Serum CS/DS, IGF-1, and IGFBP-3 as Biomarkers of Cartilage Remodeling in Juvenile Idiopathic Arthritis: Diagnostic and Therapeutic Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Biochemical Analysis
2.2.1. The Assay of the Concentration of CS/DS
2.2.2. The Assay of the Concentration of IGF-1 and IGFBP-3
2.3. The Statistical Analysis of the Results
3. Results
3.1. Serum Levels of CS/DS in Healthy Children and JIA Patients
3.2. Serum Levels of IGF-1 in Healthy Children and JIA Patients
3.3. Serum Levels of IGBP-3 in Healthy Children and JIA Patients
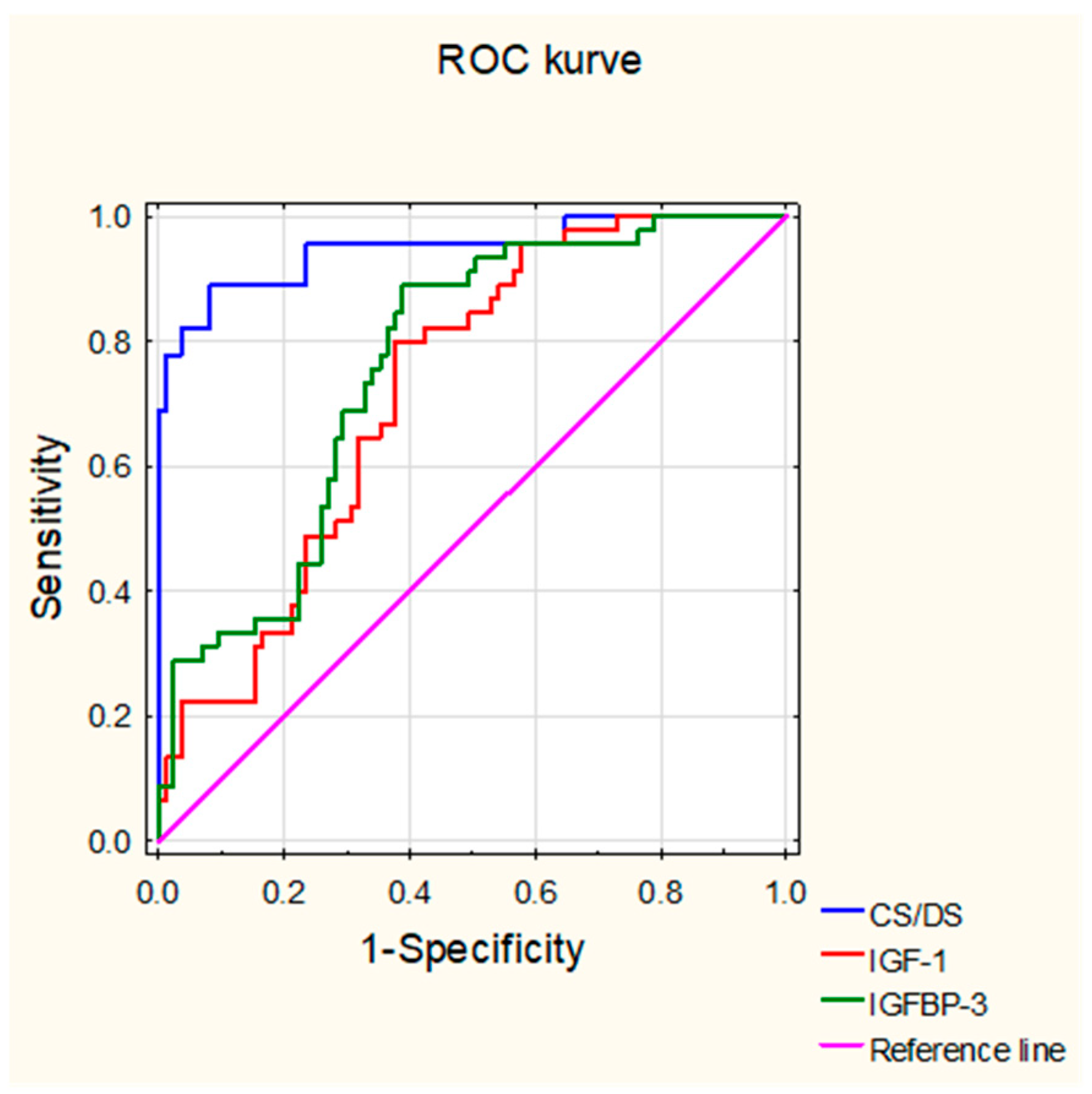
3.4. Usefulness of Levels of the Circulating CS/DS, IGF-1, and IGFBP-3 in the Diagnosis of JIA
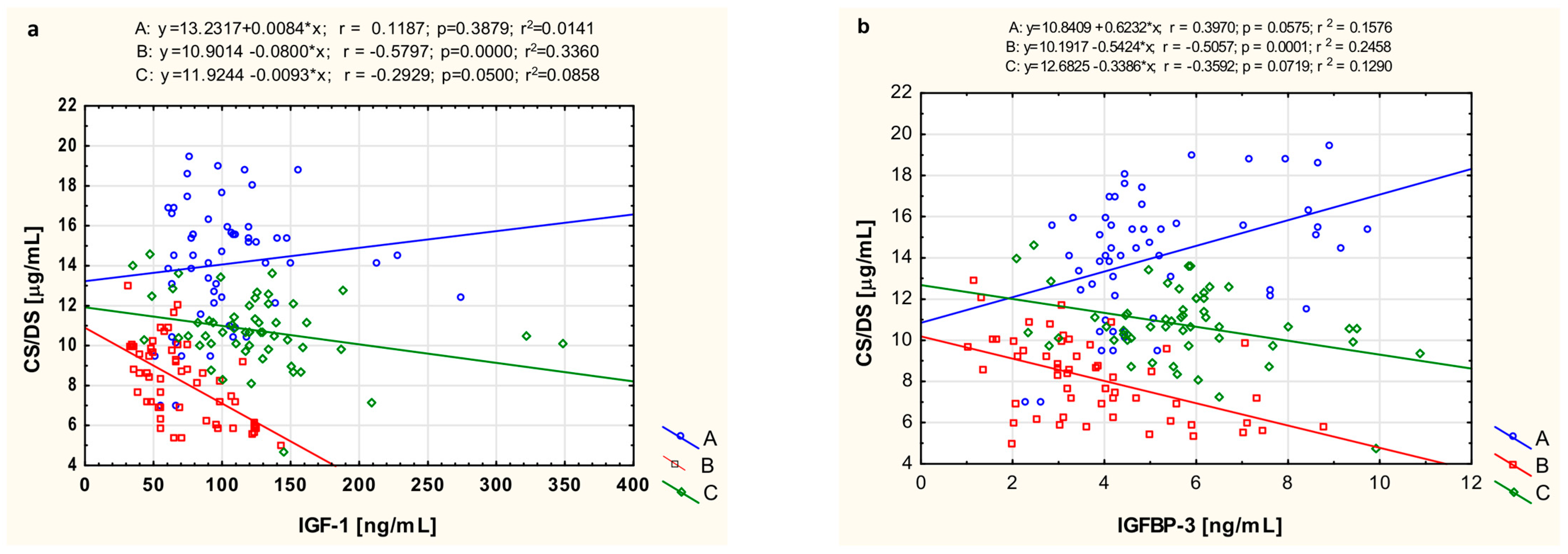
3.5. The Relationship of CS/DS with IGF-1 and IGFBP-3
3.6. The Relationship of CS/DS, IGF-1, IGFBP-3 with CRP and ESR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barut, K.; Adrovic, A.; Şahin, S.; Kasapçopur, Ö. Juvenile idiopathic arthritis. Balkan Med. J. 2017, 34, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Crayne, C.B.; Beukelman, T. Juvenile idiopathic arthritis: Oligoarthritis and polyarthritis. Pediatr. Clin. N. Am. 2018, 65, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Wojdas, M.; Dąbkowska, K.; Winsz-Szczotka, K. Alterations of extracellular matrix components in the course of juvenile idiopathic arthritis. Metabolites 2021, 25, 132. [Google Scholar] [CrossRef] [PubMed]
- Akioka, S. Interleukin-6 in juvenile idiopathic arthritis. Mod. Rheumatol. 2019, 29, 275–286. [Google Scholar] [CrossRef]
- Kostik, M.M.; Makhova, M.A.; Maletin, A.S.; Magomedova, S.M.; Sorokina, L.S.; Tsukasaki, M.; Okamoto, K.; Takayanagi, H.; Vasiliev, D.S.; Kozlova, D.I.; et al. Cytokine profile in patients with chronic non-bacterial osteomyelitis, juvenile idiopathic arthritis, and insulin-dependent diabetes mellitus. Cytokine 2021, 143, 155521. [Google Scholar] [CrossRef]
- Naba, A. Mechanisms of assembly and remodelling of the extracellular matrix. Nat. Rev. Mol. Cell Biol. 2024, 25, 865–885. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Vallet, S.D.; Clerc, O.; Ricard-Blum, S. Glycosaminoglycan-protein interactions: The first draft of the glycosaminoglycan interactome. J. Histochem. Cytochem. 2021, 69, 93–104. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Winsz-Szczotka, K.; Komosińska-Vassev, K.; Kuźnik-Trocha, K.; Siwiec, A.; Żegleń, B.; Olczyk, K. Circulating keratan sulfate as a marker of metabolic changes of cartilage proteoglycan in juvenile idiopathic arthritis; Influence of growth factors as well as proteolytic and prooxidative agents on aggrecan alterations. Clin. Chem. Lab. Med. 2015, 53, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Winsz-Szczotka, K.; Komosińska-Vassev, K.; Kuźnik-Trocha, K.; Gruenpeter, A.; Lachór-Motyka, I.; Olczyk, K. Influence of proteolytic-antiproteolytic enzymes and prooxidative-antioxidative factors on proteoglycan alterations in children with juvenile idiopathic arthritis. Clin. Biochem. 2014, 47, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Mullen, L.M.; Best, S.M.; Ghose, S.; Wardale, J.; Rushton, N.; Cameron, R.E. Bioactive IGF-1 release from collagen-GAG scaffold to enhance cartilage repair in vitro. J. Mater. Sci. Mater. Med. 2015, 26, 5325. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B. Insulin-like growth factor binding-protein-3 (IGFBP-3). Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bratulic, S.; Limeta, A.; Maccari, F.; Galeotti, F.; Volpi, N.; Levin, M.; Nielsen, J.; Gatto, F. Analysis of normal levels of free glycosaminoglycans in urine and plasma in adults. J. Biol. Chem. 2022, 298, 101575. [Google Scholar] [CrossRef]
- Jura-Półtorak, A.; Komosinska-Vassev, K.; Kotulska, A.; Kucharz, E.; Klimek, K.; Kopeć-Medrek, M.; Olczyk, K. Alterations of plasma glycosaminoglycan profile in patients with rheumatoid arthritis in relation to disease activity. Clin. Chim. Acta 2014, 433, 20–27. [Google Scholar] [CrossRef]
- Szeremeta, A.; Jura-Półtorak, A.; Koźma, E.M.; Głowacki, A.; Kucharz, E.J.; Kopeć-Mędrek, M.; Olczyk, K. Effects of a 15-month anti-TNF-α treatment on plasma levels of glycosaminoglycans in women with rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 211. [Google Scholar] [CrossRef]
- Koźma, E.M.; Kuźnik-Trocha, K.; Winsz-Szczotka, K.; Wisowski, G.; Olczyk, P.; Komosińska-Vassev, K.; Kasperczyk, M.; Olczyk, K. Significant remodeling affects the circulating glycosaminoglycan profile in adult patients with both severe and mild forms of acute pancreatitis. J. Clin. Med. 2020, 9, 1308. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, X.L.; Guo, L.; Bai, Y.P.; Tian, Y.; Luo, H. The function of the inter-alpha-trypsin inhibitors in the development of disease. Front. Med. 2024, 11, 1432224. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik-Trocha, K.; Winsz-Szczotka, K.; Lachór-Motyka, I.; Dąbkowska, K.; Wojdas, M.; Olczyk, K.; Komosińska-Vassev, K. The effects of TNF-α inhibition on the metabolism of cartilage: Relationship between KS, HA, HAPLN1 and ADAMTS4, ADAMTS5, TOS and TGF-β1 plasma concentrations in patients with juvenile idiopathic arthritis. J. Clin. Med. 2022, 11, 2013. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Ruggiero, L.; Cugat, R.; Domínguez, J.M. Proteoglycans in articular cartilage and their contribution to chondral injury and repair mechanisms. Int. J. Mol. Sci. 2023, 24, 10824. [Google Scholar] [CrossRef] [PubMed]
- Struglics, A.; Lohmander, L.S.; Last, K.; Akikusa, J.; Allen, R.; Fosang, A.J. Aggrecanase cleavage in juvenile idiopathic arthritis patients is minimally detected in the aggrecan interglobular domain but robust at the aggrecan C-terminus. Arthritis Rheum. 2012, 64, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.; Panko, N.; Rakovska, L.; Holovko, T. Connective tissue metabolism in patients with juvenile idiopathic arthritis: 10-year follow-up study. Pediatrics 2019, 6, 5–11. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Agarwal, S.; Misra, R.; Aggarwal, A. Synovial fluid RANKL and matrix metalloproteinase levels in enthesitis related arthritis subtype of juvenile idiopathic arthritis. Rheumatol. Int. 2009, 29, 907–911. [Google Scholar] [CrossRef]
- Brik, R.; Rosen, I.; Savulescu, D.; Borovoi, I.; Gavish, M.; Nagler, R. Salivary antioxidants and metalloproteinases in juvenile idiopathic arthritis. Mol. Med. 2010, 16, 122–128. [Google Scholar] [CrossRef]
- Gattorno, M.; Vignola, S.; Falcini, F.; Sabatini, F.; Buoncompagni, A.; Simonini, G.; Picco, P.; Pistoia, V. Serum and synovial fluid concentrations of matrix metalloproteinases 3 and its tissue inhibitor 1 in juvenile idiopathic arthritides. J. Rheumatol. 2002, 29, 826–831. [Google Scholar]
- Matsuyama, T. Tissue inhibitor of metalloproteinases-1 and matrix metalloproteinase-3 in Japanese healthy children and in Kawasaki disease and their clinical usefulness in juvenile rheumatoid arthritis. Pediatr. Int. 1999, 41, 239–245. [Google Scholar] [CrossRef]
- Peake, N.J.; Foster, H.E.; Khawaja, K.; Cawston, T.E.; Rowan, A.D. Assessment of the clinical significance of gelatinase activity in patients with juvenile idiopathic arthritis using quantitative protein substrate zymography. Ann. Rheum. Dis. 2006, 65, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Peake, N.J.; Khawaja, K.; Myers, A.; Jones, D.; Cawston, T.E.; Rowan, A.D.; Foster, O.N. Levels of matrix metalloproteinase (MMP)-1 in paired sera and synovial fluids of juvenile idiopathic arthritis patients: Relationship to inflammatory activity, MMP-3 and tissue inhibitor of metalloproteinases-1 in a longitudinal study. Rheumatology 2005, 44, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, V.; Myles, A.; Dayal, R.; Aggarwal, A. Levels of serum matrix metalloproteinase-3 correlate with disease activity in the enthesitis-related arthritis category of juvenile idiopathic arthritis. J. Rheumatol. 2011, 38, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Wisowski, G.; Pudełko, A.; Paul-Samojedny, M.; Komosińska-Vassev, K.; Koźma, E.M. Dermatan sulfate affects the activation of the necroptotic effector MLKL in breast cancer cell lines via the NFκB pathway and rac-mediated oxidative stress. Biomolecules 2024, 14, 829. [Google Scholar] [CrossRef]
- Wen, C.; Xu, L.; Xu, X.; Wang, D.; Liang, Y.; Duan, L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res. Ther. 2021, 30, 277. [Google Scholar] [CrossRef]
- Dixit, M.; Poudel, S.B.; Yakar, S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell Endocrinol. 2021, 519, 111052. [Google Scholar] [CrossRef]
- d’Angelo, D.M.; Di Donato, G.; Breda, L.; Chiarelli, F. Growth and puberty in children with juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2021, 19, 28. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Farquharson, C. The effect of GH and IGF1 on linear growth and skeletal development and their modulation by SOCS proteins. J. Endocrinol. 2010, 206, 249–259. [Google Scholar] [CrossRef]
- Lundell, A.C.; Erlandsson, M.; Bokarewa, M.; Liivamägi, H.; Uibo, K.; Tarraste, S.; Rebane, T.; Talvik, T.; Pruunsild, C.; Pullerits, R. Low serum IGF-1 in boys with recent onset of juvenile idiopathic arthritis. J. Immunol. Res. 2018, 2018, 3856897. [Google Scholar] [CrossRef]
- De Benedetti, F.; Meazza, C.; Oliveri, M.; Pignatti, P.; Vivarelli, M.; Alonzi, T.; Fattori, E.; Garrone, S.; Barreca, A.; Martini, A. Effect of IL-6 on IGF binding protein-3: A study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology 2001, 142, 4818–4826. [Google Scholar] [CrossRef]
- Guszczyn, T.; Rzeczycka, J.; Popko, J. IGF-I and IGF-binding proteins in articular exudates of children with post-traumatic knee damage and juvenile idiopathic arthritis. Pathobiology 2009, 76, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; MacRae, V.E.; Gracie, J.A.; McInnes, I.B.; Galea, P.; Gardner-Medwin, J.; Ahmed, S.F. Inflammatory cytokines in juvenile idiopathic arthritis: Effects on physical growth and the insulin-like-growth factor axis. Growth Horm. IGF Res. 2008, 18, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Lewander, P.; Dahle, C.; Larsson, B.; Wetterö, J.; Skogh, T. Circulating cartilage oligomeric matrix protein in juvenile idiopathic arthritis. Scand. J. Rheumatol. 2017, 46, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Tsatsoulis, A.; Siamopoulou, A.; Petsoukis, C.; Challa, A.; Bairaktari, E.; Seferiadis, K. Study of growth hormone secretion and action in growth-retarded children with juvenile chronic arthritis (JCA). Growth Horm. Igf Res. 1999, 9, 143–149. [Google Scholar] [CrossRef]
- Bechtold, S.; Ripperger, P.; Dalla Pozza, R.; Bonfig, W.; Hefner, R.; Michels, H.; Schwarz, P. Growth hormone increases final height in patients with juvenile idiopathic arthritis: Data from a randomized controlled study. J. Clin. Endocrinol. Metab. 2007, 92, 3013–3018. [Google Scholar] [CrossRef]
- Cirillo, F.; Lazzeroni, P.; Sartori, C.; Street, M.E. Inflammatory diseases and growth: Effects on the GH-IGF axis and on growth plate. Int. J. Mol. Sci. 2017, 18, 1878. [Google Scholar] [CrossRef]
- Choukair, D.; Hügel, U.; Sander, A.; Uhlmann, L.; Tönshoff, B. Inhibition of IGF-I-related intracellular signaling pathways by proinflammatory cytokines in growth plate chondrocytes. Pediatr. Res. 2014, 76, 245–251. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kashihara, N.; Makino, H.; Kanwar, H.S. Modulation of glomerular proteoglycans by insulin-like growth factor-1. Kidney Int. 1992, 41, 1262–1273. [Google Scholar] [CrossRef]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef]
- Fowlkes, J.L.; Serra, D.M. Characterization of glycosaminoglycan-binding domains present in insulin-like growth factor-binding protein-3. J. Biol. Chem. 1996, 271, 14676–14679. [Google Scholar] [CrossRef]
- Forbes, B.E.; Blyth, A.; Wit, J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol. Cell Endocrinol. 2020, 518, 111035. [Google Scholar] [CrossRef]
- Żuber, Z. Juvenile idiopathic arthritis. Pediatr. Dyplom 2012, 2, 23–32. [Google Scholar]
| Parameter | Control Subjects (n = 45) | Untreated JIA Patients (n = 55) | JIA Patients After Treatment (n = 55) |
|---|---|---|---|
| Age (years) | 8.25 ± 4.03 | 7.31 ± 4.86 | 8.28 ± 4.71 |
| Sex, female/male | 33/12 | 41/14 | 41/14 |
| JADAS-27 | - | 17.50 (15.50–21.50) | 1.00 (1.00–1.50) b |
| WBC (103/μL, by fluorescent flow cytometry method) | 5.35 ± 2.19 | 11.21 ± 2.98 a | 7.25 ± 2.57 b |
| RBC (106/μL, by impedance method) | 4.95 ± 0.35 | 3.85 ± 0.93 a | 4.54 ± 0.67 |
| Hb (g/dL, by colorimetric method) | 13.85 ± 0.94 | 11.43 ± 3.02 a | 13.06 ± 4.31 b |
| PLT (103/μL, by impedance method) | 283.47 ± 73.83 | 349.19 ± 58.89 | 316.91 ± 57.76 |
| GPT (U/L, by spectrophotometric measurement) | 18.58 ± 12.82 | 22.54 ± 11.01 | 25.52 ± 14.13 a,b |
| GOT (U/L, by spectrophotometric measurement) | 23.70 ± 12.64 | 25.85 ± 12.47 | 28.98 ± 15.15 a,b |
| Creatinine (mg/dL, by Jaffe method) | 0.68 ± 0.15 | 0.65 ± 0.21 | 0.78 ± 0.43 |
| ESR (mm/h, by Westergren technique) | 9.02 ± 3.03 | 41.47 ± 16.29 a | 13.88 ± 11.05 b |
| CRP (mg/L, by immunonephelometric assay) | 0.59 (0.27–1.28) | 30.65 (19.15–44.58) a | 2.73 (0.29–5.11) b |
| ANA (by indirect immunofluorescence assay) | - | 61% (positive) | 61% (positive) |
| RF (by latex-enhanced immunoturbidimetric test) | - | 100% (negative) | 100% (negative) |
| Control Subjects | Untreated JIA Patients | JIA Patients After Treatment | |
|---|---|---|---|
| CS/DS [µg/mL] | |||
| Total CS/DS | 14.48 (10.23–15.77) | 8.26 (6.25–9.66) * | 10.68 (10.03–11.73) *,# |
| (N) | (45) | (55) | (55) |
| Oligoarthritis | 14.48 (10.23–15.77) | 8.63 (7.36–8.24) *,$ | 11.16 (10.39–12.62) # |
| (N) | (45) | (31) | (31) |
| Polyarthritis | 14.48 (10.23–15.77) | 7.09 (5.63–8.41) * | 10.55 (10.27–11.01) *,# |
| (N) | (45) | (24) | (24) |
| Girls | 14.09 (9.66–14.89) | 7.21 (5.75–8.94) * | 10.86 (10.29–12.65) # |
| (N) | (33) | (41) | (41) |
| Boys | 12.99 (12.02–15.77) | 8.57 (8.13–9.78) * | 12.55 (10.03–13.68) # |
| (N) | (12) | (14) | (14) |
| Control Subjects | Untreated JIA Patients | JIA Patients After Treatment | |
|---|---|---|---|
| IGF-1 [ng/mL] | |||
| Total IGF-1 | 96.92 (76.04–128.59) | 66.04 (49.45–96.80) * | 120.60 (92.78–138.77) # |
| (N) | (45) | (55) | (55) |
| Oligoarthritis | 96.92 (76.04–128.59) | 57.13 (45.35–84.06) * | 95.18 (65.01–120.32) # |
| (N) | (45) | (31) | (31) |
| Polyarthritis | 96.92 (76.04–128.59) | 111.26 (94.83–178.35) | 143.75 (90.35–167.61) *,# |
| (N) | (45) | (24) | (24) |
| Girls | 90.94 (73.69–137.09) | 73.42 (54.78–115.74) | 121.16 (88.31–150.99) *,# |
| (N) | (33) | (41) | (41) |
| Boys | 118.47 (100.08–169.34) | 69.67 (48.23–98.21) * | 110.15 (63.93–154.06) # |
| (N) | (12) | (14) | (14) |
| Control Subjects | Untreated JIA Patients | JIA Patients After Treatment | |
|---|---|---|---|
| IGFBP-3 [ng/mL] | |||
| Total IGFBP-3 | 4.84 (4.21–7.75) | 3.37 (2.65–4.88) * | 5.67 (4.51–6.19) # |
| (N) | (45) | (55) | (55) |
| Oligoarthritis | 4.84 (4.21–7.75) | 3.01 (2.15–4.26) * | 5.58 (4.02–6.17) # |
| (N) | (45) | (31) | (31) |
| Polyarthritis | 4.84 (4.21–7.75) | 4.26 (3.84–5.91) | 5.69 (4.91–7.35) |
| (N) | (45) | (24) | (24) |
| Girls | 4.69 (4.10–7.07) | 3.68 (2.71–6.75) | 5.71 (4.86–7.01) # |
| (N) | (33) | (41) | (41) |
| Boys | 7.25 (5.28–7.94) | 3.07 (2.83–4.28) * | 4.55 (3.03–5.46) *,# |
| (N) | (12) | (14) | (14) |
| CS/DS | IGF-1 | IGFBP-3 | |
|---|---|---|---|
| AUC | 0.947 | 0.724 | 0.761 |
| Opitmal cut-off | 10.374 µg/mL | 75.411 ng/mL | 3.925 ng/mL |
| Youden Index | 0.807 | 0.424 | 0.501 |
| Accuracy | 0.908 | 0.685 | 0.708 |
| Sensitivity | 0.889 | 0.800 | 0.889 |
| Specificity | 0.918 | 0.624 | 0.612 |
| PPV | 0.851 | 0.529 | 0.548 |
| NPV | 0.940 | 0.855 | 0.912 |
| Parameter | CS/DS r (p) | IGF-1 r (p) | IGFBP-3 r (p) |
|---|---|---|---|
| CRP | |||
| Untreated JIA patients | |||
| 0.601 (0.0005) | −0.233 (0.215) | −0.228 (0.225) | |
| JIA’ patients after treatment | |||
| −0.318 (0.099) | −0.117 (0.368) | −0. 002(0.990) | |
| ESR | |||
| Untreated JIA’ patients | |||
| 0.283 (0.130) | −0.055 (0.774) | −0.423 (0.019) | |
| JIA’ patients after treatment | |||
| −0.216 (0.252) | −0.170 (0.370) | 0.288 (0.123) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Koźma, E.M.; Żegleń, B.; Gruenpeter, A.; Wisowski, G.; Komosińska-Vassev, K.; Olczyk, K. Serum CS/DS, IGF-1, and IGFBP-3 as Biomarkers of Cartilage Remodeling in Juvenile Idiopathic Arthritis: Diagnostic and Therapeutic Implications. Biomolecules 2024, 14, 1526. https://doi.org/10.3390/biom14121526
Winsz-Szczotka K, Kuźnik-Trocha K, Koźma EM, Żegleń B, Gruenpeter A, Wisowski G, Komosińska-Vassev K, Olczyk K. Serum CS/DS, IGF-1, and IGFBP-3 as Biomarkers of Cartilage Remodeling in Juvenile Idiopathic Arthritis: Diagnostic and Therapeutic Implications. Biomolecules. 2024; 14(12):1526. https://doi.org/10.3390/biom14121526
Chicago/Turabian StyleWinsz-Szczotka, Katarzyna, Kornelia Kuźnik-Trocha, Ewa M. Koźma, Bogusław Żegleń, Anna Gruenpeter, Grzegorz Wisowski, Katarzyna Komosińska-Vassev, and Krystyna Olczyk. 2024. "Serum CS/DS, IGF-1, and IGFBP-3 as Biomarkers of Cartilage Remodeling in Juvenile Idiopathic Arthritis: Diagnostic and Therapeutic Implications" Biomolecules 14, no. 12: 1526. https://doi.org/10.3390/biom14121526
APA StyleWinsz-Szczotka, K., Kuźnik-Trocha, K., Koźma, E. M., Żegleń, B., Gruenpeter, A., Wisowski, G., Komosińska-Vassev, K., & Olczyk, K. (2024). Serum CS/DS, IGF-1, and IGFBP-3 as Biomarkers of Cartilage Remodeling in Juvenile Idiopathic Arthritis: Diagnostic and Therapeutic Implications. Biomolecules, 14(12), 1526. https://doi.org/10.3390/biom14121526







