The Synthesis, Characterization, and Biological Evaluation of a Fluorenyl-Methoxycarbonyl-Containing Thioxo-Triazole-Bearing Dipeptide: Antioxidant, Antimicrobial, and BSA/DNA Binding Studies for Potential Therapeutic Applications in ROS Scavenging and Drug Transport
Abstract
1. Introduction
1.1. Artificial Amino Acids and Their Role in Bioactive Peptides
1.2. Synergistic Effects of Synthetic Peptides and Bioactive Plant Compounds
1.3. Aim of This Work
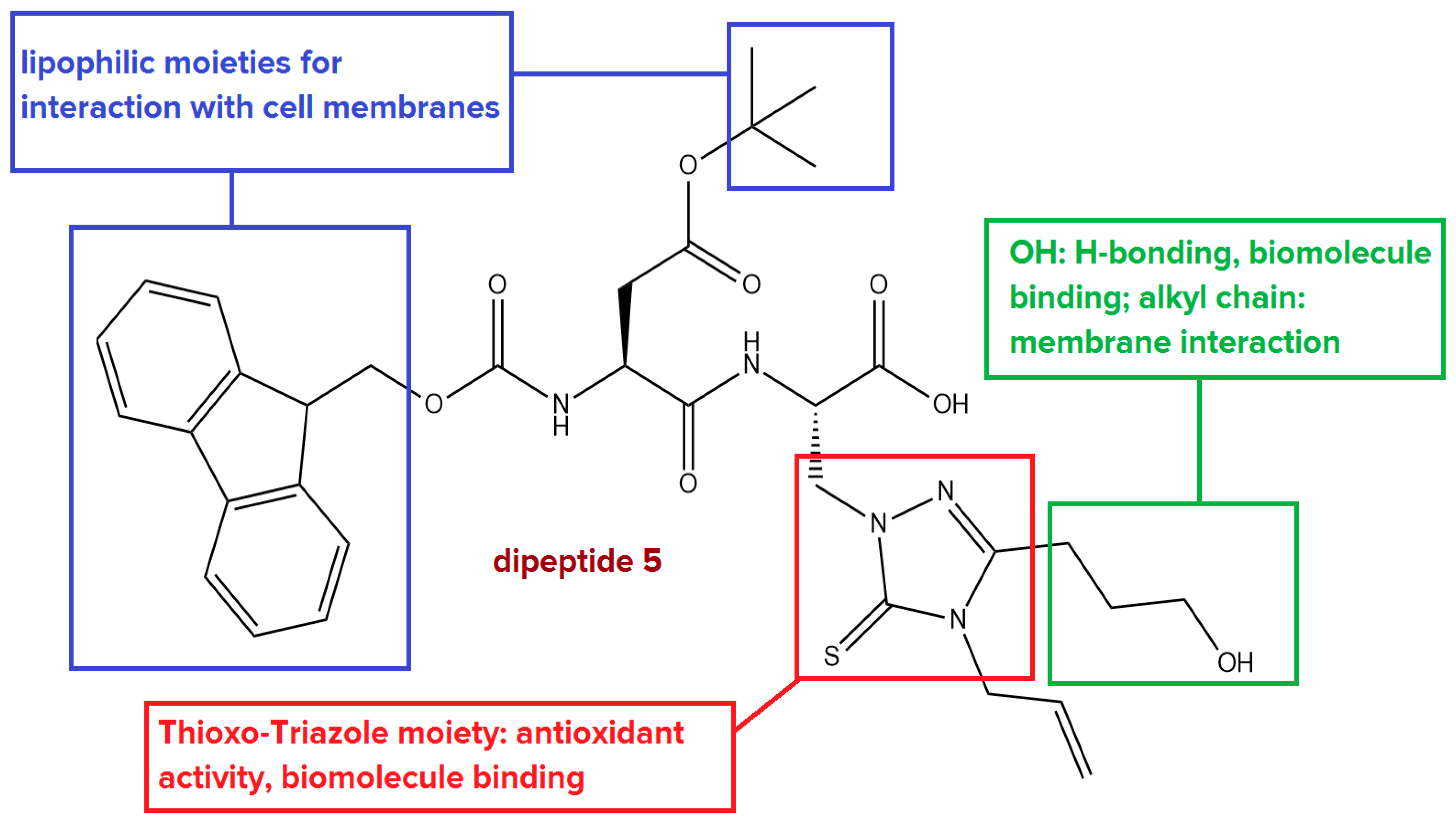
- Antioxidant activity: The thioxo-triazole moiety could neutralize reactive oxygen species (ROS) contributing to the peptide’s antioxidant properties. This feature is particularly important in combating oxidative stress.
- Biomolecular binding: The thioxo-triazole group enables binding to essential biomolecules, including proteins and nucleic acids, through metal ion coordination and other molecular interactions, potentially offering therapeutic benefits.
- Cell membrane binding: The Fmoc and tert-butyl groups contribute to the peptide’s lipophilicity, enhancing its interaction with cell membranes. These groups help to facilitate the membrane penetration required for effective cellular uptake, improving bioavailability and overall activity.
- Protection from degradation: The Fmoc and tert-butyl groups act as protective groups, not only ensuring controlled peptide synthesis but also shielding the dipeptide backbone from chemical and enzymatic degradation, thereby improving the stability and longevity of the compound in biological systems.
2. Materials and Methods
2.1. Materials
2.2. Analytical and Spectroscopic Characterization Methods
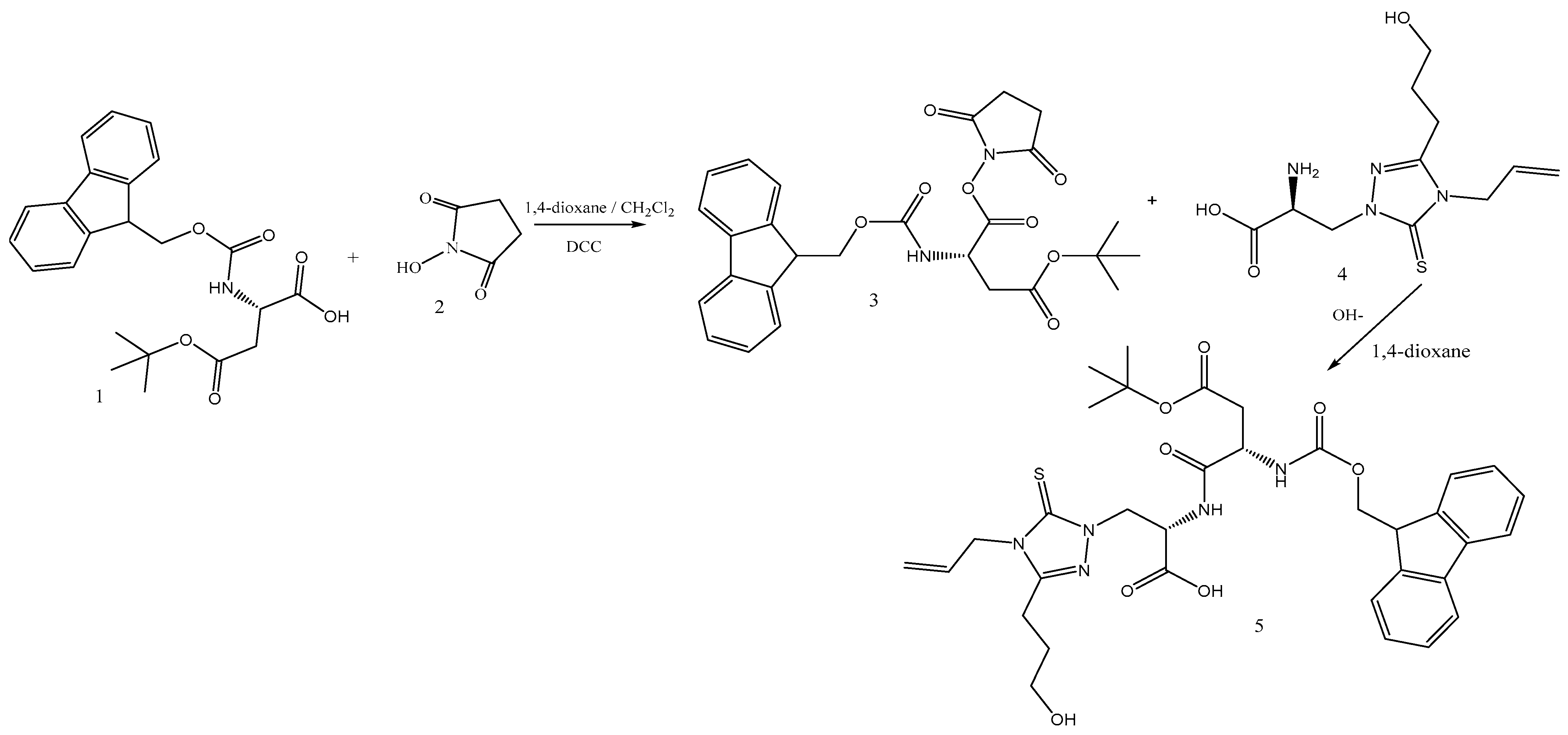
2.3. Synthesis of the N-Oxysuccinimide Ester 3
2.4. Synthesis of the Dipeptide: (S)-2-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-4-(tert-butoxy)-4-oxobutanamido)-3-(4-allyl-3-(3-hydroxypropyl)-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)propanoic acid (5)
2.5. Characterization of 5
2.6. Circular Dichroism
CD Deconvolution
2.7. Ligand Preparation and Molecular Docking
2.8. Computational Analysis of Antioxidant Properties of 5
2.9. Antioxidant and Antibacterial Activity of Peptide 5
2.9.1. Plant Material
2.9.2. Drying Processes
2.9.3. Extraction of Plant Samples
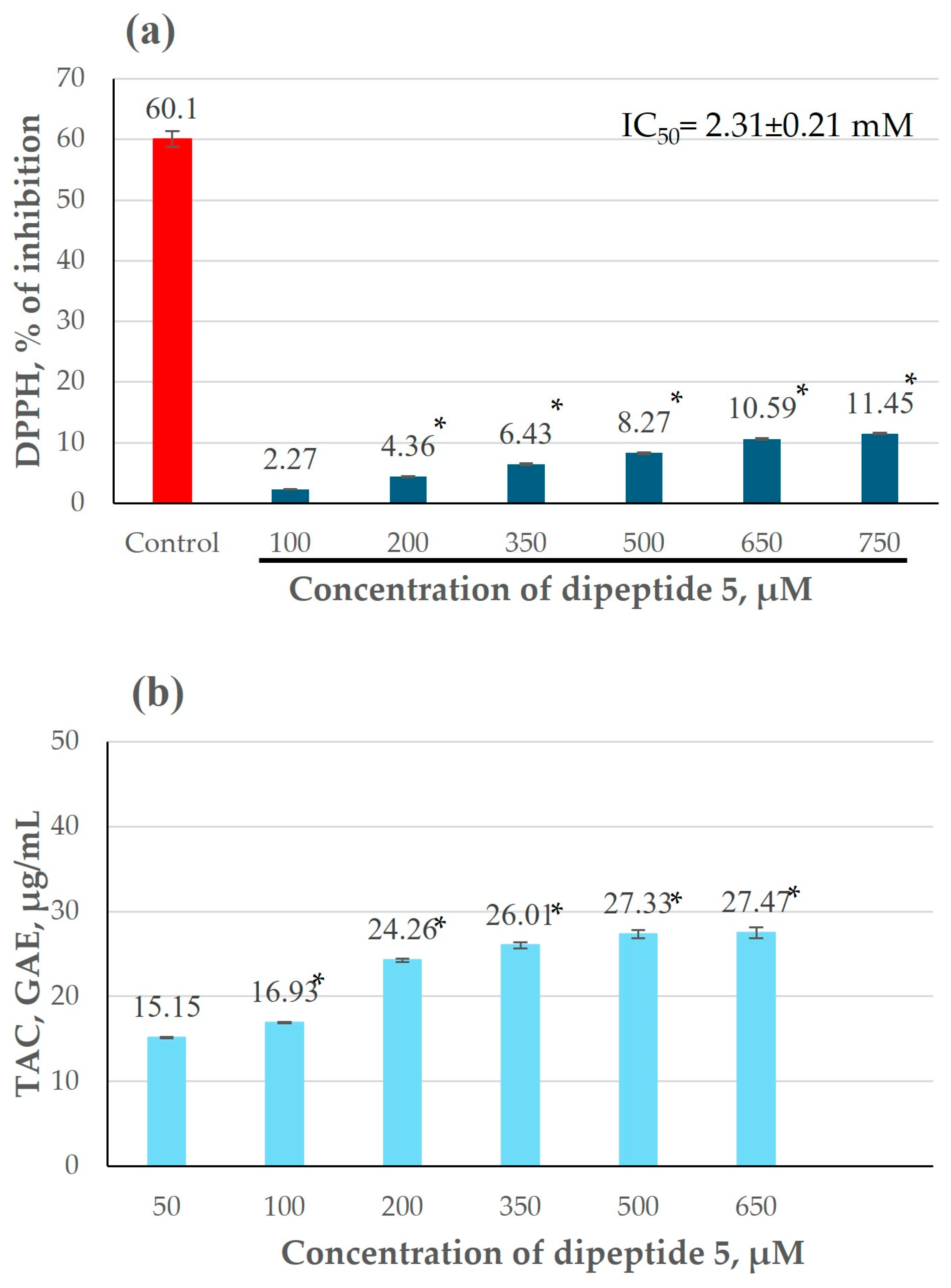
2.9.4. DPPH Radical Scavenging Activity Assay
2.9.5. Total Antioxidant Capacity (Phosphomolybdate Assay)
2.9.6. Determination of Antibacterial Activity
2.9.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of the Dipeptide
3.2. Preliminary Biological Studies
3.2.1. Antioxidant Activity
3.2.2. Preliminary Antimicrobial Studies
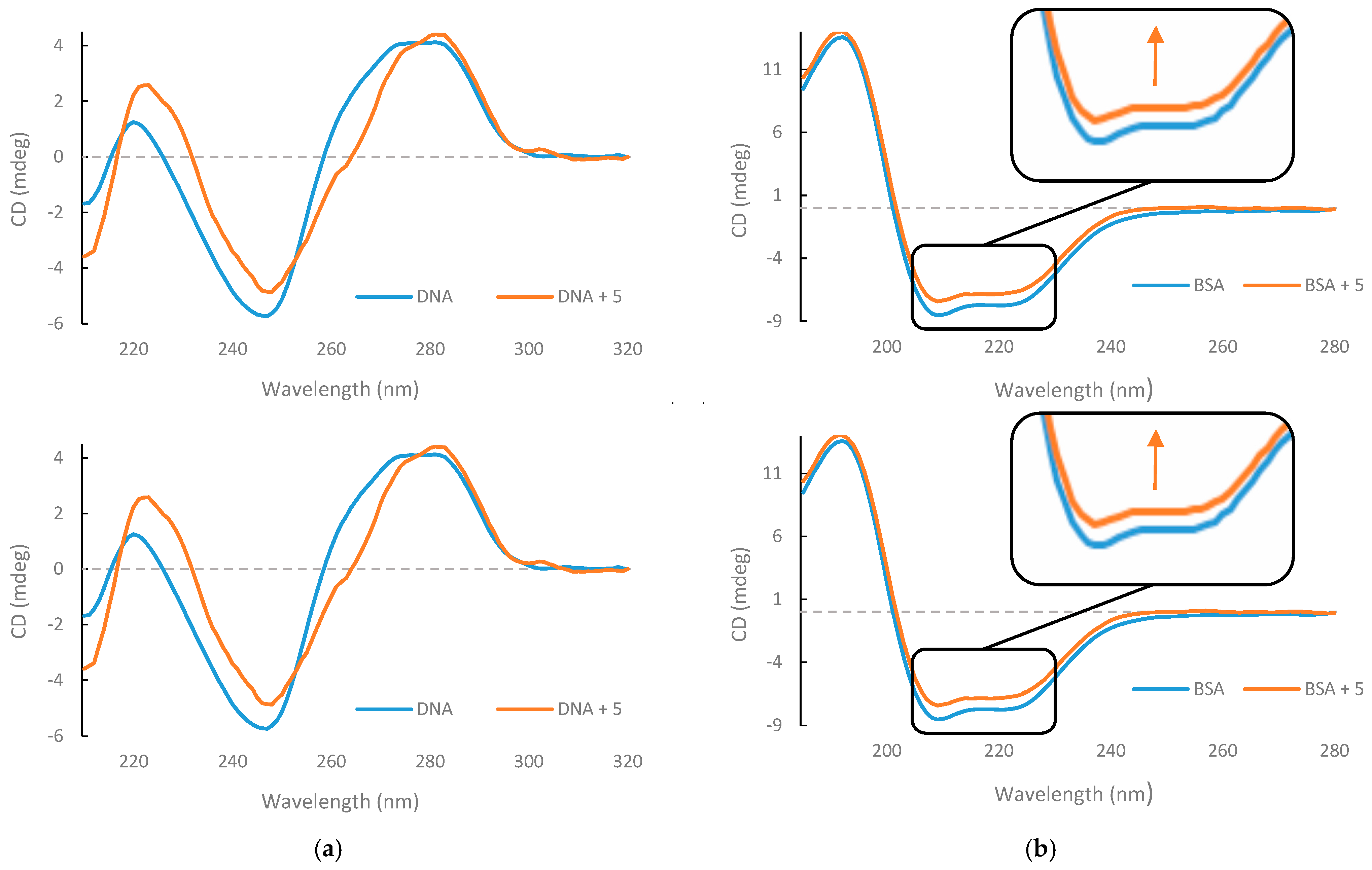
3.3. CD DNA and BSA Binding Studies
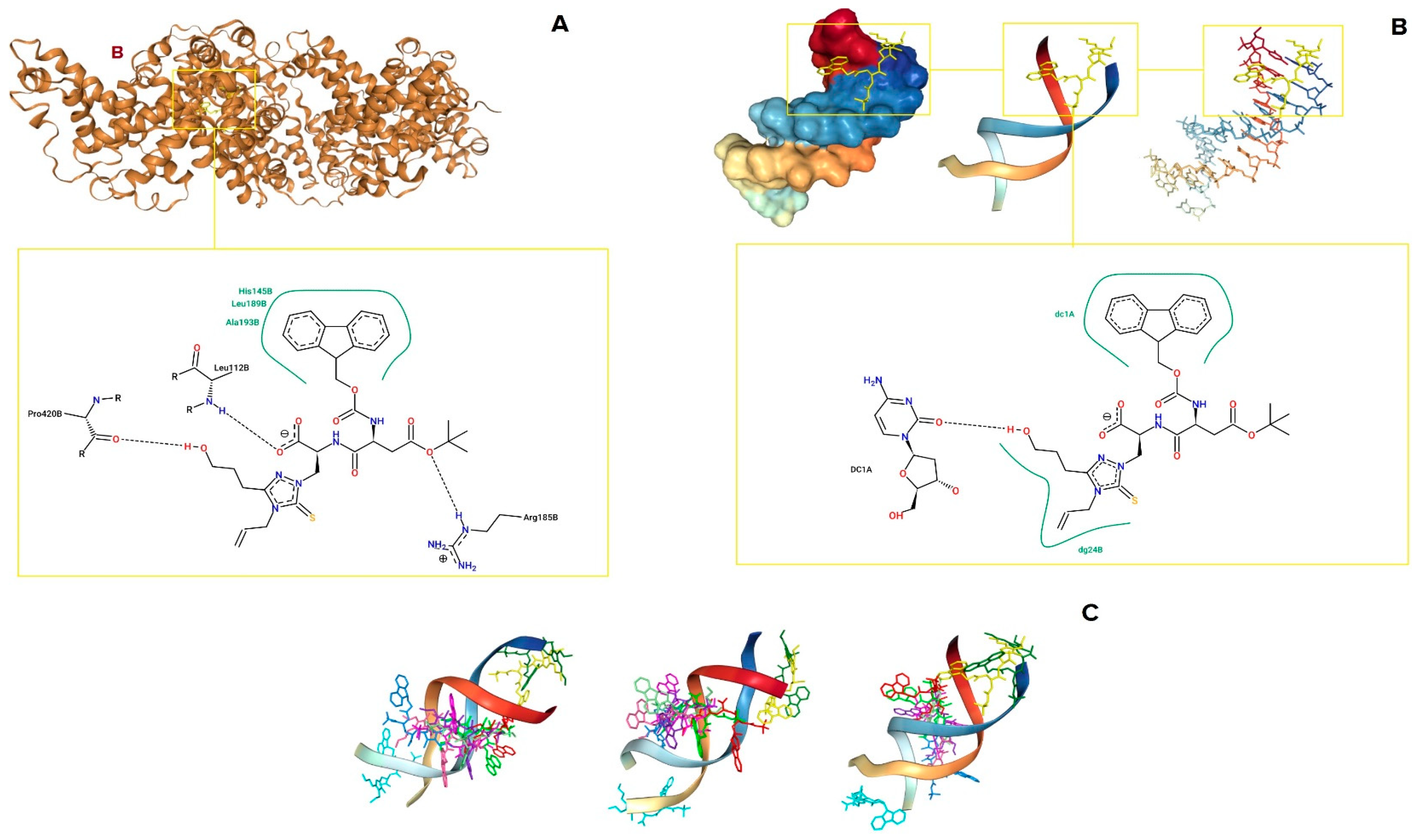
3.4. In Silico Studies
3.5. Computational Evaluation: MEPs, FMO Analysis and Global Reactivity Indexes, and Antioxidant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gómez Oliván, L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, K.H.; Ki, M.R.; Pack, S.P. Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics 2024, 13, 794. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Liu, X.Y.; Dai, X.H.; Chen, X.Z. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.-A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive Peptides: A Review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Chemtob, S.; Rosenstein, Y.; Auvynet, C. Editorial: Use of Small Peptides in the Treatment of Inflammatory Diseases. Front. Pharmacol. 2022, 13, 1090014. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the Use of Therapeutic Peptides for Cancer Treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Saeed, M.; Alharbi, A.M.; Barreto, G.E.; Ashraf, G.M.; Choi, I. Peptide-Based Therapeutics and Their Use for the Treatment of Neurodegenerative and Other Diseases. Biomed. Pharmacother. 2018, 103, 574–581. [Google Scholar] [CrossRef]
- Fetse, J.; Kandel, S.; Mamani, U.F.; Cheng, K. Recent Advances in the Development of Therapeutic Peptides. Trends Pharmacol. Sci. 2023, 44, 425–441. [Google Scholar] [CrossRef]
- Shalev, D.E. Studying Peptide-Metal Ion Complex Structures by Solution-State NMR. Int. J. Mol. Sci. 2022, 23, 15957. [Google Scholar] [CrossRef] [PubMed]
- Aiyelabola, T.O.; Isabirye, D.A.; Akinkunmi, E.O.; Ogunkunle, O.A.; Ojo, I.A.O. Synthesis, Characterization, and Antimicrobial Activities of Coordination Compounds of Aspartic Acid. J. Chem. 2016, 2016, 7317015. [Google Scholar] [CrossRef]
- Sussman, F.; Villaverde, M.C.; Domínguez, J.L.; Danielson, U.H. On the Active Site Protonation State in Aspartic Proteases: Implications for Drug Design. Curr. Pharm. Des. 2013, 19, 4257–4275. [Google Scholar] [CrossRef]
- Pająk, M.; Fichna, J.; Woźniczka, M. Protonation Constants of Endo- and Exogenous L-Amino Acids and Their Derivatives in Aqueous and Mixed Solution: Unraveling Molecular Secrets. Quater. Rev. Biophys. 2024, 57, e10. [Google Scholar] [CrossRef]
- Murcia, R.A.; Leal, S.M.; Roa, M.V.; Nagles, E.; Muñoz-Castro, A.; Hurtado, J.J. Development of Antibacterial and Antifungal Triazole Chromium (III) and Cobalt (II) Complexes: Synthesis and Biological Activity Evaluations. Molecules 2018, 23, 2013. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological Significance of Triazole Scaffold. J. Enzym. Inhib. Med. Chem. 2010, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Grabeck, J.; Mayer, J.; Miltz, A.; Casoria, M.; Quagliata, M.; Meinberger, D.; Klatt, A.R.; Wielert, I.; Maier, B.; Papini, A.M.; et al. Triazole-Bridged Peptides with Enhanced Antimicrobial Activity and Potency against Pathogenic Bacteria. ACS Infect. Dis. 2024, 10, 2717–2727. [Google Scholar] [CrossRef]
- Agouram, N.; El Hadrami, E.M.; Bentama, A. 1,2,3-Triazoles as Biomimetics in Peptide Science. Molecules 2021, 26, 2937. [Google Scholar] [CrossRef]
- Rečnik, L.-M.; Kandioller, W.; Mindt, T.L. 1,4-Disubstituted 1,2,3-Triazoles as Amide Bond Surrogates for the Stabilisation of Linear Peptides with Biological Activity. Molecules 2020, 25, 3576. [Google Scholar] [CrossRef]
- Sargsyan, T.; Stepanyan, L.; Panosyan, H.; Hakobyan, H.; Israyelyan, M.; Tsaturyan, A.; Hovhannisyan, N.; Vicidomini, C.; Mkrtchyan, A.; Saghyan, A.; et al. Synthesis and Antifungal Activity of Fmoc-Protected 1,2,4-Triazolyl-α-Amino Acids and Their Dipeptides Against Aspergillus Species. Biomolecules 2025, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, A.S.; Karapetyan, L.T.; Mkhitaryan, A.V.; Stepanyan, L.A.; Sargsyan, T.H.; Danghyan, Y.M.; Sargsyan, A.V.; Oganezova, G.G.; Hovhannisyan, N.A. Modeling, Synthesis and In Vitro Testing of Peptides Based on Unusual Amino Acids as Potential Antibacterial Agents. Biomed. Khim. 2024, 70, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.; Kostova, I. 1,2,3-Triazoles and Their Metal Chelates with Antimicrobial Activity. Front. Chem. 2023, 11, 1247805. [Google Scholar] [CrossRef]
- Staśkiewicz, A.; Ledwoń, P.; Rovero, P.; Papini, A.M.; Latajka, R. Triazole-Modified Peptidomimetics: An Opportunity for Drug Discovery and Development. Front. Chem. 2021, 9, 674705. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A. Potential Role of Bioactive Phytochemicals in Combination Therapies against Antimicrobial Activity. J. Pharmacopunct. 2022, 25, 79–87. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Bonincontro, G.; Scuderi, S.A.; Marino, A.; Simonetti, G. Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp. Pharmaceuticals 2023, 16, 1531. [Google Scholar] [CrossRef]
- Hong, D.-Y.; Zhou, S.-L. Paeonia (Paeoniaceae) in the Caucasus. Bot. J. Linn. Soc. 2003, 143, 135–150. [Google Scholar] [CrossRef]
- Roviello, G.N.; Mittova, V.; Tsetskhladze, Z.R.; Bidzinashvili, R.; Berdzenishvili, M.; Vakhania, M.; Mindiashvili, T.; Kobiashvili, M. Antioxidant Properties of Some Caucasian Medicinal Plants. Mod. Issues Med. Manag. 2024, 27, 4–15. [Google Scholar] [CrossRef]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A Pharmacological Review of Bioactive Constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch: Review of P. lactiflora Pallas and P. veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef]
- Zahra, N.; Iqbal, J.; Arif, M.; Abbasi, B.A.; Sher, H.; Nawaz, A.F.; Yaseen, T.; Ydyrys, A.; Sharifi-Rad, J.; Calina, D. A comprehensive review on traditional uses, phytochemistry and pharmacological properties of Paeonia emodi Wall. ex Royle: Current landscape and future perspectives. Chin. Med. 2023, 18, 23. [Google Scholar] [CrossRef]
- Bai, Z.-Z.; Ni, J.; Tang, J.-M.; Sun, D.-Y.; Yan, Z.-G.; Zhang, J.; Niu, L.-X.; Zhang, Y.-L. Bioactive Components, Antioxidant and Antimicrobial Activities of Paeonia rockii Fruit during Development. Food Chem. 2021, 343, 128444. [Google Scholar] [CrossRef] [PubMed]
- Marković, T.; Čutović, N.; Carević, T.; Gašić, U.; Stojković, D.; Xue, J.; Jovanović, A. Paeonia peregrina Mill Petals as a New Source of Biologically Active Compounds: Chemical Characterization and Skin Regeneration Effects of the Extracts. Int. J. Mol. Sci. 2023, 24, 11764. [Google Scholar] [CrossRef]
- Xin, Z.; Yang, W.; Niu, L.; Zhang, Y. Comprehensive Metabolite Profile Uncovers the Bioactive Components, Antioxidant and Antibacterial Activities in Wild Tree Peony Leaves. Int. J. Mol. Sci. 2023, 24, 10609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, P.; Shen, J.; Yang, K.; Wu, X.; Wang, Y.; Yuan, Y.-H.; Xiao, P.; He, C. Comprehensive Comparison of Different Parts of Paeonia ostii, a Food-Medicine Plant, Based on Untargeted Metabolomics, Quantitative Analysis, and Bioactivity Analysis. Front. Plant Sci. 2023, 14, 1243724. [Google Scholar] [CrossRef]
- Abriata, L.A. A Simple Spreadsheet Program to Simulate and Analyze the Far-UV Circular Dichroism Spectra of Proteins. J. Chem. Educ. 2011, 88, 1268–1273. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zou, X. An Iterative Knowledge-Based Scoring Function for Protein–Protein Recognition. Proteins 2008, 72, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Schöning-Stierand, K.; Diedrich, K.; Fährrolfes, R.; Flachsenberg, F.; Meyder, A.; Nittinger, E.; Steinegger, R.; Rarey, M. Proteins Plus: Interactive Analysis of Protein–Ligand Binding Interfaces. Nucleic Acids Res. 2020, 48, W48–W53. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Amić, A.; Lučić, B.; Stepanić, V.; Marković, Z.; Marković, S.; Dimitrić Marković, J.M.; Amić, D. Free radical scavenging potency of quercetin catecholic colonic metabolites: Thermodynamics of 2H⁺/2e⁻ processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Verderi, L.; Nova, N.; Borghesani, V.; Tegoni, M.; Giannetto, M.; Fortunati, S.; Ronda, L.; Pinelli, S.; Mozzoni, P.; Nicastro, M.; et al. Cytotoxic ROS-Consuming Mn (III) Synzymes: Structural Influence on Their Mechanism of Action. Int. J. Mol. Sci. 2025, 26, 150. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Xavier, S.; Periandy, S.; Carthigayan, K.; Sebastian, S. Molecular docking, TG/DTA, molecular structure, harmonic vibrational frequencies, natural bond orbital and TD-DFT analysis of diphenyl carbonate by DFT approach. J. Mol. Struct. 2016, 1125, 204–216. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- El-Sayed, D.S.; Hassan, S.S.; Jassim, L.S.; Issa, A.A.; Al-Oqaili, F.; Albayaty, M.K.; Hasoon, B.A.; Jabir, M.S.; Rasool, K.H.; Elbadawy, H.A. Structural and topological analysis of thiosemicarbazone-based metal complexes: Computational and experimental study of bacterial biofilm inhibition and antioxidant activity. BMC Chem. 2025, 19, 24. [Google Scholar] [CrossRef]
- Parr, R.G.; von Szentpály, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Kashar, T.I.; Aal, S.A. Spectral, DFT–TDDFT computational investigation and biological studies of transition metal complexes of dehydroacetic acid Schiff base. J. Iran Chem. Soc. 2021, 18, 1625–1640. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Xia, D.; Wang, S. Antioxidant properties of camphene-based thiosemicarbazones: Experimental and theoretical evaluation. Molecules 2020, 25, 1192. [Google Scholar] [CrossRef]
- Ajitha, M.J.; Mohanlal, S.; Suresh, C.H.; Jayalekshmy, A. DPPH radical scavenging activity of tricin and its conjugates isolated from “Njavara” rice bran: A density functional theory study. J. Agric. Food Chem. 2012, 60, 3693–3699. [Google Scholar] [CrossRef]
- Mittova, V.; Tsetskhladze, Z.R.; Makalatia, K.; Bidzinashvili, R.; Mindiashvili, T.; Kobiashvili, M.; Roviello, G.N. Antioxidant and antibacterial activity of root extracts of Georgian medicinal plants obtained using different extraction methods. Mod. Issues Med. Manag. 2024, 28, 17–31. [Google Scholar] [CrossRef]
- Mitra, S.; Bhesania Hodiwala, A.V.; Kar, H. Susceptibility and Synergistic Effects of Guava Plant Extract and Antimicrobial Drugs on Escherichia coli. Cureus 2024, 16, e52345. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X. Purification, Identification and Evaluation of Antioxidant Peptides from Pea Protein Hydrolysates. Molecules 2023, 28, 2952. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Mittova, V.; Pirtskhalava, M.; Bidzinashvili, R.; Vakhania, M.; Mindiashvili, T.; Kobiashvili, M. Effects of different drying, extraction methods, and solvent polarity on the antioxidant properties of Paeonia daurica subsp. mlokosewitschii leaves. Mod. Issues Med. Manag. 2023, 26, 66–77. [Google Scholar] [CrossRef]
- La, J.; Kim, M.J.; Lee, J. Evaluation of Solvent Effects on the DPPH Reactivity for Determining the Antioxidant Activity in Oil Matrix. Food Sci. Biotechnol. 2021, 30, 367–375. [Google Scholar] [CrossRef]
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. A High Correlation Indicating for an Evaluation of Antioxidant Activity and Total Phenolics Content of Various Chilli Varieties. J. Food Sci. Technol. 2015, 52, 8077–8085. [Google Scholar] [CrossRef]
- Ushie, O.A.; Neji, P.A.; Abeng, F.E.; Azuaga, T.I.; Aikhoje, E.F.; Adashu, J.M. Antioxidant Activity of Hexane, Chloroform, Acetone and Methanol Extract of Swietenia macrophylla. Int. J. Clin. Chem. Lab. Med. 2019, 5, 6–10. [Google Scholar] [CrossRef]
- Siregar, N.S.; Julianti, E.; Silalahi, J.; Sinaga, H. Antioxidant Activity of Salak Sidempuan (Salacca zalacca) Fruit with Different Solvents Using the DPPH Method. IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012097. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10-1128. [Google Scholar] [CrossRef]
- Batinić, P.; Jovanović, A.; Stojković, D.; Zengin, G.; Cvijetić, I.; Gašić, U.; Čutović, N.; Pešić, M.B.; Milinčić, D.D.; Carević, T.; et al. Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia. Pharmaceuticals 2024, 17, 518. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, Z.; Huang, X.-Y.; Chen, J.-J.; Geng, C.-A. Chemical and Biological Comparison of Different Parts of Paeonia suffruticosa (Mudan) Based on LCMS-IT-TOF and Multi-Evaluation In Vitro. Ind. Crops Prod. 2020, 144, 112028. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskienė, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of Strong Antioxidants from Paeonia officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An Update on the Potential Mechanism of Gallic Acid as an Antibacterial and Anticancer Agent. Foods 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Ji, C.; Yin, X.; Duan, H.; Liang, L. Molecular Complexes of Calf Thymus DNA with Various Bioactive Compounds: Formation and Characterization. Int. J. Biol. Macromol. 2021, 168, 775–783. [Google Scholar] [CrossRef]
- Tayyab, S.; Feroz, S.R. Serum Albumin: Clinical Significance of Drug Binding and Development as Drug Delivery Vehicle. Adv. Protein Chem. Struct. Biol. 2021, 123, 193–218. [Google Scholar] [CrossRef]
- Iemma, F.; Spizzirri, U.G.; Puoci, F.; Muzzalupo, R.; Trombino, S.; Cassano, R.; Leta, S.; Picci, N. pH-Sensitive Hydrogels Based on Bovine Serum Albumin for Oral Drug Delivery. Int. J. Pharm. 2006, 312, 151–157. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, S.Y. Modeling Protein–Protein or Protein–DNA/RNA Complexes Using the HDOCK Webserver. Methods Mol. Biol. 2020, 2165, 217–229. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of Bovine, Equine and Leporine Serum Albumin. Acta Crystallogr. D Biol. Crystallogr. 2012, 68 Pt 10, 1278–1289. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA Dodecamer: Conformation and Dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef]


| Secondary Structure 1 Component | Rate Before Ligand Addition (%) | ΔSecondary Structure (%) |
|---|---|---|
| alpha-helix | 65.49 | +10.16 |
| beta-sheet | 13.10 | −0.41 |
| turn | 4.66 | +0.12 |
| random coil | 16.76 | −9.87 |
| Target | PDB ID | HDOCK Score (Top-1 Ranked Pose) | HDOCK Score (Top 1–10 Poses, Average ± SD) | Residues Involved in Binding |
|---|---|---|---|---|
| BSA | 4F5S | −202.13 | −183.52 ± 10.68 | Leu112, Arg185, Pro420 (hydrogen bonds); His145, Leu189, Ala193 (hydrophobic interactions) |
| dsDNA | 1BNA | −171.22 | −151.40 ± 7.63 | dC1 (chain A), dG24 (chain B) (hydrogen bonds and hydrophobic interactions) |
| ωB97XD/aug-cc-pVDZ | Dipole Moment (D) | HOMO/LUMO Gap (eV) | HOMO/LUMO Gap | (eV) | (eV) | (eV−1) | (eV) | (eV) |
|---|---|---|---|---|---|---|---|---|
| Gas phase | 7.83 | 2.91 | 67.1 | 1.45 | −5.75 | 0.69 | 5.75 | 11.4 |
| MeOH | 12.1 | 4.45 | 102.6 | 2.22 | −5.30 | 0.45 | 5.30 | 6.30 |
| DMSO | 10.2 | 3.08 | 71.0 | 1.54 | −6.00 | 0.65 | 6.00 | 11.7 |
| HAT Mechanism | SET Mechanism | |||
|---|---|---|---|---|
| Energies | BDEOH (eV) | IP (eV) | ||
| Gas phase | 4.16 | 95.9 | 7.34 | 169 |
| MeOH | 4.49 | 104 | 6.20 | 143 |
| DMSO | 4.00 | 92.2 | 5.25 | 121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanyan, L.; Sargsyan, T.; Mittova, V.; Tsetskhladze, Z.R.; Motsonelidze, N.; Gorgoshidze, E.; Nova, N.; Israyelyan, M.; Simonyan, H.; Bisceglie, F.; et al. The Synthesis, Characterization, and Biological Evaluation of a Fluorenyl-Methoxycarbonyl-Containing Thioxo-Triazole-Bearing Dipeptide: Antioxidant, Antimicrobial, and BSA/DNA Binding Studies for Potential Therapeutic Applications in ROS Scavenging and Drug Transport. Biomolecules 2025, 15, 933. https://doi.org/10.3390/biom15070933
Stepanyan L, Sargsyan T, Mittova V, Tsetskhladze ZR, Motsonelidze N, Gorgoshidze E, Nova N, Israyelyan M, Simonyan H, Bisceglie F, et al. The Synthesis, Characterization, and Biological Evaluation of a Fluorenyl-Methoxycarbonyl-Containing Thioxo-Triazole-Bearing Dipeptide: Antioxidant, Antimicrobial, and BSA/DNA Binding Studies for Potential Therapeutic Applications in ROS Scavenging and Drug Transport. Biomolecules. 2025; 15(7):933. https://doi.org/10.3390/biom15070933
Chicago/Turabian StyleStepanyan, Lala, Tatevik Sargsyan, Valentina Mittova, Zurab R. Tsetskhladze, Nino Motsonelidze, Ekaterine Gorgoshidze, Niccolò Nova, Monika Israyelyan, Hayarpi Simonyan, Franco Bisceglie, and et al. 2025. "The Synthesis, Characterization, and Biological Evaluation of a Fluorenyl-Methoxycarbonyl-Containing Thioxo-Triazole-Bearing Dipeptide: Antioxidant, Antimicrobial, and BSA/DNA Binding Studies for Potential Therapeutic Applications in ROS Scavenging and Drug Transport" Biomolecules 15, no. 7: 933. https://doi.org/10.3390/biom15070933
APA StyleStepanyan, L., Sargsyan, T., Mittova, V., Tsetskhladze, Z. R., Motsonelidze, N., Gorgoshidze, E., Nova, N., Israyelyan, M., Simonyan, H., Bisceglie, F., Sahakyan, L., Ghazaryan, K., & Roviello, G. N. (2025). The Synthesis, Characterization, and Biological Evaluation of a Fluorenyl-Methoxycarbonyl-Containing Thioxo-Triazole-Bearing Dipeptide: Antioxidant, Antimicrobial, and BSA/DNA Binding Studies for Potential Therapeutic Applications in ROS Scavenging and Drug Transport. Biomolecules, 15(7), 933. https://doi.org/10.3390/biom15070933











