The Future of Tumor Markers: Advancing Early Malignancy Detection Through Omics Technologies, Continuous Monitoring, and Personalized Reference Intervals
Abstract
1. Introduction
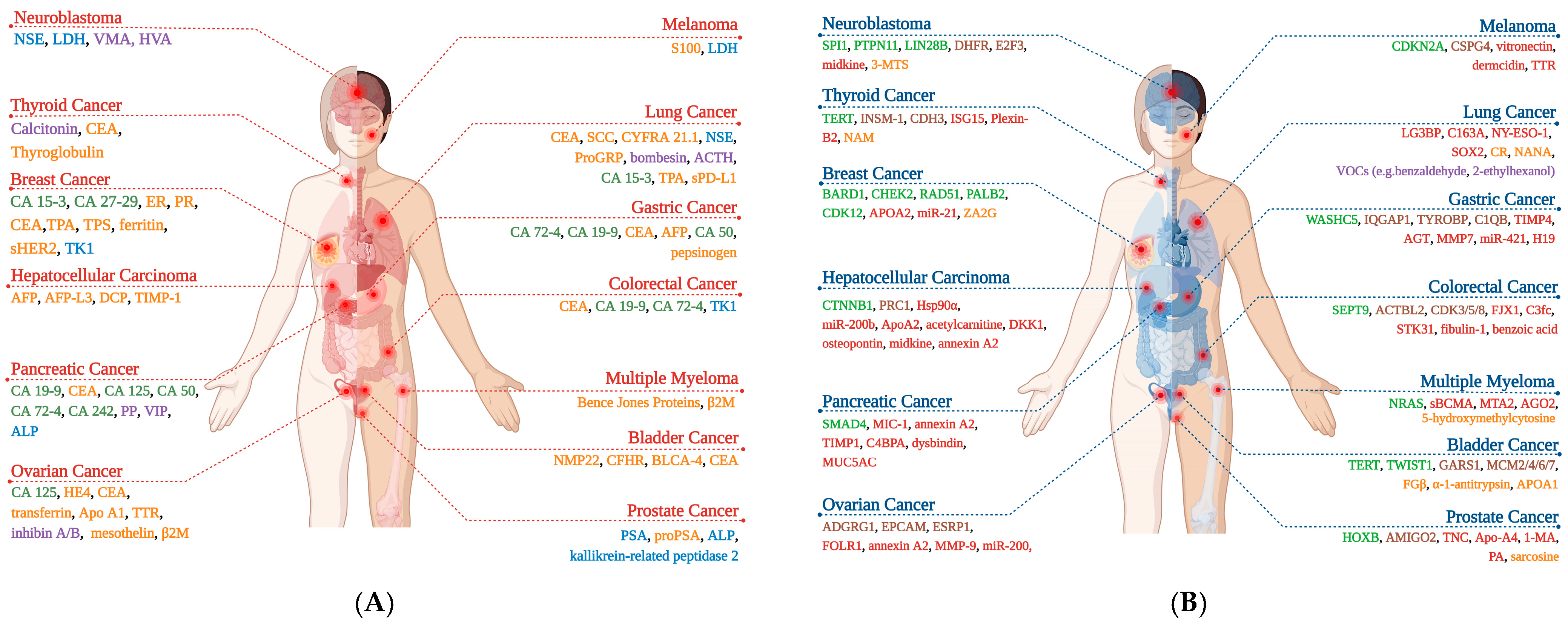
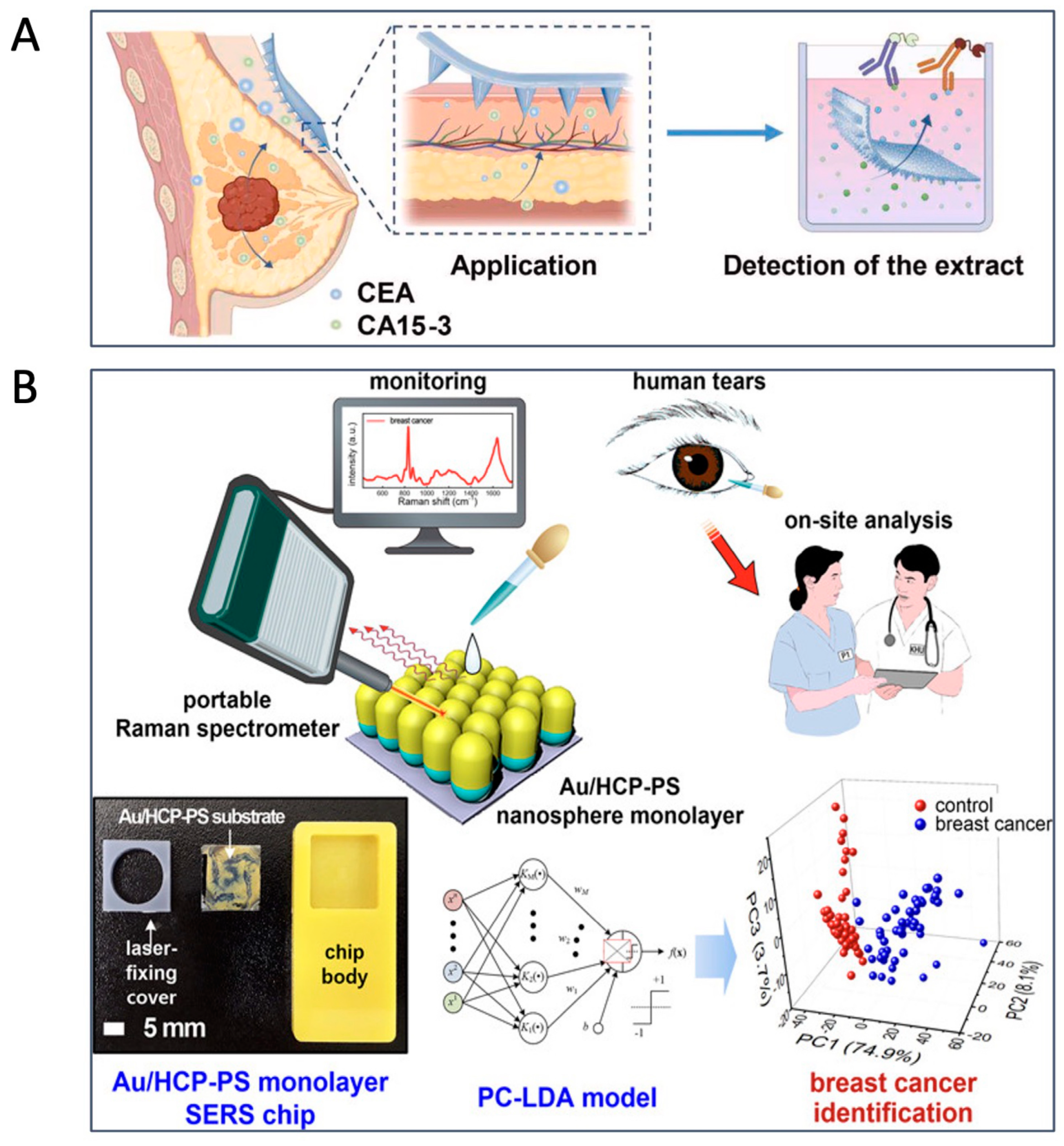
2. Tumor Markers
2.1. Proteins
2.1.1. Alpha-Fetoprotein (AFP)
2.1.2. Carcinoembryonic Antigen (CEA)
2.2. Enzymes
Prostate-Specific Antigen (PSA)
2.3. Hormones
Calcitonin
2.4. Carbohydrate Antigens
2.4.1. Carbohydrate Antigen 19-9
2.4.2. Cancer Antigen 125
2.4.3. Cancer Antigen 15-3
2.5. Circulating Tumor Cells (CTCs)
3. Strategies for Early Detection of Malignancies
3.1. Omics Technologies in Tumor Marker Discovery: Opportunities and Challenges in Malignancy Diagnosis
3.1.1. Application of Omics Approaches for Novel Tumor Marker Identification
3.1.2. Advancing Cancer Diagnostics Through Multi-Omics Integration
3.1.3. Liquid Biopsy for Omics Analysis
3.1.4. Integration of Omics Technologies and Artificial Intelligence
3.2. Continuous Monitoring of Malignancies Using Wearable Biosensors for Tumor Markers
3.2.1. Biosensors for Tumor Biomarker Analysis from Blood
3.2.2. Biosensors for Tumor Biomarker Analysis from Other Body Fluids
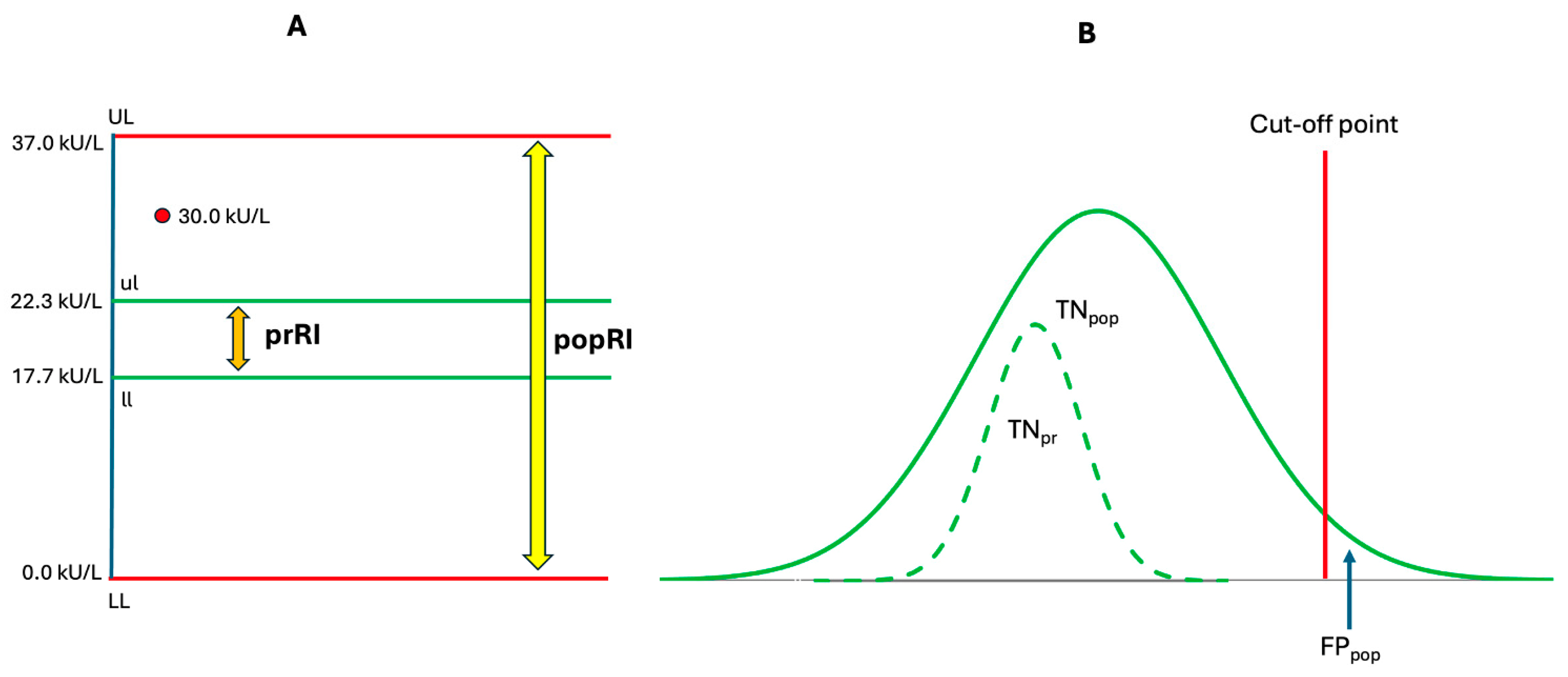
3.3. Personalization of Tumor Markers
3.3.1. Personalized Reference Intervals for Tumor Markers: A Precision Medicine Approach
3.3.2. Personalized Decision Limits for Tumor Markers in the Diagnosis of Malignant Diseases
3.3.3. Personalized Reference Change Value for Monitoring Disease Progression and Treatment Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020; Available online: http://publications.iarc.fr/586 (accessed on 1 May 2025).
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality Due to Cancer Treatment Delay: Systematic Review and Meta-Analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, N.; Gitlin, M.; Fadli, E.; Chung, K.C. Increased Healthcare Costs by Later Stage Cancer Diagnosis. BMC Health Serv. Res. 2022, 22, 1155. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Kumar, R.; Villarreal-Garza, C.; Sinha, S.; Saini, S.; Semwal, J.; Saxsena, V.; Zamre, V.; Chintamani, C.; Ray, M.; et al. Diagnostic Delays in Breast Cancer among Young Women: An Emphasis on Healthcare Providers. Breast 2024, 73, 103623. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Chen, P.; Liu, B.-F. Liquid Biopsy on Microfluidics: From Existing Endogenous to Emerging Exogenous Biomarkers Analysis. Anal. Chem. 2025, 97, 8625–8640. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Duffy, M.J.; Hofmann, B.R.; Lamerz, R.; Fritsche, H.A.; Gaarenstroom, K.; Bonfrer, J.; Ecke, T.H.; Grossman, H.B.; Hayes, P.; et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for Use of Tumor Markers in Liver, Bladder, Cervical, and Gastric Cancers. Clin. Chem. 2010, 56, e1–e48. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Duffy, M.J.; Stenman, U.H.; Lilja, H.; Brünner, N.; Chan, D.W.; Babaian, R.; Bast, R.C.; Dowell, B.; Esteva, F.J.; et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for Use of Tumor Markers in Testicular, Prostate, Colorectal, Breast, and Ovarian Cancers. Clin. Chem. 2008, 54, e11–e79. [Google Scholar] [CrossRef]
- Meany, D.L.; Sokoll, L.J.; Chan, D.W. Early Detection of Cancer: Immunoassays for Plasma Tumor Markers. Expert. Opin. Med. Diagn. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Mulshine, J.L.; Scott, F. Molecular Markers in Early Cancer Detection: New Screening Tools. Chest 1995, 107, 280S–286S. [Google Scholar] [CrossRef]
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s Lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef]
- Shackney, S.E.; McCormack, G.W.; Cuchural, G.J. Growth Rate Patterns of Solid Tumors and Their Relation to Responsiveness to Therapy: An Analytical Review. Ann. Intern. Med. 1978, 89, 107–121. [Google Scholar] [CrossRef]
- Stensjøen, A.L.; Solheim, O.; Kvistad, K.A.; Håberg, A.K.; Salvesen, Ø.; Berntsen, E.M. Growth Dynamics of Untreated Glioblastomas in Vivo. Neuro Oncol. 2015, 17, 1402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Han, D.; van der Aalst, C.M.; Lancaster, H.L.; Vonder, M.; Gratama, J.W.C.; Silva, M.; Field, J.K.; de Koning, H.J.; Heuvelmans, M.A.; et al. Lung Cancer Volume Doubling Time by Computed Tomography: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2024, 212, 114339. [Google Scholar] [CrossRef]
- Bedia, J.S.; Jacobs, I.J.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Manchanda, R.; Kalsi, J.K.; Dawnay, A.; Fallowfield, L.; et al. Estimating the Ovarian Cancer CA-125 Preclinical Detectable Phase, in-Vivo Tumour Doubling Time, and Window for Detection in Early Stage: An Exploratory Analysis of UKCTOCS. EBioMedicine 2025, 112, 105554. [Google Scholar] [CrossRef] [PubMed]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular Carcinoma Tumour Volume Doubling Time: A Systematic Review and Meta-Analysis. Gut 2021, 70, 401–407. [Google Scholar] [CrossRef]
- Furukawa, H.; Iwata, R.; Moriyama, N. Growth Rate of Pancreatic Adenocarcinoma: Initial Clinical Experience. Pancreas 2001, 22, 366–369. [Google Scholar] [CrossRef]
- Dahan, M.; Hequet, D.; Bonneau, C.; Paoletti, X.; Rouzier, R. Has Tumor Doubling Time in Breast Cancer Changed over the Past 80 Years? A Systematic Review. Cancer Med. 2021, 10, 5203–5217. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Lee, J.H.; Lee, H.J.; Kim, T.H.; Huh, Y.J.; Ahn, H.S.; Suh, Y.S.; Kong, S.H.; Kim, G.H.; Ahn, S.J.; et al. Natural History of Gastric Cancer: Observational Study of Gastric Cancer Patients Not Treated During Follow-Up. Ann. Surg. Oncol. 2019, 26, 2905–2911. [Google Scholar] [CrossRef]
- Burke, J.R.; Brown, P.; Quyn, A.; Lambie, H.; Tolan, D.; Sagar, P. Tumour Growth Rate of Carcinoma of the Colon and Rectum: Retrospective Cohort Study. BJS Open 2020, 4, 1200–1207. [Google Scholar] [CrossRef]
- Yeh, T.; Yeung, M.; Sherman, E.J.; Tuttle, R.M.; Sabra, M.M. Structural Doubling Time Predicts Overall Survival in Patients with Medullary Thyroid Cancer in Patients with Rapidly Progressive Metastatic Medullary Thyroid Cancer Treated with Molecular Targeted Therapies. Thyroid 2020, 30, 1112–1119. [Google Scholar] [CrossRef]
- Schmid, H.-P.; Mcneal, J.E.; Stamey, T.A. Observations on the Doubling Time of Prostate Cancer The Use of Serial Prostate-Specific Antigen in Patients with Untreated Disease as a Measure of Increasing Cancer Volume. Cancer 1993, 71, 2031–2040. [Google Scholar] [CrossRef]
- Klein, C.A. Parallel Progression of Primary Tumours and Metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Kwon, H.; Song, E.; Jeon, M.J.; Kim, T.Y.; Lee, J.H.; Kim, W.B.; Shong, Y.K.; Chung, K.W.; Baek, J.H.; et al. Tumor Volume Doubling Time in Active Surveillance of Papillary Thyroid Carcinoma. Thyroid 2019, 29, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Catherine, S. Tumor Markers. In Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 9th ed.; Rifai, N., Chiu, R.W.K., Young, I., Wittwer, C.T., Eds.; Elsevier: St. Louis, MO, USA, 2024; pp. 346–371. ISBN 978-0-323-93583-8. [Google Scholar]
- Coskun, A.; Ertaylan, G.; Pusparum, M.; Van Hoof, R.; Kaya, Z.Z.; Khosravi, A.; Zarrabi, A. Advancing Personalized Medicine: Integrating Statistical Algorithms with Omics and Nano-Omics for Enhanced Diagnostic Accuracy and Treatment Efficacy. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 1–86. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.B. Papers ON CHEMICAL PATHOLOGY; Prefaced by the Gulstonian Lectures, Read at the Royal College of Physicians. Lancet 1847, 50, 325–330. [Google Scholar] [CrossRef]
- Li, M.C.; Hertz, R.; Spencer, D.B. Effect of Methotrexate Therapy upon Choriocarcinoma and Chorioadenoma. Proc. Soc. Exp. Biol. Med. 1956, 93, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Abelev, G.I.; Perova, S.D.; Khramkova, N.I.; Postnikova, Z.A.; Irlin, I.S. Production of Embryonal Alpha-Globulin by Transplantable Mouse Hepatomas. Transplantation 1963, 1, 174–180. [Google Scholar] [CrossRef]
- Gold, P.; Freedman, S.O. Demonstration of Tumor-specific Antigens in Human Colonic Carcinomata by Immunological Tolerance and Absorption Techniques. J. Exp. Med. 1965, 121, 439–462. [Google Scholar] [CrossRef]
- Moore, B.W.; McGregor, D. Chromatographic and Electrophoretic Fractionation of Soluble Proteins of Brain and Liver. J. Biol. Chem. 1965, 240, 1647–1653. [Google Scholar] [CrossRef]
- Blaschko, H.; Comline, R.S.; Schneider, F.H.; Silver, M.; Smith, A.D. Secretion of a Chromaffin Granule Protein, Chromogranin, from the Adrenal Gland after Splanchnic Stimulation. Nature 1967, 215, 58–59. [Google Scholar] [CrossRef]
- Tashjian, A.H., Jr.; Melvin, K.E. Medullary Carcinoma of the Thyroid Gland: Studies of Thyrocalcitonin in Plasma and Tumor Extracts. N. Engl. J. Med. 1968, 279, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Van Herle, A.J.; Uller, R.P. Elevated Serum Thyroglobulin. A Marker of Metastases in Differentiated Thyroid Carcinomas. J. Clin. Investig. 1975, 56, 272–277. [Google Scholar] [CrossRef][Green Version]
- Kato, H.; Torigoe, T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 1977, 40, 1621–1628. [Google Scholar] [CrossRef]
- Wang, M.C.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Purification of a Human Prostate Specific Antigen. Investig. Urol. 1979, 17, 159–163. [Google Scholar] [PubMed]
- Koprowski, H.; Steplewski, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal Carcinoma Antigens Detected by Hybridoma Antibodies. Somat. Cell Genet. 1979, 5, 957–971. [Google Scholar] [CrossRef]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a Monoclonal Antibody with Human Ovarian Carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef]
- Kufe, D.; Inghirami, G.; Abe, M.; Hayes, D.; Justi-Wheeler, H.; Schlom, J. Differential Reactivity of a Novel Monoclonal Antibody (DF3) with Human Malignant versus Benign Breast Tumors. Hybridoma 1984, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lappöhn, R.E.; Burger, H.G.; Bouma, J.; Bangah, M.; Krans, M.; De Bruijn, H.W.A. Inhibin as a Marker for Granulosa-Cell Tumors. N. Engl. J. Med. 1989, 321, 790–793. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Habben, I.; Iveli, R.; Krull, N. A Major Human Epididymis-Specific CDNA Encodes a Protein with Sequence Homology to Extracellular Proteinase Inhibitors. Biol. Reprod. 1991, 45, 350–357. [Google Scholar] [CrossRef]
- Pujol, J.L.; Grenier, J.; Daurès, J.P.; Daver, A.; Pujol, H.; Michel, F.B. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993, 53, 61–66. [Google Scholar] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Meng, Y.; Cui, J. Therapeutic Strategies Targeting Metabolic Characteristics of Cancer Cells. Crit. Rev. Oncol. Hematol. 2023, 187, 104037. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, P.; Panina, Y.; Segal, J.; Yuneva, M. Adapt and Conquer: Metabolic Flexibility in Cancer Growth, Invasion and Evasion. Mol. Metab. 2020, 33, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 1–40. [Google Scholar] [CrossRef]
- Reznik, E.; Luna, A.; Aksoy, B.A.; Liu, E.M.; La, K.; Ostrovnaya, I.; Creighton, C.J.; Hakimi, A.A.; Sander, C. A Landscape of Metabolic Variation across Tumor Types. Cell Syst. 2018, 6, 301–313. [Google Scholar] [CrossRef]
- Ramírez-Carmona, W.; Díaz-Fabregat, B.; Yuri Yoshigae, A.; Musa de Aquino, A.; Scarano, W.R.; de Souza Castilho, A.C.; Avansini Marsicano, J.; Leal do Prado, R.; Pessan, J.P.; de Oliveira Mendes, L. Are Serum Ferritin Levels a Reliable Cancer Biomarker? A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 1917–1926. [Google Scholar] [CrossRef]
- Konety, B.R.; Nguyen, T.S.T.; Brenes, G.; Sholder, A.; Lewis, N.; Bastacky, S.; Getzenberg, R.H. Clinical usefulness of the novel marker BLCA-4 for the detection of bladder cancer. J. Urol. 2000, 164, 634–639. [Google Scholar] [CrossRef]
- Fontana, V.; Pistillo, M.P.; Vigani, A.; Canessa, P.A.; Berisso, G.; Giannoni, U.; Roncella, S. Potential role of serum mesothelin in predicting survival of patients with malignant pleural mesothelioma. Oncol. Lett. 2021, 21, 128. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, C.; Wang, Y.; Dai, L. Soluble PD-L1 as a Predictive Biomarker in Lung Cancer: A Systematic Review and Meta-Analysis. Future Oncol. 2022, 18, 261–273. [Google Scholar] [CrossRef]
- Tsé, C.; Gauchez, A.S.; Jacot, W.; Lamy, P.J. HER2 shedding and serum HER2 extracellular domain: Biology and clinical utility in breast cancer. Cancer Treat. Rev. 2012, 38, 133–142. [Google Scholar] [CrossRef]
- Perrier, A.; Gligorov, J.; Lefèvre, G.; Boissan, M. The Extracellular Domain of Her2 in Serum as a Biomarker of Breast Cancer. Lab. Investig. 2018, 98, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Clinical Uses of Tumor Markers: A Critical Review. Crit. Rev. Clin. Lab. Sci. 2001, 38, 225–262. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.W.; Booth, R.A.; Diamandis, E.P. Tumor Markers. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; Burtis, C.A., Ashwood, E.R., Bruns, D.E., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2006; pp. 745–795. ISBN 0-7216-0189-8. [Google Scholar]
- Głowska-Ciemny, J.; Szmyt, K.; Kuszerska, A.; Rzepka, R.; von Kaisenberg, C.; Kocyłowski, R. Fetal and Placental Causes of Elevated Serum Alpha-Fetoprotein Levels in Pregnant Women. J. Clin. Med. 2024, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-Analysis. Gastroenterology 2018, 154, 1706–1718. [Google Scholar] [CrossRef]
- Debes, J.D.; Romagnoli, P.A.; Prieto, J.; Arrese, M.; Mattos, A.Z.; Boonstra, A. Serum Biomarkers for the Prediction of Hepatocellular Carcinoma. Cancers 2021, 13, 1681. [Google Scholar] [CrossRef]
- Kudo, M. Urgent Global Need for PIVKA-II and AFP-L3 Measurements for Surveillance and Management of Hepatocellular Carcinoma. Liver Cancer 2024, 13, 113–118. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, W.; Li, X.; Zhao, H.; Wang, S. The Diagnostic Performance of AFP, AFP-L3, DCP, CA199, and Their Combination for Primary Liver Cancer. J. Hepatocell. Carcinoma 2025, 12, 513–526. [Google Scholar] [CrossRef]
- Jansen, K.; Kornfeld, L.; Lennartz, M.; Dwertmann Rico, S.; Kind, S.; Reiswich, V.; Viehweger, F.; Bawahab, A.A.; Fraune, C.; Gorbokon, N.; et al. Carcinoembryonic Antigen Expression in Human Tumors: A Tissue Microarray Study on 13,725 Tumors. Cancers 2024, 16, 4052. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, W.; Lv, Y.; Shan, L.; Xu, D.; Pan, Y.; Zhu, H.; Qi, H. Postoperative Carcinoembryonic Antigen (CEA) Levels Predict Outcomes after Resection of Colorectal Cancer in Patients with Normal Preoperative CEA Levels. Transl. Cancer Res. 2020, 9, 111–118. [Google Scholar] [CrossRef]
- Hull, M.A.; Rees, C.J.; Sharp, L.; Koo, S. A Risk-Stratified Approach to Colorectal Cancer Prevention and Diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 773–780. [Google Scholar] [CrossRef]
- Guo, J.; Yu, J.; Song, X.; Mi, H. Serum CA125, CA199 and CEA Combined Detection for Epithelial Ovarian Cancer Diagnosis: A Meta-Analysis. Open Med. 2017, 12, 1. [Google Scholar] [CrossRef]
- Wang, H.; Jin, W.; Wan, C.; Zhu, C. Diagnostic Value of Combined Detection of CA72-4, CA19-9, and Carcinoembryonic Antigen Comparing to CA72-4 Alone in Gastric Cancer: A Systematic Review and Meta-Analysis. Transl. Cancer Res. 2022, 11, 848–856. [Google Scholar] [CrossRef]
- Zhan, C.H.; Liu, G.J. Diagnostic Value of a Combined Serum α-Hydroxybutyrate Dehydrogenase, Carcinoembryonic Antigen and Glycoantigen 125 Test for Early-Stage Breast Cancer. Breast Cancer Targets Ther. 2023, 15, 617–623. [Google Scholar] [CrossRef]
- Izawa, A.; Hara, Y.; Horita, N.; Muraoka, S.; Kaneko, M.; Kaneko, A.; Somekawa, K.; Hirata, M.; Otsu, Y.; Matsumoto, H.; et al. Improved Diagnostic Accuracy with Three Lung Tumor Markers Compared to Six-Marker Panel. Transl. Lung Cancer Res. 2024, 13, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.M.; Zhao, S.H. Evaluation of the Value of Combined Detection of Tumor Markers CA724, Carcinoembryonic Antigen, CA242, and CA19-9 in Gastric Cancer. World J. Gastrointest. Oncol. 2024, 16, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Wang, Y.; Zhang, Q.; Wang, Y.; Xu, C. The Diagnostic and Prognostic Value of the Combination of Tumor M2-Pyruvate Kinase, Carcinoembryonic Antigen, and Cytokeratin 19 Fragment in Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2024, 23, 15330338241265983. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Marrades, R.M.; Augé, J.M.; Escudero, J.M.; Viñolas, N.; Reguart, N.; Ramirez, J.; Filella, X.; Molins, L.; Agustí, A. Assessment of a Combined Panel of Six Serum Tumor Markers for Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 427–437. [Google Scholar] [CrossRef]
- Nagpal, M.; Singh, S.; Singh, P.; Chauhan, P.; Zaidi, M. Tumor Markers: A Diagnostic Tool. Natl. J. Maxillofac. Surg. 2016, 7, 17. [Google Scholar] [CrossRef]
- Babkina, A.S.; Lyubomudrov, M.A.; Golubev, M.A.; Pisarev, M.V.; Golubev, A.M. Neuron-Specific Enolase—What Are We Measuring? Int. J. Mol. Sci. 2024, 25, 5040. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, T.; Deng, H. Complex Roles of Nicotinamide N-Methyltransferase in Cancer Progression. Cell Death Dis. 2022, 13, 267. [Google Scholar] [CrossRef]
- Jiang, T.; Zeng, Q.; He, J. Do Alkaline Phosphatases Have Great Potential in the Diagnosis, Prognosis, and Treatment of Tumors? Transl. Cancer Res. 2023, 12, 2932–2945. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hei, A.; Zhou, J.; He, E.; Skog, S.; Li, J. Serum Thymidine Kinase 1 Protein Concentration for Predicting Early Progression and Monitoring the Response to TACE in Hepatocellular Carcinomas: A Network Meta-Analysis. Future Sci. OA 2021, 7, FSO717. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-Specific Antigen and Prostate Cancer: Prediction, Detection and Monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.M.; Bernardino, R.M.; Marta, J.C.; Holdenrieder, S.; Guimarães, J.T. Tumour Markers in Prostate Cancer: The Post-Prostate-Specific Antigen Era. Ann. Clin. Biochem. 2022, 59, 46–58. [Google Scholar] [CrossRef]
- Benson, M.C.; Whang, I.S.; Pantuck, A.; Ring, K.; Kaplan, S.A.; Olsson, C.A.; Cooner, W.H. Prostate Specific Antigen Density: A Means of Distinguishing Benign Prostatic Hypertrophy and Prostate Cancer. J. Urol. 1992, 147, 815–816. [Google Scholar] [CrossRef]
- Kachuri, L.; Hoffmann, T.J.; Jiang, Y.; Berndt, S.I.; Shelley, J.P.; Schaffer, K.R.; Machiela, M.J.; Freedman, N.D.; Huang, W.Y.; Li, S.A.; et al. Genetically Adjusted PSA Levels for Prostate Cancer Screening. Nat. Med. 2023, 29, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Charing Cross Gestational Trophoblast Disease Service. Available online: https://www.hmole-chorio.org.uk/ (accessed on 7 April 2025).
- Pelizzo, M.R.; Mazza, E.I.; Mian, C.; Merante Boschin, I. Medullary Thyroid Carcinoma. Expert. Rev. Anticancer Ther. 2023, 23, 943–957. [Google Scholar] [CrossRef]
- Censi, S.; Manso, J.; Mian, C. Other Markers of Medullary Thyroid Cancer, Not Only Calcitonin. Eur. J. Endocrinol. 2023, 188, R1–R13. [Google Scholar] [CrossRef]
- Ni, J.; Tu, P.; Ling, Y. Gender and Tumor Size-Specific Calcitonin Cutoff Value for Diagnosing MTC in 10,618 Patients with Thyroid Nodule Surgery. Endocrine 2024, 86, 1097–1109. [Google Scholar] [CrossRef]
- Giovanella, L.; Garo, M.L.; Ceriani, L.; Paone, G.; Campenni’, A.; D’Aurizio, F. Procalcitonin as an Alternative Tumor Marker of Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2021, 106, 3634–3643. [Google Scholar] [CrossRef]
- Luo, G.; Guo, M.; Jin, K.; Liu, Z.; Liu, C.; Cheng, H.; Lu, Y.; Long, J.; Liu, L.; Xu, J.; et al. Optimize CA19-9 in Detecting Pancreatic Cancer by Lewis and Secretor Genotyping. Pancreatology 2016, 16, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in Pancreatic Cancer: Biomarker, Predictor and Promoter. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early Screening and Diagnosis Strategies of Pancreatic Cancer: A Comprehensive Review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef]
- Kaur, S.; Smith, L.M.; Patel, A.; Menning, M.; Watley, D.C.; Malik, S.S.; Krishn, S.R.; Mallya, K.; Aithal, A.; Sasson, A.R.; et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am. J. Gastroenterol. 2017, 112, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Hong, L.L.; Ling, Z.Q. MUC16/CA125 in Cancer: New Advances. Clin. Chim. Acta 2025, 565, 119981. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.; McCann, L.; Makker, S.; Mukherjee, U.; Gullapalli, S.V.N.; Erekkath, J.; Shih, S.; Mahajan, I.; Sanchez, E.; Uccello, M.; et al. Diagnostic Biomarkers in Ovarian Cancer: Advances beyond CA125 and HE4. Ther. Adv. Med. Oncol. 2024, 16, 17588359241233225. [Google Scholar] [CrossRef]
- Mukama, T.; Fortner, R.T.; Katzke, V.; Hynes, L.C.; Petrera, A.; Hauck, S.M.; Johnson, T.; Schulze, M.; Schiborn, C.; Rostgaard-Hansen, A.L.; et al. Prospective Evaluation of 92 Serum Protein Biomarkers for Early Detection of Ovarian Cancer. Br. J. Cancer 2022, 126, 1301–1309. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: Uses and Limitation as a Biomarker for Breast Cancer. Clin. Chim. Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Wang, W.; Chen, B.; Wang, L.; Zhang, N.; Wang, Z.; Yang, Q. Association of Preoperative Serum Levels of CEA and CA15-3 with Molecular Subtypes of Breast Cancer. Dis. Markers 2021, 2021, 5529106. [Google Scholar] [CrossRef]
- Ryu, J.M.; Kang, D.; Cho, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Lee, S.K.; Kim, Y.J.; Im, Y.H.; Ahn, J.S.; et al. Prognostic Impact of Elevation of Cancer Antigen 15-3 (CA15-3) in Patients With Early Breast Cancer With Normal Serum CA15-3 Level. J. Breast Cancer 2023, 26, 126. [Google Scholar] [CrossRef]
- Sekacheva, M.; Boroda, A.; Fatyanova, A.; Rozhkov, A.; Bagmet, N. Clinical Validation of the Novel CLIA-CA-62 Assay Efficacy for Early-Stage Breast Cancer Detection. Front. Oncol. 2023, 13, 1009863. [Google Scholar] [CrossRef]
- Tcherkassova, J.; Prostyakova, A.; Tsurkan, S.; Ragoulin, V.; Boroda, A.; Sekacheva, M. Diagnostic Efficacy of the New Prospective Biomarker’s Combination CA 15-3 and CA-62 for Early-Stage Breast Cancer Detection: Results of the Blind Prospective-Retrospective Clinical Study. Cancer Biomark. 2022, 35, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hayashi, N.; Iguchi, T.; Ito, S.; Eguchi, H.; Mimori, K. Clinical and Biological Significance of Circulating Tumor Cells in Cancer. Mol. Oncol. 2016, 10, 408–417. [Google Scholar] [CrossRef]
- Xu, W.; Yuan, F. Detection of Circulating Tumor Cells in the Prognostic Significance of Patients With Breast Cancer: A Retrospective Study. J. Clin. Lab. Anal. 2025, 39, e25126. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Kamalanathan, K.J.; Galeano-Garces, C.; Konety, B.R.; Antonarakis, E.S.; Parthasarathy, J.; Hong, J.; Drake, J.M. Dissemination of Circulating Tumor Cells in Breast and Prostate Cancer: Implications for Early Detection. Endocrinology 2024, 165, bqae022. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wei, S.; Lv, X. Circulating Tumor Cells: From New Biological Insights to Clinical Practice. Signal Transduct. Target. Ther. 2024, 9, 1–35. [Google Scholar] [CrossRef]
- Goldkorn, A.; Tangen, C.; Plets, M.; Bsteh, D.; Xu, T.; Pinski, J.K.; Ingles, S.; Triche, T.J.; MacVicar, G.R.; Vaena, D.A.; et al. Circulating Tumor Cell Count and Overall Survival in Patients With Metastatic Hormone-Sensitive Prostate Cancer. JAMA Netw. Open 2024, 7, e2437871. [Google Scholar] [CrossRef]
- Choi, S.-W.; Sun, A.K.; Cheung, J.P.-Y.; Ho, J.C.-Y. Circulating Tumour Cells in the Prediction of Bone Metastasis. Cancers 2024, 16, 252. [Google Scholar] [CrossRef]
- Magro, D.; Venezia, M.; Rita Balistreri, C. The Omics Technologies and Liquid Biopsies: Advantages, Limitations, Applications. Med. Omics 2024, 11, 100039. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients with Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; Accardo, M.; Carelli, E.; Angioletti, D.; Santini, M.; Di Domenico, M. Circulating Tumor Cells in Diagnosing Lung Cancer: Clinical and Morphologic Analysis. Ann. Thorac. Surg. 2015, 99, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating Tumor Cells: Clinical Validity and Utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef]
- Sewpersad, S.; Pillay, T.S. Historical Perspectives in Clinical Pathology: Bence Jones Protein—Early Urine Chemistry and the Impact on Modern Day Diagnostics. J. Clin. Pathol. 2021, 74, 212–215. [Google Scholar] [CrossRef]
- Mizuno, T.; Goto, T.; Shimojo, K.; Watanabe, N.; Tanaka, T. Clinical Utility of Tumor Markers. Open J. Pathol. 2021, 11, 38–57. [Google Scholar] [CrossRef]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef]
- Dhillon, A.; Singh, A.; Bhalla, V.K. A Systematic Review on Biomarker Identification for Cancer Diagnosis and Prognosis in Multi-Omics: From Computational Needs to Machine Learning and Deep Learning. Arch. Comput. Methods Eng. 2022, 30, 917–949. [Google Scholar] [CrossRef]
- Agarwala, P.K.; Aneja, R.; Kapoor, S. Lipidomic Landscape in Cancer: Actionable Insights for Membrane-Based Therapy and Diagnoses. Med. Res. Rev. 2022, 42, 983–1018. [Google Scholar] [CrossRef]
- Quezada, H.; Guzmán-Ortiz, A.L.; Díaz-Sánchez, H.; Valle-Rios, R.; Aguirre-Hernández, J. Omics-Based Biomarkers: Current Status and Potential Use in the Clinic. Bol. Med. Hosp. Infant. Mex. 2017, 74, 219–226. [Google Scholar] [CrossRef]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Consalvo, A.; Upadhyaya, P.; Sala, G.; Antonucci, I.; Del Boccio, P.; Stuppia, L.; De Laurenzi, V. Breast Cancer in the Era of Integrating “Omics” Approaches. Oncogenesis 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Thomas, N.E.; Edmiston, S.N.; Tsai, Y.S.; Parker, J.S.; Googe, P.B.; Busam, K.J.; Scott, G.A.; Zedek, D.C.; Parrish, E.A.; Hao, H.; et al. Utility of TERT Promoter Mutations for Cutaneous Primary Melanoma Diagnosis. Am. J. Dermatopathol. 2019, 41, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Xu, J.; Wang, Y. The Role of TERT Promoter Mutations in Postoperative and Preoperative Diagnosis and Prognosis in Thyroid Cancer. Medicine 2018, 97, e11548. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, K.; Yekula, A.; Small, J.L.; Rosh, Z.S.; Kang, K.M.; Wang, L.; Lau, S.; Zhang, H.; Lee, H.; Bettegowda, C.; et al. TERT Promoter Mutation Analysis for Blood-Based Diagnosis and Monitoring of Gliomas. Clin. Cancer Res. 2021, 27, 169–178. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, S.; Wang, M.; Lopez-Beltran, A. Biological and Clinical Perspectives of TERT Promoter Mutation Detection on Bladder Cancer Diagnosis and Management. Hum. Pathol. 2023, 133, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Ye, Y.; Cheng, W.; Xu, L.; Huang, M.; Chen, Y.; Zhu, J.; Lu, X.; Yan, F. Multi-Omics Integrative Analysis of Lung Adenocarcinoma: An in Silico Profiling for Precise Medicine. Front. Med. 2022, 9, 894338. [Google Scholar] [CrossRef]
- Yao, M.; Fu, L.; Liu, X.; Zheng, D. In-Silico Multi-Omics Analysis of the Functional Significance of Calmodulin 1 in Multiple Cancers. Front. Genet. 2022, 12, 793508. [Google Scholar] [CrossRef] [PubMed]
- Zalfa, F.; Perrone, M.G.; Ferorelli, S.; Laera, L.; Pierri, C.L.; Tolomeo, A.; Dimiccoli, V.; Perrone, G.; De Grassi, A.; Scilimati, A. Genome-Wide Identification and Validation of Gene Expression Biomarkers in the Diagnosis of Ovarian Serous Cystadenocarcinoma. Cancers 2022, 14, 3764. [Google Scholar] [CrossRef]
- Christou, C.; Stylianou, A.; Gkretsi, V. Midkine (MDK) in Hepatocellular Carcinoma: More than a Biomarker. Cells 2024, 13, 136. [Google Scholar] [CrossRef]
- Christensen, M.V.; Høgdall, C.K.; Umsen, K.M.J.; Høgdall, E.V.S. Annexin A2 and Cancer: A Systematic Review. Int. J. Oncol. 2018, 52, 5–18. [Google Scholar] [CrossRef]
- Capone, E.; Iacobelli, S.; Sala, G. Role of Galectin 3 Binding Protein in Cancer Progression: A Potential Novel Therapeutic Target. J. Transl. Med. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Makarem, A. El Diagnostic Significance of Plasma Osteopontin in Hepatitis C Virus-Related Hepatocellular Carcinoma. Ann. Hepatol. 2011, 10, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zheng, J.; Wu, F.; Kang, B.; Liang, J.; Heskia, F.; Zhang, X.; Shan, Y. OPN Is a Promising Serological Biomarker for Hepatocellular Carcinoma Diagnosis. J. Med. Virol. 2020, 92, 3596–3603. [Google Scholar] [CrossRef]
- Popovics, P.; Awadallah, W.N.; Kohrt, S.E.; Case, T.C.; Miller, N.L.; Ricke, E.A.; Huang, W.; Ramirez-Solano, M.; Liu, Q.; Vezina, C.M.; et al. Prostatic Osteopontin Expression Is Associated with Symptomatic Benign Prostatic Hyperplasia. Prostate 2020, 80, 731–741. [Google Scholar] [CrossRef]
- Fouad, S.A.; Mohamed, N.A.G.; Fawzy, M.W.; Moustafa, D.A. Plasma Osteopontin Level in Chronic Liver Disease and Hepatocellular Carcinoma. Hepat. Mon. 2015, 15, e30753. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, S.; Fatima, I.; Aslam, M.; Musharraf, S.G.; Sherman, N.E.; Moskaluk, C.; Fox, J.W.; Akhtar, M.W.; Sadaf, S. Identification of Actin Beta-like 2 (ACTBL2) as Novel, Upregulated Protein in Colorectal Cancer. J. Proteom. 2017, 152, 33–40. [Google Scholar] [CrossRef]
- Lobo, M.D.P.; Moreno, F.B.M.B.; Souza, G.H.M.F.; Verde, S.M.M.L.; Moreira, R.d.A.; Monteiro-Moreira, A.C.d.O. Label-Free Proteome Analysis of Plasma from Patients with Breast Cancer: Stage-Specific Protein Expression. Front. Oncol. 2017, 7, 218323. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zhang, P.; Hou, Q.; Feng, S.; Liu, L.; Cui, D.; Shi, H.; Fu, Y.; Luo, Y. A Novel Pan-Cancer Biomarker Plasma Heat Shock Protein 90alpha and Its Diagnosis Determinants in Clinic. Cancer Sci. 2019, 110, 2941–2959. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Fan, J.; Yang, X.R.; Tan, Y.; Zhao, W.; Xu, Y.; Wang, N.; Niu, Y.; Wu, Z.; Zhou, J.; et al. Serum DKK1 as a Protein Biomarker for the Diagnosis of Hepatocellular Carcinoma: A Large-Scale, Multicentre Study. Lancet Oncol. 2012, 13, 817–826. [Google Scholar] [CrossRef]
- Guo, X.; Lv, X.; Fang, C.; Lv, X.; Wang, F.; Wang, D.; Zhao, J.; Ma, Y.; Xue, Y.; Bai, Q.; et al. Dysbindin as a Novel Biomarker for Pancreatic Ductal Adenocarcinoma Identified by Proteomic Profiling. Int. J. Cancer 2016, 139, 1821–1829. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhao, T.; Li, Y.; Tian, L.; Zhao, J.; Zhang, J. Evaluation of Serum MUC5AC in Combination with CA19-9 for the Diagnosis of Pancreatic Cancer. World J. Surg. Oncol. 2020, 18, 1–7. [Google Scholar] [CrossRef]
- Capello, M.; Bantis, L.E.; Scelo, G.; Zhao, Y.; Li, P.; Dhillon, D.S.; Patel, N.J.; Kundnani, D.L.; Wang, H.; Abbruzzese, J.L.; et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109, djw266. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, I.; Gardeazabal, J.; Erramuzpe, A.; Sanchez-Diez, A.; Cortés, J.; García-Vázquez, M.D.; Pérez-Yarza, G.; Izu, R.; Luís Díaz-Ramón, J.; de la Fuente, I.M.; et al. Vitronectin and Dermcidin Serum Levels Predict the Metastatic Progression of AJCC I–II Early-Stage Melanoma. Int. J. Cancer 2016, 139, 1598–1607. [Google Scholar] [CrossRef]
- Greco, M.; Mitri, M.D.; Chiriacò, F.; Leo, G.; Brienza, E.; Maffia, M. Serum Proteomic Profile of Cutaneous Malignant Melanoma and Relation to Cancer Progression: Association to Tumor Derived Alpha-N-Acetylgalactosaminidase Activity. Cancer Lett. 2009, 283, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Yue, P.; Li, S.; Zhang, Y.; Mi, N.; Bai, M.; Fu, W.; Xia, Z.; Jiang, N.; et al. Identification of a Novel Bile Marker Clusterin and a Public Online Prediction Platform Based on Deep Learning for Cholangiocarcinoma. BMC Med. 2023, 21, 1–15. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic Profiles Delineate Potential Role for Sarcosine in Prostate Cancer Progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Khan, A.P.; Rajendiran, T.M.; Ateeq, B.; Asangani, I.A.; Athanikar, J.N.; Yocum, A.K.; Mehra, R.; Siddiqui, J.; Palapattu, G.; Wei, J.T.; et al. The Role of Sarcosine Metabolism in Prostate Cancer Progression. Neoplasia 2013, 15, 491-IN13. [Google Scholar] [CrossRef] [PubMed]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a Potential Prostate Cancer Biomarker—A Review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef]
- Du, X.; Hu, H. The Roles of 2-Hydroxyglutarate. Front. Cell Dev. Biol. 2021, 9, 651317. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Fu, Q.; Taly, V.; Tan, F. Integrative Analysis of Multi-Omics Data for Liquid Biopsy. Br. J. Cancer 2022, 128, 505–518. [Google Scholar] [CrossRef]
- Xu, H.; Lien, T.; Bergholtz, H.; Fleischer, T.; Djerroudi, L.; Vincent-Salomon, A.; Sørlie, T.; Aittokallio, T. Multi-Omics Marker Analysis Enables Early Prediction of Breast Tumor Progression. Front. Genet. 2021, 12, 670749. [Google Scholar] [CrossRef]
- Cowell, C.F.; Weigelt, B.; Sakr, R.A.; Ng, C.K.Y.; Hicks, J.; King, T.A.; Reis-Filho, J.S. Progression from Ductal Carcinoma in Situ to Invasive Breast Cancer: Revisited. Mol. Oncol. 2013, 7, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, X.; Li, F.; He, X.; Zhao, N. Identification of a Six Gene Prognosis Signature for Papillary Thyroid Cancer Using Multi-Omics Methods and Bioinformatics Analysis. Front. Oncol. 2021, 11, 624421. [Google Scholar] [CrossRef]
- Piga, I.; L’Imperio, V.; Capitoli, G.; Denti, V.; Smith, A.; Magni, F.; Pagni, F. Paving the Path toward Multi-Omics Approaches in the Diagnostic Challenges Faced in Thyroid Pathology. Expert Rev. Proteom. 2023, 20, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ge, W. Identification of Prognostic Biomarkers in Neuroblastoma Using WGCNA and Multi-Omics Analysis. Discover Oncol. 2024, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Xie, C.; Li, Z.; Mei, J.; Wang, K. Multi-Omics Analysis Identifies Diagnostic Circulating Biomarkers and Potential Therapeutic Targets, Revealing IQGAP1 as an Oncogene in Gastric Cancer. NPJ Precis. Oncol. 2025, 9, 1–18. [Google Scholar] [CrossRef]
- Kellogg, R.A.; Dunn, J.; Snyder, M.P. Personal Omics for Precision Health. Circ. Res. 2018, 122, 1169–1171. [Google Scholar] [CrossRef]
- Chen, R.; Mias, G.I.; Li-Pook-Than, J.; Jiang, L.; Lam, H.Y.K.; Chen, R.; Miriami, E.; Karczewski, K.J.; Hariharan, M.; Dewey, F.E.; et al. Personal Omics Profiling Reveals Dynamic Molecular and Medical Phenotypes. Cell 2012, 148, 1293–1307. [Google Scholar] [CrossRef]
- Metwally, A.A.; Zhang, T.; Wu, S.; Kellogg, R.; Zhou, W.; Contrepois, K.; Tang, H.; Snyder, M. Robust Identification of Temporal Biomarkers in Longitudinal Omics Studies. Bioinformatics 2022, 38, 3802–3811. [Google Scholar] [CrossRef]
- Luo, J.; Shen, L.; Zheng, D. Diagnostic Value of Circulating Free DNA for the Detection of EGFR Mutation Status in NSCLC: A Systematic Review and Meta-Analysis. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Palmieri, M.; Zulato, E.; Wahl, S.G.F.; Guibert, N.; Frullanti, E. Diagnostic Accuracy of Circulating Free DNA Testing for the Detection of KRAS Mutations in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Genet. 2022, 13, 1015161. [Google Scholar] [CrossRef]
- Tepus, M.; Yau, T.O. Non-Invasive Colorectal Cancer Screening: An Overview. Gastrointest. Tumors 2020, 7, 62–73. [Google Scholar] [CrossRef]
- Bardol, T.; Dujon, A.M.; Taly, V.; Dunyach-Remy, C.; Lavigne, J.P.; Costa-Silva, B.; Kurma, K.; Eslami-S, Z.; Cayrefourcq, L.; Canivet, C.; et al. Early Detection of Pancreatic Cancer by Liquid Biopsy “PANLIPSY”: A French Nation-Wide Study Project. BMC Cancer 2024, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, S.; Leal, A.; Phallen, J.; Fiksel, J.; Adleff, V.; Bruhm, D.C.; Jensen, S.Ø.; Medina, J.E.; Hruban, C.; White, J.R.; et al. Genome-Wide Cell-Free DNA Fragmentation in Patients with Cancer. Nature 2019, 570, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical Validation of a Targeted Methylation-Based Multi-Cancer Early Detection Test Using an Independent Validation Set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef]
- Schrag, D.; McDonnell, C.H.; Nadauld, L.; Dilaveri, C.A.; Klein, E.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. A Prospective Study of a Multi-Cancer Early Detection Blood Test. Ann. Oncol. 2022, 33, S961. [Google Scholar] [CrossRef]
- Bratulic, S.; Limeta, A.; Dabestani, S.; Birgisson, H.; Enblad, G.; Stålberg, K.; Hesselager, G.; Häggman, M.; Höglund, M.; Simonson, O.E.; et al. Noninvasive Detection of Any-Stage Cancer Using Free Glycosaminoglycans. Proc. Natl. Acad. Sci. USA 2022, 119, e2115328119. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Rolfo, C.; Russo, A. Liquid Biopsy for Early Stage Lung Cancer Moves Ever Closer. Nat. Rev. Clin. Oncol. 2020, 17, 523–524. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Swanton, C. Early Stage NSCLC—Challenges to Implementing CtDNA-Based Screening and MRD Detection. Nat. Rev. Clin. Oncol. 2018, 15, 9. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef]
- Mathios, D.; Johansen, J.S.; Cristiano, S.; Medina, J.E.; Phallen, J.; Larsen, K.R.; Bruhm, D.C.; Niknafs, N.; Ferreira, L.; Adleff, V.; et al. Detection and Characterization of Lung Cancer Using Cell-Free DNA Fragmentomes. Nat. Commun. 2021, 12, 5060. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Podder, P.S.; Russo, M.; Henry, C.; Cinti, S. Tailored Point-of-Care Biosensors for Liquid Biopsy in the Field of Oncology. Lab Chip 2022, 23, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhou, Y.; Ressom, H.W. MOTA: Network-Based Multi-Omic Data Integration for Biomarker Discovery. Metabolites 2020, 10, 144. [Google Scholar] [CrossRef]
- Lan, W.; Liao, H.; Chen, Q.; Zhu, L.; Pan, Y.; Chen, Y.P.P. DeepKEGG: A Multi-Omics Data Integration Framework with Biological Insights for Cancer Recurrence Prediction and Biomarker Discovery. Brief. Bioinform. 2024, 25, bbae185. [Google Scholar] [CrossRef]
- Shi, K.; Lin, W.; Zhao, X.M. Identifying Molecular Biomarkers for Diseases with Machine Learning Based on Integrative Omics. Trans. Comput. Biol. Bioinform. 2021, 18, 2514–2525. [Google Scholar] [CrossRef]
- Ozaki, Y.; Broughton, P.; Abdollahi, H.; Valafar, H.; Blenda, A.V.; Lu, L.; Kumar, A.; Ozaki, Y.; Broughton, P.; Abdollahi, H.; et al. Integrating Omics Data and AI for Cancer Diagnosis and Prognosis. Cancers 2024, 16, 2448. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Du, Y.; Ma, R.; Teng, N.; Ou, S.; Zhao, H.; Li, X. Construction of the XGBoost Model for Early Lung Cancer Prediction Based on Metabolic Indices. BMC Med. Inform. Decis. Mak. 2023, 23, 1–16. [Google Scholar] [CrossRef]
- Dessie, E.Y.; Tsai, J.J.P.; Chang, J.G.; Ng, K.L. A Novel MiRNA-Based Classification Model of Risks and Stages for Clear Cell Renal Cell Carcinoma Patients. BMC Bioinform. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Goswami, C.; Chawla, S.; Thakral, D.; Pant, H.; Verma, P.; Malik, P.S.; Jayadeva; Gupta, R.; Ahuja, G.; Sengupta, D. Molecular Signature Comprising 11 Platelet-Genes Enables Accurate Blood-Based Diagnosis of NSCLC. BMC Genom. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Gupta, R.; Kleinjans, J.; Caiment, F. Identifying Novel Transcript Biomarkers for Hepatocellular Carcinoma (HCC) Using RNA-Seq Datasets and Machine Learning. BMC Cancer 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Tran, Q.T.; Alom, M.Z.; Orr, B.A. Comprehensive Study of Semi-Supervised Learning for DNA Methylation-Based Supervised Classification of Central Nervous System Tumors. BMC Bioinform. 2022, 23, 1–17. [Google Scholar] [CrossRef]
- Tao, K.; Bian, Z.; Zhang, Q.; Guo, X.; Yin, C.; Wang, Y.; Zhou, K.; Wan, S.; Shi, M.; Bao, D.; et al. Machine Learning-Based Genome-Wide Interrogation of Somatic Copy Number Aberrations in Circulating Tumor DNA for Early Detection of Hepatocellular Carcinoma. EBioMedicine 2020, 56, 102811. [Google Scholar] [CrossRef]
- Shaukat, A.; Burke, C.A.; Chan, A.T.; Grady, W.M.; Gupta, S.; Katona, B.W.; Ladabaum, U.; Liang, P.S.; Liu, J.J.; Putcha, G.; et al. Clinical Validation of a Circulating Tumor DNA–Based Blood Test to Screen for Colorectal Cancer. JAMA 2025, 334, 56–63. [Google Scholar] [CrossRef]
- Putcha, G.; Xu, C.; Shaukat, A.; Levin, T.R. Prevention of colorectal cancer through multiomics blood testing: The PREEMPT CRC study. J. Clin. Oncol. 2022, 2022, 40. [Google Scholar] [CrossRef]
- Lin, J.; Ariazi, E.; Dzamba, M.; Hsu, T.-K.; Kothen-Hill, S.; Li, K.; Liu, T.-Y.; Mahajan, S.; Palaniappan, K.K.; Pasupathy, A.; et al. Evaluation of a sensitive blood test for the detection of colorectal advanced adenomas in a prospective cohort using a multiomics approach. J. Clin. Oncol. 2021, 39, 43. [Google Scholar] [CrossRef]
- Nagarkar, R.; Gopichand, M.; Pal, S.K.; Gupta, A.; Saquib, N.M.; Ahmad, A.; Sagar, G.; Rao, K.V.S.; Siddiqui, Z.; Longkumer, I. Development of a Serum Metabolome-Based Test for Early-Stage Detection of Multiple Cancers. Cancer Rep. 2024, 7, e70042. [Google Scholar] [CrossRef]
- Papier, K.; Atkins, J.R.; Tong, T.Y.N.; Gaitskell, K.; Desai, T.; Ogamba, C.F.; Parsaeian, M.; Reeves, G.K.; Mills, I.G.; Key, T.J.; et al. Identifying Proteomic Risk Factors for Cancer Using Prospective and Exome Analyses of 1463 Circulating Proteins and Risk of 19 Cancers in the UK Biobank. Nat. Commun. 2024, 15, 4010. [Google Scholar] [CrossRef]
- Harlid, S.; Myte, R.; Van Guelpen, B. The Metabolic Syndrome, Inflammation, and Colorectal Cancer Risk: An Evaluation of Large Panels of Plasma Protein Markers Using Repeated, Prediagnostic Samples. Mediat. Inflamm. 2017, 2017, 4803156. [Google Scholar] [CrossRef]
- Sun, X.; Shu, X.O.; Lan, Q.; Laszkowska, M.; Cai, Q.; Rothman, N.; Wen, W.; Zheng, W.; Shu, X. Prospective Proteomic Study Identifies Potential Circulating Protein Biomarkers for Colorectal Cancer Risk. Cancers 2022, 14, 3261. [Google Scholar] [CrossRef]
- Cominetti, O.; Dayon, L. Unravelling Disease Complexity: Integrative Analysis of Multi-Omic Data in Clinical Research. Expert Rev. Proteom. 2025, 22, 149–162. [Google Scholar] [CrossRef]
- Bhatt, A.A.; Niell, B. Tumor Doubling Time and Screening Interval. Radiol. Clin. N. Am. 2024, 62, 571–580. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Mahato, K.; Saha, T.; Ding, S.; Sandhu, S.S.; Chang, A.Y.; Wang, J. Hybrid Multimodal Wearable Sensors for Comprehensive Health Monitoring. Nat. Electron. 2024, 7, 735–750. [Google Scholar] [CrossRef]
- Coskun, A.; Savas, I.N.; Can, O.; Lippi, G. From Population-Based to Personalized Laboratory Medicine: Continuous Monitoring of Individual Laboratory Data with Wearable Biosensors. Crit. Rev. Clin. Lab. Sci. 2025, 62, 198–227. [Google Scholar] [CrossRef]
- Rum, L.; Sten, O.; Vendrame, E.; Belluscio, V.; Camomilla, V.; Vannozzi, G.; Truppa, L.; Notarantonio, M.; Sciarra, T.; Lazich, A.; et al. Wearable Sensors in Sports for Persons with Disability: A Systematic Review. Sensors 2021, 21, 1858–2021. [Google Scholar] [CrossRef]
- Olmedo-Aguirre, J.O.; Reyes-Campos, J.; Alor-Hernández, G.; Machorro-Cano, I.; Rodríguez-Mazahua, L.; Sánchez-Cervantes, J.L. Remote Healthcare for Elderly People Using Wearables: A Review. Biosensors 2022, 12, 73. [Google Scholar] [CrossRef]
- Sohrabi, H.; Bolandi, N.; Hemmati, A.; Eyvazi, S.; Ghasemzadeh, S.; Baradaran, B.; Oroojalian, F.; Reza Majidi, M.; de la Guardia, M.; Mokhtarzadeh, A. State-of-the-Art Cancer Biomarker Detection by Portable (Bio) Sensing Technology: A Critical Review. Microchem. J. 2022, 177, 107248. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; Tang, D. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem. 2017, 89, 5152–5160. [Google Scholar] [CrossRef]
- Chi, L.; Wang, X.; Chen, H.; Tang, D.; Xue, F. Paper-Based Photoelectrochemical Immunoassay for Ultrasensitive Screening of Carcinoembryonic Antigen on Hollow CdS/CdMoO4-Functionalized Photoanode. Talanta 2023, 254, 124176. [Google Scholar] [CrossRef]
- Tsogka, I.; Mermiga, E.; Pagkali, V.; Kokkinos, C.; Economou, A. A Simplified Lateral Flow Immunosensor for the Assay of Carcinoembryonic Antigen in Low-Resource Settings. Anal. Methods 2024, 16, 2921–2929. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Z.; Ge, S.; Mao, Y.; Gu, Y.; Cao, X.; Lu, D. A Microfluidic Chip Using Au@SiO2 Array–Based Highly SERS-Active Substrates for Ultrasensitive Detection of Dual Cervical Cancer–Related Biomarkers. Anal. Bioanal. Chem. 2022, 414, 7659–7673. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Materon, E.M.; Furuta, R.H.M.; Wilson, D.; do Nascimento, G.F.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Oliveira, O.N.; Gonçalves, D. Screen-Printed Electrodes Modified with Carbon Black and Polyelectrolyte Films for Determination of Cancer Marker Carbohydrate Antigen 19-9. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, Y.; Baryeh, K.; Takalkar, S.; Lund, M.; Zhang, X.; Liu, G. Lateral Flow Assay for Carbohydrate Antigen 19–9 in Whole Blood by Using Magnetized Carbon Nanotubes. Microchim. Acta 2017, 184, 4287–4294. [Google Scholar] [CrossRef]
- Tripathi, P.; Kumar, A.; Sachan, M.; Gupta, S.; Nara, S. Aptamer-Gold Nanozyme Based Competitive Lateral Flow Assay for Rapid Detection of CA125 in Human Serum. Biosens. Bioelectron. 2020, 165, 112368. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-Based Electrochemical System with Multi-Walled Carbon Nanotubes/Thionine/Gold Nanoparticles Modified Screen-Printed Immunosensor for Cancer Antigen 125 Detection. Microchem. J. 2022, 174, 107044. [Google Scholar] [CrossRef]
- Rebelo, T.S.C.R.; Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemical Immunosensor for Detection of CA 15-3 Biomarker in Point-of-Care. Sens. Biosensing Res. 2021, 33, 100445. [Google Scholar] [CrossRef]
- Cui, C.; Xu, M.; Guan, Q.; Wang, X.; Yang, G.; Zhang, Z.; Du, J. Electrochemical Detection of Alpha-Fetoprotein Using Graphene-Assisted Sensors: A Novel Approach for Liver Cancer Screening. Alex. Eng. J. 2025, 123, 511–518. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, L.; Chen, J.; Li, M.; Tang, D. Graded Oxygen-Doped CdS Electrode for Portable Photoelectrochemical Immunoassay of Alpha-Fetoprotein Coupling with a Digital Multimeter Readout. Sens. Actuators B Chem. 2021, 343, 130136. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, H.; Huang, S.; Yin, F.; Wang, W.; Hu, Q.; Wang, Y.; Feng, B. Rapid and Portable Detection of Hepatocellular Carcinoma Marker Alpha-Fetoprotein Using a Droplet Evaporation-Based Biosensor. Talanta 2025, 294, 128189. [Google Scholar] [CrossRef]
- He, W.; Liu, L.; Cao, Z.; Lin, Y.; Tian, Y.; Zhang, Q.; Zhou, C.; Ye, X.; Cui, T. Shrink Polymer Based Electrochemical Sensor for Point-of-Care Detection of Prostate-Specific Antigen. Biosens. Bioelectron. 2023, 228, 115193. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Banguera-Ordoñez, Y.D.; Carrascosa, J.; Maquieira, Á.; Morais, S. Portable Electrophoretic Lateral Flow Biosensing for Ultra-Sensitive Human Lactate Dehydrogenase Detection in Serum Samples. Biosens. Bioelectron. 2025, 282, 117504. [Google Scholar] [CrossRef]
- Banguera-Ordoñez, Y.D.; Sena-Torralba, A.; Quintero-Campos, P.; Maquieira, Á.; Morais, S. Smartphone-Based Lateral Flow Immunoassay for Sensitive Determination of Lactate Dehydrogenase at the Point of Care. Talanta 2025, 281, 126803. [Google Scholar] [CrossRef]
- Kim, H.M.; Jeong, D.H.; Lee, H.Y.; Park, J.H.; Lee, S.K. Design and Validation of Fiber Optic Localized Surface Plasmon Resonance Sensor for Thyroglobulin Immunoassay with High Sensitivity and Rapid Detection. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Chen, H.; Luo, X.; Guo, B.; Jia, L.; Wang, F. Internal Three-Dimensional Graphdiyne-Based Self-Powered Biosensor Integrated with External Physical Power for Portable Detection of Tumor Markers. Sens. Actuators B Chem. 2025, 426, 137114. [Google Scholar] [CrossRef]
- Srilikhit, A.; Kongkeaw, S.; Cotchim, S.; Janduang, S.; Wannapob, R.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Carcinoembryonic Antigen and Cancer Antigen 125 Simultaneously Determined Using a Fluidics-Integrated Dual Carbon Electrode. Microchem. J. 2024, 204, 110911. [Google Scholar] [CrossRef]
- Chu, T.; Wang, H.; Qiu, Y.; Luo, H.; He, B.; Wu, B.; Gao, B. 3D Printed Smart Silk Wearable Sensors. Analyst 2021, 146, 1552–1558. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Gehlot, P.; Sidapra, K.; Edwards, A.D.; Reis, N.M. Portable Smartphone Quantitation of Prostate Specific Antigen (PSA) in a Fluoropolymer Microfluidic Device. Biosens. Bioelectron. 2015, 70, 5–14. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, E.; Lee, T.-H.; Huang, C.-C.; Tai, C.-S.; Lin, Y.-R.; Chen, W.-L. Point-of-Care NSE Biosensor for Objective Assessment of Stroke Risk. Biosensors 2025, 15, 264. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, Y.; Zhuang, W.; Zhong, G.; Su, L.; Xu, T.; Zhang, X. Fully Integrated Ratiometric Fluorescence Enrichment Platform for High-Sensitivity POC Testing of Salivary Cancer Biomarkers. Anal. Chem. 2023, 95, 18739–18747. [Google Scholar] [CrossRef]
- Zhou, S.; Tu, D.; Liu, Y.; You, W.; Zhang, Y.; Zheng, W.; Chen, X. Ultrasensitive Point-of-Care Test for Tumor Marker in Human Saliva Based on Luminescence-Amplification Strategy of Lanthanide Nanoprobes. Adv. Sci. 2021, 8, 2002657. [Google Scholar] [CrossRef]
- Tofighi, F.B.; Saadati, A.; Kholafazad-kordasht, H.; Farshchi, F.; Hasanzadeh, M.; Samiei, M. Electrochemical Immunoplatform to Assist in the Diagnosis of Oral Cancer through the Determination of CYFRA 21.1 Biomarker in Human Saliva Samples: Preparation of a Novel Portable Biosensor toward Non-Invasive Diagnosis of Oral Cancer. J. Mol. Recognit. 2021, 34, e2932. [Google Scholar] [CrossRef]
- Joshi, S.; Kallappa, S.; Kumar, P.; Shukla, S.; Ghosh, R. Simple Diagnosis of Cancer by Detecting CEA and CYFRA 21-1 in Saliva Using Electronic Sensors. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Resende, M.A.C.; Ferreira, L.F. Disposable Voltammetric Immunosensor for Determination and Quantification of Biomarker CA 15-3 in Biological Specimens. Analytica 2024, 5, 74–89. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, W.; Wan, G.; Xia, M.; Chen, D.; Zhang, Y.; Wang, Y.; Guo, F.; Tan, J.; et al. Integrated Urinalysis Devices Based on Interface-Engineered Field-Effect Transistor Biosensors Incorporated With Electronic Circuits. Adv. Mater. 2022, 34, 2203224. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.G.; Lee, S.H.; Kim, W.; Bang, A.; Moon, S.W.; Song, J.; Shin, J.H.; Yu, J.S.; Choi, S. Label-Free Surface-Enhanced Raman Spectroscopy Biosensor for On-Site Breast Cancer Detection Using Human Tears. ACS Appl. Mater. Interfaces 2020, 12, 7897–7904. [Google Scholar] [CrossRef]
- Nhu, C.T.; Do Quang, L.; Jen, C.P.; Duc, T.C.; Bui, T.T.; Vu Ngoc, T. NSE Protein Detection in a Microfluidic Channel Integrated an Electrochemical Biosensor. Biomed. Phys. Eng. Express 2024, 11, 015047. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Bai, S.; Gao, Y.; Metcalfe, G.; Cheng, W.; Zhu, Y. Multiplexed Detection of Cancer Biomarkers Using a Microfluidic Platform Integrating Single Bead Trapping and Acoustic Mixing Techniques. Nanoscale 2018, 10, 20196–20206. [Google Scholar] [CrossRef]
- Oliveira, D.; Romaguera Barcelay, Y.; Moreira, F.T.C. An Electrochemically Synthesized Molecularly Imprinted Polymer for Highly Selective Detection of Breast Cancer Biomarker CA 15-3: A Promising Point-of-Care Biosensor. RSC Adv. 2024, 14, 15347–15357. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing Analytes in Biofluids for Peripheral Biochemical Monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Wei, K.; Cao, Z.; Zhu, Z.; Chen, R. Sweat as a Source of Non-Invasive Biomarkers for Clinical Diagnosis: An Overview. Talanta 2024, 273, 125865. [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent Developments in Biosensors for Healthcare and Biomedical Applications: A Review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Jayanthi, V.S.P.K.S.A.; Das, A.B.; Saxena, U. Recent Advances in Biosensor Development for the Detection of Cancer Biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef]
- Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Vikartovska, A.; Tanvir, A.; Kasak, P.; Tkac, J. Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes 2020, 8, 580. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–Organic Frameworks (MOFs) Based Electrochemical Biosensors for Early Cancer Diagnosis in Vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- Kappen, J.; Skorupa, M.; Krukiewicz, K. Conducting Polymers as Versatile Tools for the Electrochemical Detection of Cancer Biomarkers. Biosensors 2023, 13, 31. [Google Scholar] [CrossRef]
- Afshari Babazad, M.; Foroozandeh, A.; Abdouss, M.; SalarAmoli, H.; Babazad, R.A.; Hasanzadeh, M. Recent Progress and Challenges in Biosensing of Carcinoembryonic Antigen. TrAC 2024, 180, 117964. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer Biomarkers and Their Biosensors: A Comprehensive Review. TrAC 2023, 158, 116813. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J. Recent Advances in Hydrogel-Based Biosensors for Cancer Detection. ACS Appl. Mater. Interfaces 2024, 16, 46988–47002. [Google Scholar] [CrossRef]
- Gu, X.; She, Z.; Ma, T.; Tian, S.; Kraatz, H.B. Electrochemical Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2018, 102, 610–616. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Zafeirakis, A.; Kalogeropoulou, K.; Giannakos, G.; Skevis, A.; Kintzios, S. An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum. Sensors 2018, 18, 3834. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibáñez, E.; Cifuentes, A.; Lu, W. Microfluidic Biosensors for Biomarker Detection in Body Fluids: A Key Approach for Early Cancer Diagnosis. Biomark. Res. 2024, 12, 1–29. [Google Scholar] [CrossRef]
- Le, L.T.P.; Nguyen, A.H.Q.; Phan, L.M.T.; Ngo, H.T.T.; Wang, X.; Cunningham, B.; Valera, E.; Bashir, R.; Taylor-Robinson, A.W.; Do, C.D. Current Smartphone-Assisted Point-of-Care Cancer Detection: Towards Supporting Personalized Cancer Monitoring. TrAC 2024, 174, 117681. [Google Scholar] [CrossRef]
- Chanarsa, S.; Jakmunee, J.; Ounnunkad, K. A Sandwich-like Configuration with a Signal Amplification Strategy Using a Methylene Blue/Aptamer Complex on a Heterojunction 2D MoSe2/2D WSe2 Electrode: Toward a Portable and Sensitive Electrochemical Alpha-Fetoprotein Immunoassay. Front. Cell. Infect. Microbiol. 2022, 12, 916357. [Google Scholar] [CrossRef]
- Foroozandeh, A.; Babazad, M.A.; Jouybar, S.; Abdouss, M.; Salar Amoli, H.; Dashtian, K.; Hasanzadeh, M. Recent Advancements in Biosensors for Diagnosis of Ovarian Cancer: Analytical Approaches. TrAC 2025, 183, 118119. [Google Scholar] [CrossRef]
- Wang, L.; Balasubramanian, P.; Chen, A.P.; Kummar, S.; Evrard, Y.A.; Kinders, R.J. Promise and Limits of the CellSearch Platform for Evaluating Pharmacodynamics in Circulating Tumor Cells. Semin. Oncol. 2016, 43, 464–475. [Google Scholar] [CrossRef]
- Dizdar, L.; Fluegen, G.; van Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of Circulating Tumor Cells in Colorectal Cancer Patients Using the GILUPI CellCollector: Results from a Prospective, Single-Center Study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef]
- Dong, Y.; Mao, S.; Chen, S.; Ma, J.; Jaffrezic-Renault, N.; Guo, Z. Opportunities and challenges of microneedle electrochemical sensors for interstitial fluid detection. TrAC 2024, 180, 117891. [Google Scholar] [CrossRef]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Krishnakumar, R.; Sinha, A.; Poorey, K.; Williams, K.P.; et al. Extraction and Biomolecular Analysis of Dermal Interstitial Fluid Collected with Hollow Microneedles. Commun. Biol. 2018, 1, 173. [Google Scholar] [CrossRef]
- Merzougui, C.; Yang, X.; Meng, D.; Huang, Y.; Zhao, X. Microneedle Array-Based Dermal Interstitial Fluid Biopsy for Cancer Diagnosis: Advances and Challenges. Adv. Healthc. Mater. 2025, 14, 2404420. [Google Scholar] [CrossRef]
- Huang, H.; Qu, M.; Zhou, Y.; Cao, W.; Huang, X.; Sun, J.; Sun, W.; Zhou, X.; Xu, M.; Jiang, X. A Microneedle Patch for Breast Cancer Screening via Minimally Invasive Interstitial Fluid Sampling. Chem. Eng. J. 2023, 472, 145036. [Google Scholar] [CrossRef]
- Maqsood, Q.; Sumrin, A.; Saleem, Y.; Wajid, A.; Mahnoor, M. Exosomes in Cancer: Diagnostic and Therapeutic Applications. Clin. Med. Insights Oncol. 2024, 18, 11795549231215966. [Google Scholar] [CrossRef]
- Park, W.; Maeng, S.W.; Mok, J.W.; Choi, M.; Cha, H.J.; Joo, C.K.; Hahn, S.K. Hydrogel Microneedles Extracting Exosomes for Early Detection of Colorectal Cancer. Biomacromolecules 2023, 24, 1445–1452. [Google Scholar] [CrossRef]
- Bratei, A.A.; Stefan-van Staden, R.I.; Ilie-Mihai, R.M.; Gheorghe, D.C. Simultaneous Assay of CA 72-4, CA 19-9, CEA and CA 125 in Biological Samples Using Needle Three-Dimensional Stochastic Microsensors. Sensors 2023, 23, 8046. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Cheng, Y.; Huang, W.; Xiang, L. Electrochemical Biosensors Based on Saliva Electrolytes for Rapid Detection and Diagnosis. J. Mater. Chem. B 2022, 11, 33–54. [Google Scholar] [CrossRef]
- AlAli, A.M.; Walsh, T.; Maranzano, M. CYFRA 21-1 and MMP-9 as Salivary Biomarkers for the Detection of Oral Squamous Cell Carcinoma: A Systematic Review of Diagnostic Test Accuracy. Int. J. Oral Maxillofac. Surg. 2020, 49, 973–983. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, H. Next-Generation Sequencing in Liquid Biopsy: Cancer Screening and Early Detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Jafari, M.; Hasanzadeh, M. Non-Invasive Bioassay of Cytokeratin Fragment 21.1 (Cyfra 21.1) Protein in Human Saliva Samples Using Immunoreaction Method: An Efficient Platform for Early-Stage Diagnosis of Oral Cancer Based on Biomedicine. Biomed. Pharmacother. 2020, 131, 110671. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Yadav, B.K.; Malhotra, B.D. Highly Sensitive Protein Functionalized Nanostructured Hafnium Oxide Based Biosensing Platform for Non-Invasive Oral Cancer Detection. Sens. Actuators B Chem. 2016, 235, 1–10. [Google Scholar] [CrossRef]
- Chan, K.M.; Gleadle, J.M.; O’Callaghan, M.; Vasilev, K.; MacGregor, M. Prostate Cancer Detection: A Systematic Review of Urinary Biosensors. Prostate Cancer Prostatic Dis. 2022, 25, 39–46. [Google Scholar] [CrossRef]
- Cao, X.; Gao, Y.; Ding, W.; Li, C. A Wearable Electrochemical Sensor for Testing Carcinoembryonic Antigen in Sweat. In Proceedings of the 2023 4th International Symposium on Artificial Intelligence for Medicine Science, Chengdu, China, 20–22 October 2023; pp. 722–727. [Google Scholar] [CrossRef]
- Nandi, S.K.; Singh, D.; Upadhay, J.; Gupta, N.; Dhiman, N.; Mittal, S.K.; Mahindroo, N. Identification of Tear-Based Protein and Non-Protein Biomarkers: Its Application in Diagnosis of Human Diseases Using Biosensors. Int. J. Biol. Macromol. 2021, 193, 838–846. [Google Scholar] [CrossRef]
- Evans, V.; Vockler, C.; Friedlander, M.; Walsh, B.; Willcox, M.D.P. Lacryglobin in Human Tears, a Potential Marker for Cancer. Clin. Exp. Ophthalmol. 2001, 29, 161–163. [Google Scholar] [CrossRef]
- Daily, A.; Ravishankar, P.; Harms, S.; Klimberg, V.S. Using Tears as a Non-Invasive Source for Early Detection of Breast Cancer. PLoS ONE 2022, 17, e0267676. [Google Scholar] [CrossRef] [PubMed]
- Dikovskaya, M.A.; Trunov, A.N.; Chernykh, V.V.; Korolenko, T.A. Cystatin C and lactoferrin concentrations in biological fluids as possible prognostic factors in eye tumor development. Int. J. Circumpolar Health 2013, 72, 21087. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.C.; Dincer, C. Wearable Breath Analysis. Nat. Rev. Bioeng. 2023, 1, 80–82. [Google Scholar] [CrossRef]
- Gashimova, E.; Temerdashev, A.; Perunov, D.; Porkhanov, V.; Polyakov, I. Diagnosis of Lung Cancer Through Exhaled Breath: A Comprehensive Study. Mol. Diagn. Ther. 2024, 28, 847–860. [Google Scholar] [CrossRef]
- VA, B.; Mathew, P.; Thomas, S.; Mathew, L. Detection of Lung Cancer and Stages via Breath Analysis Using a Self-Made Electronic Nose Device. Expert. Rev. Mol. Diagn. 2024, 24, 341–353. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, J.; Fu, W.; Muhammad, K.G.; Li, Y.; Liu, W.; Xu, L.; Dong, H.; Wang, D.; Liu, J.; et al. Smartphone-Based Platforms for Clinical Detections in Lung-Cancer-Related Exhaled Breath Biomarkers: A Review. Biosensors 2022, 12, 223. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef]
- Zompanti, A.; Finamore, P.; Longo, F.; Grasso, S.; Frasca, L.; Celoro, F.; Santonico, M.; Cenerini, C.; La Monica, L.; Sabatini, A.; et al. Sensor Technology Advancement Enhancing Exhaled Breath Portability: Device Set up and Pilot Test in the Longitudinal Study of Lung Cancer. Sens. Actuators B Chem. 2025, 423, 136735. [Google Scholar] [CrossRef]
- Picciariello, A.; Dezi, A.; Vincenti, L.; Spampinato, M.G.; Zang, W.; Riahi, P.; Scott, J.; Sharma, R.; Fan, X.; Altomare, D.F. Colorectal Cancer Diagnosis through Breath Test Using a Portable Breath Analyzer—Preliminary Data. Sensors 2024, 24, 2343. [Google Scholar] [CrossRef]
- Acevedo, D.; Carrillo Gómez, C.M.; Cuastumal Vasquez, J.K.; Ramos, C.A.; Prostate, J.; Manuel, C.; Carrillo Gómez, J.K.; Cuastumal Vasquez, C.A.; Ramos, J. Prostate Cancer Detection in Colombian Patients through E-Senses Devices in Exhaled Breath and Urine Samples. Chemosensors 2024, 12, 11. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, J.; Li, L.; Zeng, Y.; Zhao, J.; Li, G. Electrochemical Biosensors for Cancer Diagnosis: Multitarget Analysis to Present Molecular Characteristics of Tumor Heterogeneity. JACS Au 2024, 4, 4655–4672. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Wang, M.; Min, J.; Tay, R.Y.; Lukas, H.; Sempionatto, J.R.; Li, J.; Xu, C.; Gao, W. A Wearable Aptamer Nanobiosensor for Non-Invasive Female Hormone Monitoring. Nat. Nanotechnol. 2023, 19, 330–337. [Google Scholar] [CrossRef]
- Fang, L.; Ren, H.; Mao, X.; Zhang, S.; Cai, Y.; Xu, S.; Zhang, Y.; Li, L.; Ye, X.; Liang, B. Differential Amperometric Microneedle Biosensor for Wearable Levodopa Monitoring of Parkinson’s Disease. Biosensors 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Tai, L.C.; Liaw, T.S.; Lin, Y.; Nyein, H.Y.Y.; Bariya, M.; Ji, W.; Hettick, M.; Zhao, C.; Zhao, J.; Hou, L.; et al. Wearable Sweat Band for Noninvasive Levodopa Monitoring. Nano Lett. 2019, 19, 6346–6351. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Du, W.; Zhang, L.; Suh, E.; Lin, D.; Marcus, C.; Ozkan, L.; Ahuja, A.; Fernandez, S.; Shuvo, I.I.; Sadat, D.; et al. Conformable Ultrasound Breast Patch for Deep Tissue Scanning and Imaging. Sci. Adv. 2023, 9, eadh5325. [Google Scholar] [CrossRef]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and Their Widespread Impact on Human Health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Sheydaei, O.; Khajehsharifi, H.; Rajabi, H.R. Rapid and Selective Diagnose of Sarcosine in Urine Samples as Prostate Cancer Biomarker by Mesoporous Imprinted Polymeric Nanobeads Modified Electrode. Sens. Actuators B Chem. 2020, 309, 127559. [Google Scholar] [CrossRef]
- Anıl, İ.U.; Sezgintürk, M.K. MIP-Based Sensing Strategies for the Diagnosis of Prostate and Lung Cancers. Talanta Open 2025, 11, 100432. [Google Scholar] [CrossRef]
- Coskun, A.; Lippi, G. The Impact of Physiological Variations on Personalized Reference Intervals and Decision Limits: An in-Depth Analysis. Clin. Chem. Lab. Med. 2024, 62, 2140–2147. [Google Scholar] [CrossRef]
- Coskun, A.; Lippi, G. Personalized Laboratory Medicine in the Digital Health Era: Recent Developments and Future Challenges. Clin. Chem. Lab. Med. 2024, 62, 402–409. [Google Scholar] [CrossRef]
- Fraser, C.G. Biological Variation: From Principles to Practice; AACC Press: Washington, DC, USA, 2001. [Google Scholar]
- Sandberg, S.; Carobene, A.; Bartlett, B.; Coskun, A.; Fernandez-Calle, P.; Jonker, N.; Díaz-Garzón, J.; Aarsand, A.K. Biological Variation: Recent Development and Future Challenges. Clin. Chem. Lab. Med. 2023, 61, 741–750. [Google Scholar] [CrossRef]
- Petersen, P.H.; Fraser, C.G.; Sandberg, S.; Goldschmidt, H. The Index of Individuality Is Often a Misinterpreted Quantity Characteristic. Clin. Chem. Lab. Med. 1999, 37, 655–661. [Google Scholar] [CrossRef]
- Coşkun, A.; Sandberg, S.; Unsal, I.; Cavusoglu, C.; Serteser, M.; Kilercik, M.; Aarsand, A.K. Personalized and Population-Based Reference Intervals for 48 Common Clinical Chemistry and Hematology Measurands: A Comparative Study. Clin. Chem. 2023, 69, 1009–1030. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, A.; Sandberg, S.; Unsal, I.; Cavusoglu, C.; Serteser, M.; Kilercik, M.; Aarsand, A.K. Personalized Reference Intervals in Laboratory Medicine: A New Model Based on Within-Subject Biological Variation. Clin. Chem. 2021, 67, 374–384. [Google Scholar] [CrossRef]
- Coskun, A.; Sandberg, S.; Unsal, I.; Serteser, M.; Aarsand, A.K. Personalized Reference Intervals: From Theory to Practice. Crit. Rev. Clin. Lab. Sci. 2022, 59, 501–516. [Google Scholar] [CrossRef]
- Coskun, A.; Sandberg, S.; Unsal, I.; Yavuz, F.G.; Cavusoglu, C.; Serteser, M.; Kilercik, M.; Aarsand, A.K. Personalized Reference Intervals—Statistical Approaches and Considerations. Clin. Chem. Lab. Med. 2022, 60, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A. Prediction Interval: A Powerful Statistical Tool for Monitoring Patients and Analytical Systems. Biochem. Med. 2024, 34, 020101. [Google Scholar] [CrossRef] [PubMed]
- Meeker, W.Q.; Hahn, G.J.; Escobar, L.A. Statistical Intervals: A Guide for Practitioners and Researchers; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9780471687177. [Google Scholar]
- Geisser, S. Predictive Inference: An Introduction; Springer: Boston, MA, USA, 1993; ISBN 978-0-412-03471-8. [Google Scholar]
- Aarsand, A.K.; Fernandez-Calle, P.; Webster, C.; Coskun, A.; Gonzales-Lao, E.; Diaz-Garzon, J.; Jonker, N.; Simon, M.; Braga, F.; Perich, C.; et al. EFLM Biological Variation Database. Available online: https://biologicalvariation.eu (accessed on 1 May 2025).
- Roberts, W.L.; McMillin, G.A.; Burtis, C.A.; Bruns, D.E. Reference information for the clinical laboratory. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th ed.; Burtis, C.A., Ashwood, E.R., Bruns, D.E., Eds.; Elsevier Sounders: St. Louis, MO, USA, 2012; pp. 2131–2188. [Google Scholar]
- Coskun, A. Diagnosis based on population data versus personalized data: The evolving paradigm in laboratory medicine. Diagnostics 2024, 14, 2135. [Google Scholar] [CrossRef]
- Ozarda, Y.; Sikaris, K.; Streichert, T.; Macri, J. Distinguishing Reference Intervals and Clinical Decision Limits—A Review by the IFCC Committee on Reference Intervals and Decision Limits. Crit. Rev. Clin. Lab. Sci. 2018, 55, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.G. Reference Change Values. Clin. Chem. Lab. Med. 2012, 50, 807–812. [Google Scholar] [CrossRef]



| Cancer | Subtype | Tumor Volume Doubling Time | Tumor Marker | II | Ref. |
|---|---|---|---|---|---|
| Lymphoid neoplasms | Burkitt’s lymphoma | 24–48 h | LDH | 0.37 * | [10] |
| Testicular cancer | Non-seminoma | 21 days † | AFP | 0.08 * | [11] |
| Brain tumor | Glioblastoma | 29.8 days ‡ | VEGF | - | [12] |
| Lung cancer | Small cell lung cancer | 73 days * ¤ | NSE | 0.66 * | [13] |
| proGRP | 0.29 | ||||
| Ovarian cancer | 90 days ‡ | CA 125 | 0.34 * | [14] | |
| Lung cancer | Squamous cell lung cancer | 140 days * ¤ | SCC | - | [13] |
| Liver cancer | Hepatocellular carcinoma | 140 days * ¤ | AFP | 0.08 * | [15] |
| Pancreatic cancer | 144 days ‡ | CA 19-9 | 0.07 * | [16] | |
| Breast cancer | 180 days * † | CA 15-3 | 0.12 | [17] | |
| Gastric cancer | 186 days (T1) † | CEA | 0.11 * | [18] | |
| CA 19-9 | 0.07 * | ||||
| Colorectal cancer | 211 days ‡ | CEA | 0.11 * | [19] | |
| Lung cancer | Adenocarcinoma | 223 days * ¤ | CEA | 0.11 * | [13] |
| CYFRA 21.1 | 0.67 | ||||
| Thyroid cancer | Medullary thyroid carcinoma | 1.6 years † | Calcitonin | 0.2 * | [20] |
| Thyroglobulin | 0.14 * | ||||
| Prostate cancer | >2 year (89% of patients) | PSA | 0.16 * | [21,22] | |
| Thyroid cancer | Papillary thyroid carcinoma | >5 year (71.8% of patients) | Thyroglobulin | 0.14 * | [23] |
| Tumor Markers | Method | Primary Malignancy | Other Malignancies | Non-Malignant Conditions | Year | Ref. |
|---|---|---|---|---|---|---|
| Bence Jones Protein | IFE, SFLC | Multiple myeloma * | Non-Hodgkin’s lymphoma, Waldenström’s macroglobulinemia | Pre-malignant plasma cell disorders | 1847 | [27] |
| hCG | ECLIA, CLIA | Germ cell and testicular tumors, gestastional trophoblastic neoplasia * | Lung cancer | Hyperthyroidism, chronic renal failure | 1956 | [28] |
| AFP | ECLIA, CLIA | Hepatocellular carcinoma *, germ cell tumors * | Gastric, colorectal, bilary, pancreatic, and lung cancer | Liver regeneration, viral hepatitis, pregnancy | 1963 | [29] |
| CEA | ECLIA, CLIA | Colorectal cancer | Breast, lung, gastric, pancreatic, bladder, cervical, thyroid, and hepatic cancers, lymphoma and melanoma | Ulcerative pancreatitis, cirrhosis, colitis, hypothyroidism, Crohn’s disease, COPD | 1965 | [30] |
| NSE | ECLIA, TRACE | Neuroendocrine tumors (neuroblastoma, small cell lung cancer) | Medullary thyroid carcinoma, melanoma, pancreatic endocrine tumors | Tuberculosis, COPD, alveolar proteinosis, acute respiratory distress syndrome, silicosis, neurological deficits, ischemia reperfusion, brain injury | 1965 | [31] |
| Chromo-granin A | TRACE | Neuroendocrine tumors | Presence of neuroendocrine cells in non-endocrine tumors | Atrophic gastritis, chronic renal injury, chronic heart failure, hypertension, rheumatoid arthritis | 1967 | [32] |
| Calcitonin | ECLIA, ICMA | Medullary thyroid carcinoma * | Lung, breast, kidney, and liver cancer | Pulmonary disease, pancreatitis, hyperparathyroidis, pernicious anemia | 1968 | [33] |
| Thyro-globulin | LC-MS/MS | Thyroid cancer | None | Graves’ disease, Hashimoto’s disease, and thyroiditis | 1975 | [34] |
| SCCA | TRACE | Squamous cell carcinoma (cervical, lung, skin, head and neck) | Esophageal adenocarcinoma, hepatocellular carcinoma | Inverted papilloma, non-malignant pulmonary disease, chronic hepatitis, atopic dermatitis | 1977 | [35] |
| PSA | ECLIA, CLIA | Prostate cancer * | None | Urinary tract infections, prostatisis, benign prostatic hyperplasia | 1979 | [36] |
| CA 19-9 | ECLIA, CLIA | Pancreatic cancer * | Colorectal, biliary tract, liver, gastric, and lung cancer, cholangiocarcinoma, mesothelioma | Liver damage, bile duct obstruction and inflammation, pancreatitis, interstitial pulmonary disease, pulmonary fibrosis, collagen vascular diseases, hypothyroidism, gastric ulcer | 1979 | [37] |
| CA 125 | ECLIA | Ovarian cancer * | Breast, endometrial, cervix, peritoneal, uterus, lung, and pancreatic cancer, non-Hodgkin lymphoma, hepatocellular carcinoma | Idiopathic pulmonary fibrosis, ovarian cyst, endometriosis, adenomyosis, pelvic inflammation, uterine fibroids, rheumatoid arthritis-related interstitial lung disease | 1981 | [38] |
| CA 15-3 | ECLIA | Breast cancer | Pancreatic, lung, ovarian, colorectal, and liver cancer | Benign liver and breast diseases | 1984 | [39] |
| Inhibin A Inhibin B | ICMA ELISA | Ovarian granulosa cell, mucinous epithelial ovarian and testicular tumors | Endometrial carcinoma, adrenal tumors | Preeclampsia, ovarian cysts | 1989 | [40] |
| HE4 | ECLIA | Ovarian cancer | Lung cancer, pulmonary adenocarcinoma | Chronic kidney disease, renal failure, kidney fibrosis | 1991 | [41] |
| Cyfra 21.1 | ECLIA | Lung cancer | Breast, bladder, and pancreatic cancer, hepatocellular carcinoma | Renal failure, liver cirrhosis, benign lung diseases | 1993 | [42] |
| Biofluid | Biomarker | Method | Detection Limit | Assay Time | Measurement Procedures | Ref. |
|---|---|---|---|---|---|---|
| Serum | CEA | Optical (fluorescence quenching) | 6.7 pg/mL | 80 min | Paper-based device with mesoporous silica NP and quantum dot signal generation via glucose-triggered fluorescence quenching. | [193] |
| CEA | Photoelectrochemical | 11.3 pg/mL | ~35 min | Paper-based immunoassay platform integrating shell–shell structured photoactive materials. | [194] | |
| CEA | Optical (scanned image analysis) | 0.45 ng/mL | 15 min | Lateral flow strip with Au-NP probes and nitrocellulose membranes. Office-type scanner used for quantification. | [195] | |
| CEA | Optical (SERS) | 0.36 pg/mL | ~30 min | Pump-free microfluidic chip using a Au-NP-modified SiO2 microsphere. | [196] | |
| SCCA | 0.45 pg/mL | |||||
| CA 19-9 | Electrochemical (DPV) | 0.07 U/mL | ~25 min | Flexible SP carbon electrode modified with carbon black-polyelectrolyte multilayer films. | [197] | |
| CA 19-9 | Optical | 30 U/mL | 35 min | Lateral flow sensor integrating magnetized CNT for low-cost, visual detection on disposable strips. | [198] | |
| CA 125 | Optical (colorimetric) | 5.21 U/mL | 20 min | Lateral flow platform utilizing Au nanozyme-labeled probes for low-cost, home-usable, and visually quantitative detection. | [199] | |
| CA 125 | Electrochemical (DPV) | 2 mU/mL | ~25 min | Smartphone-integrated system combining a miniaturized detector and SP electrodes modified with CNT and Au-NP. | [200] | |
| CA 15-3 | Electrochemical, (SWV) | 0.95 U/mL | ~30 min | Disposable chip device based on NP-modified SP electrodes. | [201] | |
| AFP | Electrochemical, (SWV) | 0.03 ng/mL | 35 s | POC biosensor integrating multi-functionalized graphene nanocomposites for rapid biomarker detection. | [202] | |
| AFP | Photoelectrochemical | 74.8 pg mL | 1.5 h | Biosensor combining oxygen-doped semiconductor photoelectrodes with digital multimeter readout for simple and low-cost biomarker analysis. | [203] | |
| AFP | Optical | 1.27 ng/mL | 2 h | Droplet evaporation-based biosensor utilizing surfactant-modified patterns on plastic substrates for simple, label-free detection. | [204] | |
| PSA | Electrochemical (DPV) | 0.38 fg/mL | 20 min | Miniaturized sensor integrating shrink polymer-based electrodes with smartphone-controlled operation. | [205] | |
| LDH | Optical (colorimetric) | 70 pg mL | 50 min | Electrophoretic lateral flow sensor integrating battery-powered microfluidics, Au-NP signal transduction, and smartphone-based signal quantification. Small benchtop set-up needed. | [206] | |
| LDH | Optical (colorimetric) | 86 ng/mL (LOQ) | 10 min | Smartphone-based lateral flow biosensor using carbon NP for visual detection on disposable strips. | [207] | |
| Thyroglobulin | Optical (LSPR) | 93.11 fg/mL | 10 min | Fiber optic localized surface plasmon resonance biosensor integrating Au-NP-coated fibers within a microfluidic channel for simplified detection. | [208] | |
| miRNA 21 | Electrical (direct current) | 0.0028 fM | 1.5 h | Self-powered platform employing graphdiyne-modified electrodes, physical signal amplification, and smartphone readout. | [209] | |
| Whole blood | CEA | Electrochemical (linear sweep voltammetry) | 0.15 ng/mL | ~25 min | Fluidic-integrated dual carbon electrode platform fabricated by stencil printing. | [210] |
| CA 125 | 0.6 U/mL | |||||
| CEA | Optical (fluorescence) | 10 ng/mL | ~10 min | Microfluidic silk patch fabricated by 3D printing for flexible sensing. | [211] | |
| AFP | 10 ng/mL | |||||
| PSA | Optical (fluorescence) | 0.08 ng/mL | 13–22 min | Power-free and flexible, fluoropolymer microcapillary film device integrated with a smartphone. | [212] | |
| NSE | EIS | 1.15 ng/mL | 5 min | Disposable chip-like device, enabling simplified detection using mouse model samples without clinical validation. | [213] | |
| Saliva | CEA | Optical (fluorescence) | 0.012 ng/mL | ~5 min | Fully integrated platform combining acoustic enrichment and smartphone-based visual detection for easy home monitoring. | [214] |
| CEA | Optical (time-resolved photoluminescence) | 1.47 pg/mL | 10 min | Lab-in-syringe platform integrating lanthanide nanoprobes with dissolution-enhanced luminescence for easy on-site detection. | [215] | |
| CYFRA 21-1 | Electrochemical (DPV, chronoamperometry) | 0.025 ng/mL (LLOQ) | 4 h | Paper-based platform with silver nano-ink printed electrodes. | [216] | |
| CEA | Electronic (direct current measurement) | 0.148 pg/mL | 1 h | Label-free biosensor integrating rGO/melamine-modified electrodes with wired electronic readout system. | [217] | |
| CYFRA 21-1 | 0.04 pg/mL | |||||
| CA 15-3 | Electrochemical (DPV) | 0.56 U/mL | 1 h | Immunosensor integrating SP paper electrodes modified with AuNPs for simple detection on disposable platforms. | [218] | |
| Urine | NMP22, CA9, CD47, CK8, CK18 | Electrical (FET) | ≪pg/mL | ~5 min | IGZO FET-based urinalysis device integrated with wireless data transfer and smartphone interface for the simultaneous detection of five bladder cancer markers. | [219] |
| Tears | Raman spectral profile | Optical (SERS) | 100 fM | ~5 min | Label-free Au/HCP-PS biosensor combined with a hand-held Raman spectrometer enables the detection of breast cancer with 96% classification accuracy. | [220] |
| Artificial sample | NSE | EIS | 1.005 ng/mL | 5 min | Microfluidic chip incorporating Au-modified electrodes for simplified detection, without clinical validation. | [221] |
| CEA | Optical (fluorescence) | 3.1 ng/mL | 20 min | Microfluidic device combining magnetic single-bead trapping with acoustic micro-mixing. Small benchtop set-up needed. | [222] | |
| PSA | 0.028 ng/mL | |||||
| CA 15-3 | Electrochemical (SWV) | 0.909 mU/mL | 20 min | Disposable sensor platform utilizing MIPs as alternative to natural sensing elements for stable and selective detection. | [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savas, I.N.; Coskun, A. The Future of Tumor Markers: Advancing Early Malignancy Detection Through Omics Technologies, Continuous Monitoring, and Personalized Reference Intervals. Biomolecules 2025, 15, 1011. https://doi.org/10.3390/biom15071011
Savas IN, Coskun A. The Future of Tumor Markers: Advancing Early Malignancy Detection Through Omics Technologies, Continuous Monitoring, and Personalized Reference Intervals. Biomolecules. 2025; 15(7):1011. https://doi.org/10.3390/biom15071011
Chicago/Turabian StyleSavas, Irem Nur, and Abdurrahman Coskun. 2025. "The Future of Tumor Markers: Advancing Early Malignancy Detection Through Omics Technologies, Continuous Monitoring, and Personalized Reference Intervals" Biomolecules 15, no. 7: 1011. https://doi.org/10.3390/biom15071011
APA StyleSavas, I. N., & Coskun, A. (2025). The Future of Tumor Markers: Advancing Early Malignancy Detection Through Omics Technologies, Continuous Monitoring, and Personalized Reference Intervals. Biomolecules, 15(7), 1011. https://doi.org/10.3390/biom15071011


_Kwok.png)




