–837. [Google Scholar] [CrossRef]
- Kubala-Kukuś, A.; Banaś, D.; Braziewicz, J.; Majewska, U.; Pajek, M.; Wudarczyk-Moćko, J.; Antczak, G.; Borkowska, B.; Góźdź, S.; Smok-Kalwat, J. Analysis of Copper Concentration in Human Serum by Application of Total Reflection X-Ray Fluorescence Method. Biol. Trace Elem. Res. 2014, 158, 22–28. [Google Scholar] [CrossRef]
- Kubala-Kukuś, A.; Kuternoga, E.; Braziewicz, J.; Pajek, M. Log-Stable Concentration Distributions of Trace Elements in Biomedical Samples. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 1711–1716. [Google Scholar] [CrossRef]
- Akyol, N.; Deger, O.; Keha, E.E.; Kilic, S. Aqueous Humour and Serum Zinc and Copper Concentrations of Patients with Glaucoma and Cataract. Br. J. Ophthalmol. 1990, 74, 661–662. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, K.-A.; Yoon, Y.-J.; Fujikado, T.; Joo, C.-K. Inhibition of Cyclooxygenase-2 Expression by Zinc-Chelator in Retinal Ischemia. Vision. Res. 2006, 46, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.H. Protection by Pyruvate of Rat Retinal Cells against Zinc Toxicity In Vitro, and Pressure-Induced Ischemia In Vivo. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Peng, J.; Zhang, E.; Ji, D.; Gao, Z.; Tang, Y.; Yao, X.; Xia, X. Pathologically High Intraocular Pressure Disturbs Normal Iron Homeostasis and Leads to Retinal Ganglion Cell Ferroptosis in Glaucoma. Cell Death Differ. 2023, 30, 69–81. [Google Scholar] [CrossRef]
- Dammak, A.; Sanchez Naves, J.; Huete-Toral, F.; Carracedo, G. New Biomarker Combination Related to Oxidative Stress and Inflammation in Primary Open-Angle Glaucoma. Life 2023, 13, 1455. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, H.; Li, G.; Zhang, Y. Higher TGF-Β1, TGF-Β2, MMP-2, and TIMP-1 Levels in the Aqueous Humor of Patients with Acute Primary Angle Closure. Ophthalmic Res. 2021, 64, 62–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, R.; Xu, S.; Chen, J.; Zhong, Y. Matrix Metalloproteinases in Glaucoma: An Updated Overview. Semin. Ophthalmol. 2023, 38, 703–712. [Google Scholar] [CrossRef]
- Li, Y.; Andereggen, L.; Yuki, K.; Omura, K.; Yin, Y.; Gilbert, H.-Y.; Erdogan, B.; Asdourian, M.S.; Shrock, C.; de Lima, S.; et al. Mobile Zinc Increases Rapidly in the Retina after Optic Nerve Injury and Regulates Ganglion Cell Survival and Optic Nerve Regeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E209–E218. [Google Scholar] [CrossRef]
- Osborne, N.N.; Wood, J.P.M. The β-Adrenergic Receptor Antagonist Metipranolol Blunts Zinc-Induced Photoreceptor and RPE Apoptosis. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3178. [Google Scholar] [CrossRef]
- Seyyar, S.A.; Tokuc, E.O.; Güngör, K. Evaluation of Serum Iron Status Indicators in Patients with Primary Open Angle Glaucoma. Eur. J. Ophthalmol. 2024, 34, 175–180. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Yuan, H.; Li, A.; Wang, J.; Zhu, X.; Xiu, W.; Zhang, G.; Chen, Y.; Chen, L.; et al. Association of Plasma Lactoferrin Levels with Disease Severity in Glaucoma Patients. Front. Med. 2024, 11, 1385358. [Google Scholar] [CrossRef]
- Ashok, A.; Chaudhary, S.; Kritikos, A.E.; Kang, M.H.; McDonald, D.; Rhee, D.J.; Singh, N. TGFβ2-Hepcidin Feed-Forward Loop in the Trabecular Meshwork Implicates Iron in Glaucomatous Pathology. Invest. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Xu, K.; Wang, G.; Zhang, F. Copper Homeostasis and Neurodegenerative Diseases. Neural Regen. Res. 2025, 20, 3124–3143. [Google Scholar] [CrossRef] [PubMed]
- McGahan, M.C.; Bito, L.Z.; Myers, B.M. The Pathophysiology of the Ocular Microenvironment. II. Copper-Induced Ocular Inflammation and Hypotony. Exp. Eye Res. 1986, 42, 595–605. [Google Scholar] [CrossRef]
- Cui, J.Z.; Wang, X.-F.; Hsu, L.; Matsubara, J.A. Inflammation Induced by Photocoagulation Laser Is Minimized by Copper Chelators. Lasers Med. Sci. 2009, 24, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Bito, L.Z.; Baroody, R.A. Ocular Trace Metal Kinetics and Toxicology. I. The Distribution of Intravitreally Injected 67Cu++ within Intraocular Compartments and Its Loss from the Globe. Invest. Ophthalmol. Vis. Sci. 1987, 28, 101–105. [Google Scholar]
- Lqbal, Z.; Muhammad, Z.; Shah, M.T.; Bashir, S.; Khan, T.; Khan, M.D. Relationship between the Concentration of Copper and Iron in the Aqueous Humour and Intraocular Pressure in Rabbits Treated with Topical Steroids. Clin. Exp. Ophthalmol. 2002, 30, 28–35. [Google Scholar] [CrossRef]
- Rahman, N.; O’Neill, E.; Irnaten, M.; Wallace, D.; O’Brien, C. Corneal Stiffness and Collagen Cross-Linking Proteins in Glaucoma: Potential for Novel Therapeutic Strategy. J. Ocul. Pharmacol. Ther. 2020, 36, 582–594. [Google Scholar] [CrossRef]
- Urban, Z.; Agapova, O.; Hucthagowder, V.; Yang, P.; Starcher, B.C.; Hernandez, M.R. Population Differences in Elastin Maturation in Optic Nerve Head Tissue and Astrocytes. Invest. Ophthalmol. Vis. Sci. 2007, 48, 3209. [Google Scholar] [CrossRef]
- NikhalaShree, S.; Karthikkeyan, G.; George, R.; Shantha, B.; Vijaya, L.; Ratra, V.; Sulochana, K.N.; Coral, K. Lowered Decorin With Aberrant Extracellular Matrix Remodeling in Aqueous Humor and Tenon’s Tissue From Primary Glaucoma Patients. Invest. Ophthalmol. Vis. Sci. 2019, 60, 4661. [Google Scholar] [CrossRef]
- Li, C.; Xiao, C.; Tao, H.; Tang, X. Research Progress of Iron Metabolism in Retinal Diseases. Adv. Ophthalmol. Pract. Res. 2023, 3, 93–100. [Google Scholar] [CrossRef]
- Abdukarimov, N.; Kokabi, K.; Kunz, J. Ferroptosis and Iron Homeostasis: Molecular Mechanisms and Neurodegenerative Disease Implications. Antioxidants 2025, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Han, Z.; Wang, B.; Zheng, W.; Wei, L. Ferroptosis: A Novel Mechanism of Cell Death in Ophthalmic Conditions. Front. Immunol. 2024, 15, 1440309. [Google Scholar] [CrossRef] [PubMed]
- Kravchik, M.V.; Novikov, I.A.; Petrov, S.Y.; Avetisov, S.E. Bioinorganic Chemistry of Open-Angle Glaucoma: A Review. J. Trace Elem. Med. Biol. 2020, 62, 126652. [Google Scholar] [CrossRef] [PubMed]
- Panteli, V.S.; Kanellopoulou, D.G.; Gartaganis, S.P.; Koutsoukos, P.G. Application of Anodic Stripping Voltammetry for Zinc, Copper, and Cadmium Quantification in the Aqueous Humor: Implications of Pseudoexfoliation Syndrome. Biol. Trace Elem. Res. 2009, 132, 9–18. [Google Scholar] [CrossRef]
- Liu, T.; Bolea-Fernandez, E.; Vanhaecke, F. Analysis of Microsamples of Biological Fluids via Inductively Coupled Plasma-Tandem Mass Spectrometry Using a Micro-Flow Injection System Coupled to a High-Efficiency Sample Introduction Setup. Spectrochim. Acta Part B At. Spectrosc. 2022, 198, 106546. [Google Scholar] [CrossRef]
- Hamanaka, T.; Kasahara, K.; Takemura, T. Histopathology of the Trabecular Meshwork and Schlemm’s Canal in Primary Angle-Closure Glaucoma. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8849. [Google Scholar] [CrossRef]
- Fine, B.S.; Yanoff, M.; Stone, R.A. A Clinicopathologic Study of Four Cases of Primary Open-Angle Glaucoma Compared to Normal Eyes. Am. J. Ophthalmol. 1981, 91, 88–105. [Google Scholar] [CrossRef]
- Rohen, J.W.; Lütjen-Drecoll, E.; Flügel, C.; Meyer, M.; Grierson, I. Ultrastructure of the Trabecular Meshwork in Untreated Cases of Primary Open-Angle Glaucoma (POAG). Exp. Eye Res. 1993, 56, 683–692. [Google Scholar] [CrossRef]
- Gottanka, J.; Johnson, D.H.; Martus, P.; Lütjen-Drecoll, E. Severity of Optic Nerve Damage in Eyes with POAG Is Correlated with Changes in the Trabecular Meshwork. J. Glaucoma 1997, 6, 123–132. [Google Scholar] [CrossRef]
- Buffault, J.; Labbé, A.; Hamard, P.; Brignole-Baudouin, F.; Baudouin, C. The Trabecular Meshwork: Structure, Function and Clinical Implications. A Review of the Literature. J. Français D’ophtalmologie 2020, 43, e217–e230. [Google Scholar] [CrossRef]
- AZOPT-Brinzolamide Suspension/Drops—Novartis Pharmaceuticals Corporation. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=acc89925-3ee3-4632-98ae-38a546848346&type=display (accessed on 26 June 2025).
- METHAZOLAMIDE- Methazolamide Tablet—ANI Pharmaceuticals, Inc. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=c26c8834-961e-4c9c-9ad4-5db2d9419938&type=display (accessed on 26 June 2025).
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent Advances in the Medicinal Chemistry of Carbonic Anhydrase Inhibitors. Eur. J. Med. Chem. 2021, 209, 112923. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, X.-S.; Ren, K.-D.; Peng, J.; Luo, X.-J. Restoration of Metal Homeostasis: A Potential Strategy against Neurodegenerative Diseases. Ageing Res. Rev. 2023, 87, 101931. [Google Scholar] [CrossRef] [PubMed]
- DeToma, A.S.; Dengler-Crish, C.M.; Deb, A.; Braymer, J.J.; Penner-Hahn, J.E.; van der Schyf, C.J.; Lim, M.H.; Crish, S.D. Abnormal Metal Levels in the Primary Visual Pathway of the DBA/2J Mouse Model of Glaucoma. BioMetals 2014, 27, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Han, J.; Wu, S.; Liu, C.; Zhang, Q.; Tang, J.; Liu, Z.; Yang, J.; Chen, Y.; Zhuo, Y.; et al. Reduced Zn2+ Promotes Retinal Ganglion Cells Survival and Optic Nerve Regeneration after Injury through Inhibiting Autophagy Mediated by ROS/Nrf2. Free Radic. Biol. Med. 2024, 212, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Bigot, K.; Gondouin, P.; Bénard, R.; Montagne, P.; Youale, J.; Piazza, M.; Picard, E.; Bordet, T.; Behar-Cohen, F. Transferrin Non-Viral Gene Therapy for Treatment of Retinal Degeneration. Pharmaceutics 2020, 12, 836. [Google Scholar] [CrossRef]
- Cui, Q.N.; Bargoud, A.R.; Ross, A.G.; Song, Y.; Dunaief, J.L. Oral Administration of the Iron Chelator Deferiprone Protects against Loss of Retinal Ganglion Cells in a Mouse Model of Glaucoma. Exp. Eye Res. 2020, 193, 107961. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Benzoic Acid Derivative-Modified Chitosan-g-Poly(N-Isopropylacrylamide): Methoxylation Effects and Pharmacological Treatments of Glaucoma-Related Neurodegeneration. J. Control. Release 2020, 317, 246–258. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, H.; Chen, Y.; Li, Y.; Jin, W.; Yang, J.; Zhuo, Y.; Gao, Z.; Hu, X.; Li, Y. TPEN Loaded Poly (Lactide-Co-Glycolide) Nanoparticles Promotes Neuroprotection and Optic Nerve Regeneration. Mater. Today Bio 2025, 32, 101670. [Google Scholar] [CrossRef]
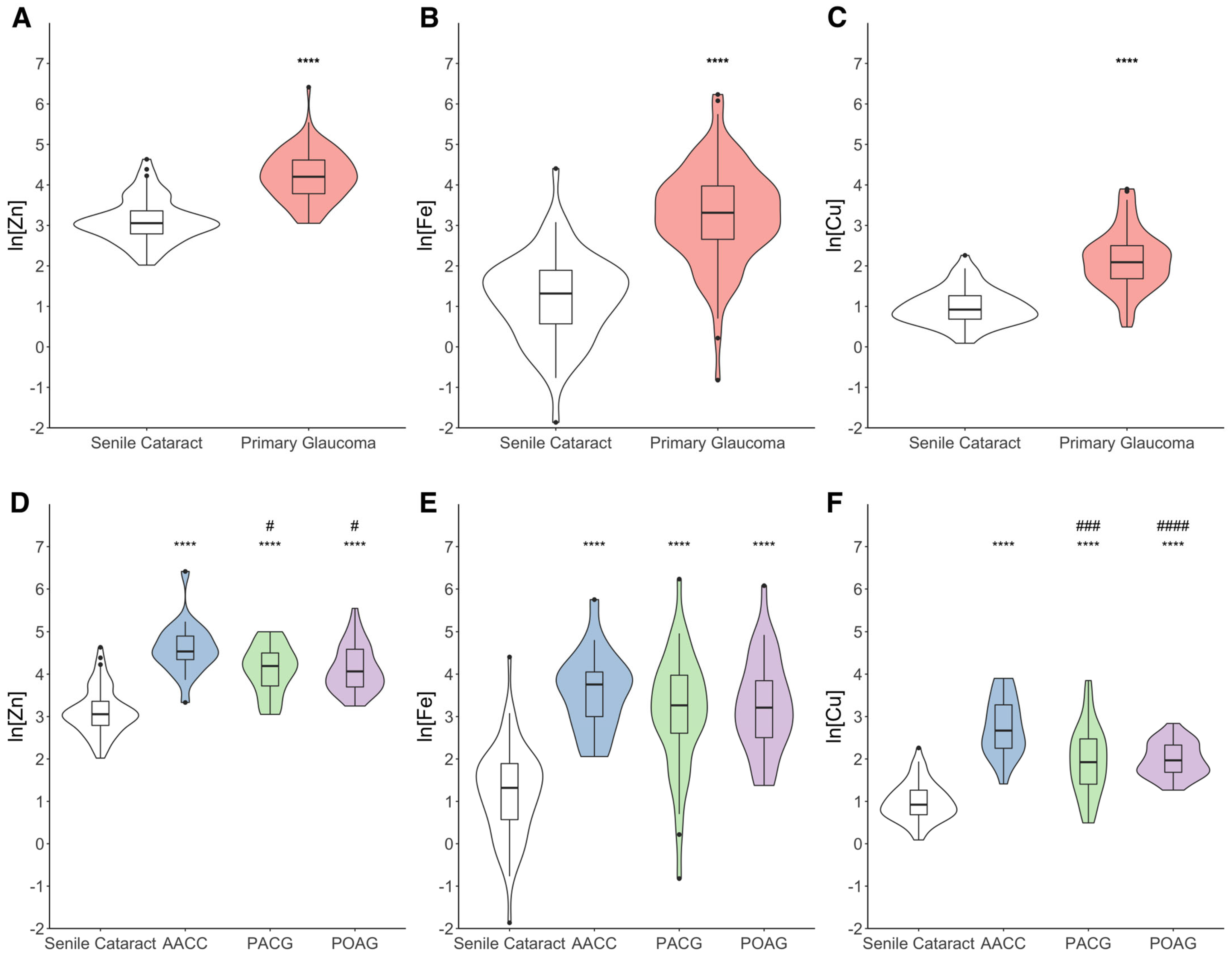
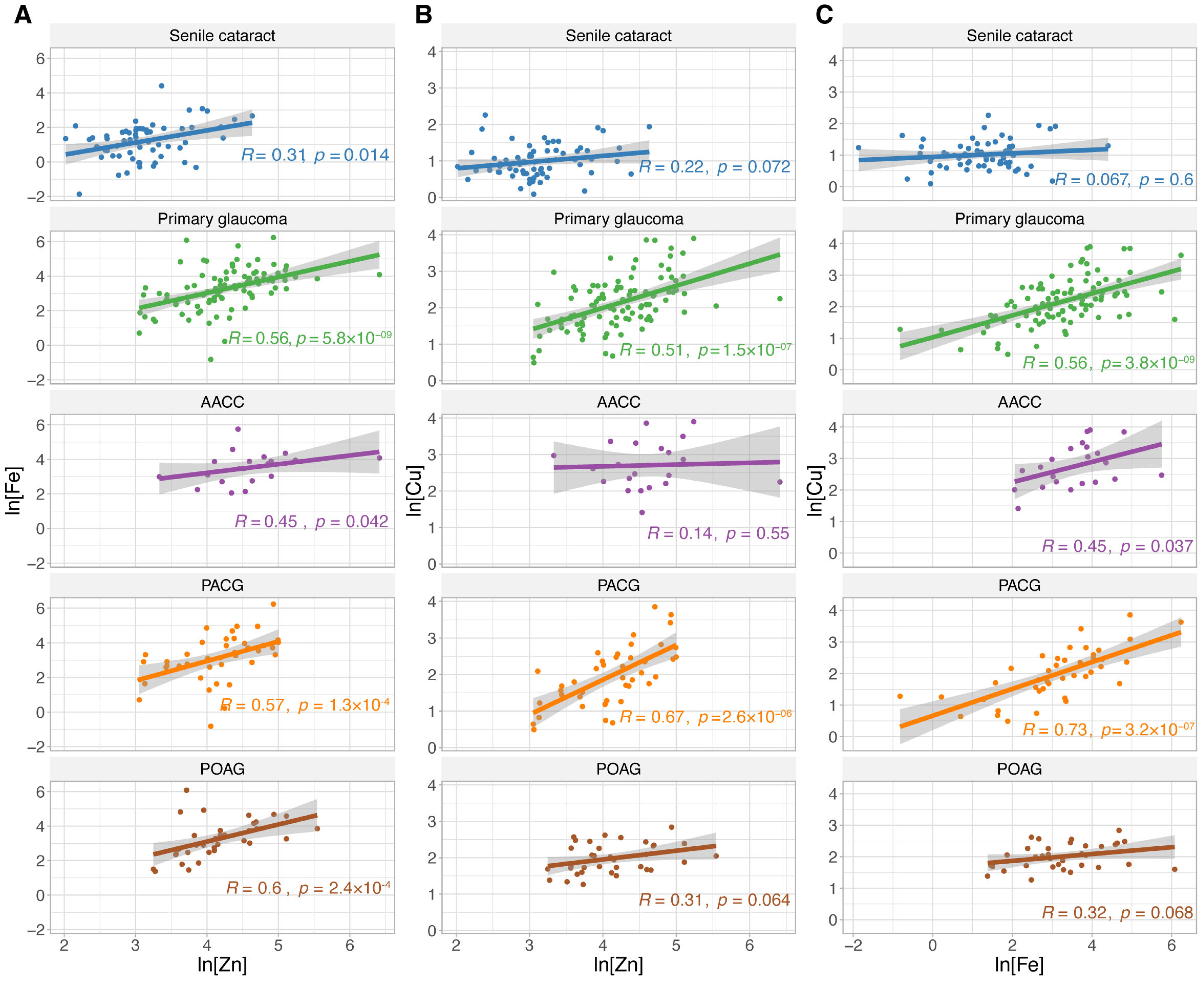
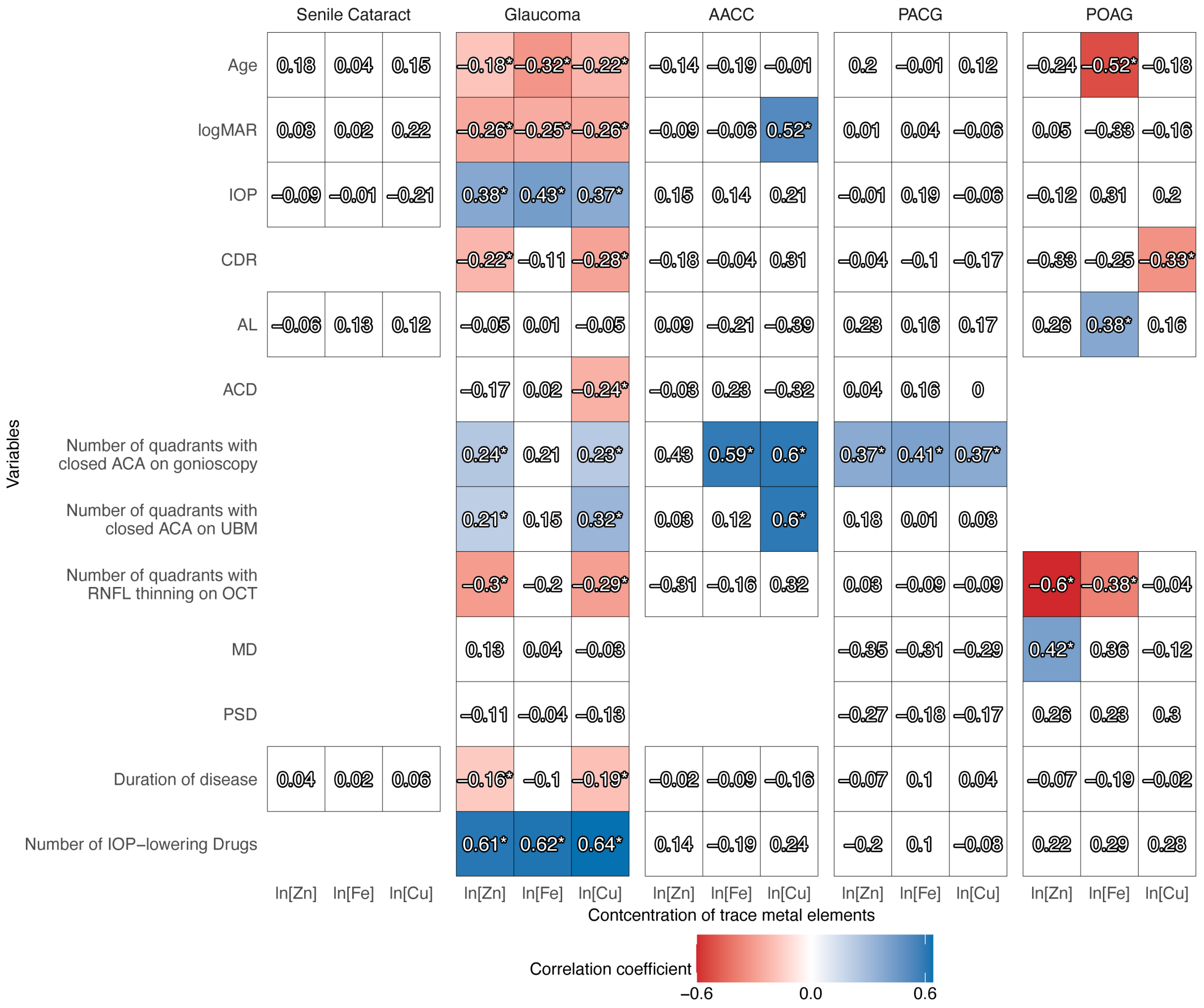

| Characteristics | Senile Cataract | Primary Glaucoma | p Value * | |||
|---|---|---|---|---|---|---|
| Total | AACC | PACG | POAG | |||
| Number of cases, n | 67 | 100 | 22 | 42 | 36 | |
| Sex, male/female | 32/35 † | 58/42 | 7/15 ‡ | 18/24 § | 33/3 †,‡,§ | <0.001 |
| Age, median (IQR), y | 67.00 (63.00, 72.00) † | 61.00 (50.00, 67.00) | 60.50 (53.75, 68.75) | 64.00 (60.25, 68.75) ¶ | 52.00 (40.75, 61.25) †,¶ | <0.001 |
| logMAR, median (IQR) | 1.00 (0.52, 1.30) †,¶,** | 0.40 (0.22, 0.82) | 0.40 (0.30, 0.67) ** | 0.40 (0.20, 0.82) † | 0.46 (0.22, 1.00) ¶ | <0.001 |
| IOP, median (IQR), mmHg | 12.70 (11.00, 15.00) †,‡,§ | 20.30 (15.00, 27.25) | 18.05 (12.25, 34.67) † | 21.85 (17.08, 28.37) ‡ | 19.45 (14.38, 24.18) § | <0.001 |
| CDR, median (IQR) | - | 0.80 (0.70, 0.90) | 0.50 (0.30, 0.70) †,¶ | 0.80 (0.70, 0.90) ¶,** | 0.90 (0.80, 0.91) †,** | <0.001 |
| AL, median (IQR), mm | 23.14 (22.76, 23.90) †,¶ | 23.07 (22.46, 23.88) | 22.65 (22.24, 23.18) ‡ | 22.58 (22.05, 23.07) †,§ | 23.98 (23.66, 25.58) ‡,§,¶ | <0.001 |
| ACD, median (IQR), mm | - | 2.13 (1.90, 2.76) | 1.74 (1.56, 1.91) † | 2.02 (1.90, 2.34) † | - | <0.001 |
| Number of quadrants with closed ACA on gonioscopy, median (IQR) | - | 0.50 (0.00, 3.00) | 3.00 (0.50, 4.00) | 2.00 (2.00, 3.00) | - | 0.564 |
| Number of quadrants with closed ACA on UBM, median (IQR) | - | 2.00 (0.00, 3.00) | 4.00 (2.25, 4.00) † | 3.00 (2.00, 3.00) † | - | 0.023 |
| Number of quadrants with RNFL thinning on OCT, median (IQR) | - | 2.00 (0.38, 3.50) | 0.00 (0.00, 0.00) †,‡ | 2.00 (1.00, 3.50) † | 3.00 (2.00, 4.00) ‡ | <0.001 |
| MD, median (IQR), dB | - | −21.37 (−28.41, −7.02) | - | −21.37 (−28.16, −16.91) | −26.44 (−30.27, −16.24) | 0.305 |
| PSD, median (IQR), dB | - | 6.20 (2.26, 9.70) | - | 8.09 (2.89, 10.76) | 7.30 (2.44, 9.91) | 0.628 |
| Duration of disease, median (IQR), y | 1.00 (1.00, 2.00) † | 1.00 (0.40, 3.00) | 0.08 (0.00, 0.44) †,‡,§ | 1.00 (0.50, 5.00) ‡ | 2.00 (0.88, 3.00) § | <0.001 |
| Number of IOP-lowering drugs, median (IQR) | - | 4.00 (2.00, 4.00) | 3.50 (2.00, 4.75) | 3.00 (1.25, 4.00) | 4.00 (2.00, 4.25) | 0.227 |
| Trace Metal | Percentile | Senile Cataract | Primary Glaucoma | |||
|---|---|---|---|---|---|---|
| Total | AACC | PACG | POAG | |||
| Zn (μg/L) | 0% | 7.54 | 21.14 | 27.99 | 21.14 | 25.76 |
| 25% | 16.31 | 43.99 | 76.92 | 41.33 | 40.36 | |
| 50% | 21.23 | 66.80 | 93.17 | 66.14 | 58.28 | |
| 75% | 28.78 | 100.89 | 133.98 | 90.03 | 98.14 | |
| 100% | 102.78 | 610.35 | 610.35 | 148.30 | 255.70 | |
| ln[Zn] | 0% | 2.02 | 3.05 | 3.33 | 3.05 | 3.25 |
| 25% | 2.79 | 3.78 | 4.34 | 3.72 | 3.70 | |
| 50% | 3.06 | 4.20 | 4.53 | 4.19 | 4.07 | |
| 75% | 3.36 | 4.61 | 4.90 | 4.50 | 4.59 | |
| 100% | 4.63 | 6.41 | 6.41 | 5.00 | 5.54 | |
| Cu (μg/L) | 0% | 1.09 | 1.64 | 4.10 | 1.64 | 3.54 |
| 25% | 1.98 | 5.38 | 9.50 | 4.07 | 5.39 | |
| 50% | 2.51 | 8.09 | 14.45 | 6.85 | 7.15 | |
| 75% | 3.54 | 12.19 | 26.52 | 11.86 | 10.26 | |
| 100% | 9.58 | 49.39 | 49.39 | 46.95 | 17.07 | |
| ln[Cu] | 0% | 0.09 | 0.49 | 1.41 | 0.49 | 1.27 |
| 25% | 0.69 | 1.68 | 2.25 | 1.40 | 1.69 | |
| 50% | 0.92 | 2.09 | 2.67 | 1.92 | 1.97 | |
| 75% | 1.26 | 2.50 | 3.28 | 2.47 | 2.33 | |
| 100% | 2.26 | 3.90 | 3.90 | 3.85 | 2.84 | |
| Fe (μg/L) | 0% | 0.155 | 0.44 | 7.82 | 0.44 | 3.96 |
| 25% | 1.77 | 14.24 | 20.07 | 13.56 | 12.23 | |
| 50% | 3.74 | 27.44 | 42.78 | 26.16 | 24.81 | |
| 75% | 6.63 | 53.20 | 57.55 | 53.20 | 46.77 | |
| 100% | 81.78 | 509.75 | 314.11 | 509.75 | 436.35 | |
| ln[Fe] | 0% | −1.87 | −0.82 | 2.06 | −0.82 | 1.38 |
| 25% | 0.57 | 2.66 | 3.00 | 2.61 | 2.50 | |
| 50% | 1.32 | 3.31 | 3.76 | 3.26 | 3.21 | |
| 75% | 1.89 | 3.97 | 4.05 | 3.97 | 3.85 | |
| 100% | 4.40 | 6.23 | 5.75 | 6.23 | 6.08 | |
| Ratios | Senile Cataract | Primary Glaucoma | p Value * | |||
|---|---|---|---|---|---|---|
| Total | AACC | PACG | POAG | |||
| Zn/Fe | 6.07 (9.77) †,‡,§ | 2.61 (3.00) | 2.86 (3.00) § | 2.57 (2.80) † | 2.72 (3.03) ‡ | <0.001 |
| Zn/Cu | 8.38 (6.49) | 8.19 (7.24) | 6.33 (8.02) | 9.03 (7.32) | 8.27 (6.09) | 0.456 |
| Fe/Cu | 1.15 (1.84) †,‡ | 2.78 (4.48) | 1.95 (3.26) | 3.40 (3.82) † | 3.59 (4.84) ‡ | <0.001 |
| Concentration of Metals | Regression Coefficient | Model Summary | |||||
|---|---|---|---|---|---|---|---|
| Variable | Coefficient | p Value | Adjusted R2 | p Value | N | ||
| B | SEM | ||||||
| Primary Glaucoma * | |||||||
| ln[Zn] | Constant | 4.225 | 0.080 | <0.001 | 0.070 | 0.017 | 67 |
| Duration of disease | −0.056 | 0.023 | 0.017 | ||||
| ln[Cu] | Constant | 2.956 | 0.309 | <0.001 | 0.163 | 0.001 | 68 |
| CDR | −1.033 | 0.386 | 0.009 | ||||
| Duration of disease | −0.063 | 0.027 | 0.024 | ||||
| AACC * | |||||||
| ln[Fe] | Constant | 2.884 | 0.288 | <0.001 | 0.291 | 0.022 | 15 |
| Number of quadrants with closed ACA on gonioscopy | 0.262 | 0.101 | 0.022 | ||||
| ln[Cu] | Constant | 2.467 | 0.240 | <0.001 | 0.303 | 0.020 | 15 |
| Number of quadrants with closed ACA on gonioscopy | 0.223 | 0.084 | 0.020 | ||||
| PACG * | |||||||
| ln[Zn] | Constant | 3.645 | 0.226 | <0.001 | 0.105 | 0.037 | 33 |
| Number of quadrants with closed ACA on gonioscopy | 0.197 | 0.091 | 0.037 | ||||
| ln[Cu] | Constant | 1.256 | 0.293 | <0.001 | 0.116 | 0.030 | 33 |
| Number of quadrants with closed ACA on gonioscopy | 0.268 | 0.117 | 0.030 | ||||
| POAG * | |||||||
| ln[Zn] | Constant | 4.812 | 0.265 | <0.001 | 0.215 | 0.011 | 25 |
| Number of quadrants with RNFL thinning on OCT | −0.244 | 0.089 | 0.011 | ||||
| ln[Fe] | Constant | 5.036 | 0.625 | <0.001 | 0.227 | 0.007 | 27 |
| Age | −0.035 | 0.012 | 0.007 | ||||
| ln[Cu] | Constant | 3.258 | 0.540 | <0.001 | 0.118 | 0.023 | 36 |
| CDR | −1.456 | 0.610 | 0.023 | ||||
| Trace Metal | Threshold [X] (ln[X]) (μg/L) | Youden Index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Zn | 30.31 (3.412) | 0.691 | 0.916 | 0.776 | 0.867 | 0.852 |
| Fe | 11.84 (2.471) | 0.735 | 0.810 | 0.919 | 0.944 | 0.740 |
| Cu | 3.93 (1.370) | 0.715 | 0.879 | 0.836 | 0.895 | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, Z.; Li, Z.; Zhu, Y.; Liang, S.; Liu, H.; Xue, J.; Lin, J.; Deng, Y.; Deng, C.; et al. Levels of Zinc, Iron, and Copper in the Aqueous Humor of Patients with Primary Glaucoma. Biomolecules 2025, 15, 962. https://doi.org/10.3390/biom15070962
Li Y, Liu Z, Li Z, Zhu Y, Liang S, Liu H, Xue J, Lin J, Deng Y, Deng C, et al. Levels of Zinc, Iron, and Copper in the Aqueous Humor of Patients with Primary Glaucoma. Biomolecules. 2025; 15(7):962. https://doi.org/10.3390/biom15070962
Chicago/Turabian StyleLi, Yangjiani, Zhe Liu, Zhidong Li, Yingting Zhu, Shuxin Liang, Hongtao Liu, Jingfei Xue, Jicheng Lin, Ye Deng, Caibin Deng, and et al. 2025. "Levels of Zinc, Iron, and Copper in the Aqueous Humor of Patients with Primary Glaucoma" Biomolecules 15, no. 7: 962. https://doi.org/10.3390/biom15070962
APA StyleLi, Y., Liu, Z., Li, Z., Zhu, Y., Liang, S., Liu, H., Xue, J., Lin, J., Deng, Y., Deng, C., Zeng, S., Zhuo, Y., & Li, Y. (2025). Levels of Zinc, Iron, and Copper in the Aqueous Humor of Patients with Primary Glaucoma. Biomolecules, 15(7), 962. https://doi.org/10.3390/biom15070962


_Kwok.png)



