A Comprehensive Review of the Role of the Microbiota–Gut–Brain Axis via Neuroinflammation: Advances and Therapeutic Implications for Ischemic Stroke
Abstract
1. Introduction
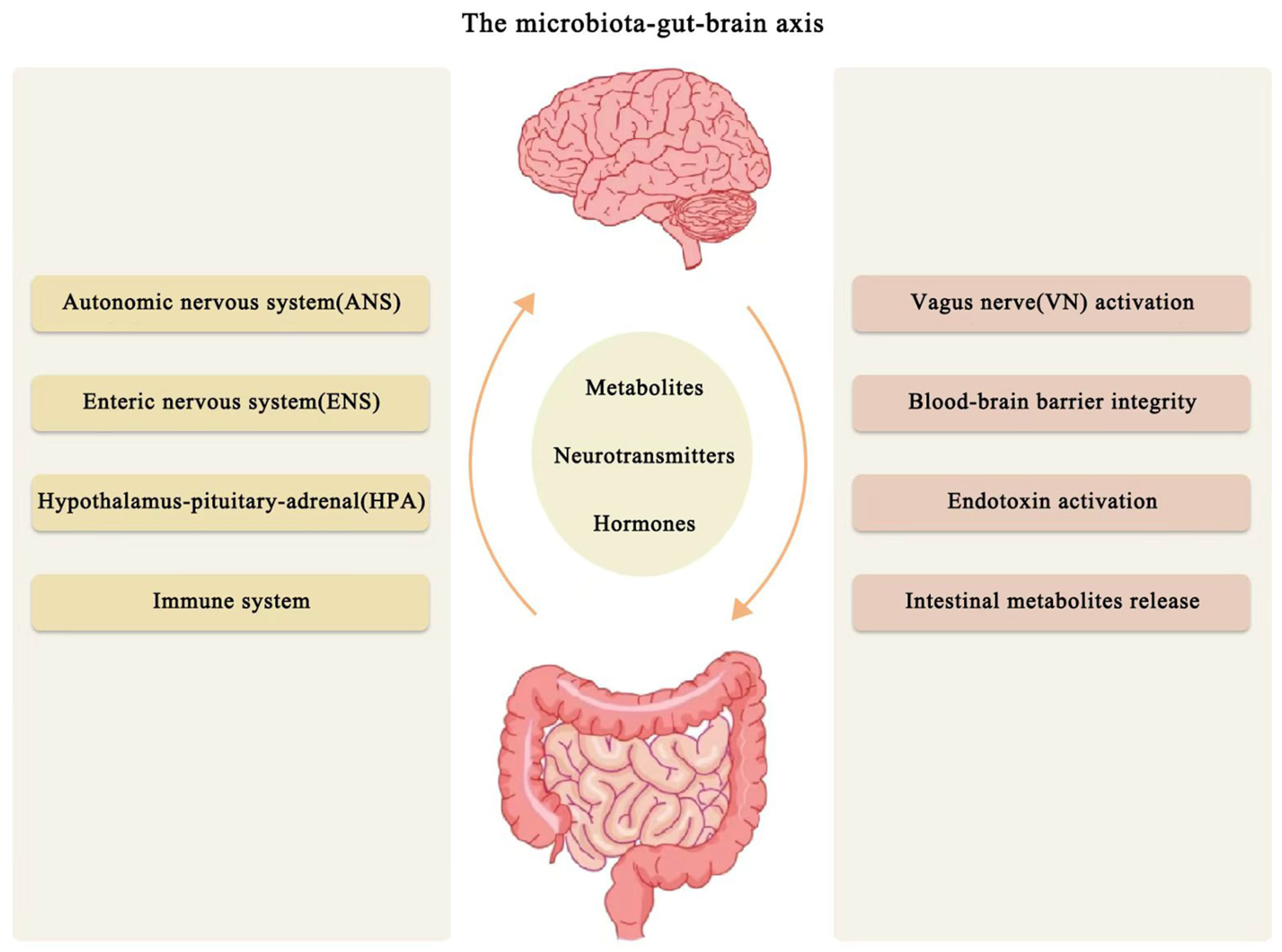
Microbiome–Gut–Brain Axis
2. Pathophysiology of Acute Ischemic Stroke
3. Inflammation and Immune Response After Ischemic Stroke
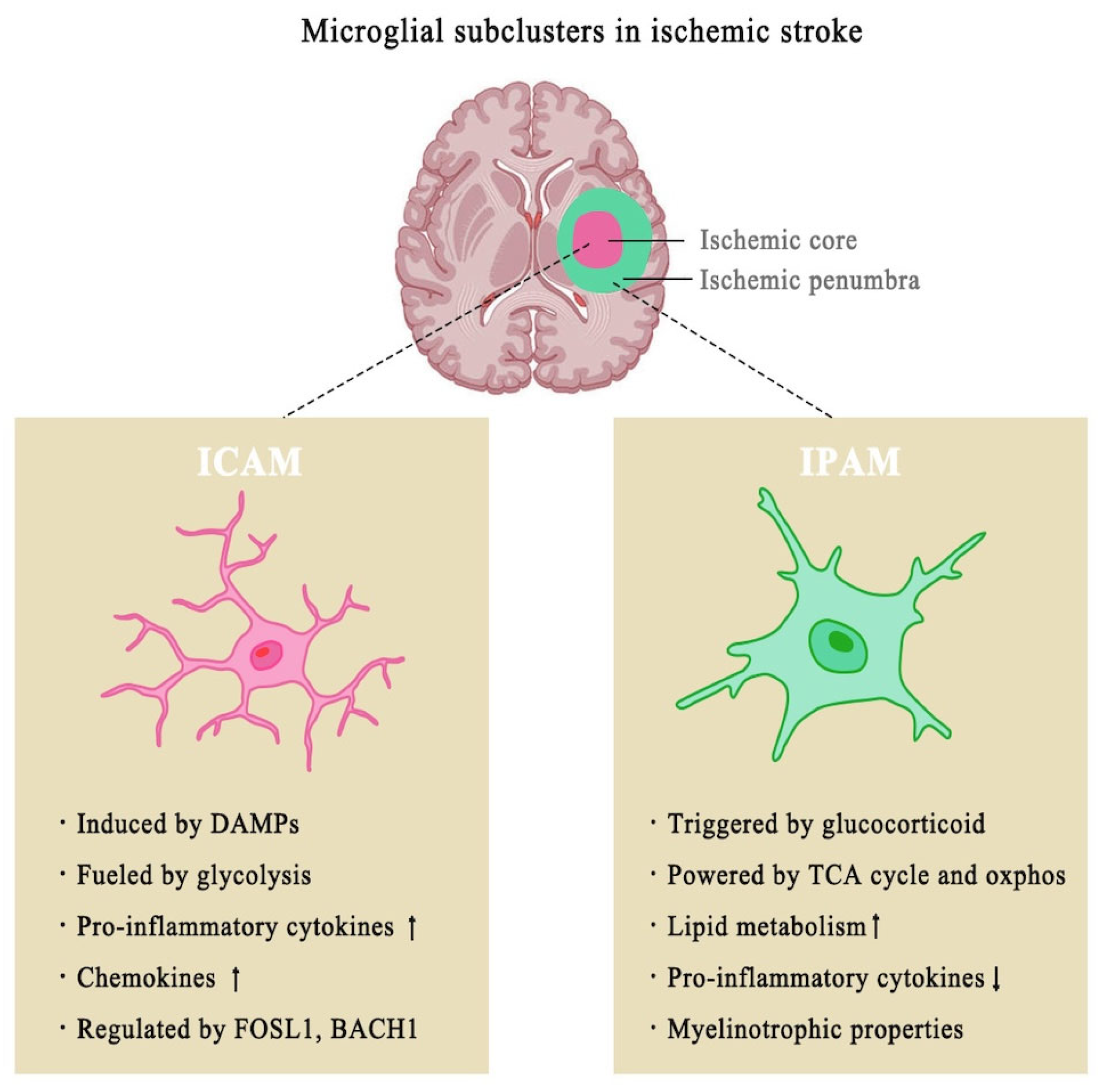
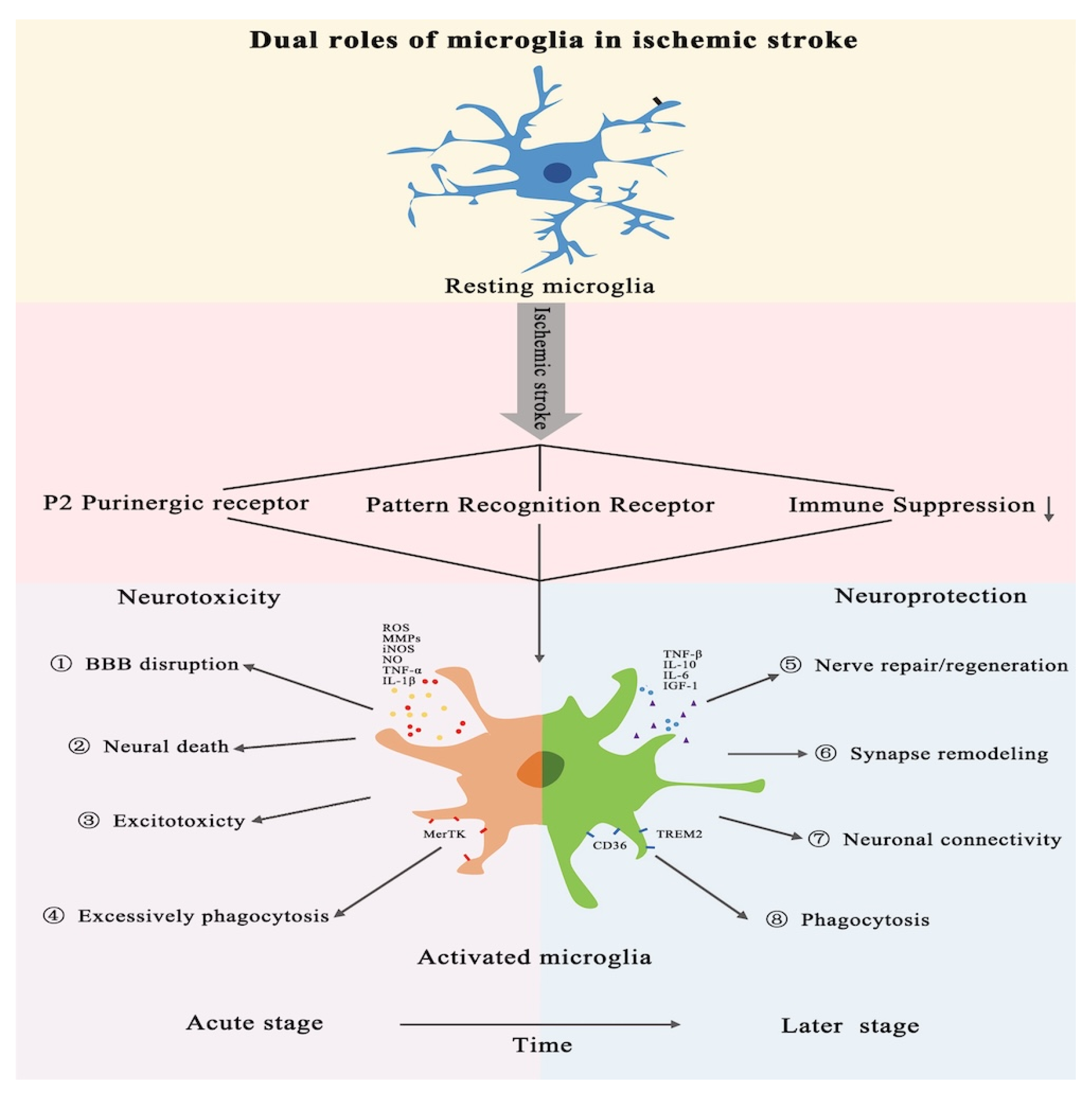
3.1. Role of Microglia in Ischemic Stroke
3.2. Microglial Activation and Its Dual Role in Post-Ischemic Stroke Inflammation
3.3. Other Immune Participants in Ischemic Stroke
4. The Microbiome–Gut–Brain Axis in Ischemic Stroke
4.1. Gut Microbiota-Derived Metabolites in Ischemic Stroke
4.1.1. SCFAs Mitigate Neuroinflammation and Post-Stroke Recovery
4.1.2. Trimethylamine N-Oxide Promotes Neuroinflammation and Worsens Stroke Outcome
4.1.3. Lipopolysaccharide Disrupting the Intestinal Barrier and the Blood–Brain Barrier via Neuroinflammation
4.1.4. Tryptophan and Indole Derivatives Modulate Neuroinflammation
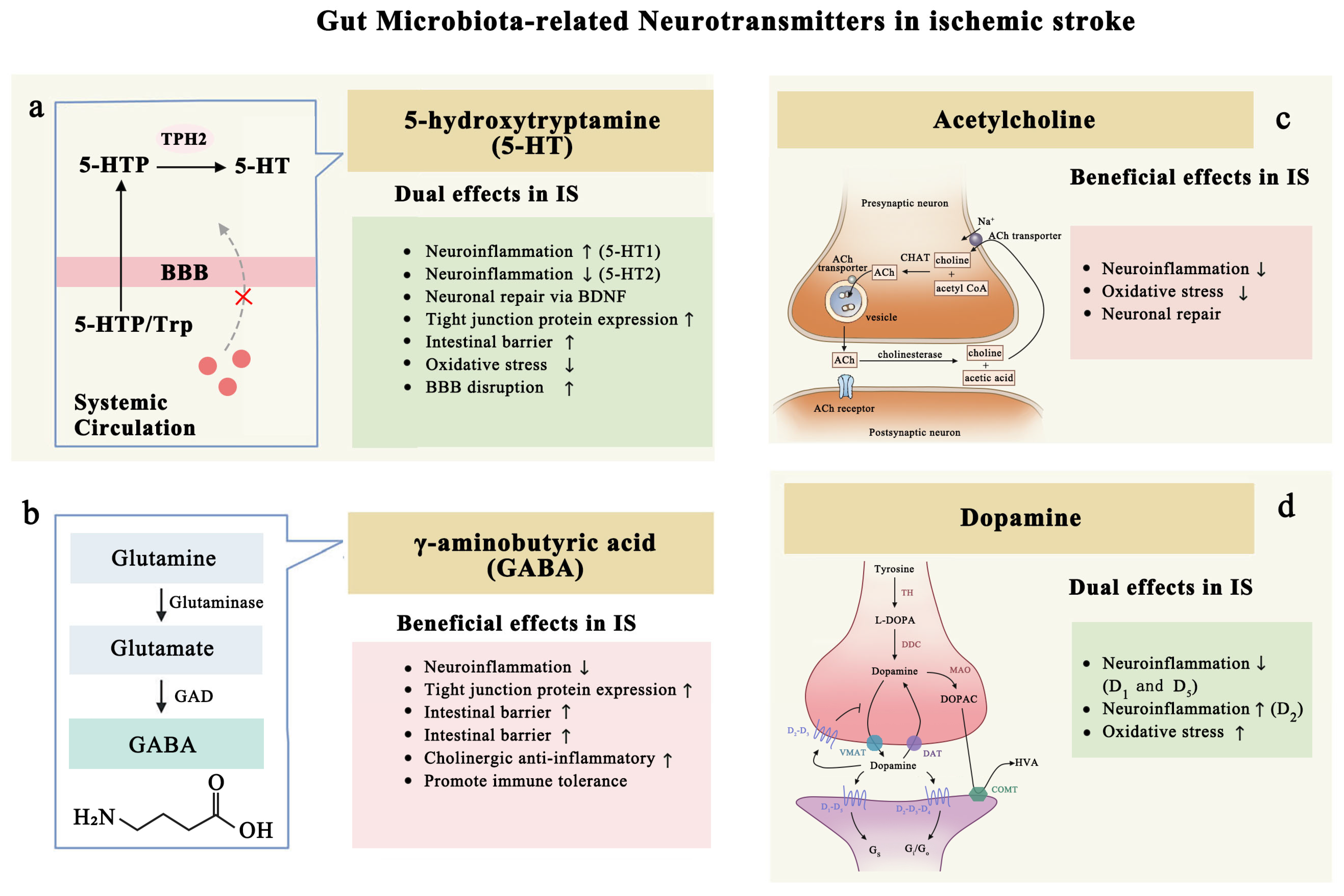
4.2. Gut Microbiota-Related Neurotransmitters in Ischemic Stroke
4.2.1. Serotonin: Inflammation and Protection
4.2.2. GABA: Post-Stroke Neurofunctional Recovery
4.2.3. Acetylcholine: Anti-Inflammation and Neuronal Regeneration
4.2.4. Dopamine (DA): Dual Roles After Ischemic Stroke
5. Points of Intervention to Improve Microbiome–Gut–Brain Axis
5.1. Intestinal Barrier Restoration
5.2. Blood–Brain Barrier Restoration
6. Stroke Treatment Based on Microbiota–Gut–Brain Axis
6.1. Diet and Stroke
6.2. Antibiotic and Probiotics or Prebiotics Therapy
6.3. Fecal Microbiota Transplantation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MGBA | microbiota–gut–brain axis |
| BBB | blood–brain barrier |
| AIS | acute ischemic stroke |
| HPA | hypothalamic–pituitary–adrenal |
| ANS | autonomic nervous system |
| SCFAs | short-chain fatty acids |
| TMAO | trimethylamine N-oxide |
| LPS | lipopolysaccharides |
| ACh | Acetylcholine |
| MCAO | middle cerebral artery occlusion |
| FMT | fecal microbiome transplantation |
| GPCRs | G protein-coupled receptors |
| GABA | Gamma-Aminobutyric Acid |
| AHR | aryl hydrocarbon receptor |
References
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cai, L.; Chen, P.; Kuang, W. A study of the correlation between stroke and gut microbiota over the last 20years: A bibliometric analysis. Front. Microbiol. 2023, 14, 1191758. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Wang, T.; Pan, C.; Xie, C.; Chen, L.; Song, Z.; Liao, H.; Xin, C. Microbiota Metabolites and Immune Regulation Affect Ischemic Stroke Occurrence, Development, and Prognosis. Mol. Neurobiol. 2023, 60, 6176–6187. [Google Scholar] [CrossRef]
- Durgan, D.J.; Lee, J.; McCullough, L.D.; Bryan, R.M., Jr. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 2019, 50, 2270–2277. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Rubsamen, N.; Grefkes, C.; Rost, N.S.; Berger, K.; Karch, A. Development and Validation of Prediction Models for Severe Complications After Acute Ischemic Stroke: A Study Based on the Stroke Registry of Northwestern Germany. J. Am. Heart Assoc. 2022, 11, e023175. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.H.; You, C.; Gao, X.X.; Zeng, X.L.; Zhu, J.J.; Xu, K.Y.; Tan, C.H.; Xu, R.T.; Wu, Q.H.; Zhou, H.W.; et al. Stroke Dysbiosis Index (SDI) in Gut Microbiome Are Associated With Brain Injury and Prognosis of Stroke. Front. Neurol. 2019, 10, 397. [Google Scholar] [CrossRef]
- Hu, W.; Kong, X.; Wang, H.; Li, Y.; Luo, Y. Ischemic stroke and intestinal flora: An insight into brain-gut axis. Eur. J. Med. Res. 2022, 27, 73. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhao, H.; Cao, R.; Dang, Y.; Yu, B. Imbalance of Microbacterial Diversity Is Associated with Functional Prognosis of Stroke. Neural Plast. 2023, 2023, 6297653. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef]
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Wlodarczyk, M.; Zielinska, A.; Poleszak, E.; Fichna, J.; Wlaz, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, L.; Guo, Y.; Xiao, J.; Zhang, J.; Xu, S. New Insights into Stroke Prevention and Treatment: Gut Microbiome. Cell. Mol. Neurobiol. 2022, 42, 455–472. [Google Scholar] [CrossRef]
- Nakhal, M.M.; Yassin, L.K.; Alyaqoubi, R.; Saeed, S.; Alderei, A.; Alhammadi, A.; Alshehhi, M.; Almehairbi, A.; Al Houqani, S.; BaniYas, S.; et al. The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review. Life 2024, 14, 1234. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef]
- Yassin, L.K.; Nakhal, M.M.; Alderei, A.; Almehairbi, A.; Mydeen, A.B.; Akour, A.; Hamad, M.I.K. Exploring the microbiota-gut-brain axis: Impact on brain structure and function. Front. Neuroanat. 2025, 19, 1504065. [Google Scholar] [CrossRef]
- Ho, J.P.; Powers, W.J. Contemporary Management of Acute Ischemic Stroke. Annu. Rev. Med. 2025, 76, 417–429. [Google Scholar] [CrossRef]
- Scheldeman, L.; Wouters, A.; Dupont, P.; Christensen, S.; Boutitie, F.; Cheng, B.; Ebinger, M.; Endres, M.; Fiebach, J.B.; Gerloff, C.; et al. Reversible Edema in the Penumbra Correlates With Severity of Hypoperfusion. Stroke 2021, 52, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, N.A.; Murtala, B.; Muis, M.; Ilyas, M.I.; Abdul Hamid, H.B.; As’ad, S.; Tammasse, J.; Wuysang, A.D.; Soraya, G.V. Non-Contrast MRI Sequences for Ischemic Stroke: A Concise Overview for Clinical Radiologists. Vasc. Health Risk Manag. 2024, 20, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, G.; Simonsen, C.Z.; Boutitie, F.; Andersen, G.; Berthezene, Y.; Cheng, B.; Cheripelli, B.; Cho, T.H.; Fazekas, F.; Fiehler, J.; et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N. Engl. J. Med. 2018, 379, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Feil, K.; Reidler, P.; Kunz, W.G.; Küpper, C.; Heinrich, J.; Laub, C.; Müller, K.; Vöglein, J.; Liebig, T.; Dieterich, M.; et al. Addressing a real-life problem: Treatment with intravenous thrombolysis and mechanical thrombectomy in acute stroke patients with an extended time window beyond 4.5 h based on computed tomography perfusion imaging. Eur. J. Neurol. 2020, 27, 168–174. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Kukulska-Pawluczuk, B.; Piec, K.; Jarek, D.J.; Motolko, K.; Szewczyk-Golec, K.; Woźniak, A. Intersecting Pathways: The Role of Metabolic Dysregulation, Gastrointestinal Microbiome, and Inflammation in Acute Ischemic Stroke Pathogenesis and Outcomes. J. Clin. Med. 2024, 13, 4258. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Zeng, J.; Bao, T.; Yang, K.; Zhu, X.; Wang, S.; Xiang, W.; Ge, A.; Zeng, L.; Ge, J. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: A review. Front. Immunol. 2022, 13, 1047550. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J. Animal models of stroke. Anim. Model. Exp. Med. 2021, 4, 204–219. [Google Scholar] [CrossRef]
- Bano, N.; Khan, S.; Ahamad, S.; Kanshana, J.S.; Dar, N.J.; Khan, S.; Nazir, A.; Bhat, S.A. Microglia and gut microbiota: A double-edged sword in Alzheimer’s disease. Ageing Res. Rev. 2024, 101, 102515. [Google Scholar] [CrossRef]
- Li, H.; Liu, P.; Zhang, B.; Yuan, Z.; Guo, M.; Zou, X.; Qian, Y.; Deng, S.; Zhu, L.; Cao, X.; et al. Acute ischemia induces spatially and transcriptionally distinct microglial subclusters. Genome Med. 2023, 15, 109. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Ament, Z.; Patki, A.; Bhave, V.M.; Chaudhary, N.S.; Garcia Guarniz, A.L.; Kijpaisalratana, N.; Judd, S.E.; Cushman, M.; Long, D.L.; Irvin, M.R.; et al. Gut microbiota-associated metabolites and risk of ischemic stroke in REGARDS. J. Cereb. Blood Flow Metab. 2023, 43, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yang, C.; Jia, X.; Yu, Z.; Wang, C.; Zhao, J.; Chen, Y.; Xie, B.; Zhuang, H.; Sun, C.; et al. High-fat diet consumption promotes adolescent neurobehavioral abnormalities and hippocampal structural alterations via microglial overactivation accompanied by an elevated serum free fatty acid concentration. Brain Behav. Immun. 2024, 119, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Miyazaki, H.; Wannakul, T.; Amo, E.; Saido, T.; Saito, T.; Sasaguri, H.; Maekawa, M.; Owada, Y. High-Fat Diet-Induced Excessive Accumulation of Cerebral Cholesterol Esters and Microglial Dysfunction Exacerbate Alzheimer’s Disease Pathology in APPNL-G-F mice. Mol. Neurobiol. 2025, 1–21. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938. [Google Scholar] [CrossRef]
- Deczkowska, A.; Amit, I.; Schwartz, M. Microglial immune checkpoint mechanisms. Nat. Neurosci. 2018, 21, 779–786. [Google Scholar] [CrossRef]
- Sadler, R.; Cramer, J.V.; Heindl, S.; Kostidis, S.; Betz, D.; Zuurbier, K.R.; Northoff, B.H.; Heijink, M.; Goldberg, M.P.; Plautz, E.J.; et al. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J. Neurosci. 2020, 40, 1162–1173. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Hu, S.; Yang, B.; Zhang, H. Host-microbiota interactions: The aryl hydrocarbon receptor in the acute and chronic phases of cerebral ischemia. Front. Immunol. 2022, 13, 967300. [Google Scholar] [CrossRef]
- Dong, F.; Perdew, G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes 2020, 12, 1859812. [Google Scholar] [CrossRef]
- Wang, M.; Pan, W.; Xu, Y.; Zhang, J.; Wan, J.; Jiang, H. Microglia-Mediated Neuroinflammation: A Potential Target for the Treatment of Cardiovascular Diseases. J. Inflamm. Res. 2022, 15, 3083–3094. [Google Scholar] [CrossRef]
- Garofalo, S.; Cocozza, G.; Bernardini, G.; Savage, J.; Raspa, M.; Aronica, E.; Tremblay, M.-E.; Ransohoff, R.M.; Santoni, A.; Limatola, C. Blocking immune cell infiltration of the central nervous system to tame Neuroinflammation in Amyotrophic lateral sclerosis. Brain Behav. Immun. 2022, 105, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H. Intestinal M cells. J. Biochem. 2016, 159, 151–160. [Google Scholar] [CrossRef]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Malone, K.; Amu, S.; Moore, A.C.; Waeber, C. The immune system and stroke: From current targets to future therapy. Immunol. Cell Biol. 2019, 97, 5–16. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, R. Four Decades of Ischemic Penumbra and Its Implication for Ischemic Stroke. Transl. Stroke Res. 2021, 12, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; d’Aigle, J.; Atadja, L.; Quaicoe, V.; Honarpisheh, P.; Ganesh, B.P.; Hassan, A.; Graf, J.; Petrosino, J.; Putluri, N.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Poststroke Recovery in Aged Mice. Circ. Res. 2020, 127, 453–465. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Zhu, W.; Romano, K.A.; Li, L.; Buffa, J.A.; Sangwan, N.; Prakash, P.; Tittle, A.N.; Li, X.S.; Fu, X.; Androjna, C.; et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 2021, 29, 1199–1208.e5. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shan, W.; Lai, Z.; Zuo, Z. Gut microbiota of old mice worsens neurological outcome after brain ischemia via increased valeric acid and IL-17 in the blood. Microbiome 2023, 11, 204. [Google Scholar] [CrossRef]
- Stuckey, S.M.; Ong, L.K.; Collins-Praino, L.E.; Turner, R.J. Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke? Int. J. Mol. Sci. 2021, 22, 13101. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, F.; Zhang, B.; Luo, Y.; Xing, X.; Wang, M.; Chen, R.; Sun, G.; Sun, X. Cellular Immune Signal Exchange From Ischemic Stroke to Intestinal Lesions Through Brain-Gut Axis. Front. Immunol. 2022, 13, 688619. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Zhong, S.; Liu, C.; Jolkkonen, J.; Zhang, X.; Liang, Y.; Liu, Z.; Zhao, C. Communications Between Peripheral and the Brain-Resident Immune System in Neuronal Regeneration After Stroke. Front. Immunol. 2020, 11, 1931. [Google Scholar] [CrossRef]
- Chai, Z.; Zheng, J.; Shen, J. Mechanism of ferroptosis regulating ischemic stroke and pharmacologically inhibiting ferroptosis in treatment of ischemic stroke. CNS Neurosci. Ther. 2024, 30, e14865. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.-W.; Lee, S.-Y.; Manzanero, S.; Chunduri, P.; Sobey, C.G.; Arumugam, T.V. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res. Rev. 2013, 12, 941–966. [Google Scholar] [CrossRef]
- Huang, Q.; Xia, J. Influence of the gut microbiome on inflammatory and immune response after stroke. Neurol. Sci. 2021, 42, 4937–4951. [Google Scholar] [CrossRef]
- Yan, C.; Liu, Z.; Xie, W.; Zhang, T.; Zhang, J.; Li, G.; Xu, X.; Ye, L.; Gong, J. Cornuside protects against ischemic stroke in rats by suppressing the IL-17F/TRAF6/NF-κB pathway via the brain-gut axis. Exp. Neurol. 2024, 373, 114672. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, Y.; Liu, Z.; Liao, Y.; Sun, S.; Kong, X.; Hu, J.; Tang, Y. The role of IL-23/IL-17 axis in ischemic stroke from the perspective of gut-brain axis. Neuropharmacology 2023, 231, 109505. [Google Scholar] [CrossRef]
- Huang, A.; Ji, L.; Li, Y.; Li, Y.; Yu, Q. Gut microbiome plays a vital role in post-stroke injury repair by mediating neuroinflammation. Int. Immunopharmacol. 2023, 118, 110126. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xie, J.; Chen, X.; Qin, B.; Kong, D.; Luo, J. Fecal microbiota transplantation alleviates neuronal Apoptosis, necroptosis and reactive microglia activation after ischemic stroke. Neuroscience 2025, 564, 299–305. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, M.; Qian, J.; Wang, C.; Zhang, J. The Bridge Between Ischemic Stroke and Gut Microbes: Short-Chain Fatty Acids. Cell. Mol. Neurobiol. 2023, 43, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Hu, J.; Deng, Y.; Zou, J.; Ding, W.; Peng, Q.; Duan, R.; Sun, J.; Zhu, J. Berberine Mediates the Production of Butyrate to Ameliorate Cerebral Ischemia via the Gut Microbiota in Mice. Nutrients 2023, 16, 9. [Google Scholar] [CrossRef]
- Conesa, M.P.B.; Blixt, F.W.; Peesh, P.; Khan, R.; Korf, J.; Lee, J.; Jagadeesan, G.; Andersohn, A.; Das, T.K.; Tan, C.; et al. Stabilizing histamine release in gut mast cells mitigates peripheral and central inflammation after stroke. J. Neuroinflamm. 2023, 20, 230. [Google Scholar] [CrossRef]
- Xu, K.; Gao, X.; Xia, G.; Chen, M.; Zeng, N.; Wang, S.; You, C.; Tian, X.; Di, H.; Tang, W.; et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 2021, 70, 1486–1494. [Google Scholar] [CrossRef]
- Cui, W.; Xu, L.; Huang, L.; Tian, Y.; Yang, Y.; Li, Y.; Yu, Q. Changes of gut microbiota in patients at different phases of stroke. CNS Neurosci. Ther. 2023, 29, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium Butyrate Ameliorates Oxidative Stress-Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage through AMPK-Mitophagy Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cao, S.; Bashir, M.E.H.; Hesser, L.A.; Su, Y.; Hong, S.M.C.; Thompson, A.; Culleen, E.; Sabados, M.; Dylla, N.P.; et al. Treatment of peanut allergy and colitis in mice via the intestinal release of butyrate from polymeric micelles. Nat. Biomed. Eng. 2023, 7, 38–55. [Google Scholar] [CrossRef]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Bisanz, J.E. Roles of the gut microbiome in weight management. Nat. Rev. Microbiol. 2023, 21, 535–550. [Google Scholar] [CrossRef]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Wong, T.S.; Li, G.; Li, S.; Gao, W.; Chen, G.; Gan, S.; Zhang, M.; Li, H.; Wu, S.; Du, Y. G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders. Signal Transduct. Target. Ther. 2023, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Song, L.; Sun, Q.; Zheng, H.; Zhang, Y.; Wang, Y.; Liu, S.; Duan, L. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, e2200164. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Li, J.; Liang, J.; Yang, M.; Xia, G.; Ren, Y.; Zhou, H.; Wu, Q.; He, Y.; et al. Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J. Neuroinflamm. 2022, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S. Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Fang, Q.; Yin, X. Gut Microbe-Generated Metabolite Trimethylamine-N-Oxide and Ischemic Stroke. Biomolecules 2024, 14, 1463. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Li, R.; Liu, R.; Yu, Z.; Zhang, Z.; Wan, Z. Trimethylamine N-oxide aggravated cognitive impairment from APP/PS1 mice and protective roles of voluntary exercise. Neurochem. Int. 2023, 162, 105459. [Google Scholar] [CrossRef]
- Wang, Q.J.; Shen, Y.E.; Wang, X.; Fu, S.; Zhang, X.; Zhang, Y.N.; Wang, R.T. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging 2020, 12, 628–649. [Google Scholar] [CrossRef]
- Dolkar, P.; Deyang, T.; Anand, N.; Rathipriya, A.G.; Hediyal, T.A.; Chandrasekaran, V.; Krishnamoorthy, N.K.; Gorantla, V.R.; Bishir, M.; Rashan, L.; et al. Trimethylamine-N-oxide and cerebral stroke risk: A review. Neurobiol. Dis. 2024, 192, 106423. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Xie, L.; Zhao, B.X.; Li, Y.; Qiu, B.; Zhu, F.; Li, G.F.; He, M.; Wang, Y.; Wang, B.; et al. Serum Trimethylamine N-Oxide Concentration Is Positively Associated With First Stroke in Hypertensive Patients. Stroke 2018, 49, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Aldeguer, X.; Vivas, D.; Manzano Fernández, S.; Gonzalez Caballero, E.; Garcia Martín, A.; Barrios, V.; Freixa-Pamias, R. The gut microbiota and its role in the development of cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2025, 23, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Bai, H.; Han, Y.; Wu, Y.; Peng, R.; Zhang, X.; Liang, B.; Zhao, Q.; Ma, M.; Zhang, P.; et al. Association of the gut microbe-dependent trimethylamine N-oxide and its precursors with risk of hypertension: A cross-sectional study in rural northeastern China. Nutr. Metab. Cardiovasc. Dis. 2025, 104032. [Google Scholar] [CrossRef]
- Wu, C.; Xue, F.; Lian, Y.; Zhang, J.; Wu, D.; Xie, N.; Chang, W.; Chen, F.; Wang, L.; Wei, W.; et al. Relationship between elevated plasma trimethylamine N-oxide levels and increased stroke injury. Neurology 2020, 94, e667–e677. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, X.Y.; Huang, R. Gut Microbiota in Ischemic Stroke: Role of Gut Bacteria-Derived Metabolites. Transl. Stroke Res. 2023, 14, 811–828. [Google Scholar] [CrossRef]
- Klimiec, E.; Pera, J.; Chrzanowska-Wasko, J.; Golenia, A.; Slowik, A.; Dziedzic, T. Plasma endotoxin activity rises during ischemic stroke and is associated with worse short-term outcome. J. Neuroimmunol. 2016, 297, 76–80. [Google Scholar] [CrossRef]
- Kurita, N.; Yamashiro, K.; Kuroki, T.; Tanaka, R.; Urabe, T.; Ueno, Y.; Miyamoto, N.; Takanashi, M.; Shimura, H.; Inaba, T.; et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2020, 40, 2505–2520. [Google Scholar] [CrossRef]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: Roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.J.; Stroes, E.S.G.; Boekholdt, S.M.; Wareham, N.J.; Murphy, A.J.; Nieuwdorp, M.; Hazen, S.L.; Hanssen, N.M.J. Tryptophan metabolites and incident cardiovascular disease: The EPIC-Norfolk prospective population study. Atherosclerosis 2023, 387, 117344. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hong, Y.; Chen, Z.; Ma, Y.; Xia, S.; Gu, S.; Zuo, H. The Tryptophan Index Is Associated with Risk of Ischemic Stroke: A Community-Based Nested Case-Control Study. Nutrients 2024, 16, 1544. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. Int. Rev. J. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; He, H.; Peng, M.; Zeng, M.; Sun, H. The role of the indoles in microbiota-gut-brain axis and potential therapeutic targets: A focus on human neurological and neuropsychiatric diseases. Neuropharmacology 2023, 239, 109690. [Google Scholar] [CrossRef]
- Honarpisheh, P.; Lee, J.; Banerjee, A.; Korf, J.; Ko, K.A.; Blasco-Conesa, M.P.; Honarpisheh, P.; Bryan, R.; McCullough, L.; Ganesh, B.P. Abstract WP240: Beneficial Gut Microbiome-Derived Ligands Can Outcompete Detrimental Brain-Derived Ligands Of Aryl Hydrocarbon Receptor After Stroke. Stroke 2022, 53, AWP240. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Mordyl, B.; Siwek, A.; Głuch-Lutwin, M.; Karcz, T.; Gawalska, A.; Sapa, M.; Bucki, A.; Szafrańska, K.; Pomierny, B.; et al. Dual Molecules Targeting 5-HT6 and GABA-A Receptors as a New Approach to Combat Depression Associated with Neuroinflammation. ACS Chem. Neurosci. 2023, 14, 1474–1489. [Google Scholar] [CrossRef]
- Sharma, A.; Castellani, R.J.; Smith, M.A.; Muresanu, D.F.; Dey, P.K.; Sharma, H.S. 5-Hydroxytryptophan: A precursor of serotonin influences regional blood-brain barrier breakdown, cerebral blood flow, brain edema formation, and neuropathology. Int. Rev. Neurobiol. 2019, 146, 1–44. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, C.N.; Xia, X.; Heng, C.W.; Tan, Y.S.; Lee, D.P.S.; Fam, J.; Kim, J.E. The impact of 5-hydroxytryptophan supplementation on sleep quality and gut microbiota composition in older adults: A randomized controlled trial. Clin. Nutr. 2024, 43, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Bruijn, N.; van Lohuizen, R.; Boron, M.; Fitzek, M.; Gabriele, F.; Giuliani, G.; Melgarejo, L.; Řehulka, P.; Sebastianelli, G.; Triller, P.; et al. Influence of metabolic state and body composition on the action of pharmacological treatment of migraine. J. Headache Pain 2024, 25, 20. [Google Scholar] [CrossRef]
- Petersen, C.L.; Hougaard, A.; Gaist, D.; Hallas, J. Risk of Stroke and Myocardial Infarction Among Initiators of Triptans. JAMA Neurol. 2024, 81, 248–254. [Google Scholar] [CrossRef]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, epigenetics and depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [CrossRef]
- Mortensen, J.K.; Kraglund, K.L.; Johnsen, S.P.; Mors, O.; Andersen, G.; Buttenschøn, H.N. The Serotonin Transporter Gene Polymorphisms and Risk of Ischemic Stroke. Cerebrovasc. Dis. 2018, 45, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Szyf, M.; Benkelfat, C.; Provençal, N.; Turecki, G.; Caramaschi, D.; Côté, S.M.; Vitaro, F.; Tremblay, R.E.; Booij, L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE 2012, 7, e39501. [Google Scholar] [CrossRef]
- Ansorge, M.S.; Zhou, M.; Lira, A.; Hen, R.; Gingrich, J.A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004, 306, 879–881. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, E.H.; Kim, J.W.; Kim, S.W.; Shin, I.S.; Kim, J.T.; Park, M.S.; Cho, K.H.; Han, J.S.; Lyoo, I.K.; et al. Association of SLC6A4 methylation with long-term outcomes after stroke: Focus on the interaction with suicidal ideation. Sci. Rep. 2021, 11, 2710. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, A.; Li, C.; Han, J.; Wang, J.; Luo, L.; Chang, X.; Tian, Z.; Bai, Y. Corticostriatal Projections Relying on GABA Levels Mediate Exercise-Induced Functional Recovery in Cerebral Ischemic Mice. Mol. Neurobiol. 2023, 60, 1836–1853. [Google Scholar] [CrossRef] [PubMed]
- Lamtahri, R.; Hazime, M.; Gowing, E.K.; Nagaraja, R.Y.; Maucotel, J.; Alasoadura, M.; Quilichini, P.P.; Lehongre, K.; Lefranc, B.; Gach-Janczak, K.; et al. The Gliopeptide ODN, a Ligand for the Benzodiazepine Site of GABAA Receptors, Boosts Functional Recovery after Stroke. J. Neurosci. 2021, 41, 7148–7159. [Google Scholar] [CrossRef]
- Chabriat, H.; Bassetti, C.L.; Marx, U.; Audoli-Inthavong, M.L.; Sors, A.; Lambert, E.; Wattez, M.; Hermann, D.M. Safety and efficacy of GABAA α5 antagonist S44819 in patients with ischaemic stroke: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2020, 19, 226–233. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Petitfils, C.; Maurel, S.; Payros, G.; Hueber, A.; Agaiz, B.; Gazzo, G.; Marrocco, R.; Auvray, F.; Langevin, G.; Motta, J.P.; et al. Identification of bacterial lipopeptides as key players in IBS. Gut 2023, 72, 939–950. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Wang, F.; Xie, X.; Xing, X.; Sun, X. Excitatory Synaptic Transmission in Ischemic Stroke: A New Outlet for Classical Neuroprotective Strategies. Int. J. Mol. Sci. 2022, 23, 9381. [Google Scholar] [CrossRef]
- Andersen, J.V. The Glutamate/GABA-Glutamine Cycle: Insights, Updates, and Advances. J. Neurochem. 2025, 169, e70029. [Google Scholar] [CrossRef]
- Van Harreveld, A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J. Neurochem. 1959, 3, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Djali, S.; Gonzales, C.; Vinegra, M.A.; Zaleska, M.M. Characterization of transient focal ischemia-induced increases in extracellular glutamate and aspartate in spontaneously hypertensive rats. Brain Res. Bull. 2000, 53, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Okiyama, K.; Smith, D.H.; Gennarelli, T.A.; Simon, R.P.; Leach, M.; McIntosh, T.K. The sodium channel blocker and glutamate release inhibitor BW1003C87 and magnesium attenuate regional cerebral edema following experimental brain injury in the rat. J. Neurochem. 1995, 64, 802–809. [Google Scholar] [CrossRef]
- Lai, K.; Pritišanac, I.; Liu, Z.Q.; Liu, H.W.; Gong, L.N.; Li, M.X.; Lu, J.F.; Qi, X.; Xu, T.L.; Forman-Kay, J.; et al. Glutamate acts on acid-sensing ion channels to worsen ischaemic brain injury. Nature 2024, 631, 826–834. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, X.; Yao, M.W.; Li, Z.; Xu, X. Research advances on the cholinergic inflammatory reflex and inflammation resolution. Zhonghua Shao Shang Za Zhi 2021, 37, 885–889. [Google Scholar] [CrossRef]
- Capcha, J.M.C.; Rodrigues, C.E.; Moreira, R.S.; Silveira, M.D.; Dourado, P.; Dos Santos, F.; Irigoyen, M.C.; Jensen, L.; Garnica, M.R.; Noronha, I.L.; et al. Wharton’s jelly-derived mesenchymal stem cells attenuate sepsis-induced organ injury partially via cholinergic anti-inflammatory pathway activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R135–R147. [Google Scholar] [CrossRef]
- Yu, L.; Huang, B.; Po, S.S.; Tan, T.; Wang, M.; Zhou, L.; Meng, G.; Yuan, S.; Zhou, X.; Li, X.; et al. Low-Level Tragus Stimulation for the Treatment of Ischemia and Reperfusion Injury in Patients With ST-Segment Elevation Myocardial Infarction: A Proof-of-Concept Study. JACC Cardiovasc. Interv. 2017, 10, 1511–1520. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, M.; Ren, J.; Sun, X.; Zhang, Z.; Luo, Y.; Sun, X. New insight into gut microbiota and their metabolites in ischemic stroke: A promising therapeutic target. Biomed. Pharmacother. 2023, 162, 114559. [Google Scholar] [CrossRef]
- Winek, K.; Soreq, H.; Meisel, A. Regulators of cholinergic signaling in disorders of the central nervous system. J. Neurochem. 2021, 158, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, R.H.; Wang, H.; Long, C.L.; Wang, H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol. Sin. 2015, 36, 1416–1425. [Google Scholar] [CrossRef]
- Tan, E.C.K.; Johnell, K.; Garcia-Ptacek, S.; Haaksma, M.L.; Fastbom, J.; Bell, J.S.; Eriksdotter, M. Acetylcholinesterase inhibitors and risk of stroke and death in people with dementia. Alzheimers Dement. 2018, 14, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, L.; Tan, X.; Liu, B.; Zhang, Y.; Li, C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J. Neurochem. 2015, 134, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.D.; Malley, K.M.; Danaphongse, T.T.; Ahmad, F.N.; Beltran, C.M.; White, M.L.; Baghdadi, S.; Pruitt, D.T.; Rennaker, R.L., II; Kilgard, M.P.; et al. Vagus Nerve Stimulation Must Occur During Tactile Rehabilitation to Enhance Somatosensory Recovery. Neuroscience 2023, 532, 79–86. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Papp, L.; Stoll, G.; Blum, R.; Volkmann, J.; Fluri, F. Mesencephalic Electrical Stimulation Reduces Neuroinflammation after Photothrombotic Stroke in Rats by Targeting the Cholinergic Anti-Inflammatory Pathway. Int. J. Mol. Sci. 2021, 22, 1254. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Y.; Wang, B.; Li, S.; Ma, C.; Liu, X.; Moynagh, P.N.; Zhou, J.; Yang, S. Dopamine Uses the DRD5-ARRB2-PP2A Signaling Axis to Block the TRAF6-Mediated NF-κB Pathway and Suppress Systemic Inflammation. Mol. Cell 2020, 78, 42–56.e46. [Google Scholar] [CrossRef]
- Retzlaff, C.L.; Kussrow, A.; Schorkopf, T.; Saetear, P.; Bornhop, D.J.; Hardaway, J.A.; Sturgeon, S.M.; Wright, J.; Blakely, R.D. Metallo-β-lactamase Domain-Containing Protein 1 (MBLAC1) Is a Specific, High-Affinity Target for the Glutamate Transporter Inducer Ceftriaxone. ACS Chem. Neurosci. 2017, 8, 2132–2138. [Google Scholar] [CrossRef]
- Moody, A.S.; Sharma, B. Multi-metal, Multi-wavelength Surface-Enhanced Raman Spectroscopy Detection of Neurotransmitters. ACS Chem. Neurosci. 2018, 9, 1380–1387. [Google Scholar] [CrossRef]
- Gorgoraptis, N.; Mah, Y.H.; Machner, B.; Singh-Curry, V.; Malhotra, P.; Hadji-Michael, M.; Cohen, D.; Simister, R.; Nair, A.; Kulinskaya, E.; et al. The effects of the dopamine agonist rotigotine on hemispatial neglect following stroke. Brain 2012, 135, 2478–2491. [Google Scholar] [CrossRef]
- Ford, G.A.; Bhakta, B.B.; Cozens, A.; Hartley, S.; Holloway, I.; Meads, D.; Pearn, J.; Ruddock, S.; Sackley, C.M.; Saloniki, E.C.; et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019, 18, 530–538. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, M.; Wang, L.; Zhang, L.; Xu, D.; Cao, P.; Wang, F.; Herzog, H.; Song, S.; Zhan, C. A Vagal-NTS Neural Pathway that Stimulates Feeding. Curr. Biol. 2020, 30, 3986–3998.e5. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, D.; Ciglieri, E.; Biglari, N.; Brandt, C.; Cremer, A.L.; Backes, H.; Tittgemeyer, M.; Wunderlich, F.T.; Bruning, J.C.; Fenselau, H. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab. 2021, 33, 1466–1482.e7. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef]
- Yamashiro, K.; Kurita, N.; Urabe, T.; Hattori, N. Role of the Gut Microbiota in Stroke Pathogenesis and Potential Therapeutic Implications. Ann. Nutr. Metab. 2021, 77 (Suppl. 2), 36–44. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; D’Antongiovanni, V.; Antonioli, L.; Bernardini, N.; Derkinderen, P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol. Hepatol. 2023, 8, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiao, J.; Li, S.; Guo, Y.; Fu, R.; Hua, S.; Du, Y.; Xu, S. The interaction between intestinal microenvironment and stroke. CNS Neurosci. Ther. 2023, 29 (Suppl. 1), 185–199. [Google Scholar] [CrossRef]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Khan, R.; Di Gesù, C.M.; Lee, J.; McCullough, L.D. The contribution of age-related changes in the gut-brain axis to neurological disorders. Gut Microbes 2024, 16, 2302801. [Google Scholar] [CrossRef]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Kong, Y.; Ye, T.; Yu, Q.; Kumaran Satyanarayanan, S.; Su, K.P.; Liu, J. Microbiota-derived metabolite Indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav. Immun. 2022, 106, 76–88. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Zeng, M.; Peng, M.; Liang, J.; Sun, H. The Role of Gut Microbiota in Blood-Brain Barrier Disruption after Stroke. Mol. Neurobiol. 2023, 61, 9735–9755. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef]
- Hoyles, L.; Pontifex, M.G.; Rodriguez-Ramiro, I.; Anis-Alavi, M.A.; Jelane, K.S.; Snelling, T.; Solito, E.; Fonseca, S.; Carvalho, A.L.; Carding, S.R.; et al. Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 2021, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Knausenberger, T.B.; Shah, S.N.; Hoyles, L.; McArthur, S. A host-gut microbial amino acid co-metabolite, p-cresol glucuronide, promotes blood-brain barrier integrity in vivo. Tissue Barriers 2023, 11, 2073175. [Google Scholar] [CrossRef]
- Sun, N.; Hu, H.; Wang, F.; Li, L.; Zhu, W.; Shen, Y.; Xiu, J.; Xu, Q. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav. Immun. 2021, 92, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience 2021, 43, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.M.; Quan, W.; Zhou, Y.; Niu, G.Y.; Hong, H.; Wu, J.; Zhao, L.P.; Li, T.; Cui, C.; Zhao, W.J.; et al. Orally Induced High Serum Level of Trimethylamine N-oxide Worsened Glial Reaction and Neuroinflammation on MPTP-Induced Acute Parkinson’s Disease Model Mice. Mol. Neurobiol. 2023, 60, 5137–5154. [Google Scholar] [CrossRef]
- Quan, W.; Qiao, C.M.; Niu, G.Y.; Wu, J.; Zhao, L.P.; Cui, C.; Zhao, W.J.; Shen, Y.Q. Trimethylamine N-Oxide Exacerbates Neuroinflammation and Motor Dysfunction in an Acute MPTP Mice Model of Parkinson’s Disease. Brain Sci. 2023, 13, 790. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Deng, Y.; Zou, J.; Hong, Y.; Peng, Q.; Fu, X.; Duan, R.; Chen, J.; Chen, X. Higher Circulating Trimethylamine N-Oxide Aggravates Cognitive Impairment Probably via Downregulating Hippocampal SIRT1 in Vascular Dementia Rats. Cells 2022, 11, 3650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, J.; Sun, Y.; Xu, P.; Liu, J.L.; Sun, S.; Zhu, B.; Wu, H. Dietary choline metabolite TMAO impairs cognitive function and induces hippocampal synaptic plasticity declining through the mTOR/P70S6K/4EBP1 pathway. Food Funct. 2023, 14, 2881–2895. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pinky, P.D.; Steinke, I.; Bloemer, J.; Ramesh, S.; Kariharan, T.; Rella, R.T.; Bhattacharya, S.; Dhanasekaran, M.; Suppiramaniam, V.; et al. Gut Metabolite TMAO Induces Synaptic Plasticity Deficits by Promoting Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2020, 13, 138. [Google Scholar] [CrossRef]
- Tan, B.Y.Q.; Paliwal, P.R.; Sharma, V.K. Gut Microbiota and Stroke. Ann. Indian Acad. Neurol. 2020, 23, 155–158. [Google Scholar] [CrossRef]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; Katzke, V.; Kuhn, T.; Boeing, H.; et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: A prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef]
- Chiu, T.H.T.; Chang, H.R.; Wang, L.Y.; Chang, C.C.; Lin, M.N.; Lin, C.L. Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 2020, 94, e1112–e1121. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Rexrode, K.M. Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef] [PubMed]
- El Masri, J.; Finge, H.; Baroud, T.; Ajaj, N.; Houmani, M.; Ghazi, M.; Younes, M.; Salameh, P.; Hosseini, H. Adherence to Dietary Approaches to Stop Hypertension (DASH) Diet as a Protective Factor for Ischemic Stroke and Its Influence on Disability Level: A Case-Control Study in Lebanon. Nutrients 2024, 16, 3179. [Google Scholar] [CrossRef]
- Challa, H.J.; Ameer, M.A.; Uppaluri, K.R. DASH Diet to Stop Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ibsen, D.B.; Christiansen, A.H.; Olsen, A.; Tjonneland, A.; Overvad, K.; Wolk, A.; Mortensen, J.K.; Dahm, C.C. Adherence to the EAT-Lancet Diet and Risk of Stroke and Stroke Subtypes: A Cohort Study. Stroke 2022, 53, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell. Neurosci. 2021, 15, 722028. [Google Scholar] [CrossRef]
- Monda, A.; La Torre, M.E.; Messina, A.; Di Maio, G.; Monda, V.; Moscatelli, F.; De Stefano, M.; La Marra, M.; Padova, M.D.; Dipace, A.; et al. Exploring the ketogenic diet’s potential in reducing neuroinflammation and modulating immune responses. Front. Immunol. 2024, 15, 1425816. [Google Scholar] [CrossRef]
- Morris, G.; Puri, B.K.; Maes, M.; Olive, L.; Berk, M.; Carvalho, A.F. The role of microglia in neuroprogressive disorders: Mechanisms and possible neurotherapeutic effects of induced ketosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109858. [Google Scholar] [CrossRef]
- Smyth, A.; Hankey, G.J.; Langhorne, P.; Reddin, C.; Ryglewicz, D.; Rosengren, A.; Xavier, D.; Canavan, M.; Oveisgharan, S.; Wang, X.; et al. Tea and coffee consumption and risk of acute stroke: The INTERSTROKE Study. Int. J. Stroke 2024, 19, 1053–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef]
- Smyth, A.; Hankey, G.J.; Damasceno, A.; Iversen, H.K.; Oveisgharan, S.; Alhussain, F.; Langhorne, P.; Xavier, D.; Jaramillo, P.L.; Oguz, A.; et al. Carbonated Beverage, Fruit Drink, and Water Consumption and Risk of Acute Stroke: The INTERSTROKE Case-Control Study. J. Stroke 2024, 26, 391–402. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Y.; Zeng, Z.; Jin, C.; Wu, S.; Wang, Y.; Fu, Z. From the Cover: Exposure to Oral Antibiotics Induces Gut Microbiota Dysbiosis Associated with Lipid Metabolism Dysfunction and Low-Grade Inflammation in Mice. Toxicol. Sci. 2016, 154, 140–152. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef] [PubMed]
- Winek, K.; Engel, O.; Koduah, P.; Heimesaat, M.M.; Fischer, A.; Bereswill, S.; Dames, C.; Kershaw, O.; Gruber, A.D.; Curato, C.; et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke 2016, 47, 1354–1363. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Cannavale, C.N.; Bailey, M.A.; Khan, N.A.; Holscher, H.D. Prebiotic Consumption Alters Microbiota but Not Biological Markers of Stress and Inflammation or Mental Health Symptoms in Healthy Adults: A Randomized, Controlled, Crossover Trial. J. Nutr. 2023, 153, 1283–1296. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis. 2020, 11, 1029–1045. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Hou, Y.; Song, X.; Yu, H.; Tan, J.; Zhou, Y.; Zhang, H.T. β-Glucan attenuates cognitive impairment of APP/PS1 mice via regulating intestinal flora and its metabolites. CNS Neurosci. Ther. 2023, 29, 1690–1704. [Google Scholar] [CrossRef]
- Zou, X.; Wang, L.; Xiao, L.; Wang, S.; Zhang, L. Gut microbes in cerebrovascular diseases: Gut flora imbalance, potential impact mechanisms and promising treatment strategies. Front. Immunol. 2022, 13, 975921. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, K.E.; Kim, J.K.; Kim, D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Gao, X.; Peng, Y.; Wu, Q.; Zhu, J.; Tan, C.; Xia, G.; You, C.; Xu, R.; Pan, S.; et al. Higher Risk of Stroke Is Correlated With Increased Opportunistic Pathogen Load and Reduced Levels of Butyrate-Producing Bacteria in the Gut. Front. Cell. Infect. Microbiol. 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Yuan, X.; Yang, J.; Li, K. Effect of early enteral nutrition combined with probiotics in patients with stroke: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2022, 76, 592–603. [Google Scholar] [CrossRef]
- Guha, D.; Banerjee, A.; Mukherjee, R.; Pradhan, B.; Peneva, M.; Aleksandrov, G.; Suklabaidya, S.; Senapati, S.; Aich, P. A probiotic formulation containing Lactobacillus bulgaricus DWT1 inhibits tumor growth by activating pro-inflammatory responses in macrophages. J. Funct. Foods 2019, 56, 232–245. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.C.; Du, J.; Zhang, L.M.; Yin, H.H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit. Care 2011, 15, R290. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Fisk, H.L.; Wootton, M.; Lown, M.; Owen-Jones, E.; Lau, M.; Lowe, R.; Hood, K.; Gillespie, D.; Hobbs, F.D.R.; et al. Combination of the Probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis, BB-12 Has Limited Effect on Biomarkers of Immunity and Inflammation in Older People Resident in Care Homes: Results From the Probiotics to Reduce Infections iN CarE home reSidentS Randomized, Controlled Trial. Front. Immunol. 2021, 12, 643321. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiotics Health 2017, 5, 159. [Google Scholar] [CrossRef]
- Wade, H.; Pan, K.; Duan, Q.; Kaluzny, S.; Pandey, E.; Fatumoju, L.; Saraswathi, V.; Wu, R.; Harris, E.N.; Su, Q. Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145. J. Biomed. Sci. 2023, 30, 38. [Google Scholar] [CrossRef]
- He, K.Y.; Lei, X.Y.; Wu, D.H.; Zhang, L.; Li, J.Q.; Li, Q.T.; Yin, W.T.; Zhao, Z.L.; Liu, H.; Xiang, X.Y.; et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef]
- Mancini, N.L.; Rajeev, S.; Jayme, T.S.; Wang, A.; Keita, Å.V.; Workentine, M.L.; Hamed, S.; Söderholm, J.D.; Lopes, F.; Shutt, T.E.; et al. Crohn’s Disease Pathobiont Adherent-Invasive E coli Disrupts Epithelial Mitochondrial Networks With Implications for Gut Permeability. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 551–571. [Google Scholar] [CrossRef]
- Bustamante, P.; Ramos-Corominas, M.N.; Martinez-Medina, M. Contribution of Toxin-Antitoxin Systems to Adherent-Invasive E. coli Pathogenesis. Microorganisms 2024, 12, 1158. [Google Scholar] [CrossRef]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, e002699. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, Y.; Kan, P.; Cai, Y.; Wang, Z.; Wu, Z.; Yang, P. Analysis of intestinal microbial communities of cerebral infarction and ischemia patients based on high throughput sequencing technology and glucose and lipid metabolism. Mol. Med. Rep. 2017, 16, 5413–5417. [Google Scholar] [CrossRef]
- Xu, N.; Kan, P.; Yao, X.; Yang, P.; Wang, J.; Xiang, L.; Zhu, Y. Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J. Microbiol. 2018, 56, 838–846. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, K.; Zhou, H. Characteristics of gut virome and microbiome in patients with stroke. Nan Fang. Yi Ke Da Xue Xue Bao 2021, 41, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]

| Cell Types | Mechanisms | |
|---|---|---|
| Microglia | Pro-inflammation |
|
| Anti-inflammation |
| |
| Astrocyte | Pro-inflammation |
|
| Anti-inflammation |
| |
| Dendritic cell | Pro-inflammation |
|
| Anti-inflammation |
| |
| Other immune components | Pro-inflammation |
|
| Anti-inflammation |
| |
| Parameter | Core Infarction | Penumbra Region | References | |
|---|---|---|---|---|
| Pathology | Irreversible | Reversible | [46] | |
| Neuroimaging | MR | 1. DWI: Hyperintense signal (ADC < 620 × 10−6 mm2/s) 2. FLAIR: hyperintense > 24 h | 1. PWI-DWI mismatch: a mismatch volume on PWI and DWI lesion (PWI-Tmax > 6 s) 2. FLAIR: Negative | [22] [23] |
| CT | 1. CBV < 40%; CBF < 30% 2. Tmax: No flow (unmeasurable) | 1. CBF-CBV mismatch (CBF < 30%; CBV normal/mildly↑) 2. Tmax > 6 s | [24] | |
| Neuro- inflammatory Biomarkers | ROS | Severe ↑ (Nox2-driven, persistent) | Moderate↑ (transient, Nrf2-inducible) | [45,46] [55] |
| Cytokines | TNF-α↑↑ IL-1β↑↑ (dominant cytokines) | IL-10↑, TGF-β↑ (mixed kinds of both destructive and protective cytokines) | [30] | |
| Microglia | M1-polarized (pro-inflammatory) | M2-polarized (anti-inflammatory) | [30,31] | |
| Cell Death | Necroptosis | Apoptosis | [46] | |
| Clinical Implications | High risk with reperfusion therapy (hemorrhagic transformation) | Primary target for reperfusion therapy (thrombectomy/thrombolysis) | [22,23,24,43] | |
| Outcomes | Functional Loss | Neuro/angiogenesis | [46] | |
| Researchers | Year | Methods | Study Subjects | Key Findings | References |
|---|---|---|---|---|---|
| Tammy Y N Tong | 2020 | Personal habitual intake questionnaires | N = 418,329 European Perspective Investigation into Cancer and Nutrition Study | Higher intake of fruits, vegetables, fiber, and dairy products was associated with a reduced risk of ischemic stroke | [181] |
| Tina H T Chiu | 2020 | Food frequency questionnaires (Vegetarian status was defined by avoidance of meat and fish) | N = 13,352 1. Tzu Chi Health Study (N = 5050) 2. Tzu Chi Vegetarian Study (N = 8302) | 1. Taiwanese vegetarian diet was associated with a lower risk of stroke 2. Vitamin B12 intake may modify the association between vegetarian diet and stroke | [182] |
| Megu Y Baden | 2021 | Plant-based diet index (PDI) Healthy PDI: nutritionally rich plant foods (fruit juices, refined grains) Unhealthful PDI: less nutritious plant foods (fries, sugary drinks) | N = 209,508 1. Nurses’ Health Study (NHS) 2. Health Professionals Follow-Up Study | Lower risk of total stroke was observed by those who adhered to a healthful plant-based diet | [183] |
| Yuan Zhang | 2021 | Questionnaires and interviews | N = 365,682 UK Biobank | 1. Consumption of coffee and tea either separately or together was associated with reduced risks of stroke and dementia 2. Drinking coffee alone or combined with tea was also linked to lower risk of post-stroke dementia. | [192] |
| Daniel B Ibsen | 2022 | Food frequency questionnaires Assessments: EAT-Lancet diet score Alternative Healthy Eating Index-2010 | N = 55,016 Danish Diet, Cancer and Health Study | 1. Adherence to the EAT-Lancet diet in midlife was associated with a lower risk of subarachnoid stroke 2. AHEI (Alternate health eating index-2010) was associated with a lower risk of total stroke, mainly ischemic stroke and intracerebral hemorrhage | [186] |
| Andrew Smyth | 2024 | Beverage intake groups None, 1 to 2 cups/day, 3 to 4 cups/day, or > 4 cups/day for each beverage | N = 26,950 INTERSTROKE (a large international matched case–control study of first stroke from 32 countries) | 1. High coffee consumption was associated with higher odds of ischemic stroke 2. Tea consumption was associated with lower odds of ischemic stroke | [191] |
| Jad El Masri | 2024 | DASH diet Adherence assessment: DASH diet index (ranging from 0 (lowest) to 11 (highest)) | N = 428 A case-control study in Lebanese people in 2023 Ischemic cases: 214 Health controls: 214 | The DASHA diet was protective against such strokes and associated with less disability | [184] |
| Martin J O’Donnell | 2024 | Food frequency questionnaires | N = 6000 INTERSTROKE | 1. Carbonated beverages were linked to higher odds of stroke 2. High water intake correlated with lower stroke odds, with notable regional variations | [193] |
| Interventions | Study Subjects | Curative Effects | |
|---|---|---|---|
| Antibiotics | Broad-spectrum antibiotic | C57BL/6J mice [197] | Broad-spectrum antibiotic reduced gut microbial populations. It did not affect brain damage within the first day post-stroke, but may suppress systemic immunity, leading to increased mortality between days 5 and 7 following stroke. |
| Amoxicillin | C57BL/6J mice [48] | Gut bacterial-primed intestinal dendritic cells drive local Treg expansion, which subsequently suppresses pro-inflammatory IL-17+ γδ T cell responses. | |
| Probiotics | Bifidobacterium longum +Lactobacillus bulgaricus +Streptococcus thermophilus | Human [211] | Daily prophylactic administration of probiotics could attenuate the deviated Th1/Th2 response induce by traumatic brain injury. |
| Bidobacterium longum NK46 | 5xFAD mice [205] | 1. Inhibited LPS-induced NF-κB activation in the colon and hippocampus; 2. Increased brain-derived neurotrophic factor expression. | |
| Lactobacillus salivarius | 5xFAD mice [206] | 1.Reduced gut leakage; Reduced the levels of IL-6 in the brain; 2. Reduced oxidative stress in the cortex and the hippocampus | |
| Prebiotics | FOS+GOS | Human [200] | 1. FOS+GOS did not affect biological markers of stress and inflammation or mental health symptoms in healthy adults; 2. FOS+GOS increased Bifidobacterium. |
| Mannan oligosaccharide (MOS) | 5xFAD mice [201] | 1. Increased the relative abundance of butyrate-producing bacteria; 2. Increased the level of butyrate in feces and serum; 3. Reduced serum LPS level and oxidative stress in the brain. | |
| Xylooligosaccharides (XOS) | APP/PS1 mice [202] | 1. Restored the integrity of intestinal barrier and BBB via increased expression of tight junction proteins in intestine; 2. Reduced the expression of pro-inflammatory cytokines (IL-1β and IL-6) and immunosuppressive cytokine (IL-10) in colon and hippocampus. | |
| β-Glucan | APP/PS1 mice [203] | 1. Increased the levels of SCFAs (propionate, butyrate, and valerate) in colon; 2. Reduced microglial and astrocytic activation in hippocampus; 3. Reduced the expression of pro-inflammatory cytokines (IL-6 and IL-1β), NF-κB and NLRP3 in hippocampus and cerebral cortex. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Tang, X.; He, X.; Weng, Y.; Zhang, Q.; Fang, Q.; Zhang, L. A Comprehensive Review of the Role of the Microbiota–Gut–Brain Axis via Neuroinflammation: Advances and Therapeutic Implications for Ischemic Stroke. Biomolecules 2025, 15, 920. https://doi.org/10.3390/biom15070920
Guo H, Tang X, He X, Weng Y, Zhang Q, Fang Q, Zhang L. A Comprehensive Review of the Role of the Microbiota–Gut–Brain Axis via Neuroinflammation: Advances and Therapeutic Implications for Ischemic Stroke. Biomolecules. 2025; 15(7):920. https://doi.org/10.3390/biom15070920
Chicago/Turabian StyleGuo, Hui, Xiang Tang, Xinyi He, Yizhen Weng, Quanquan Zhang, Qi Fang, and Lulu Zhang. 2025. "A Comprehensive Review of the Role of the Microbiota–Gut–Brain Axis via Neuroinflammation: Advances and Therapeutic Implications for Ischemic Stroke" Biomolecules 15, no. 7: 920. https://doi.org/10.3390/biom15070920
APA StyleGuo, H., Tang, X., He, X., Weng, Y., Zhang, Q., Fang, Q., & Zhang, L. (2025). A Comprehensive Review of the Role of the Microbiota–Gut–Brain Axis via Neuroinflammation: Advances and Therapeutic Implications for Ischemic Stroke. Biomolecules, 15(7), 920. https://doi.org/10.3390/biom15070920





