Application of Free and Encapsulated DNA Tracers in Surface Water Studies in Lithuanian Climatic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Free DNA Tracer and Analysis
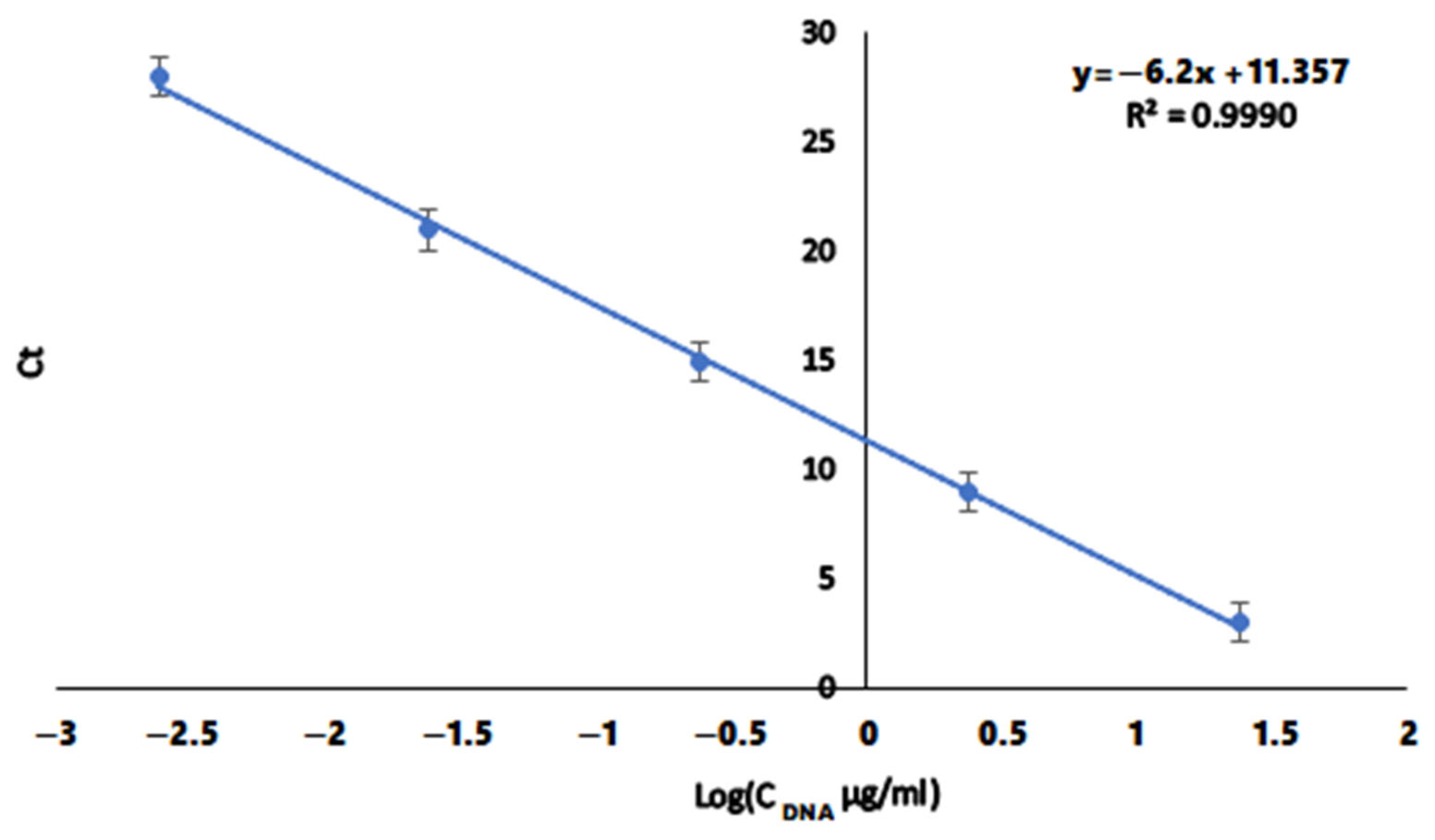
2.2. Determination of DNA Concentration
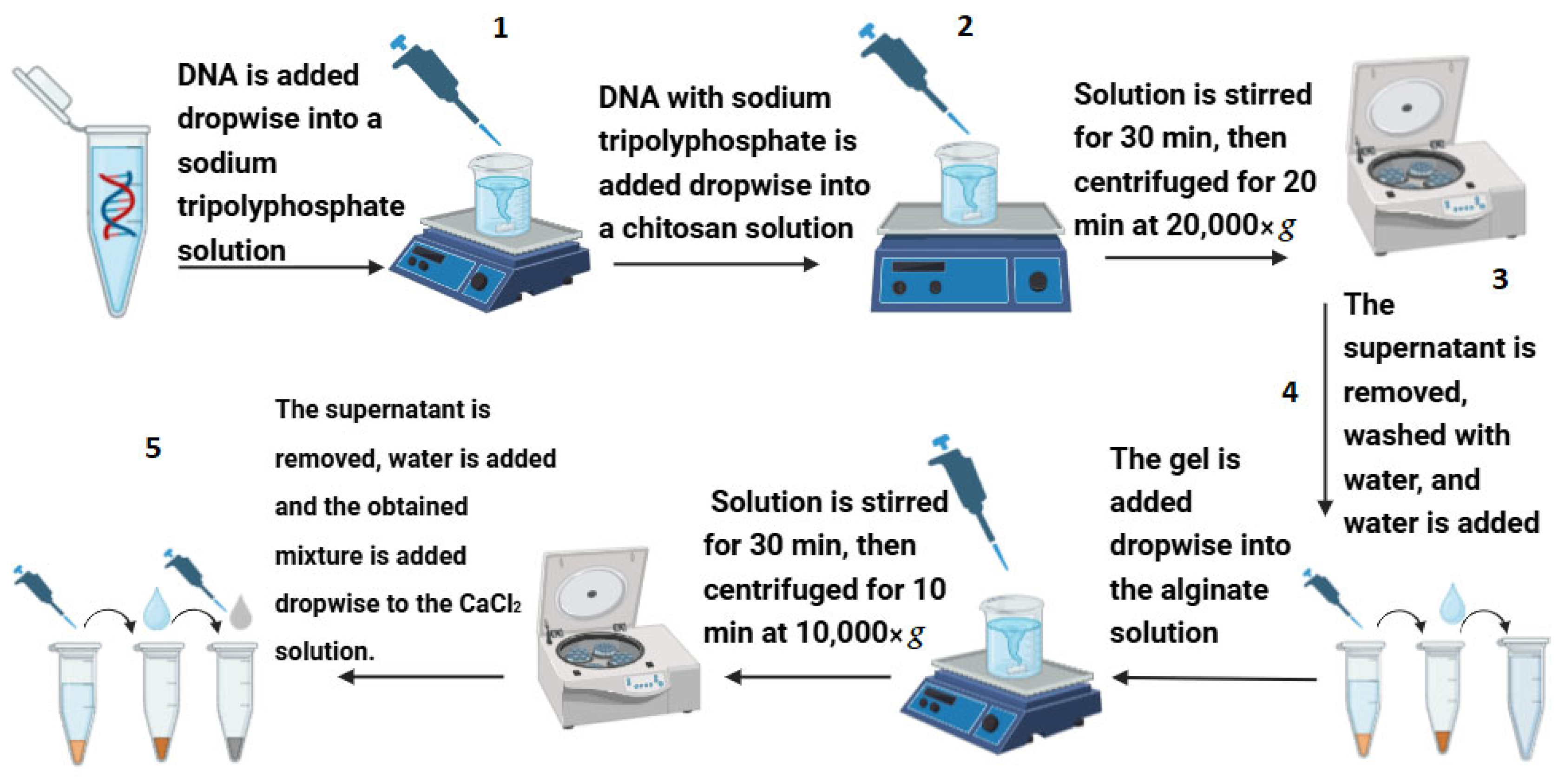
2.3. DNA Encapsulation and Control Experiments
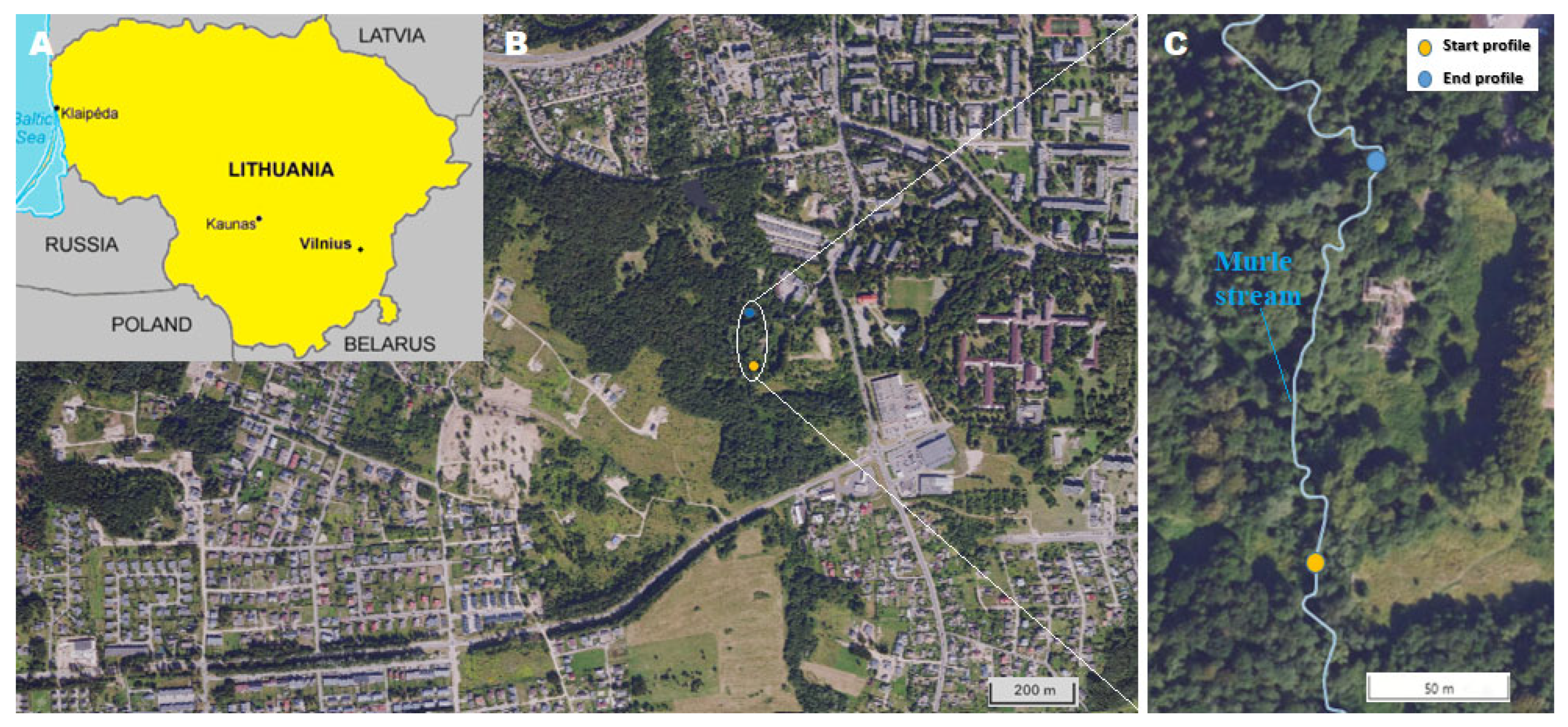
2.4. Field Experiments
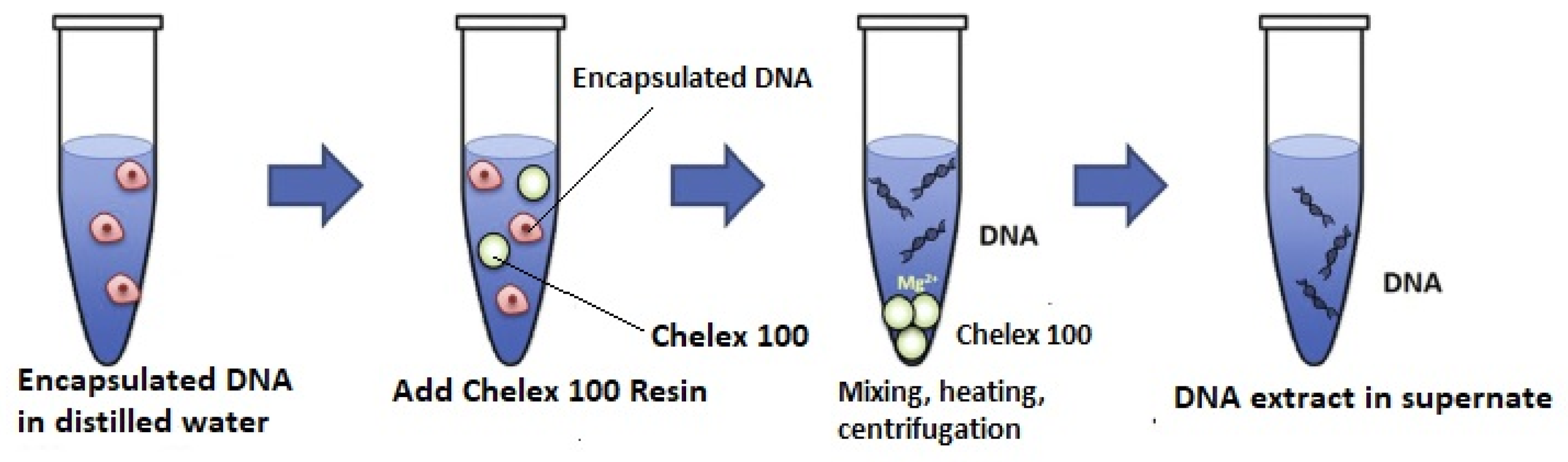
2.5. Disruption of DNA Capsules and Tracer Analysis of Free DNA
3. Results and Discussion
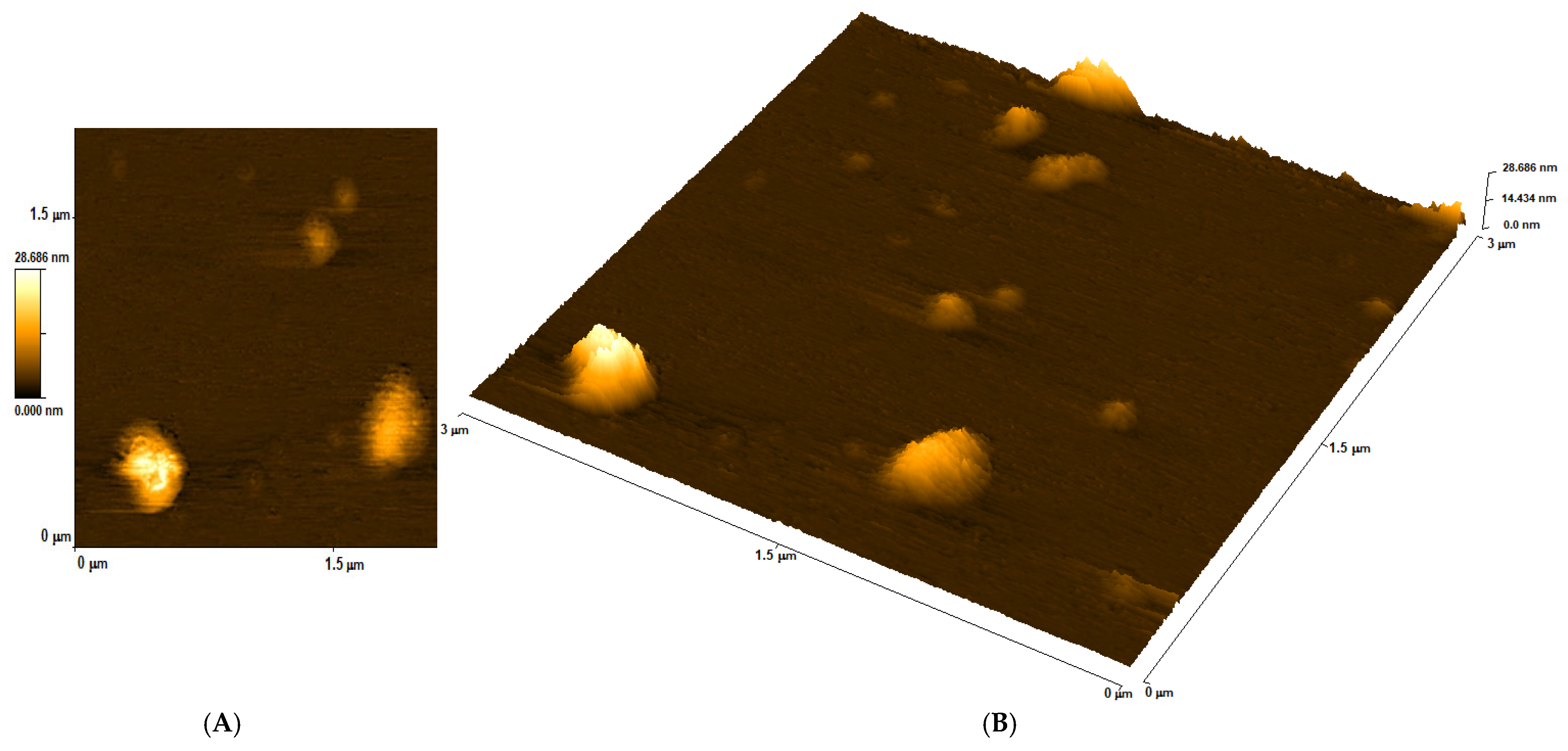
3.1. Determining the Size of DNA Capsules
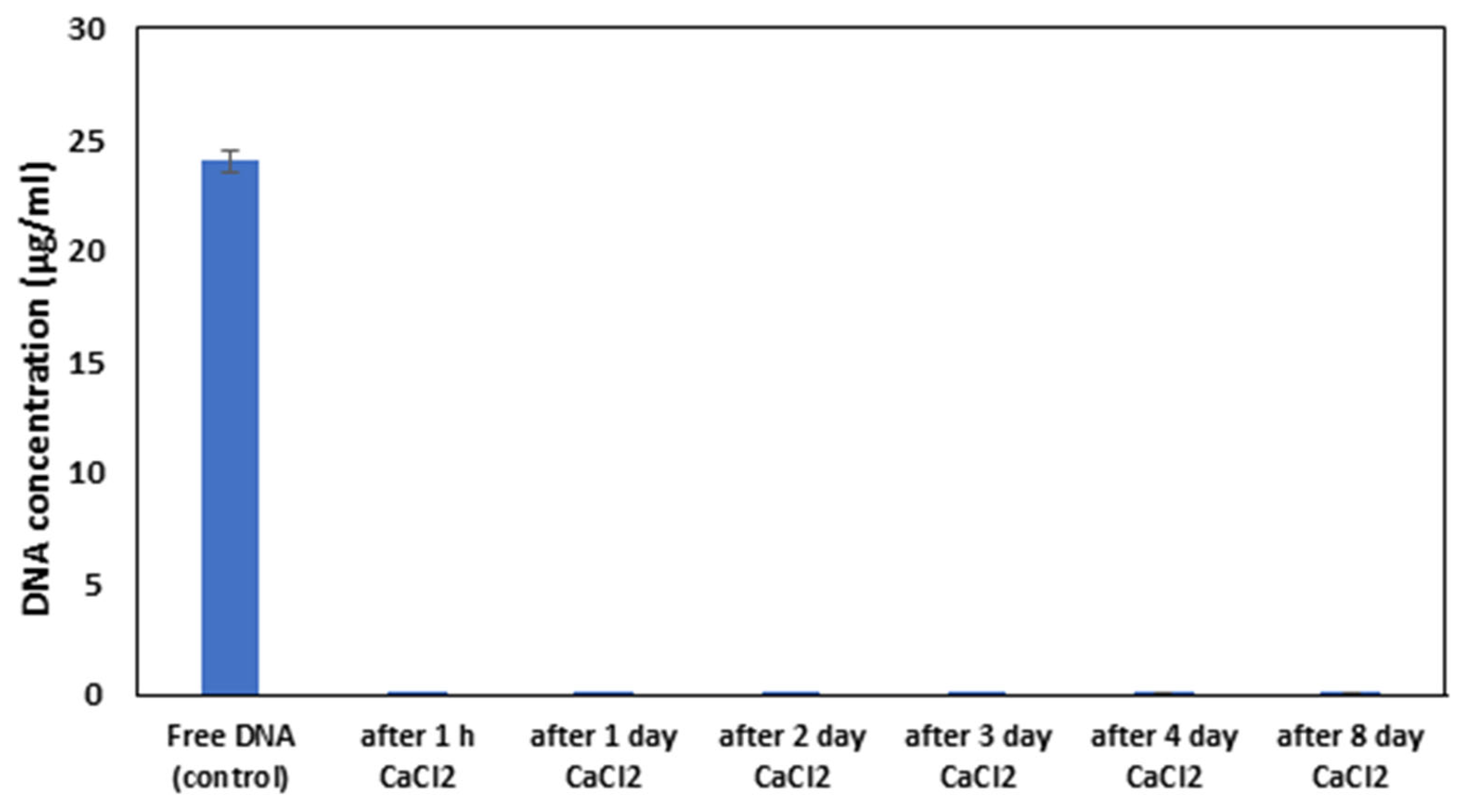
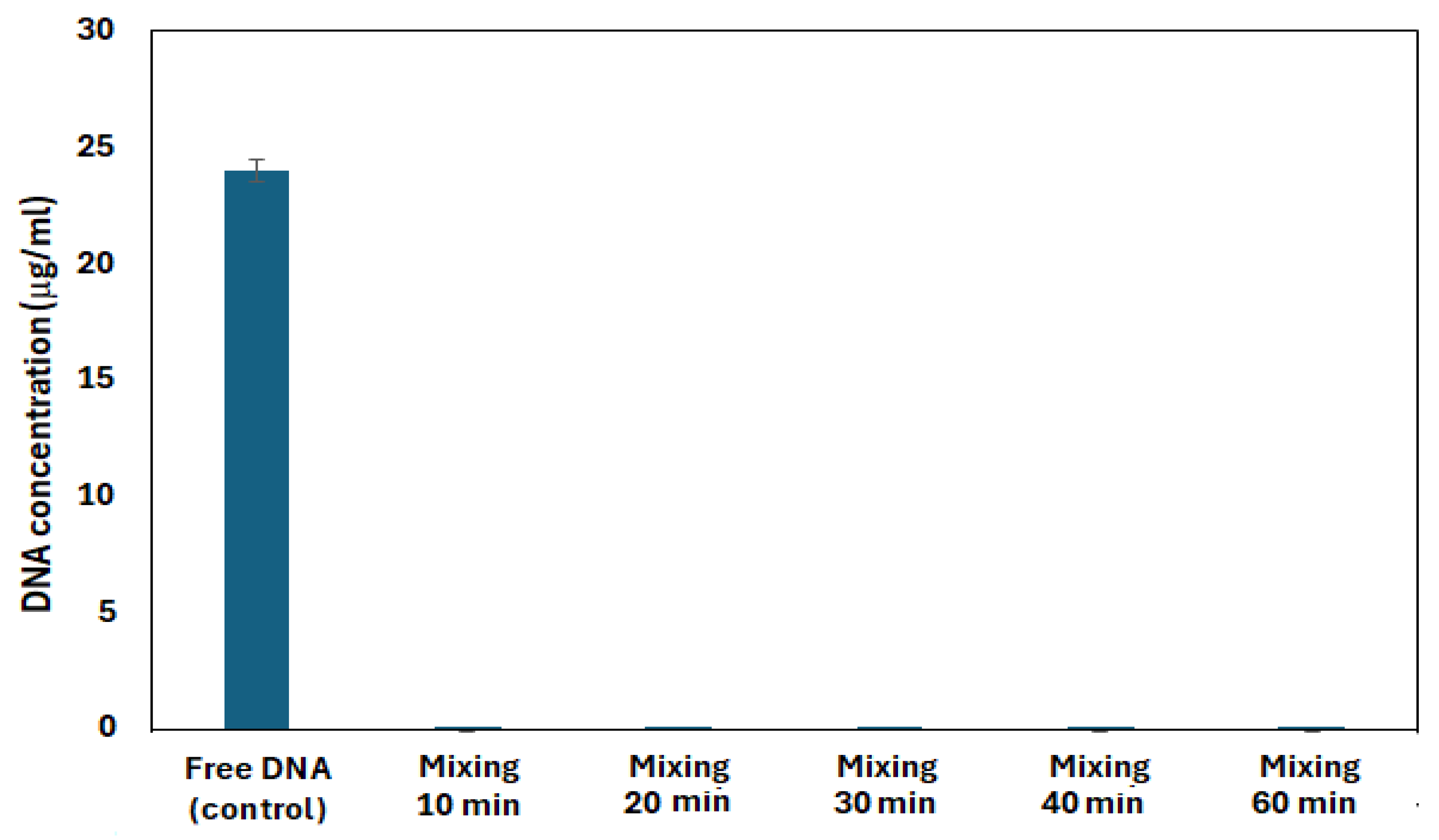
3.2. Permeability and Stability of DNA Capsules
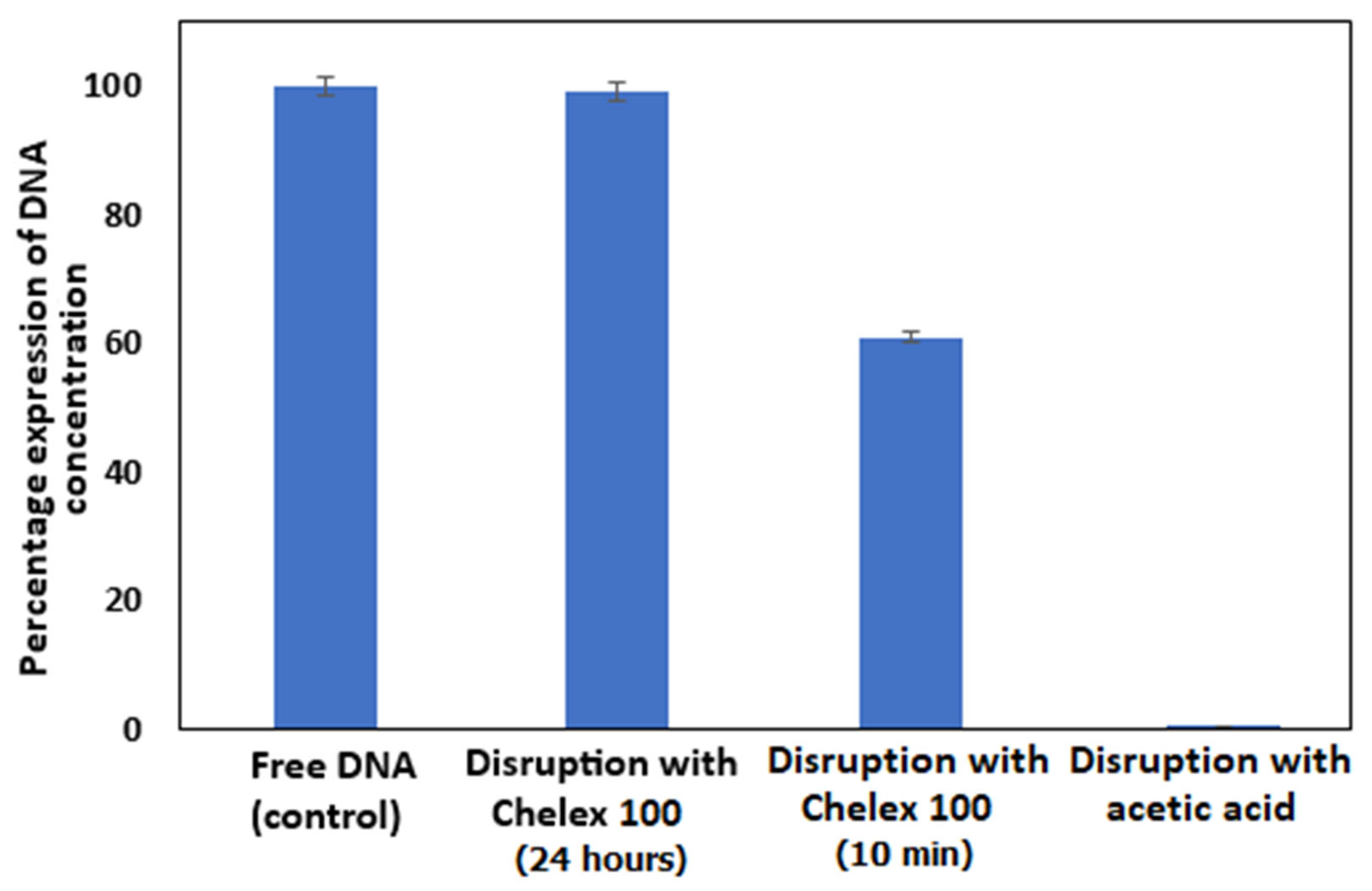
3.3. Disruption of DNA Capsules Under Laboratory Conditions
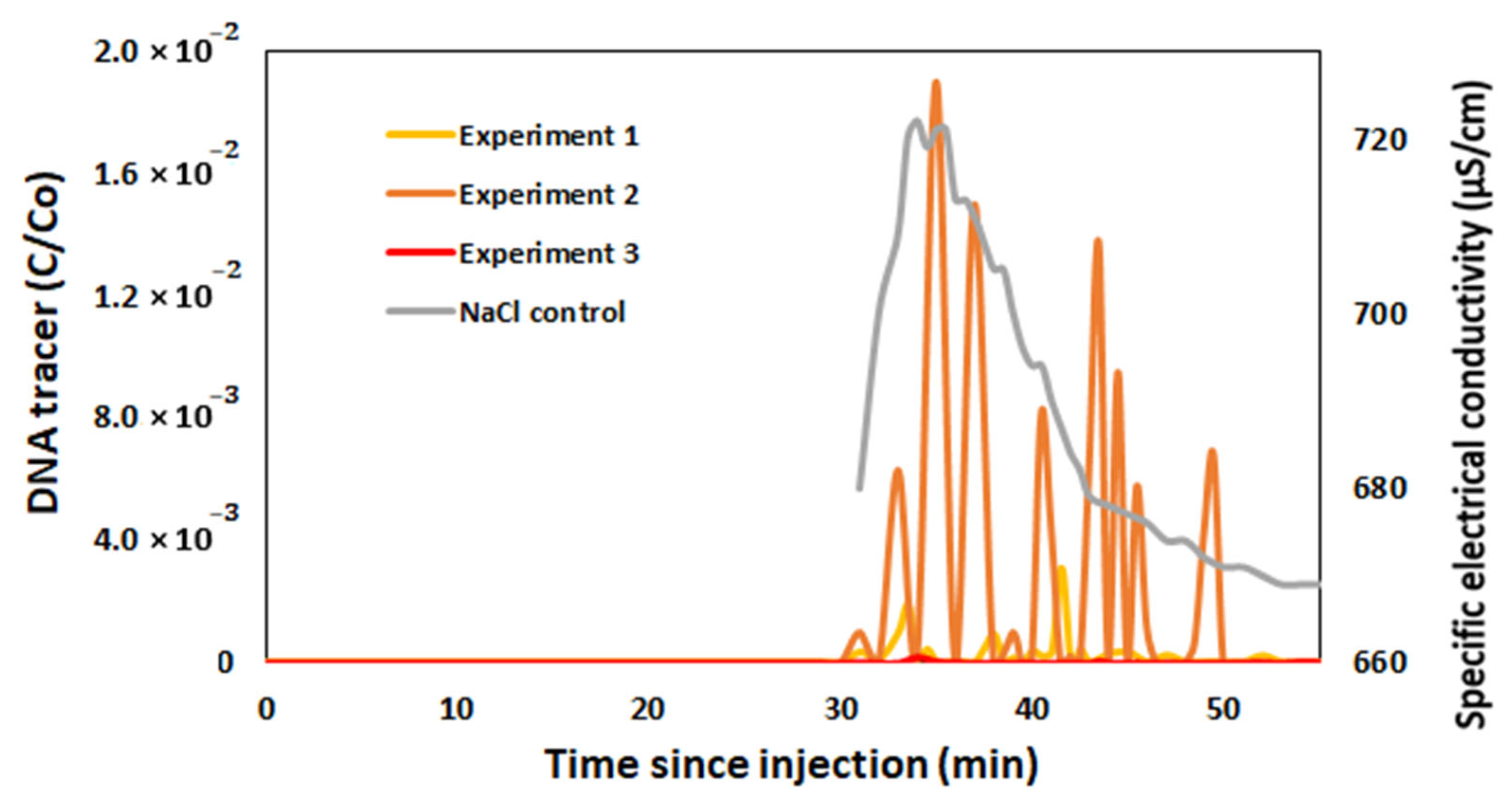
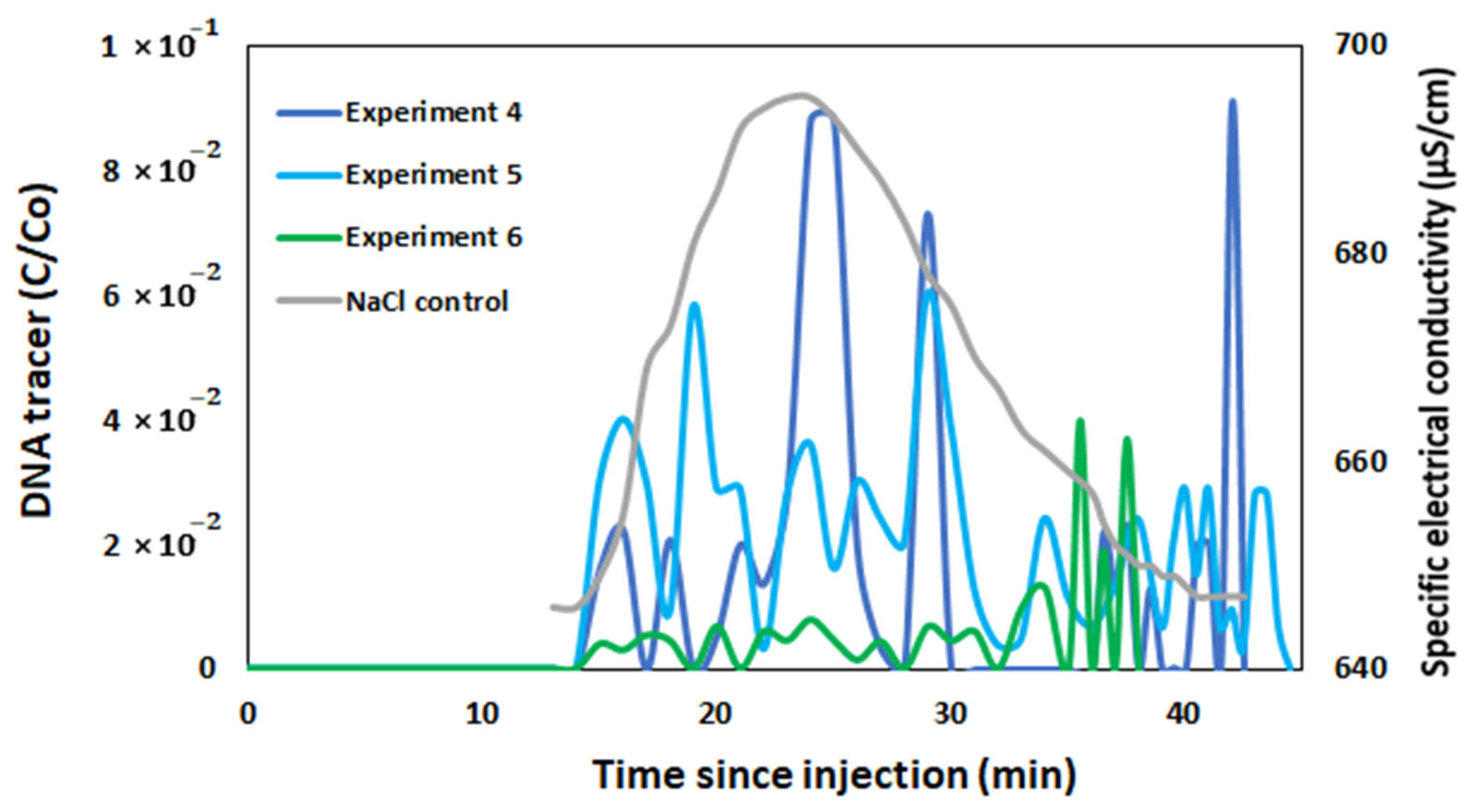
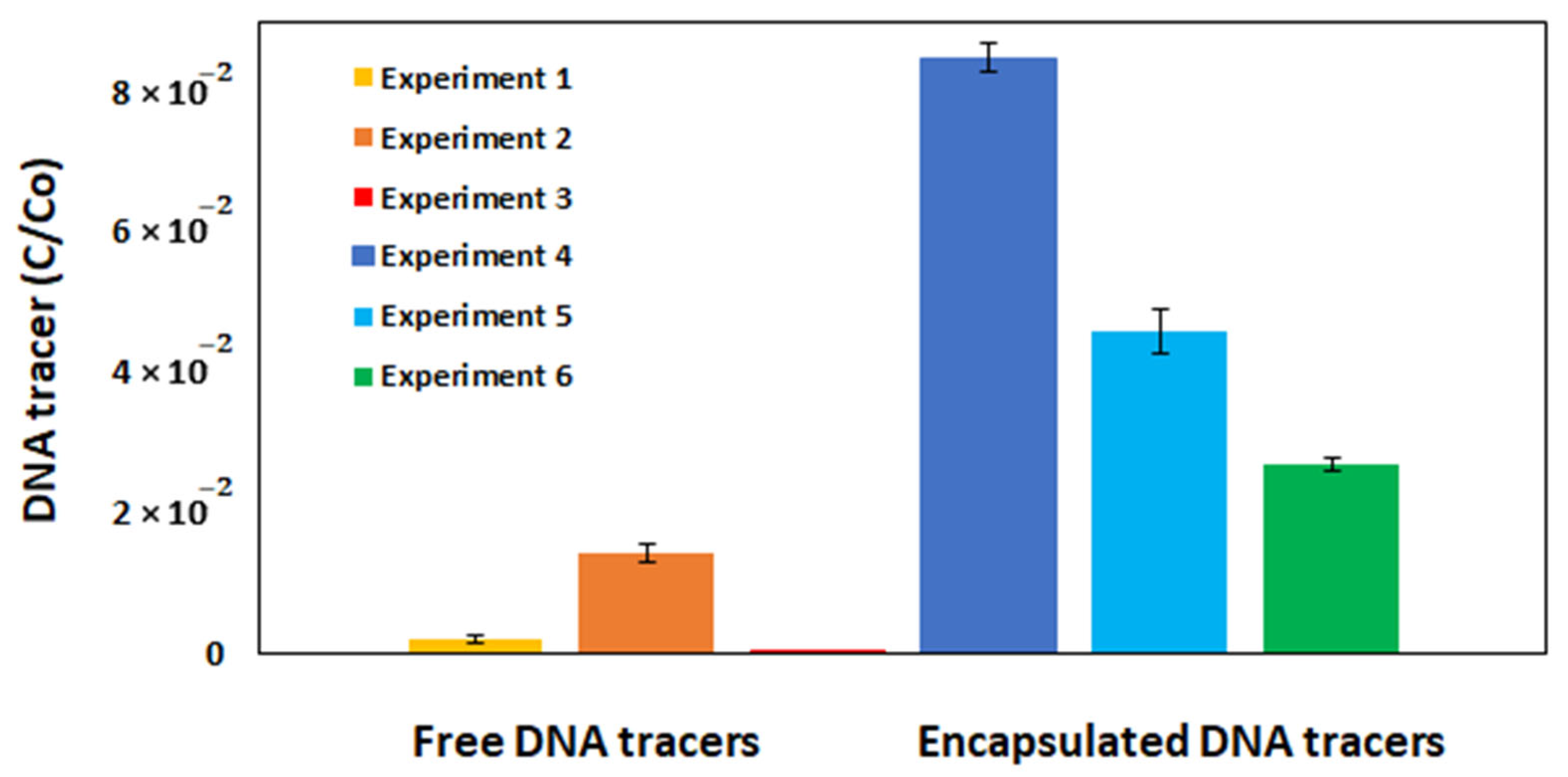
3.4. Field Experiments with Free and Encapsulated DNA Tracers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, R.; Yang, P.; Wu, W.; Luo, D.; Yang, D. A DNA Tracer System for Hydrological Environment Investigations. Environ. Sci. Technol. 2018, 52, 1695–1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, T. DNA-based tracers for the characterization of hydrogeological systems—Recent advances and new frontiers. Water 2022, 14, 3545. [Google Scholar] [CrossRef]
- Foppen, J.W. Artificial DNA in hydrology. WIREs Water 2023, 10, e1681. [Google Scholar] [CrossRef]
- McCluskey, J.; Flores, M.E.; Hinojosa, J.; Jafarzadeh, A.; Moghadam, S.V.; Phan, D.C.; Green, R.T.; Kapoor, V. Tracking water with synthetic DNA tracers using droplet digital PCR. ACS EST Water 2021, 1, 1177–1183. [Google Scholar] [CrossRef]
- Stuart, J.D.; Wickenkamp, N.R.; Davis, K.A.; Meyer, C.; Kading, R.C.; Snow, C.D. Scalable combinatorial assembly of synthetic DNA for tracking applications. Int. J. Mol. Sci. 2023, 24, 2549. [Google Scholar] [CrossRef] [PubMed]
- Georgakakos, C.B.; Richards, P.L.; Walter, M.T. Tracing septic pollution sources using synthetic DNA tracers: Proof of concept. Air Soil Water Res. 2019, 12, 1178622119863794. [Google Scholar] [CrossRef]
- Wang, C.; Liu, G.; McNew, C.P.; Volkmann, T.H.M.; Pangle, L.; Troch, P.A.; Lyon, S.W.; Kim, M.; Huo, Z.; Dahlke, H.E. Simulation of experimental synthetic DNA tracer transport through the vadose zone. Water Res. 2022, 223, 119009. [Google Scholar] [CrossRef] [PubMed]
- Mikutis, G.; Deuber, C.A.; Schmid, L.; Kittilä, A.; Lobsiger, N.; Puddu, M.; Asgeirsson, D.O.; Grass, R.N.; Saar, M.O.; Stark, W.J. Silica-encapsulated DNA-based tracers for aquifer characterization. Environ. Sci. Technol. 2018, 52, 12142–12152. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohsumi, S.; Makino, K. Mechanistic studies on depurination and apurinic site chain breakage in oligodeoxyribonucleotides. Nucleic Acids Res. 1994, 22, 4997–5003. [Google Scholar] [CrossRef]
- Allentoft, M.E.; Collins, M.; Harker, D.; Haile, J.; Oskam, C.L.; Hale, M.L.; Campos, P.F.; Samaniego, J.A.; Gilbert, M.T.P.; Willerslev, E.; et al. The half-life of DNA in bone: Measuring decay kinetics in 158 dated fossils. Proc. R. Soc. B 2012, 279, 4724–4733. [Google Scholar] [CrossRef]
- Hansen, H.B.; Damgaard, P.B.; Margaryan, A.; Stenderup, J.; Lynnerup, N.; Willerslev, E.; Allentoft, M.E. Comparing ancient DNA preservation in petrous bone and tooth Cementum. PLoS ONE 2017, 12, e0170940. [Google Scholar] [CrossRef] [PubMed]
- Weiß, C.L.; Schuenemann, V.J.; Devos, J.; Shirsekar, G.; Reiter, E.; Gould, B.A.; Stinchcombe, J.R.; Krause, J.; Burbano, H.A. Temporal patterns of damage and decay kinetics of DNA retrieved from plant herbarium specimens. R. Soc. Open Sci. 2016, 3, 160239. [Google Scholar] [CrossRef]
- Mikutis, G.; Schmid, L.; Stark, W.J.; Grass, R.N. Length-dependent DNA degradation kinetic model: Decay compensation in DNA tracer concentration measurements. AIChE J. 2019, 65, 40–48. [Google Scholar] [CrossRef]
- Dahlke, H.E.; Williamson, A.G.; Georgakakos, C.; Leung, S.; Sharma, A.N.; Lyon, S.W.; Walter, M.T. Using concurrent DNA tracer injections to infer glacial flow pathways. Hydrol. Process. 2015, 29, 5257–5274. [Google Scholar] [CrossRef]
- Paunescu, D.; Mora, C.A.; Puddu, M.; Krumeich, F.; Grass, R.N. DNA protection against ultraviolet irradiation by encapsulation in a multilayered SiO2/TiO2 assembly. J. Mater. Chem. B 2014, 2, 8504–8509. [Google Scholar] [CrossRef]
- McNew, C.P.; Wang, C.; Walter, M.T.; Dahlke, H.E. Fabrication, detection, and analysis of DNA-labeled PLGA particles for environmental transport studies. J. Colloid Interface Sci. 2018, 526, 207–219. [Google Scholar] [CrossRef]
- Sah, E.; Sah, H. Recent trends in preparation of poly (lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J. Nanomater. 2015, 16, 794601. [Google Scholar] [CrossRef]
- Niu, X.; Zou, W.; Liu, C.; Zhang, N.; Fu, C. Modified nanoprecipitation method to fabricate DNA-loaded PLGA nanoparticles. Drug Dev. Ind. Pharm. 2009, 35, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Díez, S.; de Ilarduya, C.T. Versatility of biodegradable poly (d, l-lactic-co-glycolic acid) microspheres for plasmid DNA delivery. Eur. J. Pharm. Biopharm. 2006, 63, 188–197. [Google Scholar] [CrossRef]
- Pang, L.; Abeysekera, G.; Hanning, K.; Premaratne, A.; Robson, B.; Abraham, P.; Sutton, R.; Hanson, C.; Hadfield, J.; Heiligenthal, L.; et al. Water tracking in surface water, groundwater and soils using free and alginate-chitosan encapsulated synthetic DNA tracers. Water Res. 2020, 184, 116192. [Google Scholar] [CrossRef]
- Deepak, S.A.; Kottapalli, K.R.; Rakwal, R.; Oros, G.; Rangappa, K.S.; Iwahashi, H.; Masuo, Y.; Agrawal, G.K. Real-time PCR: Revolutionizing detection and expression analysis of genes. Curr. Genom. 2007, 8, 234–251. [Google Scholar] [CrossRef]
- Maddocks, S.; Jenkins, R. Quantitative PCR: Things to consider. Underst. PCR 2017, 4, 45–52. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Lorenz, P.; Tuomi, J.M.; Hecker, M.; van den Hoff, M.J.B. Fluorescent-increase kinetics of different fluorescent reporters used for qPCR depend on monitoring chemistry, targeted sequence, type of DNA input and PCR efficiency. Mikrochim. Acta 2014, 181, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Analysis and normalization of real-time polymerase chain reaction (PCR) experimental data. Cold Spring Harb. Protoc. 2018, 10, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Cobbs, G. Stepwise kinetic equilibrium models of quantitative polymerase chain reaction. BMC Bioinform. 2012, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.W.; Binet, R.; Brown, E.W.; Cao, G.; Deng, X.; Grim, C.; Hammack, T.S.; Hoffmann, M.; Miller, J.; Pettengill, J.; et al. Molecular techniques in foodborne disease surveillance. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Elsevier Inc.: Cambridge, MA, USA, 2023; Volume 4, pp. 61–85. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Gill, P.; Bleka, Ø.; Fonneløp, A.E. Limitations of qPCR to estimate DNA quantity: An RFU method to facilitate inter-laboratory comparisons for activity level, and general applicability. Forensic Sci. Int. Genet. 2022, 61, 102777. [Google Scholar] [CrossRef]
- Galvonaitė, A.; Valiukas, D.; Kilpys, J.; Kitrienė, Z.; Misiūnienė, M. Climate Atlas of Lithuania; Lithuanian Hydrometeorological Service under the Ministry of Environment: Vilnius, Lithuania, 2013; p. 175. Available online: https://www.meteo.lt/app/uploads/2024/10/Klimato-Atlasas.pdf (accessed on 7 May 2025).
- Jakimavičiūtė-Maselienė, V.; Samalavičius, V.; Ašipauskaitė, A.; Guščo, A. Estimation of hydrogeological parameters of porous media in a radially convergent flow field in Kairėnai polygon, SE Lithuania. Baltica 2022, 35, 140–146. [Google Scholar] [CrossRef]
- Cidzikienė, V.; Jakimavičiūtė-Maselienė, V.; Girgždienė, R.; Ivanec-Goranina, R. An application of fluorescent tracer to groundwater tracking. Open Chem. 2015, 13, 497–501. [Google Scholar] [CrossRef]
- Štuopis, A.; Juodkazis, V.; Mokrik, R. Quaternary aquifer system flow modelling using chemical and tritium isotope data: The case of south-east Lithuania. Baltica 2012, 25, 91–98. [Google Scholar] [CrossRef]
- Gombert, P.; Biaudet, H.; de Seze, R.; Pandard, P.; Carré, J. Toxicity of fluorescent tracers and their degradation byproducts. Int. J. Speleol. 2017, 46, 23–31. [Google Scholar] [CrossRef]
- Meghdadi, A.; Javar, N. Quantification of spatial and seasonal variations in the proportional contribution of nitrate sources using a multi-isotope approach and Bayesian isotope mixing model. Environ. Pollut. 2018, 235, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, L.; Clementi, F.; Landolfo, S.; Nanni, T.; Palpacelli, S.; Tazioli, A. A DNA tracer used in column tests for hydrogeology applications. Environ. Earth Sci. 2013, 70, 3143–3154. [Google Scholar] [CrossRef]
- Timken, M.D.; Swango, K.L.; Orrego, C.; Chong, M.D.; Buoncristiani, M.R. Quantitation of DNA for Forensic DNA Typing by qPCR (quantitative PCR): Singleplex and Multiplex Modes for Nuclear and Mitochondrial Genomes, and the Y Chromosome; U.S. Department of Justice Office of Justice Programs: Washington, DC, USA, 2005; Volume 210302, p. 89. Available online: https://www.ojp.gov/pdffiles1/nij/grants/210302.pdf (accessed on 21 February 2024).
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 2013, 54, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A. DNA Isolation by Chelex Method. In DNA and RNA Isolation Techniques for Non-Experts; Kalyuzhny, A.E., Ed.; Springer: Cham, Switzerland, 2022; pp. 79–84. [Google Scholar] [CrossRef]
- Giraldo, J.D.; Rivas, B.L. Direct ionization and solubility of chitosan in aqueous solutions with acetic acid. Polym. Bull. 2021, 78, 1465–1488. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Gershon, Z.; Nussinovitch, M. Temperature stable liquid core hydrocolloid capsules. Food Hydrocoll. 1997, 11, 209–215. [Google Scholar] [CrossRef]
- Danilowicz, C.; Lee, C.H.; Coljee, V.W.; Prentiss, M. Effects of temperature on the mechanical properties of single stranded DNA. Phys. Rev. 2007, 75 Pt 1, 030902. [Google Scholar] [CrossRef]

| Description, Units | Free DNA | Encapsulated DNA | ||||
|---|---|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 3 | Exp 4 | Exp 5 | Exp 6 | |
| DNA tracer volume, mL | 1 | 1 | 1 | 1 | 1 | 1 |
| DNA input, μg/mL | 120 | 120 | 120 | 120 | 120 | 120 |
| NaCl tracer, L | 5 | 5 | 5 | 5 | 5 | 5 |
| NaCl input, g/L | 100 | 100 | 100 | 100 | 100 | 100 |
| Date, month day | 1 August | 17 August | 19 October | 14 March | 29 March | 11 April |
| Temperature, °C | 16.4 | 17.8 | 6.9 | 3.9 | 8.1 | 10.1 |
| pH | 6.61 | 6.72 | 6.57 | 6.71 | 6.9 | 6.63 |
| Base conductivity, μS/cm | 670 | 674 | 672 | 648 | 645 | 647 |
| Stream flow rate, m/s | 0.113 | 0.133 | 0.138 | 0.230 | 0.186 | 0.189 |
| Stream discharge, L/s | 11.4 | 12.4 | 23.1 | 47.5 | 32.6 | 32.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švedaitė, D.; Kriučkova, A.; Morkvėnas, A.; Karabanovas, V.; Stankūnavičius, G.; Klima, V.; Urbonavičius, J.; Ivanec-Goranina, R. Application of Free and Encapsulated DNA Tracers in Surface Water Studies in Lithuanian Climatic Conditions. Biomolecules 2025, 15, 889. https://doi.org/10.3390/biom15060889
Švedaitė D, Kriučkova A, Morkvėnas A, Karabanovas V, Stankūnavičius G, Klima V, Urbonavičius J, Ivanec-Goranina R. Application of Free and Encapsulated DNA Tracers in Surface Water Studies in Lithuanian Climatic Conditions. Biomolecules. 2025; 15(6):889. https://doi.org/10.3390/biom15060889
Chicago/Turabian StyleŠvedaitė, Dominyka, Anastasija Kriučkova, Augustas Morkvėnas, Vitalijus Karabanovas, Gintautas Stankūnavičius, Vigilija Klima, Jaunius Urbonavičius, and Rūta Ivanec-Goranina. 2025. "Application of Free and Encapsulated DNA Tracers in Surface Water Studies in Lithuanian Climatic Conditions" Biomolecules 15, no. 6: 889. https://doi.org/10.3390/biom15060889
APA StyleŠvedaitė, D., Kriučkova, A., Morkvėnas, A., Karabanovas, V., Stankūnavičius, G., Klima, V., Urbonavičius, J., & Ivanec-Goranina, R. (2025). Application of Free and Encapsulated DNA Tracers in Surface Water Studies in Lithuanian Climatic Conditions. Biomolecules, 15(6), 889. https://doi.org/10.3390/biom15060889







