The Role of Macrophage-Derived Netrin-1 in Inflammatory Diseases
Abstract
1. Introduction
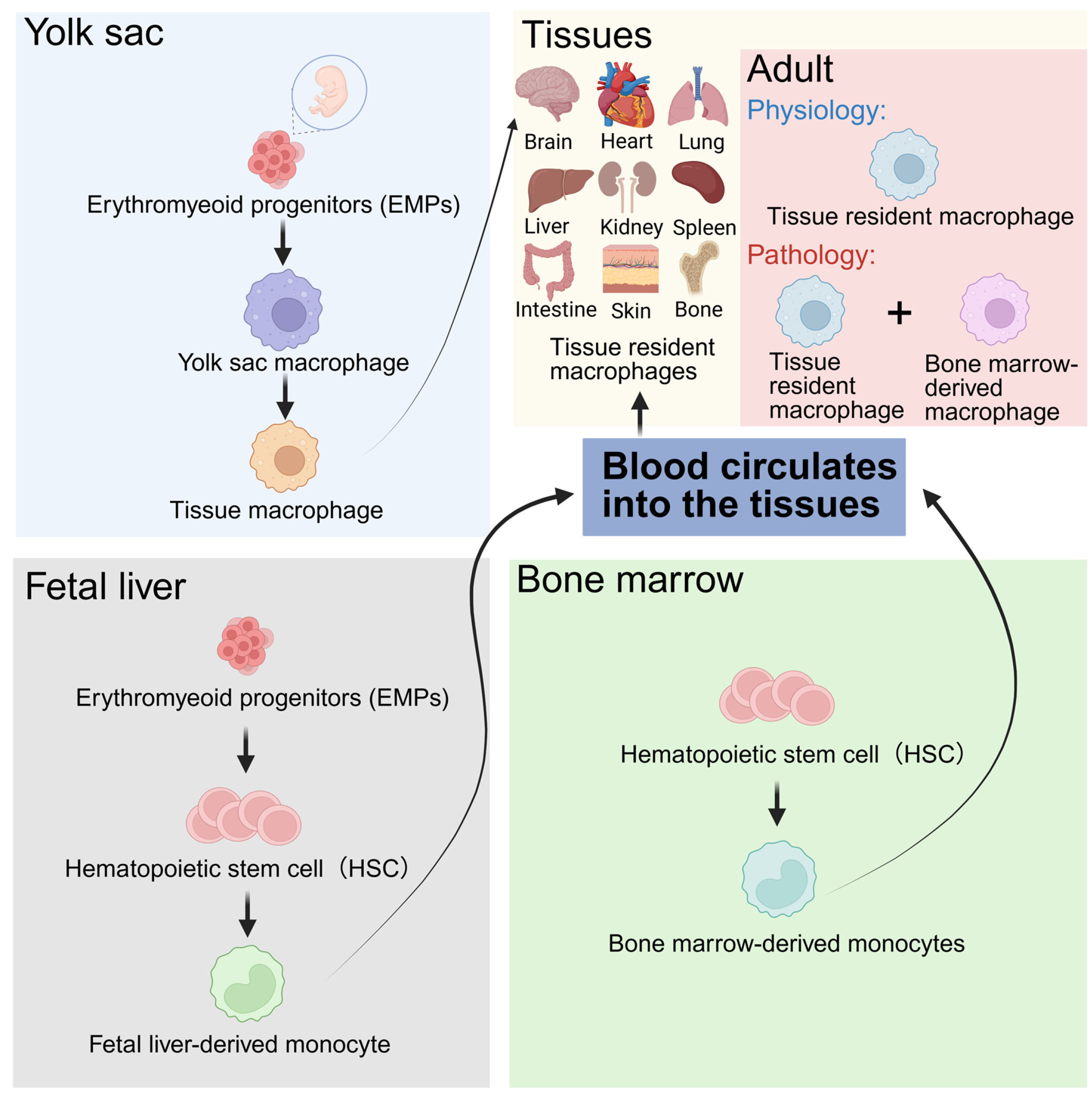
2. Origin, Polarization, and Function of Macrophages
| Phenotypes | Markers | Cytokines and Chemokines | Functions |
|---|---|---|---|
| M1 | CD80, CD86, CD68, MHCⅡ, TLR2/4, IL-1R, CD163 lo*, and CD40 hi* | TNF-α, IL-1α, IL-1β, IL-6, IL-12, IL-23, COX-2, and iNOS | Promote the Th1 response and the inflammatory response |
| M2a | CD206 hi, IL-1Rα, IL-1RⅡ, CD163 lo, HLA-DR hi, Arg-1, FIZZ1, and Ym1/2 | IL-10, TGF-β, CCL17/18, and CXCL13 | Anti-inflammatory and maintain tissue homeostasis |
| M2b | CD163 lo, HLA-DR lo, and CD86 | IL-10 hi, IL-12 lo, IL-1β, IL-6, and TNF-α | Promote the Th2 response |
| M2c | CD86 lo, CD163 hi, TLR1/8, and MerTK | IL-10, TGF-β, CCL16/18, and CXCL13 | Phagocyte apoptotic cells |
| M2d | CD86 lo, CD163 hi, VEGF, IL-10, and TGF-β | VEGF, IL-10 hi, and IL-12 lo | Angiogenesis and tumor progression |
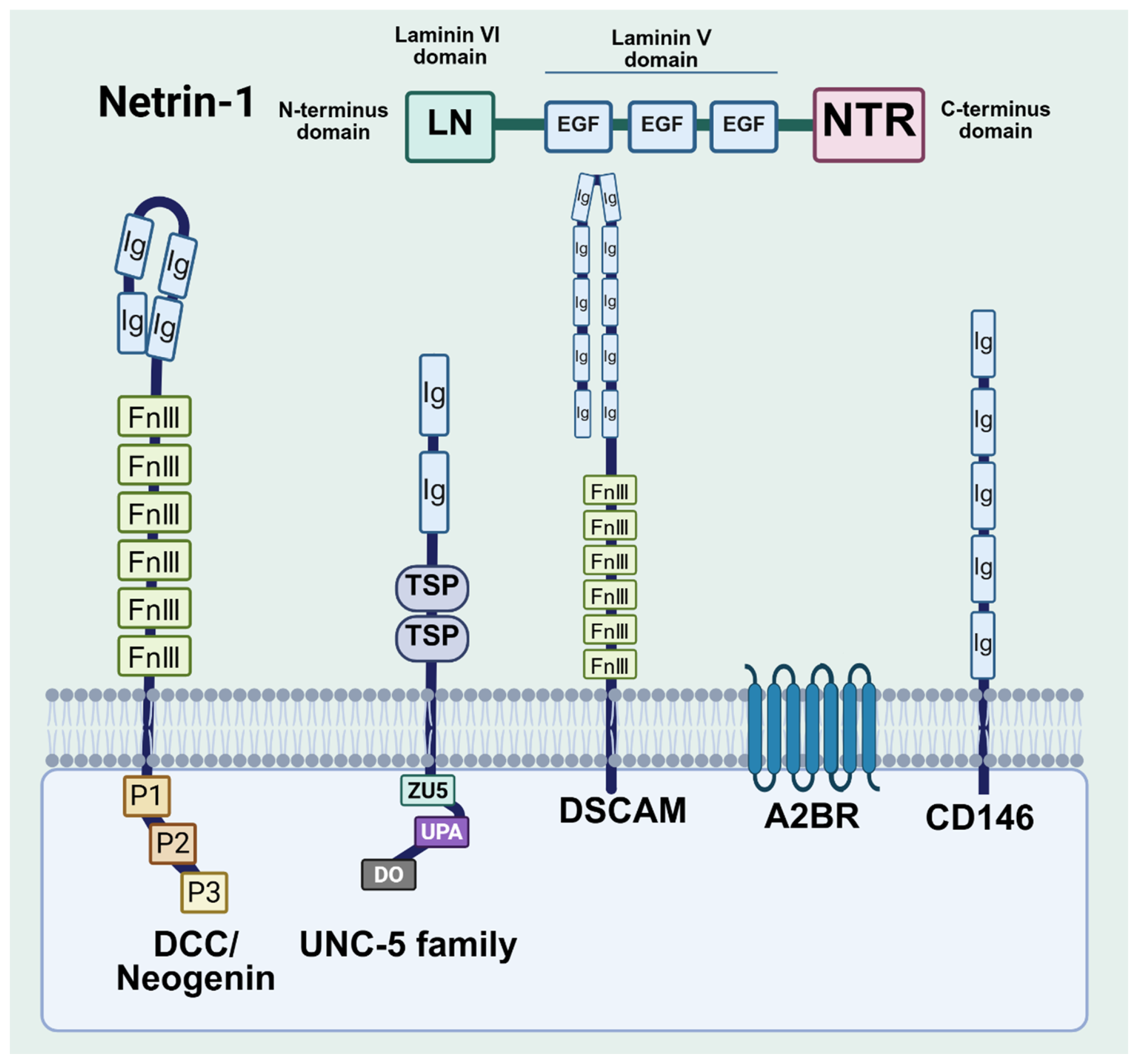
3. Structure of Netrin-1 and Its Receptors
4. Acute Inflammation
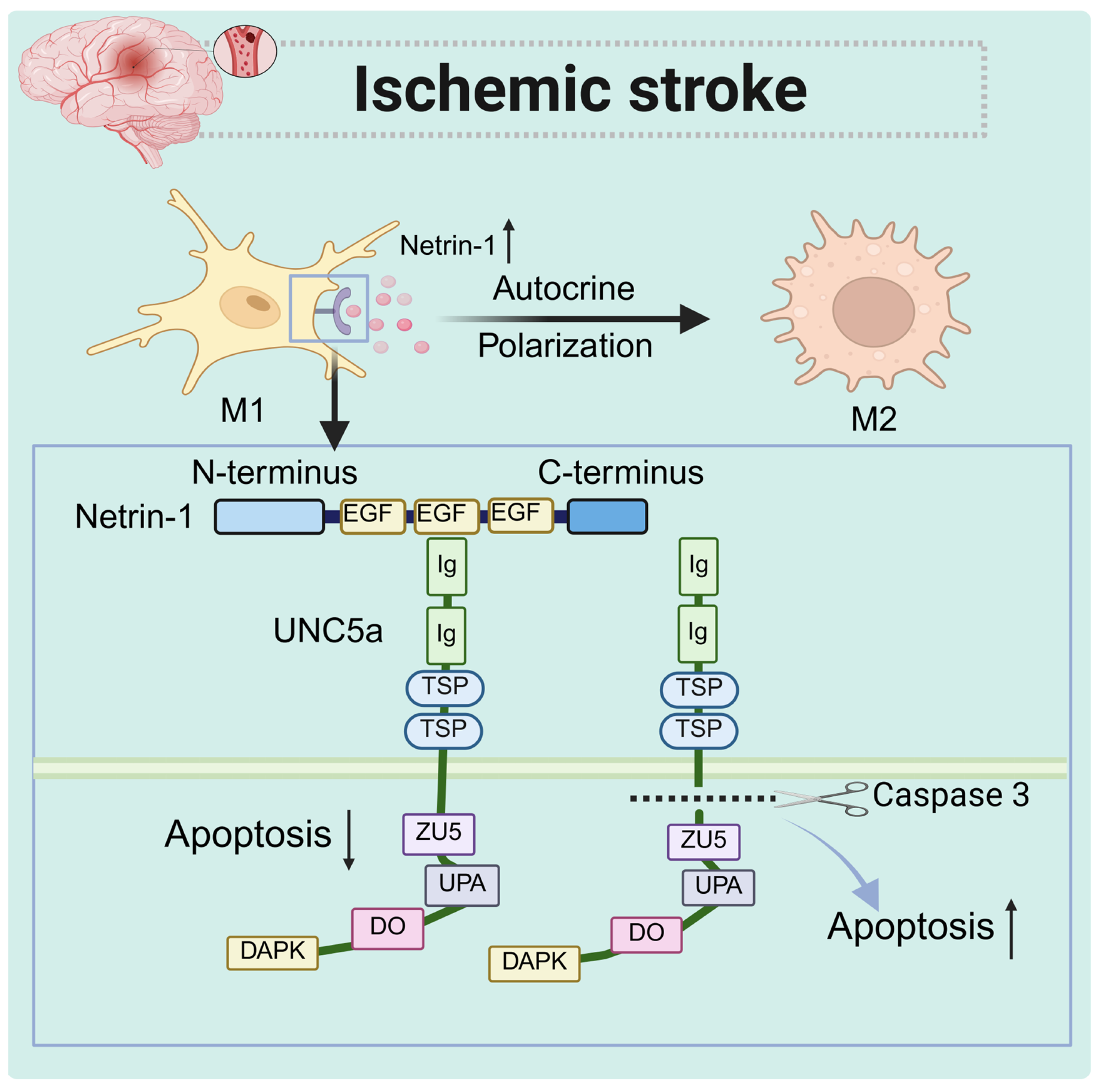
4.1. Acute Ischemic Stroke
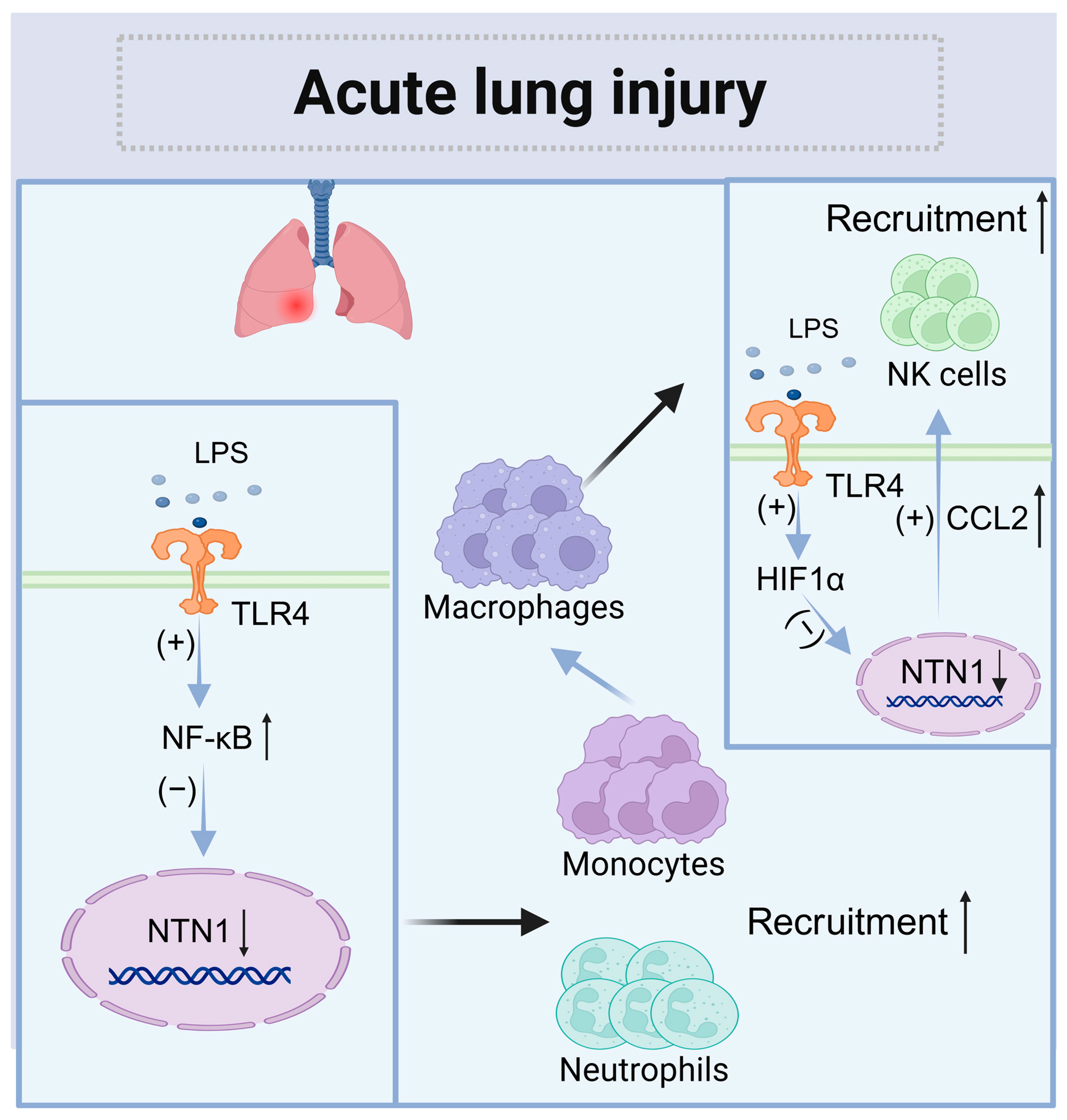
4.2. Acute Lung Injury (ALI)
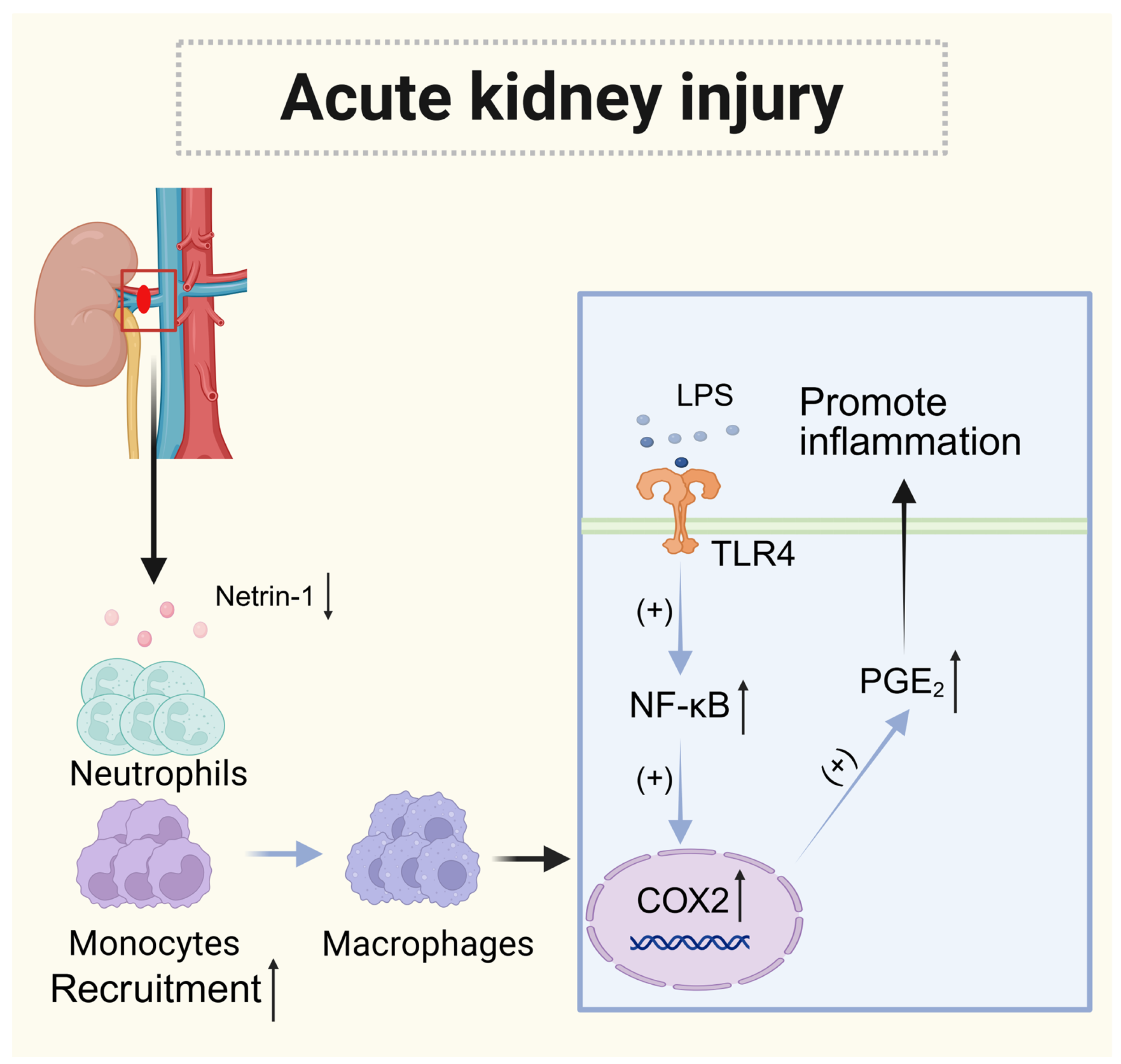
4.3. Acute Kidney Injury (AKI)
5. Chronic Inflammation
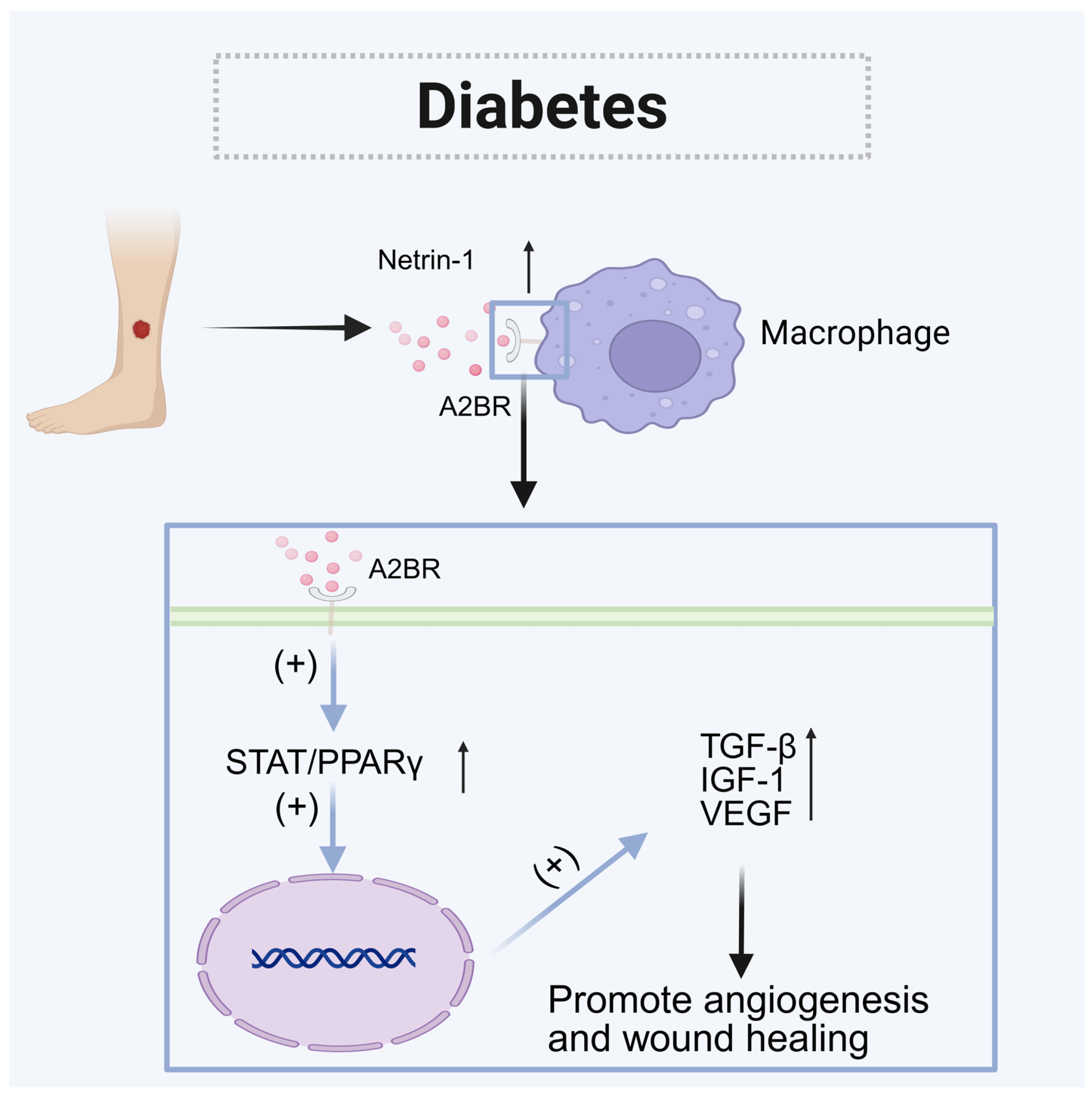
5.1. Diabetes
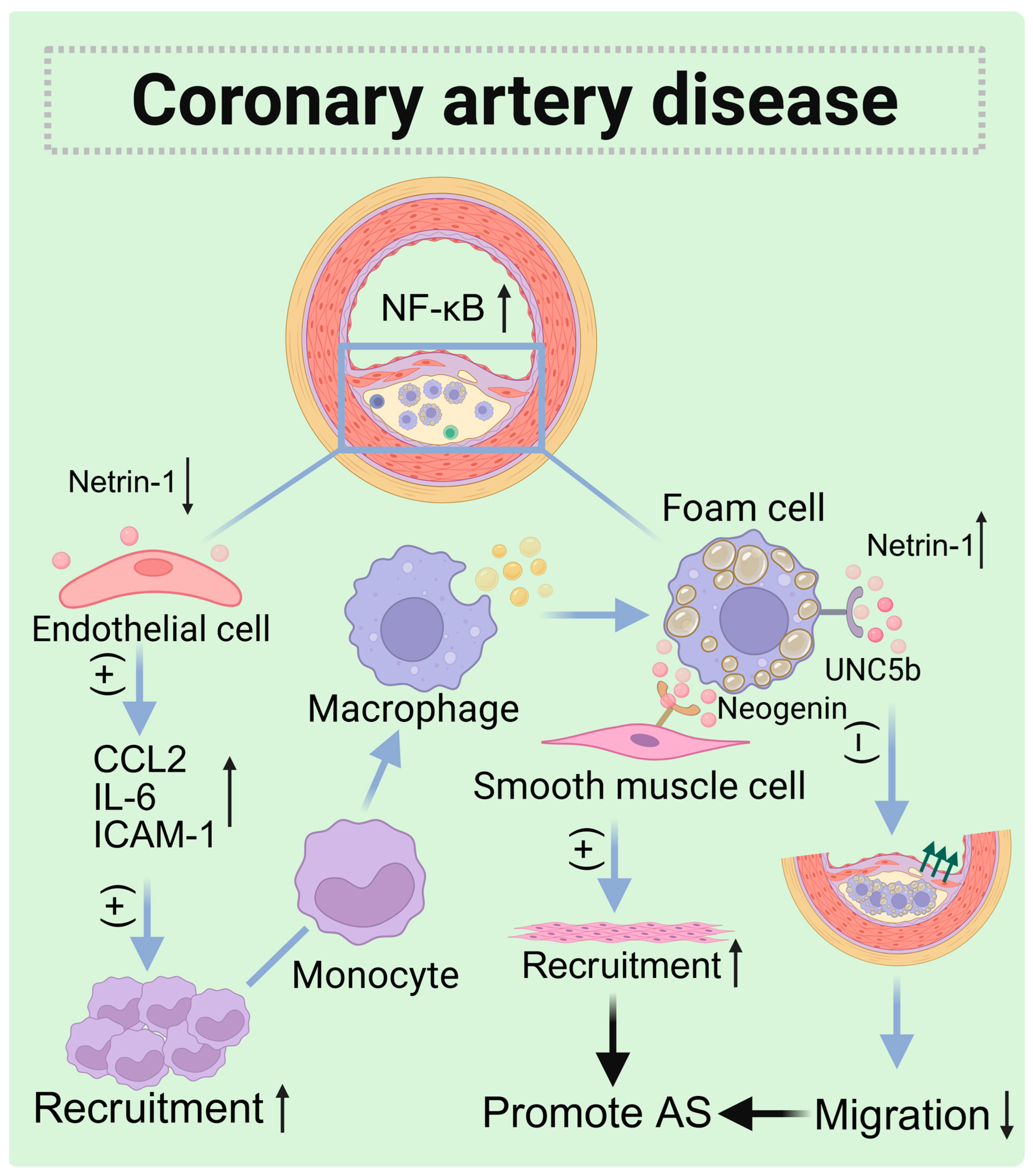
5.2. Coronary Artery Disease (CAD)
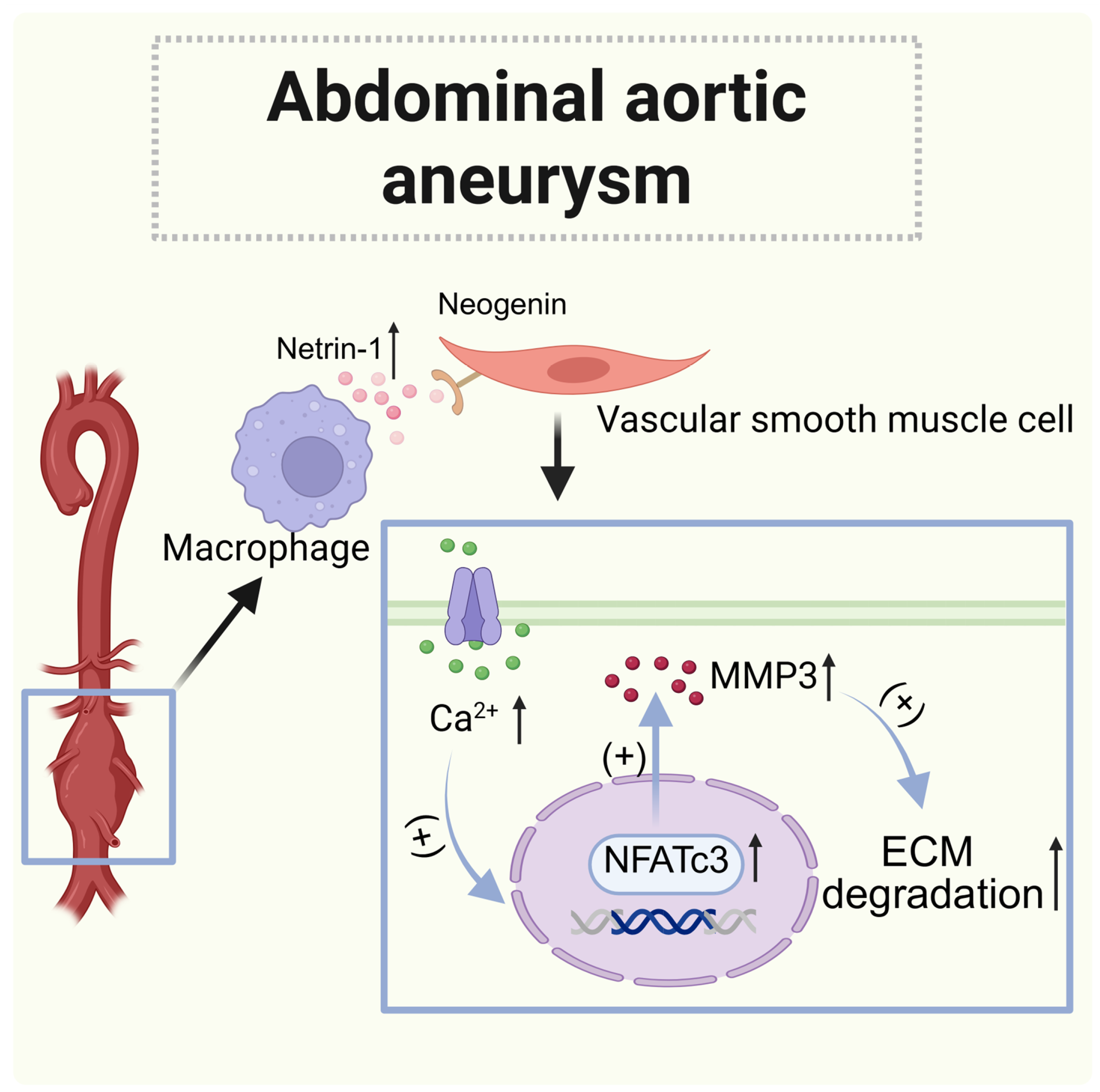
5.3. Abdominal Aortic Aneurysm (AAA)
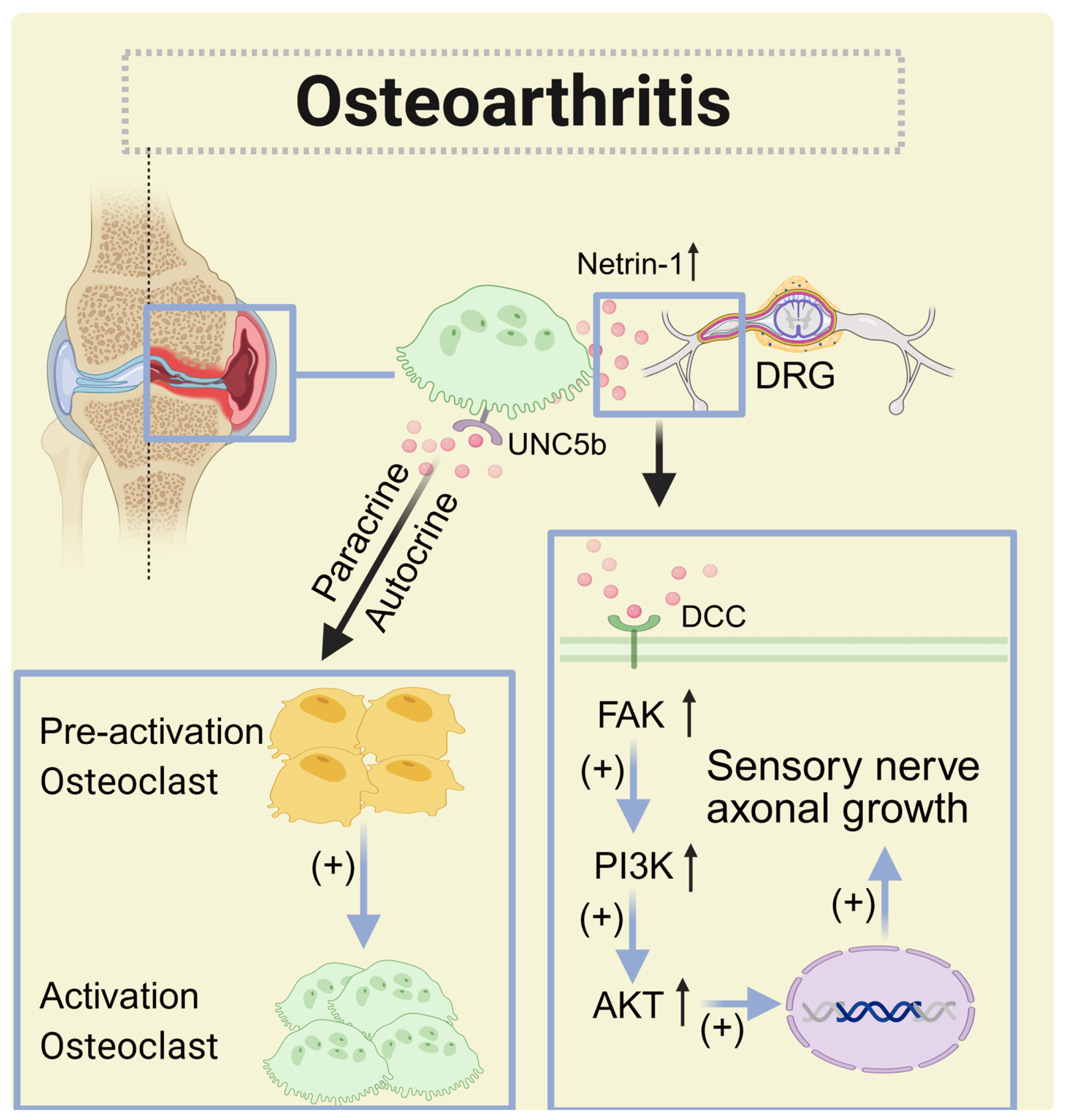
5.4. Osteoarthritis(OA)
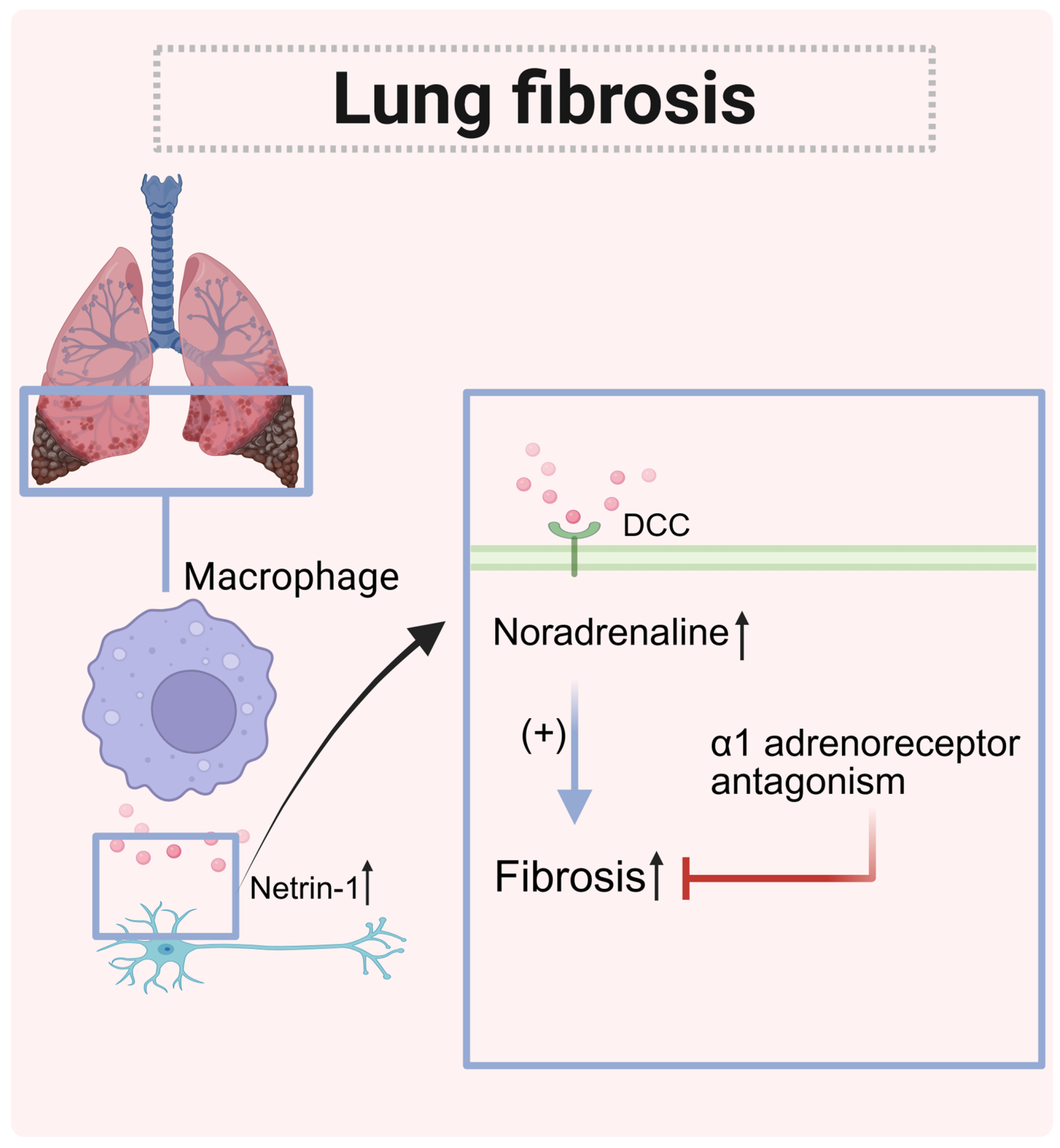
5.5. Pulmonary Fibrosis
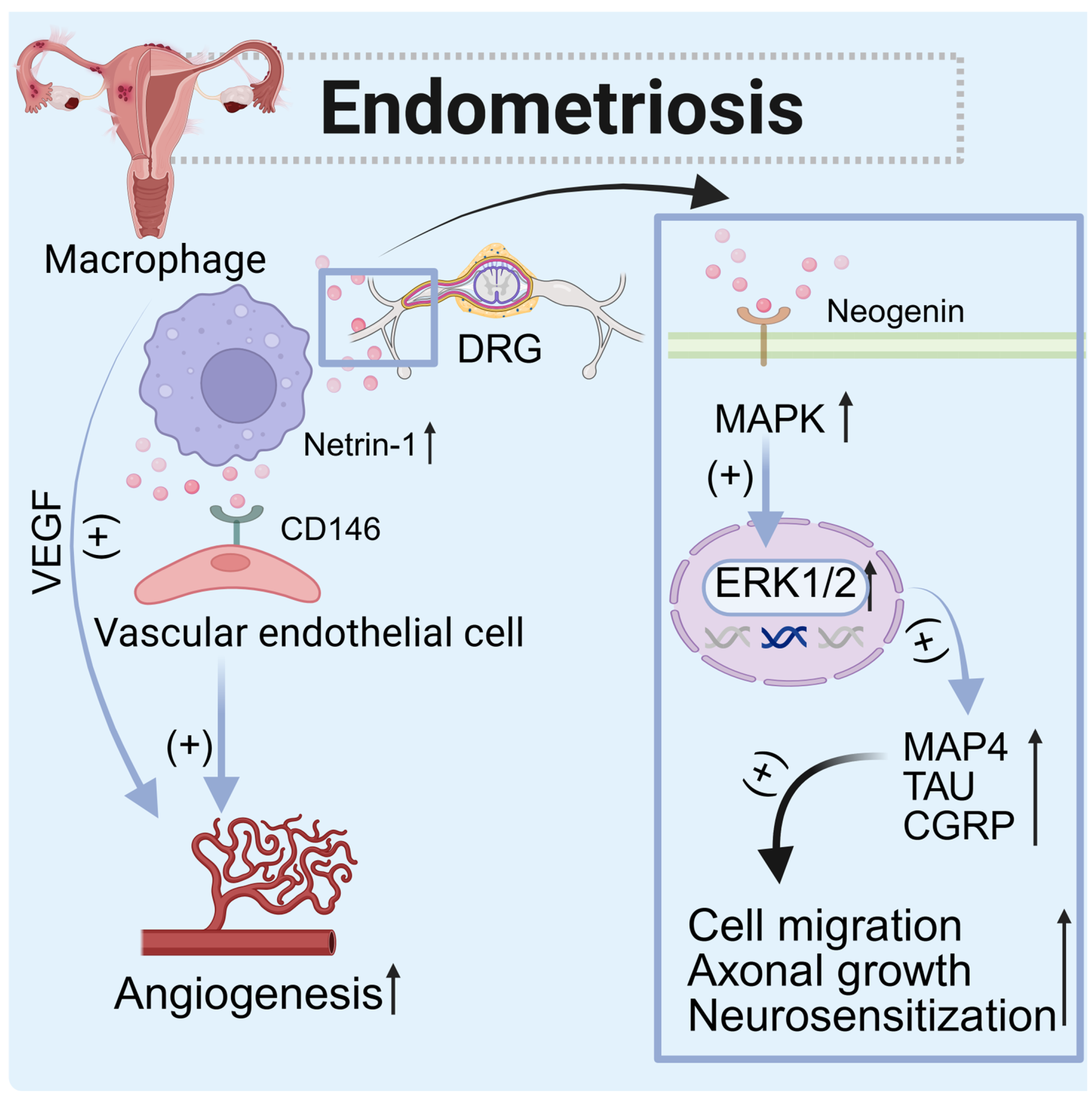
5.6. Endometriosis
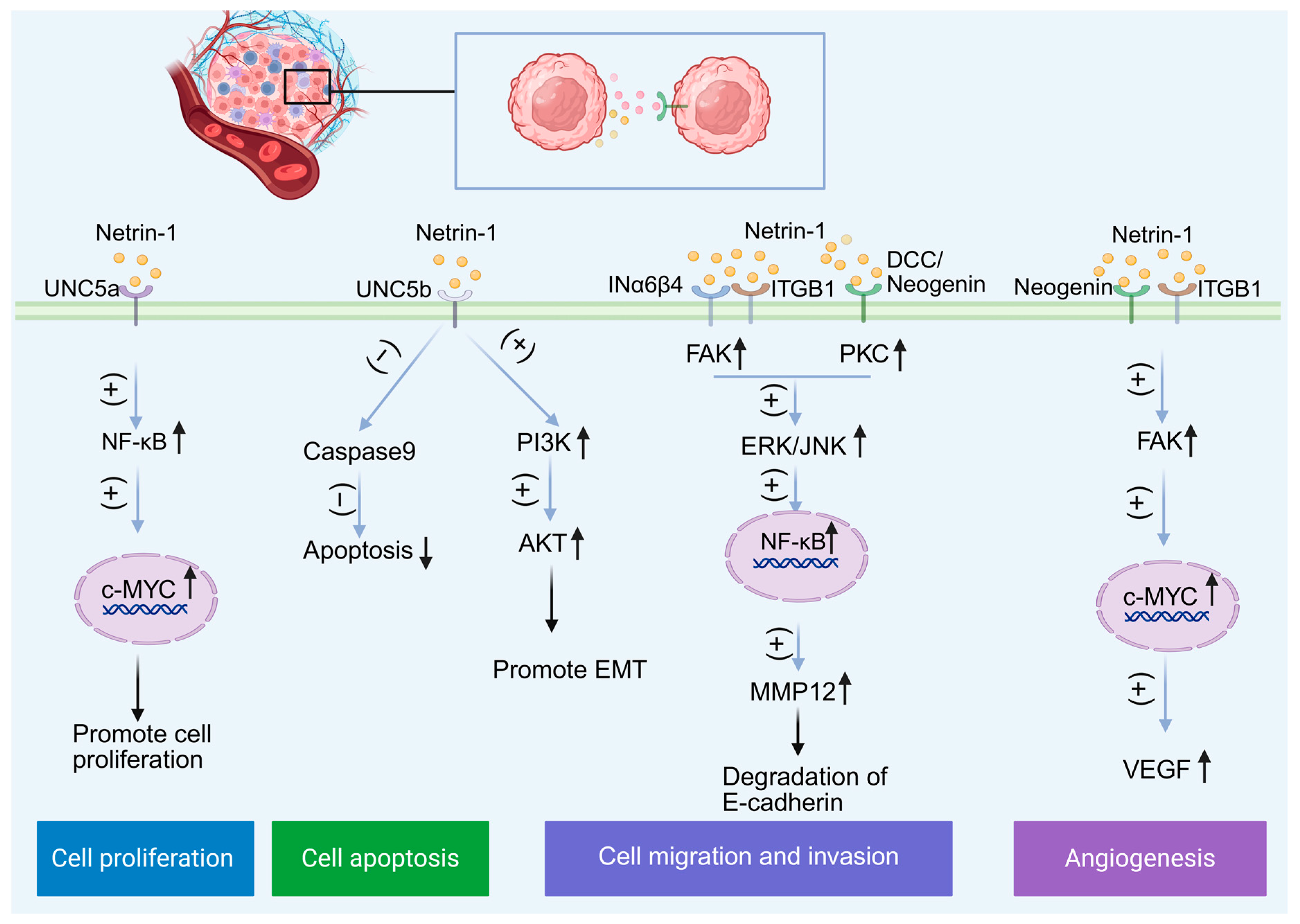
6. Cancer
6.1. Glioblastoma (GBM)
6.2. Non-Small Cell Lung Cancer (NSCLC)
6.3. Other Cancers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziegon, L.; Schlegel, M. Netrin-1: A Modulator of Macrophage Driven Acute and Chronic Inflammation. Int. J. Mol. Sci. 2021, 23, 275. [Google Scholar] [CrossRef]
- Honeycutt, S.E.; N’Guetta, P.Y.; Hardesty, D.M.; Xiong, Y.; Cooper, S.L.; Stevenson, M.J.; O’Brien, L.L. Netrin 1 directs vascular patterning and maturity in the developing kidney. Development 2023, 150, dev201886. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, H.; Ye, K.; Chen, G.; Zhang, Z. Netrin-1 signaling pathway mechanisms in neurodegenerative diseases. Neural Regen. Res. 2025, 20, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Navaridas, R.; Bellina, M.; Rama, N.; Ducarouge, B.; Hernandez-Vargas, H.; Delord, J.P.; Lengrand, J.; Paradisi, A.; Fattet, L.; et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 2023, 620, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Lengrand, J.; Pastushenko, I.; Vanuytven, S.; Song, Y.; Venet, D.; Sarate, R.M.; Bellina, M.; Moers, V.; Boinet, A.; Sifrim, A.; et al. Pharmacological targeting of netrin-1 inhibits EMT in cancer. Nature 2023, 620, 402–408. [Google Scholar] [CrossRef]
- Schlegel, M.; Sharma, M.; Brown, E.J.; Newman, A.A.C.; Cyr, Y.; Afonso, M.S.; Corr, E.M.; Koelwyn, G.J.; van Solingen, C.; Guzman, J.; et al. Silencing Myeloid Netrin-1 Induces Inflammation Resolution and Plaque Regression. Circ. Res. 2021, 129, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, J.; Li, S.; Yang, X.; Huo, N.; Chen, Q.; Wang, W.; Yang, N.; Wang, Y.; Zhou, N. Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair. Sci. Adv. 2024, 10, eadm8454. [Google Scholar] [CrossRef]
- Claro, V.; Ferro, A. Netrin-1: Focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc. Dis. 2020, 9, 2048004020959574. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Bian, Z.; Gong, Y.; Huang, T.; Lee, C.Z.W.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering human macrophage development at single-cell resolution. Nature 2020, 582, 571–576. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front. Cell Dev. Biol. 2020, 8, 617879. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, T.; Juarez-Carreño, S.; Cox, N.; Geissmann, F. Physiology and diseases of tissue-resident macrophages. Nature 2023, 618, 698–707. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut 2021, 70, 1383–1395. [Google Scholar] [CrossRef]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Guilliams, M.; Scott, C.L. Liver macrophages in health and disease. Immunity 2022, 55, 1515–1529. [Google Scholar] [CrossRef]
- Mehla, K.; Singh, P.K. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer 2019, 5, 822–834. [Google Scholar] [CrossRef]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.J.; Ramnath, D.; Singhal, A.; Kapetanovic, R. Inducible antibacterial responses in macrophages. Nat. Rev. Immunol. 2025, 25, 92–107. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Xian, C.; Chen, M.; Luo, D.; Zheng, J.; Zhao, S.; Li, X.G. Single-cell and bulk transcriptomics reveals M2d macrophages as a potential therapeutic strategy for mucosal healing in ulcerative colitis. Int. Immunopharmacol. 2023, 121, 110509. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Coulton, A.; Murai, J.; Qian, D.; Thakkar, K.; Lewis, C.E.; Litchfield, K. Using a pan-cancer atlas to investigate tumour associated macrophages as regulators of immunotherapy response. Nat. Commun. 2024, 15, 5665. [Google Scholar] [CrossRef]
- Anders, C.B.; Lawton, T.M.W.; Smith, H.L.; Garret, J.; Doucette, M.M.; Ammons, M.C.B. Use of integrated metabolomics, transcriptomics, and signal protein profile to characterize the effector function and associated metabotype of polarized macrophage phenotypes. J. Leukoc. Biol. 2022, 111, 667–693. [Google Scholar] [CrossRef]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef]
- Xia, X.; Hu, Z.; Wang, S.; Yin, K. Netrin-1: An emerging player in inflammatory diseases. Cytokine Growth Factor. Rev. 2022, 64, 46–56. [Google Scholar] [CrossRef]
- Gao, X.; Ye, J.; Huang, X.; Huang, S.; Luo, W.; Zeng, D.; Li, S.; Tang, M.; Mai, R.; Li, Y.; et al. Research progress of the netrins and their receptors in cancer. J. Cell. Mol. Med. 2024, 28, e18241. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.P.; Lee, J.; Li, W.; Guan, K.L. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J. Neurosci. 2004, 24, 10826–10834. [Google Scholar] [CrossRef]
- Finci, L.I.; Krüger, N.; Sun, X.; Zhang, J.; Chegkazi, M.; Wu, Y.; Schenk, G.; Mertens, H.D.T.; Svergun, D.I.; Zhang, Y.; et al. The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron 2014, 83, 839–849. [Google Scholar] [CrossRef]
- Xu, K.; Wu, Z.; Renier, N.; Antipenko, A.; Tzvetkova-Robev, D.; Xu, Y.; Minchenko, M.; Nardi-Dei, V.; Rajashankar, K.R.; Himanen, J.; et al. Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science 2014, 344, 1275–1279. [Google Scholar] [CrossRef]
- Grandin, M.; Meier, M.; Delcros, J.G.; Nikodemus, D.; Reuten, R.; Patel, T.R.; Goldschneider, D.; Orriss, G.; Krahn, N.; Boussouar, A.; et al. Structural Decoding of the Netrin-1/UNC5 Interaction and its Therapeutical Implications in Cancers. Cancer Cell 2016, 29, 173–185. [Google Scholar] [CrossRef]
- Bányai, L.; Patthy, L. The NTR module: Domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 1999, 8, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Untiveros, G.; Raskind, A.; Linares, L.; Dotti, A.; Strizzi, L. Netrin-1 Stimulates Migration of Neogenin Expressing Aggressive Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 12751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, X.; Zhou, X.H.; Liu, J.H.; Wu, J.; Zhang, Y.; Wang, J.H. N-terminal horseshoe conformation of DCC is functionally required for axon guidance and might be shared by other neural receptors. J. Cell Sci. 2013, 126, 186–195. [Google Scholar] [CrossRef]
- Finci, L.; Zhang, Y.; Meijers, R.; Wang, J.H. Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Prog. Biophys. Mol. Biol. 2015, 118, 153–160. [Google Scholar] [CrossRef]
- Kazuko Keino-Masu, M.M.; Lindsay Hinck, E.D.L.; Shirley, S.-Y.; Chan, J.G.C.; Tessier-Lavigne, A.M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 1996, 87, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Zou, Y.; Poo, M.; Tessier-Lavigne, M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science 2001, 291, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Han, W.; Zhou, L.; Gao, J.; Zhao, J.; Song, N.; Hu, B.; Yao, Q.; Liu, Y.; Xu, D.; et al. An imbalance of netrin-1 and DCC during nigral degeneration in experimental models and patients with Parkinson’s disease. CNS Neurosci. Ther. 2023, 29, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Schlienger, S.; Yam, P.T.; Balekoglu, N.; Ducuing, H.; Michaud, J.F.; Makihara, S.; Kramer, D.K.; Chen, B.; Fasano, A.; Berardelli, A.; et al. Genetics of mirror movements identifies a multifunctional complex required for Netrin-1 guidance and lateralization of motor control. Sci. Adv. 2023, 9, eadd5501. [Google Scholar] [CrossRef]
- Hong, K.; Nishiyama, M.; Henley, J.; Tessier-Lavigne, M.; Poo, M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 2000, 403, 93–98. [Google Scholar] [CrossRef]
- Liu, G.; Beggs, H.; Jürgensen, C.; Park, H.T.; Tang, H.; Gorski, J.; Jones, K.R.; Reichardt, L.F.; Wu, J.; Rao, Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 2004, 7, 1222–1232. [Google Scholar] [CrossRef]
- Kang, D.S.; Yang, Y.R.; Lee, C.; Park, B.; Park, K.I.; Seo, J.K.; Seo, Y.K.; Cho, H.; Lucio, C.; Suh, P.G. Netrin-1/DCC-mediated PLCγ1 activation is required for axon guidance and brain structure development. EMBO Rep. 2018, 19, e46250. [Google Scholar] [CrossRef]
- Geisbrecht, B.V.; Dowd, K.A.; Barfield, R.W.; Longo, P.A.; Leahy, D.J. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J. Biol. Chem. 2003, 278, 32561–32568. [Google Scholar] [CrossRef]
- Bedogni, F.; Hevner, R.F. Cell-Type-Specific Gene Expression in Developing Mouse Neocortex: Intermediate Progenitors Implicated in Axon Development. Front. Mol. Neurosci. 2021, 14, 686034. [Google Scholar] [CrossRef]
- Lemieux, M.; Thiry, L.; Laflamme, O.D.; Bretzner, F. Role of DSCAM in the Development of Neural Control of Movement and Locomotion. Int. J. Mol. Sci. 2021, 22, 8511. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Blin, M.G.; Bachelier, R.; Bardin, N.; Blot-Chabaud, M.; Dignat-George, F. CD146 (Cluster of Differentiation 146). Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.P.; Parkinson, D.B. Role of Netrin-1 Signaling in Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 491. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, J.; Bai, W.; Li, J.; Shan, X. Netrin-1 prevents the attachment of monocytes to endothelial cells via an anti-inflammatory effect. Mol. Immunol. 2018, 103, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Huang, H.; Xiao, S.; Xia, Z.; Zheng, Y. Netrin-1 co-cross-linked hydrogel accelerates diabetic wound healing in situ by modulating macrophage heterogeneity and promoting angiogenesis. Bioact. Mater. 2024, 39, 302–316. [Google Scholar] [CrossRef]
- Rehman, S.; Nadeem, A.; Akram, U.; Sarwar, A.; Quraishi, A.; Siddiqui, H.; Malik, M.A.J.; Nabi, M.; Ul Haq, I.; Cho, A.; et al. Molecular Mechanisms of Ischemic Stroke: A Review Integrating Clinical Imaging and Therapeutic Perspectives. Biomedicines 2024, 12, 812. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Jia, J.; Yang, L.; Chen, Y.; Zheng, L.; Chen, Y.; Xu, Y.; Zhang, M. The Role of Microglial Phagocytosis in Ischemic Stroke. Front. Immunol. 2021, 12, 790201. [Google Scholar] [CrossRef]
- Smolders, S.M.; Kessels, S.; Vangansewinkel, T.; Rigo, J.M.; Legendre, P.; Brone, B. Microglia: Brain cells on the move. Prog. Neurobiol. 2019, 178, 101612. [Google Scholar] [CrossRef]
- Zheng, Y.; He, R.; Wang, P.; Shi, Y.; Zhao, L.; Liang, J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater. Sci. 2019, 7, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 2020, 21, 2006–2018. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Huang, L.; Enkhjargal, B.; Reis, C.; Wan, W.; Tang, J.; Cheng, Y.; Zhang, J.H. Recombinant Netrin-1 binding UNC5B receptor attenuates neuroinflammation and brain injury via PPARgamma/NFkappaB signaling pathway after subarachnoid hemorrhage in rats. Brain Behav. Immun. 2018, 69, 190–202. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Zhong, W.; Li, Y.; Zhang, W. Netrin-1 controls inflammation in response to ischemic stroke through altering microglia phenotype. Front. Immunol. 2023, 14, 1178638. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Z.; Miao, M.; Kong, C. The intracellular domain of UNC5B facilities proliferation and metastasis of bladder cancer cells. J. Cell. Mol. Med. 2021, 25, 2121–2135. [Google Scholar] [CrossRef]
- Yang, G.; Fan, X.; Mazhar, M.; Guo, W.; Zou, Y.; Dechsupa, N.; Wang, L. Neuroinflammation of microglia polarization in intracerebral hemorrhage and its potential targets for intervention. Front. Mol. Neurosci. 2022, 15, 1013706. [Google Scholar] [CrossRef]
- Wang, J.; Xing, H.; Wan, L.; Jiang, X.; Wang, C.; Wu, Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed. Pharmacother. 2018, 105, 518–525. [Google Scholar] [CrossRef]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef]
- Yang, M. Acute Lung Injury in aortic dissection: New insights in anesthetic management strategies. J. Cardiothorac. Surg. 2023, 18, 147. [Google Scholar] [CrossRef]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Jian, Z.; Zou, M.; Tong, H.; Wan, P. Netrin-1 mitigates acute lung injury by preventing the activation of the Toll-like receptor 4/nuclear factor-κB (TLR4/NF-κB) signaling. Aging 2024, 16, 2978–2988. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xuan, Y.; Chen, Y.; Wu, T.; Chen, L.; Guan, H.; Yang, S.; He, J.; Shi, D.; Wang, Y. Netrin-1 alleviates subarachnoid haemorrhage-induced brain injury via the PPARγ/NF-KB signalling pathway. J. Cell. Mol. Med. 2019, 23, 2256–2262. [Google Scholar] [CrossRef]
- Boknik, P.; Eskandar, J.; Hofmann, B.; Zimmermann, N.; Neumann, J.; Gergs, U. Role of Cardiac A(2A) Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2020, 11, 627838. [Google Scholar] [CrossRef]
- Berg, N.K.; Li, J.; Kim, B.; Mills, T.; Pei, G.; Zhao, Z.; Li, X.; Zhang, X.; Ruan, W.; Eltzschig, H.K.; et al. Hypoxia-inducible factor-dependent induction of myeloid-derived netrin-1 attenuates natural killer cell infiltration during endotoxin-induced lung injury. FASEB J. 2021, 35, e21334. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Di, G.; Qi, X.; Zhou, Q.; Gao, H. Netrin-1 promotes diabetic corneal wound healing through molecular mechanisms mediated via the adenosine 2B receptor. Sci. Rep. 2018, 8, 5994. [Google Scholar] [CrossRef]
- Turgut, F.; Awad, A.S.; Abdel-Rahman, E.M. Acute Kidney Injury: Medical Causes and Pathogenesis. J. Clin. Med. 2023, 12, 375. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef]
- Tadagavadi, R.K.; Wang, W.; Ramesh, G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J. Immunol. 2010, 185, 3750–3758. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Zhong, Y.; Chen, W.; Sun, J.; Liu, B.; Dong, J. Modified Bushen Yiqi Formula mitigates pulmonary inflammation and airway remodeling by inhibiting neutrophils chemotaxis and IL17 signaling pathway in rats with COPD. J. Ethnopharmacol. 2024, 321, 117497. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Zhang, D.; Hong, Q.; Yan, L.; Han, Q.; Shao, F.; Cai, G.; Chen, X.; Zhu, H. Netrin-1 works with UNC5B to regulate angiogenesis in diabetic kidney disease. Front. Med. 2020, 14, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, Y.; He, Q.; Geng, Y.; Xu, J. LncRNA-Cox2 regulates macrophage polarization and inflammatory response through the CREB-C/EBPβ signaling pathway in septic mice. Int. Immunopharmacol. 2021, 101, 108347. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Fu, Y.J.; Shi, Y.F.; Wang, L.Y.; Zhao, Y.F.; Wang, R.K.; Li, K.; Zhang, S.T.; Zha, X.J.; Wang, W.; Zhao, X.; et al. All-Natural Immunomodulatory Bioadhesive Hydrogel Promotes Angiogenesis and Diabetic Wound Healing by Regulating Macrophage Heterogeneity. Adv. Sci. 2023, 10, e2206771. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2023, 20, 561–573. [Google Scholar] [CrossRef]
- Li, Y.; Chai, J.L.; Shi, X.; Feng, Y.; Li, J.J.; Zhou, L.N.; Cao, C.; Li, K.R. Gαi1/3 mediate Netrin-1-CD146-activated signaling and angiogenesis. Theranostics 2023, 13, 2319–2336. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef]
- Lu, W.; Zeng, M.; Liu, W.; Ma, T.; Fan, X.; Li, H.; Wang, Y.; Wang, H.; Hu, Y.; Xie, J. Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration. Mater. Today Bio 2023, 19, 100569. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.K.; Chen, Q.; Lin, Y.; Stevens, M.M. Void-free 3D Bioprinting for In-situ Endothelialization and Microfluidic Perfusion. Adv. Funct. Mater. 2020, 30, 1909009. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Z.; Jiang, X.; Wang, C.; Tang, T.; Xu, T.; Chen, H.; Li, X.; Du, X.; Cui, W. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023, 157, 175–186. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yang, J.; Yang, Y.; Ding, H.; Yu, B.; Ma, K.; Chen, M. Gelatin methacryloyl (GelMA) loaded with concentrated hypoxic pretreated adipose-derived mesenchymal stem cells(ADSCs) conditioned medium promotes wound healing and vascular regeneration in aged skin. Biomater. Res. 2023, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Potier, L.; Roussel, R.; Marre, M.; Bjornstad, P.; Cherney, D.Z.; El Boustany, R.; Fumeron, F.; Venteclef, N.; Gautier, J.F.; Hadjadj, S.; et al. Plasma Copeptin and Risk of Lower-Extremity Amputation in Type 1 and Type 2 Diabetes. Diabetes Care 2019, 42, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, J.; Cui, C.; Peng, Z.; Yang, S.; Lei, J.; Li, B.; Yang, X.; Qin, J.; Yin, M.; et al. Netrin1-Enriched Exosomes From Genetically Modified ADSCs as a Novel Treatment for Diabetic Limb Ischemia. Adv. Healthc. Mater. 2025, 14, e2403521. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Bruikman, C.S.; van Gils, J.M. Netrin-1 in coronary artery disease (CAD): Mechanism of action and potential as a therapeutic target. Expert. Opin. Ther. Targets 2019, 23, 729–731. [Google Scholar] [CrossRef]
- Bruikman, C.S.; Vreeken, D.; Hoogeveen, R.M.; Bom, M.J.; Danad, I.; Pinto-Sietsma, S.J.; van Zonneveld, A.J.; Knaapen, P.; Hovingh, G.K.; Stroes, E.S.G.; et al. Netrin-1 and the Grade of Atherosclerosis Are Inversely Correlated in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 462–472. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Chen, L.; Yuan, Z.; Qin, X.; Wu, Q.; Shen, D.; He, H.; Yu, C. The role of UNC5b in ox-LDL inhibiting migration of RAW264.7 macrophages and the involvement of CCR7. Biochem. Biophys. Res. Commun. 2018, 505, 637–643. [Google Scholar] [CrossRef]
- Crucet, M.; Wüst, S.J.; Spielmann, P.; Lüscher, T.F.; Wenger, R.H.; Matter, C.M. Hypoxia enhances lipid uptake in macrophages: Role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis 2013, 229, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chaudhary, O.; Rodríguez-Morales, P.; Sun, X.; Chen, D.; Zappasodi, R.; Xu, Z.; Pinto, A.F.M.; Williams, A.; Schulze, I.; et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity 2021, 54, 1561–1577.e1567. [Google Scholar] [CrossRef]
- Kim, S.; Cho, W.; Kim, I.; Lee, S.H.; Oh, G.T.; Park, Y.M. Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J. Mol. Med. 2020, 98, 973–983. [Google Scholar] [CrossRef]
- Guo, H.Z.; Feng, R.X.; Zhang, Y.J.; Yu, Y.H.; Lu, W.; Liu, J.J.; Yang, S.X.; Zhao, C.; Zhang, Z.L.; Yu, S.H.; et al. A CD36-dependent non-canonical lipid metabolism program promotes immune escape and resistance to hypomethylating agent therapy in AML. Cell Rep. Med. 2024, 5, 101592. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Mills, T.; Doursout, M.F.; Evans, S.E.; Vidal Melo, M.F.; Eltzschig, H.K. Alternative adenosine Receptor activation: The netrin-Adora2b link. Front. Pharmacol. 2022, 13, 944994. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, X.; Zhai, J.; Yin, J.; Ma, K.; Wang, R.; Qin, X.; Li, Y.; Dong, X.; Wang, S. Isoflurane and Netrin-1 combination therapy enhances angiogenesis and neurological recovery by improving the expression of HIF-1α-Netrin-1-UNC5B/VEGF cascade to attenuate cerebral ischemia injury. Exp. Neurol. 2022, 352, 114028. [Google Scholar] [CrossRef]
- Trogan, E.; Feig, J.E.; Dogan, S.; Rothblat, G.H.; Angeli, V.; Tacke, F.; Randolph, G.J.; Fisher, E.A. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3781–3786. [Google Scholar] [CrossRef]
- van Gils, J.M.; Derby, M.C.; Fernandes, L.R.; Ramkhelawon, B.; Ray, T.D.; Rayner, K.J.; Parathath, S.; Distel, E.; Feig, J.L.; Alvarez-Leite, J.I.; et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012, 13, 136–143. [Google Scholar] [CrossRef]
- Bruikman, C.S.; Vreeken, D.; Zhang, H.; van Gils, M.J.; Peter, J.; van Zonneveld, A.J.; Hovingh, G.K.; van Gils, J.M. The identification and function of a Netrin-1 mutation in a pedigree with premature atherosclerosis. Atherosclerosis 2020, 301, 84–92. [Google Scholar] [CrossRef]
- Wang, Q.; Wadsworth, W.G. The C domain of netrin UNC-6 silences calcium/calmodulin-dependent protein kinase- and diacylglycerol-dependent axon branching in Caenorhabditis elegans. J. Neurosci. 2002, 22, 2274–2282. [Google Scholar] [CrossRef]
- Schlegel, M.; Moore, K.J. A heritable netrin-1 mutation increases atherogenic immune responses. Atherosclerosis 2020, 301, 82–83. [Google Scholar] [CrossRef] [PubMed]
- 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011, 124, 2020–2045. [CrossRef]
- Silvestro, M.; Rivera, C.F.; Alebrahim, D.; Vlahos, J.; Pratama, M.Y.; Lu, C.; Tang, C.; Harpel, Z.; Sleiman Tellaoui, R.; Zias, A.L.; et al. The Nonproteolytic Intracellular Domain of Membrane-Type 1 Matrix Metalloproteinase Coordinately Modulates Abdominal Aortic Aneurysm and Atherosclerosis in Mice-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1244–1253. [Google Scholar] [CrossRef]
- Hadi, T.; Boytard, L.; Silvestro, M.; Alebrahim, D.; Jacob, S.; Feinstein, J.; Barone, K.; Spiro, W.; Hutchison, S.; Simon, R.; et al. Macrophage-derived netrin-1 promotes abdominal aortic aneurysm formation by activating MMP3 in vascular smooth muscle cells. Nat. Commun. 2018, 9, 5022. [Google Scholar] [CrossRef] [PubMed]
- Ramkhelawon, B.; Hennessy, E.J.; Ménager, M.; Ray, T.D.; Sheedy, F.J.; Hutchison, S.; Wanschel, A.; Oldebeken, S.; Geoffrion, M.; Spiro, W.; et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 2014, 20, 377–384. [Google Scholar] [CrossRef]
- Sun, D.; Du, Y. O304 alleviates abdominal aortic aneurysm formation via AMPK/mTOR/MMP pathway activation. Front. Pharmacol. 2024, 15, 1457817. [Google Scholar] [CrossRef]
- Qian, W.; Hadi, T.; Silvestro, M.; Ma, X.; Rivera, C.F.; Bajpai, A.; Li, R.; Zhang, Z.; Qu, H.; Tellaoui, R.S.; et al. Microskeletal stiffness promotes aortic aneurysm by sustaining pathological vascular smooth muscle cell mechanosensation via Piezo1. Nat. Commun. 2022, 13, 512. [Google Scholar] [CrossRef]
- Romac, J.M.; Shahid, R.A.; Swain, S.M.; Vigna, S.R.; Liddle, R.A. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat. Commun. 2018, 9, 1715. [Google Scholar] [CrossRef]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e797. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuang, J.; Sun, D.; Ding, Q.; Zheng, H.; Li, H.; Zhang, X.; Du, Y.; Ma, T.; Meng, Q. Netrin-1 Monoclonal Antibody-Functionalized Nanoparticle Loaded with Metformin Prevents the Progression of Abdominal Aortic Aneurysms. Int. J. Nanomed. 2023, 18, 627–639. [Google Scholar] [CrossRef]
- Duong, V.; Oo, W.M.; Ding, C.; Culvenor, A.G.; Hunter, D.J. Evaluation and Treatment of Knee Pain: A Review. JAMA 2023, 330, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, C.; Monteagudo, S.; Lories, R.J. Promising targets for therapy of osteoarthritis: A review on the Wnt and TGF-β signalling pathways. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720x211006959. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Hu, Z.; Jiang, H.; Li, C.; Guo, H.; Fang, W.; Long, X. The expression of Netrin-1 in the MIA-induced osteoarthritic temporomandibular joint in mice. Sci. Rep. 2021, 11, 15695. [Google Scholar] [CrossRef]
- Ni, S.; Ling, Z.; Wang, X.; Cao, Y.; Wu, T.; Deng, R.; Crane, J.L.; Skolasky, R.; Demehri, S.; Zhen, G.; et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat. Commun. 2019, 10, 5643. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, B.; Gao, Y.; Xie, J.; Han, H.; Wu, Q.; Yang, D. Cinnamaldehyde attenuates streptozocin-induced diabetic osteoporosis in a rat model by modulating netrin-1/DCC-UNC5B signal transduction. Front. Pharmacol. 2024, 15, 1367806. [Google Scholar] [CrossRef]
- Bashaw, G.J.; Klein, R. Signaling from axon guidance receptors. Cold Spring Harb. Perspect. Biol. 2010, 2, a001941. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Li, X.; Liu, Y.; Meijers, R.; Zhang, Y.; Wang, J.H. The binding of DCC-P3 motif and FAK-FAT domain mediates the initial step of netrin-1/DCC signaling for axon attraction. Cell Discov. 2018, 4, 8. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Aspects Med. 2019, 65, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Juan-Guardela, B.M.; Tzouvelekis, A.; Herazo-Maya, J.D. Precision medicine advances in idiopathic pulmonary fibrosis. EBioMedicine 2023, 95, 104766. [Google Scholar] [CrossRef]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef]
- Gao, R.; Peng, X.; Perry, C.; Sun, H.; Ntokou, A.; Ryu, C.; Gomez, J.L.; Reeves, B.C.; Walia, A.; Kaminski, N.; et al. Macrophage-derived netrin-1 drives adrenergic nerve-associated lung fibrosis. J. Clin. Investig. 2021, 131, e136542. [Google Scholar] [CrossRef]
- Brunet, I.; Gordon, E.; Han, J.; Cristofaro, B.; Broqueres-You, D.; Liu, C.; Bouvrée, K.; Zhang, J.; del Toro, R.; Mathivet, T.; et al. Netrin-1 controls sympathetic arterial innervation. J. Clin. Investig. 2014, 124, 3230–3240. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Li, Q.; Xu, L.; Zhang, Y.; Li, D.; Ma, J.; Mao, X. Netrin-1 overexpression in bone marrow mesenchymal stem cells promotes functional recovery in a rat model of peripheral nerve injury. J. Biomed. Res. 2015, 29, 380–389. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Vincent, K.; Brawn, J.; Zondervan, K.T.; Becker, C.M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 2014, 20, 717–736. [Google Scholar] [CrossRef]
- Guo, X.; Ding, S.; Li, T.; Wang, J.; Yu, Q.; Zhu, L.; Xu, X.; Zou, G.; Peng, Y.; Zhang, X. Macrophage-derived netrin-1 is critical for neuroangiogenesis in endometriosis. Int. J. Biol. Macromol. 2020, 148, 226–237. [Google Scholar] [CrossRef]
- Chen, T.; Ye, B.; Tan, J.; Yang, H.; He, F.; Khalil, R.A. (CD146+)Mesenchymal stem cells treatment improves vascularization, muscle contraction and VEGF expression, and reduces apoptosis in rat ischemic hind limb. Biochem. Pharmacol. 2021, 190, 114530. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Zhang, C.; Yan, H.; Luo, Y.; Kong, R.; Wen, P.; Ye, Z.; Chen, J.; Feng, J.; Liu, F.; et al. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015, 25, 275–287. [Google Scholar] [CrossRef]
- Yamashita, T. Neogenin is a Determining Factor for Regenerating Neurons Following Spinal Cord Injury. Neuroscience 2019, 408, 448–449. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Yi, S.; Liu, Q.; Zhang, R.; Wang, P.; Qian, T.; Li, S. The microRNAs let-7 and miR-9 down-regulate the axon-guidance genes Ntn1 and Dcc during peripheral nerve regeneration. J. Biol. Chem. 2019, 294, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Taïb, S.; Lamandé, N.; Martin, S.; Coulpier, F.; Topilko, P.; Brunet, I. Myelinating Schwann cells and Netrin-1 control intra-nervous vascularization of the developing mouse sciatic nerve. Elife 2022, 11, e64773. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, X.; Sánchez-Gómez, P.; Palma, V. Netrin-1 in Glioblastoma Neovascularization: The New Partner in Crime? Int. J. Mol. Sci. 2021, 22, 8248. [Google Scholar] [CrossRef]
- Boneschansker, L.; Nakayama, H.; Eisenga, M.; Wedel, J.; Klagsbrun, M.; Irimia, D.; Briscoe, D.M. Netrin-1 Augments Chemokinesis in CD4+ T Cells In Vitro and Elicits a Proinflammatory Response In Vivo. J. Immunol. 2016, 197, 1389–1398. [Google Scholar] [CrossRef]
- Kitayama, M.; Ueno, M.; Itakura, T.; Yamashita, T. Activated microglia inhibit axonal growth through RGMa. PLoS ONE 2011, 6, e25234. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.A.; Sanchez-Gomez, P.; Muñoz-Palma, E.; Puvogel, S.; Casas, B.S.; Arriagada, C.; Peña-Villalobos, I.; Lois, P.; Ramírez Orellana, M.; Lubieniecki, F.; et al. The Netrin-1-Neogenin-1 signaling axis controls neuroblastoma cell migration via integrin-β1 and focal adhesion kinase activation. Cell Adh Migr. 2021, 15, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Zuccarini, M.; Giuliani, P.; Ziberi, S.; Carluccio, M.; Iorio, P.D.; Caciagli, F.; Ciccarelli, R. The Role of Wnt Signal in Glioblastoma Development and Progression: A Possible New Pharmacological Target for the Therapy of This Tumor. Genes 2018, 9, 105. [Google Scholar] [CrossRef]
- Chen, J.Y.; He, X.X.; Ma, C.; Wu, X.M.; Wan, X.L.; Xing, Z.K.; Pei, Q.Q.; Dong, X.P.; Liu, D.X.; Xiong, W.C.; et al. Netrin-1 promotes glioma growth by activating NF-κB via UNC5A. Sci. Rep. 2017, 7, 5454. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, Y.H.; Oh, S.Y.; Yong, M.S.; Ryu, J.M.; Han, H.J. Netrin-1 induces MMP-12-dependent E-cadherin degradation via the distinct activation of PKCα and FAK/Fyn in promoting mesenchymal stem cell motility. Stem Cells Dev. 2014, 23, 1870–1882. [Google Scholar] [CrossRef]
- Wu, S.; Guo, X.; Zhou, J.; Zhu, X.; Chen, H.; Zhang, K.; Lu, Y.; Chen, Y. High expression of UNC5B enhances tumor proliferation, increases metastasis, and worsens prognosis in breast cancer. Aging 2020, 12, 17079–17098. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Remon, J.; Faivre-Finn, C.; Garassino, M.C.; Heymach, J.V.; Kerr, K.M.; Tan, D.S.W.; Veronesi, G.; Reck, M. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2024, 10, 71. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, S.; Wu, W.; Zhang, N.; Wang, J. Regulatory mechanisms and therapeutic targeting of vasculogenic mimicry in hepatocellular carcinoma. Pharmacol. Res. 2021, 166, 105507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, P.; Ding, B.; Guo, Y.; Han, K.; Li, J.; Chen, H.; Zhang, W. Netrin-1 elicits metastatic potential of non-small cell lung carcinoma cell by enhancing cell invasion, migration and vasculogenic mimicry via EMT induction. Cancer Gene Ther. 2018, 25, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luan, H.; Chai, H.; Yan, L.; Zhang, J.; Wang, Q.; Cao, L. Netrin-1 interference potentiates epithelial-to-mesenchymal transition through the PI3K/AKT pathway under the hypoxic microenvironment conditions of non-small cell lung cancer. Int. J. Oncol. 2019, 54, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Decourcelle, A.; Very, N.; Djouina, M.; Loison, I.; Thévenet, J.; Body-Malapel, M.; Lelièvre, E.; Coqueret, O.; Leprince, D.; El Yazidi-Belkoura, I.; et al. O-GlcNAcylation Links Nutrition to the Epigenetic Downregulation of UNC5A during Colon Carcinogenesis. Cancers 2020, 12, 3168. [Google Scholar] [CrossRef]
- Yuan, M.; Xie, F.; Xia, X.; Zhong, K.; Lian, L.; Zhang, S.; Yuan, L.; Ye, J. UNC5C-knockdown enhances the growth and metastasis of breast cancer cells by potentiating the integrin α6/β4 signaling pathway. Int. J. Oncol. 2020, 56, 139–150. [Google Scholar] [CrossRef]
- El-Gamal, R.; Mokhtar, N.; Ali-El-Dein, B.; Baiomy, A.A.; Aboazma, S.M. Netrin-1: A new promising diagnostic marker for muscle invasion in bladder cancer. Urol. Oncol. 2020, 38, 640.e1–640.e12. [Google Scholar] [CrossRef]
- Dudgeon, C.; Casabianca, A.; Harris, C.; Ogier, C.; Bellina, M.; Fiore, S.; Bernet, A.; Ducarouge, B.; Goldschneider, D.; Su, X.; et al. Netrin-1 feedforward mechanism promotes pancreatic cancer liver metastasis via hepatic stellate cell activation, retinoid, and ELF3 signaling. Cell Rep. 2023, 42, 113369. [Google Scholar] [CrossRef]

| Diseases | Sources | Expression | Receptors | Modes | Effects |
|---|---|---|---|---|---|
| Acute ischemic stroke | Brain tissue (microglia) | Increased | UNC5a | Autocrine | Promote microglia M2 polarization and reduce cell apoptosis, invasion, and migration |
| Acute lung injury (ALI) | Lung | Decreased | Neogenin (Neutrophils and monocytes) | Paracrine | Promote the recruitment of neutrophils, monocytes, and NK cells |
| Acute kidney injury (AKI) | Kidney | Decreased | Neogenin (Neutrophils and monocytes) | Paracrine | Promote the recruitment of neutrophils and monocytes |
| Diabetes | Diabetic wound | Increased | A2BR (Macrophages) | Paracrine | Promote angiogenesis and wound healing |
| Atherosclerosis | Artery (foam cells) | Increased | UNC5b (foam cells) and Neogenin (SMCs) | Autocrine and paracrine | Inhibit macrophages migration and induce SMC recruitment to the intima |
| Abdominal aortic aneurysms (AAA) | Abdominal aorta (macrophages) | Increased | Neogenin (vascular smooth muscle cells) | Paracrine | Promote transcription and calcium mobilization of matrix metalloproteinase 3 (MMP3) |
| Osteoarthritis | Bone tissue (osteoclasts) | Increased | DCC (nerve cells), UNC5b (osteoclasts), and A2BR | Autocrine and paracrine | Promote DRG neuron axon growth, subchondral bone sensory innervation, and osteoclast differentiation |
| Pulmonary Fibrosis | Lung tissue (macrophages) | Increased | DCC (nerve cells) | Paracrine | Remodeling of the adrenergic nerve and progression of fibrosis |
| Endometriosis | Uterine tissue (peritoneal macrophages) | Increased | CD146 (endothelial cells), Neogenin (nerve cells), DCC, and UNC5b (Schwann cells) | Paracrine | Promote angiogenesis and peripheral nerve regeneration and induce neuron regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liu, Z.; Xu, P.; Yin, K.; Wang, S. The Role of Macrophage-Derived Netrin-1 in Inflammatory Diseases. Biomolecules 2025, 15, 921. https://doi.org/10.3390/biom15070921
Wu Y, Liu Z, Xu P, Yin K, Wang S. The Role of Macrophage-Derived Netrin-1 in Inflammatory Diseases. Biomolecules. 2025; 15(7):921. https://doi.org/10.3390/biom15070921
Chicago/Turabian StyleWu, Yi, Zhiying Liu, Peiqi Xu, Kai Yin, and Shengjun Wang. 2025. "The Role of Macrophage-Derived Netrin-1 in Inflammatory Diseases" Biomolecules 15, no. 7: 921. https://doi.org/10.3390/biom15070921
APA StyleWu, Y., Liu, Z., Xu, P., Yin, K., & Wang, S. (2025). The Role of Macrophage-Derived Netrin-1 in Inflammatory Diseases. Biomolecules, 15(7), 921. https://doi.org/10.3390/biom15070921








