Caffeic Acid Phenethyl Ester Alleviates Alcohol-Induced Inflammation Associated with Pancreatic Secretion and Gut Microbiota in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Feeding and Grouping Experiment
2.2. Sample Collection and Preservation and Biochemical Analysis of Serum Inflammation Factors
2.3. Transcriptomic Analysis of Zebrafish Liver Sample
2.4. Intestinal Microbial DNA Extraction and 16S rRNA Sequencing
2.5. Molecular Docking for CCKAR
2.6. Data Analysis
3. Results and Analysis
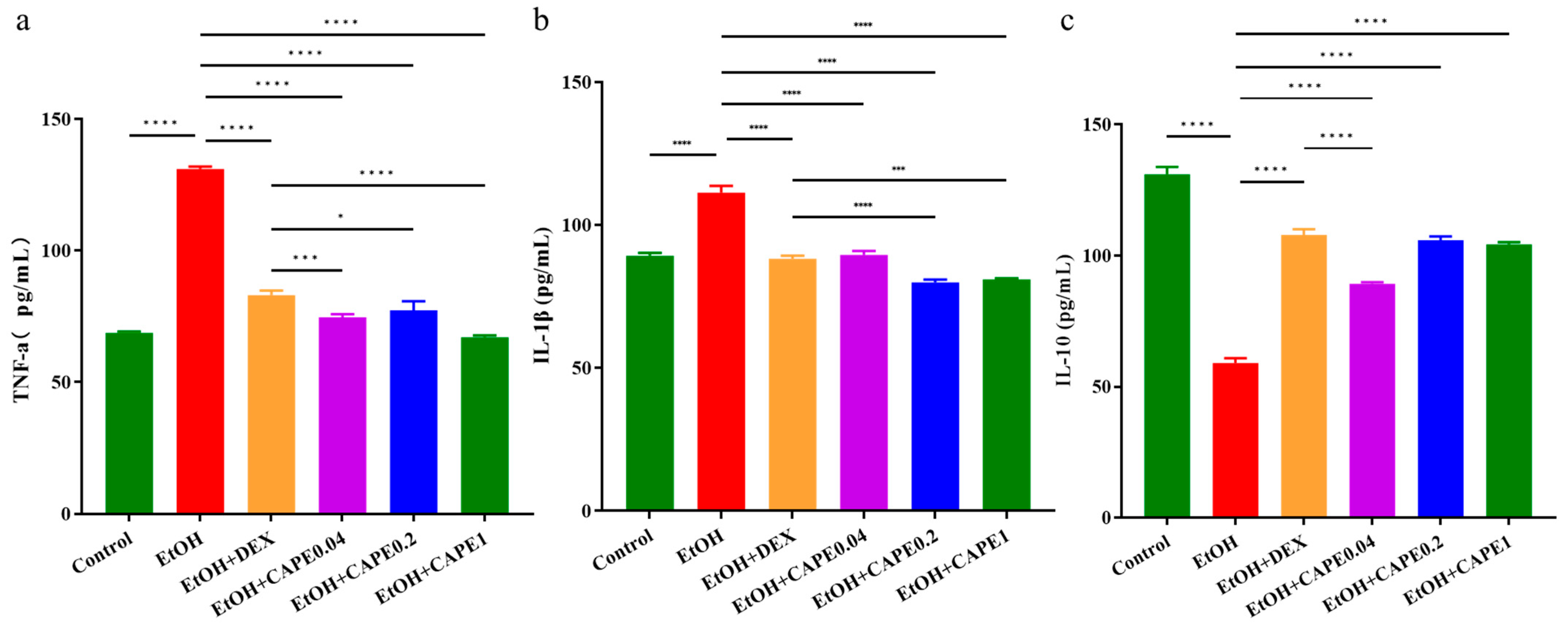
3.1. CAPE Ameliorates Alcohol-Induced Liver Inflammation in Zebrafish
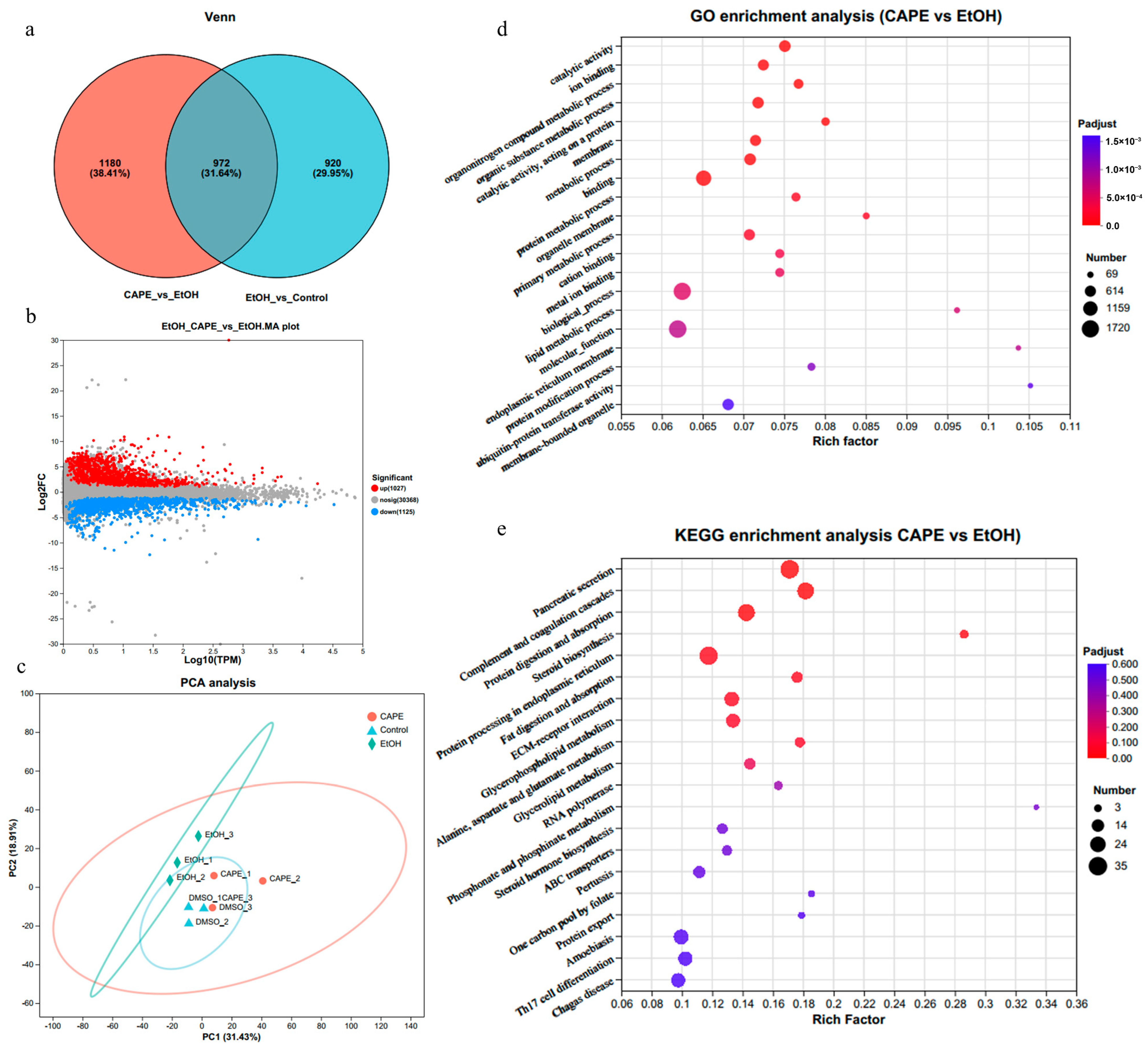
3.2. Effects of CAPE on Inflammation and Related Pathways in the Liver of Zebrafish Based on Transcriptomic Analysis
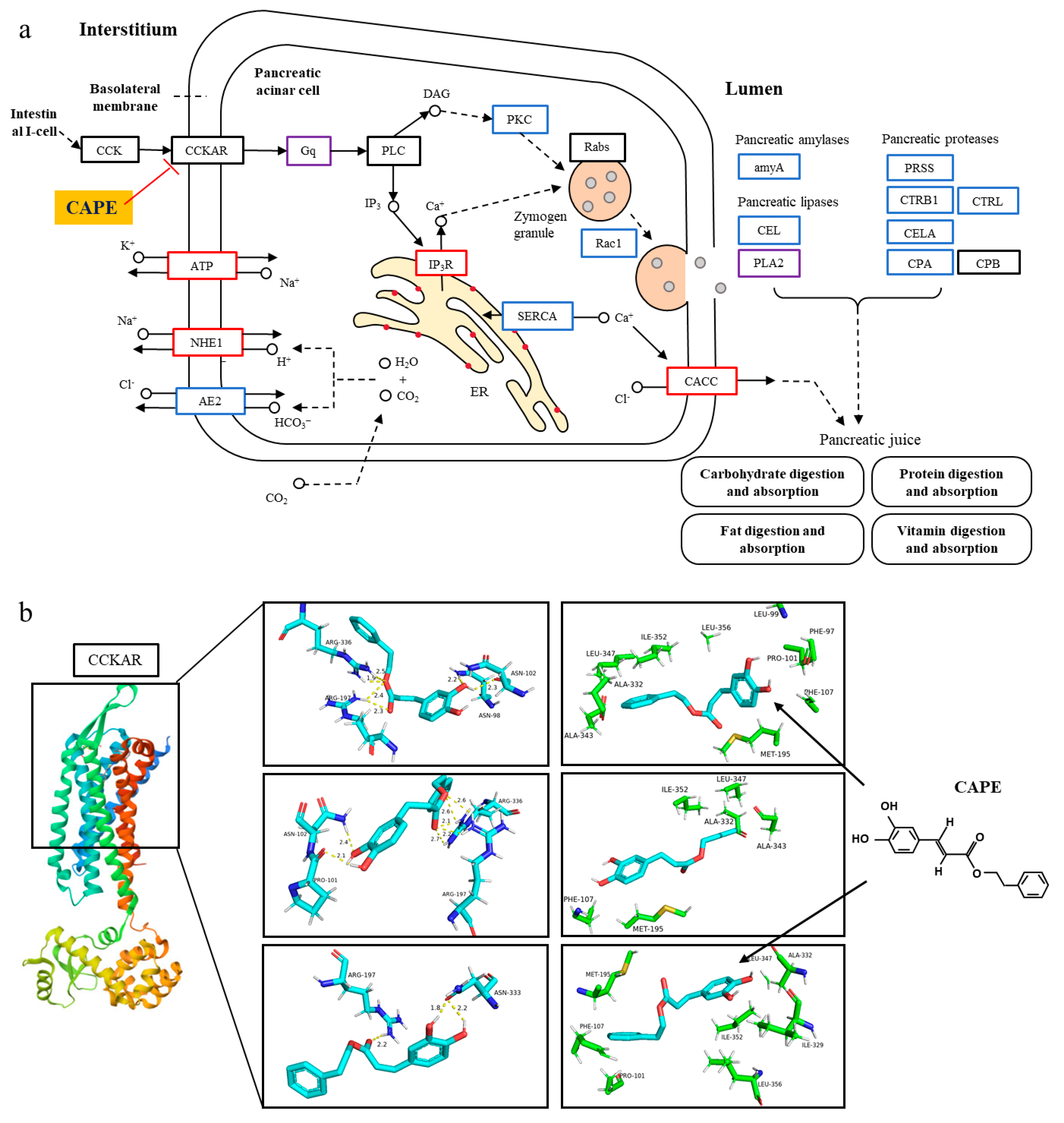
3.3. CAPE Attenuates Pancreatic Exocrine Secretion and Inflammation Through CCKAR Modulation
3.4. Effect of CAPE on Gut Microbiota Associated with Inflammation in Zebrafish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Ju, J.; Diao, H.; Song, J.; Bian, Y.; Yang, B. Innovative pharmacotherapy for hepatic metabolic and chronic inflammatory diseases in China. Br. J. Pharmacol. 2024; early view. [Google Scholar] [CrossRef]
- Khazaei, M.; Ardeshir, R.A. Protective effects of sulfated polysaccharides from Enteromorpha intestinalis on oxidative stress, liver iron overload and Ferroptosis in Zebra fish exposed to ethanol. Biomed. Pharmacother. 2024, 181, 117715. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.K.; DeRossi, C.; Alter, I.L.; Nayar, S.; Giri, M.; Zhang, C.; Cho, J.H.; Chu, J. Single-cell transcriptomics reveals conserved cell identities and fibrogenic phenotypes in zebrafish and human liver. Hepatol. Commun. 2022, 6, 1711–1724. [Google Scholar] [CrossRef]
- Ellis, J.L.; Yin, C. Histological Analyses of Acute Alcoholic Liver Injury in Zebrafish. Jove-J. Vis. Exp. 2017, 123, e55630. [Google Scholar] [CrossRef]
- Passeri, M.J.; Cinaroglu, A.; Gao, C.; Sadler, K.C. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology 2009, 49, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, M.; Wang, J.; Ma, C.; Yi, X. Analysis of the Mechanism of Ethanol on Liver Injury of Danio rerio Based on RNA-seq Technique. Genom. Appl. Biol. 2022, 41, 1391–1402. [Google Scholar]
- Holley, R.W.; Apgar, J.; Everett, G.A.; Madison, J.T.; Marquisee, M.; Merrill, S.H.; Penswick, J.R.; Zamir, A. Structure of a Ribonucleic Acid. Science 1965, 147, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Lin, M.; Chen, K.; Wu, Y.; Le, T.N.; Zhang, J.; Zhao, M. Dose-specific amelioration of caffeic acid phenethyl ester on high-fat diet-induced obesity based on intestinal FXR signaling and bile acid regulation. Food Biosci. 2025, 68, 106628. [Google Scholar] [CrossRef]
- Yordanov, Y. Caffeic acid phenethyl ester (CAPE): Cornerstone pharmacological studies and drug delivery systems. Pharmacia 2019, 66, 223–231. [Google Scholar] [CrossRef]
- Göçer, H.; Gülçin, İ. Caffeic acid phenethyl ester (CAPE): Correlation of structure and antioxidant properties. Int. J. Food Sci. Nutr. 2011, 62, 821–825. [Google Scholar] [CrossRef]
- Juman, S.; Yasui, N.; Okuda, H.; Ueda, A.; Negishi, H.; Miki, T.; Ikeda, K. Caffeic acid phenethyl ester inhibits differentiation to adipocytes in 3T3-L1 mouse fibroblasts. Biol. Pharm. Bull. 2010, 33, 1484–1488. [Google Scholar] [CrossRef]
- Shin, S.H.; Seo, S.G.; Min, S.; Yang, H.; Lee, E.; Son, J.E.; Kwon, J.Y.; Yue, S.; Chung, M.-Y.; Kim, K.-H. Caffeic acid phenethyl ester, a major component of propolis, suppresses high fat diet-induced obesity through inhibiting adipogenesis at the mitotic clonal expansion stage. J. Agric. Food Chem. 2014, 62, 4306–4312. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, C.; Chen, R.-D.; Lin, M.-H.; Sun, R.; Cai, C.-G.; Cai, H.-Y.; Zhao, M.-J. Regulatory effect of caffeic acid phenethyl ester on oxidative stress and lipid metabolism in HepG2 cells. Food Sci. 2023, 44, 86–93. [Google Scholar]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic acid phenethyl ester (propolis extract) ameliorates insulin resistance by inhibiting JNK and NF-κB inflammatory pathways in diabetic mice and HepG2 cell models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Saluja, A.K.; Bhagat, L. Pathophysiology of alcohol-induced pancreatic injury. Pancreas 2003, 27, 327–331. [Google Scholar] [CrossRef]
- Chandra, R.; Liddle, R.A. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 63–67. [Google Scholar] [CrossRef]
- Pandol, S.J.; Periskic, S.; Gukovsky, I.; Zaninovic, V.; Jung, Y.; Zong, Y.; Solomon, T.E.; Gukovskaya, A.S.; Tsukamoto, H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 1999, 117, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Carangelo, R.; Miller, L.J.; Gorelick, F. Effect of ethanol on cholecystokinin-stimulated zymogen conversion in pancreatic acinar cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 1996, 270, G171–G175. [Google Scholar] [CrossRef]
- Saluja, A. Ethanol induced increase in plasma cholecystokinin level is mediated by the release of a CCK-releasing peptide into the duodenum. Gastroenterology 1993, 104, A333. [Google Scholar]
- Chen, J.; Liu, X.; Zou, Y.; Gong, J.; Ge, Z.; Lin, X.; Zhang, W.; Huang, H.; Zhao, J.; Saw, P.E. A high-fat diet promotes cancer progression by inducing gut microbiota–mediated leucine production and PMN-MDSC differentiation. Proc. Natl. Acad. Sci. USA 2024, 121, e2306776121. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Yan, H.; Pan, Y.; Zhou, J.; Zhong, H.; Wang, J.; Cai, H.; Feng, F.; Zhao, M. A comprehensive review of medium chain monoglycerides on metabolic pathways, nutritional and functional properties, nanotechnology formulations and applications in food system. Crit. Rev. Food Sci. Nutr. 2025, 65, 2943–2964. [Google Scholar] [CrossRef]
- GB/T 35823-2018; Laboratory Animals—General Requirements for Animal Experiments. China Standard Press: Beijing, China, 2018.
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H. Majorbio Cloud 2024: Update single-cell and multiomics workflows. Imeta 2024, 3, e217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Su, Q.; Wu, H.Q.; Cai, C.G.; Ninh, L.; Cai, H.Y. Elucidating Bile Acid Tolerance in Effects on Sterol Biosynthesis and Transport Protein Expression. Foods 2024, 13, 3405. [Google Scholar] [CrossRef]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bai, X.; Luan, X.; Min, J.; Tian, X.; Li, H.; Li, H.; Sun, W.; Liu, W.; Fan, W. Delicate regulation of IL-1β-mediated inflammation by cyclophilin A. Cell Rep. 2022, 38, 110513. [Google Scholar] [CrossRef]
- Levi, M. Inflammation and coagulation. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; Wiley: Hoboken, NJ, USA, 2017; pp. 833–860. [Google Scholar]
- Tsantes, A.G.; Parastatidou, S.; Tsantes, E.A.; Bonova, E.; Tsante, K.A.; Mantzios, P.G.; Vaiopoulos, A.G.; Tsalas, S.; Konstantinidi, A.; Houhoula, D. Sepsis-induced coagulopathy: An update on pathophysiology, biomarkers, and current guidelines. Life 2023, 13, 350. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, D.; Zhuang, Y.; Croll, T.I.; Cai, X.; Dai, A.; He, X.; Duan, J.; Yin, W.; Ye, C. Ligand recognition and G-protein coupling selectivity of cholecystokinin A receptor. Nat. Chem. Biol. 2021, 17, 1238–1244. [Google Scholar] [CrossRef]
- Teng, B.; Huang, C.; Cheng, C.-L.; Udduttula, A.; Yu, X.-F.; Liu, C.; Li, J.; Yao, Z.-Y.; Long, J.; Miao, L.-F.; et al. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor, GPRC6A. J. Hepatol. 2020, 73, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Xin, Z.; Gao, W.; Guo, C. Brazilian green propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int. J. Environ. Res. Public Health 2016, 13, 498. [Google Scholar] [CrossRef]
- Williams, J.A. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu. Rev. Physiol. 2001, 63, 77–97. [Google Scholar] [CrossRef]
- Li, S.; Ni, Z.; Cong, B.; Gao, W.; Xu, S.; Wang, C.; Yao, Y.; Ma, C.; Ling, Y. CCK-8 inhibits LPS-induced IL-1β production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-κB pathway. Shock 2007, 27, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, U.; Wang, M.; Hu, J.C. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Liu, T.; Fu, A.; Ruan, S.; Zhong, H.; Tang, J.; Zhao, M.; Li, Y.; Zhu, S.; Cai, H. Novel gut microbiota patterns involved in the attenuation of dextran sodium sulfate-induced mouse colitis mediated by glycerol monolaurate via inducing anti-inflammatory responses. Mbio 2021, 12, e0214821. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.-Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef]
- Aderinto, N.; Abdulbasit, M.O.; Tangmi, A.D.E.; Okesanya, J.O.; Mubarak, J.M. Unveiling the growing significance of metabolism in modulating immune cell function: Exploring mechanisms and implications: A review. Ann. Med. Surg. 2023, 85, 5511–5522. [Google Scholar] [CrossRef]
- Prasad, N.R.; Jeyanthimala, K.; Ramachandran, S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J. Photochem. Photobiol. B: Biol. 2009, 95, 196–203. [Google Scholar] [CrossRef]
- Arias-Jayo, N.; Abecia, L.; Alonso-Sáez, L.; Ramirez-Garcia, A.; Rodriguez, A.; Pardo, M.A. High-fat diet consumption induces microbiota dysbiosis and intestinal inflammation in zebrafish. Microb. Ecol. 2018, 76, 1089–1101. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.A.; Liu, T.; Tang, J.; Zhang, X.; Feng, F.; Cai, H.; Zhao, M. Food additive glycerol monocaprylate modulated systemic inflammation and gut microbiota without stimulating metabolic dysfunction in high-fat diet fed mice. Food Res. Int. 2023, 167, 112734. [Google Scholar] [CrossRef]
- Baseman, J.B.; Tully, J.G. Mycoplasmas: Sophisticated, reemerging, and burdened by their notoriety. Emerg. Infect. Dis. 1997, 3, 21. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Dong, H.T.; Hirono, I.; Kondo, H.; Senapin, S.; Rodkhum, C. Comparative genome analysis of fish pathogen Flavobacterium columnare reveals extensive sequence diversity within the species. Infect. Genet. Evol. 2017, 54, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, J.; Pan, L.; Zhang, M.; Chen, Z.; Lu, M. Immunotoxicity and the mechanisms of aflatoxin B1-induced growth retardation in shrimp and alleviating effects of bile acids. J. Hazard. Mater. 2023, 459, 132266. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, J.; Liu, C.; Le, T.N.; Lu, Y.; Feng, F.; Zhao, M. High-fat diet-induced decreased circulating bile acids contribute to obesity associated with gut microbiota in mice. Foods 2024, 13, 699. [Google Scholar] [CrossRef]


| KEGG Pathways | Gene Name | Control | EtOH | CAPE | FC (EtOH/CAPE) | padjust |
|---|---|---|---|---|---|---|
| Coagulation cascade | F9 | 34.48 | 118.63 | 28.40 | 4.18 | 0.020 |
| F8 | 0.56 | 3.42 | 0.28 | 12.37 | 0.000 | |
| F10 | 4.01 | 7.72 | 4.14 | 1.87 | 0.009 | |
| F5 | 137.91 | 191.25 | 115.55 | 1.66 | 0.020 | |
| F2 | 755.30 | 1130.58 | 464.18 | 2.44 | 0.028 | |
| Complement cascade | C3 | 302.59 | 629.66 | 141.02 | 4.46 | 0.000 |
| C5 | 52.43 | 232.52 | 36.94 | 6.29 | 0.002 | |
| C7 | 246.62 | 453.23 | 23.85 | 19.00 | 0.001 | |
| C8A | 172.93 | 329.74 | 140.05 | 2.35 | 0.013 | |
| C9 | 1290.50 | 3452.56 | 706.43 | 4.89 | 0.001 | |
| Steroid biosynthesis | EGR27 | 11.53 | 16.97 | 5.88 | 3.61 | 0.031 |
| DHCR24 | 6.13 | 22.80 | 2.52 | 9.80 | 0.000 | |
| DHCR7 | 8.05 | 16.53 | 5.96 | 2.40 | 0.045 | |
| CEL1 | 13.92 | 41.15 | 11.78 | 4.93 | 0.000 | |
| CEL2 | 17.19 | 84.36 | 22.93 | 5.75 | 0.002 | |
| SOAT1 | 5.68 | 14.24 | 5.30 | 2.92 | 0.028 | |
| CYP24A1 | 26.31 | 248.35 | 14.38 | 23.81 | 0.000 | |
| Glycerolipid metabolism | TGL2 | 112.44 | 239.34 | 115.32 | 2.81 | 0.024 |
| ALDH3A2B | 1.88 | 14.67 | 1.47 | 12.50 | 0.000 | |
| MOGAT3A | 40.47 | 61.94 | 17.98 | 4.93 | 0.011 | |
| PLPP2A | 0.32 | 2.80 | 0.17 | 20.41 | 0.000 | |
| LPIN | 0.27 | 1.18 | 0.43 | 3.73 | 0.030 | |
| LPL | 81.40 | 124.00 | 65.99 | 2.56 | 0.014 | |
| DGAT2 | 13.58 | 27.54 | 4.58 | 8.06 | 0.000 | |
| Fat digestion and absorption | NPC1L1 | 4.06 | 7.33 | 4.15 | 3.61 | 0.004 |
| ABCG5 | 5.14 | 10.31 | 4.27 | 3.05 | 0.019 | |
| ABCA1B | 2.13 | 6.61 | 1.44 | 8.40 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Guo, X.; Xu, X.; Chang, C.; Le, T.N.; Cai, H.; Zhao, M. Caffeic Acid Phenethyl Ester Alleviates Alcohol-Induced Inflammation Associated with Pancreatic Secretion and Gut Microbiota in Zebrafish. Biomolecules 2025, 15, 918. https://doi.org/10.3390/biom15070918
Lin M, Guo X, Xu X, Chang C, Le TN, Cai H, Zhao M. Caffeic Acid Phenethyl Ester Alleviates Alcohol-Induced Inflammation Associated with Pancreatic Secretion and Gut Microbiota in Zebrafish. Biomolecules. 2025; 15(7):918. https://doi.org/10.3390/biom15070918
Chicago/Turabian StyleLin, Menghui, Xiaogang Guo, Xinyu Xu, Chao Chang, Thanh Ninh Le, Haiying Cai, and Minjie Zhao. 2025. "Caffeic Acid Phenethyl Ester Alleviates Alcohol-Induced Inflammation Associated with Pancreatic Secretion and Gut Microbiota in Zebrafish" Biomolecules 15, no. 7: 918. https://doi.org/10.3390/biom15070918
APA StyleLin, M., Guo, X., Xu, X., Chang, C., Le, T. N., Cai, H., & Zhao, M. (2025). Caffeic Acid Phenethyl Ester Alleviates Alcohol-Induced Inflammation Associated with Pancreatic Secretion and Gut Microbiota in Zebrafish. Biomolecules, 15(7), 918. https://doi.org/10.3390/biom15070918








