Impact of Long-Term Plasma Storage on Cell-Free DNA Epigenetic Biomarker Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. cfDNA Extraction
2.3. cfDNA Quality and Integrity Assessment
2.4. cfDNA 5hmC Analysis by Nano-hmC-Seal-Seq Assay
2.5. Statistical Analyses
3. Results
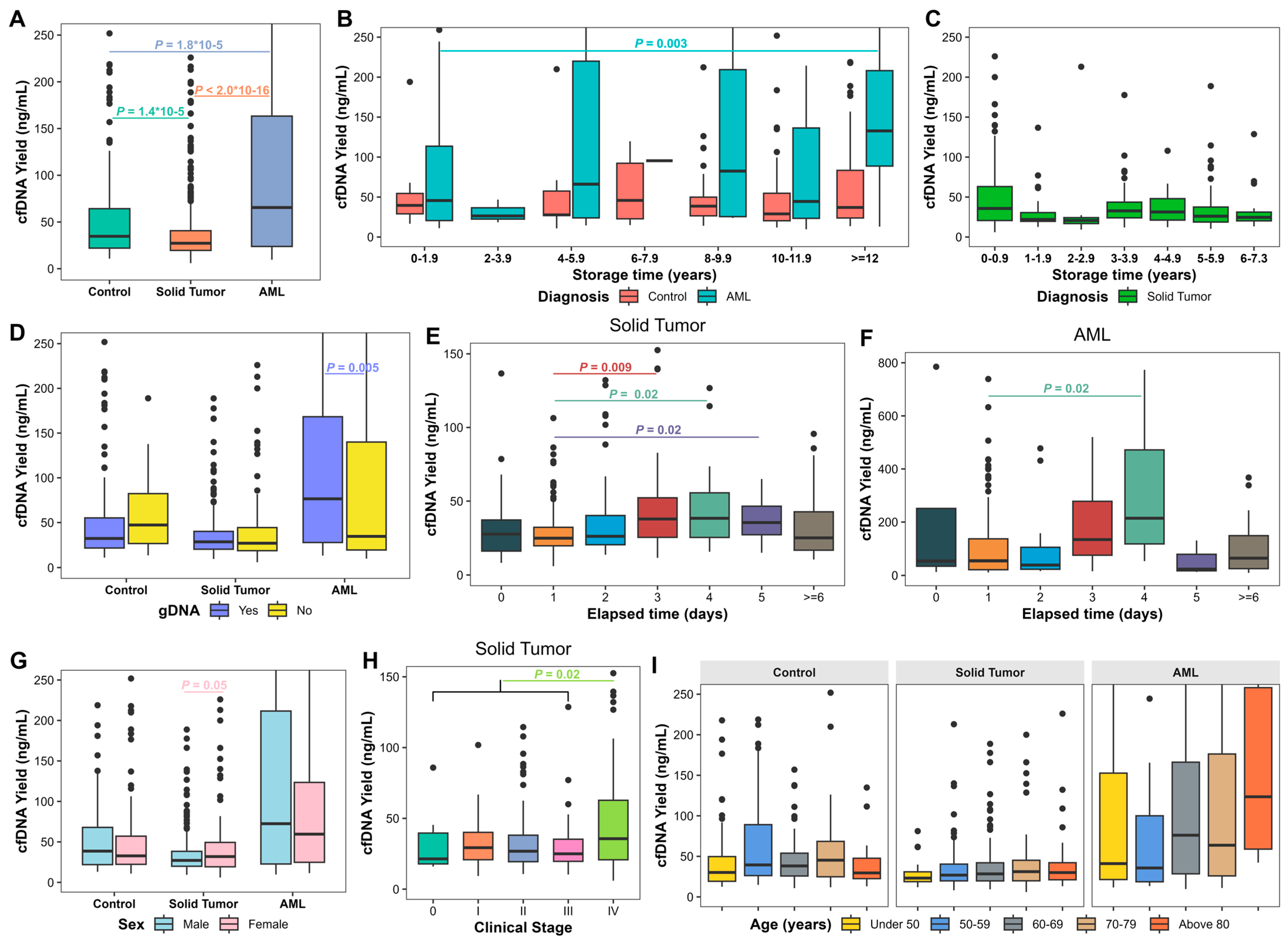
3.1. cfDNA Yield in Long-Term Stored Plasma Samples
3.2. Factors Associated with Plasma cfDNA Yield
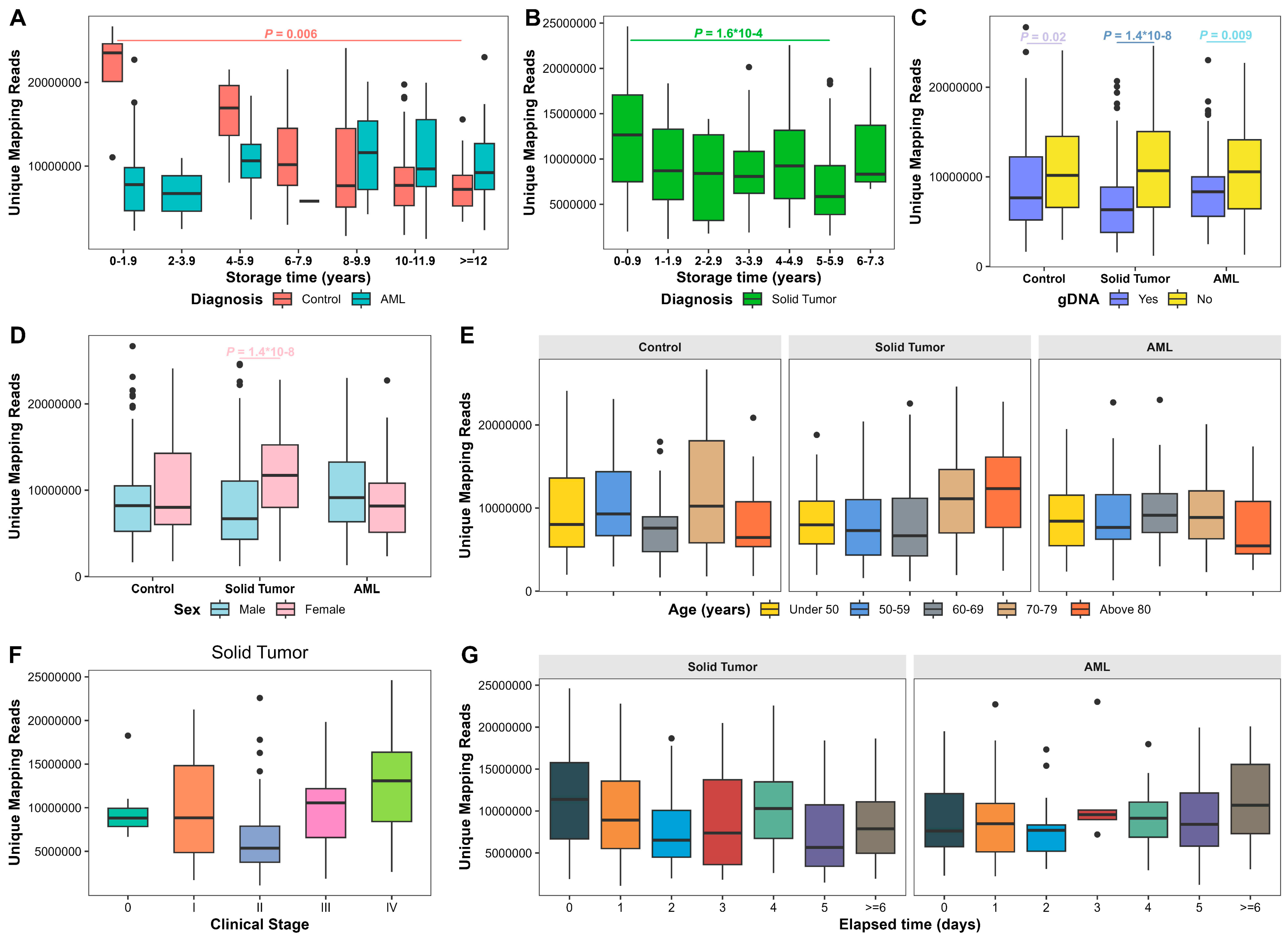
3.3. Factors Associated with cfDNA Fragment Presence
3.4. Factors Associated with gDNA Contamination
3.5. Factors Associated with Successful DNA Library Preparation for 5hmc Analysis
3.6. Factors Associated with 5hmC Sequencing Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Pardo, M.; Czarnecka-Kujawa, K.; Law, J.H.; Salvarrey, A.M.; Fernandes, R.; Fan, Z.J.; Waddell, T.K.; Yasufuku, K.; Liu, G.; Donahoe, L.L.; et al. Association of Circulating Tumor DNA Testing Before Tissue Diagnosis With Time to Treatment Among Patients With Suspected Advanced Lung Cancer: The ACCELERATE Nonrandomized Clinical Trial. JAMA Netw. Open 2023, 6, e2325332. [Google Scholar] [CrossRef]
- West-Szymanski, D.C.; Zhang, Z.; Cui, X.L.; Kowitwanich, K.; Gao, L.; Deng, Z.; Dougherty, U.; Williams, C.; Merkle, S.; He, C.; et al. 5-Hydroxymethylated Biomarkers in Cell-Free DNA Predict Occult Colorectal Cancer up to 36 Months Before Diagnosis in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. JCO Precis. Oncol. 2024, 8, e2400277. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Auinger, L.; Speicher, M.R. Cell-Free DNA and Apoptosis: How Dead Cells Inform About the Living. Trends Mol. Med. 2020, 26, 519–528. [Google Scholar] [CrossRef]
- van der Pol, Y.; Mouliere, F. Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell 2019, 36, 350–368. [Google Scholar] [CrossRef]
- Greytak, S.R.; Engel, K.B.; Parpart-Li, S.; Murtaza, M.; Bronkhorst, A.J.; Pertile, M.D.; Moore, H.M. Harmonizing Cell-Free DNA Collection and Processing Practices through Evidence-Based Guidance. Clin. Cancer Res. 2020, 26, 3104–3109. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, N.L.; Boyd, M.A.A.; Henry, C.E.; Werner, B.; Ford, C.E.; Warton, K. Circulating cell-free DNA undergoes significant decline in yield after prolonged storage time in both plasma and purified form. Clin. Chem. Lab. Med. 2022, 60, 1287–1298. [Google Scholar] [CrossRef]
- Clausen, F.B.; Barrett, A.N.; Advani, H.V.; Choolani, M.; Dziegiel, M.H. Impact of long-term storage of plasma and cell-free DNA on measured DNA quantity and fetal fraction. Vox Sang. 2020, 115, 586–594. [Google Scholar] [CrossRef]
- Peng, H.; Pan, M.; Zhou, Z.; Chen, C.; Xing, X.; Cheng, S.; Zhang, S.; Zheng, H.; Qian, K. The impact of preanalytical variables on the analysis of cell-free DNA from blood and urine samples. Front. Cell Dev. Biol. 2024, 12, 1385041. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Nakashima, C.; Abe, T.; Kato, J.; Hirai, M.; Nakamura, T.; Komiya, K.; Kimura, S.; Sueoka, E.; Sueoka-Aragane, N. Investigation of appropriate pre-analytical procedure for circulating free DNA from liquid biopsy. Oncotarget 2018, 9, 31904–31914. [Google Scholar] [CrossRef]
- Zeng, C.; Stroup, E.K.; Zhang, Z.; Chiu, B.C.; Zhang, W. Towards precision medicine: Advances in 5-hydroxymethylcytosine cancer biomarker discovery in liquid biopsy. Cancer Commun 2019, 39, 12. [Google Scholar] [CrossRef]
- Li, J.J.N.; Liu, G.; Lok, B.H. Cell-Free DNA Hydroxymethylation in Cancer: Current and Emerging Detection Methods and Clinical Applications. Genes 2024, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, Y.; Chen, L.; Bissessur, A.S.; Chen, J.; Mao, M.; Ju, S.; Chen, L.; Chen, C.; Li, Z.; et al. Deoxyribonucleic Acid 5-Hydroxymethylation in Cell-Free Deoxyribonucleic Acid, a Novel Cancer Biomarker in the Era of Precision Medicine. Front. Cell Dev. Biol. 2021, 9, 744990. [Google Scholar] [CrossRef] [PubMed]
- Kriukiene, E.; Tomkuviene, M.; Klimasauskas, S. 5-Hydroxymethylcytosine: The many faces of the sixth base of mammalian DNA. Chem. Soc. Rev. 2024, 53, 2264–2283. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Garcia-Aranda, M.; Chaves, P.; Alba, E.; Cobo-Dols, M.; Onieva, J.L.; Barragan, I. Emerging noninvasive methylation biomarkers of cancer prognosis and drug response prediction. Semin. Cancer Biol. 2022, 83, 584–595. [Google Scholar] [CrossRef]
- Lu, D.; Wu, X.; Wu, W.; Wu, S.; Li, H.; Zhang, Y.; Yan, X.; Zhai, J.; Dong, X.; Feng, S.; et al. Plasma cell-free DNA 5-hydroxymethylcytosine and whole-genome sequencing signatures for early detection of esophageal cancer. Cell Death Dis. 2023, 14, 843. [Google Scholar] [CrossRef]
- Chen, B.; Wang, D.; Xu, Y.; Guo, Q.; Pan, J.; Yu, S.; Fang, Y.; Xiao, S.; Ruan, Y.; Yang, S.; et al. 5-Hydroxymethylcytosines in circulating cell-free DNA as a diagnostic biomarker for nasopharyngeal carcinoma. Eur. J. Cancer 2024, 210, 114294. [Google Scholar] [CrossRef]
- Shao, J.; Wang, S.; West-Szymanski, D.; Karpus, J.; Shah, S.; Ganguly, S.; Smith, J.; Zu, Y.; He, C.; Li, Z. Cell-free DNA 5-hydroxymethylcytosine is an emerging marker of acute myeloid leukemia. Sci. Rep. 2022, 12, 12410. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Shah, S.; Ganguly, S.; Zu, Y.; He, C.; Li, Z. Classification of Acute Myeloid Leukemia by Cell-Free DNA 5-Hydroxymethylcytosine. Genes 2023, 14, 1180. [Google Scholar] [CrossRef]
- Shao, J.; Bernicker, E.; He, C.; Li, Z. Cell-free DNA 5-hydroxymethylcytosine as a marker for common cancer detection. Clin. Transl. Discov. 2022, 2, e136. [Google Scholar] [CrossRef]
- Shao, J.; Olsen, J.O.; Kasparian, S.; He, C.; Bernicker, E.; Li, Z. Cell-Free DNA 5-Hydroxymethylcytosine Signatures for Lung Cancer Prognosis. Cells 2024, 13, 298. [Google Scholar] [CrossRef]
- Shao, J.; Shah, S.; Ganguly, S.; Zu, Y.; He, C.; Li, Z. Cell-free DNA 5-hydroxymethylcytosine is highly sensitive for MRD assessment in acute myeloid leukemia. Clin. Epigenetics 2023, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xu, Y.; Olsen, R.J.; Kasparian, S.; Sun, K.; Mathur, S.; Zhang, J.; He, C.; Chen, S.H.; Bernicker, E.H.; et al. 5-Hydroxymethylcytosine in Cell-Free DNA Predicts Immunotherapy Response in Lung Cancer. Cells 2024, 13, 715. [Google Scholar] [CrossRef]
- Weigert, M.; Cui, X.L.; West-Szymanski, D.; Yu, X.; Bilecz, A.J.; Zhang, Z.; Dhir, R.; Kehoe, M.; Zhang, W.; He, C.; et al. 5-Hydroxymethylcytosine signals in serum are a predictor of chemoresistance in high-grade serous ovarian cancer. Gynecol. Oncol. 2024, 182, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, A.; Bronkhorst, A.J.; Uhlig, C.; Ungerer, V.; Holdenrieder, S. Tracing the Origin of Cell-Free DNA Molecules through Tissue-Specific Epigenetic Signatures. Diagnostics 2022, 12, 1834. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, C.; Lu, X.; Song, Y.; Nie, J.; Ran, R.; Zhang, Z.; He, C.; Zhang, W.; Liu, S.M. 5-Hydroxymethylcytosines in Circulating Cell-Free DNA Reveal Vascular Complications of Type 2 Diabetes. Clin. Chem. 2019, 65, 1414–1425. [Google Scholar] [CrossRef]
- Tong, X.; Chen, W.; Ye, L.; Xiong, Y.; Xu, Y.; Luo, Y.; Xia, X.; Xu, Z.; Lin, Y.; Zhu, X.; et al. 5-Hydroxymethylcytosine in circulating cell-free DNA as a potential diagnostic biomarker for SLE. Lupus Sci. Med. 2024, 11, e001286. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, C.; Yang, K.; Xu, S.; Zhang, Z.; Cai, Q.; He, C.; Zhang, W.; Liu, S.M. Genome-wide Analysis Reflects Novel 5-Hydroxymethylcytosines Implicated in Diabetic Nephropathy and the Biomarker Potential. Extracell. Vesicles Circ. Nucl. Acids 2022, 3, 49–60. [Google Scholar] [CrossRef]
- Chu, J.L.; Bi, S.H.; He, Y.; Ma, R.Y.; Wan, X.Y.; Wang, Z.H.; Zhang, L.; Zheng, M.Z.; Yang, Z.Q.; Du, L.W.; et al. 5-Hydroxymethylcytosine profiles in plasma cell-free DNA reflect molecular characteristics of diabetic kidney disease. Front. Endocrinol. 2022, 13, 910907. [Google Scholar] [CrossRef]
- Dong, C.; Chen, J.; Zheng, J.; Liang, Y.; Yu, T.; Liu, Y.; Gao, F.; Long, J.; Chen, H.; Zhu, Q.; et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic and predictive biomarkers for coronary artery disease. Clin. Epigenetics 2020, 12, 17. [Google Scholar] [CrossRef]
- Chen, H.Y.; Li, X.X.; Li, C.; Zhu, H.C.; Hou, H.Y.; Zhang, B.; Cheng, L.M.; Hu, H.; Lu, Z.X.; Liu, J.X.; et al. 5-Hydroxymethylcytosine Signatures in Circulating Cell-Free DNA as Early Warning Biomarkers for COVID-19 Progression and Myocardial Injury. Front. Cell Dev. Biol. 2021, 9, 781267. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Xu, S.; Yu, H.; Pei, S.; Zhang, Y.; He, X.; Wang, Q.; Li, D. 5-Hydroxymethylcytosine Signatures in Circulating Cell-Free DNA as Diagnostic Biomarkers for Late-Onset Alzheimer’s Disease. J. Alzheimers Dis. 2022, 85, 573–585. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Tsering, T.; Nadeau, A.; Wu, T.; Dickinson, K.; Burnier, J.V. Extracellular vesicle-associated DNA: Ten years since its discovery in human blood. Cell Death Dis. 2024, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Ben-Ami, R.; Shai, E.; Gal-Rosenberg, O.; Kalish, Y.; Klochendler, A.; Cann, G.; Glaser, B.; Arad, A.; Shemer, R.; et al. Megakaryocyte- and erythroblast-specific cell-free DNA patterns in plasma and platelets reflect thrombopoiesis and erythropoiesis levels. Nat. Commun. 2023, 14, 7542. [Google Scholar] [CrossRef]
- Thakral, D.; Gupta, R.; Sahoo, R.K.; Verma, P.; Kumar, I.; Vashishtha, S. Real-Time Molecular Monitoring in Acute Myeloid Leukemia With Circulating Tumor DNA. Front. Cell Dev. Biol. 2020, 8, 604391. [Google Scholar] [CrossRef]
- Lei, S.; Jia, S.; Takalkar, S.; Chang, T.C.; Ma, X.; Szlachta, K.; Xu, K.; Cheng, Z.; Hui, Y.; Koo, S.C.; et al. Genomic profiling of circulating tumor DNA for childhood cancers. Leukemia 2024, 39, 420–430. [Google Scholar] [CrossRef]
- Geeurickx, E.; Hendrix, A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol. Aspects Med. 2020, 72, 100828. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Guo, Q.R.; Wang, F.H.; Adhikari, R.; Zhu, Z.Y.; Zhang, H.Y.; Zhou, W.M.; Yu, H.; Li, J.Q.; Zhang, J.Y. Cell-Free DNA: Hope and Potential Application in Cancer. Front. Cell Dev. Biol. 2021, 9, 639233. [Google Scholar] [CrossRef]
- Gross, J.A.; Pacis, A.; Chen, G.G.; Barreiro, L.B.; Ernst, C.; Turecki, G. Characterizing 5-hydroxymethylcytosine in human prefrontal cortex at single base resolution. BMC Genom. 2015, 16, 672. [Google Scholar] [CrossRef] [PubMed]
| Total | Solid Tumor | Bladder | Breast | Colorectal | Kidney | Lung | Ovary | Prostate | Uteri | AML | Control | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | 1070 | 622 | 45 | 71 | 67 | 63 | 125 | 10 | 227 | 14 | 237 | 211 |
| (male) | (612) | (392) | (27) | (1) | (44) | (40) | (53) | (0) | (227) | (0) | (123) | (97) |
| Age (years) range | 20–95 | 24–95 | 37–91 | 24–91 | 40–95 | 35–88 | 46–92 | 29–83 | 40–84 | 37–86 | 27–94 | 20–93 |
| (median) | (65) | (66) | (74) | (54) | (66) | (67) | (70) | (63) | (63) | (70.5) | (63) | (60) |
| Male | 20–95 | 35–95 | 37–91 | 82–82 | 46–95 | 35–88 | 53–92 | NA | 40–84 | NA | 31–88 | 20–90 |
| (65) | (66) | (76) | (82) | (65) | (67) | (70) | (63) | (66) | (64) | |||
| Female | 21–94 | 24–91 | 51–88 | 24–91 | 40–89 | 49–82 | 46–90 | 29–83 | NA | 37–86 | 27–94 | 21–93 |
| (63) | (65) | (71.5) | (53.5) | (68) | (65) | (70) | (63) | (70.5) | (61.5) | (59) | ||
| Stage 0 | 18 | 18 | 10 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Stage I | 178 | 178 | 12 | 30 | 11 | 39 | 32 | 1 | 49 | 4 | NA | NA |
| Stage II | 190 | 190 | 7 | 11 | 6 | 11 | 11 | 5 | 154 | 1 | NA | NA |
| Stage III | 61 | 61 | 3 | 7 | 8 | 11 | 11 | 5 | 15 | 1 | NA | NA |
| Stage IV | 87 | 87 | 8 | 2 | 5 | 3 | 63 | 3 | 2 | 1 | NA | NA |
| Stage NA | 536 | 88 | 5 | 14 | 36 | 7 | 11 | 1 | 7 | 7 | 237 | 211 |
| Plasma storage years | ≤14 | ≤7 | 1–6 | 2–7 | 4–7 | 2–7 | ≤7 | 2–4 | 1–6 | 1–6 | ≤14 | ≤13 |
| (median) | (5.3) | (5.1) | (5.1) | (5.3) | (5.7) | (5.9) | (0.4) | (3.5) | (5) | (3.7) | (1.3) | (10.7) |
| Elapsed days | 0–36 | 0–36 | 0–9 | 0–5 | 0–0 | 0–1 | 0–36 | 1–6 | 0–30 | 1–8 | 0–27 | 0–4 |
| (median) | (1) | (2) | (2) | (1) | (0) | (0) | (1) | (1.5) | (2) | (3) | (1) | (1.5) |
| Sample size with elapsed time | 643 | 415 | 43 | 6 | 2 | 5 | 112 | 10 | 223 | 14 | 220 | 8 |
| p Value (Coefficient) | |||
|---|---|---|---|
| Quantile | 25th | 50th | 75th |
| AML | |||
| Age | 0.14 (0.21) | 0.02 # (0.94) | 0.76 (0.43) |
| Sex | 0.97 (0.15) | 0.38 (−11.14) | 0.13 (−73.60) |
| Storage time | 0.35 (1.35) | 0.008 # (5.58) | 0.39 (4.50) |
| gDNA contamination | 0.04 # (9.70) | 0.48 (−8.2) | 0.96 (1.85) |
| Elapsed days | 0.74 (−0.67) | 0.58 (−2.56) | 0.72 (4.34) |
| Solid Tumor | |||
| Age | 0.53 (0.03) | 0.15 (0.13) | 0.28 (−0.29) |
| Sex | 0.40 (1.90) | 0.12 (4.75) | 0.13 (9.52) |
| Storage time | 0.79 (−0.11) | 0.80 (−0.18) | 0.42 (−1.08) |
| gDNA contamination | 0.07 (2.51) | 0.07 (4.07) | 0.83 (0.96) |
| Stage IV | 0.73 (1.64) | 0.17 (10.03) | 0.48 (14.50) |
| Elapsed days | 0.44 (−0.13) | 0.93 (0.008) | 0.12 (1.51) |
| Control | |||
| Age | 0.07 (0.12) | 0.48 (0.09) | 0.60 (−0.18) |
| Sex | 0.26 (−3.24) | 0.33 (−5.90) | 0.26 (−12.56) |
| Storage time | 0.34 (−0.38) | 0.51 (−0.53) | 0.34 (1.50) |
| gDNA contamination | 0.28 (−3.86) | 0.21 (−9.01) | 0.16 (−23.89) |
| p Value (Coefficient) | |||
|---|---|---|---|
| cfDNA Fragment Presence | gDNA Contamination | Successful DNA Library Preparation | |
| AML | |||
| Age | 0.96 (8.6 × 10−4) | 0.15 (0.01) | 0.07 (−0.03) |
| Sex | 0.69 (−0.17) | 0.52 (0.18) | 0.76 (0.15) |
| Storage time | 0.97 (−0.002) | 0.05 # (0.06) | 0.19 (0.10) |
| cfDNA Yield | - | - | 4.1 × 10−4 # (0.05) |
| gDNA contamination | 0.33 (0.43) | - | 0.18 (0.64) |
| Elapsed days | 0.29 (−0.06) | 0.94 (−0.004) | 0.26 (0.24) |
| Solid Tumor | |||
| Age | 0.15 (0.02) | 0.02 # (−0.03) | 0.24 (0.02) |
| Sex | 0.41 (0.36) | 0.007 # (−0.77) | 8.6 × 10−4 # (2.49) |
| Storage time | 0.08 (−0.17) | 4.9 × 10−4 # (0.23) | 0.49 (0.07) |
| cfDNA Yield | - | - | 0.004 # (0.04) |
| gDNA contamination | 0.01 # (−0.81) | - | 0.008 # (−0.99) |
| Stage I | 0.90 (0.11) | 0.39 (−0.63) | 0.99 (−14.57) |
| Stage II | 0.75 (−0.26) | 1.0 (−6.9 × 10−4) | 0.98 (−15.43) |
| Stage III | 0.50 (0.69) | 0.33 (−0.77) | 0.98 (−15.45) |
| Stage IV | 0.57 (0.56) | 0.42 (−0.64) | 0.99 (−13.05) |
| Elapsed days | 0.70 (−0.01) | 0.004 # (0.15) | 0.84 (0.009) |
| Control | |||
| Age | 0.09 (0.02) | 0.27 (−0.01) | 0.11 (0.02) |
| Sex | 0.19 (−0.54) | 0.05 # (−0.66) | 0.25 (−0.53) |
| Storage time | 0.51 (0.05) | 0.92 (0.006) | 0.92 (0.007) |
| cfDNA Yield | - | - | 0.02 # (0.04) |
| gDNA contamination | 0.99 (−18.21) | - | 0.30 (−0.58) |
| p Value (Coefficient) | |||
|---|---|---|---|
| Quantile | 25th | 50th | 75th |
| AML | |||
| Age | 0.58 (13,759.06) | 0.50 (−27,088.05) | 0.63 (18,415.88) |
| Sex | 0.29 (−788,370.44) | 0.03 # (−2,096,216.92) | 0.15 (−1,466,351.94) |
| Storage time | 0.02 # (187,012.66) | 0.35 (108,174.20) | 0.33 (117,107.39) |
| cfDNA Yield | 0.01 # (2853.62) | 0.34 (1807.65) | 0.12 (3090.76) |
| gDNA contamination | 0.15 (−1,429,295.73) | 8.6 × 10−4 # (−2,992,290.16) | 0.006 # (−3,297,202.95) |
| Elapsed days | 0.98 (−6300.18) | 0.22 (295,514.60) | 0.38 (185,534.03) |
| Solid Tumor | |||
| Age | 0.91 (4456.51) | 0.27 (32,412.75) | 0.38 (43,104.00) |
| Sex | 0.01 # (2,723,525.42) | 0.01 # (2,658,007.93) | 0.005 # (3,424,488.43) |
| Storage time | 0.71 (−79,012.44) | 0.62 (−101,407.76) | 0.29 (−279,531.37) |
| cfDNA Yield | 0.46 (−7055.65) | 0.97 (363.78) | 0.88 (2039.74) |
| gDNA contamination | 0.008 # (−1,817,579.10) | 0.001 # (−3,147,950.68) | 0.01 # (−3,084,351.72) |
| Stage I | 0.13 (−1,972,118.07) | 0.26 (−2,262,700.55) | 0.54 (1,320,602.73) |
| Stage II | 0.07 (−2,346,599.28) | 0.009 # (−4,603,701.28) | 0.09 (−3,241,460.25) |
| Stage III | 0.57 (−962,704.24) | 0.17 (−2,825,400.68) | 0.66 (−1,139,338.78) |
| Stage IV | 0.96 (−74,919.75) | 0.93 (−174,490.45) | 0.22 (2,792,144.28) |
| Elapsed days | 0.35 (94,737.29) | 0.50 (81,313.49) | 0.15 (221,878.45) |
| Control | |||
| Age | 0.71 (−10,035.71) | 0.82 (−6904.97) | 0.31 (−32,792.94) |
| Sex | 0.95 (58,911.68) | 0.73 (312,244.64) | 0.71 (535,284.47) |
| Storage time | 0.003 # (−827,786.97) | 1.5 × 10−9 # (−1,274,748.90) | 5.6 × 10−10 # (−1,283,080.89) |
| cfDNA Yield | 0.004 # (20,045.15) | 0.03 # (15,720.95) | 0.43 (9131.48) |
| gDNA contamination | 0.18 (−1,436,569.53) | 0.19 (−1,320,681.69) | 0.04 # (−3,128,848.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Nguyen, T.; Li, Z. Impact of Long-Term Plasma Storage on Cell-Free DNA Epigenetic Biomarker Studies. Biomolecules 2025, 15, 927. https://doi.org/10.3390/biom15070927
Shao J, Nguyen T, Li Z. Impact of Long-Term Plasma Storage on Cell-Free DNA Epigenetic Biomarker Studies. Biomolecules. 2025; 15(7):927. https://doi.org/10.3390/biom15070927
Chicago/Turabian StyleShao, Jianming, Thao Nguyen, and Zejuan Li. 2025. "Impact of Long-Term Plasma Storage on Cell-Free DNA Epigenetic Biomarker Studies" Biomolecules 15, no. 7: 927. https://doi.org/10.3390/biom15070927
APA StyleShao, J., Nguyen, T., & Li, Z. (2025). Impact of Long-Term Plasma Storage on Cell-Free DNA Epigenetic Biomarker Studies. Biomolecules, 15(7), 927. https://doi.org/10.3390/biom15070927






