Emerging Insights into the Relationship Between Amino Acid Metabolism and Diabetic Cardiomyopathy
Abstract
1. Introduction
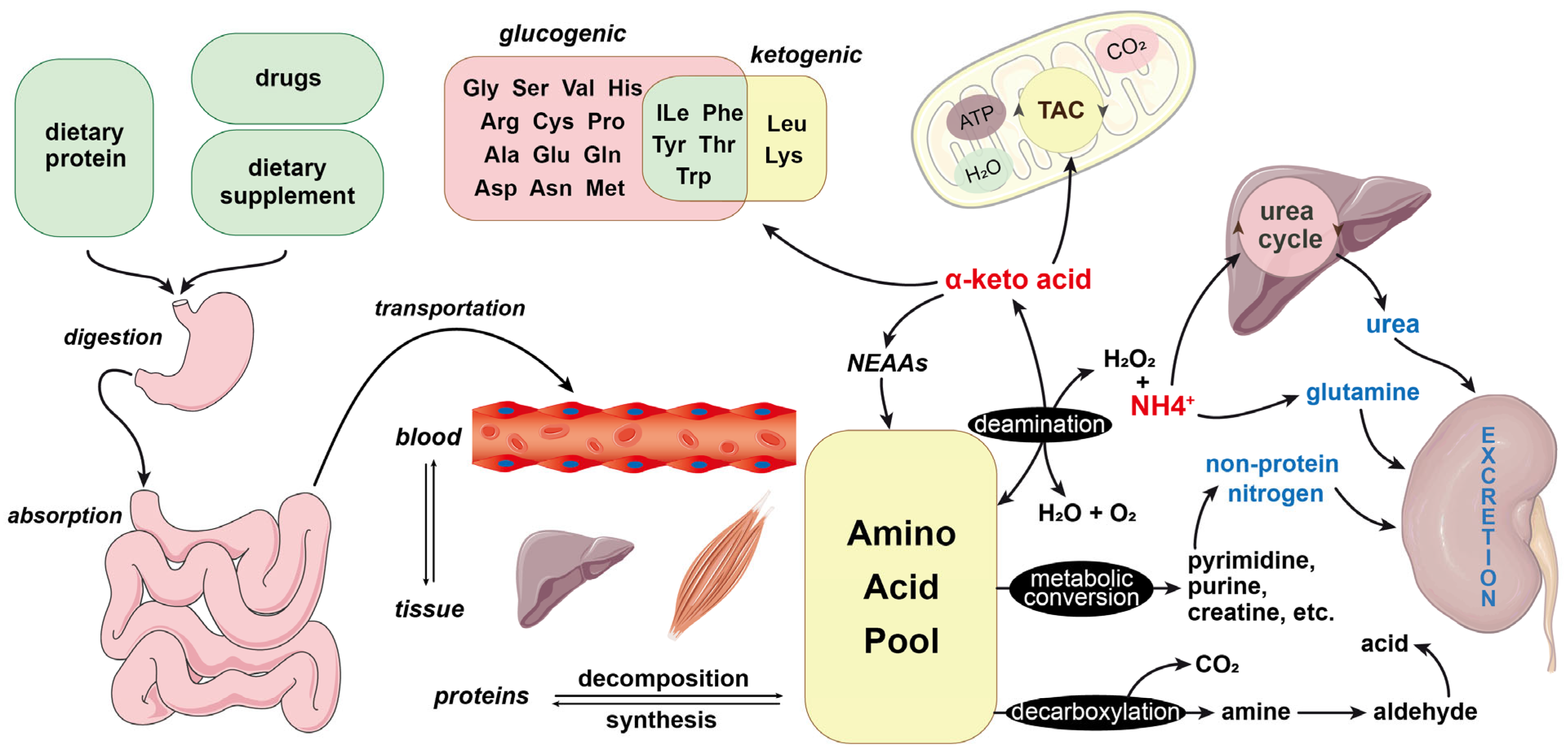
2. Overview of Amino Acids and Their Metabolism
3. Amino Acid Anabolism
4. Amino Acid Catabolism
4.1. Deamination of Amino Acids
4.2. Metabolism of Blood Ammonia
4.3. Metabolism of Individual Amino Acids
4.3.1. Aromatic Amino Acids
4.3.2. Histidine
4.3.3. Serine
4.3.4. Cysteine and Cystine
4.3.5. Arginine
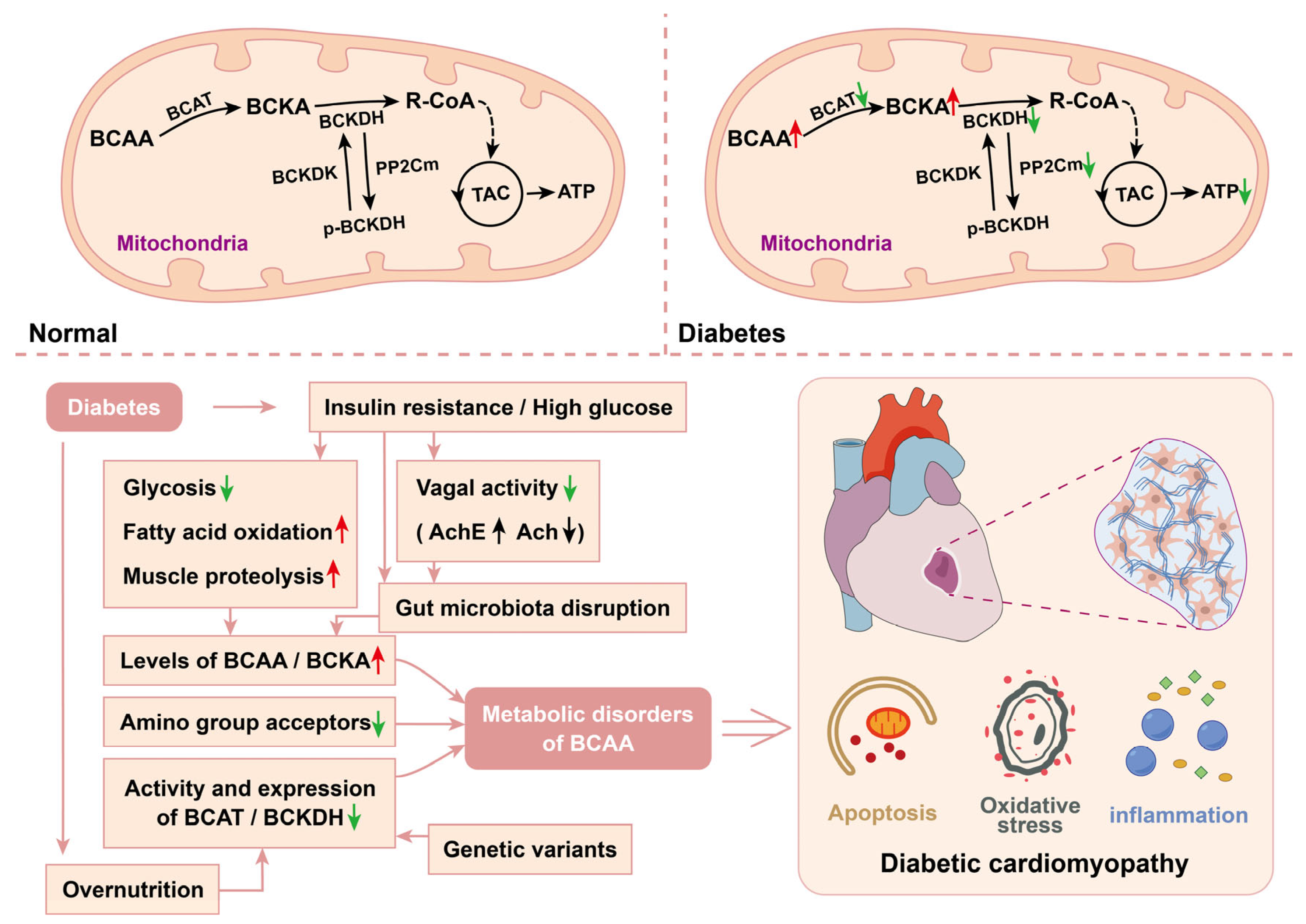
4.3.6. Branched-Chain Amino Acids
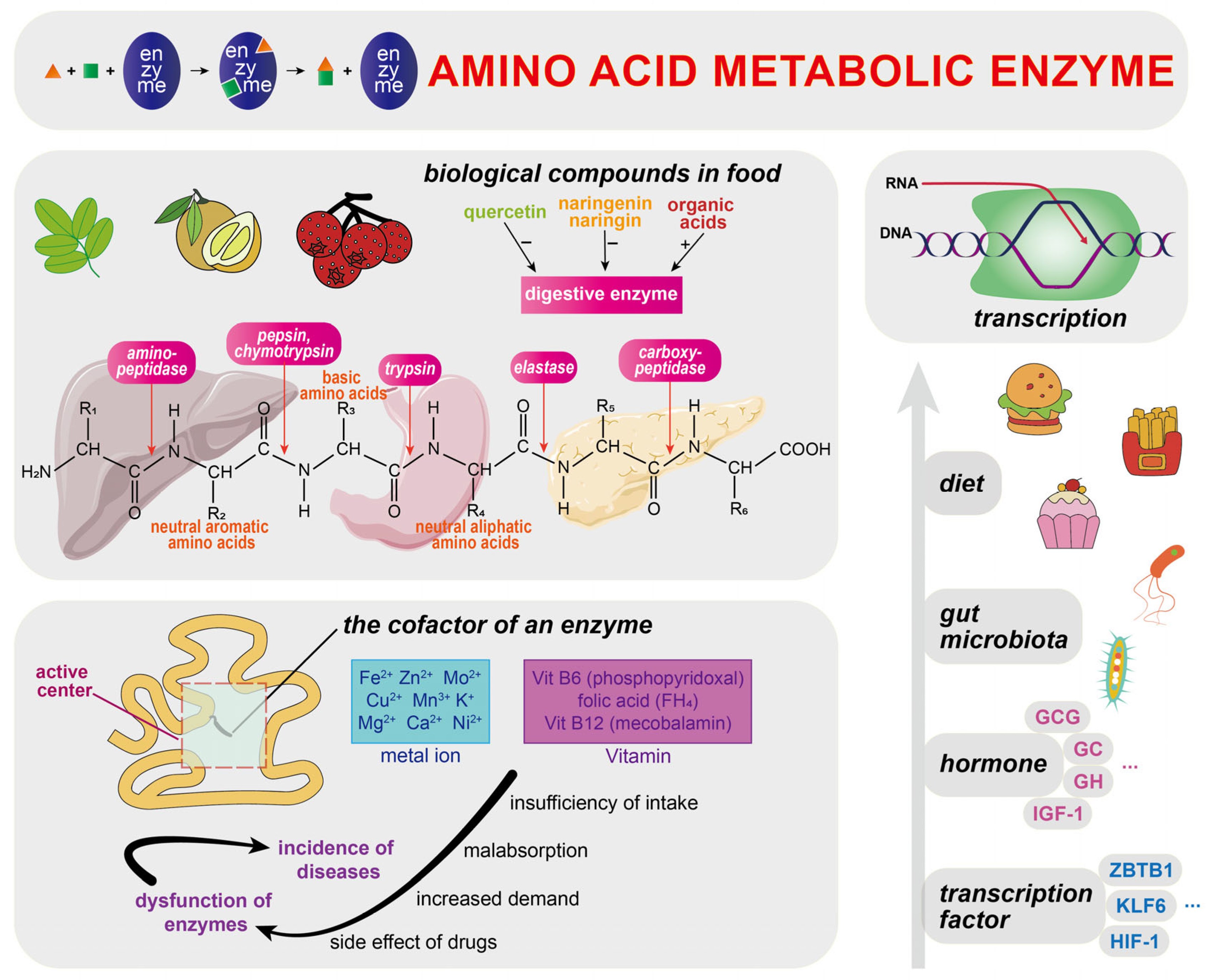
5. Regulation of Amino Acid Metabolism
5.1. Amino Acid Metabolic Enzymes
5.2. Diet
5.3. Amino Acid Transporters
6. Amino Acid Metabolism in Diabetic Cardiomyopathy
6.1. Branched-Chain Amino Acids in DCM
6.2. Aromatic Amino Acids in DCM
6.3. Other Amino Acids in DCM
6.3.1. Glycine
6.3.2. Serine
6.3.3. Methionine
6.3.4. Cysteine
6.3.5. Glutamic Acid and Glutamine
6.3.6. Lysine
6.3.7. Arginine
6.3.8. Alanine
6.3.9. Aspartic Acid and Asparagine
6.3.10. Proline
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT2B | 5-hydroxytryptamine receptor 2B |
| AAA | aromatic amino acid |
| AADE | amino acid-degrading enzyme |
| AAT | amino acid transporter |
| AGE | advanced glycation end product |
| AKT | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ARNI | angiotensin receptor-neprilysin inhibitor |
| AT1R | angiotensin II type 1 receptor |
| B0AT1 | broad neutral amino acid transporter 1 |
| BCAA | branched-chain amino acid |
| BCAT | branched-chain aminotransferase |
| BCKA | branched-chain α-keto acid |
| BCKDH | branched-chain keto acid dehydrogenase |
| BCKDHA | branched-chain keto acid dehydrogenase E1 subunit alpha |
| BCKDHB | branched-chain keto acid dehydrogenase E1 subunit beta |
| BCKDK | branched-chain keto acid dehydrogenase kinase |
| BT2 | 3:6-dichlorobenzo[b]thiophene-2-carboxylic acid |
| CVD | cardiovascular disease |
| DCM | diabetic cardiomyopathy |
| DIO | diet-induced obesity |
| DM | diabetes mellitus |
| EAA | essential amino acid |
| EF | ejection fraction |
| EGF | epidermal growth factor |
| FGF | fibroblast growth factor |
| H1R | histamine receptor 1 |
| H3K4me3 | trimethylated histone H3 at lysine 4 |
| HFD | high-fat diet |
| HFrEF | heart failure with reduced ejection fraction |
| HHCy | hyperhomocysteinemia |
| IGF-1 | insulin-like growth factor 1 |
| IIS | insulin and insulin-like growth factor signaling |
| IR | insulin resistance |
| LAT | large neutral amino acid transporter |
| LC-MS | liquid chromatography-mass spectrometry |
| MetS | metabolic syndrome |
| mTOR | mammalian target of rapamycin |
| NaPB | sodium phenylbutyrate |
| NEAA | non-essential amino acid |
| RAAS | renin-angiotensin-aldosterone system |
| RAGE | advanced glycation end product (AGE) cellular receptor |
| ROS | reactive oxygen species |
| SCFA | short-chain fatty acid |
| SGLT-2 | sodium-glucose cotransporter 2 |
| Sirt1 | sirtuin 1 |
| SLC1A1 | solute carrier family 1 member 1 |
| TAC | tricarboxylic acid |
| T1DM | type 1 diabetes mellitus |
| TGF-β | transforming growth factor beta |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Paulus, W.J.; Rosano, G.; Polovina, M.; Petrie, M.C.; Jhund, P.S.; Tschöpe, C.; Sattar, N.; Piepoli, M.; Papp, Z.; et al. Diabetic myocardial disorder. A clinical consensus statement of the Heart Failure Association of the ESC and the ESC Working Group on Myocardial & Pericardial Diseases. Eur. J. Heart Fail. 2024, 26, 1893–1903. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef]
- Rivas-Tumanyan, S.; Pacheco, L.S.; Haslam, D.E.; Morou-Bermudez, E.; Liang, L.; Tucker, K.L.; Joshipura, K.J.; Bhupathiraju, S.N. Branched-Chain and Aromatic Amino Acids, Type 2 Diabetes, and Cardiometabolic Risk Factors among Puerto Rican Adults. Nutrients 2024, 16, 2562. [Google Scholar] [CrossRef]
- Wang, M.; Ou, Y.; Yuan, X.-L.; Zhu, X.-F.; Niu, B.; Kang, Z.; Zhang, B.; Ahmed, A.; Xing, G.-Q.; Su, H. Heterogeneously elevated branched-chain/aromatic amino acids among new-onset type-2 diabetes mellitus patients are potentially skewed diabetes predictors. World J. Diabetes 2024, 15, 53–71. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Lopaschuk, G.D. Branched-Chain Amino Acid Metabolism in the Failing Heart. Cardiovasc. Drugs Ther. 2023, 37, 413–420. [Google Scholar] [CrossRef]
- Czibik, G.; Mezdari, Z.; Murat Altintas, D.; Bréhat, J.; Pini, M.; d’Humières, T.; Delmont, T.; Radu, C.; Breau, M.; Liang, H.; et al. Dysregulated Phenylalanine Catabolism Plays a Key Role in the Trajectory of Cardiac Aging. Circulation 2021, 144, 559–574. [Google Scholar] [CrossRef]
- Lund, A.; Nordrehaug, J.E.; Slettom, G.; Solvang, S.-E.H.; Pedersen, E.K.R.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Nygård, O.; Giil, L.M. Plasma kynurenines and prognosis in patients with heart failure. PLoS ONE 2020, 15, e0227365. [Google Scholar] [CrossRef]
- Zeng, F.; Zhou, P.; Wang, M.; Xie, L.; Huang, X.; Wang, Y.; Huang, J.; Shao, X.; Yang, Y.; Liu, W.; et al. ACMSD mediated de novo NAD+ biosynthetic impairment in cardiac endothelial cells as a potential therapeutic target for diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2023, 206, 111014. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W. Serine biosynthesis and transport defects. Mol. Genet. Metab. 2016, 118, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Kilberg, M.S.; Bussolati, O. Asparagine Synthetase in Cancer: Beyond Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Hanson, R.W. Resurgence of serine: An often neglected but indispensable amino Acid. J. Biol. Chem. 2012, 287, 19786–19791. [Google Scholar] [CrossRef]
- Bröer, S.; Gauthier-Coles, G. Amino Acid Homeostasis in Mammalian Cells with a Focus on Amino Acid Transport. J. Nutr. 2022, 152, 16–28. [Google Scholar] [CrossRef]
- Hushmandi, K.; Einollahi, B.; Saadat, S.H.; Lee, E.H.C.; Farani, M.R.; Okina, E.; Huh, Y.S.; Nabavi, N.; Salimimoghadam, S.; Kumar, A.P. Amino acid transporters within the solute carrier superfamily: Underappreciated proteins and novel opportunities for cancer therapy. Mol. Metab. 2024, 84, 101952. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar]
- Segawa, H.; Fukasawa, Y.; Miyamoto, K.; Takeda, E.; Endou, H.; Kanai, Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J. Biol. Chem. 1999, 274, 19745–19751. [Google Scholar] [CrossRef]
- Gauthier-Coles, G.; Fairweather, S.J.; Bröer, A.; Bröer, S. Do Amino Acid Antiporters Have Asymmetric Substrate Specificity? Biomolecules 2023, 13, 301. [Google Scholar] [CrossRef]
- Oparija, L.; Rajendran, A.; Poncet, N.; Verrey, F. Anticipation of food intake induces phosphorylation switch to regulate basolateral amino acid transporter LAT4 (SLC43A2) function. J. Physiol. 2019, 597, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Poncet, N.; Oparija-Rogenmozere, L.; Herzog, B.; Verrey, F. Tissue-specific deletion of mouse basolateral uniporter LAT4 (Slc43a2) reveals its crucial role in small intestine and kidney amino acid transport. J. Physiol. 2020, 598, 5109–5132. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, R.; Guan, X.; Feng, T.; Zhao, Y.; Lu, J. L-lysine dietary supplementation for childhood and adolescent growth: Promises and precautions. J. Adv. Res. 2025, 70, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, Y.; Song, T.; Park, S.; Kim, H.-J.; Koh, J.-H.; Cho, Y.; Park, S.-Y.; Sadayappan, S.; Kwak, H.-B.; et al. Free essential amino acid feeding improves endurance during resistance training via DRP1-dependent mitochondrial remodelling. J. Cachexia Sarcopenia Muscle 2024, 15, 1651–1663. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Peng, J.; Liang, X.; Yang, Q.; Bai, X.; Li, Y.; Li, J.; Dong, W.; Wang, Y.; et al. Amelioration of hepatic steatosis by dietary essential amino acid-induced ubiquitination. Mol. Cell 2022, 82, 1528–1542.e10. [Google Scholar] [CrossRef]
- Knaus, L.S.; Basilico, B.; Malzl, D.; Gerykova Bujalkova, M.; Smogavec, M.; Schwarz, L.A.; Gorkiewicz, S.; Amberg, N.; Pauler, F.M.; Knittl-Frank, C.; et al. Large neutral amino acid levels tune perinatal neuronal excitability and survival. Cell 2023, 186, 1950–1967.E25. [Google Scholar] [CrossRef]
- Hu, D.; Liu, J.; Yu, W.; Li, C.; Huang, L.; Mao, W.; Lu, Z. Tryptophan intake, not always the more the better. Front. Nutr. 2023, 10, 1140054. [Google Scholar] [CrossRef]
- Skvorak, K.; Liu, J.; Kruse, N.; Mehmood, R.; Das, S.; Jenne, S.; Chng, C.; Lao, U.L.; Duan, D.; Asfaha, J.; et al. Oral enzyme therapy for maple syrup urine disease (MSUD) suppresses plasma leucine levels in intermediate MSUD mice and healthy nonhuman primates. J. Inherit. Metab. Dis. 2023, 46, 1089–1103. [Google Scholar] [CrossRef]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2024, 37, 273–286. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Salas-Garrucho, F.M.; Carrillo-Moreno, A.; Contreras, L.M.; Rodríguez-Vico, F.; Clemente-Jiménez, J.M.; Las Heras-Vázquez, F.J. Exploring the Kinetics and Thermodynamics of a Novel Histidine Ammonia-Lyase from Geobacillus kaustophilus. Int. J. Mol. Sci. 2024, 25, 163. [Google Scholar] [CrossRef]
- Boros, K.; Moisă, M.E.; Nagy, C.L.; Paizs, C.; Toşa, M.I.; Bencze, L.C. Robust, site-specifically immobilized phenylalanine ammonia-lyases for the enantioselective ammonia addition of cinnamic acids. Catal. Sci. Technol. 2021, 11, 5553–5563. [Google Scholar] [CrossRef]
- Qian, L.; Li, N.; Lu, X.-C.; Xu, M.; Liu, Y.; Li, K.; Zhang, Y.; Hu, K.; Qi, Y.-T.; Yao, J.; et al. Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression. Nat. Metab. 2023, 5, 1159–1173. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Z.; Yan, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-α pathway-dependent fatty acid oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Park, S.J.; Nam, M.; Kang, J.; Kim, K.; Yeo, J.H.; Kim, J.-K.; Heo, Y.; Lee, H.S.; Lee, M.Y.; et al. A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab. 2020, 31, 267–283.E12. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Mori, Y.; Nara, M.; Kotani, Y.; Nagai, E.; Kawada, H.; Kitamura, M.; Hirano, R.; Shimokawa, H.; Nakagawa, A.; et al. Gut bacterial aromatic amine production: Aromatic amino acid decarboxylase and its effects on peripheral serotonin production. Gut Microbes. 2022, 14, 2128605. [Google Scholar] [CrossRef]
- Gabrawy, M.M.; Westbrook, R.; King, A.; Khosravian, N.; Ochaney, N.; DeCarvalho, T.; Wang, Q.; Yu, Y.; Huang, Q.; Said, A.; et al. Dual treatment with kynurenine pathway inhibitors and NAD+ precursors synergistically extends life span in Drosophila. Aging Cell 2024, 23, e14102. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ching, J.; Wee, H.N.; Liu, S.; Gurung, R.L.; Lee, J.; M, Y.; Zheng, H.; Lee, L.S.; Ang, K.; et al. Plasma Tryptophan-Kynurenine Pathway Metabolites and Risk for Progression to End-Stage Kidney Disease in Patients With Type 2 Diabetes. Diabetes Care 2023, 46, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Qin, Z.-S.; Zheng, Y.; Xie, J.-Y.; Liang, S.-S.; Zhang, J.-L.; Feng, Y.-B.; Zhang, Z.-J. Minocycline, a classic antibiotic, exerts psychotropic effects by normalizing microglial neuroinflammation-evoked tryptophan-kynurenine pathway dysregulation in chronically stressed male mice. Brain Behav. Immun. 2023, 107, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 2023, 44, 442–456. [Google Scholar] [CrossRef]
- Liang, H.; Zhan, J.; Chen, Y.; Xing, Z.; He, Z.N.T.; Liu, Y.; Li, X.; Chen, Y.; Li, Z.; Kuang, C.; et al. Tryptophan deficiency induced by indoleamine 2,3-dioxygenase 1 results in glucose transporter 1-dependent promotion of aerobic glycolysis in pancreatic cancer. MedComm 2024, 5, e555. [Google Scholar] [CrossRef]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; Gil de Biedma-Elduayen, L.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Yang, X.; Zhu, B.; Xiong, Y.; Song, Z.; Yang, X.; Zheng, Y. Histamine deficiency deteriorates LPS-induced periodontal diseases in a murine model via NLRP3/Caspase-1 pathway. Int. Immunopharmacol. 2023, 115, 109630. [Google Scholar] [CrossRef]
- Pham, V.N.; Bruemmer, K.J.; Toh, J.D.W.; Ge, E.J.; Tenney, L.; Ward, C.C.; Dingler, F.A.; Millington, C.L.; Garcia-Prieto, C.A.; Pulos-Holmes, M.C.; et al. Formaldehyde regulates S-adenosylmethionine biosynthesis and one-carbon metabolism. Science 2023, 382, eabp9201. [Google Scholar] [CrossRef]
- McBride, M.J.; Hunter, C.J.; Zhang, Z.; TeSlaa, T.; Xu, X.; Ducker, G.S.; Rabinowitz, J.D. Glycine homeostasis requires reverse SHMT flux. Cell Metab. 2024, 36, 103–115.E4. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, Y.; Sun, J.; Ji, B.; Luo, S.; Wu, B.; Zheng, C.; Wang, P.; Xu, F.; Cheng, K.; et al. New possible silver lining for pancreatic cancer therapy: Hydrogen sulfide and its donors. Acta Pharm. Sin. B 2021, 11, 1148–1157. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jain, M.K. Circadian regulation of cardiac metabolism. J. Clin. Investig. 2021, 131, e148276. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef] [PubMed]
- Söllinger, A.; Séneca, J.; Borg Dahl, M.; Motleleng, L.L.; Prommer, J.; Verbruggen, E.; Sigurdsson, B.D.; Janssens, I.; Peñuelas, J.; Urich, T.; et al. Down-regulation of the bacterial protein biosynthesis machinery in response to weeks, years, and decades of soil warming. Sci. Adv. 2022, 8, eabm3230. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; Navrotska, V. Extension of life span by down-regulation of enzymes catalyzing tryptophan conversion into kynurenine: Possible implications for mechanisms of aging. Exp. Biol. Med. (Maywood) 2023, 248, 573–577. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Chen, D.; Zhou, Y.; Yu, S.; Lin, H.; Liao, C.K.; Lin, H.; Xu, P.; Huang, M. Dual effects of quercetin on protein digestion and absorption in the digestive tract. Food Chem. 2021, 358, 129891. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wu, X.; Xu, R.; Ma, X.; Zhang, C.; Song, Z.; Peng, Y.; Ni, T.; Xu, Y. Exploring the interactions of naringenin and naringin with trypsin and pepsin: Experimental and computational modeling approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119859. [Google Scholar] [CrossRef]
- Wang, K.; Luo, L.; Xu, X.; Chen, X.; He, Q.; Zou, Z.; Wang, S.; Liang, S. LC-MS-based plasma metabolomics study of the intervention effect of different polar parts of hawthorn on gastrointestinal motility disorder rats. Biomed. Chromatogr. 2021, 35, e5076. [Google Scholar] [CrossRef]
- Nieraad, H.; Pannwitz, N.; Bruin, N.d.; Geisslinger, G.; Till, U. Hyperhomocysteinemia: Metabolic Role and Animal Studies with a Focus on Cognitive Performance and Decline-A Review. Biomolecules 2021, 11, 1546. [Google Scholar] [CrossRef]
- Torres, N.; Tobón-Cornejo, S.; Velazquez-Villegas, L.A.; Noriega, L.G.; Alemán-Escondrillas, G.; Tovar, A.R. Amino Acid Catabolism: An Overlooked Area of Metabolism. Nutrients 2023, 15, 3378. [Google Scholar] [CrossRef]
- Alam, M.S.; Liang, X.-F.; Liu, L.; He, S.; Kuang, Y.; Hoseinifar, S.H.; Dawar, F.U. Growth and Metabolic Response of Chinese Perch to Different Dietary Protein-to-Energy Ratios in Artificial Diets. Int. J. Mol. Sci. 2019, 20, 5983. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ai, C.; Luo, C.; Yuan, J. Effect of Dietary Crude Protein and Apparent Metabolizable Energy Levels on Growth Performance, Nitrogen Utilization, Serum Parameter, Protein Synthesis, and Amino Acid Metabolism of 1- to 10-Day-Old Male Broilers. Int. J. Mol. Sci. 2024, 25, 7431. [Google Scholar] [CrossRef] [PubMed]
- Heibel, S.K.; McGuire, P.J.; Haskins, N.; Majumdar, H.D.; Rayavarapu, S.; Nagaraju, K.; Hathout, Y.; Brown, K.; Tuchman, M.; Caldovic, L. AMP-activated protein kinase signaling regulated expression of urea cycle enzymes in response to changes in dietary protein intake. J. Inherit. Metab. Dis. 2019, 42, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Elmelund, E.; Galsgaard, K.D.; Johansen, C.D.; Trammell, S.A.J.; Bomholt, A.B.; Winther-Sørensen, M.; Hunt, J.E.; Sørensen, C.M.; Kruse, T.; Lau, J.F.; et al. Opposing effects of chronic glucagon receptor agonism and antagonism on amino acids, hepatic gene expression, and alpha cells. iScience 2022, 25, 105296. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Malkawi, A.K.; Wang, X.; Colak, D.; Mujamammi, A.H.; Sabi, E.M.; Li, L.; Dasouki, M.; Abdel Rahman, A.M. Dexamethasone-Induced Perturbations in Tissue Metabolomics Revealed by Chemical Isotope Labeling LC-MS analysis. Metabolites 2020, 10, 42. [Google Scholar] [CrossRef]
- Grøfte, T.; Wolthers, T.; Jensen, S.A.; Møller, N.; Jørgensen, J.O.; Tygstrup, N.; Orskov, H.; Vilstrup, H. Effects of growth hormone and insulin-like growth factor-I singly and in combination on in vivo capacity of urea synthesis, gene expression of urea cycle enzymes, and organ nitrogen contents in rats. Hepatology 1997, 25, 964–969. [Google Scholar] [CrossRef]
- Williams, R.T.; Guarecuco, R.; Gates, L.A.; Barrows, D.; Passarelli, M.C.; Carey, B.; Baudrier, L.; Jeewajee, S.; La, K.; Prizer, B.; et al. ZBTB1 Regulates Asparagine Synthesis and Leukemia Cell Response to L-Asparaginase. Cell Metab. 2020, 31, 852–861.e6. [Google Scholar] [CrossRef]
- Piret, S.E.; Guo, Y.; Attallah, A.A.; Horne, S.J.; Zollman, A.; Owusu, D.; Henein, J.; Sidorenko, V.S.; Revelo, M.P.; Hato, T.; et al. Krüppel-like factor 6-mediated loss of BCAA catabolism contributes to kidney injury in mice and humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2024414118. [Google Scholar] [CrossRef]
- Warnhoff, K.; Bhattacharya, S.; Snoozy, J.; Breen, P.C.; Ruvkun, G. Hypoxia-inducible factor induces cysteine dioxygenase and promotes cysteine homeostasis in Caenorhabditis elegans. Elife 2024, 12, RP89173. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, X.; Zhou, G.; Li, C. Dietary soy, pork and chicken proteins induce distinct nitrogen metabolism in rat liver. Food Chem. Mol. Sci. 2021, 3, 100050. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, C.; Ran, T.; Jiao, J.; Liu, Y.; Tan, Z.; Tang, S.; Kang, J.; Xie, J.; Chen, L.; et al. Dietary amylose:amylopectin ratio influences the expression of amino acid transporters and enzyme activities for amino acid metabolism in the gastrointestinal tract of goats. Br. J. Nutr. 2022, 127, 1121–1131. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, Q.; Li, Y.; Wang, R.; Xu, W.; Tang, X.; Li, X.; Lv, X.; Kong, X.; Cai, L.; et al. Longevity extension in rats via improved redox homeostasis with high carbohydrate diet intervention from weaning to adulthood: A comprehensive multi-omics study. Food Funct. 2024, 15, 7920–7935. [Google Scholar] [CrossRef]
- Liu, L.; Tian, X.; Li, W. Mechanistic study of the anti-excitatory amino acid toxicity of Bushen Zhichan decoction for Parkinson’s disease based on the transcriptional regulation of EAAT1 by YY1. J. Ethnopharmacol. 2024, 325, 117857. [Google Scholar] [CrossRef]
- Gan, Z.; Guo, Y.; Zhao, M.; Ye, Y.; Liao, Y.; Liu, B.; Yin, J.; Zhou, X.; Yan, Y.; Yin, Y.; et al. Excitatory amino acid transporter supports inflammatory macrophage responses. Sci. Bull. 2024, 69, 2405–2419. [Google Scholar] [CrossRef]
- Pajarillo, E.; Digman, A.; Nyarko-Danquah, I.; Son, D.-S.; Soliman, K.F.A.; Aschner, M.; Lee, E. Astrocytic transcription factor REST upregulates glutamate transporter EAAT2, protecting dopaminergic neurons from manganese-induced excitotoxicity. J. Biol. Chem. 2021, 297, 101372. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Yuan, F.; Zhang, L.; Wang, Y.; Wang, L.; Mao, Z.; Luo, J.; Zhang, H.; Zhu, W.-G.; et al. Increased Amino Acid Uptake Supports Autophagy-Deficient Cell Survival upon Glutamine Deprivation. Cell Rep. 2018, 23, 3006–3020. [Google Scholar] [CrossRef]
- Augusto, L.; Amin, P.H.; Wek, R.C.; Sullivan, W.J. Regulation of arginine transport by GCN2 eIF2 kinase is important for replication of the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2019, 15, e1007746. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Z.; Li, Z.; Ke, Q.; Ma, X.; Li, N.; Zhao, X.; Zou, Q.; Sun, L.; Song, T. TFE3-SLC36A1 axis promotes resistance to glucose starvation in kidney cancer cells. J. Biol. Chem. 2024, 300, 107270. [Google Scholar] [CrossRef]
- Marchingo, J.M.; Sinclair, L.V.; Howden, A.J.; Cantrell, D.A. Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. Elife 2020, 9, e53725. [Google Scholar] [CrossRef]
- Davalos, V.; Esteller, M. Cancer epigenetics in clinical practice. CA Cancer J. Clin. 2023, 73, 376–424. [Google Scholar] [CrossRef]
- Alam, M.A.; Datta, P.K. Epigenetic Regulation of Excitatory Amino Acid Transporter 2 in Neurological Disorders. Front. Pharmacol. 2019, 10, 1510. [Google Scholar] [CrossRef]
- Simner, C.; Novakovic, B.; Lillycrop, K.A.; Bell, C.G.; Harvey, N.C.; Cooper, C.; Saffery, R.; Lewis, R.M.; Cleal, J.K. DNA methylation of amino acid transporter genes in the human placenta. Placenta 2017, 60, 64–73. [Google Scholar] [CrossRef]
- Tümer, E.; Bröer, A.; Balkrishna, S.; Jülich, T.; Bröer, S. Enterocyte-specific regulation of the apical nutrient transporter SLC6A19 (B(0)AT1) by transcriptional and epigenetic networks. J. Biol. Chem. 2013, 288, 33813–33823. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Q.; Zhu, L.; Li, Y.; Li, J.; Zhang, Y.; Zheng, G.; Han, T.; Sun, S.; Feng, F. Involvement of methylation of MicroRNA-122, -125b and -106b in regulation of Cyclin G1, CAT-1 and STAT3 target genes in isoniazid-induced liver injury. BMC Pharmacol. Toxicol. 2018, 19, 11. [Google Scholar] [CrossRef]
- Warmbrunn, M.V.; Attaye, I.; Aron-Wisnewsky, J.; Rampanelli, E.; van der Vossen, E.W.J.; Hao, Y.; Koopen, A.; Bergh, P.-O.; Stols-Gonçalves, D.; Mohamed, N.; et al. Oral histidine affects gut microbiota and MAIT cells improving glycemic control in type 2 diabetes patients. Gut Microbes. 2024, 16, 2370616. [Google Scholar] [CrossRef]
- Howe, C.G.; Zhou, M.; Wang, X.; Pittman, G.S.; Thompson, I.J.; Campbell, M.R.; Bastain, T.M.; Grubbs, B.H.; Salam, M.T.; Hoyo, C.; et al. Associations between Maternal Tobacco Smoke Exposure and the Cord Blood DNA Methylome. Environ. Health Perspect. 2019, 127, 047009. [Google Scholar] [CrossRef]
- Panda, S.K.; Kim, D.-H.; Desai, P.; Rodrigues, P.F.; Sudan, R.; Gilfillan, S.; Cella, M.; Van Dyken, S.J.; Colonna, M. SLC7A8 is a key amino acids supplier for the metabolic programs that sustain homeostasis and activation of type 2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2215528119. [Google Scholar] [CrossRef]
- Nath, P.; Alfarsi, L.H.; El-Ansari, R.; Masisi, B.K.; Erkan, B.; Fakroun, A.; Ellis, I.O.; Rakha, E.A.; Green, A.R. The amino acid transporter SLC7A11 expression in breast cancer. Cancer Biol. Ther. 2024, 25, 2291855. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Zhao, X.; Zhang, L.; Zhou, L.; Li, X.; Ge, C.; Zhao, F.; Chen, T.; Xie, H.; et al. STAT5A modulates CDYL2/SLC7A6 pathway to inhibit the proliferation and invasion of hepatocellular carcinoma by targeting to mTORC1. Oncogene 2022, 41, 2492–2504. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, W.; Zhang, Y. Loss of Slc38a4 imprinting is a major cause of mouse placenta hyperplasia in somatic cell nuclear transferred embryos at late gestation. Cell Rep. 2022, 38, 110407. [Google Scholar] [CrossRef]
- Tomblin, J.K.; Arthur, S.; Primerano, D.A.; Chaudhry, A.R.; Fan, J.; Denvir, J.; Salisbury, T.B. Aryl hydrocarbon receptor (AHR) regulation of L-Type Amino Acid Transporter 1 (LAT-1) expression in MCF-7 and MDA-MB-231 breast cancer cells. Biochem. Pharmacol. 2016, 106, 94–103. [Google Scholar] [CrossRef]
- Paullin, T.; Powell, C.; Menzie, C.; Hill, R.; Cheng, F.; Martyniuk, C.J.; Westerheide, S.D. Spheroid growth in ovarian cancer alters transcriptome responses for stress pathways and epigenetic responses. PLoS ONE 2017, 12, e0182930. [Google Scholar] [CrossRef]
- Bacci, M.; Lorito, N.; Ippolito, L.; Ramazzotti, M.; Luti, S.; Romagnoli, S.; Parri, M.; Bianchini, F.; Cappellesso, F.; Virga, F.; et al. Reprogramming of Amino Acid Transporters to Support Aspartate and Glutamate Dependency Sustains Endocrine Resistance in Breast Cancer. Cell Rep. 2019, 28, 104–118.E8. [Google Scholar] [CrossRef]
- Sengupta, D.; Cassel, T.; Teng, K.-Y.; Aljuhani, M.; Chowdhary, V.K.; Hu, P.; Zhang, X.; Fan, T.W.M.; Ghoshal, K. Regulation of hepatic glutamine metabolism by miR-122. Mol. Metab. 2020, 34, 174–186. [Google Scholar] [CrossRef]
- Liu, X.; Nishikubo, K.; Ohgaki, R.; Okanishi, H.; Okuda, S.; Xu, M.; Kanai, Y. Identification of tumor-suppressive miRNAs that target amino acid transporter LAT1 and exhibit anti-proliferative effects on cholangiocarcinoma cells. J. Pharmacol. Sci. 2024, 154, 301–311. [Google Scholar] [CrossRef]
- Yi, W.; Tu, M.-J.; Liu, Z.; Zhang, C.; Batra, N.; Yu, A.-X.; Yu, A.-M. Bioengineered miR-328-3p modulates GLUT1-mediated glucose uptake and metabolism to exert synergistic antiproliferative effects with chemotherapeutics. Acta Pharm. Sin. B 2020, 10, 159–170. [Google Scholar] [CrossRef]
- Xiong, G.; Liu, C.; Yang, G.; Feng, M.; Xu, J.; Zhao, F.; You, L.; Zhou, L.; Zheng, L.; Hu, Y.; et al. Long noncoding RNA GSTM3TV2 upregulates LAT2 and OLR1 by competitively sponging let-7 to promote gemcitabine resistance in pancreatic cancer. J. Hematol. Oncol. 2019, 12, 97. [Google Scholar] [CrossRef]
- Oda, K.; Lee, Y.; Wiriyasermkul, P.; Tanaka, Y.; Takemoto, M.; Yamashita, K.; Nagamori, S.; Nishizawa, T.; Nureki, O. Consensus mutagenesis approach improves the thermal stability of system xc—transporter, xCT, and enables cryo-EM analyses. Protein Sci. 2020, 29, 2398–2407. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, X.; Lei, J.; Zhou, Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature 2019, 568, 127–130. [Google Scholar] [CrossRef]
- Jeckelmann, J.-M.; Fotiadis, D. Sub-Nanometer Cryo-EM Density Map of the Human Heterodimeric Amino Acid Transporter 4F2hc-LAT2. Int. J. Mol. Sci. 2020, 21, 7094. [Google Scholar] [CrossRef]
- Ohno, H.; Nakatsu, Y.; Sakoda, H.; Kushiyama, A.; Ono, H.; Fujishiro, M.; Otani, Y.; Okubo, H.; Yoneda, M.; Fukushima, T.; et al. 4F2hc stabilizes GLUT1 protein and increases glucose transport activity. Am. J. Physiol. Cell Physiol. 2011, 300, C1047–C1054. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Camargo, S.M.R.; Vuille-Dit-Bille, R.N.; Meier, C.F.; Verrey, F. ACE2 and gut amino acid transport. Clin. Sci. 2020, 134, 2823–2833. [Google Scholar] [CrossRef]
- Yan, R.; Li, Y.; Shi, Y.; Zhou, J.; Lei, J.; Huang, J.; Zhou, Q. Cryo-EM structure of the human heteromeric amino acid transporter b0,+AT-rBAT. Sci. Adv. 2020, 6, eaay6379. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.; Mo, S.; Zhang, Y.; Mao, X.; Chen, J.; Liu, Y.; Tong, W.-M.; Lu, Z.; Yu, S.; et al. Targeting N-glycosylation of 4F2hc mediated by glycosyltransferase B3GNT3 sensitizes ferroptosis of pancreatic ductal adenocarcinoma. Cell Death Differ. 2023, 30, 1988–2004. [Google Scholar] [CrossRef]
- Chen, Z.-Z.; Gerszten, R.E. Metabolomics and Proteomics in Type 2 Diabetes. Circ. Res. 2020, 126, 1613–1627. [Google Scholar] [CrossRef]
- Xiong, R.-Q.; Li, Y.-P.; Lin, L.-P.; Yao, J.-Y. Identification of potential biomarkers for diabetic cardiomyopathy using LC-MS-based metabolomics. Endocr. Connect. 2024, 13, e230384. [Google Scholar] [CrossRef]
- Lu, Q.-B.; Fu, X.; Liu, Y.; Wang, Z.-C.; Liu, S.-Y.; Li, Y.-C.; Sun, H.-J. Disrupted cardiac fibroblast BCAA catabolism contributes to diabetic cardiomyopathy via a periostin/NAP1L2/SIRT3 axis. Cell. Mol. Biol. Lett. 2023, 28, 93. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; He, X.; Wu, Q.; Li, D.-L.; Zang, W.-J. Pyridostigmine Protects Against Diabetic Cardiomyopathy by Regulating Vagal Activity, Gut Microbiota, and Branched-Chain Amino Acid Catabolism in Diabetic Mice. Front. Pharmacol. 2021, 12, 647481. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Hou, K.; Xin, C.; Zeng, D.; Cheng, C.; Zhao, H.; Wang, Z.; Wang, L. Portulaca oleracea L. Extract Alleviated Type 2 Diabetes Via Modulating the Gut Microbiota and Serum Branched-Chain Amino Acid Metabolism. Mol. Nutr. Food Res. 2022, 66, e2101030. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Pei, J.; Liu, Y.; Niu, W.; Sun, H. Targeting BCAA metabolism to potentiate metformin’s therapeutic efficacy in the treatment of diabetes in mice. Diabetologia 2023, 66, 2139–2153. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gao, C.; Yu, J.; Ren, S.; Wang, M.; Wynn, R.M.; Chuang, D.T.; Wang, Y.; Sun, H. Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure. J. Am. Heart Assoc. 2019, 8, e011625. [Google Scholar] [CrossRef] [PubMed]
- Lian, K.; Guo, X.; Wang, Q.; Liu, Y.; Wang, R.-T.; Gao, C.; Li, C.-Y.; Li, C.-X.; Tao, L. PP2Cm overexpression alleviates MI/R injury mediated by a BCAA catabolism defect and oxidative stress in diabetic mice. Eur. J. Pharmacol. 2020, 866, 172796. [Google Scholar] [CrossRef]
- Li, Z.; Xia, H.; Sharp, T.E.; LaPenna, K.B.; Elrod, J.W.; Casin, K.M.; Liu, K.; Calvert, J.W.; Chau, V.Q.; Salloum, F.N.; et al. Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure. Circ. Res. 2022, 131, 222–235. [Google Scholar] [CrossRef]
- Acevedo, A.; Jones, A.E.; Danna, B.T.; Turner, R.; Montales, K.P.; Benincá, C.; Reue, K.; Shirihai, O.S.; Stiles, L.; Wallace, M.; et al. The BCKDK inhibitor BT2 is a chemical uncoupler that lowers mitochondrial ROS production and de novo lipogenesis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Crossland, H.; Smith, K.; Idris, I.; Phillips, B.E.; Atherton, P.J.; Wilkinson, D.J. Phenylbutyrate, a branched-chain amino acid keto dehydrogenase activator, promotes branched-chain amino acid metabolism and induces muscle catabolism in C2C12 cells. Exp. Physiol. 2021, 106, 585–592. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Xie, X.; Tie, R.; Shang, X.; Zhao, Q.; Xu, J.; Jin, L.; Zhang, J.; Ye, P. Empagliflozin ameliorates diabetic cardiomyopathy via regulated branched-chain amino acid metabolism and mTOR/p-ULK1 signaling pathway-mediated autophagy. Diabetol. Metab. Syndr. 2023, 15, 93. [Google Scholar] [CrossRef]
- Samms, R.J.; Zhang, G.; He, W.; Ilkayeva, O.; Droz, B.A.; Bauer, S.M.; Stutsman, C.; Pirro, V.; Collins, K.A.; Furber, E.C.; et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol. Metab. 2022, 64, 101550. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.; Zhou, J.; Lin, H.; Wu, Z.; Liu, N.; Xie, W.; Guo, H.; Chi, J. Functional components of Chinese rice wine can ameliorate diabetic cardiomyopathy through the modulation of autophagy, apoptosis, gut microbiota, and metabolites. Front. Cardiovasc. Med. 2022, 9, 940663. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zuo, J.; Cheng, Y.; Zhang, Y.; Zhang, Z.; Wu, M.; Yang, Y.; Tong, H. Ethanol extract of Sargarsum fusiforme alleviates HFD/STZ-induced hyperglycemia in association with modulation of gut microbiota and intestinal metabolites in type 2 diabetic mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef]
- Wang, S.; Schianchi, F.; Neumann, D.; Wong, L.-Y.; Sun, A.; van Nieuwenhoven, F.A.; Zeegers, M.P.; Strzelecka, A.; Col, U.; Glatz, J.F.C.; et al. Specific amino acid supplementation rescues the heart from lipid overload-induced insulin resistance and contractile dysfunction by targeting the endosomal mTOR-v-ATPase axis. Mol. Metab. 2021, 53, 101293. [Google Scholar] [CrossRef]
- Hussein, A.M.; Eid, E.A.; Taha, M.; Elshazli, R.M.; Bedir, R.F.; Lashin, L.S. Comparative Study of the Effects of GLP1 Analog and SGLT2 Inhibitor against Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Biomedicines 2020, 8, 43. [Google Scholar] [CrossRef]
- Hussein, A.M.; Eid, E.A.; Bin-Jaliah, I.; Taha, M.; Lashin, L.S. Exercise and Stevia Rebaudiana (R) Extracts Attenuate Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Endocr. Metab. Immune. Disord. Drug Targets 2020, 20, 1117–1132. [Google Scholar] [CrossRef]
- Yehya, Y.M.; Hussein, A.M.; Ezam, K.; Eid, E.A.; Ibrahim, E.M.; Sarhan, M.A.F.E.; Elsayed, A.; Sarhan, M.E. Blockade of Renin Angiotensin System Ameliorates the Cardiac Arrhythmias and Sympathetic Neural Remodeling in Hearts of Type 2 DM Rat Model. Endocr. Metab. Immune. Disord. Drug Targets 2020, 20, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Sefidgari-Abrasi, S.; Roshangar, L.; Karimi, P.; Morshedi, M.; Rahimiyan-Heravan, M.; Saghafi-Asl, M. From the gut to the heart: L. plantarum and inulin administration as a novel approach to control cardiac apoptosis via 5-HT2B and TrkB receptors in diabetes. Clin. Nutr. 2021, 40, 190–201. [Google Scholar] [CrossRef]
- Peng, M.; Xia, T.; Zhong, Y.; Zhao, M.; Yue, Y.; Liang, L.; Zhong, R.; Zhang, H.; Li, C.; Cao, X.; et al. Integrative pharmacology reveals the mechanisms of Erzhi Pill, a traditional Chinese formulation, against diabetic cardiomyopathy. J. Ethnopharmacol. 2022, 296, 115474. [Google Scholar] [CrossRef]
- Kakoki, M.; Ramanathan, P.V.; Hagaman, J.R.; Grant, R.; Wilder, J.C.; Taylor, J.M.; Charles Jennette, J.; Smithies, O.; Maeda-Smithies, N. Cyanocobalamin prevents cardiomyopathy in type 1 diabetes by modulating oxidative stress and DNMT-SOCS1/3-IGF-1 signaling. Commun. Biol. 2021, 4, 775. [Google Scholar] [CrossRef]
- Ilkhanizadeh, B.; Shirpoor, A.; Khadem Ansari, M.H.; Nemati, S.; Rasmi, Y. Protective Effects of Ginger (Zingiber officinale) Extract against Diabetes-Induced Heart Abnormality in Rats. Diabetes Metab. J. 2016, 40, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, K.-F.; Chang, Y.-H.; Wang, C.; Chen, Y.; Wang, M.-J.; Zhu, Y.-C. S-Propargyl-Cysteine Attenuates Diabetic Cardiomyopathy in db/db Mice Through Activation of Cardiac Insulin Receptor Signaling. Front. Cardiovasc. Med. 2021, 8, 737191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, M.; Li, H.; Li, Q.; Liu, N.; Dong, S.; Zhao, Y.; Pang, K.; Huang, J.; Ren, C.; et al. Exogenous H2S promotes ubiquitin-mediated degradation of SREBP1 to alleviate diabetic cardiomyopathy via SYVN1 S-sulfhydration. J. Cachexia Sarcopenia Muscle. 2023, 14, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, F.; Yu, X.; Wang, B.; Chen, J.; Lu, F.; Peng, S.; Sun, X.; Yu, M.; Chen, H.; et al. Exogenous H2S Promoted USP8 Sulfhydration to Regulate Mitophagy in the Hearts of db/db Mice. Aging Dis. 2020, 11, 269–285. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Yang, T.; Nie, L.; Liu, S.; Zhou, J.; Chen, J.; Jiang, Z.; Xiao, T.; Yang, J.; et al. Gaseous signal molecule SO2 regulates autophagy through PI3K/AKT pathway inhibits cardiomyocyte apoptosis and improves myocardial fibrosis in rats with type II diabetes. Korean J. Physiol. Pharmacol. 2022, 26, 541–556. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Wang, X.; Li, P.; Zhang, S.; Wu, T.; Yan, Y.; Zhan, Y.; Ren, Y.; Rong, X.; et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem. Biol. Interact. 2019, 310, 108754. [Google Scholar] [CrossRef]
- Cai, W.; Chong, K.; Huang, Y.; Huang, C.; Yin, L. Empagliflozin improves mitochondrial dysfunction in diabetic cardiomyopathy by modulating ketone body metabolism and oxidative stress. Redox. Biol. 2024, 69, 103010. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Ge, Q.; Zhao, L.; Ren, X.-M.; Ye, P.; Hu, Z.-Y. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp. Biol. Med. (Maywood). 2019, 244, 1028–1039. [Google Scholar] [CrossRef]
- Gbr, A.A.; Abdel Baky, N.A.; Mohamed, E.A.; Zaky, H.S. Cardioprotective effect of pioglitazone and curcumin against diabetic cardiomyopathy in type 1 diabetes mellitus: Impact on CaMKII/NF-κB/TGF-β1 and PPAR-γ signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 349–360. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanian, H.; Djazayery, A.; Javanbakht, M.H.; Eshraghian, M.R.; Mirshafiey, A.; Jahanabadi, S.; Ghadbeigi, S.; Zarei, M.; Alvandi, E.; Djalali, M. Vitamin D downregulates key genes of diabetes complications in cardiomyocyte. J. Cell Physiol. 2019, 234, 21352–21358. [Google Scholar] [CrossRef]
- Baumgardt, S.L.; Paterson, M.; Leucker, T.M.; Fang, J.; Zhang, D.X.; Bosnjak, Z.J.; Warltier, D.C.; Kersten, J.R.; Ge, Z.-D. Chronic Co-Administration of Sepiapterin and L-Citrulline Ameliorates Diabetic Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury in Obese Type 2 Diabetic Mice. Circ. Heart Fail. 2016, 9, e002424. [Google Scholar] [CrossRef] [PubMed]
- Kambis, T.N.; Tofilau, H.M.N.; Gawargi, F.I.; Chandra, S.; Mishra, P.K. Regulating Polyamine Metabolism by miRNAs in Diabetic Cardiomyopathy. Curr. Diab. Rep. 2021, 21, 52. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Li, S.; Zhang, X.; Guo, Z.; Hu, J.; Shao, X.; Song, N.; Zhao, Y.; Li, H.; et al. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox. Biol. 2020, 32, 101514. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.R.; Nachane, S.S.; Tupe, R.S. Alleviation of albumin glycation-induced diabetic cardiomyopathy by L-Arginine: Insights into Nrf-2 signaling. Int. J. Biol. Macromol. 2024, 264, 130478. [Google Scholar] [CrossRef]
- Kumawat, V.S.; Kaur, G. Cannabinoid 2 receptor agonist and L-arginine combination attenuates diabetic cardiomyopathy in rats via NF-ĸβ inhibition. Can. J. Physiol. Pharmacol. 2022, 100, 259–271. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Zhang, L.; Yao, X.; Zhong, X.; Cheng, G.; Wang, L.; Wan, Q. Spiraeoside protects human cardiomyocytes against high glucose-induced injury, oxidative stress, and apoptosis by activation of PI3K/Akt/Nrf2 pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22548. [Google Scholar] [CrossRef]
- Zhou, F.; Sheng, C.; Ma, X.; Li, T.; Ming, X.; Wang, S.; Tan, J.; Yang, Y.; Sun, H.; Lu, J.; et al. BCKDH kinase promotes hepatic gluconeogenesis independent of BCKDHA. Cell Death Dis. 2024, 15, 736. [Google Scholar] [CrossRef]
- Nishi, K.; Yoshii, A.; Abell, L.; Zhou, B.; Frausto, R.; Ritterhoff, J.; McMillen, T.S.; Sweet, I.; Wang, Y.; Gao, C.; et al. Branched-chain keto acids inhibit mitochondrial pyruvate carrier and suppress gluconeogenesis in hepatocytes. Cell Rep. 2023, 42, 112641. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M.; Fingrová, R. Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes. Biomolecules 2020, 10, 1475. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Qiao, H.; Wang, Z.; Wang, H.; Han, M.; Zhang, W.; Zhou, Y.; Hassan, H.M.; Zhao, W.; Qin, T. Taohe Chengqi decoction alleviated metabolic-associated fatty liver disease by boosting branched chain amino acids catabolism in the skeletal muscles of type 2 diabetes mellitus. Phytomedicine 2024, 126, 155315. [Google Scholar] [CrossRef] [PubMed]
- Karusheva, Y.; Strassburger, K.; Markgraf, D.F.; Zaharia, O.-P.; Bódis, K.; Kössler, T.; Tura, A.; Pacini, G.; Burkart, V.; Roden, M.; et al. Branched-Chain Amino Acids Associate Negatively With Postprandial Insulin Secretion in Recent-Onset Diabetes. J. Endocr. Soc. 2021, 5, bvab067. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Liu, Y.; Peng, X.; Hou, Y.; Zhang, X.; Sun, H.; Shan, C. Serum branched chain amino acids: An effective indicator of diabetic kidney disease. Front. Endocrinol. 2023, 14, 1269633. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- Bai, X.; Long, X.; Song, F.; Chen, B.; Sheng, C.; Tang, C.; Li, L.; Zhang, J.; Zhang, R.; Zhang, J.; et al. High doses of rosuvastatin induce impaired branched-chain amino acid catabolism and lead to insulin resistance. Exp. Physiol. 2023, 108, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Patel, A.; Devaraj, S.; Adams-Huet, B. Metabolites that activate the inflammasome in nascent metabolic syndrome. J. Diabetes Complicat. 2021, 35, 107836. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Gogg, S.; Lotta, L.A.; Stančáková, A.; Nerstedt, A.; Boren, J.; Blüher, M.; Ferrannini, E.; Langenberg, C.; Wareham, N.J.; et al. Elevated Plasma Levels of 3-Hydroxyisobutyric Acid Are Associated With Incident Type 2 Diabetes. EBioMedicine 2018, 27, 151–155. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Li, C.; Wang, D.; Shen, Y.; Lu, J.; Zhao, L.; Li, X.; Gao, H. BCAA mediated microbiota-liver-heart crosstalk regulates diabetic cardiomyopathy via FGF21. Microbiome. 2024, 12, 157. [Google Scholar] [CrossRef]
- Ogawa, T.; Kouzu, H.; Osanami, A.; Tatekoshi, Y.; Sato, T.; Kuno, A.; Fujita, Y.; Ino, S.; Shimizu, M.; Toda, Y.; et al. Downregulation of extramitochondrial BCKDH and its uncoupling from AMP deaminase in type 2 diabetic OLETF rat hearts. Physiol. Rep. 2023, 11, e15608. [Google Scholar] [CrossRef] [PubMed]
- Tso, S.-C.; Gui, W.-J.; Wu, C.-Y.; Chuang, J.L.; Qi, X.; Skvora, K.J.; Dork, K.; Wallace, A.L.; Morlock, L.K.; Lee, B.H.; et al. Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain α-ketoacid dehydrogenase kinase. J. Biol. Chem. 2014, 289, 20583–20593. [Google Scholar] [CrossRef] [PubMed]
- Burrage, L.C.; Jain, M.; Gandolfo, L.; Lee, B.H.; Nagamani, S.C.S. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol. Genet. Metab. 2014, 113, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Osaka, S.; Nakano, S.; Mizuno, T.; Hiraoka, Y.; Minowa, K.; Hirai, S.; Mizutani, A.; Sabu, Y.; Miura, Y.; Shimizu, T.; et al. A randomized trial to examine the impact of food on pharmacokinetics of 4-phenylbutyrate and change in amino acid availability after a single oral administration of sodium 4-phenylbutyrarte in healthy volunteers. Mol. Genet. Metab. 2021, 132, 220–226. [Google Scholar] [CrossRef]
- Crossland, H.; Smith, K.; Idris, I.; Phillips, B.E.; Atherton, P.J.; Wilkinson, D.J. Exploring mechanistic links between extracellular branched-chain amino acids and muscle insulin resistance: An in vitro approach. Am. J. Physiol. Cell Physiol. 2020, 319, C1151–C1157. [Google Scholar] [CrossRef]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Kitaura, Y.; Shindo, D.; Ogawa, T.; Sato, A.; Shimomura, Y. Antihypertensive drug valsartan as a novel BDK inhibitor. Pharmacol. Res. 2021, 167, 105518. [Google Scholar] [CrossRef]
- Moellmann, J.; Klinkhammer, B.M.; Droste, P.; Kappel, B.; Haj-Yehia, E.; Maxeiner, S.; Artati, A.; Adamski, J.; Boor, P.; Schütt, K.; et al. Empagliflozin improves left ventricular diastolic function of db/db mice. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165807. [Google Scholar] [CrossRef]
- Ye, Y.; Bajaj, M.; Yang, H.-C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs. Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef]
- Rivera, M.E.; Lyon, E.S.; Vaughan, R.A. Effect of metformin on myotube BCAA catabolism. J. Cell Biochem. 2020, 121, 816–827. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Andreadou, I.; Aragno, M.; Beauloye, C.; Bertrand, L.; Lazou, A.; Falcão-Pires, I.; Bell, R.; Zuurbier, C.J.; Pagliaro, P.; et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia-reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br. J. Pharmacol. 2020, 177, 5312–5335. [Google Scholar] [CrossRef]
- Andreadou, I.; Daiber, A.; Baxter, G.F.; Brizzi, M.F.; Di Lisa, F.; Kaludercic, N.; Lazou, A.; Varga, Z.V.; Zuurbier, C.J.; Schulz, R.; et al. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Radic. Biol. Med. 2021, 166, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Role of Impaired Glycolysis in Perturbations of Amino Acid Metabolism in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 1724. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Li, M.; Yu, Y.; Wang, Z.; Chen, S. Improvement of cardiac dysfunction by bilateral surgical renal denervation in animals with diabetes induced by high fructose and high fat diet. Diabetes Res. Clin. Pract. 2016, 115, 140–149. [Google Scholar] [CrossRef]
- Huo, J.-Y.; Jiang, W.-Y.; Zhang, S.-G.; Lyu, Y.-T.; Geng, J.; Chen, M.; Chen, Y.-Y.; Jiang, Z.-X.; Shan, Q.-J. Renal denervation ameliorates cardiac metabolic remodeling in diabetic cardiomyopathy rats by suppressing renal SGLT2 expression. Lab. Investig. 2022, 102, 341–351. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Chakraborty, A.D.; Light, C.M.; Tian, R.; Sadoshima, J.; Shi, X.; Gu, H.; Lee, C.F. NAD+ Redox Imbalance in the Heart Exacerbates Diabetic Cardiomyopathy. Circ. Heart Fail. 2021, 14, e008170. [Google Scholar] [CrossRef]
- Alves, A.; Lamarche, F.; Lefebvre, R.; Drevet Mulard, E.; Bassot, A.; Chanon, S.; Loizon, E.; Pinteur, C.; Bloise, A.M.N.d.L.G.; Godet, M.; et al. Glycine Supplementation in Obesity Worsens Glucose Intolerance through Enhanced Liver Gluconeogenesis. Nutrients 2022, 15, 96. [Google Scholar] [CrossRef]
- Handzlik, M.K.; Gengatharan, J.M.; Frizzi, K.E.; McGregor, G.H.; Martino, C.; Rahman, G.; Gonzalez, A.; Moreno, A.M.; Green, C.R.; Guernsey, L.S.; et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature 2023, 614, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Shahshahan, H.R.; Kambis, T.N.; Yadav, S.K.; Li, Z.; Lefer, D.J.; Mishra, P.K. Hydrogen Sulfide Ameliorates Homocysteine-Induced Cardiac Remodeling and Dysfunction. Front. Physiol. 2019, 10, 598. [Google Scholar] [CrossRef]
- Mishra, P.K.; Givvimani, S.; Metreveli, N.; Tyagi, S.C. Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 2010, 401, 175–181. [Google Scholar] [CrossRef]
- Tao, H.; Shi, P.; Xuan, H.-Y.; Ding, X.-S. DNA methyltransferase-1 inactivation of androgen receptor axis triggers homocysteine induced cardiac fibroblast autophagy in diabetic cardiac fibrosis. Arch. Biochem. Biophys. 2020, 692, 108521. [Google Scholar] [CrossRef]
- Guo, X.; Chen, K.; Ji, L.; Wang, S.; Ye, X.; Xu, L.; Feng, L. Ultrasound-targeted microbubble technology facilitates SAHH gene delivery to treat diabetic cardiomyopathy by activating AMPK pathway. iScience 2024, 27, 108852. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, Y.; Guasch-Ferré, M.; Ruiz-Canela, M.; Toledo, E.; Clish, C.; Liang, L.; Razquin, C.; Corella, D.; Estruch, R.; et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: Case-cohort study within the PREDIMED trial. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Leandro, J.; Houten, S.M. The lysine degradation pathway: Subcellular compartmentalization and enzyme deficiencies. Mol. Genet. Metab. 2020, 131, 14–22. [Google Scholar] [CrossRef]
- Razquin, C.; Ruiz-Canela, M.; Clish, C.B.; Li, J.; Toledo, E.; Dennis, C.; Liang, L.; Salas-Huetos, A.; Pierce, K.A.; Guasch-Ferré, M.; et al. Lysine pathway metabolites and the risk of type 2 diabetes and cardiovascular disease in the PREDIMED study: Results from two case-cohort studies. Cardiovasc. Diabetol. 2019, 18, 151. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, C.-B.; Liu, C.; Fan, Y.; Zhu, H.-Y.; Wu, X.-W.; Hu, L.; Li, Q.-P. Upregulation of arginase activity contributes to intracellular ROS production induced by high glucose in H9c2 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 2728–2736. [Google Scholar]
- Zhu, Z.-D.; Ye, J.-M.; Fu, X.-M.; Wang, X.-C.; Ye, J.-Y.; Wu, X.-R.; Hua, P.; Liao, Y.-Q.; Xuan, W.; Duan, J.-L.; et al. DDAH2 alleviates myocardial fibrosis in diabetic cardiomyopathy through activation of the DDAH/ADMA/NOS/NO pathway in rats. Int. J. Mol. Med. 2019, 43, 749–760. [Google Scholar] [CrossRef]
- Wei, C.; Song, T.; Yuan, H.; Li, X.; Zhang, X.; Liang, X.; Fan, Y. Transcriptomics Coupled to Proteomics Reveals Novel Targets for the Protective Role of Spermine in Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 5909378. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, X.; Zhang, X.; Shao, X.; Wang, Y.; Chen, J.; Zhao, B.; Li, S.; Xu, C.; Wei, C. Exogenous spermine attenuates myocardial fibrosis in diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress and the canonical Wnt signaling pathway. Cell Biol. Int. 2020, 44, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Cerasuolo, F.A.; Avvisato, R.; Buonaiuto, A.; Maisto, M.; Bianco, A.; D’Argenio, V.; Mone, P.; Perrino, C.; D’Apice, S.; et al. L-Arginine supplementation as mitochondrial therapy in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2024, 23, 450. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, S.; Chen, J.; Murphy, C.; Martin, L.; Chiazza, F.; Collotta, D.; Yaqoob, M.M.; Collino, M.; Thiemermann, C. Linagliptin Attenuates the Cardiac Dysfunction Associated With Experimental Sepsis in Mice With Pre-existing Type 2 Diabetes by Inhibiting NF-κB. Front. Immunol. 2018, 9, 2996. [Google Scholar] [CrossRef]
- Wen, C.; Liu, C.; Li, Y.; Xia, T.; Zhang, X.; Xue, S.; Olatunji, O.J. Ameliorative potentials of the ethanolic extract from Lycium chinense leaf extract against diabetic cardiomyopathy. Insight into oxido-inflammatory and apoptosis modulation. Biomed. Pharmacother. 2022, 154, 113583. [Google Scholar] [CrossRef]
- Liza; Hussain, G.; Malik, A.; Akhtar, S.; Anwar, H. Artemisia vulgaris Extract as a Novel Therapeutic Approach for Reversing Diabetic Cardiomyopathy in a Rat Model. Pharmaceuticals 2024, 17, 46. [Google Scholar] [CrossRef]
- Asghari, A.A.; Mahmoudabady, M.; Shabab, S.; Niazmand, S. Anti-inflammatory, anti-oxidant and anti-apoptotic effects of olive leaf extract in cardiac tissue of diabetic rats. J. Pharm. Pharmacol. 2022, 74, 961–972. [Google Scholar] [CrossRef]
- Abdi, T.; Mahmoudabady, M.; Marzouni, H.Z.; Niazmand, S.; Khazaei, M. Ginger (Zingiber officinale Roscoe) Extract Protects the Heart Against Inflammation and Fibrosis in Diabetic Rats. Can. J. Diabetes 2021, 45, 220–227. [Google Scholar] [CrossRef]
- Yao, P.-A.; Wei, K.-Z.; Feng, J.-H.; Liu, X.-N.; Xu, X.; Cui, H.-Y.; Zhang, X.-C.; Gao, J.-P. Sodium houttuyfonate protects against cardiac injury by regulating cardiac energy metabolism in diabetic rats. Eur. J. Pharmacol. 2022, 932, 175236. [Google Scholar] [CrossRef]
- Farazandeh, M.; Mahmoudabady, M.; Asghari, A.A.; Niazmand, S. Diabetic cardiomyopathy was attenuated by cinnamon treatment through the inhibition of fibro-inflammatory response and ventricular hypertrophy in diabetic rats. J. Food Biochem. 2022, 46, e14206. [Google Scholar] [CrossRef]
- Gur, F.M.; Aktas, I. The ameliorative effects of thymoquinone and beta-aminoisobutyric acid on streptozotocin-induced diabetic cardiomyopathy. Tissue Cell. 2021, 71, 101582. [Google Scholar] [CrossRef]
- Shabab, S.; Mahmoudabady, M.; Gholamnezhad, Z.; Fouladi, M.; Asghari, A.A. Diabetic cardiomyopathy in rats was attenuated by endurance exercise through the inhibition of inflammation and apoptosis. Heliyon 2024, 10, e23427. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Lin, H.; Wang, G.; Xu, Y.; Zhao, X.; Hu, C.; Zhang, Y.; Zheng, R.; Hu, R.; et al. Amino acids, microbiota-related metabolites, and the risk of incident diabetes among normoglycemic Chinese adults: Findings from the 4C study. Cell Rep. Med. 2022, 3, 100727. [Google Scholar] [CrossRef]
- Mathew, A.V.; Jaiswal, M.; Ang, L.; Michailidis, G.; Pennathur, S.; Pop-Busui, R. Impaired Amino Acid and TCA Metabolism and Cardiovascular Autonomic Neuropathy Progression in Type 1 Diabetes. Diabetes 2019, 68, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Li, X.; Deng, X.; Kong, Y.; Wang, W.; Zhou, Y. Machine learning of plasma metabolome identifies biomarker panels for metabolic syndrome: Findings from the China Suboptimal Health Cohort. Cardiovasc. Diabetol. 2022, 21, 288. [Google Scholar] [CrossRef]
- Wu, J.; Subbaiah, K.C.V.; Xie, L.H.; Jiang, F.; Khor, E.-S.; Mickelsen, D.; Myers, J.R.; Tang, W.H.W.; Yao, P. Glutamyl-Prolyl-tRNA Synthetase Regulates Proline-Rich Pro-Fibrotic Protein Synthesis During Cardiac Fibrosis. Circ. Res. 2020, 127, 827–846. [Google Scholar] [CrossRef] [PubMed]
- Vanweert, F.; Neinast, M.; Tapia, E.E.; van de Weijer, T.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Blair, M.C.; Bornstein, M.R.; Hesselink, M.K.C.; Schrauwen, P.; et al. A randomized placebo-controlled clinical trial for pharmacological activation of BCAA catabolism in patients with type 2 diabetes. Nat. Commun. 2022, 13, 3508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Qorbani, M.; Heshmat, R.; Larijani, B.; Hosseini, S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015, 31, 119–126. [Google Scholar] [CrossRef]
- Zhang, Y. The essential role of glutamine metabolism in diabetic cardiomyopathy: A review. Medicine 2023, 102, e36299. [Google Scholar] [CrossRef]
- Arslan, A.K.; Yagin, F.H.; Algarni, A.; Karaaslan, E.; Al-Hashem, F.; Ardigò, L.P. Enhancing type 2 diabetes mellitus prediction by integrating metabolomics and tree-based boosting approaches. Front. Endocrinol. 2024, 15, 1444282. [Google Scholar] [CrossRef] [PubMed]
- Wamil, M.; Goncalves, M.; Rutherford, A.; Borlotti, A.; Pellikka, P.A. Multi-modality cardiac imaging in the management of diabetic heart disease. Front. Cardiovasc. Med. 2022, 9, 1043711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, Z.; Wang, J.; Cheung, C.Y.; Zheng, Y.; Lim, L.-L.; Lim, C.C.; Ruamviboonsuk, P.; Raman, R.; Corsino, L.; et al. Integrated image-based deep learning and language models for primary diabetes care. Nat. Med. 2024, 30, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
| Name (Abbreviation, Symbol) | Nutritional Classification | Metabolic Transformation | Important Nitrogenous Derivative | Main AADEs (Abbreviation) |
|---|---|---|---|---|
| Alanine (Ala, A) | NEAA | Glucogenic | - | Glutamic pyruvate transaminase (GPT) |
| Cysteine (Cys, C) | NEAA | Glucogenic | Taurine | Cysteine dioxygenase 1 (CDO1) |
| Aspartic acid (Asp, D) | NEAA | Glucogenic | Purine base, pyrimidine base | Glutamic-oxaloacetic transaminase 1 (GOT1) |
| Glutamic acid (Glu, E) | NEAA | Glucogenic | GABA | Glutamate dehydrogenase 1 (GLUD1) |
| Phenylalanine (Phe, F) | EAA | Glucogenic and ketogenic | CA, thyroxine, melanin | Phenylalanine hydroxylase (PAH) |
| Glycine (Gly, G) | NEAA | Glucogenic | Purine base, porphyrin, creatine, creatine phosphate | Serine hydroxymethyltransferase 1 (SHMT1) |
| Histidine (His, H) | EAA | Glucogenic | Histamine | Histidine ammonia-lyase (HAL) |
| Isoleucine (Ile, I) | EAA | Glucogenic and ketogenic | - | Branched-chain amino acid transaminase (BCATc, BCATm) |
| Branched-chain keto acid dehydrogenase (BCKDH) | ||||
| Lysine (Lys, K) | EAA | Ketogenic | Crotonyl-CoA | Glutaryl-CoA dehydrogenase (GCDH) |
| Leucine (Leu, L) | EAA | Ketogenic | - | see Isoleucine |
| Methionine (Met, M) | EAA | Glucogenic | Spermidine, spermine, creatine, creatine phosphate | Methionine adenosyl transferase 1A (MAT1A) |
| Asparagine (Asn, N) | NEAA | Glucogenic | - | Asparaginase (ASPG) |
| Proline (Pro, P) | NEAA | Glucogenic | - | Proline dehydrogenase 1 (PRODH1) |
| Glutamine (Gln, Q) | NEAA | Glucogenic | Purine base | Glutaminase 1 (GLS1) |
| Glutaminase 2 (GLS2) | ||||
| Arginine (Arg, R) | NEAA | Glucogenic | NO, Creatine, creatine phosphate | Arginase 1 (ARG1) |
| Serine (Ser, S) | NEAA | Glucogenic | - | Serine dehydratase (SDS) |
| Threonine (Thr, T) | EAA | Glucogenic and ketogenic | - | Serine dehydratase like (SDSL) |
| Valine (Val, V) | EAA | Glucogenic | - | see Isoleucine |
| Tryptophan (Trp, W) | EAA | Glucogenic and ketogenic | 5-HT, nicotinic acid | Tryptophan 2,3-dioxygenase (TDO2) |
| Aminocarboxymuconate semialdehyde decarboxylase (ACMSD) | ||||
| Tryptophan hydroxylase 1 (TPH1) | ||||
| Tyrosine (Tyr, Y) | NEAA | Glucogenic and ketogenic | CA, thyroxine, melanin | Tyrosinase (TYR) |
| Tyrosine hydroxylase (TH) | ||||
| Tyrosine aminotransferase (TAT) |
| Target | Drug | Model | Main Findings | Refs |
|---|---|---|---|---|
| BCAT2, PP2Cm | Glucosyringic acid (GA) | Male C57BL/6 J mice induced by HFD and SFZ | GA restored normal BCAA metabolism in diabetic mouse heart via targeting and inhibiting the periostin/NAP1L2/SIRT3 axis | [111] |
| BCAT2, PP2Cm, BCKDK, BCKDH | Pyridostigmine | Male C57BL/6 J mice induced by HFD and SFZ | Pyridostigmine improved disrupted BCAA metabolic enzymes and intestinal microbiota homeostasis, and enhanced vagal nerve activity | [112] |
| PP2Cm, BCKDK, BCKDH | Portulaca oleracea L. extracts (PE) | Mice induced by HFD and STZ | PE improved disrupted BCAA metabolic enzymes and intestinal microbiota homeostasis | [113] |
| BCKDK | 3,6-dichlorobenzo[b] thiophene-2-carboxylic acid (BT2) | Male ob/ob mice, wild-type C57BL/6 J mice, leptin gene mutant ob/ob mice and BCKDK-Alb cre+ mice, induced by HFD | BT2 inhibited BCKDK, reduced the level of p-BCKDH and thus enhanced BCAA catabolism, and potentiated the hypoglycemic effect of metformin | [114] |
| BCKDK | BT2 | Male C57BL/6 N mice performed with TAC | BT2 enhanced BCAA catabolism, and improved TAC-induced cardiac contractile dysfunction and pathological state | [115] |
| BCKDK | BT2 | C57BL/6 mice induced by HFD and SFZ | BT2 enhanced BCAA catabolism, ameliorated the impaired heart function and reduced the infarction area in diabetic mice with myocardial infarction | [116] |
| BCKDK | BT2 | 3-MST KO mice and C57BL/6 J mice performed with TAC | BT2 enhanced BCAA catabolism and ameliorated the severity of TAC-induced heart failure in 3-MST KO mice | [117] |
| The oxidative respiration process within the mitochondria | BT2 | NRVMs and iPSC-derived cardiomyocytes | Independent of the inhibitory effect on BCKDK, BT2 decreased mitochondrial membrane potential, increased proton conductance across the mitochondrial inner membrane, and reduced the production of ROS | [118] |
| IRS1/Akt signaling pathway | Sodium Phenylbutyrate (NaPB) | Murine C2C12 myoblasts, passage 6–8, treated with elevated (4×) media BCAA concentrations | Under high BCAA conditions, NaPB treatment elevated Akt and AS160 phosphorylation, while decreased glycogen synthesis and BCAA concentrations | [119] |
| PP2Cm and mTOR/p-ULK1 signaling pathway | Empagliflozin | Male KK-Ay mice aged 8 weeks induced by HFD | Empagliflozin promoted BCAA degradation through the upregulation of PP2Cm and inhibited mTOR/p-ULK1 to enhance autophagy | [120] |
| BCAA/BCKA metabolism in BAT | Tirzepatide | Male C57BL/6 J mice induced by HFD | Tirzepatide stimulated catabolism of BCAAs/BCKAs in BAT, as demonstrated by increased BCAA/BCKA-derived metabolites | [121] |
| PAH in livers | Tetrahydrobiopterin | Global p21−/− mice backcrossed to C57BL/6 J background for at least 10 generations | In naturally aged mice, consistent with siRNA-mediated p21 knockdown, tetrahydrobiopterin treatment restored healthy cardiac structure and function through reviving hepatic PAH activity and normalizing plasma phenylalanine levels | [11] |
| Gut microbiota | Rice wine polyphenols and polypeptides within Chinese rice wine | Male db/db and db/m mice | Functional components of Chinese rice wine provided a cardioprotective effect against DCM via increasing tryptophan metabolism-associated metabolites and reducing serum phenylalanine by modulating the composition and metabolic function of the gut microbiota | [122] |
| Gut microbiota | Ethanol extract of S. fusiforme (EE) | Male ICR mice aged 8 weeks induced by HFD and SFZ | EE altered the composition of gut microbiota, reduced the levels of BCAAs and AAAs, and improved glucose tolerance as well as pathological changes in the heart | [123] |
| mTORC1-v-ATPase axis, adaptor protein Ragulator, and SLC38A9 | Specific cocktail of amino acids (lysine/leucine/arginine) | Male Lewis rats induced by HFD, and cardiomyocyte models (aRCMs, HL-1 and hiPSC-CM) | Lysine/leucine/arginine stimulated mTORC1-v-ATPase axis, reinternalized CD36, and reduced cardiac lipid uptake | [124] |
| Myocardial NE and TH | Liraglutide and dapagliflozin | Male SD rats induced by HFD and SFZ | Both liraglutide and dapagliflozin significantly reduced TH density and myocardial NE contents, and dapagliflozin exhibited more reduction than liraglutide | [125] |
| Myocardial TH | Stevia Rebaudiana (R) extracts | Male SD rats induced by HFD and SFZ | Stevia R extracts significantly attenuated myocardial TH density | [126] |
| Myocardial NE and TH | RAAS blockers (enalapril and losartan) | Male SD rats induced by HFD and SFZ | Blockade of RAAS attenuated myocardial TH density and NE contents | [127] |
| 5-HT and its cardiac receptor (5-HT2B receptor) | L. plantarum and inulin | Male Wistar rats induced by HFD and SFZ | Increase in intestinal and serum 5-HT as well as decrease in cardiac 5-HT and 5-HT2B receptor were observed in diabetic rats, which were reversed by L. plantarum and insulin administration | [128] |
| Amino acid metabolism and AMPK and PI3K/Akt/FoxO3a signaling pathways in the heart tissue | Erzhi Pill | Male SD rats induced by HFD and SFZ | Erzhi Pill balanced amino acid metabolism similar to glutamic acid and glycine, and regulated the AMPK and PI3K/Akt/FoxO3a signaling pathways | [129] |
| SAMe and DNMT-SOCS1/3-IGF-1 signaling | Vitamin B12 | Male C57BL/6 J mice carrying Elmo1H/H and Ins2Akita/+ genes | High oral dose of vitamin B12 normalized the decreased levels of SAMe and DNMTs, modulated oxidative stress, and improved the echocardiographic indices | [130] |
| Hcy, etc. | Ginger extract | Male Wistar rats induced by SFZ | Ginger extract restored the increased levels of Hcy and alleviated heart structural abnormalities | [131] |
| Cardiac CSE and H2S as well as insulin receptor and Akt/GSK-3β signaling | S-Propargyl-Cysteine (SPRC) | Male C57BLKS/J db/db mice | SPRC increased CSE expression and H2S content, activated cardiac insulin receptor and Akt/GSK-3β signaling | [132] |
| Synoviolin-1 (SYVN1/Hrd1) | NaHS and the novel hydrogen sulfide-releasing molecule GYY4137 | Female db/db mice and HL-1 cells treated with palmitate and oleate | Exogenous H2S improved H2S levels in cardiomyocytes, prevented LDs formation by restoring SYVN1 S sulfhydration and promoting SREBP1 ubiquitination | [133] |
| USP8/parkin signaling pathway | NaHS | Male and female db/db mice | Exogenous H2S activated USP8 S sulfhydration, promoted parkin-dependent mitophagy and ameliorated cardiac impairment | [134] |
| PI3K/Akt pathway | Exogenous SO2 donor (Na2SO3/NaHSO3) | Male SD rats induced by high-fat high-sucrose diet (HFHSD) and SFZ | SO2 activated autophagy to antagonize cardiomyocyte apoptosis and fibrosis by downregulating the excessive activation of PI3K/Akt pathway | [135] |
| Nrf2/HO-1 and NF-κB pathways | Piceatannol (PIC) | Male SD rats induced by SFZ, and HG-induced H9C2 cardiac myoblasts | PIC suppressed HG-induced NF-κB activation by upregulating Nrf2 and HO-1 expression, and alleviated inflammation and oxidative stress in DCM rats | [136] |
| Nrf2/ARE signaling pathway | Empagliflozin | Male db/db mice | Empagliflozin inhibited oxidative stress via activating Nrf2/ARE signaling, modulated ketone body metabolism, and improved mitochondrial dysfunction in DCM | [137] |
| Nrf2/ARE signaling and TGF-β/Smad pathway | Empagliflozin | KK-Ay mice induced by HFD | Empagliflozin attenuated oxidative stress and fibrosis in diabetic heart by activating Nrf2/ARE and suppressing TGF-β/Smad signaling | [138] |
| JNK/p38 MAPK and NF-κB pathways | LCZ696 (an ARNI) | Male C57BL/6 mice induced by SFZ, and HG-induced H9C2 cardiomyocytes | LCZ696 inhibited inflammation and oxidative stress by suppressing JNK/p38 MAPK phosphorylation and NF-κB nuclear translocation | [139] |
| CaMKII/NF-κB/TGF-β1 and PPAR-γ signaling pathways | Pioglitazone and curcumin (Pio/Cur) | Male adult SD rats induced by SFZ | Pio/Cur treatment ameliorated DCM in T1DM via inhibition of CaMKII/NF-κB/TGF-β1 and activation of PPAR-γ pathways | [140] |
| AMPK/Nrf2 pathways | Sulforaphane (SFN) | Engineered cardiac tissue and AMPKα2-KO mice induced by HFD and SFZ | SFN prevented ferroptosis and associated cardiac pathogenesis via AMPK-mediated Nrf2 activation | [141] |
| RAGE, OGT, and GFAT and NF-κB in heart tissue | Vitamin D | Male SD rats induced by SFZ | Vitamin D alleviated DCM by down-regulating the RAGE expression and HBP-mediated O-glycosylation, while reducing NF-κB activity | [142] |
| BH4/eNOS/NO pathway | Sepiapterin (SEP) and L-citrulline (L-Cit) | db/db mice and HG-induced ECs stimulating I/R or H/R conditions | Coadministration of SEP and L-Cit protected diabetic heart, via improvements in coronary arterial endothelial function, cardiac BH4 concentrations, and eNOS function | [143] |
| Nrf2-ROS-p53-MuRF1 axis | Spermine | Male Wistar rats induced by SFZ | Exogenous spermine attenuated DCM by suppressing ROS-p53 mediated downregulation of cell membrane calcium-sensitive receptor | [144] |
| Wnt/β-catenin signaling | Spermine | Male Wistar rats induced by SFZ, and HG-induced CFs from neonatal Wistar rats | Exogenous spermine attenuated myocardial fibrosis by inhibiting ERS and the canonical Wnt/β-catenin signaling pathway | [145] |
| Nrf2 signaling | L-Arginine | Neonatal rat cardiomyocytes H9c2 (2-1) cell line, incubated with MGO to stimulate glycation | L-Arginine exerted protective effects in DCM due to the inhibition of HSA glycation as well as the activation and nuclear translocation of Nrf2 | [146] |
| NF-κβ pathway | β-caryophyllene (BCP) and L-Arginine (LA) | Male SD rats induced by SFZ | Coadministration of BCP and LA led to a reduction in collagen deposition and cardiac fibrosis via NF-ĸβ inhibition | [147] |
| PI3K/Akt/Nrf2 pathway | Spiraeoside | HG-induced AC16 cells | Spiraeoside protected HG-stimulated cardiomyocytes through its antioxidant and antiapoptotic activities via the activation of PI3K/Akt/Nrf2 pathway | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Ma, X.; Mei, S.; Wuyun, Q.; Yan, J. Emerging Insights into the Relationship Between Amino Acid Metabolism and Diabetic Cardiomyopathy. Biomolecules 2025, 15, 916. https://doi.org/10.3390/biom15070916
Wen Y, Ma X, Mei S, Wuyun Q, Yan J. Emerging Insights into the Relationship Between Amino Acid Metabolism and Diabetic Cardiomyopathy. Biomolecules. 2025; 15(7):916. https://doi.org/10.3390/biom15070916
Chicago/Turabian StyleWen, Yi, Xiaozhu Ma, Shuai Mei, Qidamugai Wuyun, and Jiangtao Yan. 2025. "Emerging Insights into the Relationship Between Amino Acid Metabolism and Diabetic Cardiomyopathy" Biomolecules 15, no. 7: 916. https://doi.org/10.3390/biom15070916
APA StyleWen, Y., Ma, X., Mei, S., Wuyun, Q., & Yan, J. (2025). Emerging Insights into the Relationship Between Amino Acid Metabolism and Diabetic Cardiomyopathy. Biomolecules, 15(7), 916. https://doi.org/10.3390/biom15070916







