The Connection Between Lipid Metabolism in the Heart and Liver of Wuzhishan Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA-Sequencing
2.3. Metabolome Analysis
2.3.1. Metabolite Extraction from Tissues
2.3.2. LC-MS Analysis
2.3.3. Data Processing and Metabolite Identification
2.3.4. Metabolites Analysis
2.4. Blood Biochemical Parameters Test
2.5. Histological Examination
3. Results
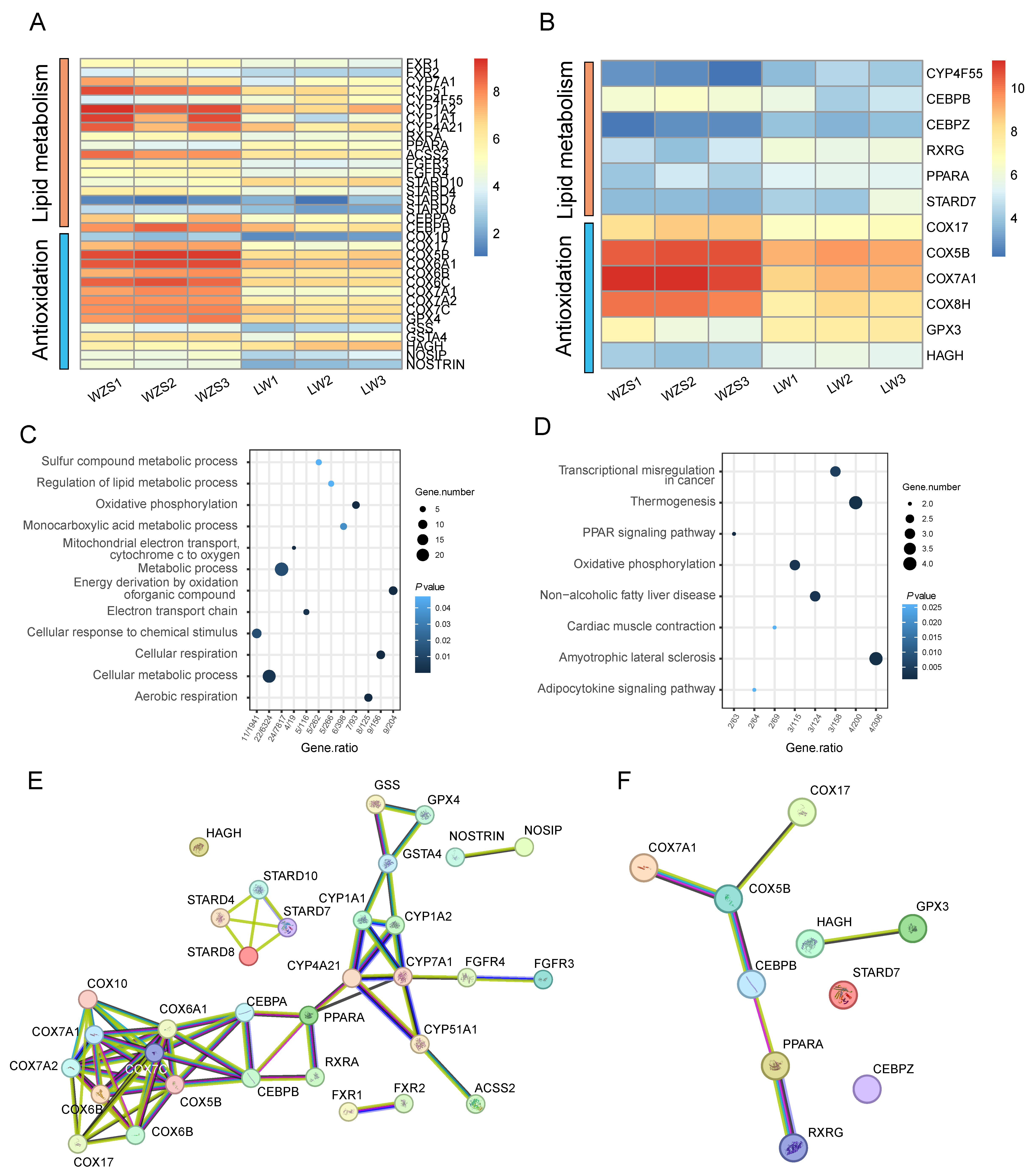
3.1. Gene Expressions
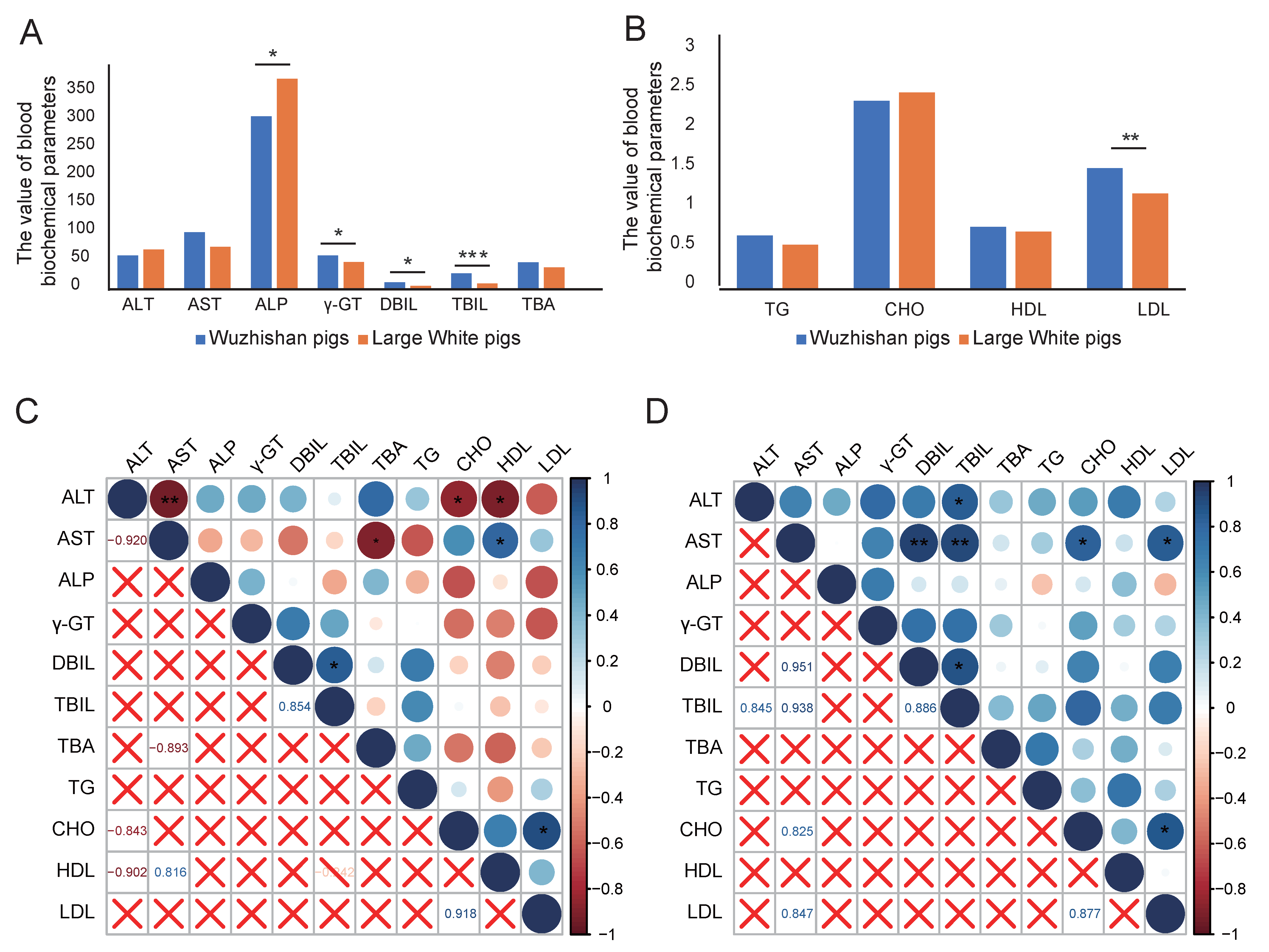
3.2. Blood Biochemical Parameters Analysis
3.3. Metabolite Analysis of the Liver and the Heart
3.4. The Correlations Between Blood Biochemical Parameters and Tissue Metabolites
3.5. Histological Examination Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodayan, A.; Coggan, A.R.; Peterson, L.R. A “PET” area of interest: Myocardial metabolism in human systolic heart failure. Heart Fail. Rev. 2013, 18, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wen, J.; Zhou, Z.; Dai, Q.; Huang, Y.; Yang, N.; Guo, J.; Zhang, J.; Ren, F.; Zhou, X.; et al. Effect of hydroxy-α-sanshool on lipid metabolism in liver and hepatocytes based on AMPK signaling pathway. Phytomedicine 2024, 132, 155849. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.A.; Smith, T.K. Lipid metabolism in Trypanosoma cruzi: A review. Mol. Biochem. Parasitol. 2020, 240, 111324. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M. Domain 4 (D4) of perfringolysin o to visualize cholesterol in cellular membranes-the update. Sensors 2017, 17, 504. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol review: A metabolically important molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.; Sinturel, F.; Riezman, H.; Dibner, C. Lipid metabolism around the body clocks. Prog. Lipid. Res. 2023, 91, 101235. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L.; et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 2022, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, F.; Kan, M.; Jin, C.; Jones, R.B.; Weinstein, M.; Deng, C.X.; McKeehan, W.L. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 2000, 275, 15482–15489. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; Wu, D.; Qin, L.; He, Y.; Tan, D. Gypensapogenin a-liposomes efficiently ameliorates hepatocellular lipid accumulation via activation of FXR receptor. Molecules 2024, 29, 4080. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, D.; Kong, B.; Taylor, R.E.; Brinker, A.; Goedken, M.; Buckley, B.; Guo, G.L. Bile acid homeostasis in female mice deficient in Cyp7a1 and Cyp27a1. Acta Pharm. Sin. B 2021, 11, 38473856. [Google Scholar] [CrossRef] [PubMed]
- Da Dalt, L.; Moregola, A.; Svecla, M.; Pedretti, S.; Fantini, F.; Ronzio, M.; Uboldi, P.; Dolfini, D.; Donetti, E.; Baragetti, A.; et al. The inhibition of inner mitochondrial fusion in hepatocytes reduces non-alcoholic fatty liver and improves metabolic profile during obesity by modulating bile acid conjugation. Cardiovasc. Res. 2024, 119, 2917–2929. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, N.N.; Peng, Z.G. Effect of bicyclol on blood biomarkers of NAFLD: A systematic review and meta-analysis. BMJ Open 2020, 10, e039700. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; He, L.; Sun, J.; Wenbo, J.; Li, X.; Chen, J.; Wang, C. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Foucquier, J.; Younossi, Z.M.; Harrison, S.A.; Newsome, P.N.; Chan, W.K.; Yilmaz, Y.; De Ledinghen, V.; Costentin, C.; Zheng, M.H.; et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J. Hepatol. 2023, 78, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Qiao, J.; Zhang, H. Mildly elevated serum bilirubin and its correlations with lipid levels among male patients undergoing health checkups. Lipids Health Dis. 2023, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- El-Sebaey, A.M.; Abramov, P.N. Hepatocyte-derived canine familiaris-microRNAs as serum biomarkers of hepatic steatosis or fibrosis as implicated in the pathogenesis of canine cholecystolithiasis. Vet. Clin. Pathol. 2022, 50 (Suppl. 1), 37–46. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Zeng, Z.; Zhang, Q.; Du, G.; Guo, X.; Wei, Y. The LOX-1 receptor ectopically expressed in the liver alleviates atherosclerosis by clearing Ox-LDL from the circulation. Mol. Med. 2022, 28, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Su, J.; Chen, H.; Zhao, P.; Qian, H.; Gao, X.; Ye, Q.; Zhang, G.; Li, X. Associations of blood metals with liver function: Analysis of NHANES from 2011 to 2018. Chemosphere 2023, 317, 137854. [Google Scholar] [CrossRef] [PubMed]
- Cross-Najafi, A.A.; Lopez, K.; Isidan, A.; Park, Y.; Zhang, W.; Li, P.; Yilmaz, S.; Akbulut, S.; Ekser, B. Current barriers to clinical liver xenotransplantation. Front. Immunol. 2022, 13, 827535. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MSJ. Nat. Protoc. 2012, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shrestha, R.; Kim, S.; Kim, J.A.; Lee, J.; Jeong, T.C.; Kim, J.H.; Lee, S. In vitro characterization of glycyrol metabolites in human liver microsomes using HR-resolution MS spectrometer coupled with tandem mass spectrometry. Xenobiotica 2020, 50, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Lee, G.; Cavanaugh, K.E.; Chang, J.W.; Gardel, M.L.; Moellering, R.E. High throughput discovery of functional protein modifications by Hotspot Thermal Profiling. Nat. Methods 2019, 16, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; Yang, R. An assessment of AcquireX and Compound Discoverer software 3.3 for non-targeted metabolomics. Sci. Rep. 2024, 14, 4841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, S.; Li, K.; Xiong, P.; Qin, S.; Cai, W. Systematic screening of chemical constituents in the traditional chinese medicine arnebiae radix by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules 2022, 27, 2631. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.; Lu, X.; Lin, X.; Xu, G. A data preprocessing strategy for metabolomics to reduce the mask effect in data analysis. Front. Mol. Biosci. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H.; et al. Majorbio Cloud 2024: Update single-cell and multiomics workflows. iMeta 2024, 3, e217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nanduri, R.; Bhagyaraj, E.; Kalra, R.; Ahuja, N.; Chacko, A.P.; Tiwari, D.; Sethi, K.; Saini, A.; Chandra, V.; et al. Vitamin D3-VDR-PTPN6 axis mediated autophagy contributes to the inhibition of macrophage foam cell formation. Autophagy 2021, 17, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jang, H.J.; Kim, S.; Choi, S.S.; Khim, K.W.; Eom, H.J.; Hyun, J.; Shin, K.J.; Chae, Y.C.; Kim, H.; et al. Hepatic MIR20B promotes nonalcoholic fatty liver disease by suppressing PPARA. eLife 2021, 10, e70472. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Luo, Y.; Yan, N.; Hamada, K.; Zhao, N.; Xia, Y.; Wang, P.; Zhao, C.; Qi, D.; Yang, S.; et al. Intestinal peroxisome proliferator-activated receptor α-fatty acid-binding protein 1 axis modulates nonalcoholic steatohepatitis. Hepatology 2023, 77, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Liu, L.; Hao, N.; Zou, S.; Jiang, Q.; Liang, Y.; Ma, N.; Feng, S.; Wang, X.; et al. Sirtuin 1 is involved in oleic acid-induced calf hepatocyte steatosis via alterations in lipid metabolism-related proteins. J. Anim. Sci. 2021, 99, skab250. [Google Scholar] [CrossRef] [PubMed]
- Toh, R. Assessment of HDL Cholesterol Removal Capacity: Toward Clinical Application. J. Atheroscler. Thromb. 2019, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Tian, M.; Zhang, X.; Jin, X.; Jiang, Y.; Sun, X.; Wang, Y.; Peng, P.; Liu, J.; Xia, C.; et al. Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2014681118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Kuna, R.S.; Pinheiro, L.V.; Nguyen, P.T.T.; Welles, J.E.; Drummond, J.M.; Murali, N.; Sharma, P.V.; Supplee, J.G.; Shiue, M.; et al. Bempedoic acid suppresses diet-induced hepatic steatosis independently of ATP-citrate lyase. Cell Metab. 2025, 37, 239–254.e7. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Geng, Y.; Yang, Y.; Seim, I.; Yang, G. Oxidative stress drives divergent evolution of the glutathione peroxidase (GPX) gene family in mammals. Integr. Zool. 2021, 16, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Zhao, W.; Tang, S.Y.; Levin, M.G.; Ibrahim, A.; Yang, Y.; Roberts, E.; Lai, L.; Li, J.; Assoian, R.K.; et al. Endothelial lipid droplets suppress eNOS to link high fat consumption to blood pressure elevation. J. Clin. Investig. 2023, 133, e173160. [Google Scholar] [CrossRef] [PubMed]
- Milewski, K.; Czarnecka, A.M.; Albrecht, J.; Zielińska, M. Decreased expression and uncoupling of endothelial nitric oxide synthase in the cerebral cortex of rats with thioacetamide-induced acute liver failure. Int. J. Mol. Sci. 2021, 22, 6662. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, A.B.; Gohil, V.M. The role of COA6 in the mitochondrial copper delivery pathway to cytochrome c oxidase. Biomolecules 2022, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gao, M.; Wang, H.; Chen, Q.; Liu, X.; Mo, Q.; Huang, X.; Ye, X.; Zhang, D. Jingangteng capsules ameliorate liver lipid disorders in diabetic rats by regulating microflora imbalances, metabolic disorders, and farnesoid X receptor. Phytomedicine 2024, 132, 155806. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, X.; Xu, Q.; Li, R.; Yao, L.; Zhang, A.; Zhou, Q.; Xiao, Z.; Li, S.; Meng, X.; et al. Total Sanghuangporus vaninii extract inhibits hepatocyte ferroptosis and intestinal microbiota disturbance to attenuate liver fibrosis in mice. J. Ethnopharmacol. 2025, 345, 119571. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Wang, F.; Sun, R.; Zheng, X.; Lin, Y.; Chao, Z. The Connection Between Lipid Metabolism in the Heart and Liver of Wuzhishan Pigs. Biomolecules 2025, 15, 1024. https://doi.org/10.3390/biom15071024
Ren Y, Wang F, Sun R, Zheng X, Lin Y, Chao Z. The Connection Between Lipid Metabolism in the Heart and Liver of Wuzhishan Pigs. Biomolecules. 2025; 15(7):1024. https://doi.org/10.3390/biom15071024
Chicago/Turabian StyleRen, Yuwei, Feng Wang, Ruiping Sun, Xinli Zheng, Yanning Lin, and Zhe Chao. 2025. "The Connection Between Lipid Metabolism in the Heart and Liver of Wuzhishan Pigs" Biomolecules 15, no. 7: 1024. https://doi.org/10.3390/biom15071024
APA StyleRen, Y., Wang, F., Sun, R., Zheng, X., Lin, Y., & Chao, Z. (2025). The Connection Between Lipid Metabolism in the Heart and Liver of Wuzhishan Pigs. Biomolecules, 15(7), 1024. https://doi.org/10.3390/biom15071024





