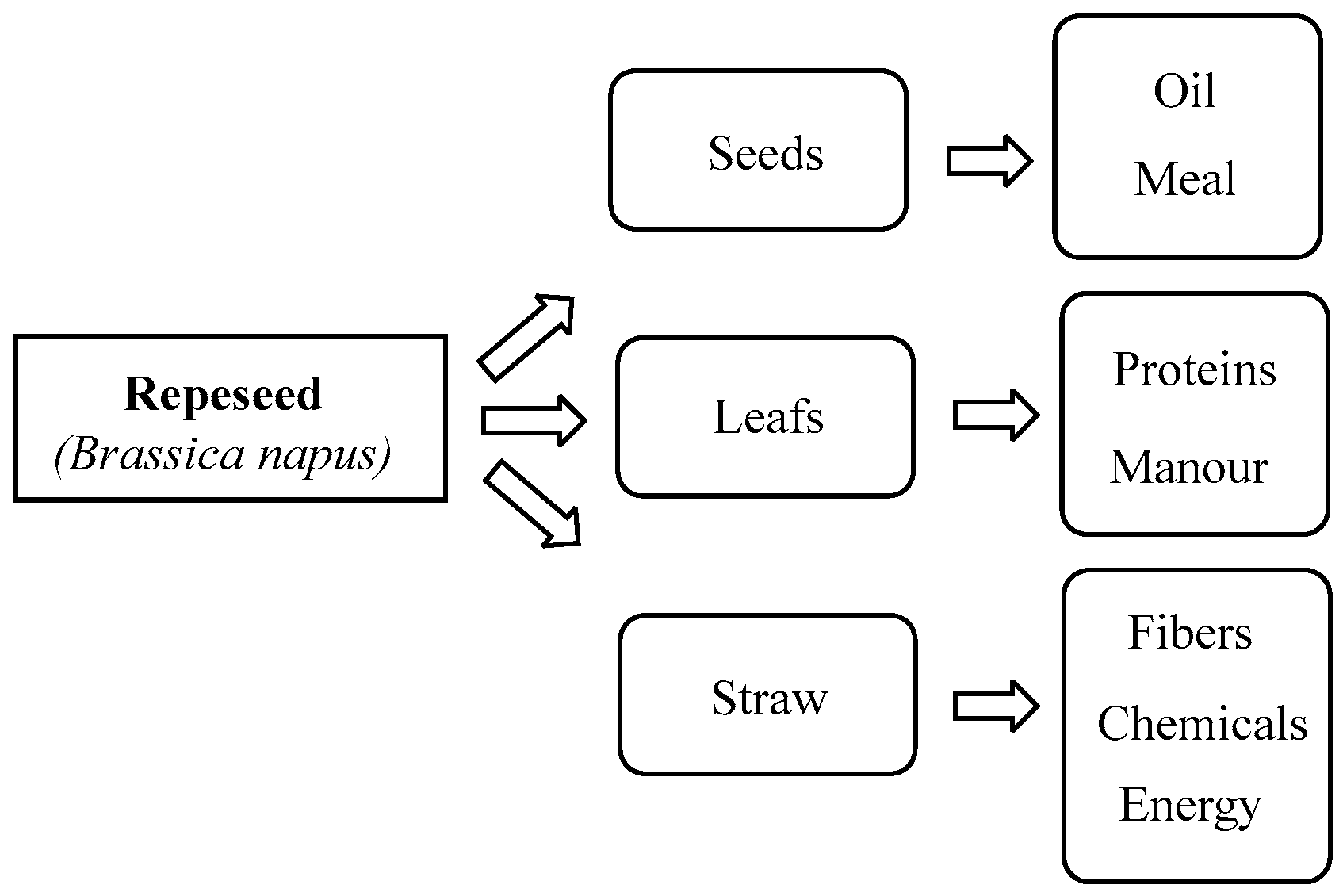
Application of Microorganisms for the Valorization of Side-Products of Rapeseed De-Oiling
Abstract
1. Introduction
2. Procedures for De-Oiling Rapeseed
| Bacterial Genus/Species | Key Volatile Compounds Produced | Compound Class/Origin | Notes | Ref. |
|---|---|---|---|---|
| Pseudomonas spp. | Alkanes, alkenes, aldehydes, ketones, alcohols | Fatty acid degradation products | Common rapeseed colonizers producing typical off-flavor compounds | [57,59] |
| Bacillus spp. | Sulfur-containing compounds (e.g., dimethyl disulfide), pyrazines | Sulfur volatiles, Maillard reaction products | Contribute to pungent and nutty aromas; pyrazines formed during seed roasting and storage | [59] |
| Microbacterium spp. | Aldehydes (hexanal, nonanal), ketones | Lipid oxidation products | Associated with oxidation of unsaturated fatty acids | [56,57] |
| Staphylococcus spp. | Alcohols, aldehydes, ketones | Fatty acid and amino acid metabolism | Influence aroma profile through diverse VOCs | [58,59] |
| Enterobacter spp. | Sulfides, nitriles | Glucosinolate degradation products | Linked to pungent aroma via GLS breakdown | [57,58] |
| Pantoea spp. | Pyrazines, furans | Maillard reaction products | Important for nutty and roasted aroma notes | [56] |
3. Cake and Meal
4. Antinutrient Components
| Type of Components | Meal Obtained from Yellow Seed [%] | Meal Obtained from Black Seeds [%] | References |
|---|---|---|---|
| Protein [38] | 35.46 | 30.29 | [38] |
| sucrose | 7.88 | 7.29 | [38,94] |
| dietary fiber | 26.19 | 34.63 | [95] |
| crude fiber | 4.56 | 8.86 | [89] |
| Glucosinolates | 22.18 | 28.19 | [88,89] |
| phytic acid | 4.98 | 5.60 | [38,89] |
| total polyphenols | 2.67 | 2.82 | [38,89,90] |
4.1. Glucosinolates
4.2. Erucic Acid
4.3. Sinapine and Other Polyphenolic Derivatives
4.4. Fibers
4.5. Phytic Acid
5. Application of Microorganisms for Valorization of Rapeseed Meal and Cake
5.1. Substrate for Enzyme Production
5.1.1. Lipase
5.1.2. Phytase
5.1.3. Alkaline Protease
5.1.4. Nattokinase
5.2. Enzymes Degrading Polysaccharides
Xylanase (Beta Glucanase)
5.3. Multienzyme Producers
5.4. Microbiota Supported Organic Synthesis
5.4.1. Whisky Lactone
5.4.2. Surfactin Analogues
5.4.3. 1,3-Propandiol
5.4.4. Omega-3 Docosahexaenoic Acid
5.5. Neutralization of Antifeedant and Enhancing Nutritional Value of Rapeseed Meal by Treatment with Microrganizms
5.6. Interactions Between Rapeseed and Soil Microorganisms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EUBIA | European Biomass Industry Association |
| FAOSTAT | Food and Agriculture Organization Statistics |
| UPLC | Ultra-performance liquid chromatography |
| NRRL | Northern Regional Research Laboratory |
References
- Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries. Foods 2025, 14, 1444. [Google Scholar] [CrossRef]
- Di Lena, G.; Del Pulgar, J.S.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.M.; Aguzzi, A.; Nicoli, S.F.; Casini, I.; et al. Valorization potentials of rapeseed meal in a biorefinery perspective: Focus on nutritional and bioactive components. Molecules 2021, 26, 6787. [Google Scholar] [CrossRef] [PubMed]
- Wongsirichot, P.; Gonzalez-Miquel, M.; Winterburn, J. Rapeseed meal biorefining: Fractionation, valorization and integration approaches. Biocatal. Agric. Biotechnol. 2024, 62, 103460. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, C.; François, J.M.; Wan, X.; Deng, Q.; Feng, D.; Deng, S.; Chen, S.; Huang, F.; Chen, W.; et al. A Two-step Strategy for High-Value-Added Utilization of Rapeseed Meal by Concurrent Improvement of Phenolic Extraction and Protein Conversion for Microbial Iturin A Production. Front. Bioeng. Biotechnol. 2021, 9, 735714. [Google Scholar] [CrossRef]
- Tiwari, A.; Srivastav, N.; Srivastava, P. Utilization of natural dyes extracted from mustard flower (Genus: Brassica, Species: Napus) as photosensitizer for DSSC: Experimental and computational studies. Results Opt. 2024, 15, 100631. [Google Scholar] [CrossRef]
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica napus Rapeseed. In The Brassica napus Genome; Liu, S., Snowdon, R., Chalhoub, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. ISBN 978-3-319-43694-4. [Google Scholar]
- Schiessl, S.V.; Mason, A.S. Ancient and Recent Polyploid Evolution in Brassica. In Brassica Improvement; Wani, S., Thakur, A., Jeshima Khan, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–66. ISBN 978-3-030-34694-2. [Google Scholar]
- Zając, T.; Klimek-Kopyra, A.; Oleksy, A.; Lorenc-Kozik, A.; Ratajczak, K. Analysis of yield and plant traits of oilseed rape (Brassica napus L.) cultivated in temperate region in light of the possibilities of sowing in arid areas. Acta Agrobot. 2016, 69, 1696. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Li, Z.; Cowling, W.A. Center of origin and centers of diversity in an ancient crop, brassica rapa (turnip rape). J. Hered. 2014, 105, 555–565. [Google Scholar] [CrossRef]
- Kole, C. Genome Mapping and Molecular Breeding in Plants: Technical Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 2, ISBN 978-3-540-34531-2. [Google Scholar]
- Reiner, H.; Holzner, W.; Ebermann, R. The Development of Turnip-Type and Oilseed-Type Brassica Rapa Crops from the Wild-Type in Europe-an Overview of Botanical, Historical and Linguistic Facts. Rapeseed Today Tomorrow 1995, 4, 1066–1069. [Google Scholar]
- Hohmann, N.; Wolf, E.M.; Lysak, M.A.; Koch, M.A. A time-calibrated road map of brassicaceae species radiation and evolutionary history. Plant Cell 2015, 27, 2770–2784. [Google Scholar] [CrossRef]
- Kiefer, M.; Schmickl, R.; German, D.A.; Mandáková, T.; Lysak, M.A.; Al-Shehbaz, I.A.; Franzke, A.; Mummenhoff, K.; Stamatakis, A.; Koch, M.A. BrassiBase: Introduction to a novel knowledge database on brassicaceae evolution. Plant Cell Physiol. 2014, 55, e3. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yang, D.; Zhao, Y.; Yang, X.; Zhou, Z.; Sun, X.; Kong, X.; Li, X.; Wang, G.; Duan, Y.; et al. Differences in pseudogene evolution contributed to the contrasting flavors of turnip and Chiifu, two Brassica rapa subspecies. Plant Commun. 2023, 4, 100427. [Google Scholar] [CrossRef] [PubMed]
- The Genomic Variation Database of Brassica napus. Available online: http://rapeseed.biocloud.net/home (accessed on 25 July 2022).
- Yan, T.; Yao, Y.; Wu, D.; Jiang, L. BnaGVD: A Genomic Variation Database of Rapeseed (Brassica napus). Plant Cell Physiol. 2021, 62, 378–383. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, X.; Feng, Z.; Chen, M.; Qiu, X.; Sun, J.; Wu, M.; He, J. Protein from rapeseed for food applications: Extraction, sensory quality, functional and nutritional properties. Food Chem. 2024, 439, 138109. [Google Scholar] [CrossRef] [PubMed]
- Łuczkiewicz, T. Nasiennictwo, Rozmnażanie Materiału Siewnego, Tom 2; PWRiL: Poznań, Poland, 2000; ISBN 8309017332. [Google Scholar]
- Callihan, B.; Brennan, J.; Miller, T.; Brown, J.; Moore, M. Guide to Identification of Canola Mustard Rapeseed and Related Weeds; Ag Publication: Moscow, Russia, 2000; pp. 1–23. [Google Scholar]
- Alford, D.V. Biocontrol of Oilseed Rape Pests; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780470750988. [Google Scholar]
- Lopez, F.B.; Barclay, G.F.; Badal, S. Plant anatomy and physiology. In Pharmacognosy: Fundamentals, Applications, and Strategies, 2nd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 29–48. ISBN 9780443186578. [Google Scholar]
- Feed Industry Guide, 6th Edition; Canola Meal Feeding Guide; The Canola Council of Canada: Winnipeg, MB, Canada, 2019.
- Agnihotri, A.; Kumar, M.; Kilam, D.; Aneja, J.K. Genomic and transcriptomic approaches for quality improvement in oilseed brassicas. In Plant OMICS and Crop Breeding; Apple Academic Press: Burlington, ON, Canada, 2017; pp. 31–48. ISBN 9781771884563. [Google Scholar]
- Gaber, M.A.F.M.; Tujillo, F.J.; Mansour, M.P.; Juliano, P. Improving Oil Extraction from Canola Seeds by Conventional and Advanced Methods. Food Eng. Rev. 2018, 10, 198–210. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- European Biomass Industry Association EUBIA Biodiesel Market. Available online: https://www.eubia.org/cms/wiki-biomass/biofuels/biodiesel/ (accessed on 6 July 2022).
- Burdge, G.C.; Calder, P.C. α-Linolenic acid metabolism in adult humans: The effects of gender and age on conversion to longer-chain polyunsaturated fatty acids. Eur. J. Lipid Sci. Technol. 2005, 107, 426–439. [Google Scholar] [CrossRef]
- Gu, H.; Li, J.; Lu, Z.; Li, X.; Cong, R.; Ren, T.; Lu, J. Effects of combined application of nitrogen and potassium on oil concentration and fatty acid component of oilseed rape (Brassica napus L.). Field Crop. Res. 2024, 306, 109229. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Chen, J.; Jing, B.; Zhang, L.; Yu, X. Characterization of Differences in Flavor in Virgin Rapeseed Oils by Using Gas Chromatography—Mass Spectrometry, Electronic Nose, and Sensory Analysis. Eur. J. Lipid Sci. Technol. 2020, 122, 1900205. [Google Scholar] [CrossRef]
- Yin, N.W.; Wang, S.X.; Jia, L.D.; Zhu, M.C.; Yang, J.; Zhou, B.J.; Yin, J.M.; Lu, K.; Wang, R.; Li, J.N.; et al. Identification and characterization of major constituents in different-colored rapeseed petals by uplc-hesi-ms/ms. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Elshout, P.M.F.; van der Velde, M.; van Zelm, R.; Steinmann, Z.J.N.; Huijbregts, M.A.J. Comparing greenhouse gas footprints and payback times of crop-based biofuel production worldwide. Biofuels 2022, 13, 55–61. [Google Scholar] [CrossRef]
- F.A.O. FAOSTAT Statistics Database 1998. Available online: https://www.fao.org/faostat/en/#home (accessed on 6 July 2022).
- Rathke, G.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agric. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar] [CrossRef]
- Tofanica, B.M. Rapeseed—A Valuable Renewable Bioresource. Cellul. Chem. Technol. 2019, 53, 837–849. [Google Scholar] [CrossRef]
- Tofanica, B.M.; Puitel, A.C. Optimization and design of alkaline pulping of rapeseed (Brassica napus) stalks. Chem. Eng. Commun. 2019, 206, 378–386. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A comprehensive review on activated carbon from pyrolysis of lignocellulosic biomass: An application for energy and the environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Xie, T.; Rong, H.; Li, A.; Fang, Y.; Wang, Y.; McPhee, D.J. Metabolic characteristics in meal of black rapeseed and yellow-seeded progeny of brassica napus-sinapis alba hybrids. Molecules 2015, 20, 21204–21213. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, J.; Wang, C.; Blinov, O.A.; Asiedu Kumi, M.; Liu, W.; Chu, X.; Teng, Y.; Liu, H.; Yang, Z.; et al. Integrating Agricultural and Ecotourism Development: A Crop Cultivation Suitability Framework Considering Tourists’ Landscape Preferences in Qinghai Province, China. Remote Sens. 2023, 15, 4685. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, Y. Polyphenolic compounds from rapeseeds (Brassica napus L.): The major types, biofunctional roles, bioavailability, and the influences of rapeseed oil processing technologies on the content. Food Res. Int. 2023, 163, 112282. [Google Scholar] [CrossRef]
- Oroian, M. A new perspective regarding the adulteration detection of cold-pressed oils. LWT 2024, 198, 116025. [Google Scholar] [CrossRef]
- Vidal, N.P.; Roman, L.; Swaraj, V.J.S.; Ragavan, K.V.; Simsek, S.; Rahimi, J.; Kroetsch, B.; Martinez, M.M. Enhancing the nutritional value of cold-pressed oilseed cakes through extrusion cooking. Innov. Food Sci. Emerg. Technol. 2022, 77, 102956. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Prior, E.M.; Vadke, V.S.; Sosulski, F.W. Effect of heat treatments on canola press oils. I. Non-triglyceride components. J. Am. Oil Chem. Soc. 1991, 68, 401–406. [Google Scholar] [CrossRef]
- Unger, E.H. Commercial Processing of Canola and Rapeseed: Crushing and Oil Extraction. In Canola and Rapeseed; Shahidi, F., Ed.; Springer: Boston, MA, USA, 1990; pp. 235–249. ISBN 978-1-4615-3912-4. [Google Scholar]
- Smithard, R. Full-fat rapeseed for pig and poultry diets. Feed Compd. 1993, 13, 35–38. [Google Scholar]
- Daun, J.K.; Eskin, M.N.A.; Hickling, D. Canola: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 0128043482. [Google Scholar]
- Li, H.; Wu, J.; Rempel, C.B.; Thiyam, U. Effect of operating parameters on oil and phenolic extraction using supercritical CO2. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1081–1089. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, Z.; Xu, S.Y. Downstream processes for aqueous enzymatic extraction of rapeseed oil and protein hydrolysates. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 693–700. [Google Scholar] [CrossRef]
- Cherstva, A.; Lastovetska, A.; Nosenko, T. Using of enzymes to extract of rapeseed oil by pressing. Ukr. J. Food Sci. 2016, 4, 85–93. [Google Scholar]
- Gaber, M.A.F.M.; Trujillo, F.J.; Mansour, M.P.; Taylor, C.; Juliano, P. Megasonic-assisted aqueous extraction of canola oil from canola cake. J. Food Eng. 2019, 252, 60–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Lu, X.; Sun, H.; Wang, F. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Res. Int. 2021, 150, 110791. [Google Scholar] [CrossRef]
- Pathak, P.K.; Agrawal, Y.C.; Singh, B.P.N. Effect of elevated drying temperature on rapeseed oil quality. J. Am. Oil Chem. Soc. 1991, 68, 580–582. [Google Scholar] [CrossRef]
- Siger, A.; Józefiak, M.; Górnas, P. Cold-pressed and hot-pressed rapeseed oil: The effects of roasting and seed moisture on the antioxidant activity, canolol, and tocopherol level. Acta Sci. Pol. Technol. Aliment. 2017, 16, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Khalid, W.; Maggiolino, A.; Kour, J.; Arshad, M.S.; Aslam, N.; Afzal, M.F.; Meghwar, P.; Zafar, K.-W.; De Palo, P.; Korma, S.A. Dynamic alterations in protein, sensory, chemical, and oxidative properties occurring in meat during thermal and non-thermal processing techniques: A comprehensive review. Front. Nutr. 2023, 9, 1057457. [Google Scholar] [CrossRef]
- Wagner, C.; Bonte, A.; Brühl, L.; Niehaus, K.; Bednarz, H.; Matthäus, B. Micro-organisms growing on rapeseed during storage affect the profile of volatile compounds of virgin rapeseed oil. J. Sci. Food Agric. 2018, 98, 2147–2155. [Google Scholar] [CrossRef]
- Guo, F.; Ma, M.; Yu, M.; Bian, Q.; Hui, J.; Pan, X.; Su, X.; Wu, J. Classification of chinese fragrant rapeseed oil based on sensory evaluation and gas chromatography-olfactometry. Front. Nutr. 2022, 9, 945144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Zhao, X.; Chen, W.; Du, S.; Yu, X. Key volatile compound formation of rapeseed oil induced via the Maillard reaction during seed roasting. Food Chem. 2022, 388, 132992. [Google Scholar] [CrossRef] [PubMed]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of secondary and tertiary volatile compounds resulting from the lipid oxidation of rapeseed oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Zou, Y.; He, R.; Ju, X.; Yuan, J. Effect of static-state fermentation on volatile composition in rapeseed meal. J. Sci. Food Agric. 2020, 100, 2145–2152. [Google Scholar] [CrossRef]
- Bos, C.; Airinei, G.; Mariotti, F.; Benamouzig, R.; Bérot, S.; Evrard, J.; Fénart, E.; Tomé, D.; Gaudichon, C. The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans. J. Nutr. 2007, 137, 594–600. [Google Scholar] [CrossRef]
- Fleddermann, M.; Fechner, A.; Rößler, A.; Bähr, M.; Pastor, A.; Liebert, F.; Jahreis, G. Nutritional evaluation of rapeseed protein compared to soy protein for quality, plasma amino acids, and nitrogen balance—A randomized cross-over intervention study in humans. Clin. Nutr. 2013, 32, 519–526. [Google Scholar] [CrossRef]
- Škvorová, P.; Kulma, M.; Božik, M.; Kurečka, M.; Plachý, V.; Slavíková, D.; Šebelová, K.; Kouřimská, L. Evaluation of rapeseed cake as a protein substitute in the feed of edible crickets: A case study using Gryllus assimilis. Food Chem. 2024, 441, 138254. [Google Scholar] [CrossRef]
- Raikos, V.; Neacsu, M.; Duthie, G.; Nicol, F.; Reid, M.; Cantlay, L.L.; Ranawana, V. Proteomic and Glucosinolate Profiling of Rapeseed Isolates from Meals Produced by Different Oil Extraction Processes. J. Food Process. Preserv. 2017, 41, e13060. [Google Scholar] [CrossRef]
- Nietzel, T.; Dudkina, N.V.; Haase, C.; Denolf, P.; Semchonok, D.A.; Boekema, E.J.; Braun, H.-P.; Sunderhaus, S. The Native Structure and Composition of the Cruciferin Complex in Brassica napus. J. Biol. Chem. 2013, 288, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, A.; Kozłowska, M.; Rachwał, D.; Wnukowski, P.; Amarowicz, R.; Nebesny, E.; Rosicka-Kaczmarek, J. Canola/rapeseed protein—Nutritional value, functionality and food application: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3836–3856. [Google Scholar] [CrossRef]
- Aluko, R.E.; McIntosh, T. Polypeptide profile and functional properties of defatted meals and protein isolates of canola seeds. J. Sci. Food Agric. 2001, 81, 391–396. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wada, Y.; Wäsche, A. Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem. 2008, 107, 32–39. [Google Scholar] [CrossRef]
- Tandang, M.R.G.; Adachi, M.; Utsumi, S. Cloning and expression of rapeseed procruciferin in Escherichia coli and crystallization of the purified recombinant protein. Biotechnol. Lett. 2004, 26, 385–391. [Google Scholar] [CrossRef]
- Tandang-Silvas, M.R.G.; Fukuda, T.; Fukuda, C.; Prak, K.; Cabanos, C.; Kimura, A.; Itoh, T.; Mikami, B.; Utsumi, S.; Maruyama, N. Conservation and divergence on plant seed 11S globulins based on crystal structures. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 1432–1442. [Google Scholar] [CrossRef]
- Rico, M.; Bruix, M.; González, C.; Monsalve, R.I.; Rodríguez, R. 1H NMR assignment and global fold of napin BnIb, a representative 2S albumin seed protein. Biochemistry 1996, 35, 15672–15682. [Google Scholar] [CrossRef]
- Perera, S.; McIntosh, T.; Wanasundara, J. Structural Properties of Cruciferin and Napin of Brassica napus (Canola) Show Distinct Responses to Changes in pH and Temperature. Plants 2016, 5, 36. [Google Scholar] [CrossRef]
- Tzen, J.T.C. Integral Proteins in Plant Oil Bodies. ISRN Bot. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and functions of oleosomes (oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, P.; Boulard, C.; Bellamy, A.; Larré, C.; Barre, M.; Rogniaux, H.; D’Andréa, S.; Chardot, T.; Nesi, N. Protein composition of oil bodies from mature Brassica napus seeds. Proteomics 2009, 9, 3268–3284. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, B.; Chung, T.Y.; Chen, C.S.; Jiang, P.L.; Tzen, J.T.C. Identification of steroleosin in oil bodies of pine megagametophytes. Plant Physiol. Biochem. 2016, 101, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Faber, I.; Berton-Carabin, C.C.; Nikiforidis, C.V.; van der Linden, E.; Sagis, L.M.C. Foams and air-water interfaces stabilised by mildly purified rapeseed proteins after defatting. Food Hydrocoll. 2021, 112, 106270. [Google Scholar] [CrossRef]
- De Chirico, S.; di Bari, V.; Romero Guzmán, M.J.; Nikiforidis, C.V.; Foster, T.; Gray, D. Assessment of rapeseed oil body (oleosome) lipolytic activity as an effective predictor of emulsion purity and stability. Food Chem. 2020, 316, 126355. [Google Scholar] [CrossRef]
- Ntone, E.; Bitter, J.H.; Nikiforidis, C.V. Not sequentially but simultaneously: Facile extraction of proteins and oleosomes from oilseeds. Food Hydrocoll. 2020, 102, 105598. [Google Scholar] [CrossRef]
- Boulard, C.; Bardet, M.; Chardot, T.; Dubreucq, B.; Gromova, M.; Guillermo, A.; Miquel, M.; Nesi, N.; Yen-Nicolaÿ, S.; Jolivet, P. The structural organization of seed oil bodies could explain the contrasted oil extractability observed in two rapeseed genotypes. Planta 2015, 242, 53–68. [Google Scholar] [CrossRef]
- Dingyuan, F.; Jianjun, Z. Nutritional and anti-nutritional composition of rapeseed meal and its utilization as a feed ingredient for animal. Int. Consult. Gr. Res. Rapeseed 2007, Volume V, 265–270. [Google Scholar]
- Bell, J.M. Factors affecting the nutritional value of canola meal: A review. Can. J. Anim. Sci. 1993, 73, 689–697. [Google Scholar] [CrossRef]
- United States-Canadian Tables of Feed Composition; National Academies Press: Washington, DC, USA, 1982; ISBN 978-0-309-03245-2.
- Bojanowska, M. Changes in chemical composition of rapeseed meal during storage, influencing nutritional value of its protein and lipid fractions. J. Anim. Feed Sci. 2017, 26, 157–164. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; McIntosh, T.C.; Perera, S.P.; Withana-Gamage, T.S.; Mitra, P. Canola/rapeseed protein-functionality and nutrition. OCL-Oilseeds Fats Crop. Lipids 2016, 23, D407. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Spinks, E.A.; Wilkinson, A.P.; Heaney, R.K.; Legoy, M.A. Effect of processing on the antinutrient content of rapeseed. J. Sci. Food Agric. 1986, 37, 735–741. [Google Scholar] [CrossRef]
- Wnęk-Auguścik, K.; Witeska, M.; Niemiec, T.; Piotrowska, I.; Fajkowska, M.; Gomułka, P.; Kondera, E.; Łozicki, A.; Zglińska, K.; Rzepkowska, M. The effects of diets containing rapeseed meal on Siberian sturgeon (Acipenser baerii) growth, muscle composition, and physiological performance. Aquac. Rep. 2024, 34, 101891. [Google Scholar] [CrossRef]
- Ochodzki, P.; Piotrowska, A. Właściwości fizyczne i skład chemiczny nasion rzepaku ozimego o różnym kolorze okrywy nasiennej. Rośliny Oleiste-Oilseed Crop. 2002, 23, 235–241. [Google Scholar]
- Hernacki, B. Rzepak żółtonasienny—Aktualny stan badań w skali światowej, problemy i zagadnienia. Rośliny Oleiste-Oilseed Crop. 2007, 28, 125–150. [Google Scholar]
- Smulikowska, S.; Święch, E.; Czerwiński, J. Wartość paszowa żółtonasiennych roślin oleistych z rodzaju Brassica dla drobiu i świń*. Rośliny Oleiste-Oilseed Crop. 2008, 29, 231–242. [Google Scholar]
- Qu, C.; Yin, N.; Chen, S.; Wang, S.; Chen, X.; Zhao, H.; Shen, S.; Fu, F.; Zhou, B.; Xu, X.; et al. Comparative Analysis of the Metabolic Profiles of Yellow- versus Black-Seeded Rapeseed Using UPLC–HESI–MS/MS and Transcriptome Analysis. J. Agric. Food Chem. 2020, 68, 3033–3049. [Google Scholar] [CrossRef] [PubMed]
- Theodoridou, K.; Zhang, X.; Vail, S.; Yu, P. Magnitude Differences in Bioactive Compounds, Chemical Functional Groups, Fatty Acid Profiles, Nutrient Degradation and Digestion, Molecular Structure, and Metabolic Characteristics of Protein in Newly Developed Yellow-Seeded and Black-Seeded Canola Lines. J. Agric. Food Chem. 2015, 63, 5476–5484. [Google Scholar] [CrossRef]
- Myszka, K.; Boros, D.; Piotrowska, A.; Bartkowiak-Broda, I. Porównanie składu chemicznego śrut rzepakowych uzyskanych z rzepaku ozimego (Brassica napus L.) o zróżnicowanej barwie nasion. Rośliny Oleiste-Oilseed Crop. 2011, 32, 257–268. [Google Scholar]
- Slominski, B.A.; Meng, X.; Jia, W.; Nyachoti, M.; Jones, O.; Rakow, G. Chemical composition and nutritive value of yellow-seeded Brassica napus canola. Feed Ind. Raw Mater. Feed 2004, 253–255. [Google Scholar]
- Slominski, B.A.; Jia, W.; Rogiewicz, A.; Nyachoti, C.M.; Hickling, D. Low-fiber canola. Part 1. Chemical and nutritive composition of the meal. J. Agric. Food Chem. 2012, 60, 12225–12230. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [PubMed]
- Schnug, E.; Haneklaus, S. Glucosinolates—The Agricultural Story. Adv. Bot. Res. 2016, 80, 281–302. [Google Scholar] [CrossRef]
- McGregor, D.I. Glucosinolate content of developing rapeseed (Brassica napus L. ’Midas’) Seedlings. Can. J. Plant Sci. 1988, 68, 367–380. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, C.; Bilal, M.; Wang, P.; Yang, R. Screening of glucosinolates degrading lactic acid bacteria and their utilization in rapeseed meal fermentation. Grain Oil Sci. Technol. 2024, 7, 168–176. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J. Glucosinolates and their breakdown products in food and food plants. CRC Crit. Rev. Food Sci. Nutr. 1982, 18, 123–201. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Kumar, V.; Kumar, V. Enzymes of aldoxime–nitrile pathway for organic synthesis. Rev. Environ. Sci. Bio/Technol. 2018, 17, 229–239. [Google Scholar] [CrossRef]
- Mavratzotis, M.; Cassel, S.; Montaut, S.; Rollin, P. ω-methylsulfanylalkyl glucosinolates: A general synthetic pathway. Molecules 2018, 23, 786. [Google Scholar] [CrossRef]
- Ibrahim, N.; Allart-Simon, I.; De Nicola, G.R.; Iori, R.; Renault, J.H.; Rollin, P.; Nuzillard, J.M. Advanced NMR-Based Structural Investigation of Glucosinolates and Desulfoglucosinolates. J. Nat. Prod. 2018, 81, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ribaillier, D.; Quinsac, A. Glucosinolates of rapeseed: What is the present status of their determination? Rev. Fr. des Corps Gras 1985, 32, 159–162. [Google Scholar]
- Elfakir, C.; Lafosse, M.; Viaud, M.C.; Dreux, M. New artificial standards for the HPLC analysis of natural glucosinolates. J. High Resolut. Chromatogr. 1992, 15, 392–398. [Google Scholar] [CrossRef]
- Lange, R.; Petrzika, M. Beiträge zur Charakterisierung der schwefelhaltigen Glucoside des Rapssamens—Thermospray-Technik/Massenspektrometrie. Lipid/Fett 1991, 93, 284–288. [Google Scholar] [CrossRef]
- Leoni, O.; Iori, R.; Palmieri, S. Immobilization of Myrosinase on Membrane for Determining the Glucosinolate Content of Cruciferous Material. J. Agric. Food Chem. 1991, 39, 2322–2326. [Google Scholar] [CrossRef]
- Olsen, C.E.; Huang, X.C.; Hansen, C.I.C.; Cipollini, D.; Ørgaard, M.; Matthes, A.; Geu-Flores, F.; Koch, M.A.; Agerbirk, N. Glucosinolate diversity within a phylogenetic framework of the tribe Cardamineae (Brassicaceae) unraveled with HPLC-MS/MS and NMR-based analytical distinction of 70 desulfoglucosinolates. Phytochemistry 2016, 132, 33–56. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Halkier, B.A. General Introduction to Glucosinolates. Adv. Bot. Res. 2016, 80, 1–14. [Google Scholar]
- Zhao, J.; Meng, J. Detection of loci controlling seed glucosinolate content and their association with Sclerotinia resistance in Brassica napus. Plant Breed. 2003, 122, 19–23. [Google Scholar] [CrossRef]
- Lüthy, B.; Matile, P. The mustard oil bomb: Rectified analysis of the subcellular organisation of the myrosinase system. Biochem. Physiol. Pflanz. 1984, 179, 5–12. [Google Scholar] [CrossRef]
- Frandsen, H.B.; Sørensen, J.C.; Jensen, S.K.; Markedal, K.E.; Joehnke, M.S.; Maribo, H.; Sørensen, S.; Sørensen, H. Non-enzymatic transformations of dietary 2-hydroxyalkenyl and aromatic glucosinolates in the stomach of monogastrics. Food Chem. 2019, 291, 77–86. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielińska, M.; Jeleń, H.H. Bitter taste of Brassica vegetables: The role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.d.C.; Carvajal, M. Myrosinase in Brassicaceae: The most important issue for glucosinolate turnover and food quality. Phytochem. Rev. 2015, 14, 1045–1051. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Cottaz, S.; Driguez, H.; Iori, R.; Palmieri, S.; Henrissat, B. The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure 1997, 5, 663–676. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Cottaz, S.; Rollin, P.; Vasella, A.; Henrissat, B. High resolution x-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J. Biol. Chem. 2000, 275, 39385–39393. [Google Scholar] [CrossRef]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Virtanen, A.I. Studies on organic sulphur compounds and other labile substances in plants. Phytochemistry 1965, 4, 207–228. [Google Scholar] [CrossRef]
- Tookey, H.L. Crambe Thioglucoside Glucohydrolase (EC 3.2.3.1): Separation of a Protein Required for Epithiobutane Formation. Can. J. Biochem. 1973, 51, 1654–1660. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Liu, Z.; Xie, Y.; Wang, H.; Mu, Y.; Huang, Y.; Feng, Y. Crystal structure of the Epithiospecifier Protein, ESP from Arabidopsis thaliana provides insights into its product specificity. Biochem. Biophys. Res. Commun. 2016, 478, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Burow, M. Tipping the Scales—Specifier Proteins in Glucosinolate Hydrolysis. IUBMB Life 2007, 59, 744–751. [Google Scholar] [CrossRef]
- Eisenschmidt-Bönn, D.; Schneegans, N.; Backenköhler, A.; Wittstock, U.; Brandt, W. Structural diversification during glucosinolate breakdown: Mechanisms of thiocyanate, epithionitrile and simple nitrile formation. Plant J. 2019, 99, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Y.; Wang, K.; Dong, Y.; Wang, W.; Feng, Y. Crystal structure of the nitrile-specifier protein NSP1 from Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 488, 147–152. [Google Scholar] [CrossRef]
- McDonald, P.; Edward, R.; Greenhalgh, J.F.D.; Morgan, E. Animal Nutrition, 5th ed.; Longman Publishing Group: Harlow, UK, 2000. [Google Scholar]
- Barnaba, C.; Larcher, R.; Nardin, T.; Dellacassa, E.; Nicolini, G. Glycosylated simple phenolic profiling of food tannins using high resolution mass spectrometry (Q-Orbitrap). Food Chem. 2018, 267, 196–203. [Google Scholar] [CrossRef]
- Naczk, M.; Amarowicz, R.; Sullivan, A.; Shahidi, F. Current research developments on polyphenolics of rapeseed/canola: A review. Food Chem. 1998, 62, 489–502. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, C.; Yang, M.; Zhou, Q.; Li, W.; Liu, C.; Huang, F. Primary Metabolites and Polyphenols in Rapeseed (Brassica napus L.) Cultivars in China. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 303–317. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Zhou, Z.-X.; Tan, M.; Ye, P.-P.; Shi, J.-C.; Zhang, H.-B.; Chen, Z.-W.; Zhou, T.-L.; Shu, X.-Q.; Cui, F.-J.; et al. Fragrant rapeseed oils: A review in production, volatile flavor formation and regulation. Ind. Crops Prod. 2024, 217, 118870. [Google Scholar] [CrossRef]
- Yates, K.; Pohl, F.; Busch, M.; Mozer, A.; Watters, L.; Shiryaev, A.; Kong Thoo Lin, P. Determination of sinapine in rapeseed pomace extract: Its antioxidant and acetylcholinesterase inhibition properties. Food Chem. 2019, 276, 768–775. [Google Scholar] [CrossRef]
- Quinn, L.; Gray, S.G.; Meaney, S.; Finn, S.; Kenny, O.; Hayes, M. Sinapinic and protocatechuic acids found in rapeseed: Isolation, characterisation and potential benefits for human health as functional food ingredients. Irish J. Agric. Food Res. 2017, 56, 104–119. [Google Scholar] [CrossRef]
- Ferreres, F.; Fernandes, F.; Sousa, C.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Metabolic and bioactivity insights into brassica oleracea var. acephala. J. Agric. Food Chem. 2009, 57, 8884–8892. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Niu, Y.; Jiang, M.; Guo, M.; Wan, C.; Hu, S.; Jin, H.; Huang, F. Characterization of the Factors that Influence Sinapine Concentration in Rapeseed Meal during Fermentation. PLoS ONE 2015, 10, e0116470. [Google Scholar] [CrossRef] [PubMed]
- Djilas, S.; Canadanovic-Brunet, J.; Cetkovic, G. Antioxidants in food. Hem. Ind. 2002, 56, 105–112. [Google Scholar] [CrossRef]
- Moirangthem, L.; Praveen, N. Phytochemical analysis and antioxidant activity studies of leaf of brassica juncea var. Rugosa through sequential solvent extraction. Int. J. Pharm. Sci. Res. 2020, 11, 343. [Google Scholar]
- Zardo, I.; Rodrigues, N.P.; Sarkis, J.R.; Marczak, L.D. Extraction and identification by mass spectrometry of phenolic compounds from canola seed cake. J. Sci. Food Agric. 2020, 100, 578–586. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cassida, K.A.; Turner, K.E.; Foster, J.G.; Hesterman, O.B. Comparison of detergent fiber analysis methods for forages high in pectin. Anim. Feed Sci. Technol. 2007, 135, 283–295. [Google Scholar] [CrossRef]
- Mongeau, R.; Brooks, S.P.J. Dietary Fiber: Determination. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015; pp. 383–391. ISBN 9780123849533. [Google Scholar]
- Bedford, M.R.; Cowieson, A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012, 173, 76–85. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, Z.; Yao, Y.; Ma, Y.; Zhang, D.; Chen, M.; Ren, D. Potential reinforcing properties of silane-modified rapeseed straw fibers in friction composites: Fractal dimension and circularity characterise powder flowability. Ind. Crops Prod. 2023, 206, 117644. [Google Scholar] [CrossRef]
- Raboy, V. myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003, 64, 1033–1043. [Google Scholar] [CrossRef]
- Liu, G.; Yan, L.; Wang, S.; Yuan, H.; Zhu, Y.; Xie, C.; Wang, P.; Yang, R. A novel type of sprout food development: Effects of germination on phytic acid, glucosinolates, and lipid profiles in rapeseed. Food Biosci. 2023, 55, 102893. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J. Phytate levels in diverse rat tissues: Influence of dietary phytate. Br. J. Nutr. 2001, 86, 225–231. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Variation of InsP4, InsP5 and InsP6 levels in tissues and biological fluids depending on dietary phytate. J. Nutr. Biochem. 2001, 12, 595–601. [Google Scholar] [CrossRef]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Vats, P.; Bhattacharyya, M.S.; Banerjee, U.C. Use of Phytases (myo-Inositolhexakisphosphate Phosphohydrolases) for Combatting Environmental Pollution: A Biological Approach. Crit. Rev. Environ. Sci. Technol. 2005, 35, 469–486. [Google Scholar] [CrossRef]
- Lei, X.G.; Porres, J.M. Phytase enzymology, applications, and biotechnology. Biotechnol. Lett. 2003, 25, 1787–1794. [Google Scholar] [CrossRef]
- Sashidhar, N.; Harloff, H.J.; Potgieter, L.; Jung, C. Gene editing of three BnITPK genes in tetraploid oilseed rape leads to significant reduction of phytic acid in seeds. Plant Biotechnol. J. 2020, 18, 2241–2250. [Google Scholar] [CrossRef]
- Karthick, C.; Nanthagopal, K. A comprehensive review on ecological approaches of waste to wealth strategies for production of sustainable biobutanol and its suitability in automotive applications. Energy Convers. Manag. 2021, 239, 114219. [Google Scholar] [CrossRef]
- Vasileiadou, A. From Organic Wastes to Bioenergy, Biofuels, and Value-Added Products for Urban Sustainability and Circular Economy: A Review. Urban Sci. 2024, 8, 121. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Rozenfelde, L.; Puke, M.; Vedernikovs, N.; Scherbaka, R.; Rapoport, A. Catalytic treatment of rapeseed straw for enhanced production of furfural and glucose for bioethanol production. Process Biochem. 2021, 102, 102–107. [Google Scholar] [CrossRef]
- Gaballah, E.S.; Abomohra, A.E.F.; Xu, C.; Elsayed, M.; Abdelkader, T.K.; Lin, J.; Yuan, Q. Enhancement of biogas production from rape straw using different co-pretreatment techniques and anaerobic co-digestion with cattle manure. Bioresour. Technol. 2020, 309, 123311. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Hu, F.; Zhang, S.; Lu, J.; Liu, S. Bio-pretreatment promote hydrolysis and acidification of oilseed rape straw: Roles of fermentation broth and micro-oxygen. Bioresour. Technol. 2020, 308, 123272. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Salvatore, I.; Leue-Rüegg, R.; Beretta, C.; Müller, N. Valorisation potential and challenges of food side product streams for food applications: A review using the example of Switzerland. Futur. Foods 2024, 9, 100325. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Chander Kuhad, R.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in valorization technologies to improve utilization of bio-based waste in bioeconomy context. Renew. Sustain. Energy Rev. 2020, 131, 109965. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Theodoropoulos, C.; Yousuf, A. Techno-economic evaluation of heat integrated second generation bioethanol and furfural coproduction. Biochem. Eng. J. 2019, 144, 89–103. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Ben, M.R.; Fal, S.; El Arroussi, H.; Peng, W.; Tabatabaei, M.; et al. Progress in valorisation of agriculture, aquaculture and shellfish biomass into biochemicals and biomaterials towards sustainable bioeconomy. Chemosphere 2022, 291, 133036. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sindhu, R.; Sirohi, R.; Kumar, V.; Ahluwalia, V.; Binod, P.; Juneja, A.; Kumar, D.; Yan, B.; Sarsaiya, S.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112122. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Kumar, R.; Malyan, S.K.; Pugazhendhi, A. Microbial fuel cells as a sustainable platform technology for bioenergy, biosensing, environmental monitoring, and other low power device applications. Fuel 2019, 255, 115682. [Google Scholar] [CrossRef]
- Batog, J.; Wawro, A. Chemical and Biological Deconstruction in the Conversion Process of Sorghum Biomass for Bioethanol. J. Nat. Fibers 2022, 19, 5827–5838. [Google Scholar] [CrossRef]
- Zanellati, A.; Spina, F.; Bonaterra, M.; Dinuccio, E.; Varese, G.C.; Scarpeci, T.E. Screening and evaluation of phenols and furans degrading fungi for the biological pretreatment of lignocellulosic biomass. Int. Biodeterior. Biodegrad. 2021, 161, 105246. [Google Scholar] [CrossRef]
- Giuliano, A.; Barletta, D.; De Bari, I.; Poletto, M. Techno-economic assessment of a lignocellulosic biorefinery co-producing ethanol and xylitol or furfural. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 43, pp. 585–590. ISBN 9780444642356. [Google Scholar]
- Kuglarz, M.; Alvarado-Morales, M.; Dąbkowska, K.; Angelidaki, I. Integrated production of cellulosic bioethanol and succinic acid from rapeseed straw after dilute-acid pretreatment. Bioresour. Technol. 2018, 265, 191–199. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E.; Mussatto, S.I. Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 2018, 247, 736–743. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Ruiz, E.; Moya, M.; Castro, E. Bioethanol production from rapeseed straw at high solids loading with different process configurations. Fuel 2014, 122, 112–118. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Papagianni, M.; Nokes, S.E.; Filer, K. Production of phytase by Aspergillus niger in submerged and solid-state fermentation. Process Biochem. 1999, 35, 397–402. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Ahmad, K.; Panday, V.K.; Mohammad, U. Enzyme-assisted extraction of oil (soybean, rapeseed, corn, canola, and peanut). In Enzymes in Oil Processing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 263–278. [Google Scholar]
- Nigam, P. Utilization of agricultural and food waste and by-products by biotechnology. Agro Food Ind. Hi. Tech. 2001, 12, 26–29. [Google Scholar]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Nigam, P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999, 77, 149–162. [Google Scholar]
- Ebune, A.; Al-Asheh, S.; Duvnjak, Z. Production of phytase during solid state fermentation using Aspergillus ficuum NRRL 3135 in canola meal. Bioresour. Technol. 1995, 53, 7–12. [Google Scholar] [CrossRef]
- Wang, R.; Shaarani, S.M.; Godoy, L.C.; Melikoglu, M.; Vergara, C.S.; Koutinas, A.; Webb, C. Bioconversion of rapeseed meal for the production of a generic microbial feedstock. Enzym. Microb. Technol. 2010, 47, 77–83. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef]
- Freitas, A.C.; Castro, R.J.S.; Fontenele, M.A.; Egito, A.S.; Farinas, C.S.; Pinto, G.A.S. Canola Cake as a Potential Substrate for Proteolytic Enzymes Production by a Selected Strain of Aspergillus oryzae: Selection of Process Conditions and Product Characterization. ISRN Microbiol. 2013, 2013, 369082. [Google Scholar] [CrossRef]
- Carboué, Q.; Tranier, M.-S.; Perraud-Gaime, I.; Roussos, S. Production of Microbial Enzymes by Solid-state Fermentation for Food Applications. In Microbial Enzyme Technology in Food Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 437–451. [Google Scholar]
- Santhosh Kumar, D. Fungal Lipase Production by Solid State Fermentation-An Overview. J. Anal. Bioanal. Technol. 2015, 06, 1000230. [Google Scholar] [CrossRef]
- Monié, A.; Habersetzer, T.; Sureau, L.; David, A.; Clemens, K.; Malet-Martino, M.; Perez, E.; Franceschi, S.; Balayssac, S.; Delample, M. Modulation of the crystallization of rapeseed oil using lipases and the impact on ice cream properties. Food Res. Int. 2023, 165, 112473. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Rehman, S. Optimization of growth parameters for lipase production by Ganoderma lucidum using response surface methodology. African J. Biotechnol. 2011, 10, 5514–5523. [Google Scholar]
- Bhatti, H.N.; Amin, F. Kinetic and hydrolytic characterization of newly isolated alkaline lipase from ganoderma lucidum using canola oil cake as substrate. J. Chem. Soc. Pakistan 2013, 35, 585–592. [Google Scholar]
- Boratyński, F.; Szczepańska, E.; Grudniewska, A.; Gniłka, R.; Olejniczak, T. Improving of hydrolases biosythesis by solid-state fermentation of Penicillium camemberti on rapeseed cake. Sci. Rep. 2018, 8, 10157. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bhatti, I.A.; Asgher, M. Optimization of process parameters for enhanced production of lipase by Penicillium notatum using agricultural wastes. African J. Biotechnol. 2011, 10, 19580–19589. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Optimization of process variables for enhanced production of extracellular lipase by Pleurotus ostreatus IBL-02 in solid-state fermentation. Pak. J. Pharm. Sci. 2019, 32, 617–624. [Google Scholar] [PubMed]
- Amin, M.; Bhatti, H.N. Effect of physicochemical parameters on lipase production by Penicillium fellutanum using canola seed oil cake as substrate. Int. J. Agric. Biol. 2014, 16, 118–124. [Google Scholar]
- Amin, M.; Bhatti, H.N.; Zuber, M.; Bhatti, I.A.; Asgher, M. Potential use of agricultural wastes for the production of lipase by Aspergillus melleus under solid state fermentation. J. Anim. Plant Sci. 2014, 24, 1430–1437. [Google Scholar]
- Freitas, A.C.; Baleeiro, F.C.F.; Fonseca, R.F.; Bertucci Neto, V.; Pinto, G.A.S.; Farinas, C.S. Bioprocess development to add value to canola cake used as substrate for proteolytic enzyme production. Food Bioprod. Process. 2015, 95, 173–182. [Google Scholar] [CrossRef]
- Treichel, H.; Sbardelotto, M.; Venturin, B.; Agnol, A.D.; Mulinari, J.; Golunski, S.M.; Baldoni, D.B.; Bevilacqua, C.B.; Seminoti Jacques, R.J.; Vargas, G.D.L.P.; et al. Lipase Production from a Newly Isolated Aspergillus niger by Solid State Fermentation Using Canola Cake as Substrate. Curr. Biotechnol. 2015, 6, 295–300. [Google Scholar] [CrossRef]
- Imandi, S.B.; Karanam, S.K.; Garapati, H.R. Use of Plackett-Burman design for rapid screening of nitrogen and carbon sources for the production of lipase in solid state fermentation by Yarrowia lipolytica from mustard oil cake (Brassica napus). Brazilian J. Microbiol. 2013, 44, 915–921. [Google Scholar] [CrossRef]
- Souza, C.E.C.; Farias, M.A.; Ribeiro, B.D.; Coelho, M.A.Z. Adding Value to Agro-industrial Co-products from Canola and Soybean Oil Extraction Through Lipase Production Using Yarrowia lipolytica in Solid-State Fermentation. Waste Biomass Valorization 2017, 8, 1163–1176. [Google Scholar] [CrossRef]
- Nascimento, F.; Lemes, A.; Castro, A.; Secchi, A.; Zarur Coelho, M. A Temporal Evolution Perspective of Lipase Production by Yarrowia lipolytica in Solid-State Fermentation. Processes 2022, 10, 381. [Google Scholar] [CrossRef]
- Sparvoli, F.; Cominelli, E. Seed Biofortification and Phytic Acid Reduction: A Conflict of Interest for the Plant? Plants 2015, 4, 728–755. [Google Scholar] [CrossRef]
- Colombo, F.; Paolo, D.; Cominelli, E.; Sparvoli, F.; Nielsen, E.; Pilu, R. MRP Transporters and Low Phytic Acid Mutants in Major Crops: Main Pleiotropic Effects and Future Perspectives. Front. Plant Sci. 2020, 11, 576091. [Google Scholar] [CrossRef]
- Wyss, M.; Pasamontes, L.; Friedlein, A.; Rémy, R.; Tessier, M.; Kronenberger, A.; Middendorf, A.; Lehmann, M.; Schnoebelen, L.; Röthlisberger, U.; et al. Biophysical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): Molecular size, glycosylation pattern, and engineering of proteolytic resistance. Appl. Environ. Microbiol. 1999, 65, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.M.; Koh, K.; Rix, G.D. Structural and functional profile of phytases across the domains of life. Curr. Res. Struct. Biol. 2024, 7, 100139. [Google Scholar] [CrossRef]
- Cromwell, G.L.; Coffey, R.D.; Monegue, H.J.; Randolph, J.H. Efficacy of low-activity, microbial phytase in improving the bioavailability of phosphorus in corn-soybean meal diets for pigs. J. Anim. Sci. 1995, 73, 449–456. [Google Scholar] [CrossRef]
- Traylor, S.L.; Cromwell, G.L.; Lindemann, M.D.; Knabe, D.A. Effects of level of supplemental phytase on ileal digestibility of amino acids, calcium, and phosphorus in dehulled soybean meal for growing pigs. J. Anim. Sci. 2001, 79, 2634–2642. [Google Scholar] [CrossRef]
- Liao, S.F.; Kies, A.K.; Sauer, W.C.; Zhang, Y.C.; Cervantes, M.; He, J.M. Effect of phytase supplementation to a low- and a high-phytate diet for growing pigs on the digestibilities of crude protein, amino acids, and energy. J. Anim. Sci. 2005, 83, 2130–2136. [Google Scholar] [CrossRef]
- Newkirk, R.; Classen, H. The non-mineral nutritional impact of phytate in canola meal fed to broiler chicks. Anim. Feed Sci. Technol. 2001, 91, 115–128. [Google Scholar] [CrossRef]
- Stein, H.H.; Lagos, L.V.; Casas, G.A. Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed Sci. Technol. 2016, 218, 33–69. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, X.; Hu, S.; He, R.; Ju, X.; Wang, Z.; Aluko, R.E. Effects of phytase/ethanol treatment on aroma characteristics of rapeseed protein isolates. Food Chem. 2024, 431, 137119. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.-F.; Suriyagoda, L.D.B.; Lambers, H. Tightening the Phosphorus Cycle through Phosphorus-Efficient Crop Genotypes. Trends Plant Sci. 2020, 25, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bogar, B.; Szakacs, G.; Pandey, A.; Abdulhameed, S.; Linden, J.C.; Tengerdy, R.P. Production of Phytase by Mucor racemosus in Solid-State Fermentation. Biotechnol. Prog. 2003, 19, 312–319. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Duvnjak, Z. The effect of surfactants on the phytase production and the reduction of the phytic acid content in canola meal by Aspergillus carbonarius during a solid state fermentation process. Biotechnol. Lett. 1994, 16, 183–188. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Duvnjak, Z. Phytase production and decrease of phytic acid content in canola meal by Aspergillus carbonarius in solid-state fermentation. World J. Microbiol. Biotechnol. 1995, 11, 228–231. [Google Scholar] [CrossRef]
- Mandviwala, T.N.; Khire, J.M. Production of high activity thermostable phytase from thermotolerant Aspergillus niger in solid state fermentation. J. Ind. Microbiol. Biotechnol. 2000, 24, 237–243. [Google Scholar] [CrossRef]
- El-Batal, A.; Abdel Karem, H. Phytase production and phytic acid reduction in rapeseed meal by Aspergillus niger during solid state fermentation. Food Res. Int. 2001, 34, 715–720. [Google Scholar] [CrossRef]
- Fadel, M.; El-Batal, A.I. Studies on Activation of Amylolytic Enzymes Production by Gamma Irradiated Aspergillus niger Using Some Surfactants and Natural Oils under Solid State Fermentation. Pakistan J. Biol. Sci. 2000, 3, 1762–1768. [Google Scholar] [CrossRef]
- Herrmann, K.R.; Ruff, A.J.; Schwaneberg, U. Phytase-based phosphorus recovery process for 20 distinct press cakes. ACS Sustain. Chem. Eng. 2020, 8, 3913–3921. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gat, Y.; Arya, S.; Kumar, V.; Panghal, A.; Kumar, A. A Review on Microbial Alkaline Protease: An Essential Tool for Various Industrial Approaches. Ind. Biotechnol. 2019, 15, 69–78. [Google Scholar] [CrossRef]
- Gupta, R.; Beg, Q.K.; Khan, S.; Chauhan, B. An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl. Microbiol. Biotechnol. 2002, 60, 381–395. [Google Scholar]
- Mukhtar, H.; Haq, I. Comparative evaluation of agroindustrial byproducts for the production of alkaline protease by wild and mutant strains of bacillus subtilis in submerged and solid state fermentation. Sci. World J. 2013, 2013, 538067. [Google Scholar] [CrossRef]
- Daudi, S.; Mukhtar, H.; Rehman, A.U.; Haq, I.U. Production of rennin-like acid protease by mucor pusillus through submerged fermentation. Pakistan J. Bot. 2015, 47, 1121–1127. [Google Scholar]
- Bajpai, P. Industrial applications of thermophilic/hyperthermophilic enzymes. In Developments and Applications of Enzymes from Thermophilic Microorganisms; Academic Press: Cambridge, MA, USA, 2023; pp. 105–284. ISBN 978-0-443-19197-8. [Google Scholar]
- Yanagisawa, Y.; Chatake, T.; Chiba-Kamoshida, K.; Naito, S.; Ohsugi, T.; Sumi, H.; Yasuda, I.; Morimoto, Y. Purification, crystallization and preliminary X-ray diffraction experiment of nattokinase from Bacillus subtilis natto. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1670–1673. [Google Scholar] [CrossRef]
- Yang, L.; Cao, S.; Xie, M.; Shi, T. Virtual screening, activity evaluation, and stability of pancreatic lipase inhibitors in the gastrointestinal degradation of nattokinase. Heliyon 2024, 10, e24868. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liao, J.; Fan, L.; Yin, L. Breeding of a Producing High Vitality Bacillus natto Kinase Strains with Fermenting Rapeseed Meal. J. Anhui Agric. Sci. 2012, 40, 16567–16569. [Google Scholar]
- Neagu, D.; Destain, J.; Thonart, P.; Socaciu, C. Effects of Different Carbon Sources on Pectinase Production by Penicillium oxalicum. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Agric. 2012, 69. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Guo, Y.; Song, X.; Yin, J.; Nie, S. Exploring the partial degradation of polysaccharides: Structure, mechanism, bioactivities, and perspectives. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4831–4870. [Google Scholar] [CrossRef] [PubMed]
- Polizeli, M.L.T.M.; Rizzatti, A.C.S.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Phuyal, M.; Budhathoki, U.; Bista, D.; Shakya, S.; Shrestha, R.; Shrestha, A.K. Xylanase-Producing Microbes and Their Real-World Application. Int. J. Chem. Eng. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Gattinger, L.D.; Duvnjak, Z.; Khan, A.W. The use of canola meal as a substrate for xylanase production by Trichoderma reesei. Appl. Microbiol. Biotechnol. 1990, 33, 21–25. [Google Scholar] [CrossRef]
- Almeida, J.M.; Lima, V.A.; Giloni-Lima, P.C.; Knob, A. Canola meal as a novel substrate for β-glucosidase production by Trichoderma viride: Application of the crude extract to biomass saccharification. Bioprocess Biosyst. Eng. 2015, 38, 1889–1902. [Google Scholar] [CrossRef]
- Bandikari, R.; Katike, U.; Seelam, N.S.; Obulam, V.S.R. Trichoderma koningii izolatının tarafından merkezi kompozit tasarımı kullanılarak ksilanaz üretimi ve optimizasyonu için de yağlanmış kek Valorizasyon. Turkish J. Biochem. 2017, 42, 317–328. [Google Scholar] [CrossRef]
- Dahiya, S.; Rapoport, A.; Singh, B. Biotechnological Potential of Lignocellulosic Biomass as Substrates for Fungal Xylanases and Its Bioconversion into Useful Products: A Review. Fermentation 2024, 10, 82. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Zaworska-Zakrzewska, A.; Łodyga, D.; Józefiak, D. The Effect of Enzymatic Fermentation on the Chemical Composition and Contents of Antinutrients in Rapeseed Meal. Fermentation 2024, 10, 107. [Google Scholar] [CrossRef]
- Yeoman, K.H.; Edwards, C. Protease production by Streptomyces thermovulgaris grown on rapemeal-derived media. J. Appl. Bacteriol. 1994, 77, 264–270. [Google Scholar] [CrossRef]
- Yeoman, K.H.; Edwards, C. Purification and characterization of the protease enzymes of Streptomyces thermovulgaris grown in rapemeal-derived media. J. Appl. Microbiol. 1997, 82, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Torres, M.; Reyes, A. ARTÍCULO CORTO Characterization of Aspergillus ficuum hydrolytic enzymes produced in solid state fermentation of cold-pressed canola cake. Rev. Colomb. Biotecnología 2012, 14, 208–215. [Google Scholar]
- Costa, M.; Torres, M.; Magariños, H.; Reyes, A. Production and Partial Purification of Aspergillus ficuum Hydrolytic Enzymes in Solid State Fermentation of Agroindustrial Residues; Instituto de Biotecnología, Universidad Nacional de Colombia: Bogota, Colombia, 2010; Volume XII. [Google Scholar]
- Wilk, M.; Gąsiorek, E.; Marzec, D. Tworzenie celulaz i ksylanaz w procesie biosyntezy kwasu szczawiowego metodą hodowli w podłożu stałym z makuchu rzepakowego. Nauk. Inżynierskie Technol. 2011, 3, 245–257. [Google Scholar]
- Konkol, D.; Szmigiel, I.; Domżał-Kędzia, M.; Kułażyński, M.; Krasowska, A.; Opaliński, S.; Korczyński, M.; Łukaszewicz, M. Biotransformation of rapeseed meal leading to production of polymers, biosurfactants, and fodder. Bioorg. Chem. 2019, 93, 102865. [Google Scholar] [CrossRef]
- Shimotori, Y.; Hoshi, M.; Okabe, H.; Miyakoshi, T.; Kanamoto, T.; Nakashima, H. Synthesis, odour characteristics and antibacterial activities of the stereoisomeric forms of whisky lactone and its thiono analogues. Flavour Fragr. J. 2017, 32, 29–35. [Google Scholar] [CrossRef]
- Günther, C.; Mosandl, A. Stereoisomere Aromastoffe, XII. 3-Methyl-4-octanolid—„Quercuslacton, Whiskylacton”—Struktur und Eigenschaften der Stereoisomeren. Liebigs Ann. Chemie 1986, 1986, 2112–2122. [Google Scholar] [CrossRef]
- Boratyński, F.; Smuga, M.; Wawrzeńczyk, C. Lactones 42. Stereoselective enzymatic/microbial synthesis of optically active isomers of whisky lactone. Food Chem. 2013, 141, 419–427. [Google Scholar] [CrossRef]
- Boratyński, F.; Szczepańska, E.; Grudniewska, A.; Skalny, B.; Olejniczak, T. A Novel Approach for Microbial Synthesis of Enantiomerically Pure Whisky Lactones Based on Solid-State Fermentation. Molecules 2018, 23, 659. [Google Scholar] [CrossRef]
- Hernik, D.; Gatti, F.; Brenna, E.; Szczepańska, E.; Olejniczak, T.; Boratyński, F. Stereoselective synthesis of whisky lactone isomers catalyzed by bacteria in the genus Rhodococcus. Front. Microbiol. 2023, 14, 1117835. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Laprevote, O.; Peypoux, F. Diversity Among Microbial Cyclic Lipopeptides: Iturins and Surfactins. Activity-Structure Relationships to Design New Bioactive Agents. Comb. Chem. High Throughput Screen. 2012, 6, 541–556. [Google Scholar] [CrossRef]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The Surfactin-Like Lipopeptides from Bacillus spp.: Natural Biodiversity and Synthetic Biology for a Broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef]
- Roongsawang, N.; Washio, K.; Morikawa, M. Diversity of Nonribosomal Peptide Synthetases Involved in the Biosynthesis of Lipopeptide Biosurfactants. Int. J. Mol. Sci. 2010, 12, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Zhen, C.; Ge, X.-F.; Lu, Y.-T.; Liu, W.-Z. Chemical structure, properties and potential applications of surfactin, as well as advanced strategies for improving its microbial production. AIMS Microbiol. 2023, 9, 195–217. [Google Scholar] [CrossRef]
- Yao, D.; Ji, Z.; Wang, C.; Qi, G.; Zhang, L.; Ma, X.; Chen, S. Co-producing iturin A and poly-γ-glutamic acid from rapeseed meal under solid state fermentation by the newly isolated Bacillus subtilis strain 3-10. World J. Microbiol. Biotechnol. 2012, 28, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, X.; Li, K.; Niu, Y.; Guo, M.; Hu, C.; Wan, X.; Gong, Y.; Huang, F. Direct bio-utilization of untreated rapeseed meal for effective iturin a production by Bacillus subtilis in submerged fermentation. PLoS ONE 2014, 9, e111171. [Google Scholar] [CrossRef]
- Jin, H.; Li, K.; Niu, Y.; Guo, M.; Hu, C.; Chen, S.; Huang, F. Continuous enhancement of iturin A production by Bacillus subtilis with a stepwise two-stage glucose feeding strategy. BMC Biotechnol. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, X.; Wang, X.; Chen, S.; Rogiewicz, A.; Slominski, B.; Wan, X.; Huang, F. Establishment of a rapeseed meal fermentation model for iturin A production by Bacillus amyloliquefaciens CX-20. Microb. Biotechnol. 2019, 12, 1417–1429. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Ma, X.; Chen, S.; Kang, Y.; Yang, M.; Huang, F.; Wan, X. Simultaneous hydrolysis with lipase and fermentation of rapeseed cake for iturin A production by Bacillus amyloliquefaciens CX-20. BMC Biotechnol. 2019, 19, 98. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Gong, Y.; Deng, Q.; Zheng, M.; Chen, S.; Wan, X.; Yang, C.; Huang, F. The unconventional adverse effects of fungal pretreatment on iturin A fermentation by Bacillus amyloliquefaciens CX-20. Microb. Biotechnol. 2021, 14, 587–599. [Google Scholar] [CrossRef]
- Jajor, P.; Piłakowska-Pietras, D.; Krasowska, A.; Łukaszewicz, M. Surfactin analogues produced by Bacillus subtilis strains grown on rapeseed cake. J. Mol. Struct. 2016, 1126, 141–146. [Google Scholar] [CrossRef]
- Szymanowska-Powałowska, D. 1,3-Propanediol production from crude glycerol by Clostridium butyricum DSP1 in repeated batch. Electron. J. Biotechnol. 2014, 17, 322–328. [Google Scholar] [CrossRef]
- Tey, K.Y.; Tan, J.P.; Yeap, S.K.; He, N.; Bukhari, N.A.; Hui, Y.W.; Luthfi, A.A.I.; Abdul Manaf, S.F. Current analysis on 1,3-propanediol production from glycerol via pure wild strain fermentation. J. Environ. Chem. Eng. 2023, 11, 110998. [Google Scholar] [CrossRef]
- Drozdzyńska, A.; Pawlicka, J.; Kubiak, P.; Kośmider, A.; Pranke, D.; Olejnik-Schmidt, A.; Czaczyk, K. Conversion of glycerol to 1,3-propanediol by Citrobacter freundii and Hafnia alvei—Newly isolated strains from the Enterobacteriaceae. N. Biotechnol. 2014, 31, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jin, L.; Li, Y.; Xu, H.; Lin, Y.; Zhou, T.; Zheng, B.; Wang, M.; Wang, Z. Complete-genome sequencing and comparative genomic characterization of blaNDM-5 carrying Citrobacter freundii isolates from a patient with multiple infections. BMC Genom. 2023, 24, 506. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Aggelis, G.; Komaitis, M.; Zeng, A.P.; Papanikolaou, S. Impact of anaerobiosis strategy and bioreactor geometry on the biochemical response of Clostridium butyricum VPI 1718 during 1,3-propanediol fermentation. Bioresour. Technol. 2011, 102, 10625–10632. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Papanikolaou, S.; Kopsahelis, N.; Kachrimanidou, V.; Dorado, M.P.; Koutinas, A.A. Biorefinery development through utilization of biodiesel industry by-products as sole fermentation feedstock for 1,3-propanediol production. Bioresour. Technol. 2014, 159, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Michael-Titus, A.T. Neurological benefits of omega-3 fatty acids. NeuroMolecular Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.M.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The Effect of Dietary Supplementation with n—3 Polyunsaturated Fatty Acids on the Synthesis of Interleukin-1 and Tumor Necrosis Factor by Mononuclear Cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef]
- Ouagueni, A.; Al-Zoubi, R.M.; Zarour, A.; Al-Ansari, A.; Bawadi, H. Effects of Omega-3 Polyunsaturated Fatty Acids, Docosahexaenoic Acid and Eicosapentaenoic Acid, on Post-Surgical Complications in Surgical Trauma Patients: Mechanisms, Nutrition, and Challenges. Mar. Drugs 2024, 22, 207. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Sowmiya, G.; Jeslin, J.; Chamundeeswari, M.; Verma, M.L. Algal biotechnology: A sustainable route for omega-3 fatty acid production. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 125–145. ISBN 9789811501692. [Google Scholar]
- Gong, Y.; Liu, J.; Jiang, M.; Liang, Z.; Jin, H.; Hu, X.; Wan, X.; Hu, C. Improvement of Omega-3 Docosahexaenoic Acid Production by Marine Dinoflagellate Crypthecodinium cohnii Using Rapeseed Meal Hydrolysate and Waste Molasses as Feedstock. PLoS ONE 2015, 10, e0125368. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Bajestani, R.S.; Nemati, F.; Kolahi, S.A. Production of clavulanic acid by streptomyces clavuligerus bacteria using rapeseed meal as the source of nitrogen. Biomed. Pharmacol. J. 2016, 9, 631–637. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Wan, X.; Huang, H.; Li, J.; Nomura, C.T.; Wang, C.; Chen, S. Optimization of Inexpensive Agricultural By-Products as Raw Materials for Bacitracin Production in Bacillus licheniformis DW2. Appl. Biochem. Biotechnol. 2017, 183, 1146–1157. [Google Scholar] [CrossRef]
- Zeng, X.; Miao, W.; Zeng, H.; Zhao, K.; Zhou, Y.; Zhang, J.; Zhao, Q.; Tursun, D.; Xu, D.; Li, F. Production of natamycin by Streptomyces gilvosporeus Z28 through solid-state fermentation using agro-industrial residues. Bioresour. Technol. 2019, 273, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yao, H.; Xu, Z.; Wang, R.; Xu, Z.; Li, S.; Feng, X.; Liu, Y.; Xu, H. Production of ε-poly-lysine by Streptomyces albulus PD-1 via solid-state fermentation. Bioresour. Technol. 2017, 223, 149–156. [Google Scholar] [CrossRef]
- Sutter, S.; Thevenieau, F.; Bourdillon, A.; De Coninck, J. Immunomodulatory Properties of Filamentous Fungi Cultivated through Solid-State Fermentation on Rapeseed Meal. Appl. Biochem. Biotechnol. 2017, 182, 910–924. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Hao, S.; Jin, S.; Li, X. Improvement of the Nutritional Quality of Rapeseed Meal through Solid-State Fermentation with B. subtilis, S. cerevisiae, and B. amyloliquefaciens. Fermentation 2023, 9, 492. [Google Scholar] [CrossRef]
- Mansour, E.H.; Dworschák, E.; Lugasi, A.; Gaál, Ö.; Barna, É.; Gergely, A. Effect of processing on the antinutritive factors and nutritive value of rapeseed products. Food Chem. 1993, 47, 247–252. [Google Scholar] [CrossRef]
- Bournazel, M.; Lessire, M.; Duclos, M.J.; Magnin, M.; Même, N.; Peyronnet, C.; Recoules, E.; Quinsac, A.; Labussière, E.; Narcy, A. Effects of rapeseed meal fiber content on phosphorus and calcium digestibility in growing pigs fed diets without or with microbial phytase. Animal 2018, 12, 34–42. [Google Scholar] [CrossRef]
- Lan, S.; Mao, X.; Xiao, T.; Wang, H.; Deng, Y.; Tan, B. Detoxification Conditions of Solid-State Fermentation from Rapeseed Meal by Mixture Strains Using Response Surface Methodology. Acta Zoonutrimenta Sin. 2013, 25, 617–627. [Google Scholar]
- Hou, X.; Dai, C.; Tang, Y.; Xing, Z.; Mintah, B.K.; Dabbour, M.; Ding, Q.; He, R.; Ma, H. Thermophilic solid-state fermentation of rapeseed meal and analysis of microbial community diversity. LWT 2019, 116, 108520. [Google Scholar] [CrossRef]
- European Food Safety Authority Scientific Opinion on the safety of “rapeseed protein isolate” as a Novel Food ingredient. EFSA J. 2013, 11, 23. [CrossRef]
- Wanasundara, J.P.D.; Tan, S.; Alashi, A.M.; Pudel, F.; Blanchard, C. Proteins from Canola/Rapeseed. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–304. [Google Scholar]
- Pal Vig, A.; Walia, A. Beneficial effects of Rhizopus oligosporus fermentation on reduction of glucosinolates, fibre and phytic acid in rapeseed (Brassica napus) meal. Bioresour. Technol. 2001, 78, 309–312. [Google Scholar] [CrossRef]
- Elhussieny, N.I.; El-Refai, H.A.; Mohamed, S.S.; Shetaia, Y.M.; Amin, H.A.; Klöck, G. Rhizopus stolonifer biomass catalytic transesterification capability: Optimization of cultivation conditions. Microb. Cell Fact. 2023, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Lücke, F.K.; Fritz, V.; Tannhäuser, K.; Arya, A. Controlled fermentation of rapeseed presscake by Rhizopus, and its effect on some components with relevance to human nutrition. Food Res. Int. 2019, 120, 726–732. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Q.; Wang, T.; Huang, J.; Xia, Y.; Yao, L.; Wang, X. Screening of glucosinolate-degrading strains and its application in improving the quality of rapeseed meal. Ann. Microbiol. 2012, 62, 1013–1020. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Chen, D. Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Li, L.; Liu, J.; Liu, C.; Fu, J.; Xiao, X.; Wang, G.; Wang, J. Two-step biological approach for treatment of rapeseed meal. J. Food Sci. 2020, 85, 340–348. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C. Isolation and identification of a glucosinolates-degrading bacteria strain. Pratacultural Sci. 2013, 30, 1862–1865. [Google Scholar]
- Wang, X.-D.; Mai, K.-S.; Zhang, Y.-J.; Ai, Q.-H.; Xu, W.; Zhang, W.-B.; Hu, H.-B. Degradation of Tannins and Phytic Acid in Double-Low Rapeseed Meal by Aspergillus niger in Solid Fermentation and Optimization of Fermenting Condition. Period. Ocean Univ. China 2013, 43, 15–22. [Google Scholar]
- Rakariyatham, N.; Butr-Indr, B.; Niamsup, H.; Shank, L. Improvement of myrosinase activity of Aspergillus sp. NR4617 by chemical mutagenesis. Electron. J. Biotechnol. 2006, 9, 341–347. [Google Scholar] [CrossRef]
- Butrindr, B.; Niamsup, H.; Shank, L.; Rakariyatham, N. Myrosinase overproducing mutants of Aspergillus sp. NR463. Ann. Microbiol. 2004, 54, 493–501. [Google Scholar]
- Xu, F.Z.; Zeng, X.G.; Ding, X.L. Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian-Australasian J. Anim. Sci. 2012, 25, 1734–1741. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wei, F.; Liu, X.; Yi, C.; Zhang, Y. Improvement of the nutritional value, sensory properties and bioavailability of rapeseed meal fermented with mixed microorganisms. LWT 2019, 112, 108238. [Google Scholar] [CrossRef]
- Grewal, J.; Tiwari, R.; Khare, S.K. Secretome Analysis and Bioprospecting of Lignocellulolytic Fungal Consortium for Valorization of Waste Cottonseed Cake by Hydrolase Production and Simultaneous Gossypol Degradation. Waste and Biomass Valorization 2020, 11, 2533–2548. [Google Scholar] [CrossRef]
- Gu, B.; Ma, H.; Liu, B.; He, R.; Luo, L. Fermentation of rapeseed meal to prepare peptide by mixed strains. Sci. Technol. Food Ind. 2011, 32, 190–196. [Google Scholar]
- Huang, Q.; Huang, F.; Niu, Y.; Zhou, H. Removal of glucosinolates from rapeseed meal by solid fermentation. China Oils Fats 2011, 1, 34–37. [Google Scholar]
- Gu, B.; Ma, H.; Liu, B. Preparation of Bio-Feedstuff with Rich Peptides from Rapeseed Meal by Mixed Solid Fermentation. J. Chinese Cereal. Oils Assoc. 2011, 1, 83–87. [Google Scholar]
- Liu, L.; Guo, Y.; Qiu, S.; Zhou, H. Study on improving the nutritive value of cold-pressed rapeseed cake with microbes under solid-state fermentation. Food Sci. Technol. 2011, 8, 16–19. [Google Scholar]
- Jiang, B.; Pan, J.; Xie, R.; Qin, J.; He, R.; Ma, H. Screening of strains used for producing rapeseed peptide and degradation of glucosinolates by solid-state fermentation. Sci. Technol. Food Ind. 2015, 36, 164–168. [Google Scholar]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Liu, N.; Huang, G.; Zhou, Q. Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping. Agric. 2022, 12, 1538. [Google Scholar] [CrossRef]
- Neale, M. The regulation of natural products as crop-protection agents. Pest Manag. Sci. 2000, 56, 677–680. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014, 70, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Menkissoglu-Spiroudi, U. Pesticides of Botanical Origin: A Promising Tool in Plant Protection. In Pesticides—Formulations, Effects, Fate; InTech: London, UK, 2011. [Google Scholar]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bennett, A.J.; Hilton, S.; Bending, G.D.; Chandler, D.; Mills, P. Impact of fresh root material and mature crop residues of oilseed rape (Brassica napus) on microbial communities associated with subsequent oilseed rape. Biol. Fertil. Soils 2014, 50, 1267–1279. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A. Biofumigation potential of brassicas II. Effect of environment and ontogeny on glucosinolate production and implications for screening. Plant Soil 1998, 201, 91–101. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation potential of brassicas III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Rumberger, A.; Marschner, P. 2-Phenylethylisothiocyanate concentration and microbial community composition in the rhizosphere of canola. Soil Biol. Biochem. 2003, 35, 445–452. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Eugui, D.; Velasco, P.; Abril-Urías, P.; Escobar, C.; Gómez-Torres, Ó.; Caballero, S.; Poveda, J. Glucosinolate-extracts from residues of conventional and organic cultivated broccoli leaves (Brassica oleracea var. italica) as potential industrially-scalable efficient biopesticides against fungi, oomycetes and plant parasitic nematodes. Ind. Crops Prod. 2023, 200, 116841. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Sørensen, J.C.; Tovgaard, L.; Jørgensen, A.M.F.; Hansen, H.C.B. Degradation kinetics of glucosinolates in soil. Environ. Toxicol. Chem. 2006, 25, 2038–2044. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; Burns, I.G. Fate of nitrogen from crop residues as affected by biochemical quality and the microbial biomass. Soil Biol. Biochem. 1998, 30, 2055–2065. [Google Scholar] [CrossRef]
- Bending, G.D.; Lincoln, S.D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biol. Biochem. 2000, 32, 1261–1269. [Google Scholar] [CrossRef]
- Kaur, A.; Chaudhary, A.; Kaur, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid—A bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Pollierer, M.M.; Ferlian, O.; Scheu, S. Temporal dynamics and variation with forest type of phospholipid fatty acids in litter and soil of temperate forests across regions. Soil Biol. Biochem. 2015, 91, 248–257. [Google Scholar] [CrossRef]
- Orwin, K.H.; Dickie, I.A.; Holdaway, R.; Wood, J.R. A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions. Soil Biol. Biochem. 2018, 117, 27–35. [Google Scholar] [CrossRef]
- Hansen, J.C.; Schillinger, W.F.; Sullivan, T.S.; Paulitz, T.C. Soil microbial biomass and fungi reduced with canola introduced into long-term monoculture wheat rotations. Front. Microbiol. 2019, 10, 1488. [Google Scholar] [CrossRef]
- Baggs, E.M.; Watson, C.A.; Rees, R.M. The fate of nitrogen from incorporated cover crop and green manure residues. Nutr. Cycl. Agroecosyst. 2000, 56, 153–163. [Google Scholar] [CrossRef]
- Hansen, J.C.; Schillinger, W.F.; Sullivan, T.S.; Paulitz, T.C. Rhizosphere microbial communities of canola and wheat at six paired field sites. Appl. Soil Ecol. 2018, 130, 185–193. [Google Scholar] [CrossRef]
- Wanniarachchi, S.D.; Voroney, R.P. Phytotoxicity of canola residues: Release of water-soluble phytotoxins. Can. J. Soil Sci. 1997, 77, 535–541. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Hydrolysis products of glucosinolates in Brassica napus tissues as inhibitors of seed germination. Plant Soil 1996, 181, 307–316. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping for weed management: A review. Renew. Agric. Food Syst. 2004, 19, 187–198. [Google Scholar] [CrossRef]
- Bell, D.T.; Muller, C.H. Dominance of California Annual Grasslands by Brassica nigra. Am. Midl. Nat. 1973, 90, 277. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, M.; Shi, J.; Li, R.; Shen, Q. Adding protein materials as solid-state fermentation media for producing bio-organic fertilizers with functional microbes. Nanjing Nongye Daxue Xuebao 2014, 37, 85–91. [Google Scholar]
- Hu, X.; Roberts, D.P.; Xie, L.; Maul, J.E.; Yu, C.; Li, Y.; Zhang, S.; Liao, X. Development of a biologically based fertilizer, incorporating Bacillus megaterium A6, for improved phosphorus nutrition of oilseed rape. Can. J. Microbiol. 2013, 59, 231–236. [Google Scholar] [CrossRef]

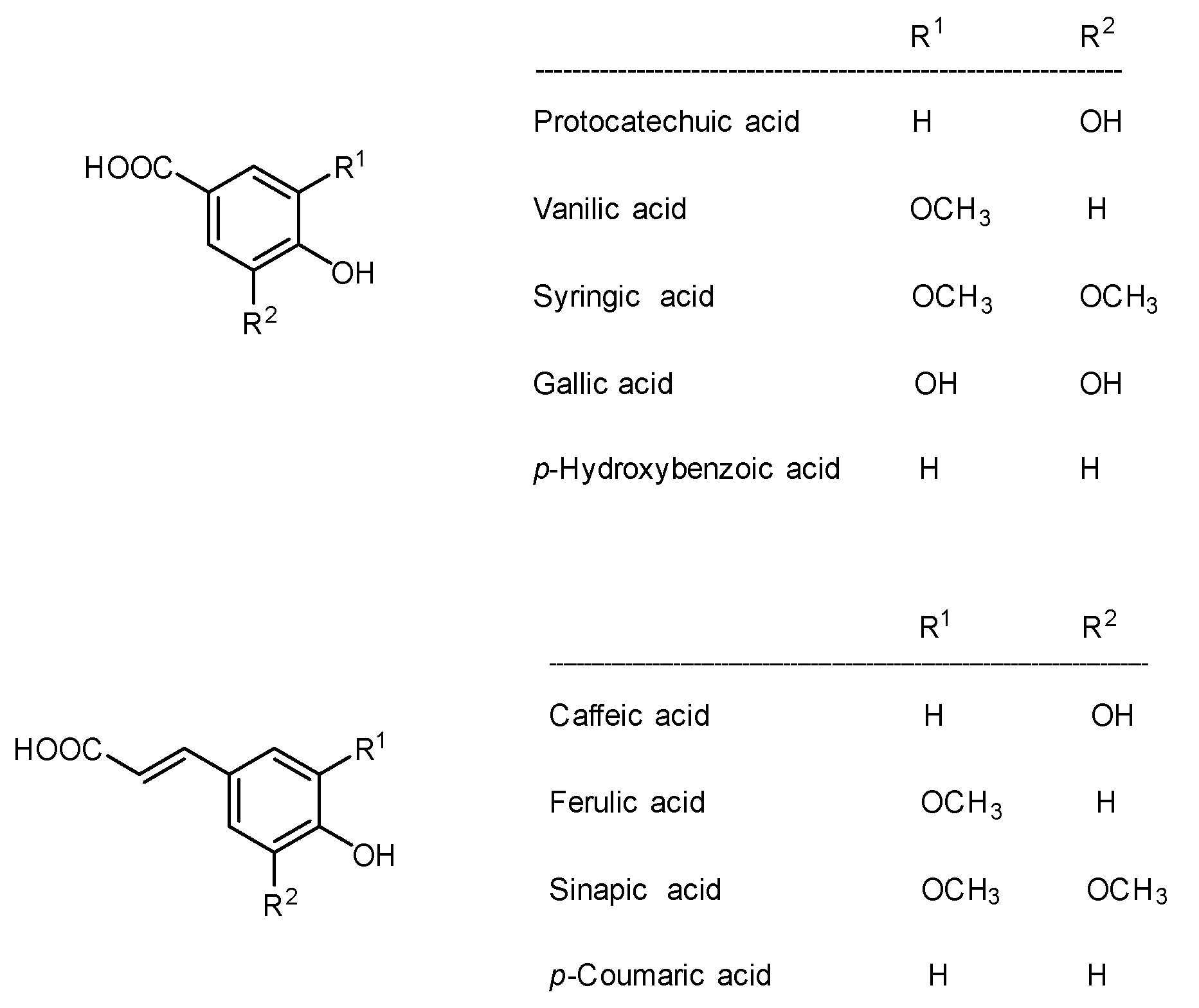
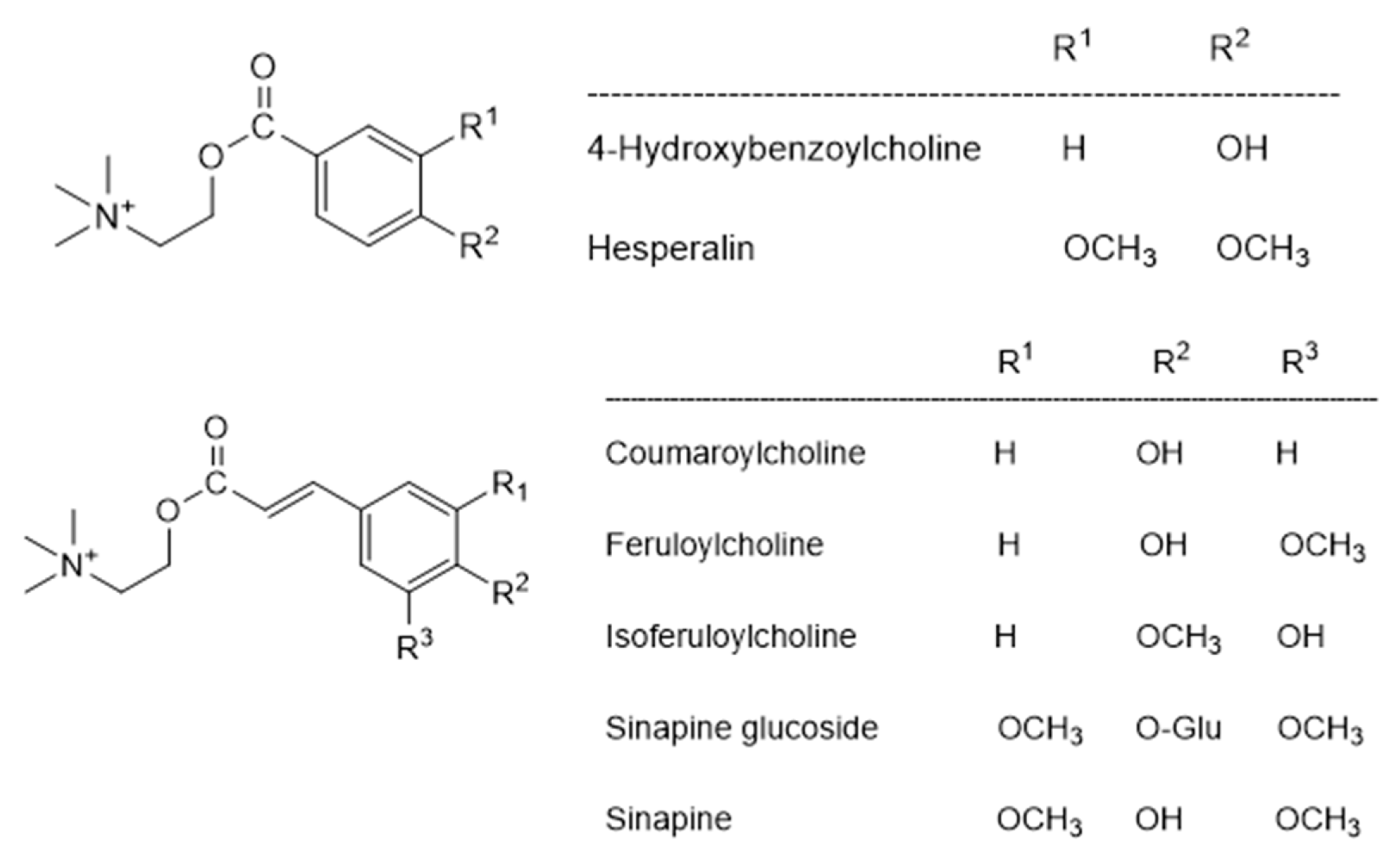
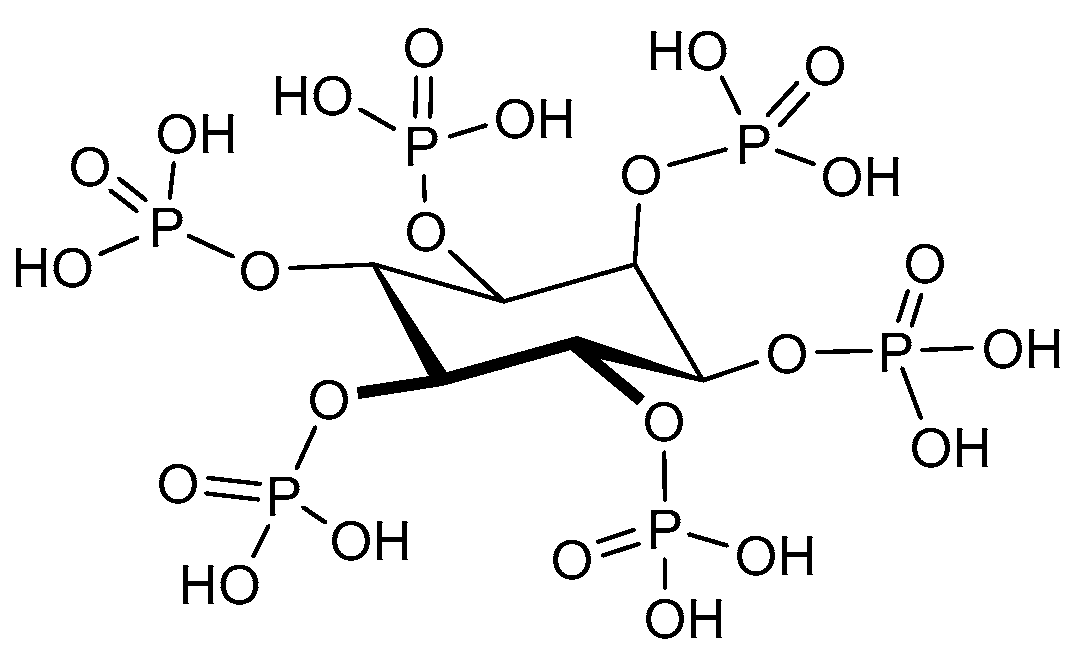
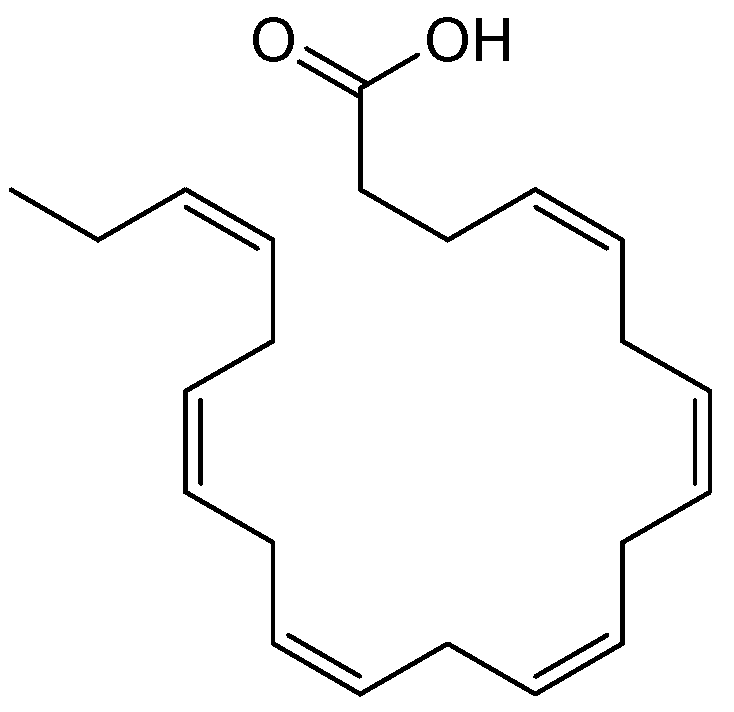
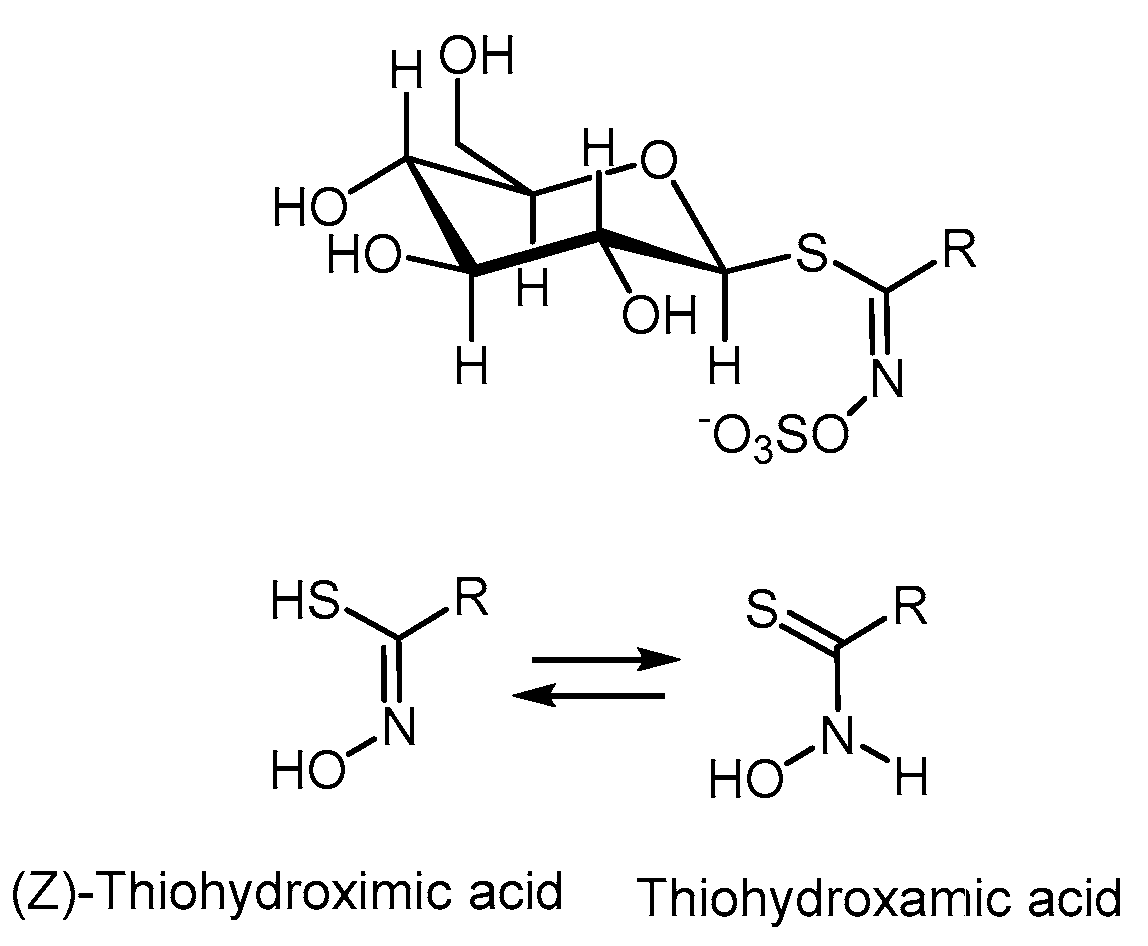
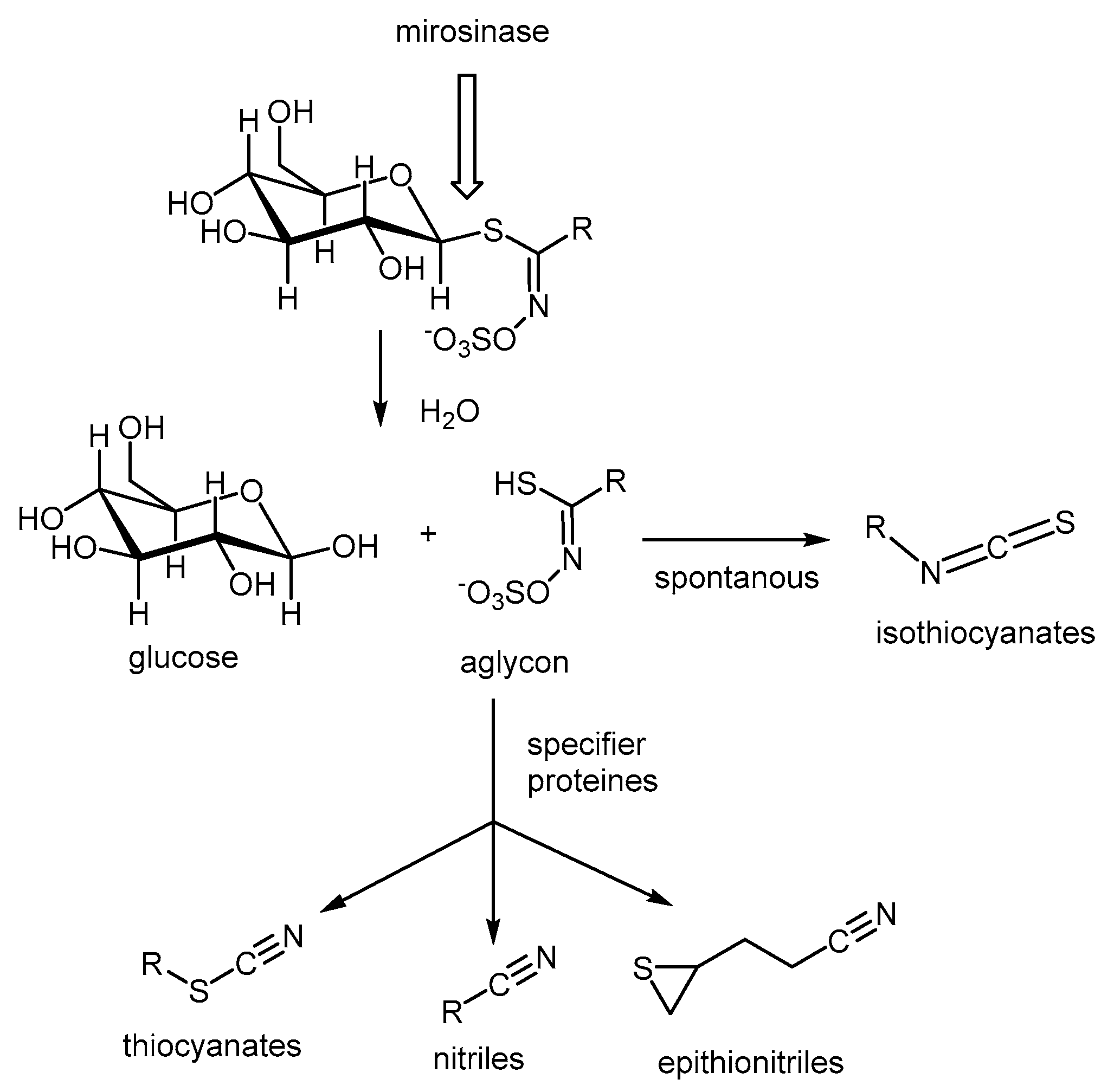
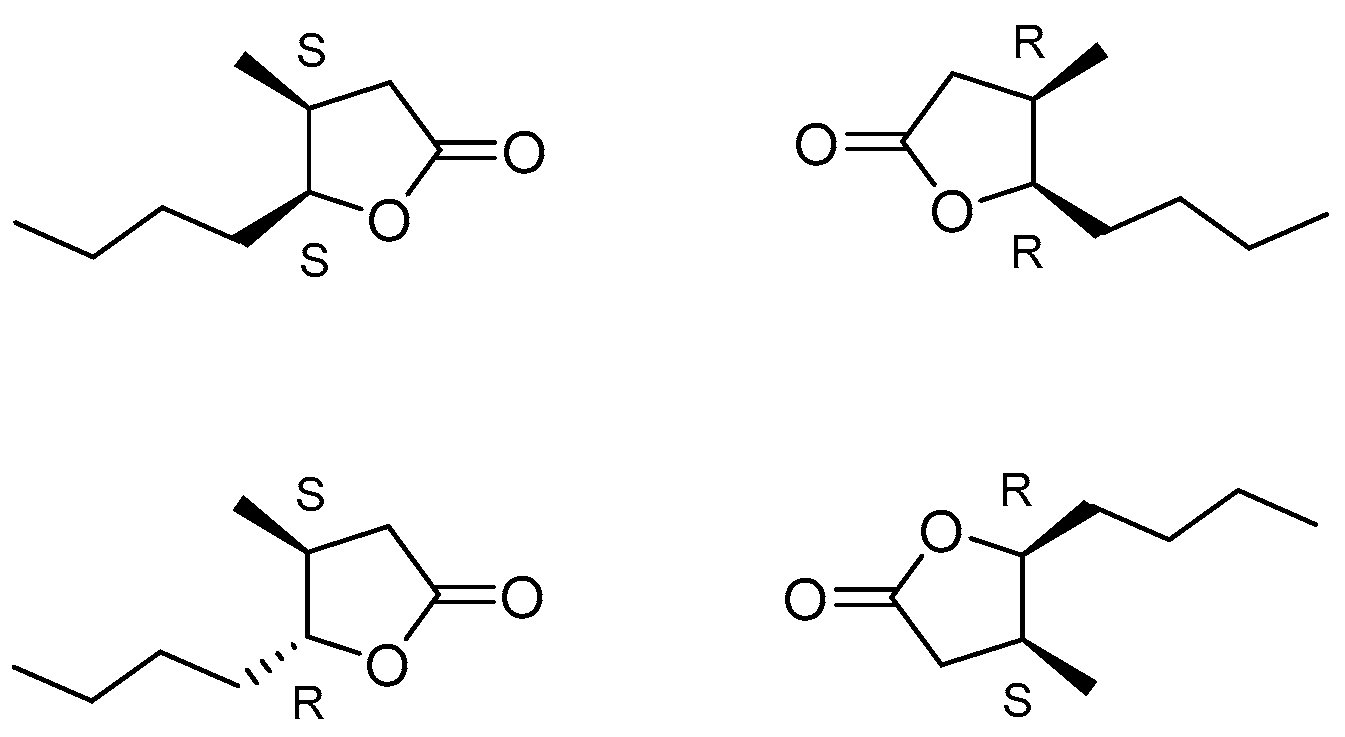
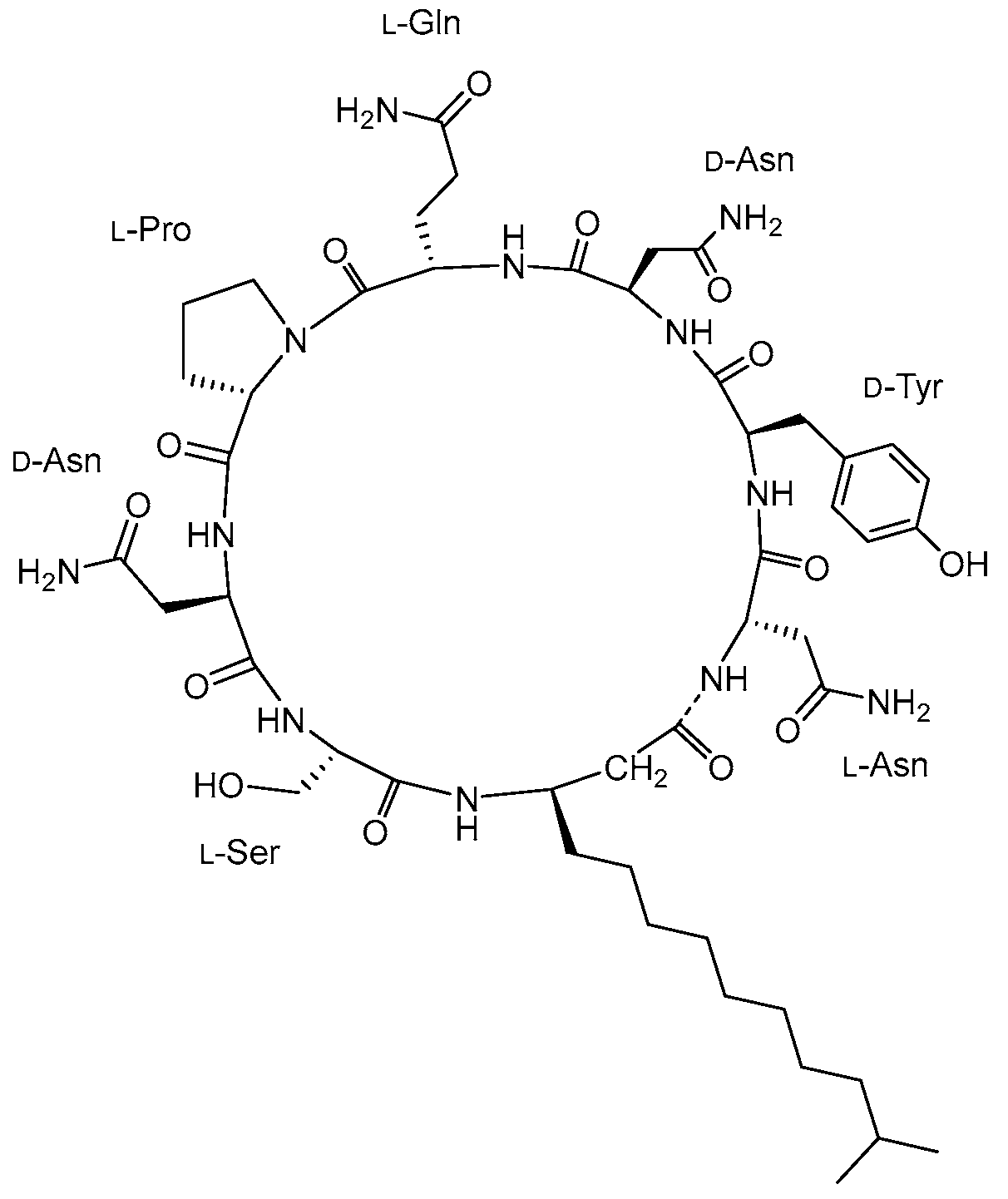
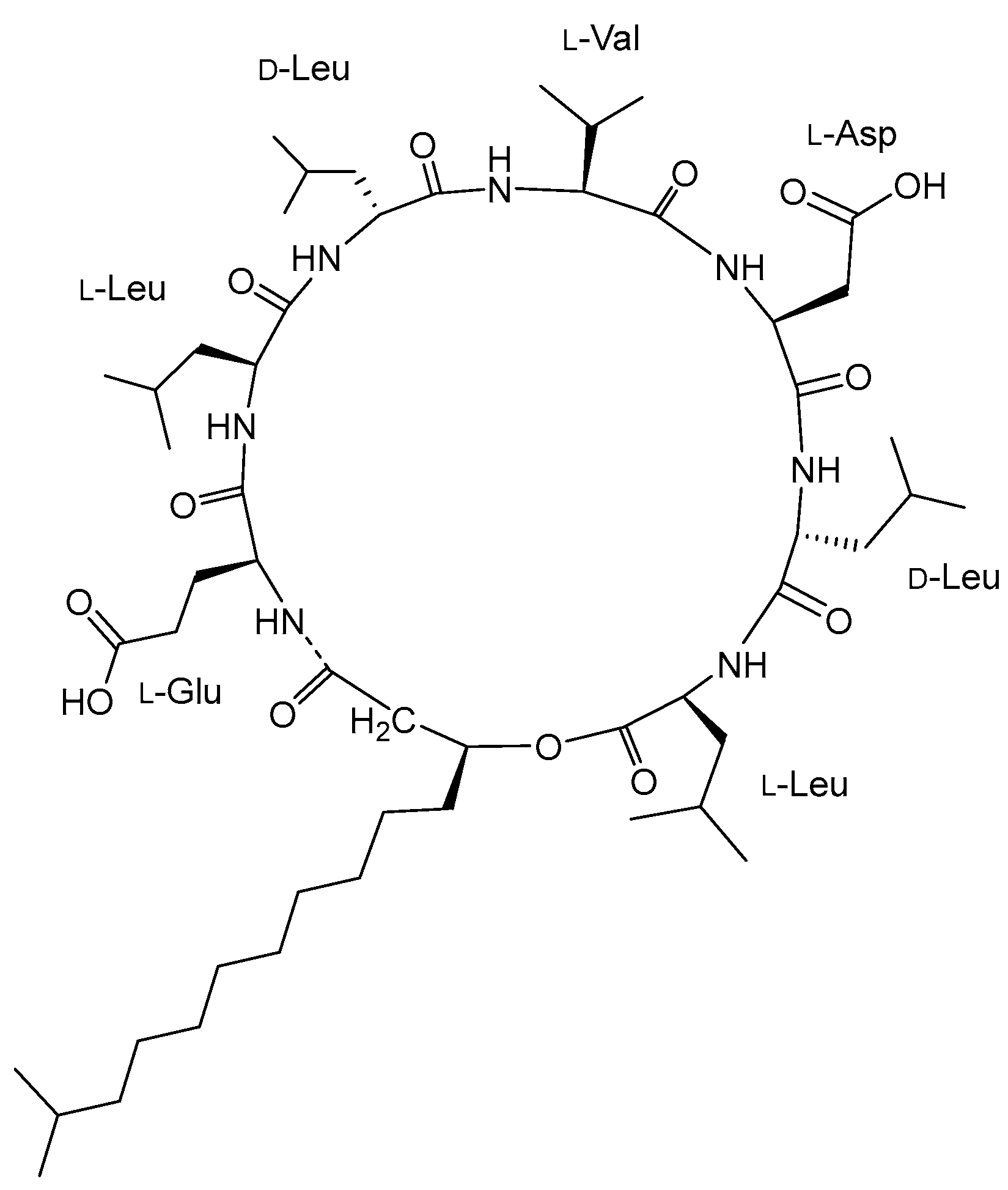
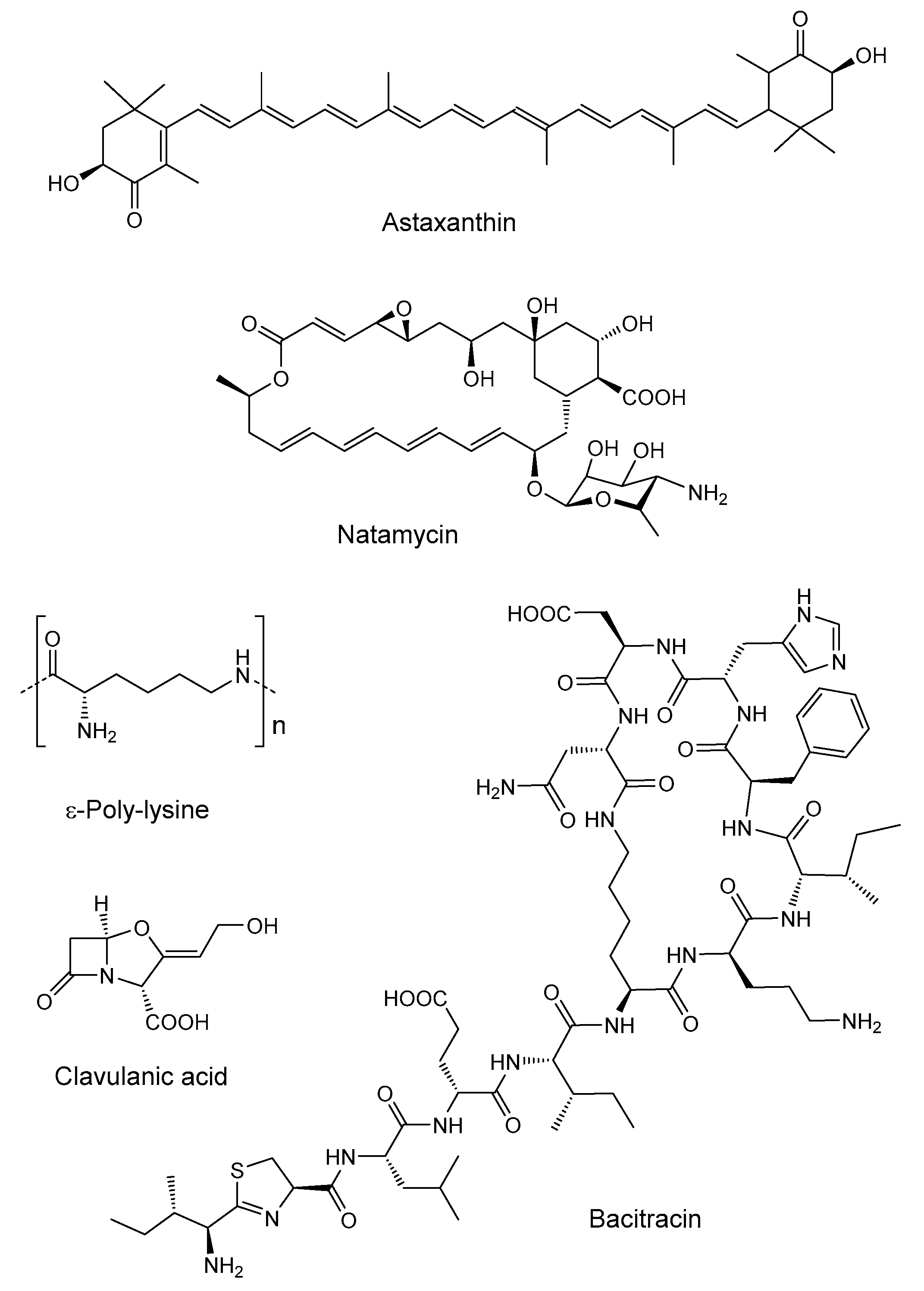
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binczarski, M.J.; Zuberek, J.; Fraczyk, J.; Kolesinska, B.; Radojčin, M.; Pavkov, I.; Wiktorowska-Sowa, E.; Piotrowski, J.; Kaminski, Z.J.; Witonska, I.A. Application of Microorganisms for the Valorization of Side-Products of Rapeseed De-Oiling. Biomolecules 2025, 15, 917. https://doi.org/10.3390/biom15070917
Binczarski MJ, Zuberek J, Fraczyk J, Kolesinska B, Radojčin M, Pavkov I, Wiktorowska-Sowa E, Piotrowski J, Kaminski ZJ, Witonska IA. Application of Microorganisms for the Valorization of Side-Products of Rapeseed De-Oiling. Biomolecules. 2025; 15(7):917. https://doi.org/10.3390/biom15070917
Chicago/Turabian StyleBinczarski, Michal Jacek, Justyna Zuberek, Justyna Fraczyk, Beata Kolesinska, Milivoj Radojčin, Ivan Pavkov, Ewa Wiktorowska-Sowa, Jan Piotrowski, Zbigniew Jerzy Kaminski, and Izabela Alina Witonska. 2025. "Application of Microorganisms for the Valorization of Side-Products of Rapeseed De-Oiling" Biomolecules 15, no. 7: 917. https://doi.org/10.3390/biom15070917
APA StyleBinczarski, M. J., Zuberek, J., Fraczyk, J., Kolesinska, B., Radojčin, M., Pavkov, I., Wiktorowska-Sowa, E., Piotrowski, J., Kaminski, Z. J., & Witonska, I. A. (2025). Application of Microorganisms for the Valorization of Side-Products of Rapeseed De-Oiling. Biomolecules, 15(7), 917. https://doi.org/10.3390/biom15070917


_Kwok.png)