Current Efficacy of Multiepitope Vaccines Against Helminths: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registry
2.2. Research Question
2.3. Eligibility Criteria
2.4. Information Sources and Search Strategy
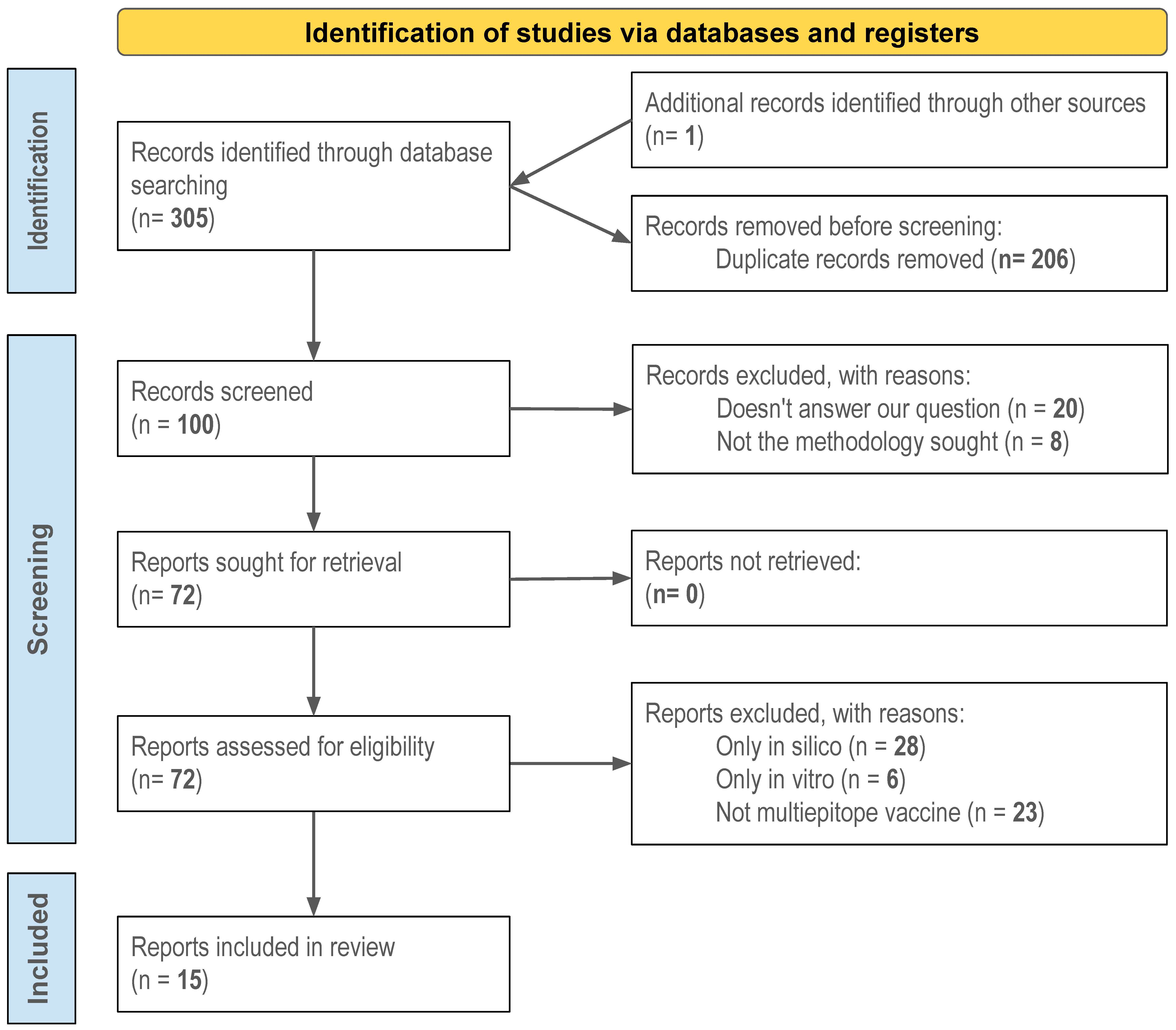
2.5. Selection Process of the Studies
2.6. Data Extraction
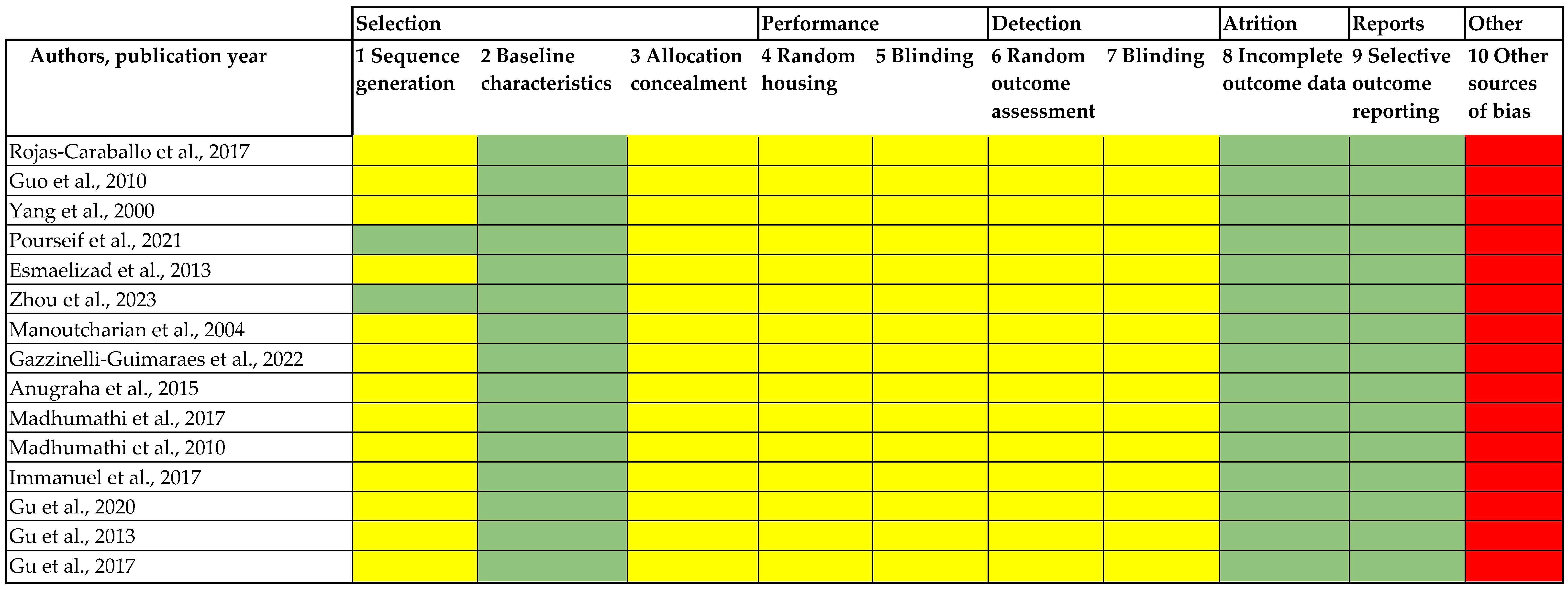
2.7. Risk of Bias
2.8. Data Synthesis and Analysis
3. Results
3.1. Study Selection
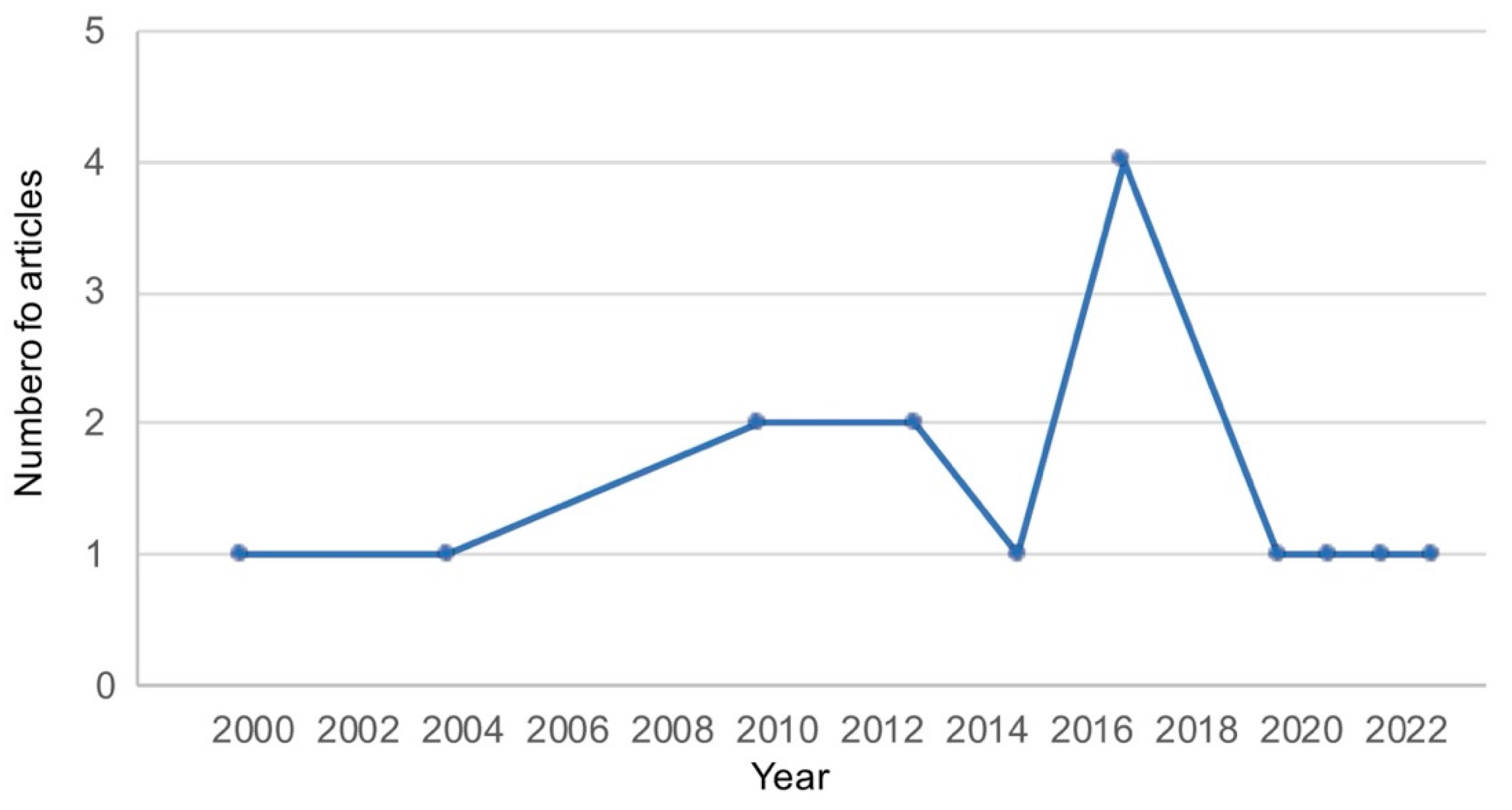
3.2. Study Characteristics
| Parasite | Vaccine Type | Animal Model | Nº Animals (nº per Group) | Country | Authors, Publication Year [Reference] |
|---|---|---|---|---|---|
| Trematodes | |||||
| F. hepatica | Mixture synthetic peptides | Mouse, CD1 | 49 (7) | Spain | Rojas-Caraballo et al., 2017 [16] |
| F. hepatica | Mixture synthetic peptides | Mouse, CD1 | 49 (7) | Spain | Rojas-Caraballo et al., 2017 [16] |
| F. hepatica | Mixture synthetic peptides | Mouse, CD1 | 49 (7) | Spain | Rojas-Caraballo et al., 2017 [16] |
| F. hepatica | Mixture synthetic peptides | Mouse, CD1 | 49 (7) | Spain | Rojas-Caraballo et al., 2017 [16] |
| S. japonicum | Plasmid pET32a | Mouse, BALB/c | 55 (11) | China | Guo et al., 2010 [25] |
| S. mansoni | Synthetic peptide epitope-based polymers | Mice, CBA, BALB/c | 72 (12) | Australia | Yang et al., 2000 [24] |
| S. mansoni | DNA vaccine encoding different epitopes in tandem | Mice CBA, BALB/c, C57BL/6J | 48 (8) or 61 (10) | Australia | Yang et al., 2000 [24] |
| Cestodes | |||||
| E. granulosus | Recombinant multiepitope (rEGVac) | Dog | 15 (3) | Iran | Pourseif et al., 2021 [20] |
| E. granulosus | Recombinant multiepitope (rEGVac) | Sheep | 15 (3) | Iran | Pourseif et al., 2021 [20] |
| E. granulosus | Recombinant fusion polypeptide (ChMEA) | Mouse, BALB/C | 20 (5) | Iran | Esmaelizad et al., 2013 [21] |
| E. multilocularis | Recombinant multiepitope (rMEV) (GILE) | Mice, SWISS, BALB/c | 12 (6) | China | Zhou et al., 2023 [22] |
| T. crassiceps | DNA fragments in phage vector (CPhV) | Mouse, BALB/cAnN | 18 (6) | Mexico | Manoutcharian et al., 2004 [23] |
| T. solium | DNA fragments in phage vector (CPhV) | Pig | 12 (3) | Mexico | Manoutcharian et al., 2004 [23] |
| T. solium | DNA fragments in phage vector (CPhV) | Pig | 12 (3) | Mexico | Manoutcharian et al., 2004 [23] |
| Nematodes | |||||
| A. suum | Recombinant multipeptide (ASCVac-1) | Mouse, BALB/c | 64 (16) | Brazil | Gazzinelli-Guimaraes et al., 2022 [26] |
| B. malayi | Chimeric epitope gene construct (FEP) | Mongolian jirds | 10 (5) | India | Anugraha et al., 2015 [12] |
| B. malayi | Recombinant multiepitope (rAEP) | Mastomys coucha | 18 (6) | India | Madhumathi et al., 2017 [13] |
| B. malayi | Conjugated synthetic peptides (PC1) | Mastomys coucha | 18 (6) | India | Madhumathi et al., 2010 [14] |
| B. malayi | Synthetic multi-antigen peptide (TT MAP) | Mongolian jird | 25 (5) | India | Immanuel et al., 2017 [15] |
| T. spiralis | Multiple antigen peptide (MAP-TB) | Mouse, BALB/c | 30 (10) | China | Gu et al., 2020 [17] |
| T. spiralis | Multiple antigen peptide (MAP-B) | Mouse, BALB/c | 30 (10) | China | Gu et al., 2020 [17] |
| T. spiralis | KLH conjugated peptides | Mouse, BALB/c | 48 (6) | China | Gu et al., 2013 [18] |
| T. spiralis | Recombinant multiepitope (rMEV) | Mouse, BALB/c | 36 (12) | China | Gu et al., 2017 [19] |
3.3. Risk of Bias Assessment
3.4. Synthesis of Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, F.E.G. History of Human Parasitology. Clin. Microbiol. Rev. 2002, 15, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Ng’etich, A.I.; Amoah, I.D.; Bux, F.; Kumari, S. Anthelmintic Resistance in Soil-Transmitted Helminths: One-Health Considerations. Parasitol. Res. 2024, 123, 62. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Vidal, R.M.; Velasco, J.; Carreño, L.J.; Torres, J.P.; Benachi O., M.A.; Tovar-Rosero, Y.-Y.; Oñate, A.A.; O’Ryan, M. Two Centuries of Vaccination: Historical and Conceptual Approach and Future Perspectives. Front. Public. Health 2024, 11, 1326154. [Google Scholar] [CrossRef]
- Claerebout, E.; Geldhof, P. Helminth Vaccines in Ruminants: From Development to Application. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 159–171. [Google Scholar] [CrossRef]
- Kuri, P.R.; Goswami, P. Current Update on Rotavirus In-Silico Multiepitope Vaccine Design. ACS Omega 2023, 8, 190–207. [Google Scholar] [CrossRef]
- Lopes, K.F.; Freire, M.L.; Murta, S.M.F.; Oliveira, E. Efficacy of Vaccines Based on Chimeric or Multiepitope Antigens for Protection against Visceral Leishmaniasis: A Systematic Review. PLoS Negl. Trop. Dis. 2024, 18, e0012757. [Google Scholar] [CrossRef] [PubMed]
- Rajneesh; Tiwari, R.; Singh, V.K.; Kumar, A.; Gupta, R.P.; Singh, A.K.; Gautam, V.; Kumar, R. Advancements and Challenges in Developing Malaria Vaccines: Targeting Multiple Stages of the Parasite Life Cycle. ACS Infect. Dis. 2023, 9, 1795–1814. [Google Scholar] [CrossRef]
- Perera, D.J.; Ndao, M. Promising Technologies in the Field of Helminth Vaccines. Front. Immunol. 2021, 12, 711650. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 3 February 2025).
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Anugraha, G.; Madhumathi, J.; Prince, P.R.; Jeya Prita, P.J.; Khatri, V.K.; Amdare, N.P.; Reddy, M.V.R.; Kaliraj, P. Chimeric Epitope Vaccine from Multistage Antigens for Lymphatic Filariasis. Scand. J. Immunol. 2015, 82, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, J.; Prince, P.R.; Rao, D.N.; Karande, A.A.; Reddy, M.V.R.; Kaliraj, P. Epitope Mapping of Brugia malayi ALT-2 and the Development of a Multi-Epitope Vaccine for Lymphatic Filariasis. J. Helminthol. 2017, 91, 43–54. [Google Scholar] [CrossRef]
- Madhumathi, J.; Prince, P.R.; Anugraha, G.; Kiran, P.; Rao, D.N.; Reddy, M.R.; Kaliraj, P. Identification and Characterization of Nematode Specific Protective Epitopes of Brugia malayi TRX towards Development of Synthetic Vaccine Construct for Lymphatic Filariasis. Vaccine 2010, 28, 5038–5048. [Google Scholar] [CrossRef] [PubMed]
- Immanuel, C.; Ramanathan, A.; Balasubramaniyan, M.; Khatri, V.K.; Amdare, N.P.; Rao, D.N.; Reddy, M.V.R.; Perumal, K. Immunoprophylaxis of Multi-Antigen Peptide (MAP) Vaccine for Human Lymphatic Filariasis. Immunol. Res. 2017, 65, 729–738. [Google Scholar] [CrossRef]
- Rojas-Caraballo, J.; López-Abán, J.; Moreno-Pérez, D.A.; Vicente, B.; Fernández-Soto, P.; Del Olmo, E.; Patarroyo, M.A.; Muro, A. Transcriptome Profiling of Gene Expression during Immunisation Trial against Fasciola hepatica: Identification of Genes and Pathways Involved in Conferring Immunoprotection in a Murine Model. BMC Infect. Dis. 2017, 17, 94. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, X.; Huang, J.; Zhan, B.; Zhu, X. A Multiple Antigen Peptide Vaccine Containing CD4+ T Cell Epitopes Enhances Humoral Immunity against Trichinella spiralis Infection in Mice. J. Immunol. Res. 2020, 2020, 2074803. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, J.; Yang, J.; Huang, J.; Yang, X.; Zhu, X. Protective Immunity against Trichinella spiralis Infection Induced by a Multi-Epitope Vaccine in a Murine Model. PLoS ONE 2013, 8, e77238. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Sun, X.; Li, B.; Huang, J.; Zhan, B.; Zhu, X. Vaccination with a Paramyosin-Based Multi-Epitope Vaccine Elicits Significant Protective Immunity against Trichinella spiralis Infection in Mice. Front. Microbiol. 2017, 8, 1475. [Google Scholar] [CrossRef]
- Pourseif, M.M.; Moghaddam, G.; Nematollahi, A.; Khordadmehr, M.; Naghili, B.; Dehghani, J.; Omidi, Y. Vaccination with rEGVac Elicits Immunoprotection against Different Stages of Echinococcus granulosus Life Cycle: A Pilot Study. Acta Trop. 2021, 218, 105883. [Google Scholar] [CrossRef]
- Esmaelizad, M.; Ahmadian, G.; Aghaiypour, K.; Shamsara, M.; Paykari, H.; Tebianian, M. Induction of Protective T-Helper 1 Immune Responses against Echinococcus granulosus in Mice by a Multi-T-Cell Epitope Antigen Based on Five Proteins. Mem. Inst. Oswaldo Cruz 2013, 108, 408–413. [Google Scholar] [CrossRef]
- Zhou, P.; Zhou, Z.; Huayu, M.; Wang, L.; Feng, L.; Xiao, Y.; Dai, Y.; Xin, M.; Tang, F.; Li, R. A Multi-Epitope Vaccine GILE against Echinococcus multilocularis Infection in Mice. Front. Immunol. 2023, 13, 1091004. [Google Scholar] [CrossRef] [PubMed]
- Manoutcharian, K.; Díaz-Orea, A.; Gevorkian, G.; Fragoso, G.; Acero, G.; González, E.; De Aluja, A.; Villalobos, N.; Gómez-Conde, E.; Sciutto, E. Recombinant Bacteriophage-Based Multiepitope Vaccine against Taenia solium Pig Cysticercosis. Vet. Immunol. Immunopathol. 2004, 99, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jackson, D.C.; Zeng, Q.; McManus, D.P. Multi-Epitope Schistosome Vaccine Candidates Tested for Protective Immunogenicity in Mice. Vaccine 2000, 19, 103–113. [Google Scholar] [CrossRef]
- Guo, F.J.; Wang, Y.; Li, Y.; Peng, J.B.; Yang, H.; Qiu, C.H.; Chen, S.; Fu, Z.Q.; Shi, Y.J.; Lin, J.J. Evaluation on the Immuno-Protective Efficacy of the Recombinant Antigen SjPGAM-SjEnol against Schistosoma japonicum in Mice. Chin. J. Parasitol. Parasit. Dis. 2010, 28, 246–251. [Google Scholar]
- Gazzinelli-Guimarães, A.C.; Nogueira, D.S.; Amorim, C.C.O.; Oliveira, F.S.; Coqueiro-Dos-Santos, A.; Carvalho, S.A.P.; Kraemer, L.; Barbosa, F.S.; Fraga, V.G.; Santos, F.V.; et al. ASCVac-1, a Multi-Peptide Chimeric Vaccine, Protects Mice Against Ascaris Suum Infection. Front. Immunol. 2021, 12, 788185. [Google Scholar] [CrossRef]
- Atere, A.O.; Mustapha, F.B.; Mustapha, M.J. Logistics of the Malaria Vaccine Roll-out in Nigeria. Lancet 2025, 405, 1815. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S. The Immunology of Parasite Infections: Grand Challenges. Front. Parasitol. 2022, 1, 1069205. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, J.; Yan, C.; Wang, D.; Pan, W. Trained Immunity: A Cutting Edge Approach for Designing Novel Vaccines against Parasitic Diseases? Front. Immunol. 2023, 14, 1252554. [Google Scholar] [CrossRef]
- Hambrook, J.R.; Hanington, P.C. Immune Evasion Strategies of Schistosomes. Front. Immunol. 2021, 11, 624178. [Google Scholar] [CrossRef]
- Zhang, W.; Molehin, A.; Patel, P.; Kim, E.; Peña, A.; Siddiqui, A.A. Testing of Schistosoma mansoni Vaccine Targets. In Schistosoma mansoni: Methods and Protocols; Timson, D.J., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2151, pp. 229–262. ISBN 978-1-0716-0634-6. [Google Scholar]
- Ortega, L.; Quesada, J.; Ruiz, A.; Conde-Felipe, M.M.; Ferrer, O.; Muñoz, M.D.C.; Molina, J.A.; Rodríguez, F.; Molina, J.M. Analysis of Protection and Immune Response against Teladorsagia circumcincta in Goats Immunised with Thiol-Binding Proteins from Adult Worms. Vaccines 2024, 12, 437. [Google Scholar] [CrossRef]
- Machín, C.; Corripio-Miyar, Y.; Hernández, J.N.; Pérez-Hernández, T.; Hayward, A.D.; Wright, H.W.; Price, D.R.G.; Matthews, J.B.; McNeilly, T.N.; Nisbet, A.J.; et al. Cellular and Humoral Immune Responses Associated with Protection in Sheep Vaccinated against Teladorsagia circumcincta. Vet. Res. 2021, 52, 89. [Google Scholar] [CrossRef] [PubMed]
- Stear, M.; Preston, S.; Piedrafita, D.; Donskow-Łysoniewska, K. The Immune Response to Nematode Infection. Int. J. Mol. Sci. 2023, 24, 2283. [Google Scholar] [CrossRef]
- Shears, R.K.; Grencis, R.K. Whipworm Secretions and Their Roles in Host-Parasite Interactions. Parasit. Vectors 2022, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, A.; Forman, R.; Smith, H.; Mair, I.; Jibril, M.; Albaqshi, M.H.; Brass, A.; Derrick, J.P.; Else, K.J. In Silico Design of a T-Cell Epitope Vaccine Candidate for Parasitic Helminth Infection. PLoS Pathog. 2020, 16, e1008243. [Google Scholar] [CrossRef] [PubMed]
- Debroy, B.; Chowdhury, S.; Pal, K. Designing a Novel and Combinatorial Multi-Antigenic Epitope-Based Vaccine “MarVax” against Marburg Virus—A Reverse Vaccinology and Immunoinformatics Approach. J. Genet. Eng. Biotechnol. 2023, 21, 143. [Google Scholar] [CrossRef]
- Jamialahmadi, H.; Khalili-Tanha, G.; Nazari, E.; Rezaei-Tavirani, M. Artificial Intelligence and Bioinformatics: A Journey from Traditional Techniques to Smart Approaches. Gastroenterol. Hepatol. Bed Bench 2024, 17, 241–252. [Google Scholar] [CrossRef]
- Abdelaziz, E.H.; Ismail, R.; Mabrouk, M.S.; Amin, E. Multi-Omics Data Integration and Analysis Pipeline for Precision Medicine: Systematic Review. Comput. Biol. Chem. 2024, 113, 108254. [Google Scholar] [CrossRef]
- Sánchez-Montejo, J.; Strilets, T.; Manzano-Román, R.; López-Abán, J.; García-Blanco, M.A.; Vicente, B.; Muro, A. Design and Expression of Fasciola Hepatica Multiepitope Constructs Using mRNA Vaccine Technology. Int. J. Mol. Sci. 2025, 26, 1190. [Google Scholar] [CrossRef]
- Das, N.C.; Gorai, S.; Gupta, P.S.S.; Panda, S.K.; Rana, M.K.; Mukherjee, S. Immune Targeting of Filarial Glutaredoxin through a Multi-Epitope Peptide-Based Vaccine: A Reverse Vaccinology Approach. Int. Immunopharmacol. 2024, 133, 112120. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Corripio-Miyar, Y.; Hayward, A.D.; Lemon, H.; Bal, X.; Cunnea, C.; Kenyon, F.; Pilkington, J.G.; Pemberton, J.M.; Nussey, D.H.; McNeilly, T.N. T-Helper Cell Phenotypes Are Repeatable, Positively Correlated, and Associated with Helminth Infection in Wild Soay Sheep. Discov. Immunol. 2025, 4, kyae017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ahmad, G.; Torben, W.; Noor, Z.; Le, L.; Damian, R.T.; Wolf, R.F.; White, G.L.; Chavez-Suarez, M.; Podesta, R.B.; et al. Sm-p80–Based DNA Vaccine Provides Baboons with Levels of Protection against Schistosoma mansoni Infection Comparable to Those Achieved by the Irradiated Cercarial Vaccine. J. Infect. Dis. 2010, 201, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.J.; McNeilly, T.N.; Price, D.R.G.; Oliver, E.M.; Bartley, Y.; Mitchell, M.; Palarea-Albaladejo, J.; Matthews, J.B. The Rational Simplification of a Recombinant Cocktail Vaccine to Control the Parasitic Nematode Teladorsagia circumcincta. Int. J. Parasitol. 2019, 49, 257–265. [Google Scholar] [CrossRef]
- Caoili, S.E.C. Comprehending B-Cell Epitope Prediction to Develop Vaccines and Immunodiagnostics. Front. Immunol. 2022, 13, 908459. [Google Scholar] [CrossRef]
- Caoili, S.E.C. Beyond B-Cell Epitopes: Curating Positive Data on Antipeptide Paratope Binding to Support Peptide-Based Vaccine Design. Protein Pept. Lett. 2021, 28, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Farnell, E.J.; Fitzsimmons, C.M.; Ryan, S.; Tukahebwa, E.; Maizels, R.M.; Dunne, D.W.; Thornton, J.M.; Furnham, N. Comparisons of Allergenic and Metazoan Parasite Proteins: Allergy the Price of Immunity. PLoS Comput. Biol. 2015, 11, e1004546. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the Host Immune System by Helminth Parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef]
- Manhani, M.N.; Tilelli, C.Q.; Ribeiro, V.D.S.; Goulart, L.R.; Costa-Cruz, J.M. Mimotope-Based Antigens as Potential Vaccine Candidates in Experimental Murine Cysticercosis. Parasitology 2020, 147, 1330–1337. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Vacca, F.; Le Gros, G. Tissue-Specific Immunity in Helminth Infections. Mucosal Immunol. 2022, 15, 1212–1223. [Google Scholar] [CrossRef]
- Fitzsimmons, C.M.; Falcone, F.H.; Dunne, D.W. Helminth Allergens, Parasite-Specific IgE, and Its Protective Role in Human Immunity. Front. Immunol. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Adam, L.; Rosenbaum, P.; Bonduelle, O.; Combadière, B. Strategies for Immunomonitoring after Vaccination and during Infection. Vaccines 2021, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Torelli, A.; Piu, P.; Bonifazi, C.; Ganfini, L.; Montomoli, E. Flow Cytometry as an Integrative Method for the Evaluation of Vaccine Immunogenicity: A Validation Approach. Biochem. Biophys. Rep. 2023, 34, 101472. [Google Scholar] [CrossRef]
- Sitali, M.C.; Schmidt, V.; Mwenda, R.; Sikasunge, C.S.; Mwape, K.E.; Simuunza, M.C.; Da Costa, C.P.; Winkler, A.S.; Phiri, I.K. Experimental Animal Models and Their Use in Understanding Cysticercosis: A Systematic Review. PLoS ONE 2022, 17, e0271232. [Google Scholar] [CrossRef]
- Sharma, R.; Rajput, V.S.; Jamal, S.; Grover, A.; Grover, S. An Immunoinformatics Approach to Design a Multi-Epitope Vaccine against Mycobacterium Tuberculosis Exploiting Secreted Exosome Proteins. Sci. Rep. 2021, 11, 13836. [Google Scholar] [CrossRef]
- Celis-Giraldo, C.; Suárez, C.F.; Agudelo, W.; Ibarrola, N.; Degano, R.; Díaz, J.; Manzano-Román, R.; Patarroyo, M.A. Immunopeptidomics of Salmonella enterica Serovar Typhimurium-Infected Pig Macrophages Genotyped for Class II Molecules. Biology 2024, 13, 832. [Google Scholar] [CrossRef] [PubMed]
- Palma, M. Epitopes and Mimotopes Identification Using Phage Display for Vaccine Development against Infectious Pathogens. Vaccines 2023, 11, 1176. [Google Scholar] [CrossRef]
- McCaffrey, P. Bioinformatic Techniques for Vaccine Development: Epitope Prediction and Structural Vaccinology. In Vaccine Design: Methods and Protocols, Volume 3. Resources for Vaccine Development; Thomas, S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2412, ISBN 978-1-0716-1891-2. [Google Scholar]
- Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef]
- Cecílio, P.; Oristian, J.; Meneses, C.; Serafim, T.D.; Valenzuela, J.G.; Cordeiro Da Silva, A.; Oliveira, F. Engineering a Vector-Based Pan-Leishmania Vaccine for Humans: Proof of Principle. Sci. Rep. 2020, 10, 18653. [Google Scholar] [CrossRef]
| Parasite | Antigen | Adjuvant | Dose/Route/Schedule | Challenge/Administration Route | Vaccine Efficacy (% Reduction) | Humoral Response | Cellular Response | Authors, Publication Year [Reference] |
|---|---|---|---|---|---|---|---|---|
| Trematodes | ||||||||
| F. hepatica | B6, T14 (B, T epitopes) | ADAD | 10 μg s.c./3 doses/2 w interval | 7 metacercariae/v.o. | 31% hepatic lesion, 57.1% survival | No study | No study | Rojas-Caraballo et al., 2017 [16] |
| F. hepatica | B1, B5, B6 (B epitopes) | ADAD | 10 μg s.c./3 doses/2 w interval | 7 metacercariae/v.o. | 14% hepatic lesion, 57.1% survival | No study | No study | Rojas-Caraballo et al., 2017 [16] |
| F. hepatica | T14, T15, T16 (T epitopes) | ADAD | 10 μg s.c./3 doses/2 w interval | 7 metacercariae/v.o. | 45% hepatic lesion, 71.4% survival | ↑IgG, IgG1 | ↑IL-12, IL-10, IL-8 | Rojas-Caraballo et al., 2017 [16] |
| F. hepatica | B1, B2, B5, B6, T14, T15, T16 (B, T epitopes) | ADAD | 10 μg s.c./3 doses/2 w interval | 7 metacercariae/v.o. | 39% hepatic lesion, 57.1% survival | No study | No study | Rojas-Caraballo et al., 2017 [16] |
| S. japonicum | SjPGAM-SjEnol | Montanide ISA 206 | 27 μg s.c./3 doses/2 w interval | 40 cercariae/i.p. | 39.7% adult worm, 64.9% liver egg | ↑IgG | No study | Guo et al., 2010 [25] |
| S. mansoni | Pmy-3, TPI-1, TPI-2, Sm23, Sm28-1, Sm28-2, Sm28-3, Smcal (Polymer-1); Pmy-1, Pmy-2, TPI-1, TPI-2, Sm23, Sm28-1, Sm28-2, Sm28-3 (Polymer-2) (B, T epitopes) | Freund’s adjuvant | 50 μg i.m./3 doses/2 w interval | 120 cercariae/p.c. | No protection | ↑IgG | No study | Yang et al., 2000 [24] |
| S. mansoni | Pmy-1, Pmy-2, TPI-1, TPI-2, Sm23, Sm28-1, Sm28-2, Sm28-3 (B, T epitopes) | No adjuvant | 100 μg i.m./3 doses/3 w interval | 150 cercariae/p.c. | No protection | No study | No study | Yang et al., 2000 [24] |
| Cestodes | ||||||||
| E. granulosus | Eg95, Eg14-3-3, EgEnolase (B, T epitopes) | Freund’s adjuvant | 0.5 mg/mL s.c./2 doses/4 w interval | 105,000 protoscoleces/v.o. | 100% intestine adults | ↑IgG, IgE | ↑IL-4 | Pourseif et al., 2021 [20] |
| E. granulosus | Eg95, Eg14-3-3, EgEnolase (B, T epitopes) | Freund’s adjuvant | 1 mg/mL s.c./2 doses/4 w interval | 2000 eggs/v.o. | 85.4% liver cysts | ↑IgG, IgE | ↑IL-4 | Pourseif et al., 2021 [20] |
| E. granulosus | EgA31, EgTrp, EgGST, Eg95, P14-3-5 (T epitopes) | Freund’s adjuvant | 50 μg s.c./3 doses/2 w interval | 500 protoscoleces/i.p. | 99.6% peritoneal cysts | No study | No study | Esmaelizad et al., 2013 [21] |
| E. multilocularis | EMY162, LAP, GLUT1 (B, T epitopes) | Freund’s adjuvant | 50 μg i.p./4 doses/1 w interval | 1000 protoscoleces/i.p. | 50.0% liver cysts, 96.9% cysts weight | ↑IgG | ↑IFN-γ, IL-4, CD4+, CD8+ | Zhou et al., 2023 [22] |
| T. crassiceps | KETc1, KETc12, GK1, KETc7 | No adjuvant | 5 × 1010 phage s.c./4 doses/0, 12, 23, 34 d | 10 cysticerci/i.p. | 65.9% peritoneal cysticerci | No study | No study | Manoutcharian et al., 2004 [23] |
| T. solium | KETc1, KETc12, GK1, KETc7 | No adjuvant | 4 × 1011–4 × 1012 phage s.c./2 doses/2 w interval | 17,000 eggs/v.o. | 95.1% muscle cysticerci | ↑IgG | ↑IFN-γ, IL-4. Lymphocyte proliferation | Manoutcharian et al., 2004 [23] |
| T. solium | KETc1, KETc12, GK1, KETc7 | No adjuvant | 4 × 1012 phage v.o./2 doses/2 w interval | 17,000 eggs/v.o. | 41.7% muscle cysticerci | = IgG | ↑IFN-γ, IL-4. Lymphocyte proliferation | Manoutcharian et al., 2004 [23] |
| Nematodes | ||||||||
| A. suum | Top 35 immunogenic (B epitopes) | BpMPLA | 25 μg s.c./2 doses/10 d interval | 2500 embryonated eggs/v.o. | 33.7% lung larvae | ↑IgG1, IgG3, IgE. Low IgG2a | ↑IL-4, IL-5, IL-13 in lung, ↑ lymphocyte, eosinophil.↓ neutrophils | Gazzinelli-Guimaraes et al., 2022 [26] |
| B. malayi | TRX, TGA, ALT-2 (B, T epitopes) | Alum | 50 μg i.p./4 doses/2 w interval | 20 L3 larvae/i.p. | 69.5% implanted larvae | ↑IgG, IgG1, IgG2a, IgG2b | ↑IL-5, IFN-γ. = IL-4, IL-2, IL-10. Splenocyte proliferation | Anugraha et al., 2015 [12] |
| B. malayi | 1–30 (EDI), 89–128 (EDII) of ALT-2 (B epitopes) | Alum | 50 μg i.p./4 doses/1 w interval | 10 L3 larvae i.p. | 74.6% implanted larvae | ↑IgG1, IgG2b. = in IgGa, IgG3, IgA, IgM | No study | Madhumathi et al., 2017 [13] |
| B. malayi | TRXP1, TRXP2 (B, T epitopes) | Alum | 100 μg i.p./4 doses/1 w interval | 10 L3 larvae i.p. | 75.1% implanted larvae | No study | ↑IL-2 IL-5. = IL-4, IL-10 and IFN-γ | Madhumathi et al., 2010 [14] |
| B. malayi | TGA, TRX P1, TRX P2 (B, T epitopes) | Alum | 100 μg i.p./4 doses/1 w interval | 10 L3 larvae/i.p. | 63.0% implanted larvae | ↑IgG1, IgM | ↑IL-2, IFN-γ, IL-4, IL-5, IL-10. Splenocyte proliferation | Immanuel et al., 2017 [15] |
| T. spiralis | T2, T5 (T epitopes), YX1 (B epitope) | Freund’s adjuvant | 30 μg s.c./3 doses, 2 w interval | 400 muscle larvae/v.o. | 35.5% muscle larvae | ↑IgG, IgG1, IgG2a | ↑IFN-γ, IL-2, IL-4, IL-5, IL-6, Tfh, GC.↓ Tfr, Treg. | Gu et al., 2020 [17] |
| T. spiralis | YX1 (B epitope) | Freund’s adjuvant | 30 μg s.c./3 doses/2 w interval | 400 muscle larvae/v.o. | 12.4% muscle larvae | ↑IgG1 ↓ IgG2a | = cytokines.↑ Tfh, GC.↓ Tfr, Treg | Gu et al., 2020 [17] |
| T. spiralis | YX1-KLH, 8F7-KLH, M7-KLH epitopes | Montanide ISA 50 V2 | 50 μg s.c./3 doses/2 w interval | 400 muscle larvae/v.o. | 35.0% muscle larvae | ↑IgG, IgG1 | No study | Gu et al., 2013 [18] |
| T. spiralis | P2, P3, P4, P5 (T epitopes), YX1 (B epitope) | Montanide ISA 50 V2 | 25 μg s.c./3 doses/2 w interval | 400 muscle larvae/v.o. | 55.4% muscle larvae | ↑IgG, IgG1, IgG2 | ↑IFN-γ, IL-4, IL-5 | Gu et al., 2017 [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo-Rodríguez, I.; López-Abán, J.; Alonso-Sardón, M.; Vicente-Santiago, B.; Muro-Álvarez, A.; Manzano-Román, R. Current Efficacy of Multiepitope Vaccines Against Helminths: A Systematic Review. Biomolecules 2025, 15, 867. https://doi.org/10.3390/biom15060867
Trujillo-Rodríguez I, López-Abán J, Alonso-Sardón M, Vicente-Santiago B, Muro-Álvarez A, Manzano-Román R. Current Efficacy of Multiepitope Vaccines Against Helminths: A Systematic Review. Biomolecules. 2025; 15(6):867. https://doi.org/10.3390/biom15060867
Chicago/Turabian StyleTrujillo-Rodríguez, Ignacio, Julio López-Abán, Montserrat Alonso-Sardón, Belén Vicente-Santiago, Antonio Muro-Álvarez, and Raúl Manzano-Román. 2025. "Current Efficacy of Multiepitope Vaccines Against Helminths: A Systematic Review" Biomolecules 15, no. 6: 867. https://doi.org/10.3390/biom15060867
APA StyleTrujillo-Rodríguez, I., López-Abán, J., Alonso-Sardón, M., Vicente-Santiago, B., Muro-Álvarez, A., & Manzano-Román, R. (2025). Current Efficacy of Multiepitope Vaccines Against Helminths: A Systematic Review. Biomolecules, 15(6), 867. https://doi.org/10.3390/biom15060867






