Retinoic-Acid-Related Orphan Receptor Alpha Is Involved in the Regulation of the Cytoskeleton of Hair Follicle Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cellular Model and Drug Treatment
2.2. Immunofluorescence
2.3. Real-Time qPCR
2.4. Western Blotting
2.5. Migration Experiment
2.6. Wound Healing Assay and Adhesion Capacity Detection
2.7. Phalloidin Staining
2.8. Cleavage Under Targets and Release Using Nuclease (CUT&RUN)
2.9. Droplet Digital PCR (ddPCR)
2.10. Super-Shift Electrophoretic Mobility Shift Assay
2.11. Statistical Analysis
3. Results
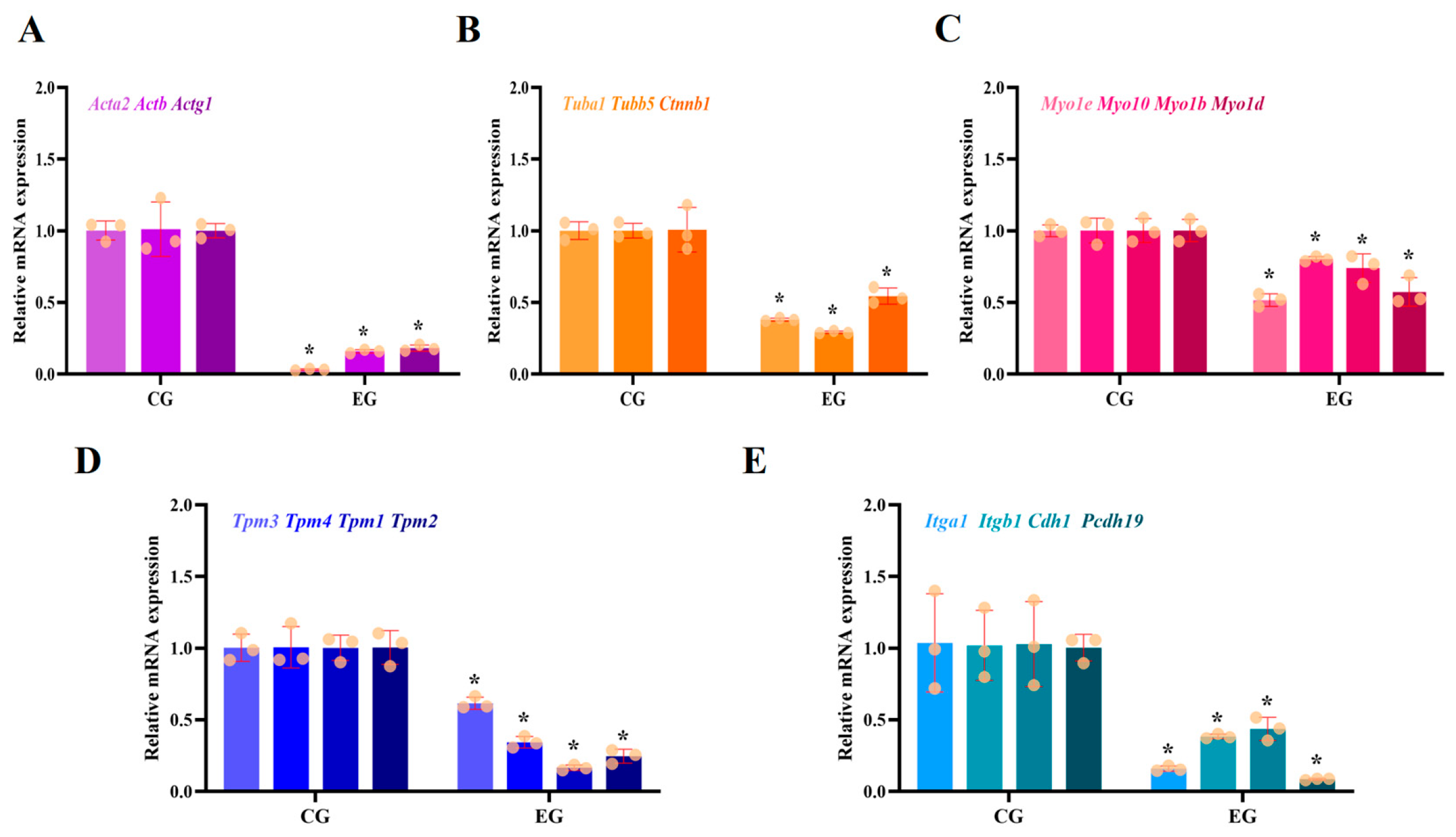
3.1. RORA Activation Inhibits mRNA Levels of Genes Related to Cytoskeletal Composition and Cell Junctions
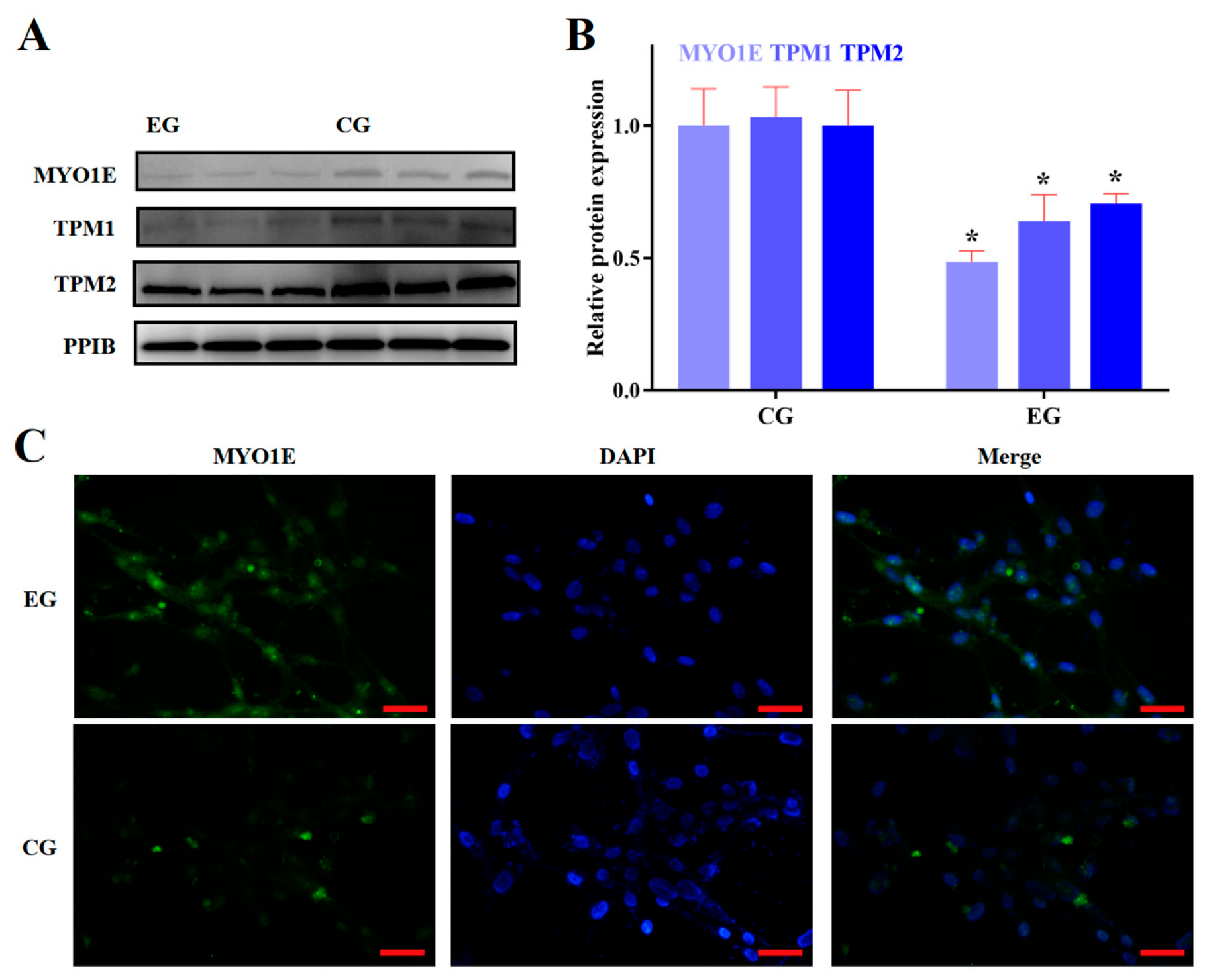
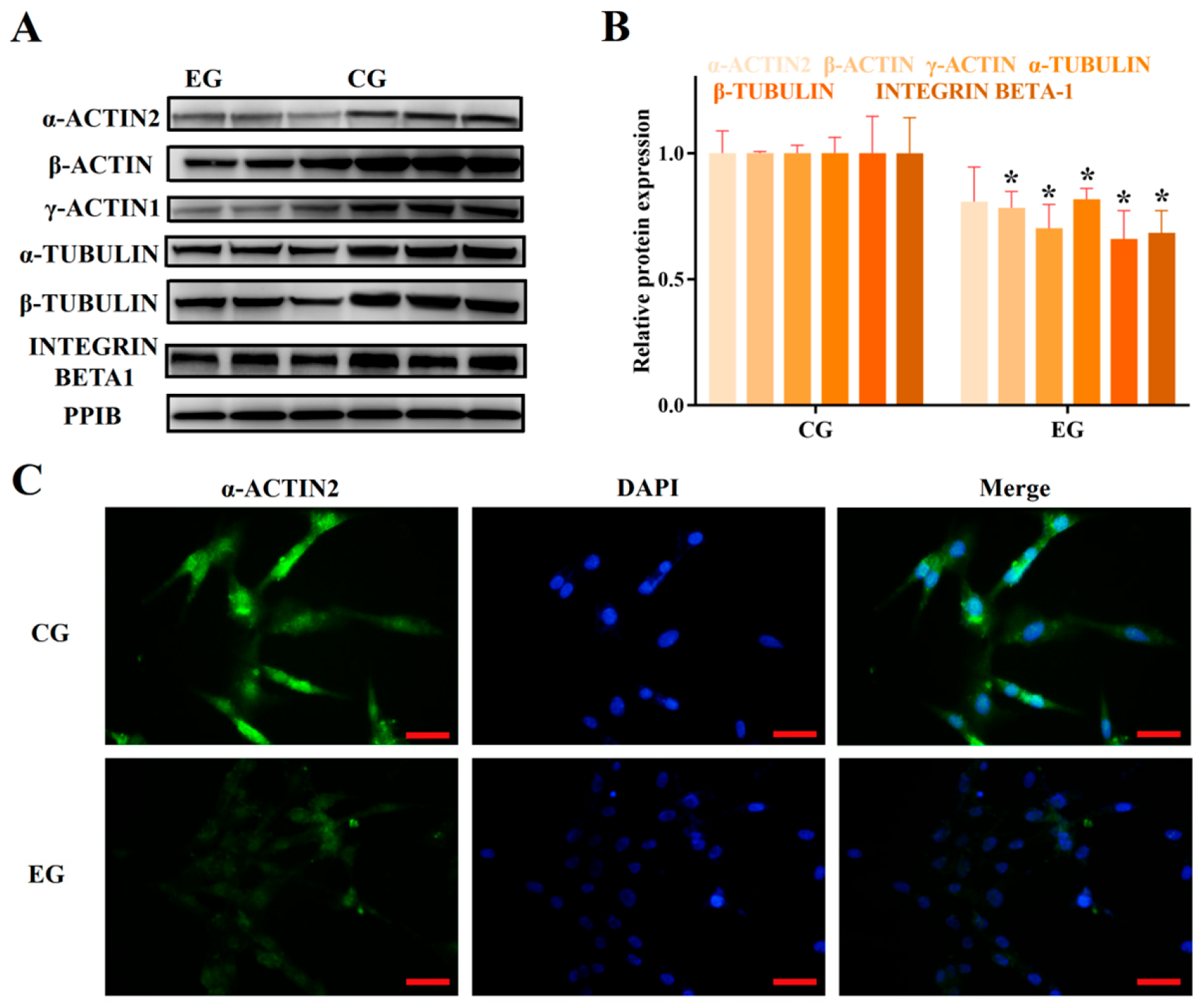
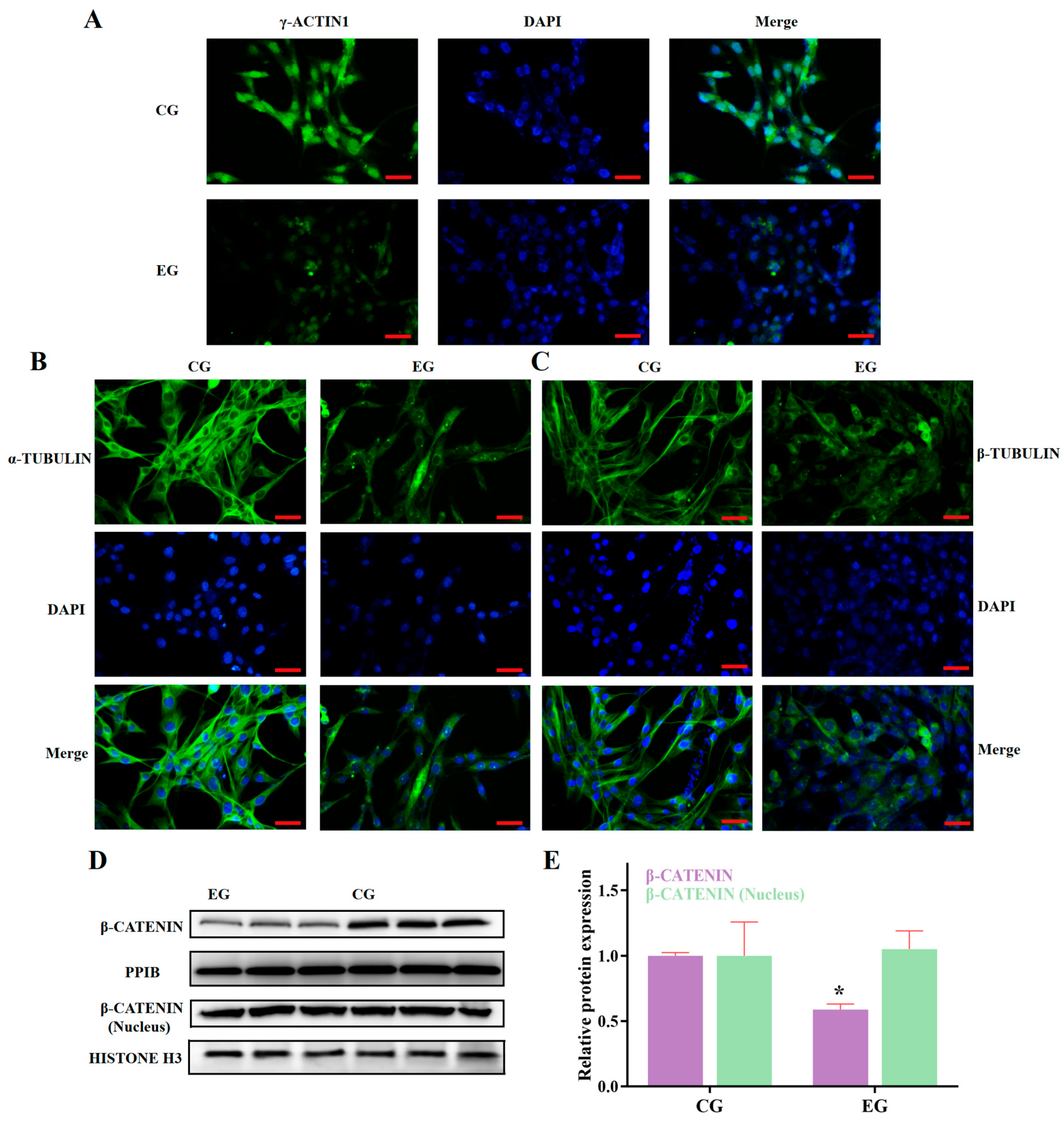
3.2. RORA Activation Inhibits the Protein Expression of Some Genes Related to Cytoskeletal Composition and Cell Adhesion
3.3. Activation of RORA Inhibits Cytoskeleton Formation, Migration, and Adhesion of HFSCs
3.4. RORA Can Bind to the Promoter Regions of the Myo1e and Actg1 Genes to Regulate Their Transcription
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Draelos, Z.D. The biology of hair care. Dermatol. Clin. 2000, 18, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.V. The Biology, Structure, and Function of Eyebrow Hair. J. Drugs Dermatol. 2014, 13, S12–S16. [Google Scholar] [PubMed]
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 2024, 25, 87–100. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Schlake, T. Determination of hair structure and shape. Semin. Cell Dev. Biol. 2007, 18, 267–273. [Google Scholar] [CrossRef]
- Wollina, U. Histochemistry of the human hair follicle. EXS 1997, 78, 31–58. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Paus, R. Molecular biology of hair morphogenesis: Development and cycling. J. Exp. Zool. B Mol. Dev. Evol. 2003, 298B, 164–180. [Google Scholar] [CrossRef]
- Plikus, M.V.; Chuong, C.-M. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J. Investig. Dermatol. 2008, 128, 1071–1080. [Google Scholar] [CrossRef]
- Billoni, N.; Buan, B.; Gautier, B.; Gaillard, O.; Mahe, Y.F.; Bernard, B.A. Thyroid hormone receptor beta1 is expressed in the human hair follicle. Br. J. Dermatol. 2000, 142, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Miranda, B.H.; Charlesworth, M.R.; Tobin, D.J.; Sharpe, D.T.; Randall, V.A. Androgens trigger different growth responses in genetically identical human hair follicles in organ culture that reflect their epigenetic diversity in life. FASEB J. 2018, 32, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Reichler, I.M.; Welle, M.; Eckrich, C.; Sattler, U.; Barth, A.; Hubler, M.; Nett-Mettler, C.S.; Joechle, W.; Arnold, S. Spaying-induced coat changes: The role of gonadotropins, GnRH and GnRH treatment on the hair cycle of female dogs. Vet. Dermatol. 2008, 19, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Schilli, M.B.; Ray, S.; Paus, R.; Obi-Tabot, E.; Holick, M.F. Control of hair growth with parathyroid hormone (7-34). J. Investig. Dermatol. 1997, 108, 928–932. [Google Scholar] [CrossRef]
- Gebbie, F.E.; Forsyth, I.A.; Arendt, J. Effects of maintaining solstice light and temperature on reproductive activity, coat growth, plasma prolactin and melatonin in goats. J. Reprod. Fertil. 1999, 116, 25–33. [Google Scholar] [CrossRef]
- Kul, B.C.; Bilgen, N.; Biskin, M.; Akkurt, M.Y.; Cildir, O.S.; Ozmen, O.; Kul, O. Seasonal Gene Expression Profile Responsible for Hair Follicle Development in Angora Goats. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 365–372. [Google Scholar] [CrossRef]
- Ji, J.; Ho, B.S.-Y.; Qian, G.; Xie, X.-M.; Bigliardi, P.L.; Bigliardi-Qi, M. Aging in hair follicle stem cells and niche microenvironment. J. Dermatol. 2017, 44, 1097–1104. [Google Scholar] [CrossRef]
- Ma, D.R.; Yang, E.N.; Lee, S.T. A review: The location, molecular characterisation and multipotency of hair follicle epidermal stem cells. Ann. Acad. Med. Sing. 2004, 33, 784–788. [Google Scholar] [CrossRef]
- Hoffman, R.M. The pluripotency of hair follicle stem cells. Cell Cycle 2006, 5, 232–233. [Google Scholar] [CrossRef]
- Joachimiak, R.; Bajek, A.; Drewa, T. Hair follicle as a novel source of stem cells. Postepy Hig. Med. Dosw. 2012, 66, 181–186. [Google Scholar] [CrossRef]
- Pang, S.F.; Pang, C.S.; Poon, A.M.; Lee, P.P.; Liu, Z.M.; Shiu, S.Y. Melatonin: A chemical photoperiodic signal with clinical significance in humans. Chin. Med. J. 1998, 111, 197–203. [Google Scholar]
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the hair follicle. J. Pineal Res. 2008, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Cao, M.; Cao, Q.; Mao, J.; Zhao, F.; Piao, J.A. Long noncoding RNA and gene expression analysis of melatonin-exposed Liaoning cashmere goat fibroblasts indicating cashmere growth. Sci. Nat. 2018, 105, 60. [Google Scholar] [CrossRef]
- Rong, Y.; Ma, R.; Zhang, Y.; Guo, Z. Melatonin’s effect on hair follicles in a goat (Capra hircus) animal model. Front. Endocrinol. 2024, 15, 1361100. [Google Scholar] [CrossRef]
- Yang, C.-H.; Xu, J.-H.; Ren, Q.-C.; Duan, T.; Mo, F.; Zhang, W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Burmeister, G.; Schmidt, H.W.; Elsner, P. Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia: Results of a pilot randomized controlled trial. Br. J. Dermatol. 2004, 150, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Trueb, R.M.; Hanggi, G.; Innocenti, M.; Elsner, P. Topical melatonin for treatment of androgenetic alopecia. Int. J. Trichol. 2012, 4, 236–245. [Google Scholar] [CrossRef]
- Nematisouldaragh, D.; Kirshenbaum, E.; Uzonna, M.; Kirshenbaum, L.; Rabinovich-Nikitin, I. The Role of Retinoic-Acid-Related Orphan Receptor (RORs) in Cellular Homeostasis. Int. J. Mol. Sci. 2024, 25, 1340. [Google Scholar] [CrossRef]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, J.; Wu, J.; Zhang, T.; Liu, J.; Mu, Q.; Wu, Z.; Zhang, Y.; Su, R.; Liu, Z.; et al. Melatonin regulates the periodic growth of secondary hair follicles through the nuclear receptor RORα. Front. Vet. Sci. 2023, 10, 1203302. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Li, S.; Xu, Y.; Bai, S.; Zhang, W. Retinoic Acid-Related Orphan Receptor Alpha May Regulate the State of Hair Follicle Stem Cells by Upregulating the Expression of BNIP3. Animals 2024, 14, 3477. [Google Scholar] [CrossRef]
- Li, M.; Peng, L.; Wang, Z.; Liu, L.; Cao, M.; Cui, J.; Wu, F.; Yang, J. Roles of the cytoskeleton in human diseases. Mol. Biol. Rep. 2023, 50, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Moujaber, O.; Stochaj, U. The Cytoskeleton as Regulator of Cell Signaling Pathways. Trends Biochem. Sci. 2020, 45, 96–107. [Google Scholar] [CrossRef]
- Bifulco, M. Role of the isoprenoid pathway in ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci. 2005, 77, 1740–1749. [Google Scholar] [CrossRef]
- Iwazaki, R.; Watanabe, S.; Otaka, K.; Ota, K.; Ono, Y.; Sato, N. The role of the cytoskeleton in migration and proliferation of a cultured human gastric cancer cell line using a new metastasis model. Cancer Lett. 1997, 119, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.; Choi, B.; Hong, K.U.; Kim, E.; Seong, Y.-S.; Bae, C.-D.; Park, J. A cytoskeleton-associated protein, TMAP/CKAP2, is involved in the proliferation of human foreskin fibroblasts. Biochem. Bioph. Res. Co. 2006, 348, 222–228. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Gao, Z.; Zhang, P.; Gao, J.; Wu, X. Regulation of Hippo Signaling by Mechanical Signals and the Cytoskeleton. DNA Cell Biol. 2020, 39, 159–166. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Wang, J.; Wang, L.; Qiu, W.; Kume, T.; Dowell, R.; Yi, R. Escape of hair follicle stem cells causes stem cell exhaustion during aging. Nat. Aging 2021, 1, 889–903. [Google Scholar] [CrossRef]
- Oshima, H.; Rochat, A.; Kedzia, C.; Kobayashi, K.; Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001, 104, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Rochat, A.; Kobayashi, K.; Barrandon, Y. Location of stem cells of human hair follicles by clonal analysis. Cell 1994, 76, 1063–1073. [Google Scholar] [CrossRef]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.-L.; Pasolli, H.A.; Sheu, S.-H.; Fan, S.M.-Y.; Shamsi, F.; Assaad, S.; Lin, E.T.-Y.; Zhang, B.; et al. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 2020, 182, 578–593.e19. [Google Scholar] [CrossRef]
- Bitsch, F.; Aichholz, R.; Kallen, J.; Geisse, S.; Fournier, B.; Schlaeppi, J.M. Identification of natural ligands of retinoic acid receptor-related orphan receptor α ligand-binding domain expressed in Sf9 cells: A mass spectrometry approach. Anal. Biochem. 2003, 323, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kamenecka, T.M.; Lyda, B.; Chang, M.R.; Griffin, P.R. Synthetic modulators of the retinoic acid receptor-related orphan receptors. MedChemComm 2013, 4, 764–776. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Burris, T.P. Action of RORs and their ligands in (patho)physiology. Trends. Endocrin. Met. 2012, 23, 619–627. [Google Scholar] [CrossRef]
- Acevedo, N.; Saaf, A.; Soderhall, C.; Melen, E.; Mandelin, J.; Pietras, C.O.; Ezer, S.; Karisola, P.; Vendelin, J.; Gennas, G.B.A.; et al. Interaction between retinoid acid receptor-related orphan receptor alpha (RORA) and neuropeptide S receptor 1 (NPSR1) in asthma. PLoS ONE 2013, 8, e60111. [Google Scholar] [CrossRef]
- Al-Zaid, F.S.; Hurley, M.J.; Dexter, D.T.; Gillies, G.E. Neuroprotective role for RORA in Parkinson’s disease revealed by analysis of post-mortem brain and a dopaminergic cell line. NPJ Park. Dis. 2023, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Caston, J.; Chianale, C.; Mariani, J. Spatial memory of heterozygous staggerer (Rora+/Rorasg) versus normal (Rora+/Rora+) mice during aging. Behav. Genet. 2004, 34, 319–324. [Google Scholar] [CrossRef]
- Doulazmi, M.; Frederic, F.; Lemaigre-Dubreuil, Y.; Hadj-Sahraoui, N.; Delhaye-Bouchaud, N.; Mariani, J. Cerebellar Purkinje cell loss during life span of the heterozygous staggerer mouse (Rora(+)/Rora(sg)) is gender-related. J. Comp. Neurol. 1999, 411, 267–273. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liu, Z.H.; Wang, L.; Xiao, H.M.; Du, C.G.; Zhang, Y.J.; Su, R.; Li, J.Q. Expression of the RORα gene in Inner Mongolian cashmere goat hair follicles. Genet. Mol. Res. 2015, 14, 380–388. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Li, S.; Xu, Y.; Bai, S.; Zhang, W. Melatonin-Mediated Circadian Rhythm Signaling Exhibits Bidirectional Regulatory Effects on the State of Hair Follicle Stem Cells. Biomolecules 2025, 15, 226. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Li, S.; Bai, S.; Zhang, W. The Retinoic-Acid-Related Orphan Receptor Alpha May Be Highly Involved in the Regulation of Seasonal Hair Molting. Int. J. Mol. Sci. 2025, 26, 1579. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; James, C.G.; Wang, G.; Dupuis, H.; Beier, F. Control of chondrocyte gene expression by actin dynamics: A novel role of cholesterol/Ror-α signalling in endochondral bone growth. J. Cell Mol. Med. 2009, 13, 3497–3516. [Google Scholar] [CrossRef]
- Sun, M.; Zaman, M.H. Modeling, signaling and cytoskeleton dynamics: Integrated modeling-experimental frameworks in cell migration. Wires. Syst. Biol. Med. 2017, 9, e1365. [Google Scholar] [CrossRef]
- Wehrle-Haller, B.; Imhof, B.A. Actin, microtubules and focal adhesion dynamics during cell migration. Int. J. Biochem. Cell B 2003, 35, 39–50. [Google Scholar] [CrossRef]
- Cleveland, D.W. Treadmilling of tubulin and actin. Cell 1982, 28, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Grazi, E. Treadmilling or fragmentation? A proposed repair mechanism for actin filaments. Ital. J. Biochem. 1986, 35, 284–286. [Google Scholar]
- Margolis, R.L.; Wilson, L. Microtubule treadmilling: What goes around comes around. BioEssays 1998, 20, 830–836. [Google Scholar] [CrossRef]
- Waterman-Storer, C.M.; Salmon, E.D. Microtubule dynamics: Treadmilling comes around again. Curr. Biol. 1997, 7, R369–R372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, X. MiR-652 serves as a prognostic biomarker in gastric cancer and promotes tumor proliferation, migration, and invasion via targeting RORA. Cancer Biomark. 2019, 26, 323–331. [Google Scholar] [CrossRef]
- Sun, X.; Dongol, S.; Qiu, C.; Xu, Y.; Sun, C.; Zhang, Z.; Yang, X.; Zhang, Q.; Kong, B. miR-652 Promotes Tumor Proliferation and Metastasis by Targeting RORA in Endometrial Cancer. Mol. Cancer Res. 2018, 16, 1927–1939. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y.; Chen, L.; Ma, Y.; Zhang, H.; Zhou, J.; Li, H.; Jing, Z. The EIF4A3/CASC2/RORA Feedback Loop Regulates the Aggressive Phenotype in Glioblastomas. Front. Oncol. 2021, 11, 699933. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, X.; Li, S.; Bai, S.; Zhang, W. Retinoic-Acid-Related Orphan Receptor Alpha Is Involved in the Regulation of the Cytoskeleton of Hair Follicle Stem Cells. Biomolecules 2025, 15, 863. https://doi.org/10.3390/biom15060863
Zhang Y, Zhao X, Li S, Bai S, Zhang W. Retinoic-Acid-Related Orphan Receptor Alpha Is Involved in the Regulation of the Cytoskeleton of Hair Follicle Stem Cells. Biomolecules. 2025; 15(6):863. https://doi.org/10.3390/biom15060863
Chicago/Turabian StyleZhang, Yu, Xuefei Zhao, Shuqi Li, Suying Bai, and Wei Zhang. 2025. "Retinoic-Acid-Related Orphan Receptor Alpha Is Involved in the Regulation of the Cytoskeleton of Hair Follicle Stem Cells" Biomolecules 15, no. 6: 863. https://doi.org/10.3390/biom15060863
APA StyleZhang, Y., Zhao, X., Li, S., Bai, S., & Zhang, W. (2025). Retinoic-Acid-Related Orphan Receptor Alpha Is Involved in the Regulation of the Cytoskeleton of Hair Follicle Stem Cells. Biomolecules, 15(6), 863. https://doi.org/10.3390/biom15060863




