The Immune Regulatory Functions of CD226 and Its Implications in Immune-Mediated Diseases
Abstract
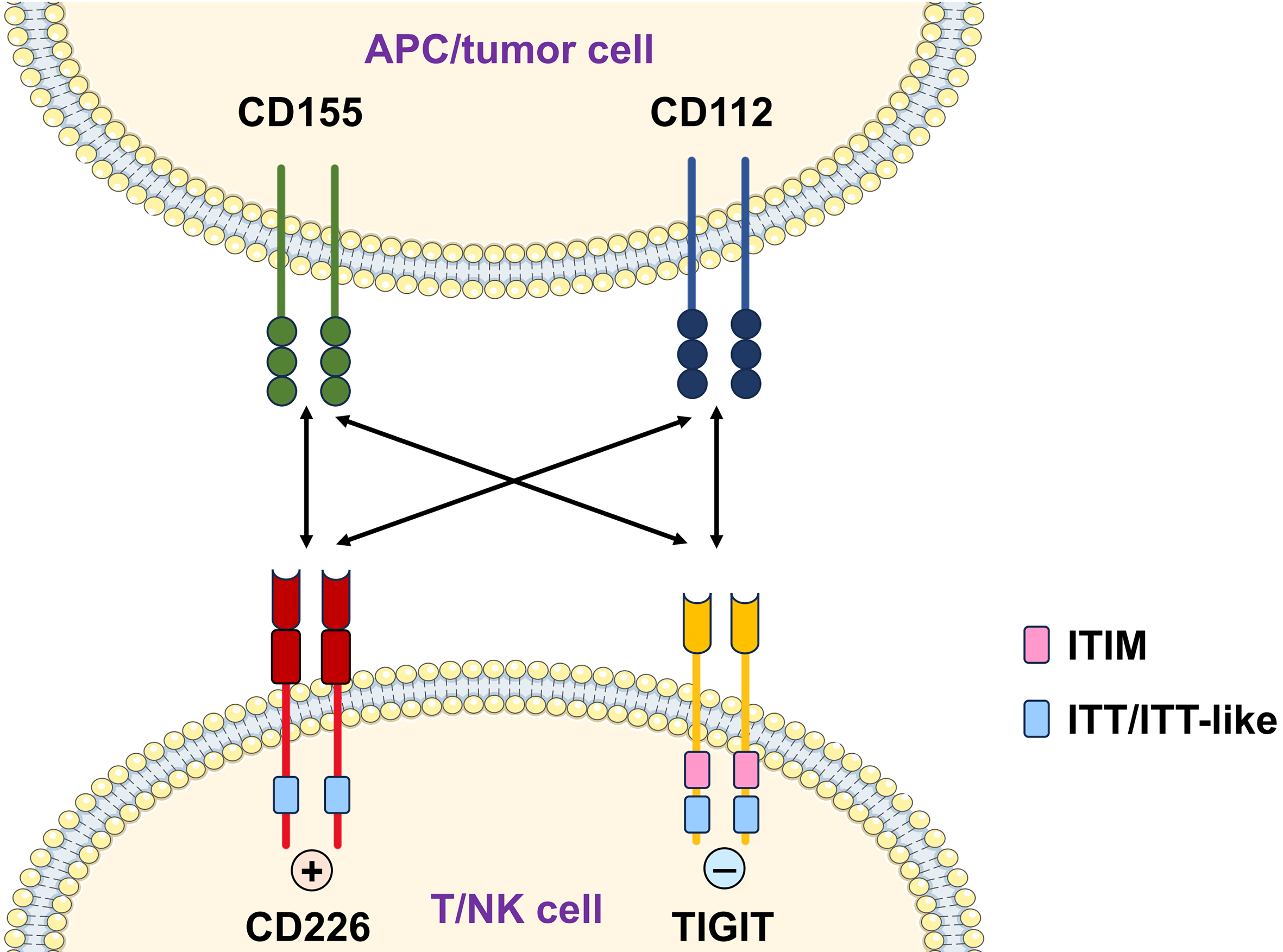
1. Introduction
2. Effects of CD226 in Immune Cells
2.1. CD226 and T Cells
2.2. CD226 and NK Cells
2.3. CD226 and Other Immune Cells
3. The Roles of CD226 in Immune-Mediated Disorders
3.1. CD226 and Tumors
3.1.1. CD226 Expression and Prognostic Significance
3.1.2. CD226 and Immune Checkpoint Interactions
3.1.3. CD226 in Tumor Immune Evasion
3.1.4. CD226 as a Therapeutic Target
3.2. CD226 and Infectious Diseases
3.3. CD226 and Allergic Diseases
3.4. CD226 and Autoimmune Diseases
3.4.1. Rheumatoid Arthritis
3.4.2. Systemic Lupus Erythematosus
3.4.3. Systemic Sclerosis
3.4.4. Primary Sjögren’s Syndrome
3.4.5. Type 1 Diabetes
3.4.6. Multiple Sclerosis and Neuromyelitis Optica
3.4.7. Other Autoimmune Diseases
4. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- de Andrade, L.F.; Smyth, M.J.; Martinet, L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol. Cell Biol. 2014, 92, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, N.; Lu, Y.; Davidson, D.; Colonna, M.; Veillette, A. DNAM-1 controls NK cell activation via an ITT-like motif. J. Exp. Med. 2015, 212, 2165–2182. [Google Scholar] [CrossRef]
- Liu, J.; Qian, X.; Chen, Z.; Xu, X.; Gao, F.; Zhang, S.; Zhang, R.; Qi, J.; Gao, G.F.; Yan, J. Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J. Immunol. 2012, 188, 5511–5520. [Google Scholar] [CrossRef]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Zhang, S.; Li, Y.; Tan, S.; Gao, G.F. Binding mode of the side-by-side two-IgV molecule CD226/DNAM-1 to its ligand CD155/Necl-5. Proc. Natl. Acad. Sci. USA 2019, 116, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Kanada, H.; Shimizu, S.; Kasama, E.; Shibuya, K.; Nakauchi, H.; Nagasawa, T.; Shibuya, A. CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. J. Biol. Chem. 2003, 278, 36748–36753. [Google Scholar] [CrossRef]
- Bachelet, I.; Munitz, A.; Mankutad, D.; Levi-Schaffer, F. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): A novel interface in the allergic process. J. Biol. Chem. 2006, 281, 27190–27196. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Georgoudaki, A.M.; Chambers, B.J.; Qiu, Q.; Kremmer, E.; Maier, M.K.; Czeloth, N.; Ravens, I.; Foerster, R.; Bernhardt, G. Heterogeneous expression of the adhesion receptor CD226 on murine NK and T cells and its function in NK-mediated killing of immature dendritic cells. J. Leukoc. Biol. 2009, 86, 91–101. [Google Scholar] [CrossRef]
- Kraus, A.K.; Chen, J.; Edenhofer, I.; Ravens, I.; Gaspert, A.; Cippa, P.E.; Mueller, S.; Wuthrich, R.P.; Segerer, S.; Bernhardt, G.; et al. The Role of T Cell Costimulation via DNAM-1 in Kidney Transplantation. PLoS ONE 2016, 11, e0147951. [Google Scholar] [CrossRef]
- Gilfillan, S.; Chan, C.J.; Cella, M.; Haynes, N.M.; Rapaport, A.S.; Boles, K.S.; Andrews, D.M.; Smyth, M.J.; Colonna, M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 2008, 205, 2965–2973. [Google Scholar] [CrossRef]
- Zeng, T.; Cao, Y.; Jin, T.; Tian, Y.; Dai, C.; Xu, F. The CD112R/CD112 axis: A breakthrough in cancer immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 285. [Google Scholar] [CrossRef]
- Shibuya, A.; Lanier, L.L.; Phillips, J.H. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor. J. Immunol. 1998, 161, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Lanier, L.L.; Phillips, J.H.; Ochs, H.D.; Shimizu, K.; Nakayama, E.; Nakauchi, H.; Shibuya, A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity 1999, 11, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; Duan, C.; Wu, S.; Xie, Y.; Yang, L.; Li, X.; Wang, Y.; Zhang, Y.; Zhuang, R. CD226 knockout reduces the development of CD8+ T by impairing the TCR sensitivity of double-positive thymocytes. Immunology 2023, 169, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Gaud, G.; Roncagalli, R.; Chaoui, K.; Bernard, I.; Familiades, J.; Colacios, C.; Kassem, S.; Monsarrat, B.; Burlet-Schiltz, O.; de Peredo, A.G.; et al. The costimulatory molecule CD226 signals through VAV1 to amplify TCR signals and promote IL-17 production by CD4(+) T cells. Sci. Signal. 2018, 11, eaar3083. [Google Scholar] [CrossRef]
- Georgiev, H.; Ravens, I.; Shibuya, A.; Forster, R.; Bernhardt, G. CD155/CD226-interaction impacts on the generation of innate CD8(+) thymocytes by regulating iNKT-cell differentiation. Eur. J. Immunol. 2016, 46, 993–1003. [Google Scholar] [CrossRef]
- Danisch, S.; Qiu, Q.; Seth, S.; Ravens, I.; Dorsch, M.; Shibuya, A.; Shibuya, K.; Forster, R.; Bernhardt, G. CD226 interaction with CD155 impacts on retention and negative selection of CD8 positive thymocytes as well as T cell differentiation to follicular helper cells in Peyer’s Patches. Immunobiology 2013, 218, 152–158. [Google Scholar] [CrossRef]
- Lozano, E.; Joller, N.; Cao, Y.; Kuchroo, V.K.; Hafler, D.A. The CD226/CD155 interaction regulates the proinflammatory (Th1/Th17)/anti-inflammatory (Th2) balance in humans. J. Immunol. 2013, 191, 3673–3680. [Google Scholar] [CrossRef]
- Dardalhon, V.; Schubart, A.S.; Reddy, J.; Meyers, J.H.; Monney, L.; Sabatos, C.A.; Ahuja, R.; Nguyen, K.; Freeman, G.J.; Greenfield, E.A.; et al. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J. Immunol. 2005, 175, 1558–1565. [Google Scholar] [CrossRef]
- Shibuya, K.; Shirakawa, J.; Kameyama, T.; Honda, S.; Tahara-Hanaoka, S.; Miyamoto, A.; Onodera, M.; Sumida, T.; Nakauchi, H.; Miyoshi, H.; et al. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J. Exp. Med. 2003, 198, 1829–1839. [Google Scholar] [CrossRef]
- Yasutomi, M.; Christiaansen, A.F.; Imai, N.; Martin-Orozco, N.; Forst, C.V.; Chen, G.; Ueno, H. CD226 and TIGIT Cooperate in the Differentiation and Maturation of Human Tfh Cells. Front. Immunol. 2022, 13, 840457. [Google Scholar] [CrossRef]
- Morandi, E.; Adoue, V.; Bernard, I.; Friebel, E.; Nunez, N.; Aubert, Y.; Masson, F.; Dejean, A.S.; Becher, B.; Astier, A.; et al. Impact of the Multiple Sclerosis-Associated Genetic Variant CD226 Gly307Ser on Human CD8 T-Cell Functions. Neurol. (R) Neuroimmunol. Neuroinflamm. 2024, 11, e200306. [Google Scholar] [CrossRef] [PubMed]
- Sunina, M.; Alnek, K.; Kisand, K.; Uibo, R. Human CD4(+) and CD8(+) T lymphocyte subpopulations have significantly different surface expression patterns of CD226 and TIGIT molecules. Scand. J. Immunol. 2021, 94, e13089. [Google Scholar] [CrossRef]
- Thirawatananond, P.; Brown, M.E.; Sachs, L.K.; Arnoletti, J.M.; Yeh, W.I.; Posgai, A.L.; Shapiro, M.R.; Chen, Y.G.; Brusko, T.M. Treg-Specific CD226 Deletion Reduces Diabetes Incidence in NOD Mice by Improving Regulatory T-Cell Stability. Diabetes 2023, 72, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamashita-Kanemaru, Y.; Abe, F.; Murata, R.; Nakamura-Shinya, Y.; Kanemaru, K.; Muratani, M.; Veillette, A.; Goto, M.; Ito, M.; et al. DNAM-1 regulates Foxp3 expression in regulatory T cells by interfering with TIGIT under inflammatory conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2021309118. [Google Scholar] [CrossRef]
- Ma, J.; Hu, W.; Liu, Y.; Duan, C.; Zhang, D.; Wang, Y.; Cheng, K.; Yang, L.; Wu, S.; Jin, B.; et al. CD226 maintains regulatory T cell phenotype stability and metabolism by the mTOR/Myc pathway under inflammatory conditions. Cell Rep. 2023, 42, 113306. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Duan, C.; Ma, J.; Hu, W.; Xie, Y.; Yang, L.; Wang, T.; Wu, S.; Li, X.; Wang, Y.; et al. Costimulatory Molecule CD226 Regulates Atopic Dermatitis in a Mouse Model. J. Investig. Dermatol. 2024, 144, 1743–1753. [Google Scholar] [CrossRef]
- Wang, T.; Qiao, W.; Xie, Y.; Ma, J.; Hu, W.; Yang, L.; Li, X.; Duan, C.; Wu, S.; Wang, Y.; et al. CD226 deficiency exacerbated intestinal immune dysregulation in mice with dinitrochlorobenzene-induced atopic dermatitis. Immunology 2023, 169, 431–446. [Google Scholar] [CrossRef]
- Wang, N.; Chen, P.; Song, Y.; Shen, Y.; Li, J.; Li, X.; Fang, L.; Chen, L. CD226 deficiency attenuates the homeostasis and suppressive capacity of Tr1 cells. Mol. Immunol. 2021, 132, 192–198. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, B.R.; Lee, H.K.; Lee, J.H.; Kim, K.H.; Choi, J.E.; Ji, A.Y.; Hong, J.T.; Kim, Y.; Han, S.B. Cd226(-/-) natural killer cells fail to establish stable contacts with cancer cells and show impaired control of tumor metastasis in vivo. Oncoimmunology 2017, 6, e1338994. [Google Scholar] [CrossRef]
- Hou, S.; Ge, K.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. CD226 protein is involved in immune synapse formation and triggers Natural Killer (NK) cell activation via its first extracellular domain. J. Biol. Chem. 2014, 289, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; de Almeida, P.; Manieri, N.; de Almeida Nagata, D.; Wu, T.D.; Harden Bowles, K.; Arumugam, V.; Schartner, J.; Cubas, R.; Mittman, S.; et al. CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1. Proc. Natl. Acad. Sci. USA 2018, 115, E11731–E11740. [Google Scholar] [CrossRef]
- Martinet, L.; Ferrari De Andrade, L.; Guillerey, C.; Lee, J.S.; Liu, J.; Souza-Fonseca-Guimaraes, F.; Hutchinson, D.S.; Kolesnik, T.B.; Nicholson, S.E.; Huntington, N.D.; et al. DNAM-1 expression marks an alternative program of NK cell maturation. Cell Rep. 2015, 11, 85–97. [Google Scholar] [CrossRef]
- Enqvist, M.; Ask, E.H.; Forslund, E.; Carlsten, M.; Abrahamsen, G.; Beziat, V.; Andersson, S.; Schaffer, M.; Spurkland, A.; Bryceson, Y.; et al. Coordinated expression of DNAM-1 and LFA-1 in educated NK cells. J. Immunol. 2015, 194, 4518–4527. [Google Scholar] [CrossRef] [PubMed]
- Iguchi-Manaka, A.; Kai, H.; Yamashita, Y.; Shibata, K.; Tahara-Hanaoka, S.; Honda, S.; Yasui, T.; Kikutani, H.; Shibuya, K.; Shibuya, A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J. Exp. Med. 2008, 205, 2959–2964. [Google Scholar] [CrossRef]
- Byemerwa, J.; Chang, C.Y.; McDonnell, D.P. The Roles of Natural Killer Cells in Breast Cancer Pathobiology and their Regulation by Estrogens. Endocr. Rev. 2025, bnaf014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Jin, J.Y.; Li, G.H.; Guo, Y.Z.; Yi, H.Y.; Zhang, J.R.; Lu, Y.J.; Zhang, J.L.; Li, C.Y.; et al. CD226 deletion improves post-infarction healing via modulating macrophage polarization in mice. Theranostics 2020, 10, 2422–2435. [Google Scholar] [CrossRef]
- Ma, J.; Hu, W.; Zhang, D.; Xie, J.; Duan, C.; Liu, Y.; Wang, Y.; Xu, X.; Cheng, K.; Jin, B.; et al. CD226 knockout alleviates high-fat diet induced obesity by suppressing proinflammatory macrophage phenotype. J. Transl. Med. 2021, 19, 477. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Li, J.; Shen, Y.; Hou, Y.; Fu, Z.; Fang, L.; Jin, B.; Chen, L. CD226 promotes renal fibrosis by regulating macrophage activation and migration. J. Leukoc. Biol. 2024, 116, 103–117. [Google Scholar] [CrossRef]
- Nagayama-Hasegawa, Y.; Honda, S.I.; Shibuya, A.; Shibuya, K. Expression and function of DNAM-1 on human B-lineage cells. Cytom. Part B Clin. Cytom. 2020, 98, 368–374. [Google Scholar] [CrossRef]
- Huang, H.; Huang, Z.; Ge, J.; Yang, J.; Chen, J.; Xu, B.; Wu, S.; Zheng, X.; Chen, L.; Zhang, X.; et al. CD226 identifies functional CD8(+)T cells in the tumor microenvironment and predicts a better outcome for human gastric cancer. Front. Immunol. 2023, 14, 1150803. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.X.; Gao, J.P.; Huang, Y.K.; Huang, H. Tumor-infiltrating CD226(+)CD8(+) T cells are associated with postoperative prognosis and adjuvant chemotherapeutic benefits in gastric cancer patients. J. Cancer Res. Clin. Oncol. 2023, 149, 4381–4389. [Google Scholar] [CrossRef] [PubMed]
- Viot, J.; Abdeljaoued, S.; Vienot, A.; Seffar, E.; Spehner, L.; Bouard, A.; Asgarov, K.; Pallandre, J.R.; Renaude, E.; Klajer, E.; et al. CD8(+) CD226(high) T cells in liver metastases dictate the prognosis of colorectal cancer patients treated with chemotherapy and radical surgery. Cell. Mol. Immunol. 2023, 20, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, L.; Wang, H.X.; Cui, S.P.; Gao, Y.; Hu, B.; Zhou, L.; Lang, R. Anti-PD-1 therapy reverses TIGIT(+)CD226(+)NK depletion in immunotherapy resistance of hepatocellular carcinoma through PVR/TIGIT pathway. Int. Immunopharmacol. 2024, 130, 111681. [Google Scholar] [CrossRef] [PubMed]
- Weulersse, M.; Asrir, A.; Pichler, A.C.; Lemaitre, L.; Braun, M.; Carrie, N.; Joubert, M.V.; Le Moine, M.; Do Souto, L.; Gaud, G.; et al. Eomes-Dependent Loss of the Co-activating Receptor CD226 Restrains CD8(+) T Cell Anti-tumor Functions and Limits the Efficacy of Cancer Immunotherapy. Immunity 2020, 53, 824–839.e10. [Google Scholar] [CrossRef] [PubMed]
- Guillamon, C.F.; Martinez-Sanchez, M.V.; Gimeno, L.; Campillo, J.A.; Server-Pastor, G.; Martinez-Garcia, J.; Martinez-Escribano, J.; Torroba, A.; Ferri, B.; Abellan, D.J.; et al. Activating KIRs on Educated NK Cells Support Downregulation of CD226 and Inefficient Tumor Immunosurveillance. Cancer Immunol. Res. 2019, 7, 1307–1317. [Google Scholar] [CrossRef]
- Sanchez-Correa, B.; Gayoso, I.; Bergua, J.M.; Casado, J.G.; Morgado, S.; Solana, R.; Tarazona, R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol. Cell Biol. 2012, 90, 109–115. [Google Scholar] [CrossRef]
- Peng, Y.P.; Xi, C.H.; Zhu, Y.; Yin, L.D.; Wei, J.S.; Zhang, J.J.; Liu, X.C.; Guo, S.; Fu, Y.; Miao, Y. Altered expression of CD226 and CD96 on natural killer cells in patients with pancreatic cancer. Oncotarget 2016, 7, 66586–66594. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Shahbaz, S.; Peters, A.C.; Gibson, S.B.; Elahi, S. Polyfunctional CD8(+)CD226(+)RUNX2(hi) effector T cells are diminished in advanced stages of chronic lymphocytic leukemia. Mol. Oncol. 2025, 19, 1347–1370. [Google Scholar] [CrossRef]
- Wang, X.; Mou, W.; Han, W.; Xi, Y.; Chen, X.; Zhang, H.; Qin, H.; Wang, H.; Ma, X.; Gui, J. Diminished cytolytic activity of gammadelta T cells with reduced DNAM-1 expression in neuroblastoma patients. Clin. Immunol. 2019, 203, 63–71. [Google Scholar] [CrossRef]
- Han, B.; Mao, F.Y.; Zhao, Y.L.; Lv, Y.P.; Teng, Y.S.; Duan, M.; Chen, W.; Cheng, P.; Wang, T.T.; Liang, Z.Y.; et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 Expression on Circulating NK Cells Is Associated with Tumor Progression in Human Gastric Cancer. J. Immunol. Res. 2018, 2018, 6248590. [Google Scholar] [CrossRef]
- Ma, P.; Sun, W. Integrated single-cell and bulk sequencing analyses with experimental validation identify the prognostic and immunological implications of CD226 in pan-cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 14597–14617. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Lv, G.; Li, J.; Zhang, J.F.; Zhou, P. CD226 rs727088A>G polymorphism increases the susceptibility to gastric cancer in Chinese populations. Gene 2015, 557, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.X.; Peng, Y.; Li, W.M. CD226 gene polymorphisms are associated with non-small-cell lung cancer in the Chinese Han population. Ther. Clin. Risk Manag. 2015, 11, 1259–1264. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, S.S.; Hong, M.J.; Choi, J.E.; Kim, S.; Kang, H.G.; Do, S.K.; Kim, J.H.; Baek, S.A.; Lee, W.K.; et al. Impact of immune checkpoint gene CD155 Ala67Thr and CD226 Gly307Ser polymorphisms on small cell lung cancer clinical outcome. Sci. Rep. 2021, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Worboys, J.D.; Vowell, K.N.; Hare, R.K.; Ambrose, A.R.; Bertuzzi, M.; Conner, M.A.; Patel, F.P.; Zammit, W.H.; Gali-Moya, J.; Hazime, K.S.; et al. TIGIT can inhibit T cell activation via ligation-induced nanoclusters, independent of CD226 co-stimulation. Nat. Commun. 2023, 14, 5016. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Ko, M.; Choi, D.S.; Kim, J.H.; Lee, D.H.; Kang, S.H.; Kim, I.; Lee, H.J.; Choi, E.K.; Kim, K.P.; et al. CD226(hi)CD8(+) T Cells Are a Prerequisite for Anti-TIGIT Immunotherapy. Cancer Immunol. Res. 2020, 8, 912–925. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Chauvin, J.M.; Ka, M.; Davar, D.; Pagliano, O.; Wang, H.; Saada, S.; Menna, C.; Amin, R.; et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 2018, 3, e121157. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, T.; Zhao, Y.; Du, J.; Chen, J.; Lai, J.; Xu, L.; Chen, S.; Zhong, X.; Wu, X.; et al. Higher TIGIT(+)CD226(−) gammadelta T cells in Patients with Acute Myeloid Leukemia. Immunol. Investig. 2022, 51, 40–50. [Google Scholar] [CrossRef]
- Braun, M.; Aguilera, A.R.; Sundarrajan, A.; Corvino, D.; Stannard, K.; Krumeich, S.; Das, I.; Lima, L.G.; Meza Guzman, L.G.; Li, K.; et al. CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8(+) T Cells. Immunity 2020, 53, 805–823. [Google Scholar] [CrossRef]
- Mantovani, S.; Varchetta, S.; Mele, D.; Maiello, R.; Donadon, M.; Soldani, C.; Franceschini, B.; Torzilli, G.; Tartaglia, G.; Maestri, M.; et al. Defective DNAM-1 Dependent Cytotoxicity in Hepatocellular Carcinoma-Infiltrating NK Cells. Cancers 2022, 14, 4060. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Ferrari de Andrade, L.; Vuckovic, S.; Miles, K.; Ngiow, S.F.; Yong, M.C.; Teng, M.W.; Colonna, M.; Ritchie, D.S.; Chesi, M.; et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J. Clin. Investig. 2015, 125, 2077–2089. [Google Scholar] [CrossRef]
- Dastouri, M.; Kilic, N.; Yilmaz, H. The apoptotic effects of NK-92 cells stimulated with an anti-CD226 antibody on MDA-MB-231 triple-negative breast cancer cells. Med. Oncol. 2023, 40, 228. [Google Scholar] [CrossRef] [PubMed]
- Sayitoglu, E.C.; Georgoudaki, A.M.; Chrobok, M.; Ozkazanc, D.; Josey, B.J.; Arif, M.; Kusser, K.; Hartman, M.; Chinn, T.M.; Potens, R.; et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Sang, L.; Zhu, J.; Han, X.; Shan, F.; Li, S.; Zhai, J.; Wang, D.; Lu, C.; et al. Enhanced therapeutic effects against murine colon carcinoma induced by a Colon 26/Ag85A-CD226 tumor cell vaccine. Oncol. Rep. 2015, 34, 1795–1804. [Google Scholar] [CrossRef][Green Version]
- Hou, S.; Zheng, X.; Wei, H.; Tian, Z.; Sun, R. Recombinant soluble CD226 protein directly inhibits cancer cell proliferation in vitro. Int. Immunopharmacol. 2014, 19, 119–126. [Google Scholar] [CrossRef]
- Takahashi, N.; Sugaya, M.; Suga, H.; Oka, T.; Kawaguchi, M.; Miyagaki, T.; Fujita, H.; Inozume, T.; Sato, S. Increased Soluble CD226 in Sera of Patients with Cutaneous T-Cell Lymphoma Mediates Cytotoxic Activity against Tumor Cells via CD155. J. Investig. Dermatol. 2017, 137, 1766–1773. [Google Scholar] [CrossRef]
- Di Pace, A.L.; Tumino, N.; Besi, F.; Alicata, C.; Conti, L.A.; Munari, E.; Maggi, E.; Vacca, P.; Moretta, L. Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor In Exosome-Mediated Cytotoxicity Against Tumor. Cancers 2020, 12, 661. [Google Scholar] [CrossRef]
- Tang, K.; Hou, Y.; Cheng, L.; Zhang, Y.; Li, J.; Qin, Q.; Zheng, X.; Jia, X.; Zhang, C.; Zhuang, R.; et al. Increased blood CD226(-) inflammatory monocytes with low antigen presenting potential correlate positively with severity of hemorrhagic fever with renal syndrome. Ann. Med. 2023, 55, 2247000. [Google Scholar] [CrossRef]
- Murillo, O.; Moreira, J.D.; Kujur, W.; Velasco-Alzate, K.; Sen Santara, S.; Konduru, N.V.; Mulik, S. Costimulatory CD226 Signaling Regulates Proliferation of Memory-like NK Cells in Healthy Individuals with Latent Mycobacterium tuberculosis Infection. Int. J. Mol. Sci. 2022, 23, 12838. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.; Fei, Q.; Shao, X.; Lv, W.; Yang, J.; Xu, F.; Shi, J. Upregulation of CD226 on subsets of T cells and NK cells is associated with upregulated adhesion molecules and cytotoxic factors in patients with tuberculosis. Int. Immunopharmacol. 2023, 120, 110360. [Google Scholar] [CrossRef]
- Redlberger-Fritz, M.; Vietzen, H.; Puchhammer-Stockl, E. Association of Severe Influenza Virus Infections With CD226 (DNAM-1) Variants. J. Infect. Dis. 2019, 220, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, J.; Scharf, L.; Frederiksen, J.; Naji, A.; Ljunggren, H.G.; Sonnerborg, A.; Lund, O.; Reyes-Teran, G.; Hecht, F.M.; Deeks, S.G.; et al. Perturbed CD8(+) T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Sci. Rep. 2017, 7, 40354. [Google Scholar] [CrossRef]
- Cella, M.; Presti, R.; Vermi, W.; Lavender, K.; Turnbull, E.; Ochsenbauer-Jambor, C.; Kappes, J.C.; Ferrari, G.; Kessels, L.; Williams, I.; et al. Loss of DNAM-1 contributes to CD8+ T-cell exhaustion in chronic HIV-1 infection. Eur. J. Immunol. 2010, 40, 949–954. [Google Scholar] [CrossRef]
- Nabekura, T.; Kanaya, M.; Shibuya, A.; Fu, G.; Gascoigne, N.R.; Lanier, L.L. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity 2014, 40, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Teijaro, J.R.; Lewicki, H.A.; Colonna, M.; Oldstone, M.B. CD8 T cell defect of TNF-alpha and IL-2 in DNAM-1 deficient mice delays clearance in vivo of a persistent virus infection. Virology 2012, 429, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sakano, Y.; Sakano, K.; Hurrell, B.P.; Helou, D.G.; Shafiei-Jahani, P.; Kazemi, M.H.; Li, X.; Shen, S.; Hilser, J.R.; Hartiala, J.A.; et al. Blocking CD226 regulates type 2 innate lymphoid cell effector function and alleviates airway hyperreactivity. J. Allergy Clin. Immunol. 2024, 153, 1406–1422. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Zhang, X.; Duan, C.; Ma, J.; Wang, Y.; Wu, Y.; Shan, N.; Cheng, K.; Zhuang, R.; et al. CD226 implicated in Akt-dependent apoptosis of CD4(+) T cell contributes to asthmatic pathogenesis. Cell Death Dis. 2024, 15, 705. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Wang, T.; Liu, Y.; Ma, J.; Wu, S.; Duan, C.; Qiao, W.; Cheng, K.; Lu, L.; et al. Ablation of CD226 on CD4(+) T cells modulates asthma progress associated with altered IL-10 response and gut microbiota. Int. Immunopharmacol. 2023, 118, 110051. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Zhu, T.; Ma, J.; Duan, C.; Yang, L.; Wang, T.; Zhuang, R.; Bian, K.; Lu, L. CD226 Deficiency Alleviates Murine Allergic Rhinitis by Suppressing Group 2 Innate Lymphoid Cell Responses. Mediat. Inflamm. 2022, 2022, 1756395. [Google Scholar] [CrossRef]
- Mosaad, Y.M.; El-Toraby, E.E.; Tawhid, Z.M.; Abdelsalam, A.I.; Enin, A.F.; Hasson, A.M.; Shafeek, G.M. Association between CD226 polymorphism and soluble levels in rheumatoid arthritis: Relationship with clinical activity. Immunol. Investig. 2018, 47, 264–278. [Google Scholar] [CrossRef]
- Du, Y.; Shen, L.X.; Yu, L.K.; Song, Y.; Zhu, J.F.; Du, R. The CD226 gene in susceptibility of rheumatoid arthritis in the Chinese Han population. Rheumatol. Int. 2012, 32, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Zakeri, Z.; Eskandari-Nasab, E.; Atabaki, M.; Pourhosseini, S.M.; Jahantigh, M.; Bahari, G.; Taheri, M. CD226 rs763361 (Gly307Ser) polymorphism is associated with susceptibility to rheumatoid arthritis in Zahedan, southeast Iran. Iran. Biomed. J. 2013, 17, 194–199. [Google Scholar] [PubMed]
- Tan, R.J.; Gibbons, L.J.; Potter, C.; Hyrich, K.L.; Morgan, A.W.; Wilson, A.G.; Isaacs, J.D.; Barton, A.; Braggss. Investigation of rheumatoid arthritis susceptibility genes identifies association of AFF3 and CD226 variants with response to anti-tumour necrosis factor treatment. Ann. Rheum. Dis. 2010, 69, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Chiocchia, G.; Marchiol, C.; Lager, F.; Renault, G.; Colonna, M.; Bernhardt, G.; Allanore, Y.; Avouac, J. Targeting CD226/DNAX accessory molecule-1 (DNAM-1) in collagen-induced arthritis mouse models. J. Inflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Ayano, M.; Kushimoto, K.; Kawano, S.; Higashioka, K.; Inokuchi, S.; Mitoma, H.; Kimoto, Y.; Akahoshi, M.; Ono, N.; et al. Association of elevated serum soluble CD226 levels with the disease activity and flares of systemic lupus erythematosus. Sci. Rep. 2021, 11, 16162. [Google Scholar] [CrossRef]
- Nakano, M.; Ayano, M.; Kushimoto, K.; Kawano, S.; Higashioka, K.; Inokuchi, S.; Mitoma, H.; Kimoto, Y.; Akahoshi, M.; Ono, N.; et al. Increased Proportion of CD226(+) B Cells Is Associated With the Disease Activity and Prognosis of Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 713225. [Google Scholar] [CrossRef]
- Du, Y.; Tian, L.; Shen, L.X.; Wang, F.; Yu, L.K.; Song, Y.; Zhu, J.F.; Du, R. Association of the CD226 single nucleotide polymorphism with systemic lupus erythematosus in the Chinese Han population. Tissue Antigens 2011, 77, 65–67. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, B.; Zheng, S.G.; Li, X.; Sun, R.; Tian, Z.; Wei, H. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J. Immunol. 2011, 186, 3421–3431. [Google Scholar] [CrossRef]
- Avouac, J.; Elhai, M.; Tomcik, M.; Ruiz, B.; Friese, M.; Piedavent, M.; Colonna, M.; Bernhardt, G.; Kahan, A.; Chiocchia, G.; et al. Critical role of the adhesion receptor DNAX accessory molecule-1 (DNAM-1) in the development of inflammation-driven dermal fibrosis in a mouse model of systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 1089–1098. [Google Scholar] [CrossRef]
- Ayano, M.; Tsukamoto, H.; Kohno, K.; Ueda, N.; Tanaka, A.; Mitoma, H.; Akahoshi, M.; Arinobu, Y.; Niiro, H.; Horiuchi, T.; et al. Increased CD226 Expression on CD8+ T Cells Is Associated with Upregulated Cytokine Production and Endothelial Cell Injury in Patients with Systemic Sclerosis. J. Immunol. 2015, 195, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Dieude, P.; Guedj, M.; Truchetet, M.E.; Wipff, J.; Revillod, L.; Riemekasten, G.; Matucci-Cerinic, M.; Melchers, I.; Hachulla, E.; Airo, P.; et al. Association of the CD226 Ser(307) variant with systemic sclerosis: Evidence of a contribution of costimulation pathways in systemic sclerosis pathogenesis. Arthritis Rheum. 2011, 63, 1097–1105. [Google Scholar] [CrossRef]
- Abbasi, F.; Mansouri, R.; Gharibdoost, F.; Aslani, S.; Mostafaei, S.; Kavosi, H.; Poursani, S.; Sobhani, S.; Mahmoudi, M. Association Study of CD226 and CD247 Genes Single Nucleotide Polymorphisms in Iranian Patients with Systemic Sclerosis. Iran. J. Allergy Asthma Immunol. 2017, 16, 471–479. [Google Scholar]
- Deng, C.; Chen, Y.; Li, W.; Peng, L.; Luo, X.; Peng, Y.; Zhao, L.; Wu, Q.; Zhang, W.; Zhang, X.; et al. Alteration of CD226/TIGIT immune checkpoint on T cells in the pathogenesis of primary Sjogren’s syndrome. J. Autoimmun. 2020, 113, 102485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cheng, W.; Liu, C.; Peng, C.; Shen, Y.; Yang, Y.; Sun, C.; Chang, X.; Wu, J. Increased proportion of CD226 + CD14 + monocytes correlates with clinical features and laboratory parameters in patients with primary Sjogren’s syndrome. Int. J. Rheum. Dis. 2023, 26, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhao, P.; Song, S.; Yang, Y.; Peng, C.; Chang, X.; Liu, C. A novel enzyme-linked immunosorbent assay tool to evaluate plasma soluble CD226 in primary Sjogren’s syndrome. Anal. Biochem. 2024, 692, 115573. [Google Scholar] [CrossRef]
- Xi, J.S.; Nie, C.L.; Wang, J.; Ma, Y.; Ma, A.H. Association of CD226 polymorphisms with the susceptibility to type 1 diabetes in Chinese children. Genet. Mol. Res. 2015, 14, 15249–15255. [Google Scholar] [CrossRef]
- Mattana, T.C.; Santos, A.S.; Fukui, R.T.; Mainardi-Novo, D.T.; Costa, V.S.; Santos, R.F.; Matioli, S.R.; da Silva, M.E. CD226 rs763361 is associated with the susceptibility to type 1 diabetes and greater frequency of GAD65 autoantibody in a Brazilian cohort. Mediat. Inflamm. 2014, 2014, 694948. [Google Scholar] [CrossRef]
- Shapiro, M.R.; Yeh, W.I.; Longfield, J.R.; Gallagher, J.; Infante, C.M.; Wellford, S.; Posgai, A.L.; Atkinson, M.A.; Campbell-Thompson, M.; Lieberman, S.M.; et al. CD226 Deletion Reduces Type 1 Diabetes in the NOD Mouse by Impairing Thymocyte Development and Peripheral T Cell Activation. Front. Immunol. 2020, 11, 2180. [Google Scholar] [CrossRef]
- Brown, M.E.; Thirawatananond, P.; Peters, L.D.; Kern, E.J.; Vijay, S.; Sachs, L.K.; Posgai, A.L.; Brusko, M.A.; Shapiro, M.R.; Mathews, C.E.; et al. Inhibition of CD226 co-stimulation suppresses diabetes development in the NOD mouse by augmenting regulatory T cells and diminishing effector T cell function. Diabetologia 2025, 68, 397–418. [Google Scholar] [CrossRef]
- Zhong, T.; Li, X.; Lei, K.; Tang, R.; Deng, Q.; Love, P.E.; Zhou, Z.; Zhao, B.; Li, X. TGF-beta-mediated crosstalk between TIGIT(+) Tregs and CD226(+)CD8(+) T cells in the progression and remission of type 1 diabetes. Nat. Commun. 2024, 15, 8894. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Bucciarelli, F.; Angles, T.; Oster, A.C.; Cauboue, P.; Laviolette, K.; Mougenot, M.; Morandi, E.; Bernard, I.; Pignolet, B.; et al. Increased levels of circulating soluble CD226 in multiple sclerosis. Mult. Scler. 2024, 30, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, S.; Jin, J.; Fang, L.; Ma, Q.; Wang, X.; Song, Y.; Chen, L. CD226 attenuates Treg suppressive capacity via CTLA-4 and TIGIT during EAE. Immunol. Res. 2019, 67, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yi, H.; Fang, L.; Jin, J.; Ma, Q.; Shen, Y.; Li, J.; Liang, S.; Xiong, J.; Li, Z.; et al. CD226 Attenuates Treg Proliferation via Akt and Erk Signaling in an EAE Model. Front. Immunol. 2020, 11, 1883. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, H.; Zhang, Y.; Chen, K.; Zhang, C.; Song, C.; Fang, L.; Xu, Z.; Yang, K.; Jin, B.; et al. CD226 ligation protects against EAE by promoting IL-10 expression via regulation of CD4+ T cell differentiation. Oncotarget 2016, 7, 19251–19264. [Google Scholar] [CrossRef]
- Chen, P.; Wu, M.; Wang, N.; Xia, F.; Du, F.; Liu, Z.; Wang, J.; Jin, J.; Jin, B.; Zhao, G.; et al. Expression of CD226 is upregulated on Tr1 cells from neuromyelitis optica spectrum disorder patients. Brain Behav. 2022, 12, e2623. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Liu, H.; Li, Y.; Li, J.; Dai, Y.; Hu, X. CD226 Gly307Ser association with neuromyelitis optica in Southern Han Chinese. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2012, 39, 488–490. [Google Scholar] [CrossRef]
- Capozzi, A.; Manganelli, V.; Riitano, G.; Caissutti, D.; Longo, A.; Garofalo, T.; Sorice, M.; Misasi, R. Advances in the Pathophysiology of Thrombosis in Antiphospholipid Syndrome: Molecular Mechanisms and Signaling through Lipid Rafts. J. Clin. Med. 2023, 12, 891. [Google Scholar] [CrossRef]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Caissutti, D.; Longo, A.; Mattei, V.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; et al. Anti-beta2-GPI Antibodies Induce Endothelial Cell Expression of Tissue Factor by LRP6 Signal Transduction Pathway Involving Lipid Rafts. Cells 2022, 11, 1288. [Google Scholar] [CrossRef]
- Shirakawa, J.; Wang, Y.; Tahara-Hanaoka, S.; Honda, S.; Shibuya, K.; Shibuya, A. LFA-1-dependent lipid raft recruitment of DNAM-1 (CD226) in CD4+ T cell. Int. Immunol. 2006, 18, 951–957. [Google Scholar] [CrossRef]
- Long, Y.; Lu, K.J.; Xia, C.S.; Feng, J.H.; Li, W.Y.; Ma, Y.T.; Sun, Y.Y.; Fan, C.H.; Li, C. Altered CD226/TIGIT expressions were associated with NK phenotypes in primary antiphospholipid syndrome and affected by IL-4/JAK pathway. Clin. Exp. Immunol. 2024, 216, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Reinards, T.H.; Albers, H.M.; Brinkman, D.M.; Kamphuis, S.S.; van Rossum, M.A.; Girschick, H.J.; Wouters, C.; Hoppenreijs, E.P.; Saurenmann, R.K.; Hinks, A.; et al. CD226 (DNAM-1) is associated with susceptibility to juvenile idiopathic arthritis. Ann. Rheum. Dis. 2015, 74, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, C.; Yang, H.; Tian, X.; Chen, L.; Liu, Q.; Gao, C.; Lu, X.; Wang, G.; Peng, Q. Upregulation of the CD155-CD226 Axis Is Associated With Muscle Inflammation and Disease Severity in Idiopathic Inflammatory Myopathies. Neurol. R Neuroimmunol. Neuroinflamm. 2023, 10, e200143. [Google Scholar] [CrossRef] [PubMed]
- Hafler, J.P.; Maier, L.M.; Cooper, J.D.; Plagnol, V.; Hinks, A.; Simmonds, M.J.; Stevens, H.E.; Walker, N.M.; Healy, B.; Howson, J.M.; et al. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009, 10, 5–10. [Google Scholar] [CrossRef]
- Qiu, Z.X.; Zhang, K.; Qiu, X.S.; Zhou, M.; Li, W.M. CD226 Gly307Ser association with multiple autoimmune diseases: A meta-analysis. Hum. Immunol. 2013, 74, 249–255. [Google Scholar] [CrossRef]
| Cell Type | Roles of CD226 |
|---|---|
| Conventional T cells | Enhances CD8+ T cell maturation and memory formation [16,17]; promotes Th1/Th17 polarization via enhanced IL-17/IFN-γ production [18,19]; facilitates early Tfh differentiation and proliferation [21] |
| Treg cells | Dual roles: impairs stability/suppressive function [24,25] vs. maintains metabolic fitness/lineage stability [26] |
| NK cells | Enhances activation, cytotoxicity and stable target cell interactions [30,31]; modulates NK cell education [34] |
| Macrophages | Drives M1 polarization [37,38,39] |
| Disease Category | Disease/Context | Roles of CD226 |
|---|---|---|
| Tumor | Multiple Tumor Types | High CD226+ TILs infiltration correlate with better prognosis [41,42,43,44,45,46,47,48,49,50,51]; tumor-induced CD226 downregulation promotes immune evasion [60,61]; polymorphisms increase cancer susceptibility and influence treatment response [53,54,55] |
| Infectious Diseases | Viral Infections | Reduced expression on immune cells impairs antiviral immunity [69,73,74,75,76] |
| Tuberculosis | Elevated expression on NK/T cells enhances effector functions [70,71] | |
| Allergic Diseases | Asthma | Upregulation in Th2/Th17/ILC2s drives inflammation [77,78] |
| Allergic Rhinitis | Deficiency reduces Th2 cytokines, eosinophil recruitment, and inflammatory responses [80] | |
| Autoimmune Diseases | Rheumatoid Arthritis | rs763361 polymorphism confers susceptibility [81,82,83]; soluble CD226 correlates with disease activity [81] |
| Systemic Lupus Erythematosus | Increased soluble CD226 and CD226+ switched-memory B cells correlate with disease activity [86,87]; rs763361 polymorphism increases susceptibility [88] | |
| Systemic Sclerosis | Overexpression in skin tissue/CD8+ T cells drives pro-inflammatory cytokines secretion [90,91]; rs763361 T allele is genetic risk in Europeans [92] | |
| Primary Sjögren’s Syndrome | Increased proportions of CD226+ CD4+ T cells/monocytes correlate with disease severity [94,95]; reduced soluble CD226 inversely associates with disease progression [96] | |
| Type 1 Diabetes | rs763361 polymorphism increases susceptibility [97,98]; blockade delays insulitis onset [99,100,101] | |
| Multiple Sclerosis and Neuromyelitis Optica | Increased soluble CD226 correlates with neuroinflammation [102]; CD226 deficiency attenuates experimental autoimmune encephalomyelitis severity [103,104,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Liu, Y.; Xiong, H.; Ning, Z. The Immune Regulatory Functions of CD226 and Its Implications in Immune-Mediated Diseases. Biomolecules 2025, 15, 1007. https://doi.org/10.3390/biom15071007
Liu K, Liu Y, Xiong H, Ning Z. The Immune Regulatory Functions of CD226 and Its Implications in Immune-Mediated Diseases. Biomolecules. 2025; 15(7):1007. https://doi.org/10.3390/biom15071007
Chicago/Turabian StyleLiu, Keyan, Yuanzhen Liu, Huabao Xiong, and Zhaochen Ning. 2025. "The Immune Regulatory Functions of CD226 and Its Implications in Immune-Mediated Diseases" Biomolecules 15, no. 7: 1007. https://doi.org/10.3390/biom15071007
APA StyleLiu, K., Liu, Y., Xiong, H., & Ning, Z. (2025). The Immune Regulatory Functions of CD226 and Its Implications in Immune-Mediated Diseases. Biomolecules, 15(7), 1007. https://doi.org/10.3390/biom15071007





