Hyaluronic Acid in Immune Response
Abstract
1. Introduction
2. HA Metabolism
3. HA Receptors
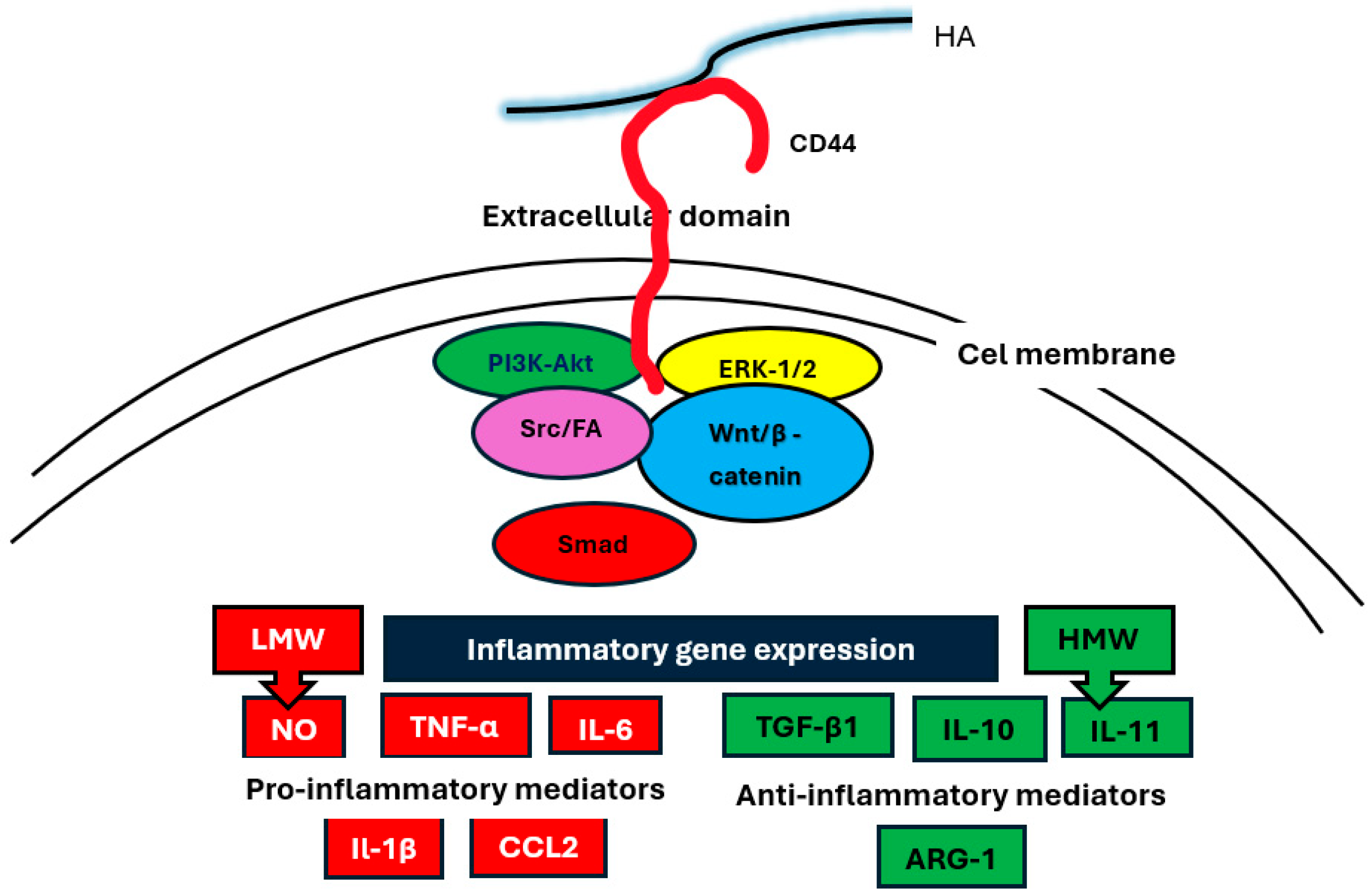
4. HA Activation of Intracellular Signaling Pathways
5. The Association of HA with the Expression of Cytokines and Chemokines
6. HA in Cytokine Storms During COVID-19
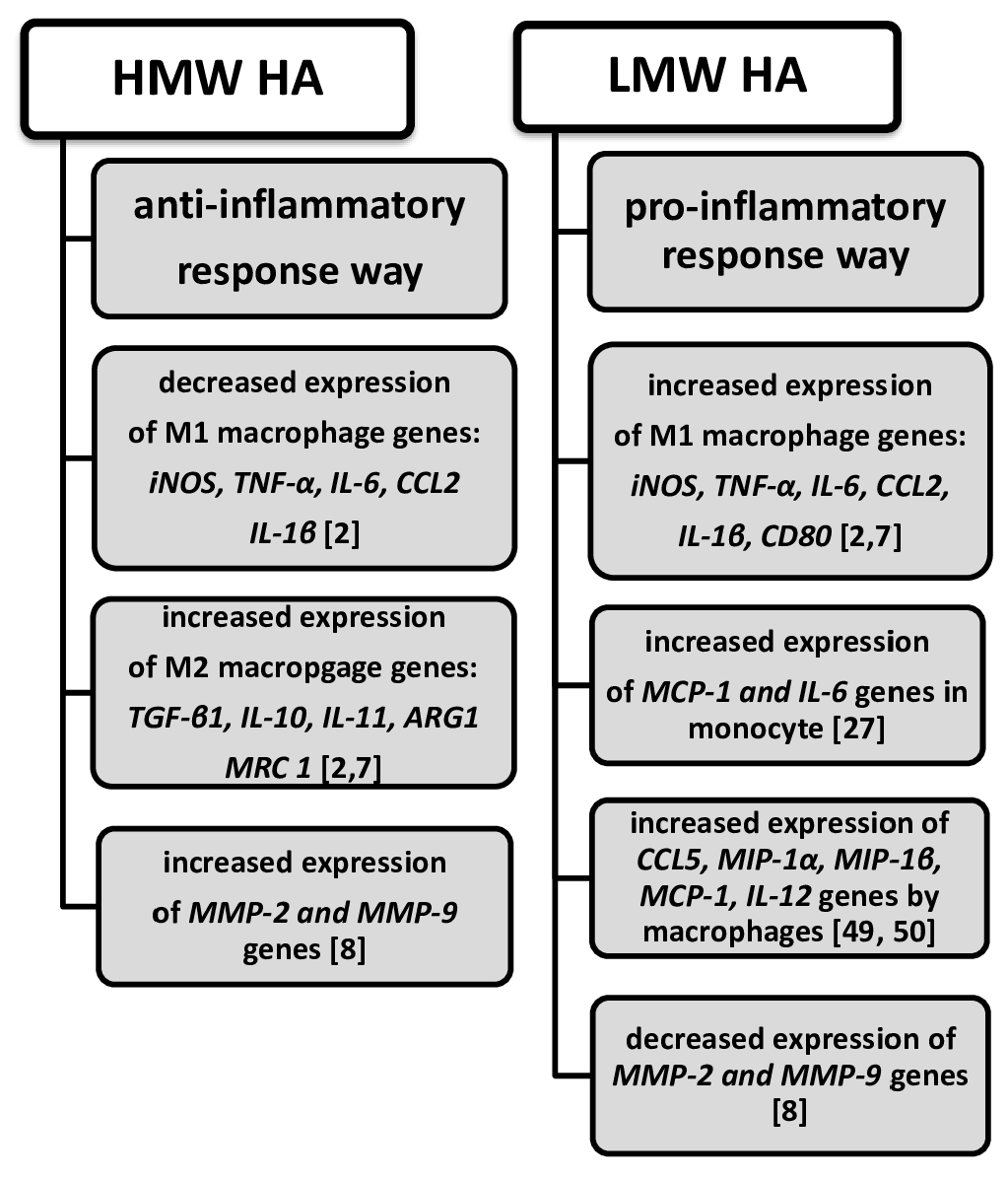
7. HA Provokes Inflammation
8. HA Provokes Tumor Growth and Progression
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.-H. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Donelan, W.; Dominguez-Gutierrez, P.R.; Kusmartsev, S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor associated immune suppression. Front. Immunol. 2022, 13, 971278. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef]
- Rosales, P.; Vitale, Z.D.; Icardi, A.; Sevic, I.; Alaniz, L. Role of Hyaluronic acid and its chemical derivatives in immunity during homeostasis, cancer and tissue regeneration. Semin. Immunopathol. 2024, 46, 15. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.; Laurent, U.B. Hyaluronan: Its nature, distribution, func- tions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef]
- Cabrera, P.V.; Blanco, G.; Alaniz, L.; Greczanik, S.; Garcia, M.; Alvarez, E.; Hajos, S.E. CD44 and hyaluronic acid regulate in vivo iNOS expression and metalloproteinase activity in murine air-pouch inflammation. Inflamm. Res. 2004, 53, 556–566. [Google Scholar] [CrossRef]
- McDonald, B.; Kubes, P. Interactions between CD44 and hyaluronan in leukocyte trafficking. Front. Immunol. 2015, 6, 68. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef]
- Johnson, P.; Arif, A.A.; Lee-Sayer, S.S.M.; Dong, Y. Hyaluronan and Its Interactions with mmune Cells in the Healthy and Inflamed Lung. Front. Immunol. 2018, 9, 2787. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Prestipino, V.; Nastasi, G.; Calatroni, A.; Campo, S. The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. Cell Biochem. 2012, 113, 1852–1867. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Veiseh, M.; Turley, E.A. Hyaluronan metabolism in remodeling extracellular matrix: Probes for imaging and therapy of breast cancer. Integr. Biol. 2011, 3, 304–315. [Google Scholar] [CrossRef]
- Moseley, R.; Waddington, R.J.; Embery, G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim. Biophys. Acta 1997, 1362, 221–231. [Google Scholar] [CrossRef]
- Niemietz, I.; Brown, K.L. Hyaluronan promotes intracellular ROS production and apoptosis in TNFα-stimulated neutrophils. Front. Immunol. 2023, 14, 1032469. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Hawkins, C.L.; Parsons, B.J.; Davies, M.J.; Phillips, G.O. Identification of radicals from hyaluronan (hyaluronic acid) and cross-linked derivatives using electron paramagnetic resonance spectroscopy. Carbohydr. Polym. 1999, 38, 17–22. [Google Scholar] [CrossRef]
- Lee-Sayer, S.S.M.; Dong, Y.; Arif, A.A.; Olsson, M.; Brown, K.L.; Johnson, P. The where, when, how, and why of hyaluronan binding by immune cells. Front. Immunol. 2015, 6, 150. [Google Scholar] [CrossRef]
- Romo, M.; López-Vicario, C.; Pérez-Romero, N.; Casulleras, M.; Martínez-Puchol, A.I.; Sánchez, B.; Flores-Costa, R.; Alcaraz-Quiles, J.; Duran-Güell, M.; Ibarzábal, A.; et al. Small fragments of hyaluronan are increased in individuals with obesity and contribute to low-grade inflammation through TLR-mediated activation of innate immune cells. Int. J. Obesity 2022, 46, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, C.L.; Mummert, M.E. Hyaluronan Synthesis Is Required for IL-2-Mediated T Cell Proliferation. J. Immunol. 2007, 179, 8191–8199. [Google Scholar] [CrossRef]
- Riehl, T.E.; Foster, L.; Stenson, W.F. Hyaluronic Acid Is Radioprotective in the iintestine through a TLR4 and COX-2-Mediated Mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G309–G316. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Jackson, D.G. Hyaluronan and its receptors: Key mediators of immune cell entry and trafficking in the lymphatic system. Cells 2021, 10, 2061. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, A.; Polette, M.; Coraux, C. Lung Hyaluronasome: Involvement of Low Molecular Weight Ha (Lmw-Ha) in Innate Immunity. Biomolecules 2022, 12, 658. [Google Scholar] [CrossRef]
- Dodd, R.J.; Blundell, C.D.; Sattelle, B.M.; Enghild, J.J.; Milner, C.M.; Day, A.J. Chemical modification of hyaluronan oligosaccharides differentially modulates hyaluronan–hyaladherin interactions. J. Biol. Chem. 2024, 300, 107668. [Google Scholar] [CrossRef]
- Yamawaki, H.; Hirohata, S.; Miyoshi, T.; Takahashi, K.; Ogawa, H.; Shinohata, R.; Demircan, K.; Kusachi, S.; Yamamoto, K.; Ninomiya, Y. Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology 2009, 19, 83–92. [Google Scholar] [CrossRef]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan Fragments Stimulate Endothelial Recognition of Injury through TLR4. J. Biol. Chem. 2004, 279, 17079–17084. [Google Scholar] [CrossRef]
- Levesque, M.C.; Haynes, B.F. Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNF-alpha and is inhibited by IL-4 and IL-13. J. Immunol. 1997, 159, 6184–6194. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronian and its Rerceptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Weigel, P.H. Systemic glycosaminoglycan clearance by HARE/Stabilin-2 activates Intracellular Signaling. Cells 2020, 29, 2366. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Wu, L.-J.; Kong, X.; Zheng, B.-Y.; Zhang, Z.; He, Z.-W. Regulation of the expression of proinflammatory cytokines induced by SARS-CoV-2. World J. Clin. Cases 2021, 9, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Jackson, D.G. Hyaluronan in the lymphatics: The key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 2019, 78–79, 219–235. [Google Scholar] [CrossRef]
- Lawrance, W.; Banerji, S.; Day, A.J.; Bhattacharjee, S.; Jackson, D.G. Binding of Hyaluronan to the Native Lymphatic Vessel Endothelial Receptor LYVE-1 Is Critically Dependent on Receptor Clustering and Hyaluronan Organization. J. Biol. Chem. 2016, 291, 8014–8030. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Banerji, S.; Lagerholm, B.C.; Jackson, D.G. Dendritic cell entry to lymphatic capillaries is orchestrated by CD44 and the hyaluronan glycocalyx. Life Sci. Alliance 2021, 4, e202000908. [Google Scholar] [CrossRef] [PubMed]
- Bono, P.; Rubin, K.; Higgins, J.M.G.; Hynes, R.O. Layilin, a novel integraal membranę protein, is a hyaluronian receptor. Mol. Biol. Cell 2017, 12, 891–900. [Google Scholar] [CrossRef]
- Avenoso, A.; Bruschetta, G.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Gullace, R.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan fragments produced during tissue injury: A signal amplifying the inflammatory response. Arch. Biochem. Biophys. 2019, 663, 228–238. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Reis, R.L.; Pashkuleva, I. Hyaluronan Receptors as Mediators and Modulators of the Tumor Microenvironment. Adv. Healthc. Mater. 2023, 12, e2202118. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Kim, D.-W.; Ahn, J.-H.; Choi, E.-J.; Kim, Y.H.; Jeun, M.; Kim, E.J. Propofol suppresses LPS-induced inflammation in amnion cells via inhibition of NF-κB activation. Tissue Eng. Regen. Med. 2019, 16, 301–309. [Google Scholar] [CrossRef]
- Day, A.; De La Motte, C.A. Hyaluronan cross-linking: A protective mechanism in inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, L.; Garcia, M.; Rizzo, M.; Piccioni, F.; Guillermo, M. Altered Hyaluronan Biosynthesis and Cancer Progression: An immunological perspective. Mini-Rev. Med. Chem. 2010, 9, 1538–1546. [Google Scholar] [CrossRef]
- Pandey, M.S.; Weigel, P.H. Hyaluronic acid receptor for endocytosis (HARE)-mediated endocytosis of hyaluronan, heparin, dermatan sulfate, and acetylated low density lipoprotein (AcLDL), but not chondroitin sulfate types A, C, D, or E, activates NF-κB-regulated gene expression. J. Biol. Chem. 2014, 289, 1756–1767. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Chu, X.; Yan, Q.; He, J.; Guo, Y.; Zhao, Z.; Zhang, Y.; Hu, D.; Ding, H.; et al. Targeting LAYN inhibits colorectal cancer metastasis and tumor-associated macrophage infiltration induced by hyaluronan oligosaccharides. Matrix Biol. 2023, 117, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and reactive oxygen Species Signaling—Novel cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- Voelcker, V.; Gebhardt, C.; Averbeck, M.; Saalbach, A.; Wolf, V.; Weih, F.; Sleeman, J.; Anderegg, U.; Simon, J. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp. Dermatol. 2008, 17, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef]
- Hodge-Dufour, J.; Noble, P.W.; Horton, M.R.; Bao, C.; Wysoka, M.; Burdick, M.D.; Strieter, R.M.; Trinchieri, G.; Puré, E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 1997, 159, 2492–2500. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef]
- Horton, M.R.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J. Immunol. 1998, 160, 3023–3030. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Mustafa, M.I.; Abdelmoneim, A.H.; Mahmoud, E.M.; Makhawi, A.M. Cytokine Storm in COVID-19 Patients, Its Impact on Organs and Potential Treatment by QTY Code-Designed Detergent-Free Chemokine Receptors. Mediat. Inflamm. 2020, 2020, 8198963. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. Dysregulation of Pulmonary Responses in Severe COVID-19. Viruses 2021, 13, 957. [Google Scholar] [CrossRef]
- Hellman, U.; Rosendal, E.; Lehrstrand, J.; Henriksson, J.; Björsell, T.; Wennemo, A.; Hahn, M.; Österberg, B.; Dorofte, L.; Nilsson, E.; et al. SARS-CoV-2 infection induces hyaluronan production in vitro and hyaluronan levels in COVID-19 patients relate to morbidity and long-term lung impairment: A prospective cohort study. mBio 2024, 15, e01303-24. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.-J.; Spicer, A.P.; Mascarenhas, M.M.; Yu, L.; Ochoa, C.D.; Garg, H.G.; Quinn, D.A. Theroleofhyaluronan synthase 3inventilator-inducedlunginjury. Am. J. Respir. Crit. Care Med. 2005, 172, 92–98. [Google Scholar] [CrossRef]
- Barnes, H.W.; Demirdjian, S.; Haddock, N.L.; Kaber, G.; Martinez, H.A.; Nagy, N.; Karmouty-Quintana, H.; Bollyky, P.L. Hyaluronan in the pathogenesis of acute and post-acute COVID-19 infection. Matrix Biol. 2023, 116, 49–66. [Google Scholar] [CrossRef]
- Dodd, R.J.; Allen, J.E.; Day, A.J. Hyaluronan in COVID-19: A matrix for understanding lung disease. mBio 2024, 15, e0260924. [Google Scholar] [CrossRef]
- Andonegui-Elguera, S.; Taniguchi-Ponciano, K.; Gonzalez-Bonilla, C.R.; Torres, J.; Mayani, H.; Herrera, L.A.; Peña-Martínez, E.; Silva-Román, G.; Vela-Patiño, S.; Ferreira-Hermosillo, A.; et al. Molecular Alterations Prompted by SARS-CoV-2 Infection: Induction of Hyaluronan, Glycosaminoglycan and Mucopolysaccharide Metabolism. Arch. Med. Res. 2020, 51, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Ontong, P.; Prachayasittikul, V. Unraveled roles of hyaluronan in severe COVID-19. EXCLI J. 2021, 20, 117–125. [Google Scholar] [PubMed]
- Cylwik, B.; Gan, K.; Kazberuk, M.; Gruszewska, E.; Panasiuk, A.; Chrostek, L. Diagnostic Usefulness of Serum Hyaluronic Acid in Patients with SARS-CoV-2 Infection. J. Clin. Med. 2024, 13, 7471. [Google Scholar] [CrossRef] [PubMed]
- Standiford, T.J.; Kunkel, S.L.; Greenberger, M.J.; Laichalk, L.L.; Strieter, R.M. Expression and regulation of chemokines in bacterial pneumonia. J. Leukoc. Biol. 1996, 59, 24–28. [Google Scholar] [CrossRef]
- Tanino, Y.; Coombe, D.R.; Gill, S.E.; Kett, W.C.; Kajikawa, O.; Proudfoot, A.E.; Wells, T.N.C.; Parks, W.C.; Wight, T.N.; Martin, T.R.; et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J. Immunol. 2010, 184, 2677–2685. [Google Scholar] [CrossRef]
- Tanino, Y. Roles of extracellular matrix in lung diseases. Fukushima J. Med. Sci. 2024, 70, 1–9. [Google Scholar] [CrossRef]
- de la Motte, C.; Nigro, J.; Vasanji, A.; Rho, H.; Kessler, S.; Bandyopadhyay, S.; Danese, S.; Fiocchi, C.; Stern, R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 2009, 174, 2254–2264. [Google Scholar] [CrossRef]
- Monzon, M.E.; Manzanares, D.; Schmid, N.; Casalino-Matsuda, S.M.; Forteza, R.M. Hyaluronidaseexpressio and activity is regulated by proinflammatory cytokines in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2008, 39, 289–295. [Google Scholar] [CrossRef]
- Petrey, A.C.; de laMotte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Westergren-Thorsson, G.; Särnstrand, B.; Fransson, L.-Å.; Malmström, A. TGF-b enhances the production of hyaluronan in human lung but not in skin fibroblasts. Exp. Cell Res. 1990, 186, 192–195. [Google Scholar] [CrossRef]
- Audam, T.N.; Howard, C.M.; Little, D.T.; Garrett, L.F.; Zheng, Y.w.; Gu, Z.; Brittian, K.R.; Gray, R.; Chariker, J.; Singhal, R.A.; et al. Hyaluronan provokes inflammation but suppresses phagocytotic function in macrophages. J. Mol. Cell Cardiol. 2025, 198, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Arif, A.; Olsson, M.; Cali, V.; Hardman, B.; Dosanjh, M.; Lauer, M.; Midura, R.J.; Hascall, V.C.; Brown, K.L.; et al. Endotoxin free hyaluronan and hyaluronan fragments do not stimulate TNF-alpha, interleukin-12 or upregulate co-stimulatory molecules in dendritic cells or macrophages. Sci. Rep. 2016, 6, 36928. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Poon, G.F.T.; Arif, A.A.; Lee-Sayer, S.S.M.; Dosanjh, M.; Johnson, P. The survival of fetal and bone marrow monocyte-derived alveolar macrophages is promoted by CD44 and its interaction with hyaluronan. Mucosal Immunol. 2018, 11, 601–614. [Google Scholar] [CrossRef]
- Jenkins, R.H.; Thomas, G.J.; Williams, J.D.; Steadman, R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J. Biol. Chem. 2004, 279, 41453–41460. [Google Scholar] [CrossRef]
- Auvinen, P.; Rilla, K.; Tumelius, R.; Tammi, M.; Sironen, R.; Soini, Y.; Kosma, V.M.; Mannermaa, A.; Viikari, J.; Tammi, R. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res. Treat. 2014, 143, 277–286. [Google Scholar] [CrossRef]
- Liu, N.; Gao, F.; Han, Z.; Xu, X.; Underhill, C.B.; Zhang, L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res. 2001, 61, 5207–5214. [Google Scholar]
- Kosaki, R.; Watanabe, K.; Yamaguchi, Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999, 59, 1141–1145. [Google Scholar] [PubMed]
- Posey, J.T.; Soloway, M.S.; Ekici, S.; Sofer, M.; Civantos, F.; Duncan, R.C.; Lokeshwar, V.B. Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res. 2003, 63, 2638–2644. [Google Scholar]
- Udabage, L.; Brownlee, G.R.; Nilsson, S.K.; Brown, T.J. The over-expression of HAS2, hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp. Cell Res. 2005, 310, 205–217. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Itano, N. Hyaluronan: A modulator of the tumor microenvironment. Cancer Lett. 2016, 375, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Irvine, K.D. The Hippo Signaling Network and Its Biological Functions. Annu. Rev. Genet. 2018, 52, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Ooki, T.; Murata-Kamiya, N.; Takahashi-Kanemitsu, A.; Wu, W.; Hatakeyama, M. High-molecular-weight hyaluronan is a hippo pathway ligand directing cel density-dependent growth inhibition via PAR1b. Dev. Cell 2019, 49, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gehlot, P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr. Opin. Pharmacol. 2009, 9, 351–369. [Google Scholar] [CrossRef]
- Shawki, S.; Ashburn, J.; Signs, S.A.; Huang, E. Colon Cancer: Inflammation-Associated Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 269–287. [Google Scholar] [CrossRef]
- O’Connor, P.; Lapointe, T.K.; Beck, P.L.; Buret, A.G. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 6, 1411–1420. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, L.; Zhang, X. NF-κB Signaling Pathway, Inflammation and Colorectal Cancer. Cell Mol. Immunol. 2009, 6, 327–334. [Google Scholar] [CrossRef]
- Pikarsky, E.; Porat, R.; Stein, I.; Abramovitch, R.; Amit, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrostek, L.; Cylwik, B. Hyaluronic Acid in Immune Response. Biomolecules 2025, 15, 1008. https://doi.org/10.3390/biom15071008
Chrostek L, Cylwik B. Hyaluronic Acid in Immune Response. Biomolecules. 2025; 15(7):1008. https://doi.org/10.3390/biom15071008
Chicago/Turabian StyleChrostek, Lech, and Bogdan Cylwik. 2025. "Hyaluronic Acid in Immune Response" Biomolecules 15, no. 7: 1008. https://doi.org/10.3390/biom15071008
APA StyleChrostek, L., & Cylwik, B. (2025). Hyaluronic Acid in Immune Response. Biomolecules, 15(7), 1008. https://doi.org/10.3390/biom15071008





