Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Isolation and Culture of Menstrual Effluent-Derived Endometrial Stromal Cells (eSCs)
2.3. Decidualization Assays
2.4. Cytotoxicity Assays
2.5. Western Blotting
2.6. Single Cell RNA Sequencing (scRNAseq) of Quercetin vs. Vehicle-Treated eSCs
2.7. Migration Assays
2.8. Assessment of Senescence Phenotype
2.9. Statistical Analyses
2.10. Patents
3. Results
3.1. Senotherapeutic Agents Have Differential Effects on eSC Decidualization
3.2. Some Senotherapeutics Are Cytotoxic
3.3. Quercetin-Treated eSCs Upregulate Gene Expression Related to Cell Migration and Cell Survival
3.4. Multiple Senotherapeutics Effectively Inhibit Cell Migration
3.5. Effects of Senotherapeutics on eSC Signaling Pathways
3.6. Elimination of Senescent Endometrial Stromal Cells (eSCs) by Senolytics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cAMP | 8-Bromoadenosine 3′,5′-cyclic monophosphate sodium salt |
| eSCs | Endometrial stromal cells |
| FBS | Fetal bovine serum (mesenchymal stem cell) |
| IGFBP1 | Insulin growth factor-binding protein 1 |
| ME | Menstrual effluent |
| ME-eSCs | Menstrual effluent-derived endometrial stromal cells |
| MPA | Medroxyprogesterone acetate |
| PALB | Palbociclib |
| PCOS | Polycystic ovary syndrome |
| PSQ | Penicillin: streptomycin, and L-glutamine |
| RT | Room temperature |
| scRNAseq | Single cell RNA sequencing |
| SASP | Senescence-associated secretory phenotype |
| UMIs | Unique molecular identities |
References
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Ortiz, G.; Duarte, L.F.; Fernandez, C.; Hernandez-Armengol, R.; Palacios, P.A.; Prado, Y.; Andrade, C.A.; Rodriguez-Guilarte, L.; Kalergis, A.M.; et al. Contribution of viral and bacterial infections to senescence and immunosenescence. Front. Cell Infect. Microbiol. 2023, 13, 1229098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- L’Hote, V.; Mann, C.; Thuret, J.Y. From the divergence of senescent cell fates to mechanisms and selectivity of senolytic drugs. Open Biol. 2022, 12, 220171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lelarge, V.; Capelle, R.; Oger, F.; Mathieu, T.; Le Calve, B. Senolytics: From pharmacological inhibitors to immunotherapies, a promising future for patients’ treatment. NPJ Aging 2024, 10, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deryabin, P.I.; Borodkina, A.V. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.J.; Adelson, R.P.; Vashistha, H.; Khalili, H.; Nayyar, A.; Puran, R.; Herrera, R.; Chatterjee, P.K.; Lee, A.T.; Truskinovsky, A.M.; et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Secomandi, L.; Borghesan, M.; Velarde, M.; Demaria, M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum. Reprod. Update 2022, 28, 172–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmieri, L.; Malvezzi, H.; Cestari, B.; Podgaec, S. Colocalization of senescent biomarkers in deep, superficial, and ovarian endometriotic lesions: A pilot study. Sci. Rep. 2022, 12, 17280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallve-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maia, H., Jr.; Haddad, C.; Coelho, G.; Casoy, J. Role of inflammation and aromatase expression in the eutopic endometrium and its relationship with the development of endometriosis. Womens Health 2012, 8, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Brosens, J.J.; Benagiano, G. The eutopic endometrium in endometriosis: Are the changes of clinical significance? Reprod. Biomed. Online 2012, 24, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lang, J.H. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med. Sci. Monit. 2011, 17, RA92–RA99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar] [PubMed]
- Jensen, J.R.; Coddington, C.C., 3rd. Evolving spectrum: The pathogenesis of endometriosis. Clin. Obstet. Gynecol. 2010, 53, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Saleem, M.I.; Yilmaz, M.; DeFranco, M.; Klein, G.; Elmaliki, K.M.; Kowalsky, E.; Chatterjee, P.K.; Xue, X.; Viswanathan, R.; et al. Menstrual Effluent Provides a Novel Diagnostic Window on the Pathogenesis of Endometriosis. Front. Reprod. Health 2020, 2, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ticconi, C.; Di Simone, N.; Campagnolo, L.; Fazleabas, A. Clinical consequences of defective decidualization. Tissue Cell 2021, 72, 101586. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.A.; Shih, A.; Renteira, S.M.; Seckin, T.; Blau, B.; Simpfendorfer, K.; Lee, A.; Metz, C.N.; Gregersen, P.K. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol. Med. 2018, 24, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barragan, F.; Irwin, J.C.; Balayan, S.; Erikson, D.W.; Chen, J.C.; Houshdaran, S.; Piltonen, T.T.; Spitzer, T.L.; George, A.; Rabban, J.T.; et al. Human Endometrial Fibroblasts Derived from Mesenchymal Progenitors Inherit Progesterone Resistance and Acquire an Inflammatory Phenotype in the Endometrial Niche in Endometriosis. Biol. Reprod. 2016, 94, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klemmt, P.A.; Carver, J.G.; Kennedy, S.H.; Koninckx, P.R.; Mardon, H.J. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil. Steril. 2006, 85, 564–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathare, A.D.S.; Loid, M.; Saare, M.; Gidlof, S.B.; Zamani Esteki, M.; Acharya, G.; Peters, M.; Salumets, A. Endometrial receptivity in women of advanced age: An underrated factor in infertility. Hum. Reprod. Update 2023, 29, 773–793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grandi, G.; Barra, F.; Ferrero, S.; Sileo, F.G.; Bertucci, E.; Napolitano, A.; Facchinetti, F. Hormonal contraception in women with endometriosis: A systematic review. Eur. J. Contracept. Reprod. Health Care 2019, 24, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: A review with comparison of 8 guidelines. BMC Womens Health 2021, 21, 397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jamali, N.; Zal, F.; Mostafavi-Pour, Z.; Samare-Najaf, M.; Poordast, T.; Dehghanian, A. Ameliorative Effects of Quercetin and Metformin and Their Combination Against Experimental Endometriosis in Rats. Reprod. Sci. 2021, 28, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.Y.; Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Yamauchi, N.; Yoshida, K.; Azumi, M.; Yoshie, M.; Tamura, K. Senolytic treatment modulates decidualization in human endometrial stromal cells. Biochem. Biophys. Res. Commun. 2021, 571, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Delenko, J.; Xue, X.; Chatterjee, P.K.; Hyman, N.; Shih, A.J.; Adelson, R.P.; Safaric Tepes, P.; Gregersen, P.K.; Metz, C.N. Quercetin enhances decidualization through AKT-ERK-p53 signaling and supports a role for senescence in endometriosis. Reprod. Biol. Endocrinol. 2024, 22, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Yoon, D.; Choi, Y.; Kim, Y.; Cha, J.S.; Yoo, J. Quercetin: Molecular Insights into Its Biological Roles. Biomolecules 2025, 15, 313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dent. Press J. Orthod. 2014, 19, 27–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doi-Tanaka, Y.; Tamura, I.; Shiroshita, A.; Fujimura, T.; Shirafuta, Y.; Maekawa, R.; Taketani, T.; Sato, S.; Sugino, N. Differential gene expression in decidualized human endometrial stromal cells induced by different stimuli. Sci. Rep. 2024, 14, 7726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrington, R.; Williamson, G.; Bennett, R.N.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, Conjugation and Efflux of the Flavonoids, Kaempferol and Galangin, Using the Intestinal CACO-2/TC7 Cell Model. J. Funct. Foods 2009, 1, 74–87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic effects of quercetin in an in vitro model of pre-adipocytes and adipocytes induced senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-kappaB axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ijima, S.; Saito, Y.; Nagaoka, K.; Yamamoto, S.; Sato, T.; Miura, N.; Iwamoto, T.; Miyajima, M.; Chikenji, T.S. Fisetin reduces the senescent tubular epithelial cell burden and also inhibits proliferative fibroblasts in murine lupus nephritis. Front. Immunol. 2022, 13, 960601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullen, M.; Nelson, A.L.; Goff, A.; Billings, J.; Kloser, H.; Huard, C.; Mitchell, J.; Hambright, W.S.; Ravuri, S.; Huard, J. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem Cells 2023, 41, 698–710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.J.; Moon, S.J.; Jeong, J.H.; Lee, S.; Lee, M.H.; Yoo, S.M.; Lee, H.S.; Kang, H.C.; Lee, J.Y.; Lee, W.S.; et al. Kaempferol targeting on the fibroblast growth factor receptor 3-ribosomal S6 kinase 2 signaling axis prevents the development of rheumatoid arthritis. Cell Death Dis. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol. Lett. 2021, 21, 192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, P.; Zhang, J.Y.; Sha, B.B.; Ma, Y.E.; Hu, T.; Ma, Y.C.; Sun, H.; Shi, J.X.; Dong, Z.M.; Li, P. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget 2017, 8, 27471–27480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestermann, K.; Giavridis, T.; Weber, J.; Rydzek, J.; Frenz, S.; Nerreter, T.; Mades, A.; Sadelain, M.; Einsele, H.; Hudecek, M. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 2019, 11, eaau5907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.G.; Hartono, M.; Ou, H.L.; Popov, A.B.; Brown, E.L.; Joseph, J.; Golinska, M.; Gonzalez-Gualda, E.; Macias, D.; Ge, J.; et al. An Indocyanine Green-Based Nanoprobe for In Vivo Detection of Cellular Senescence. Angew. Chem. Int. Ed. Engl. 2024, 63, e202404885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colom-Fernandez, B.; Kreutzman, A.; Marcos-Jimenez, A.; Garcia-Gutierrez, V.; Cuesta-Mateos, C.; Portero-Sainz, I.; Perez-Garcia, Y.; Casado, L.F.; Sanchez-Guijo, F.; Martinez-Lopez, J.; et al. Immediate Effects of Dasatinib on the Migration and Redistribution of Naive and Memory Lymphocytes Associated With Lymphocytosis in Chronic Myeloid Leukemia Patients. Front. Pharmacol. 2019, 10, 1340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.H.; Hsieh, S.C.; Yu, Y.L.; Huang, M.H.; Huang, Y.C.; Hsieh, Y.H. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. PLoS ONE 2013, 8, e71983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, D.; Li, N.; Liu, Y.; Xu, Q.; Liu, Q.; You, Y.; Wei, Z.; Jiang, Y.; Liu, M.; Guo, T.; et al. Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int. Immunopharmacol. 2018, 55, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.O.A.; Alshebremi, M.; Babiker, A.Y.; Rahmani, A.H. The Role of Quercetin, a Flavonoid in the Management of Pathogenesis Through Regulation of Oxidative Stress, Inflammation, and Biological Activities. Biomolecules 2025, 15, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergsten, T.M.; Li, K.; Lantvit, D.D.; Murphy, B.T.; Burdette, J.E. Kaempferol, a Phytoprogestin, Induces a Subset of Progesterone-Regulated Genes in the Uterus. Nutrients 2023, 15, 1407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piltonen, T.T.; Chen, J.C.; Khatun, M.; Kangasniemi, M.; Liakka, A.; Spitzer, T.; Tran, N.; Huddleston, H.; Irwin, J.C.; Giudice, L.C. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum. Reprod. 2015, 30, 1203–1215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simon, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weimar, C.H.; Macklon, N.S.; Post Uiterweer, E.D.; Brosens, J.J.; Gellersen, B. The motile and invasive capacity of human endometrial stromal cells: Implications for normal and impaired reproductive function. Hum. Reprod. Update 2013, 19, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, D.; Vigano, P.; Somigliana, E.; Vicentini, L.M.; Vignali, M.; Busacca, M.; Di Blasio, A.M. Endometrial stromal cells from women with endometriosis reveal peculiar migratory behavior in response to ovarian steroids. Fertil. Steril. 2010, 93, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, C.; Liu, W.; Guo, F.; Kou, X.; Sun, S.; Ye, T.; Li, S.; Zhao, A. Estrogen-induced FOS-like 1 regulates matrix metalloproteinase expression and the motility of human endometrial and decidual stromal cells. J. Biol. Chem. 2020, 295, 2248–2258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanchez, A.M.; Vigano, P.; Mugione, A.; Panina-Bordignon, P.; Candiani, M. The molecular connections between the cannabinoid system and endometriosis. Mol. Hum. Reprod. 2012, 18, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deryabin, P.; Griukova, A.; Nikolsky, N.; Borodkina, A. The link between endometrial stromal cell senescence and decidualization in female fertility: The art of balance. Cell Mol. Life Sci. 2020, 77, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife 2017, 6, e31274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. NPJ Aging 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arrieta, V.A.; Chen, A.X.; Kane, J.R.; Kang, S.J.; Kassab, C.; Dmello, C.; Zhao, J.; Burdett, K.B.; Upadhyayula, P.S.; Lee-Chang, C.; et al. ERK1/2 phosphorylation predicts survival following anti-PD-1 immunotherapy in recurrent glioblastoma. Nat. Cancer 2021, 2, 1372–1386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattes, K.; Berger, G.; Geugien, M.; Vellenga, E.; Schepers, H. CITED2 affects leukemic cell survival by interfering with p53 activation. Cell Death Dis. 2017, 8, e3132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rather, R.A.; Bhagat, M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020, 9, 9181–9192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.A., Jr.; Hogaboam, C.M. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirota, Y.; Daikoku, T.; Tranguch, S.; Xie, H.; Bradshaw, H.B.; Dey, S.K. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J. Clin. Investig. 2010, 120, 803–815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pemmaraju, N.; Garcia, J.S.; Perkins, A.; Harb, J.G.; Souers, A.J.; Werner, M.E.; Brown, C.M.; Passamonti, F. New era for myelofibrosis treatment with novel agents beyond Janus kinase-inhibitor monotherapy: Focus on clinical development of BCL-X(L) /BCL-2 inhibition with navitoclax. Cancer 2023, 129, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Vissers, G.; Giacomozzi, M.; Verdurmen, W.; Peek, R.; Nap, A. The role of fibrosis in endometriosis: A systematic review. Hum. Reprod. Update 2024, 30, 706–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome(R), a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
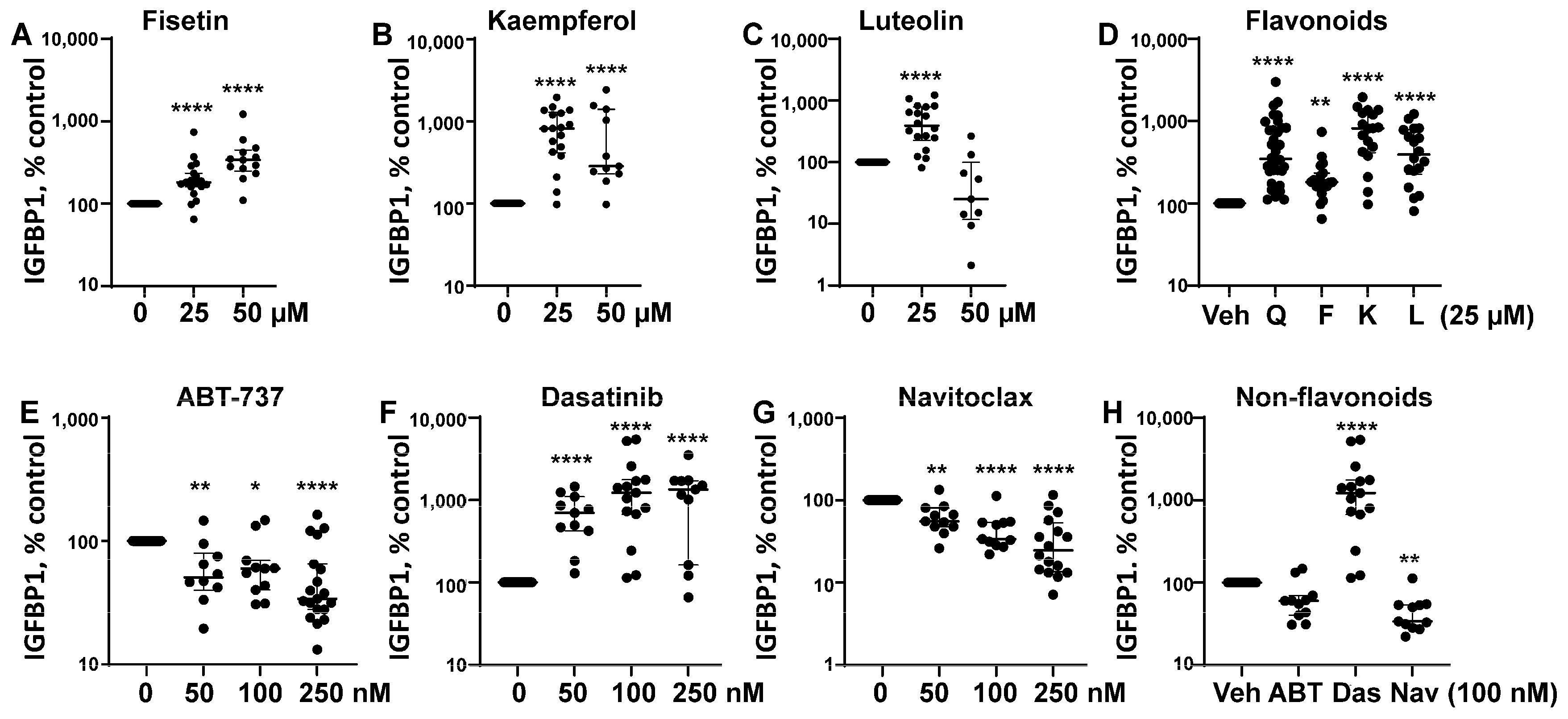



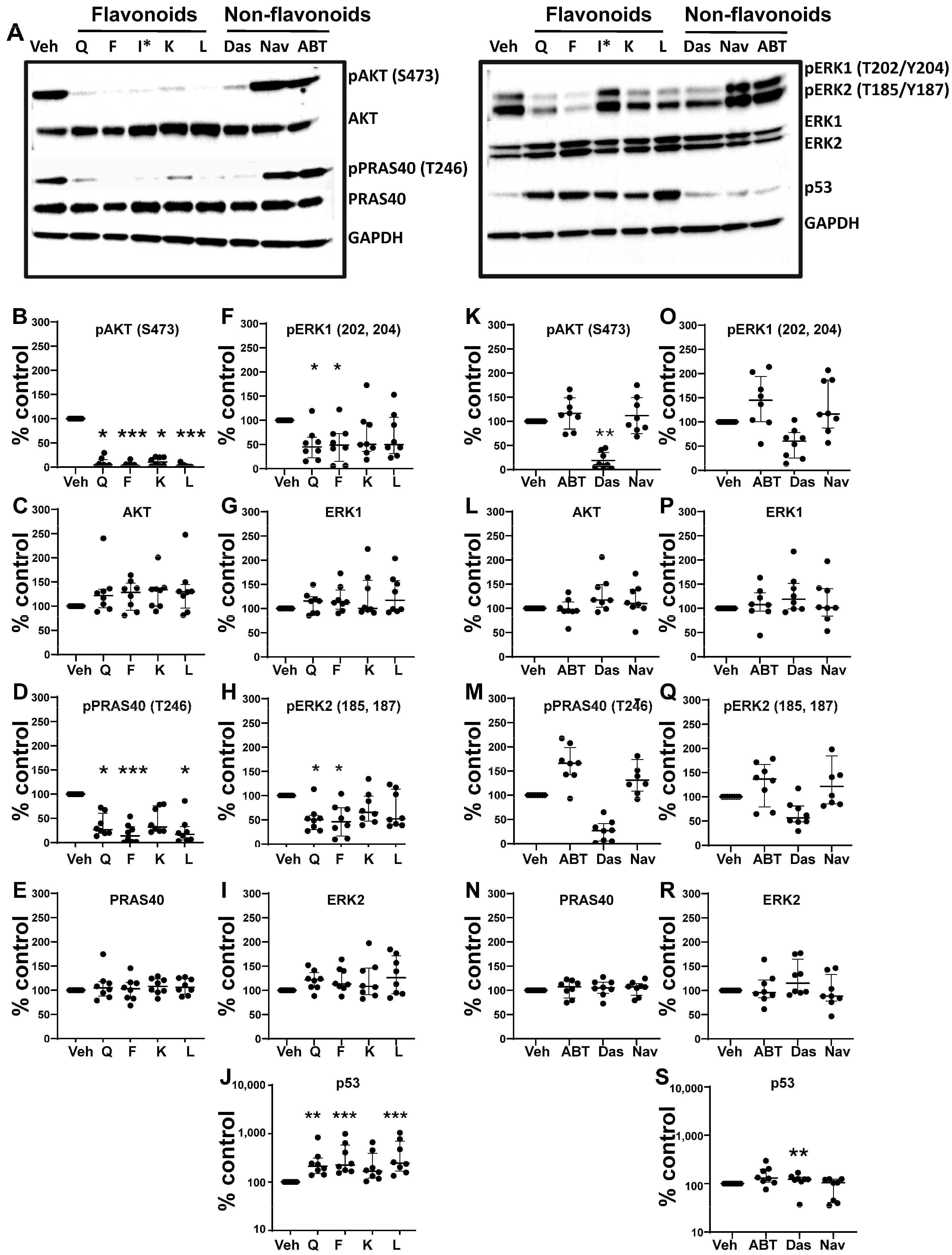

| Senotherapeutic | Flavonoid Y/N Subclass | Senescence-Related Function | Doses Used | Other Functions |
|---|---|---|---|---|
Quercetin | Yes Flavonol | Senolytic Senomorphic | 25–50 µM | Antioxidant anti-inflammatory inhibits CDKs and cyclins Kinase inhibitor (PI3K, AKT) |
Fisetin | Yes Flavonol | Senolytic Senomorphic | 25–50 µM | Antioxidant anti-inflammatory inhibits CDK6 Kinase inhibitor (PI3K, AKT) |
Kaempferol | Yes Flavonol | Senomorphic | 25–50 µM | Antioxidant anti-inflammatory; targets SASP Kinase inhibitor (AKT) |
Luteolin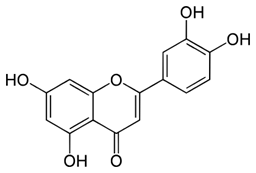 | Yes Flavone | Senolytic (weak) Senomorphic | 25–50 µM | Anti-inflammatory inhibits cyclins Kinase inhibitor (p38) |
ABT-737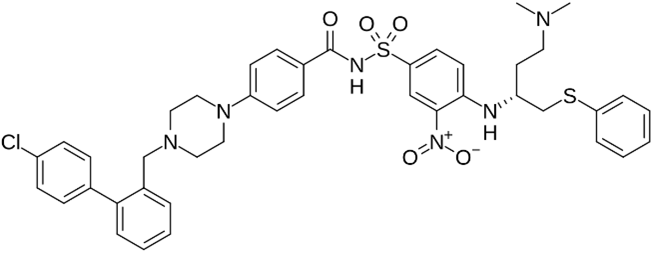 | No BH3 mimetic | Senolytic | 50–250 nM | Inhibits Bcl-2 family members (Bcl-2 and Bcl-xl) |
Dasatinib | No Tyrosine kinase inhibitor | Senolytic | 50–250 nM | Kinase inhibitor (Src/Abl) Anti-inflammatory (indirectly) |
| Navitoclax (ABT-263) 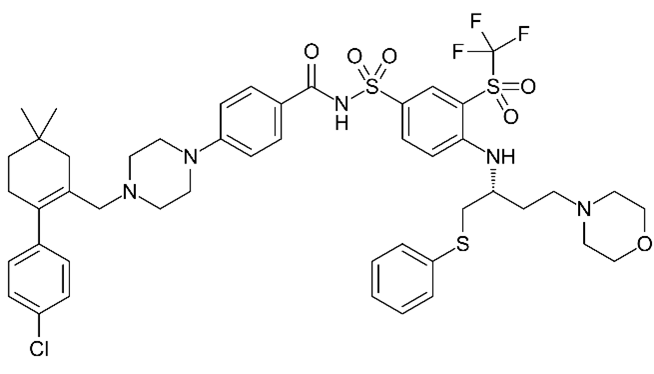 | No BH3 mimetic | Senolytic | 50–250 nM | Inhibits Bcl-2 family members (Bcl-2 and Bcl-xl) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delenko, J.; Hyman, N.; Chatterjee, P.K.; Safaric Tepes, P.; Shih, A.J.; Xue, X.; Gurney, J.; Baker, A.G.; Wei, C.; Munoz Espin, D.; et al. Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration. Biomolecules 2025, 15, 873. https://doi.org/10.3390/biom15060873
Delenko J, Hyman N, Chatterjee PK, Safaric Tepes P, Shih AJ, Xue X, Gurney J, Baker AG, Wei C, Munoz Espin D, et al. Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration. Biomolecules. 2025; 15(6):873. https://doi.org/10.3390/biom15060873
Chicago/Turabian StyleDelenko, Julia, Nathaniel Hyman, Prodyot K. Chatterjee, Polona Safaric Tepes, Andrew J. Shih, Xiangying Xue, Jane Gurney, Andrew G. Baker, Cheng Wei, Daniel Munoz Espin, and et al. 2025. "Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration" Biomolecules 15, no. 6: 873. https://doi.org/10.3390/biom15060873
APA StyleDelenko, J., Hyman, N., Chatterjee, P. K., Safaric Tepes, P., Shih, A. J., Xue, X., Gurney, J., Baker, A. G., Wei, C., Munoz Espin, D., Fruk, L., Gregersen, P. K., & Metz, C. N. (2025). Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration. Biomolecules, 15(6), 873. https://doi.org/10.3390/biom15060873





