Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Stool, Blood Plasma Sample Collection and Storage
2.1.1. Gut Microbiome Profiling
2.1.2. Bioinformatics and Statistical Analysis
2.1.3. Metadata and Sample Collection
2.1.4. Human Cytokine and Chemokine Analysis
2.1.5. Permissions and Approvals
2.1.6. Statistical Analysis
3. Results of PASC Patients
3.1. Gut Microbiota Alpha- and Beta-Diversity
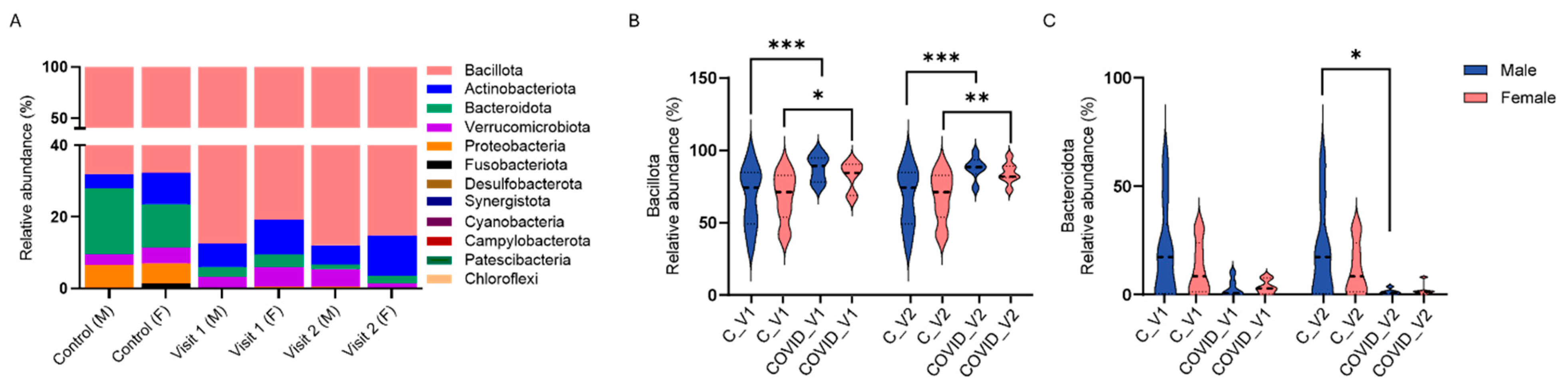
3.1.1. Gut Microbiome Alterations at the Phylum Level
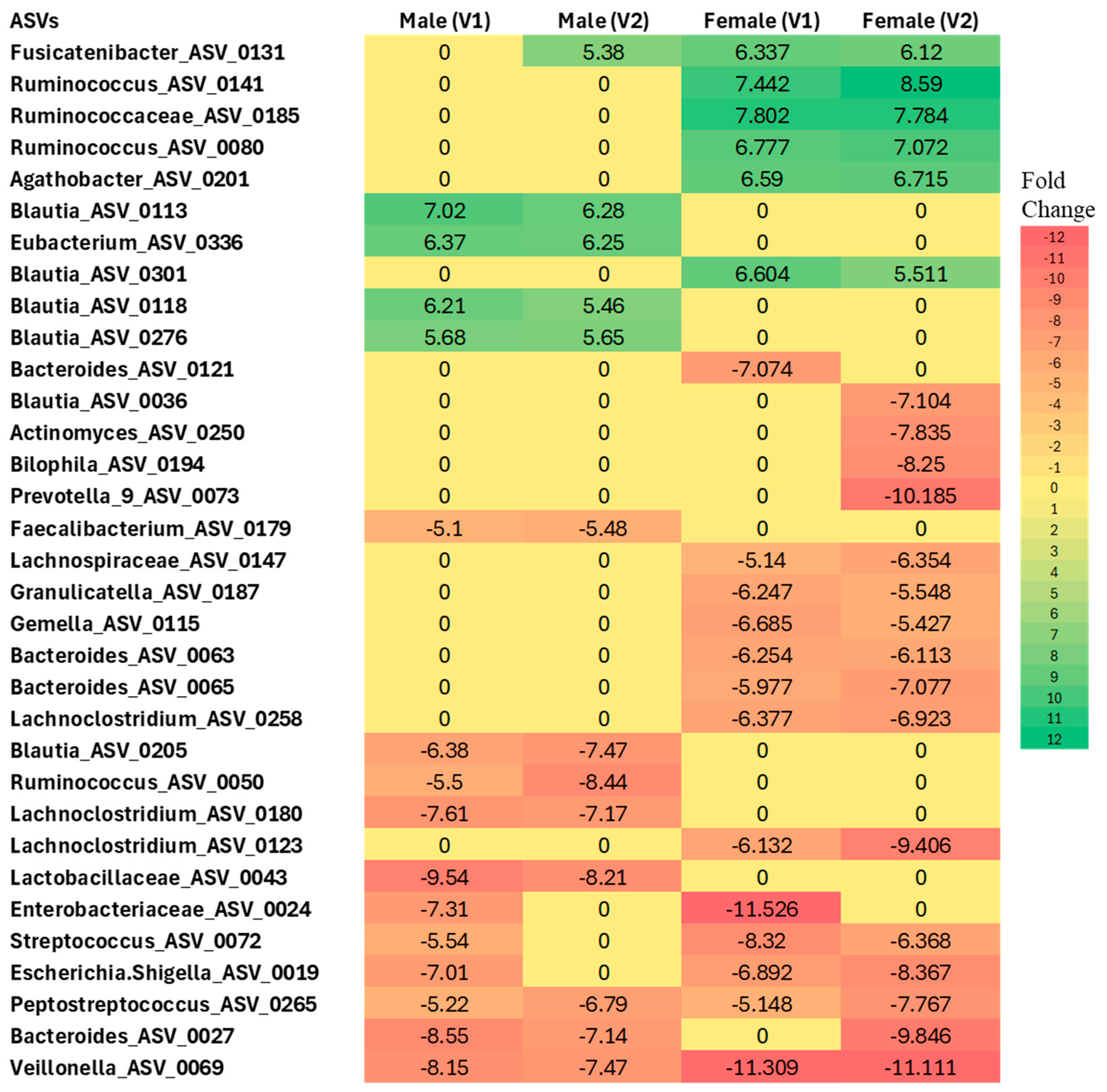
3.1.2. Differential Abundance Analysis at the ASV Level
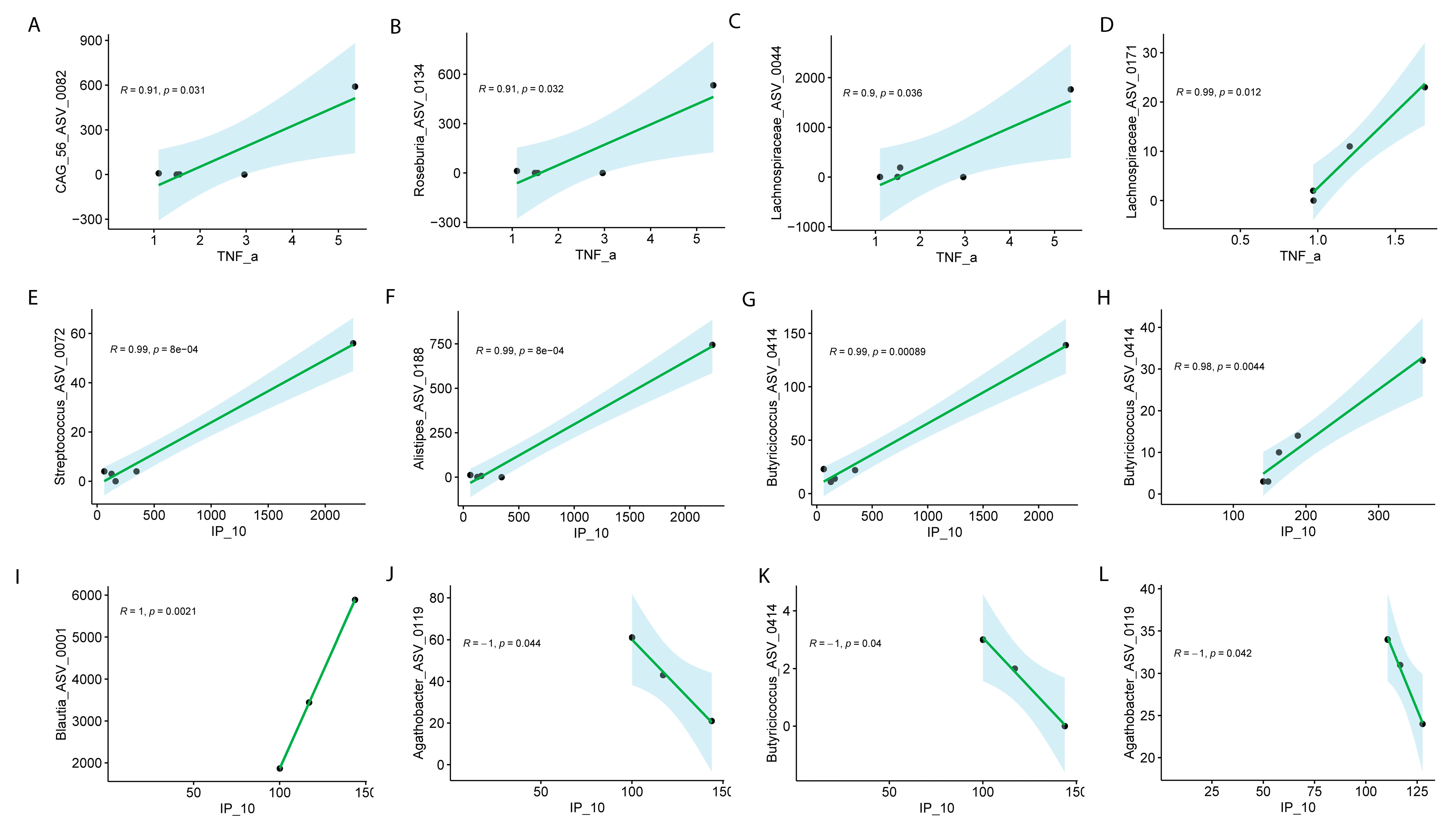
Sex-Specific Microbiome Associations with Inflammatory Markers in Long COVID
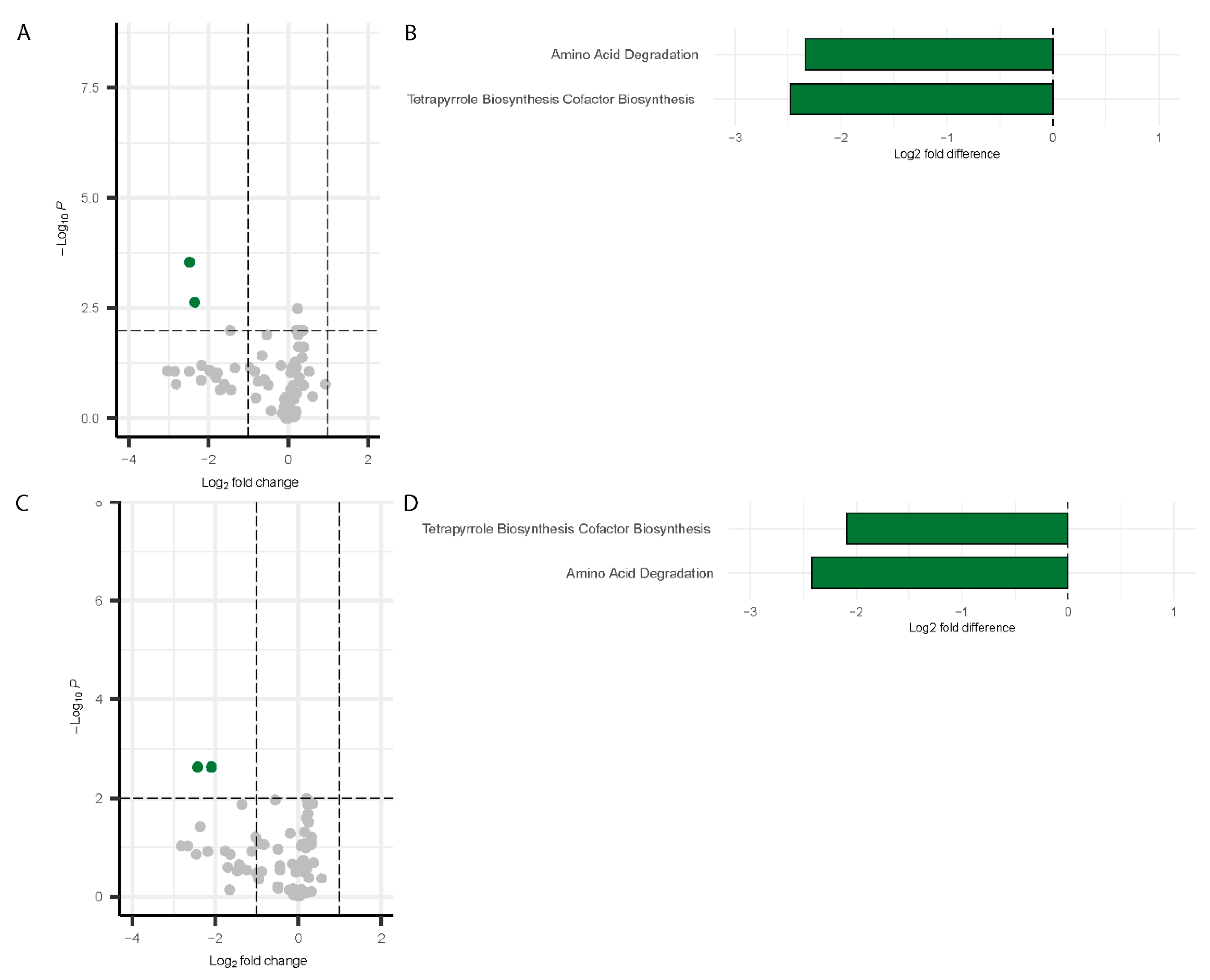
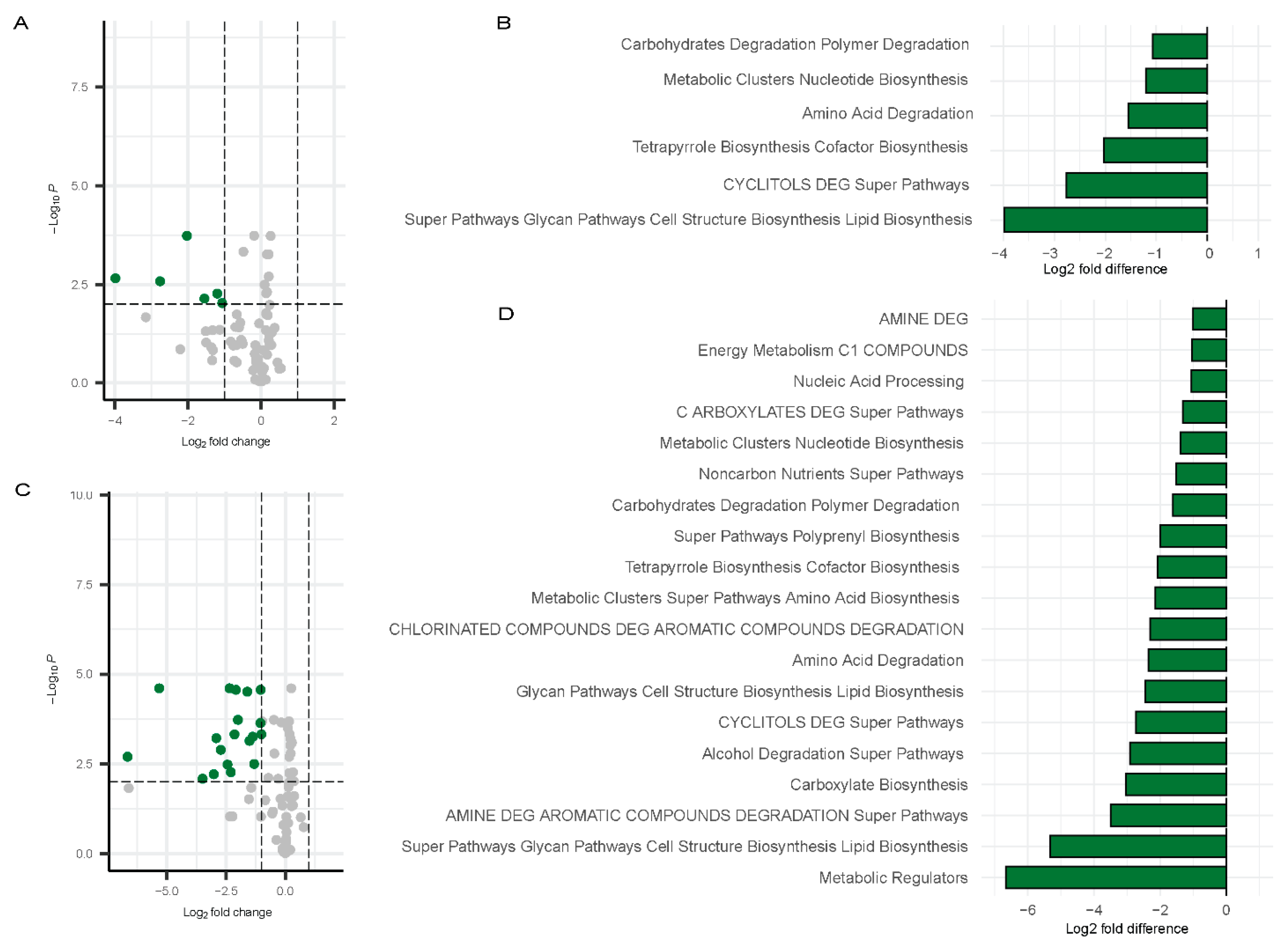
Inference of Functional Pathways Associated with COVID-19
Correlation Between Gut Microbiome and Inflammatory Markers
Inference of Functional Genes and Pathways
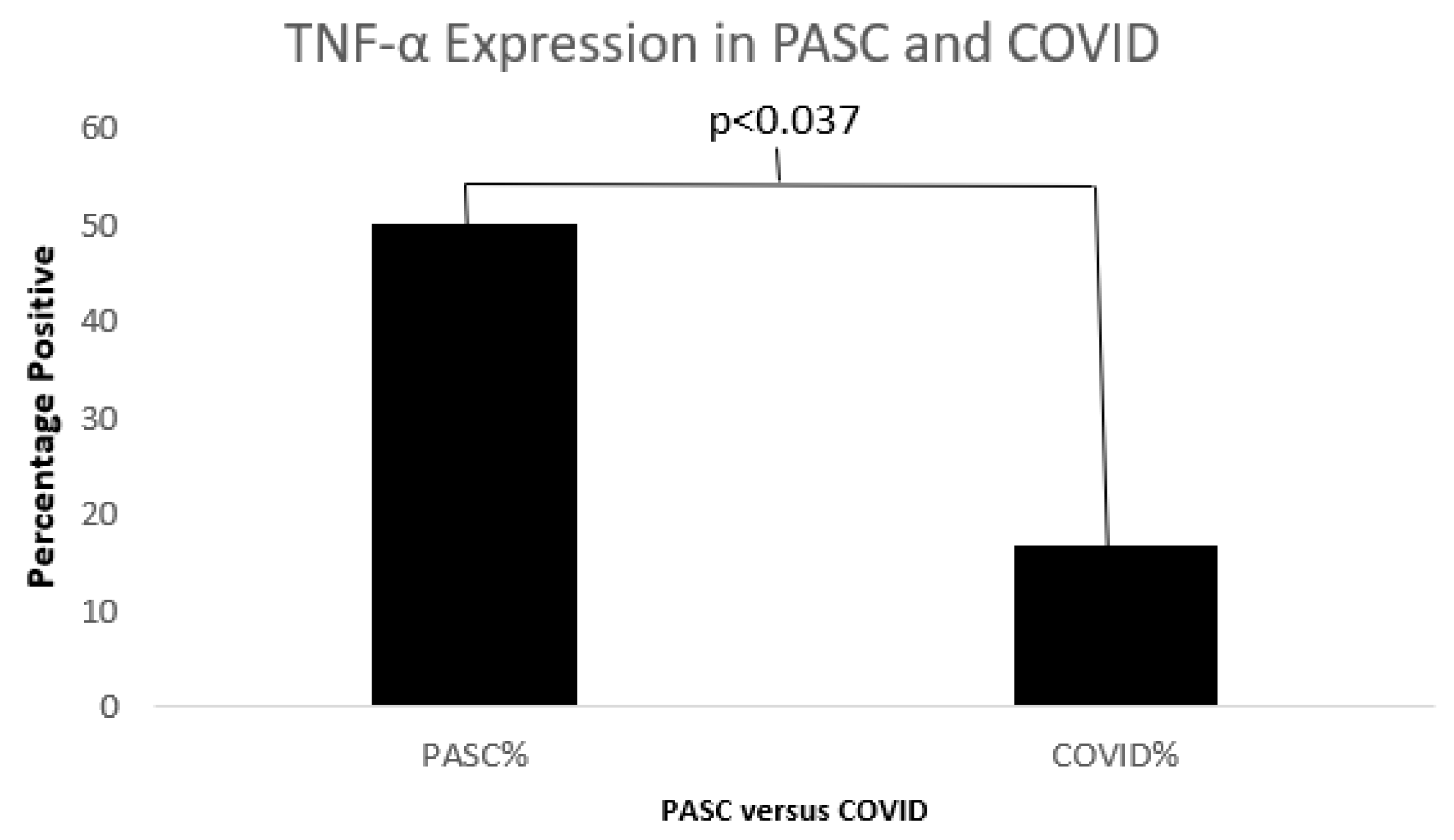
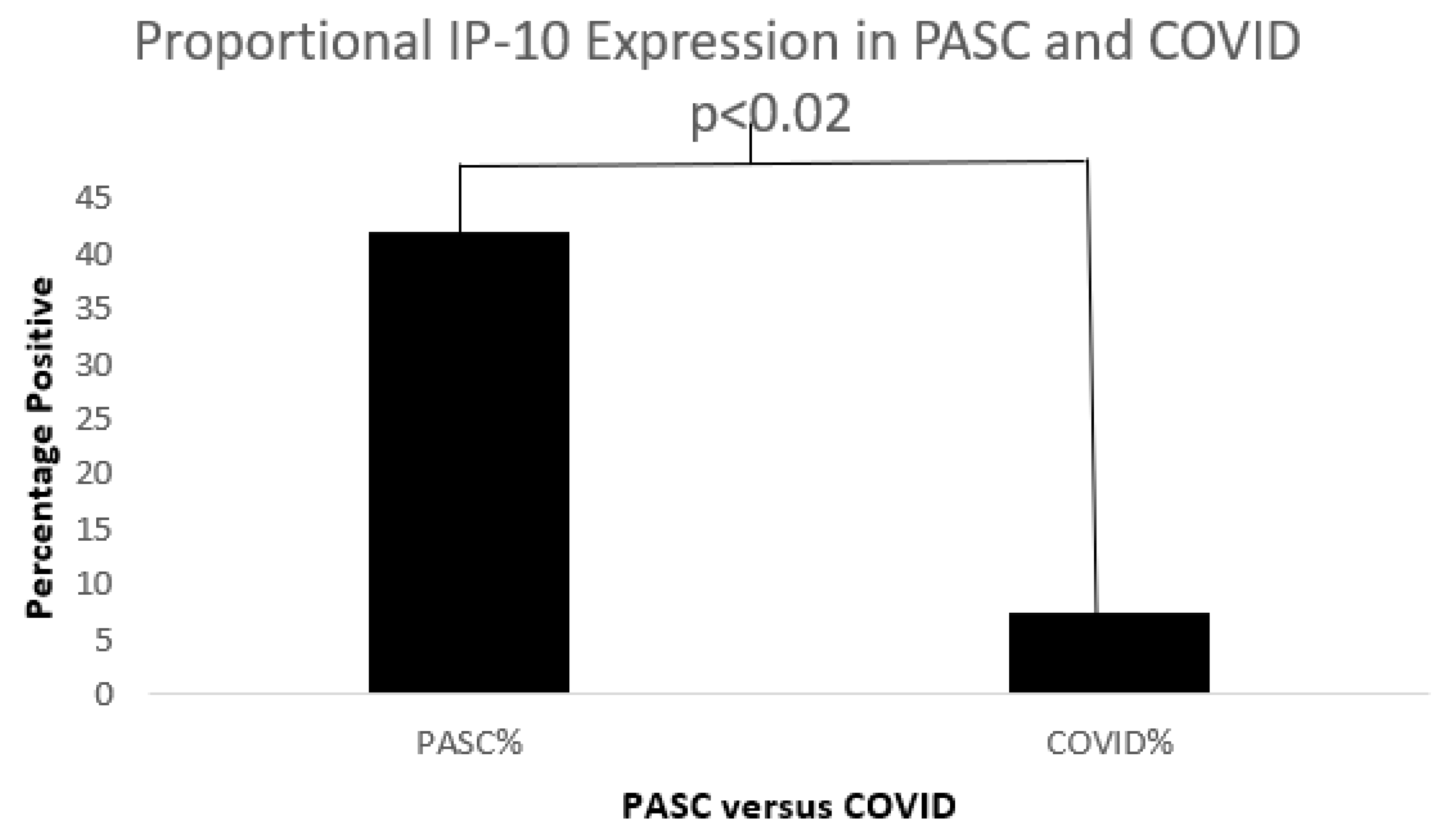
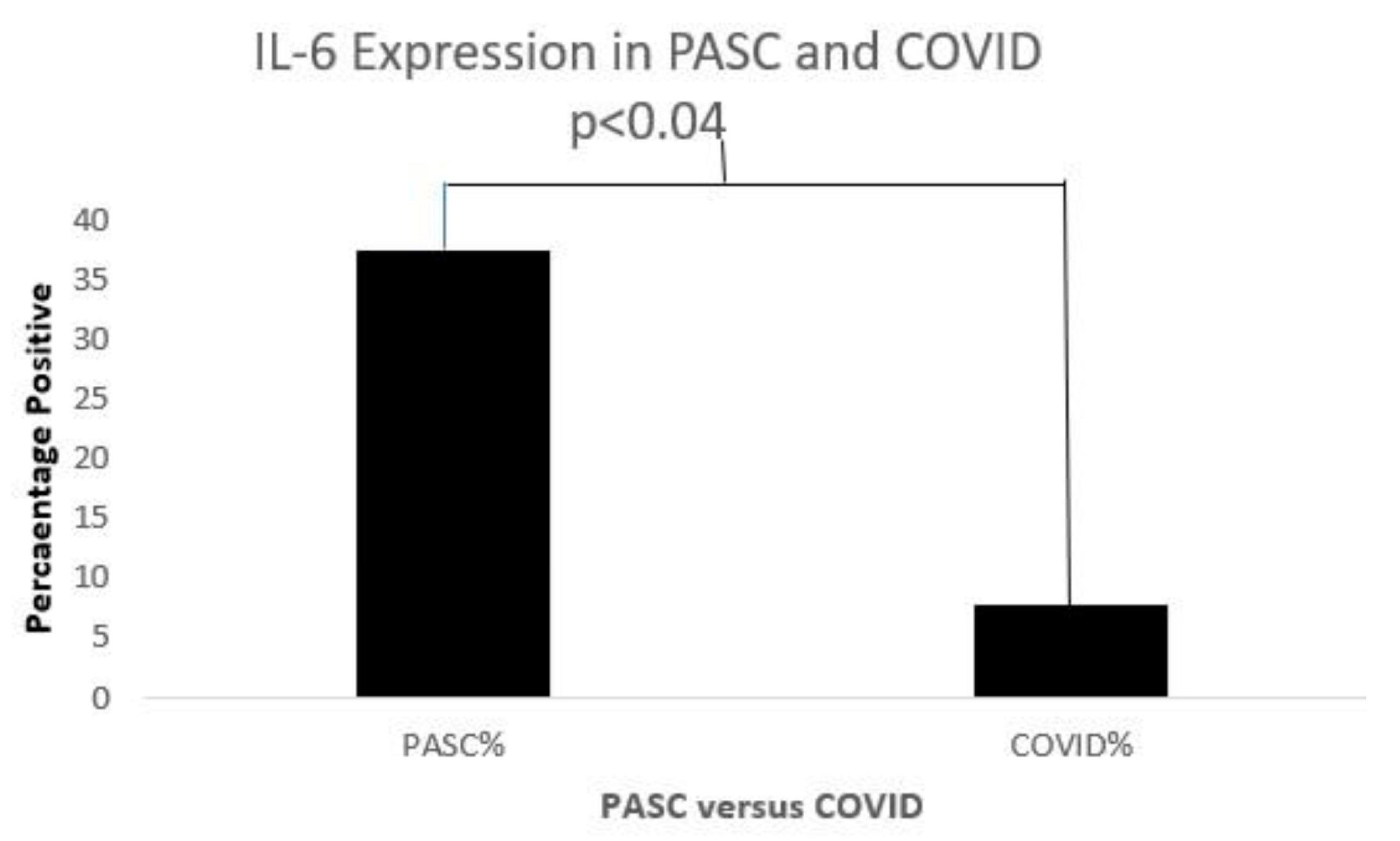
Serum Cytokines
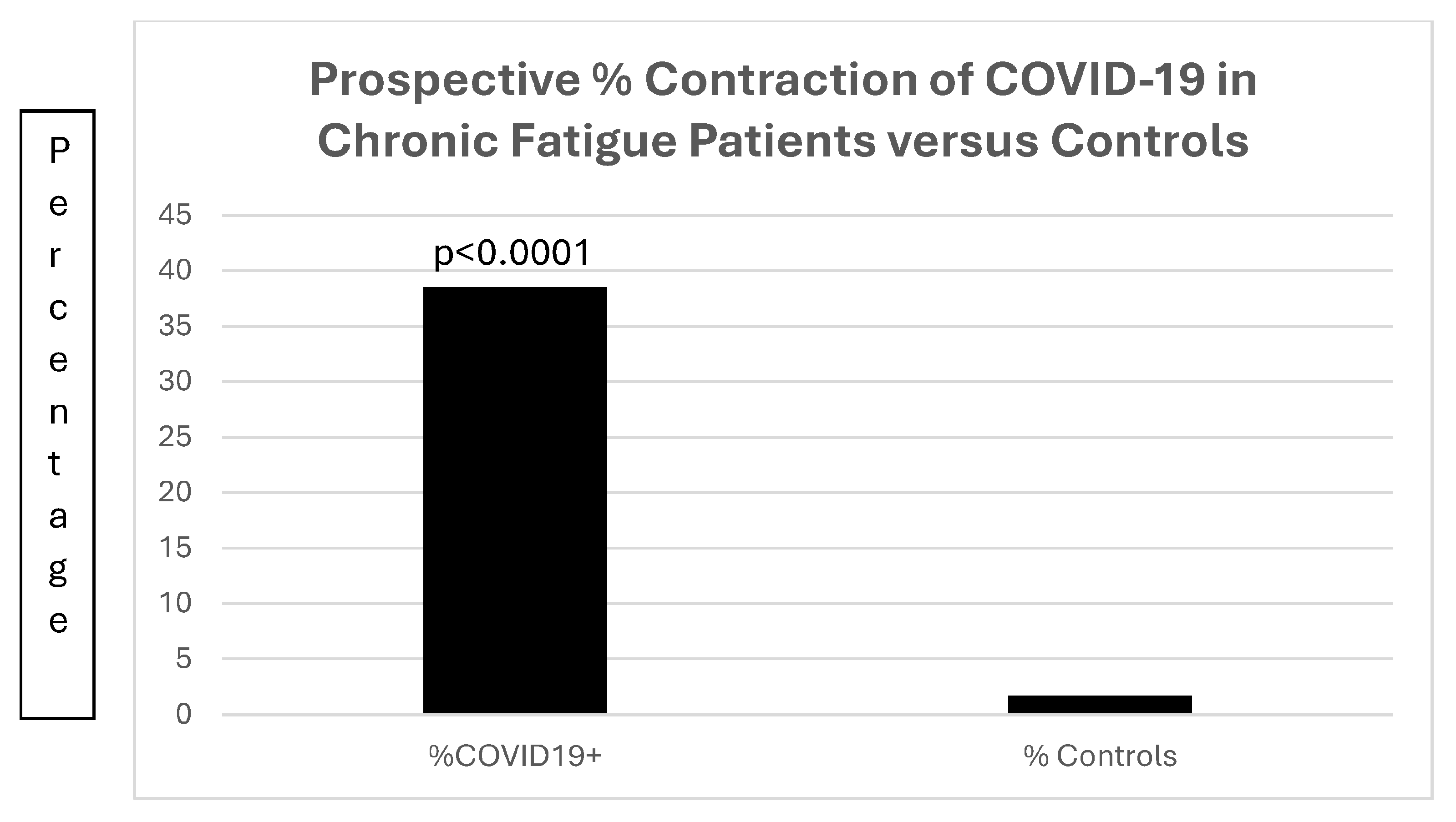
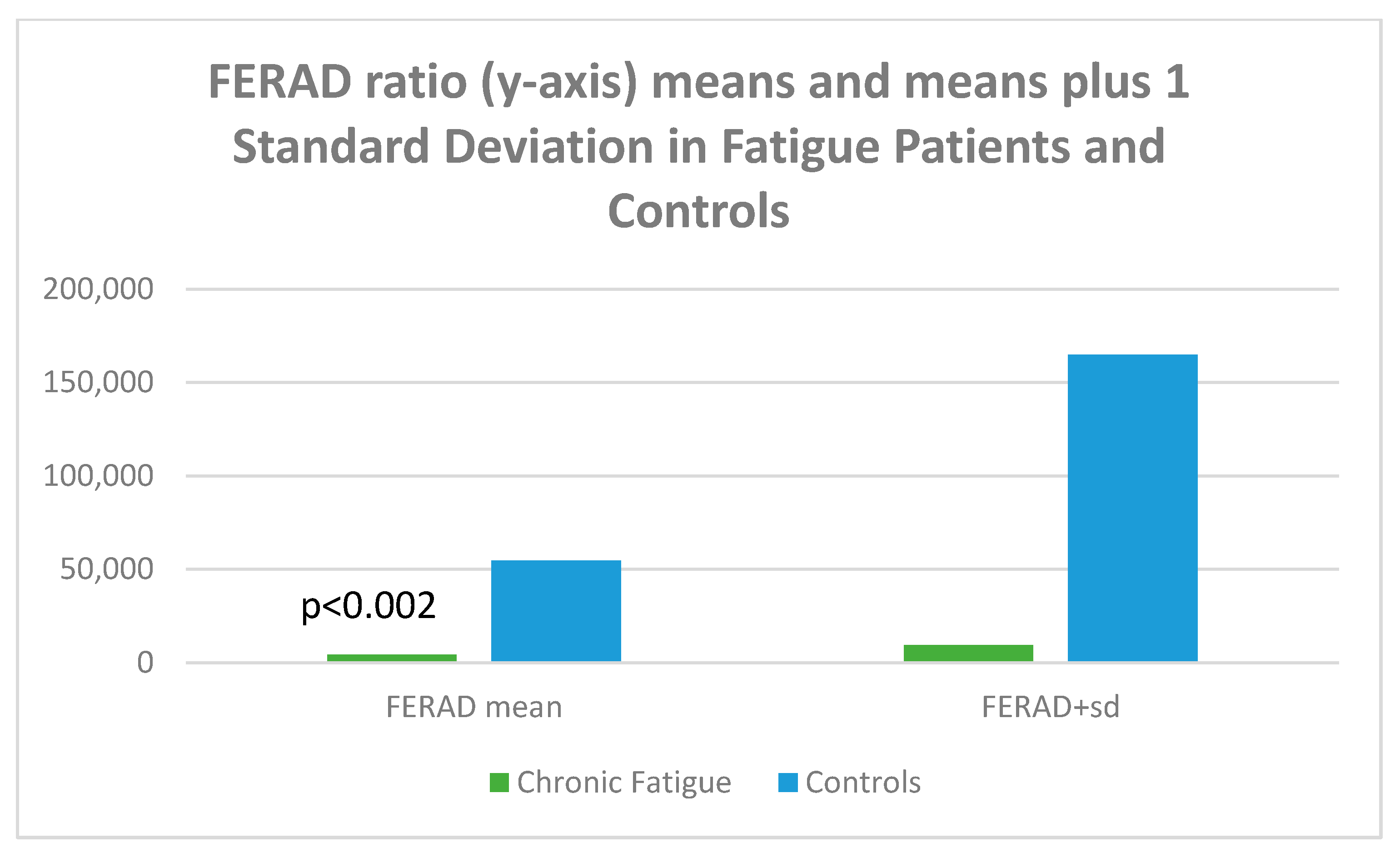
Database Correlates with Chronic Fatigue
Cognitive Testing in Long COVID
4. Discussion
- Epilogue:
- And out came the grief and woe
- We won‘t ever be rid of,
- For heaven had hidden
- That in the jar.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LaVergne, S.M.; Stromberg, S.; Baxter, B.A.; Webb, T.L.; Dutt, T.S.; Berry, K.; Tipton, M.; Haberman, J.; Massey, B.R.; McFann, K.; et al. A longitudinal SARS-CoV-2 biorepository for COVID-19 survivors with and without post-acute sequelae. BMC Infect. Dis. 2021, 21, 677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Morag, A.; Ravid, Z.; Chowers, I.; Feldman-Weiss, V.; Michaeli, Y.; Ben-Chetrit, E.; Shalit, M.; Knobler, H. Prolonged atypical illness associated with serological evidence of persistent Epstein-Barr virus infection. Lancet 1982, 1, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Morag, A.; Tobi, M.; Ravid, Z.; Revel, M.; Schattner, A. Increased (2′-5′)-oligo-A synthetase activity in patients with prolonged illness associated with serological evidence of persistent Epstein-Barr virus infection. Lancet 1982, 319, 744. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.E.; Tosato, G.; Armstrong, G.; Lawley, T.; Preble, O.T.; Henle, W.; Davey, R.; Pearson, G.; Epstein, J.; Brus, I. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann. Intern. Med. 1985, 102, 7–16. [Google Scholar] [CrossRef]
- Tobi, M.; Straus, S.E. Chronic Epstein-Barr virus disease: A workshop held by the National Institute of Allergy and Infectious Diseases. Ann. Intern. Med. 1985, 103, 951–953. [Google Scholar] [CrossRef]
- Straus, S.E.; Dale, J.K.; Tobi, M.; Lawley, T.; Preble, O.; Blaese, R.M.; Hallahan, C.; Henle, W. Acyclovir treatment of the chronic fatigue syndrome. N. Engl. J. Med. 1988, 319, 1692–1698. [Google Scholar] [CrossRef]
- Zhou, S.; Butler-Laporte, G.; Nakanishi, T.; Morrison, D.R.; Afilalo, J.; Afilalo, M.; Laurent, L.; Pietzner, M.; Kerrison, N.; Zhao, K. A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity. Nat. Med. 2021, 27, 659–667. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef]
- Buchwald, D.; Sullivan, J.L.; Komaroff, A.L. Frequency of ‘chronic active Epstein-Barr virus infection’ in a general medical practice. JAMA 1987, 257, 2303–2307. [Google Scholar] [CrossRef]
- Straus, S.E.; Dale, J.K.; Wright, R.; Metcalfe, D.D. Allergy and the chronic fatigue syndrome. J. Allergy Clin. Immunol. 1988, 81, 791–795. [Google Scholar] [CrossRef]
- Englebienne, P.; De Meirleir, K. (Eds.) Chronic Fatigue Syndrome: A Biological Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Ikuta, K.; Yamada, T.; Shimomura, T.; Kuratsune, H.; Kawahara, R.; Ikawa, S.; Ohnishi, E.; Sokawa, Y.; Fukushi, H.; Hirai, K.; et al. Diagnostic evaluation of 2′,5′-oligoadenylate synthetase activities and antibodies against Epstein-Barr virus and Coxiella burnetii in patients with chronic fatigue syndrome in Japan. Microbes Infect. 2003, 5, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Light, A.R.; Vierck, C.J.; Light, K.C. Myalgia and fatigue: Translation from mouse sensory neurons to fibromyalgia and chronic fatigue syndromes. In Translational Pain Research: From Mouse to Man; Kruger, L., Ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ruiz-Nunez, B.; Tarasse, R.; Vogelaar, E.F.; Janneke Dijck-Brouwer, D.A.; Muskiet, F.A.J. Higher Prevalence of “Low T3 Syndrome” in Patients With Chronic Fatigue Syndrome: A Case-Control Study. Front. Endocrinol. 2018, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, G.; Manning, P.; Newton, J.L. Understanding Muscle Dysfunction in Chronic Fatigue Syndrome. J. Aging Res. 2016, 2016, 2497348. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Smith, A. An investigation into the cognitive deficits associated with chronic fatigue syndrome. Open Neurol. J. 2009, 3, 13–23. [Google Scholar] [CrossRef]
- Wallis, A.; Ball, M.; McKechnie, S.; Butt, H.; Lewis, D.P.; Bruck, D. Examining clinical similarities between myalgic encephalomyelitis/chronic fatigue syndrome and D-lactic acidosis: A systematic review. J. Transl. Med. 2017, 15, 129. [Google Scholar] [CrossRef]
- Watt, T.; Oberfoell, S.; Balise, R.; Lunn, M.R.; Kar, A.K.; Merrihew, L.; Bhangoo, M.S.; Montoya, J.G. Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein-Barr virus IgG antibody titers. J. Med. Virol. 2012, 84, 1967–1974. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2021, 59, 2101341. [Google Scholar] [CrossRef]
- Walsh-Messinger, J.; Manis, H.; Vrabec, A.; Sizemore, B.; Jenna Bishof, K.; Debidda, M.; Malaspina, D.; Greenspan, N. The kids are not alright: A preliminary report of post-COVID syndrome in university students. J. Am. Coll. Health 2021, 71, 1367–1373. [Google Scholar] [CrossRef]
- Omer, H.; Foldes, J.; Toby, M.; Menczel, J. Screening for cognitive deficits in a sample of hospitalized geriatric patients: A re-evaluation of a brief mental status questionnaire. J. Am. Geriatr. Soc. 1983, 31, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Jason, L.A.; Fennell, P.A.; Taylor, R.R. Cardiac and virologic issues. In Handbook of Chronic Fatigue Syndrome; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 304–330. [Google Scholar]
- Eisen, S.A.; Kang, H.K.; Murphy, F.M.; Blanchard, M.S.; Reda, D.J.; Henderson, W.G.; Toomey, R.; Jackson, L.W.; Alpern, R.; Parks, B.J. Gulf War veterans’ health: Medical evaluation of a US cohort. Ann. Intern. Med. 2005, 142, 881–890. [Google Scholar] [CrossRef]
- Anaya, J.-M.; Rojas, M.; Salinas, M.L.; Rodríguez, Y.; Roa, G.; Lozano, M.; Rodríguez-Jiménez, M.; Montoya, N.; Zapata, E.; Monsalve, D.M. Post-COVID syndrome. A case series and comprehensive review. Autoimmun. Rev. 2021, 20, 102947. [Google Scholar] [CrossRef]
- Becker, C.; Beck, K.; Zumbrunn, S.; Memma, V.; Herzog, N.; Bissmann, B.; Gross, S.; Loretz, N.; Mueller, J.; Amacher, S.A.; et al. Long COVID 1 year after hospitalisation for COVID-19: A prospective bicentric cohort study. Swiss. Med. Wkly. 2021, 151, w30091. [Google Scholar] [CrossRef]
- Haran, J.P.; Bradley, E.; Zeamer, A.L.; Cincotta, L.; Salive, M.C.; Dutta, P.; Mutaawe, S.; Anya, O.; Meza-Segura, M.; Moormann, A.M.; et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms long COVID. JCI Insight 2021, 6, e152346. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Garcia-Abellan, J.; Padilla, S.; Fernandez-Gonzalez, M.; Garcia, J.A.; Agullo, V.; Andreo, M.; Ruiz, S.; Galiana, A.; Gutierrez, F.; Masia, M. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. J. Clin. Immunol. 2021, 41, 1490–1501. [Google Scholar] [CrossRef]
- Vanichkachorn, G.; Newcomb, R.; Cowl, C.T.; Murad, M.H.; Breeher, L.; Miller, S.; Trenary, M.; Neveau, D.; Higgins, S. Post-COVID-19 Syndrome (Long Haul Syndrome): Description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin. Proc. 2021, 96, 1782–1791. [Google Scholar] [CrossRef]
- Moreno-Perez, O.; Merino, E.; Leon-Ramirez, J.M.; Andres, M.; Ramos, J.M.; Arenas-Jimenez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021, 110, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Brian, D.A.; Shockley, L.J. Coronaviruses in tamarin and marmoset colitis. In A Primate Model for the Study of Colitis and Colonic Carcinoma the Cotton-Top Tamarin (Saguinus oedipus); CRC Press: Boca Raton, FL, USA, 2018; pp. 145–159. [Google Scholar]
- Russell, R.G.; Brian, D.A.; Lenhard, A.; Potgieter, L.N.; Gillespie, D.; Clapp, N.K. Coronavirus-like particles and Campylobacter in marmosets with diarrhea and colitis. Dig. Dis. Sci. 1985, 30, 72S–77S. [Google Scholar] [CrossRef] [PubMed]
- Talwar, H.; McVicker, B.; Tobi, M. p38gamma Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer-A Comparative Study with Humans. Vaccines 2020, 8, 720. [Google Scholar] [CrossRef]
- Munoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef]
- Chaccour, C.; Abizanda, G.; Irigoyen-Barrio, A.; Casellas, A.; Aldaz, A.; Martinez-Galan, F.; Hammann, F.; Gil, A.G. Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats. Sci. Rep. 2020, 10, 17073. [Google Scholar] [CrossRef]
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J. Ther. 2021, 28, e434–e460. [Google Scholar] [CrossRef]
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angoa-Pérez, M.; Zagorac, B.; Francescutti, D.M.; Shaffer, Z.D.; Theis, K.R.; Kuhn, D.M. Cocaine hydrochloride, cocaine methiodide and methylenedioxypyrovalerone (MDPV) cause distinct alterations in the structure and composition of the gut microbiota. Sci. Rep. 2023, 13, 13754. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Zagorac, B.; Francescutti, D.M.; Winters, A.D.; Greenberg, J.M.; Ahmad, M.M.; Manning, S.D.; Gulbransen, B.D.; Theis, K.R.; Kuhn, D.M. Effects of a high fat diet on gut microbiome dysbiosis in a mouse model of Gulf War Illness. Sci. Rep. 2020, 10, 9529. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.M.; Winters, A.D.; Zagorac, B.; Kracht, D.J.; Francescutti, D.M.; Cannella, N.; Ciccocioppo, R.; Woods, L.C.S.; Mackle, J.M.; Hardiman, G.T.; et al. Long Access Heroin Self-Administration Significantly Alters Gut Microbiome Composition and Structure. Front. Psychiatry 2024, 15, 1369783. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2019, 48, D445–D453. [Google Scholar] [CrossRef]
- Conroy, D.M.; Williams, T.J. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir. Res. 2001, 2, 150–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Huang, Y.; Ji, X.; Wu, T.; Xiao, P. CCL11 (Eotaxin) Promotes the Advancement of Aging-Related Cardiovascular Diseases. Rev. Cardiovasc. Med. 2025, 26, 26020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitaura, M.; Suzuki, N.; Imai, T.; Takagi, S.; Suzuki, R.; Nakajima, T.; Hirai, K.; Nomiyama, H.; Yoshie, O. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J. Biol. Chem. 1999, 274, 27975–27980. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious disease pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhard, S.; Hug, S.; Stratmann, A.E.P.; Erber, M.; Vidoni, L.; Knapp, C.L.; Thomaß, B.D.; Fauler, M.; Nilsson, B.; Nilsson Ekdahl, K.; et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J. Innate Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamkhioued, B.; Garcia-Zepeda, E.A.; Abi-Younes, S.; Nakamura, H.; Jedrzkiewicz, S.; Wagner, L.; Renzi, P.M.; Allakhverdi, Z.; Lilly, C.; Hamid, Q.; et al. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. Induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am. J. Respir. Crit. Care Med. 2000, 162 2 Pt 1, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Gray, P.A.; Van Damme, J.; Sozzani, S. Macrophage-derived chemokine (MDC). J. Leukoc. Biol. 2000, 68, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Tekamp-Olson, P.; Gallegos, C.; Bauer, D.; Davatelis, G.; Wolpe, S.D.; Masiarz, F.; Coit, D.; Cerami, A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J. Exp. Med. 1988, 168, 2251–2259. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Lacy, P. Eosinophil Cytokines in Allergy. In Cytokine Effector Functions in Tissues; Academic Press: Cambridge, MA, USA, 2017; pp. 173–218. ISBN 978-0-12-804214-4. [Google Scholar] [CrossRef]
- Gray, P.W.; Goeddel, D.V. Structure of the human immune interferon gene. Nature 1982, 298, 859–863. [Google Scholar] [CrossRef]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H. The role of IL-1β during human immunodeficiency virus type 1 infection. Rev. Med. Virol. 2023, 33, e2400. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010, 21, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Schwartz, D. The Expanding Role of Anti–IL-12 and/or Anti–IL-23 Antibodies in the Treatment of Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2019, 15, 255–265. [Google Scholar]
- Arenas-Ramirez, N.; Woytschak, J.; Boyman, O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015, 36, 763–777. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the brain: A cytokine to remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef]
- Raised troponin and interleukin-6 levels are associated with a poor prognosis in COVID-19. Cardiac Rhythm News, 2 April 2020.
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Horvath, A.; Habisch, H.; Prietl, B.; Pfeifer, V.; Balazs, I.; Kovacs, G.; Foris, V.; John, N.; Kleinschek, D.; Feldbacher, N.; et al. Alteration of the Gut-Lung Axis After Severe COVID-19 Infection and Modulation Through Probiotics: A Randomized, Controlled Pilot Study. Nutrients 2024, 16, 3840. [Google Scholar] [CrossRef]
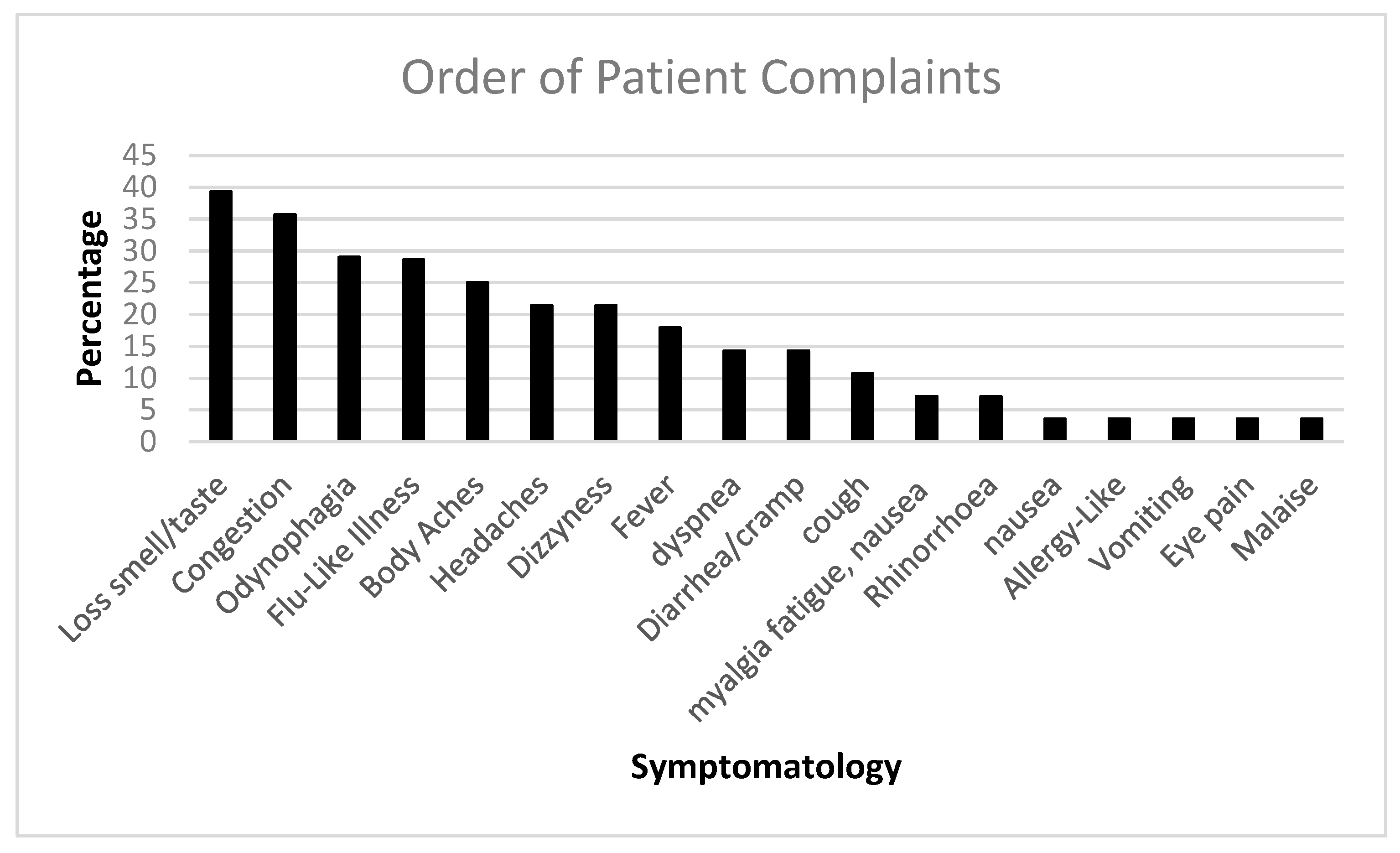
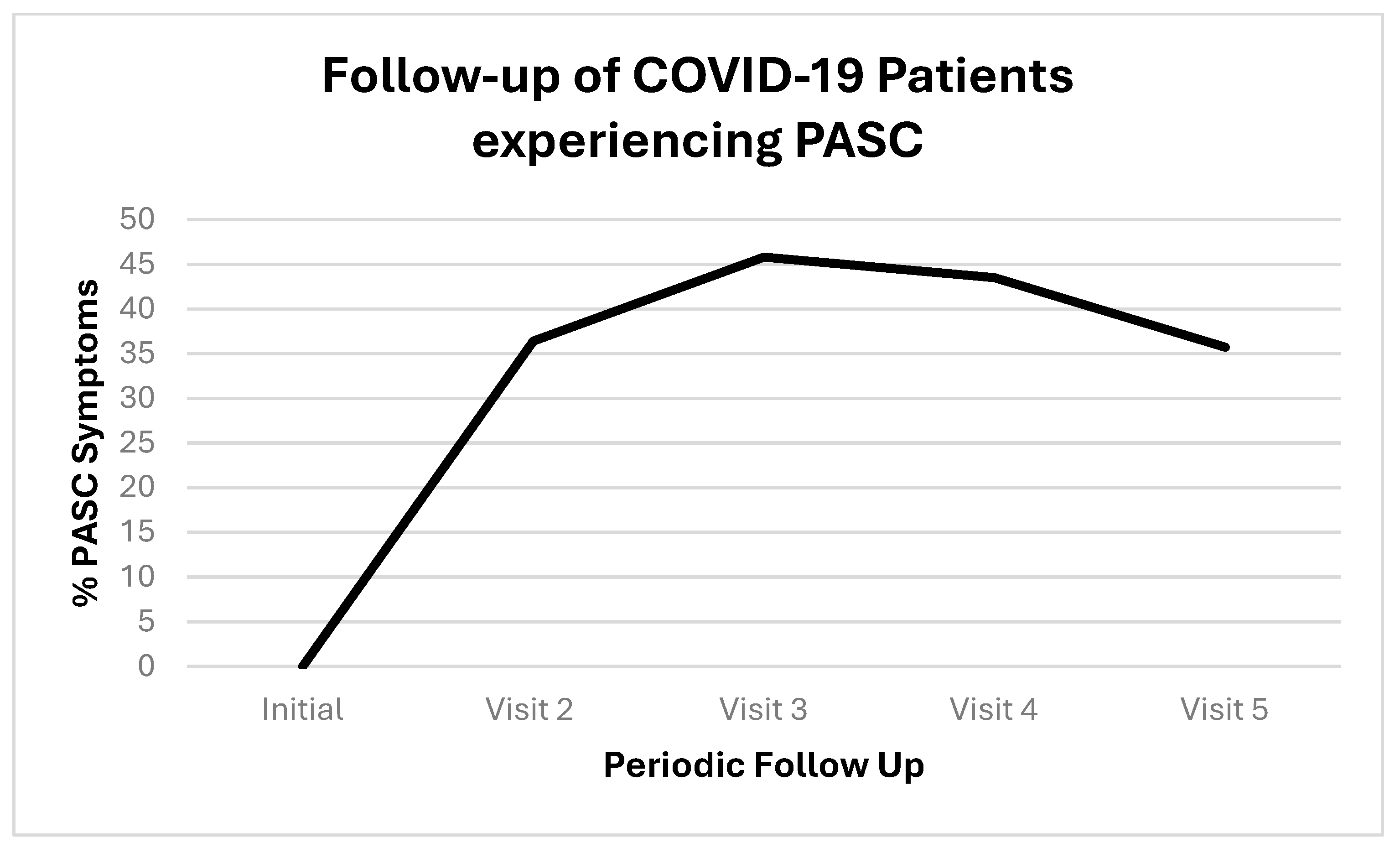
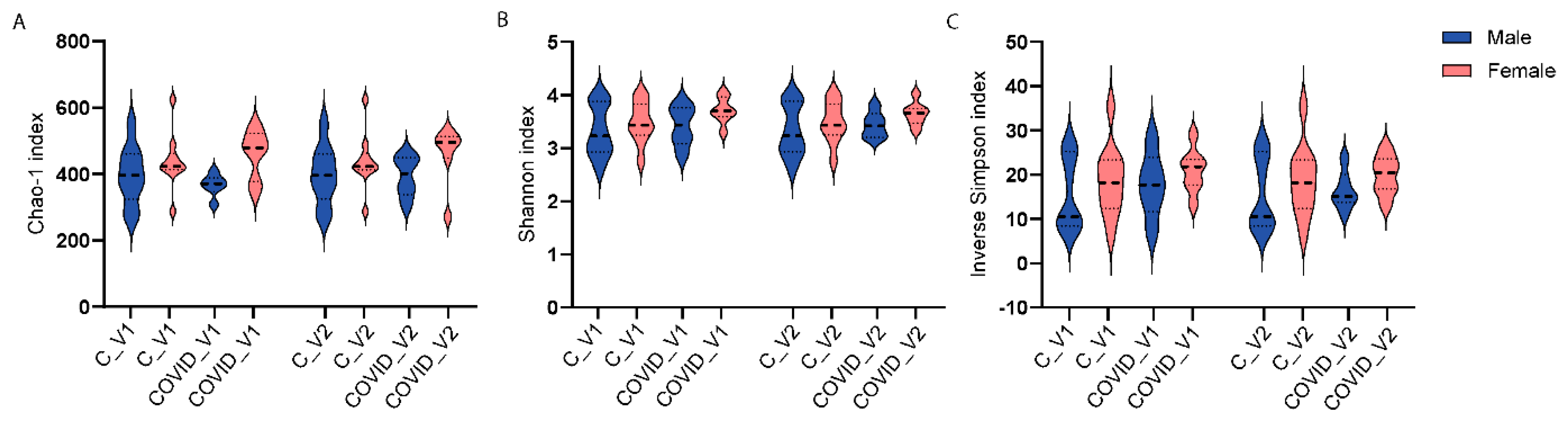
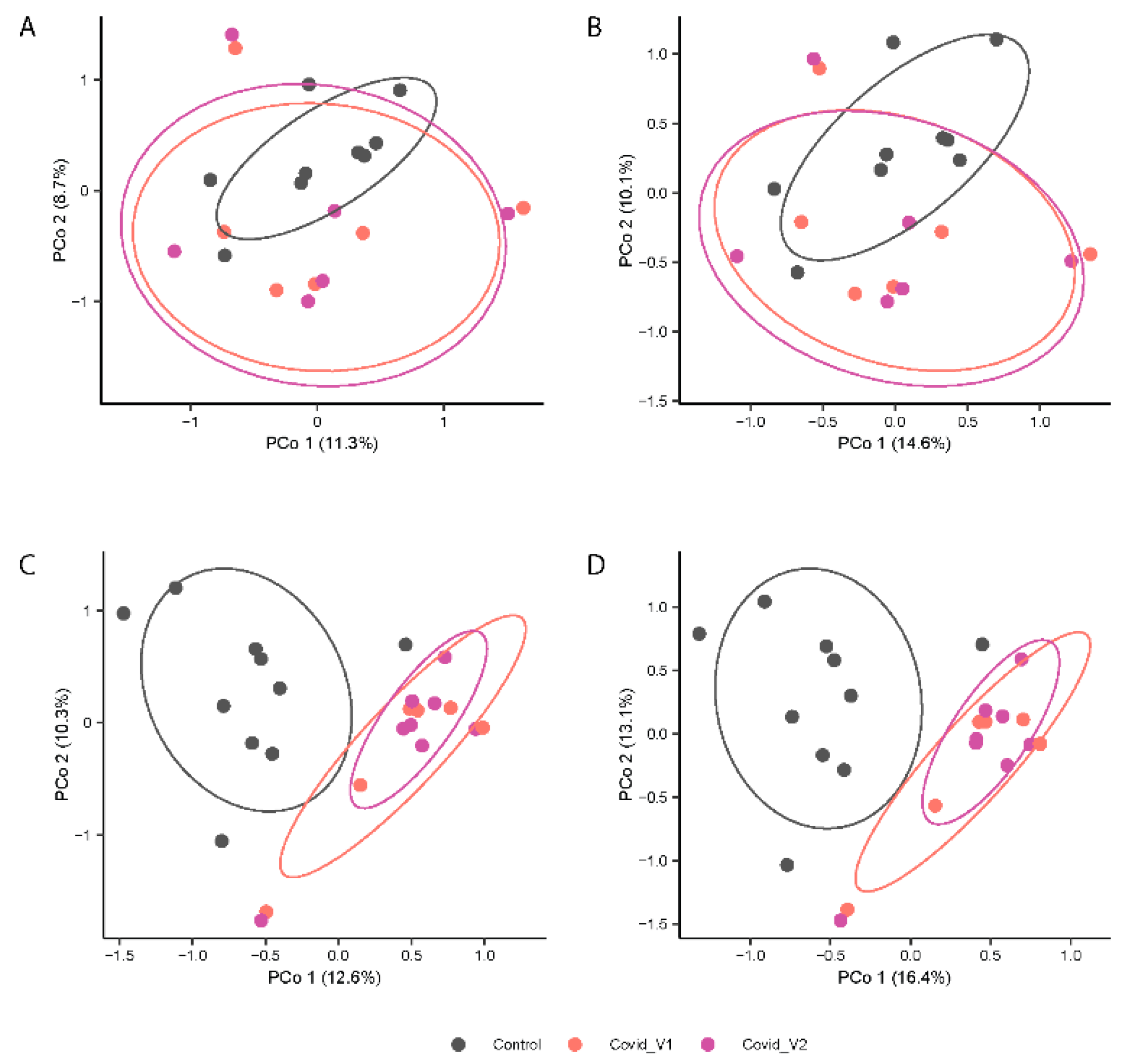

| Patient Number | Age | Sex | Ethnicity | Employed:Unemployed/R* |
|---|---|---|---|---|
| n = 28^ | 47.1 ± 15.7 yrs | M:F 17:11 | Cau:24; His:3; As:1 | 17:1/5 |
| Smoker Status | Other Household | Hospitalized | Intubated | Received Oxygen |
|---|---|---|---|---|
| 1 smoker; 27 none | Occupants 2.4 ± 1.4 | 10+14−42% | 2+22−12.5% | 9+15−37.5%; (average saturation: 9.7 ± 26.5%) |
| Other Measures/Conditions | 3+21−12.5% received convalescent serum | 1 pt expired 2 received vaccination | 5:19 diabetes 21% | 1 Expired 0.04% |
| Designation | Function | Disease Role | Conjunction | DX | SubFam | Ref. |
|---|---|---|---|---|---|---|
| Eotaxin | Eosinophil rec. | Pancreatic | HGF; MCP-1; CXCL10 | s.86 sp92 | MCP-1 | [54,55] |
| Eotaxin-3 | Eo.Chemotactic | Unknown | MIF-4α; TSC-1; IL-4 | Unknown | CCL26 | [56] |
| IP-10 | Growth, Apop | Infections | IFN γ-induced pr- | Severity Pre. | CXCL10 | [57] |
| IL-8 | Gprotein-couple | Neutro Attract | Macrophages | Inflammation | CXCL8 | [58] |
| MCP-1 | Monocyte Attrac | IFNγ induct. | CXCR3; predict: COVID | Inflammation | CCL2 | [59] |
| MCP-4 | Allergic Reaction | Asthma | TNFα&IL1β; IFN-γEo | Inflammation | CC chem | [60] |
| MDC | Amplication Type2 | Immunobiol | IL-4; IL13; IFN, CCR4 | Regulation | CCR4 chem | [61] |
| MIP-1α | Divergent signal | Inhibition | CCR5; M-tropic HIV | Inhibition HIV | CCL3 | [62] |
| MIP-1β | Related to above | Immunity | CCR5; M-tropic HIV | Immunity | CCL4 | [63] |
| TARC | Evade immunity | Melanoma | Dendritic cells | Improve Mel | CCL17 | [64] |
| IFN-γ | Viral Inhibition | Viruses | Lymphocytes | Type II IFN | Dimerized | [65] |
| IL-1β | COX2 induction | Inflammation | Dendritic/monocytes | Autoimmune | slanDC | [66] |
| IL-10 | Inhibit Inflammation | Jak; TYKinase | T-reg cells; IL-10R’s | Inhibitor DC | Class 2 | [67] |
| IL-12p70/p40 | T to Th1, NK cells | Stim IFN-γTNFα | Dendritic cells prod | Diverse functions | Het-dimeric | [68] |
| IL-2 | Infection reponse | wbc regulation | Leuco-& lymphocytes | Increase H&T cells | interleukin | [69] |
| IL-4 | IgE Class switching | Allergies | Differentiation Th cells | Mast cell stim | interleukin | [70] |
| IL-6 | Pro-inflammatory | COVID-19 prog | Activates IL-1ra&IL-10 | osteoblasts | interleukin | [71] |
| TNF-α | Pro-Inflammation | Immune-Stim | Active Tri-homotrimers | Macrophages | T-Membrane | [72] |
| Comparison | TTest/p 1-Tail t * | COVID n | PASC n | PASC R:p | COVID r:p |
|---|---|---|---|---|---|
| Eotaxin vs. Eo-3 | None; Eo 0.11*0.05 | 27 | 12 | −0.4; 0.16 | 0.006; 0.16 |
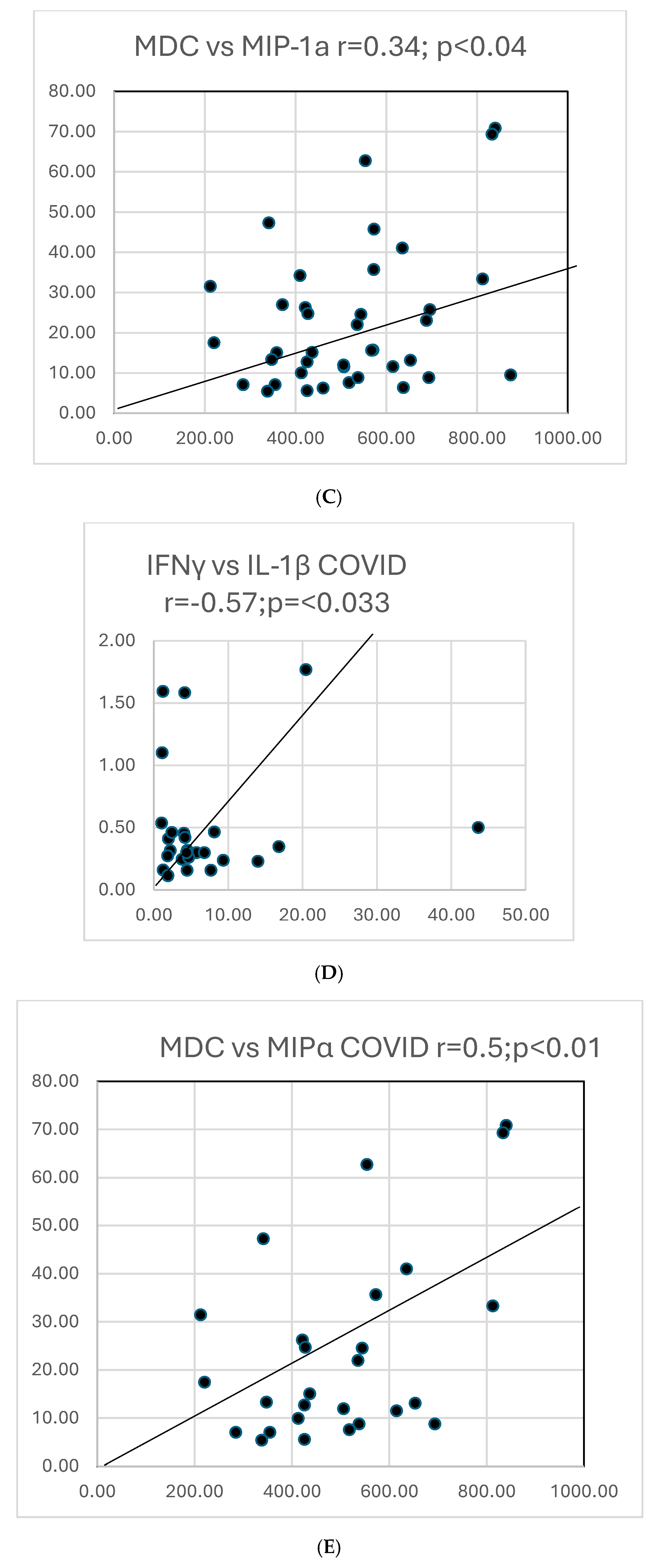
| IFN-γ vs. IL-1β | Tr0.07; r-0.1p0.6 | 25 | 16 | 0.36; 0.17 | 0.3:p0.122 |
| IL-8 vs. IP-10 | <0.02 IP10; IL8 p0.9 | 24 | 16 | 0.13:0.7 | 0.22; p0.26 |
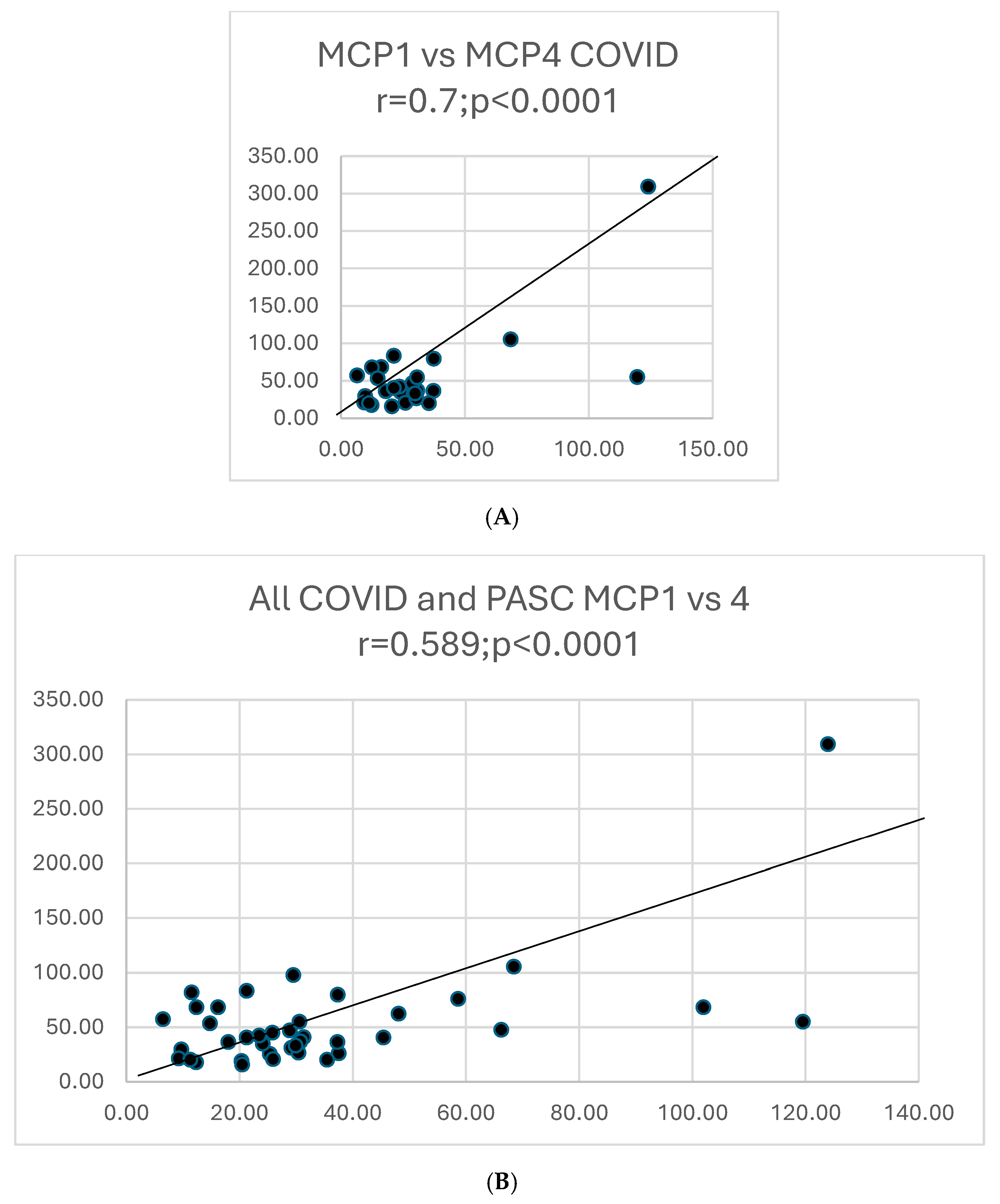
| MCP1vs. MCP4 | None; r = 0.6; p < 0.0001 | 25 | 16 | 0.2; p = 0.54 | 0.68; <0.0001 |
| IL-8 vs. TNFα | <0.02; | 25 | 16 | 0.2; p = 0.47 | −0.1; 0.62 |
| MDC vs. MIP-1α | 0.3 | 25 | 16 | −0.18; p = 0.6 | 0.5; <0.01 |
| MIP-1β vs. TARC | 0.7 | 25 | 16 | 0.6; p < 0.018 | 0.5; <0.015 |
| IL-1β vs. IL-10 | 0.07 IL-1b; | 11 il10 8 | 16/il7 | 0.13; p = 0.7 | −0.3; p = 0.3 |
| IL10 vs. IL12p70α | 0.3 | 8 | 7 | r = 0.76; p < 0.02 | r = 0.5; p = 0.9 |
| IL-2 vs. IL4 | 0.5 | 8 | 4 | 0.2; p = 0.8 | −0.1; p = 0.8 |
| Demo/Symptoms/Sign | Chronic Atypical Illness | Chronic Fatigue/ME | Long COVID |
|---|---|---|---|
| Age (years) | 24.6 ± 5.0 | Peak 20–25 | 45 ± 15 (France) 43.8 ± 13.4 (US) |
| Sex (% male) | 28.6 | 25 | 23 44 France vs. US |
| Illness Duration (months) | 19.14 ± 5.15 then ongoing | 36–108 | 1–3 subacute/ongoing >3 chronic |
| Prevalence % | Unknown | Estimated 0.1–0.76% | 32.6 persist19worse |
| Chronic Fatigue/malaise | 71.4 | 100 by definition | 39–63 |
| Mood/cognitive impaired | 28.6 | 46.89 (0.92) Illness severity scores | 23–40 |
| Myalgia/joint pain | 14.3 | 73 | 2–19.6/9–27.3 |
| Weakness | 43 | 95 | 63 |
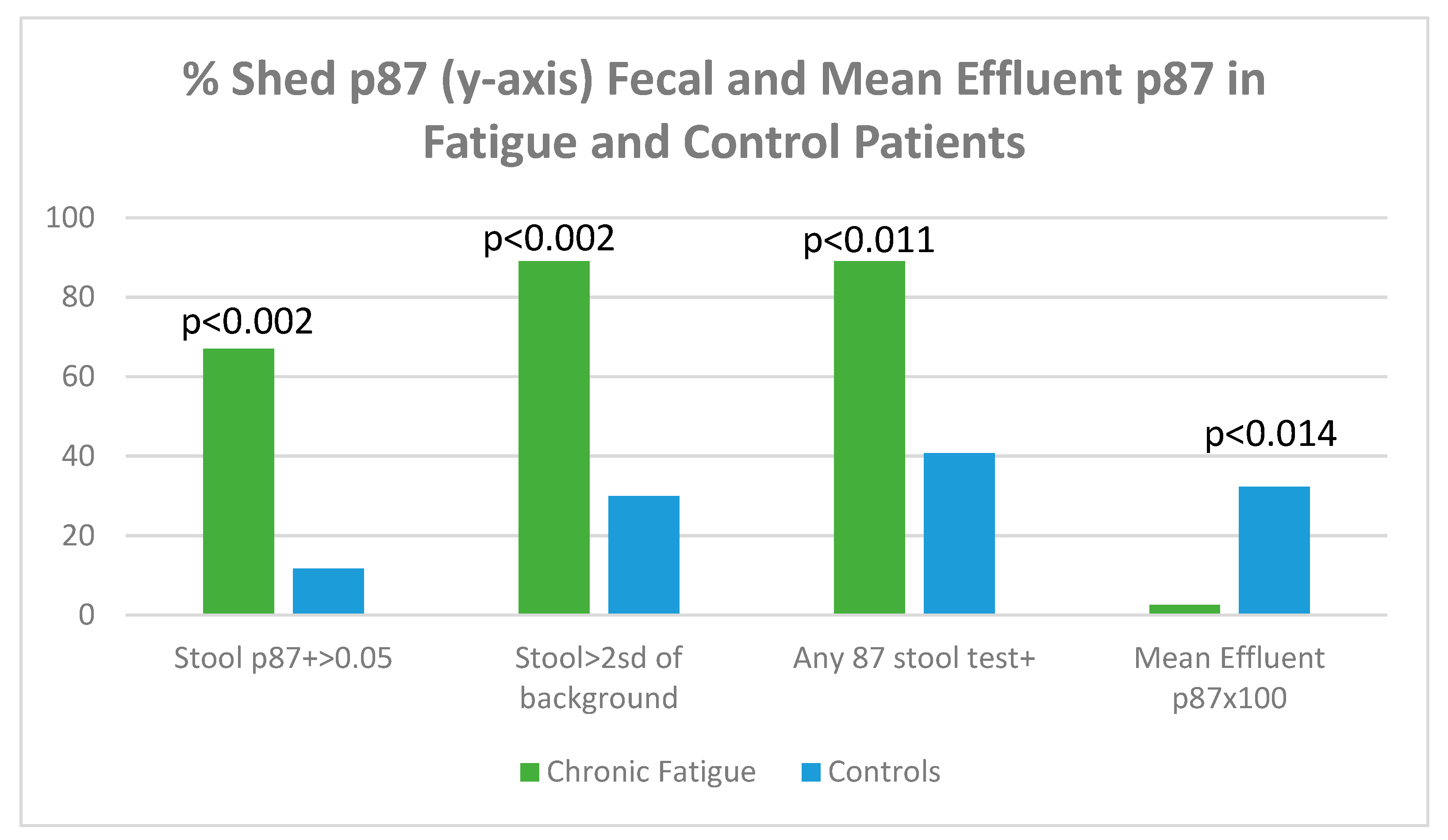
| GI symptoms % | 28.6 | 82–86 depending on criteria | 0.9–10.5 diarrhea fecal shedding/dysbiosis |
| Thyroidopathy | 28.6 | 16.3 | Worsening control |
| Allergy history | 43 | 50–75 | #OR 1.67(CI 1.04 to 2.67) |
| Lymphadenopathy % | 71.4 | 17 | Reported 13.8–19 |
| Fever-subjective | 71.4 | 46–57 low grade fever DOC | 0–0.9 |
| Hepatomegaly | 28.6 | 0 | NA |
| Splenomegaly | 71.4 | 0 | NA |
| Lymphocytosis | 71.4 | 20 | NA |
| 2–5oligoAsynthetase % | 100 | 59 | NA |
| IgM VCA EBV positive % | 100 | 57 in a special subset | 66.7 p < 0.001 |
| IgG VCA EBV positive % | 100 | 90 general population | Not associated |
| EBV early antigen R % | 100 | 33.3 | ND |
| EBV early antigen D % | 100 | 17.6 & 79(a special subset) | 66.7 p < 0.001 |
| EBV Nuclear Antigen % | 100 | 14.8 | Not associated |
| CMV IgM % | 28.6 | 0 in a special subset | Not associated |
| CMV IgG % | 100 | 30 (>1:320) | Not associated |
| Heterophile Ab % | 14.3 | NA | ND |
| References | [2,3,4,6,8,9,10] | [11,12,13,14,15,16,17,18] | [19,20,21,22] |
| Parameter | Chronic Fatigue | Controls | Statistical p Value (CI) |
|---|---|---|---|
| Number | 13 | 53 | N/A |
| Age (Years ± SD) | 44.77 ± 15.94 | 55.17 ± 7.92 | p < 0.04 |
| % African American | 53.9 | 69.2 | p = 0.34 |
| % Male | 61.5 | 82.7 | p = 0.13 |
| Overweight %BMI > 28 kg/m2 | 25 | 62.8 | OR21[0.90–490.14); p < 0.041 |
| % Survival | 92.3 | 96 | p = 0.89 |
| % Helicobacter pylori infection | 16.7 | 34.4 | p = 0.39 |
| % Smokers | 67 | 38.5 | p = 0.11 |
| % Alcohol Imbibers | 54 | 36 | p = 0.3 |
| Parameter | Chronic Fatigue | Controls | Statistical p Value (CI) |
|---|---|---|---|
| Cumulative adenomas x ± sd | 1.00 ± 1.48 | 2.89 ± 4.45 | p < 0.002 |
| Adenomas/colonoscopy x ± sd | 0.08 ± 0.28 | 0.56 ± 1.22 | p < 0.014 |
| Inflammatory field colon x ± sd | 0.417 ± 0.589 | 1.338 ± 0.555 | p < 0.045 |
| Blood Creatinine x ± sd mg/dL | 0.939 ± 0.198 | 1.129 ± 0.256 | p < 0.016 |
| Proton Pump Inhibitors taken | 50% | 18.2% | p = 0.11 |
| Calcium Channel Blockers/ACEi | 25%/37.5% | 42.8%/44.1% | p = 0.4/p = 1 |
| History of Tonsillectomy | 42.9% | 0% | OR21[0.9–490.14]; p < 0.032 |
| Paper (Cite #) | Patient Number | Publication Type | Control | Conclusions |
|---|---|---|---|---|
| Anaya et al. [27] | 100; 10Vaccinated | Cross-sectional/MA | Acute vs. LC | >Arthralgia in LC |
| Becker et al. [28] | 90; at 90 and 365 days | Prospective | LC vs. non-LC | >Severe initial; days |
| Haran et al. [29] | 164; 13<; 14 > 4 wks | Prospective Dysbiosis | LC vs. non-LC | Prevatella/Veillonella * |
| Fogarty et al. [30] | 50; median 68 days | Coagulation factors | 17 normal | Increased; older LC ** |
| García-Abellán [31] | 146; 5.5% deaths | Prospective | None | Weak ab; Ꝗ, severity |
| Vanichkachorn [32] | 100; mean 93 days | Descriptive > wks LC | None | 80% fatigue; 59%neuro |
| Moreno-Pérez [33] | 141; 34/66%mild/s | Prospective@77 days | None | No markers for LC |
| Ludvigsson [34] | 5; 4 girls; 1p-myoC | Literature review | None | Sx for 4–8 months |
| Wong [35] | 21 studies LC and CFS | Literature review | Compare Sx | 86%Sx shared ME/CFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobi, M.; Chaudhari, D.; Ryan, E.P.; Rossi, N.F.; Koka, O.; Baxter, B.; Tipton, M.; Dutt, T.S.; Tobi, Y.; McVicker, B.; et al. Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles. Biomolecules 2025, 15, 928. https://doi.org/10.3390/biom15070928
Tobi M, Chaudhari D, Ryan EP, Rossi NF, Koka O, Baxter B, Tipton M, Dutt TS, Tobi Y, McVicker B, et al. Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles. Biomolecules. 2025; 15(7):928. https://doi.org/10.3390/biom15070928
Chicago/Turabian StyleTobi, Martin, Diptaraj Chaudhari, Elizabeth P. Ryan, Noreen F. Rossi, Orena Koka, Bridget Baxter, Madison Tipton, Taru S. Dutt, Yosef Tobi, Benita McVicker, and et al. 2025. "Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles" Biomolecules 15, no. 7: 928. https://doi.org/10.3390/biom15070928
APA StyleTobi, M., Chaudhari, D., Ryan, E. P., Rossi, N. F., Koka, O., Baxter, B., Tipton, M., Dutt, T. S., Tobi, Y., McVicker, B., & Angoa-Perez, M. (2025). Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles. Biomolecules, 15(7), 928. https://doi.org/10.3390/biom15070928







