Role of SOST in Response to Mechanical Stimulation in Bone and Extraosseous Organs
Abstract
1. Introduction
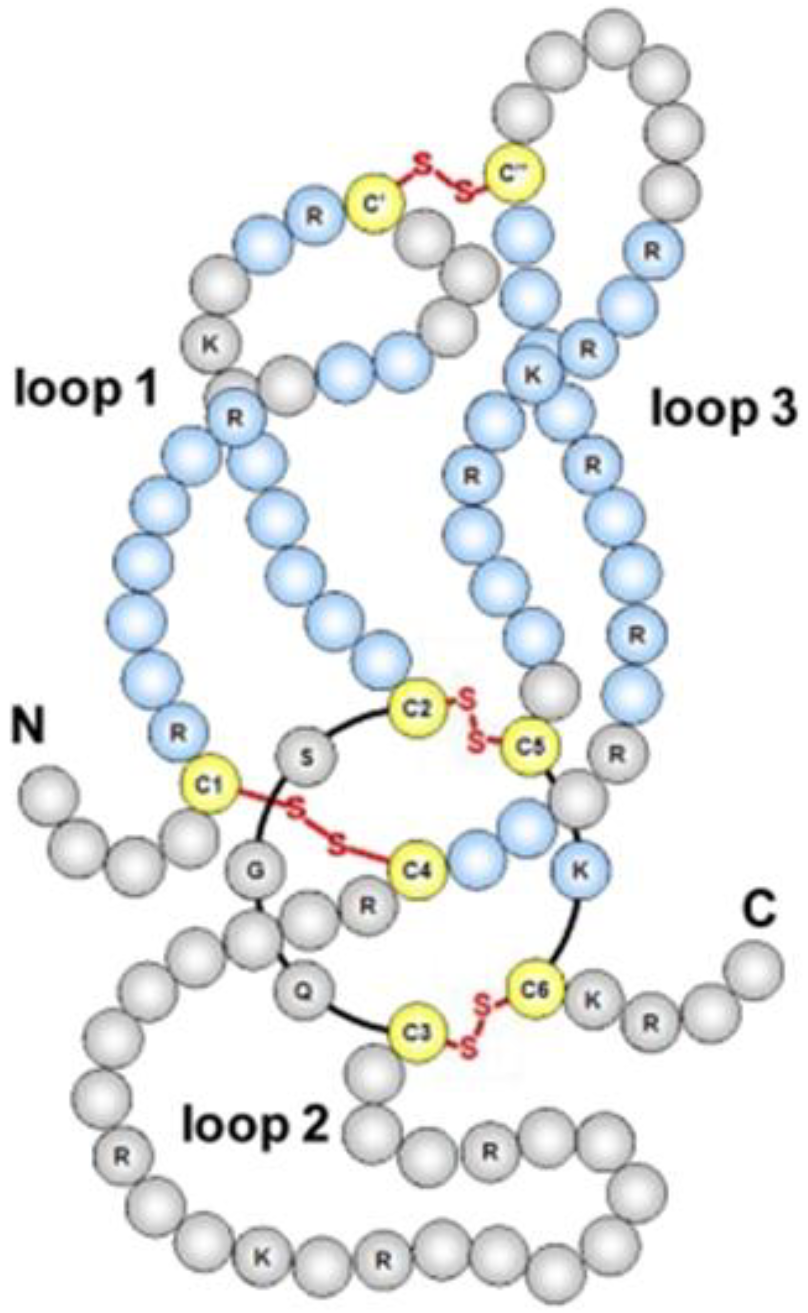
2. Structure and Function of SOST in Bone Homeostasis
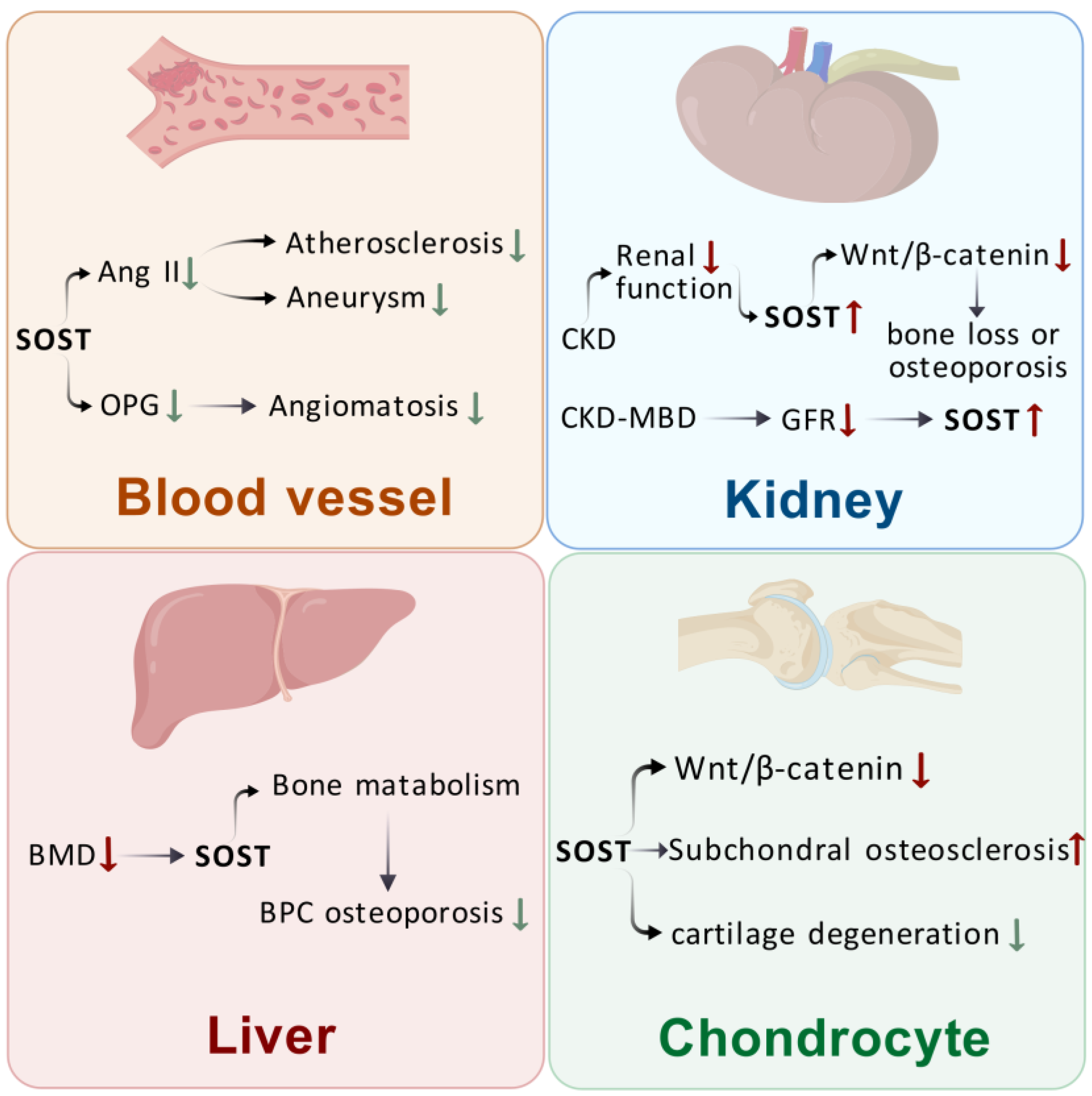
3. Effects of SOST on Extraosseous Organs
3.1. Effects of SOST on Blood Vessels
3.2. Effects of SOST on the Kidney
3.3. Effect of SOST on the Liver
3.4. Effect of SOST on the Cartilage
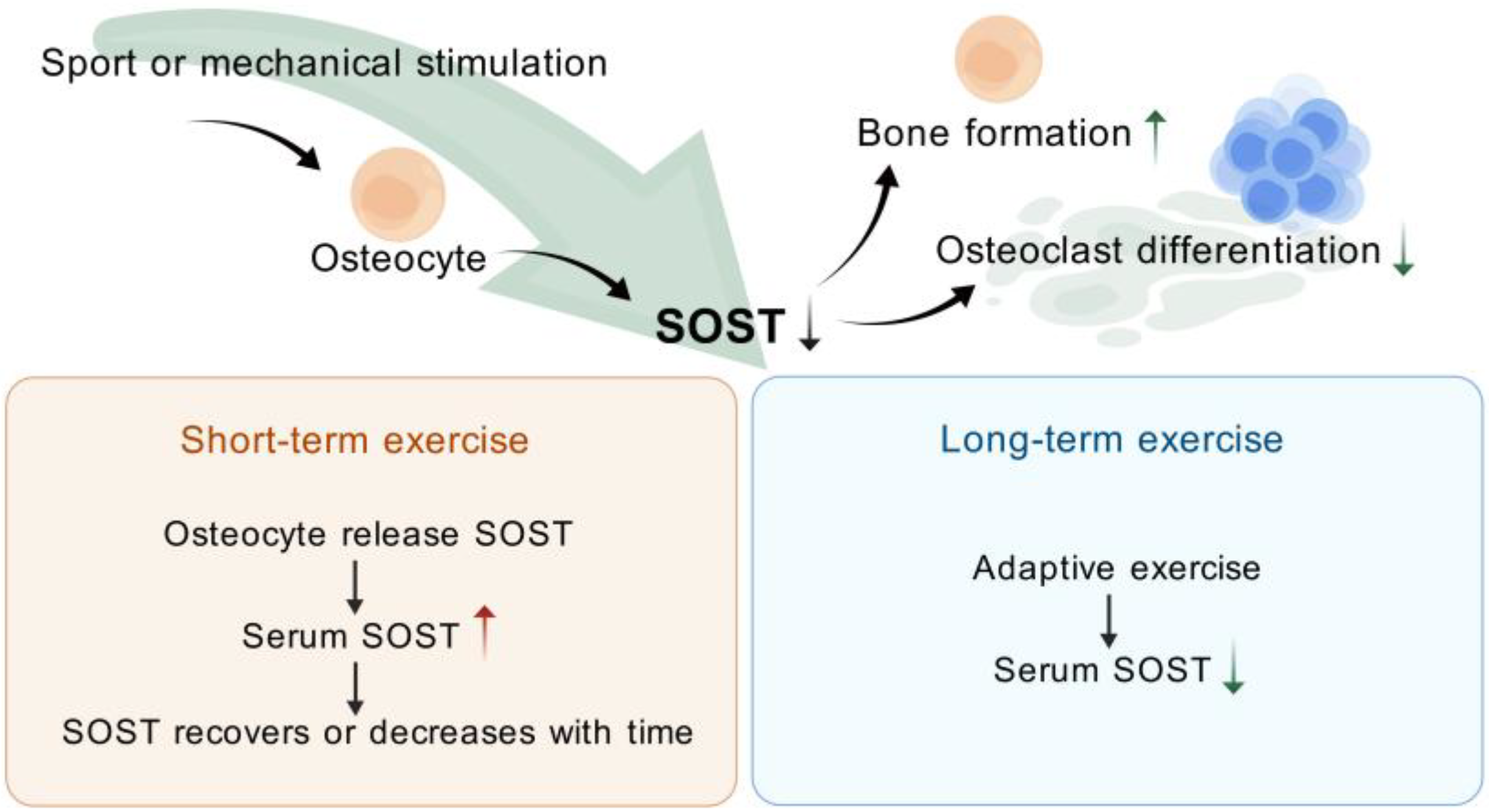
4. Exercise (or Mechanical Stimulation) Modulation of SOST
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Xue, P.; Wu, X.; Ma, J.; Wang, Y.; Li, Y. Role of sclerostin and dkk1 in bone remodeling in type 2 diabetic patients. Endocr. Res. 2018, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, R.T.; Solomon, L.B.; Yang, D.; Crotti, T.N.; Haynes, D.R.; Findlay, D.M.; Atkins, G.J. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta Biomater. 2019, 87, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Loots, G.G. Transcriptional control of Sost in bone. Bone 2017, 96, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yang, Y.S.; Xie, J.; Lee, O.; Kim, J.; Hong, J.; Boldyreff, B.; Filhol, O.; Chun, H.; Greenblatt, M.B.; et al. Regulation of sclerostin by the SIRT1 stabilization pathway in osteocytes. Cell Death Differ. 2022, 29, 1625–1638. [Google Scholar] [CrossRef]
- Galea, G.L.; Paradise, C.R.; Meakin, L.B.; Camilleri, E.T.; Taipaleenmaki, H.; Stein, G.S.; Lanyon, L.E.; Price, J.S.; van Wijnen, A.J.; Dudakovic, A. Mechanical strain-mediated reduction in RANKL expression is associated with RUNX2 and BRD2. Gene X 2020, 5, 100027. [Google Scholar] [CrossRef]
- Mankar, R.; Bueso-Ramos, C.E.; Yin, C.C.; Hidalgo-Lopez, J.E.; Berisha, S.; Kansiz, M.; Mayerich, D. Automated Osteosclerosis Grading of Clinical Biopsies Using Infrared Spectroscopic Imaging. Anal. Chem. 2020, 92, 749–757. [Google Scholar] [CrossRef]
- Omran, A.; Atanasova, D.; Landgren, F.; Magnusson, P. Sclerostin: From Molecule to Clinical Biomarker. Int. J. Mol. Sci. 2022, 23, 4751. [Google Scholar] [CrossRef]
- Futai, M.; Sun-Wada, G.H.; Wada, Y.; Matsumoto, N.; Nakanishi-Matsui, M. Vacuolar-type ATPase: A proton pump to lysosomal trafficking. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 261–277. [Google Scholar] [CrossRef]
- Daly, N.L.; Craik, D.J. Bioactive cystine knot proteins. Curr. Opin. Chem. Biol. 2011, 15, 362–368. [Google Scholar] [CrossRef]
- Aryana, I.; Rini, S.S.; Soejono, C.H. Importance of Sclerostin as Bone-Muscle Mediator Crosstalk. Ann. Geriatr. Med. Res. 2022, 26, 72–82. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Jia, X.Y.; Fang, J.Y.; Chai, H.; Huang, Q.; She, C.; Jia, P.; Geng, C.; Xu, W. Reduction of SOST gene promotes bone formation through the Wnt/β-catenin signalling pathway and compensates particle-induced osteolysis. J. Cell. Mol. Med. 2020, 24, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Mazur, C.M.; Wein, M.N. Sclerostin and Osteocalcin: Candidate Bone-Produced Hormones. Front. Endocrinol. 2021, 12, 584147. [Google Scholar] [CrossRef] [PubMed]
- Gipson, G.R.; Kattamuri, C.; Czepnik, M.; Thompson, T.B. Characterization of the different oligomeric states of the DAN family antagonists SOSTDC1 and SOST. Biochem. J. 2020, 477, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; O’Brien, C.A. Osteocyte RANKL: New insights into the control of bone remodeling. J. Bone Miner. Res. 2012, 27, 499–505. [Google Scholar] [CrossRef]
- Prideaux, M.; Loveridge, N.; Pitsillides, A.A.; Farquharson, C. Extracellular matrix mineralization promotes E11/gp38 glycoprotein expression and drives osteocytic differentiation. PLoS ONE 2012, 7, e36786. [Google Scholar] [CrossRef]
- Silvestrini, G.; Ballanti, P.; Leopizzi, M.; Sebastiani, M.; Berni, S.; Di Vito, M.; Bonucci, E. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J. Mol. Histol. 2007, 38, 261–269. [Google Scholar] [CrossRef]
- Chavassieux, P.; Chapurlat, R.; Portero-Muzy, N.; Roux, J.P.; Garcia, P.; Brown, J.P.; Libanati, C.; Boyce, R.W.; Wang, A.; Grauer, A. Bone-Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women with Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis After 2 and 12 Months of Treatment. J. Bone Miner. Res. 2019, 34, 1597–1608. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Chang, M.K.; Kramer, I.; Keller, H.; Gooi, J.H.; Collett, C.; Jenkins, D.; Ettenberg, S.A.; Cong, F.; Halleux, C.; Kneissel, M. Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity. J. Bone Miner. Res. 2014, 29, 29–42. [Google Scholar] [CrossRef]
- Zhu, D.; Mackenzie, N.C.; Millán, J.L.; Farquharson, C.; MacRae, V.E. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 2011, 6, e19595. [Google Scholar] [CrossRef]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Li, J.; Morton, S.K.; Biros, E.; Wang, Y.; Nsengiyumva, V.; Lindeman, J.H.; Loots, G.G.; et al. Wnt Signaling Pathway Inhibitor Sclerostin Inhibits Angiotensin II-Induced Aortic Aneurysm and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Morales-Santana, S.; García-Fontana, B.; García-Martín, A.; Rozas-Moreno, P.; García-Salcedo, J.A.; Reyes-García, R.; Muñoz-Torres, M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013, 36, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Leto, G.; D’Onofrio, L.; Lucantoni, F.; Zampetti, S.; Campagna, G.; Foffi, C.; Moretti, C.; Carlone, A.; Palermo, A.; Leopizzi, M.; et al. Sclerostin is expressed in the atherosclerotic plaques of patients who undergoing carotid endarterectomy. Diabetes Metab. Res. Rev. 2019, 35, e3069. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Bellone, F.; Morabito, N.; Corica, F. Sclerostin and Vascular Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4779. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Kramann, R.; Koos, R.; Krüger, T.; Schurgers, L.; Mühlenbruch, G.; Hübner, S.; Gladziwa, U.; Drechsler, C.; Ketteler, M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2013, 14, 219. [Google Scholar] [CrossRef]
- Deng, D.; Han, X.; Diao, Z.; Liu, W. Secreted Frizzled-Related Protein 5 Ameliorates Vascular Calcification in a Rat Model of Chronic Kidney Disease through the Wnt/β-Catenin Pathway. Kidney Blood Press. Res. 2021, 46, 758–767. [Google Scholar] [CrossRef]
- Liao, H.W.; Huang, T.H.; Chang, Y.H.; Liou, H.H.; Chou, Y.H.; Sue, Y.M.; Hung, P.H.; Chang, Y.T.; Ho, P.C.; Tsai, K.J. Exercise Alleviates Osteoporosis in Rats with Mild Chronic Kidney Disease by Decreasing Sclerostin Production. Int. J. Mol. Sci. 2019, 20, 2044. [Google Scholar] [CrossRef]
- Drüeke, T.B.; Massy, Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016, 89, 289–302. [Google Scholar] [CrossRef]
- Lima, F.; Monier-Faugere, M.C.; Mawad, H.; David, V.; Malluche, H.H. FGF-23 and sclerostin in serum and bone of CKD patients. Clin. Nephrol. 2023, 99, 209–218. [Google Scholar] [CrossRef]
- Zeng, C.; Guo, C.; Cai, J.; Tang, C.; Dong, Z. Serum sclerostin in vascular calcification and clinical outcome in chronic kidney disease. Diabetes Vasc. Dis. Res. 2018, 15, 99–105. [Google Scholar] [CrossRef]
- Post, T.M.; Schmidt, S.; Peletier, L.A.; de Greef, R.; Kerbusch, T.; Danhof, M. Application of a mechanism-based disease systems model for osteoporosis to clinical data. J. Pharmacokinet. Pharmacodyn. 2013, 40, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Bielesz, B.; Reiter, T.; Marculescu, R.; Gleiss, A.; Bojic, M.; Kieweg, H.; Cejka, D. Calcification Propensity of Serum is Independent of Excretory Renal Function. Sci. Rep. 2017, 7, 17941. [Google Scholar] [CrossRef]
- Maré, A.; Verhulst, A.; Cavalier, E.; Delanaye, P.; Behets, G.J.; Meijers, B.; Kuypers, D.; D’Haese, P.C.; Evenepoel, P. Clinical Inference of Serum and Bone Sclerostin Levels in Patients with End-Stage Kidney Disease. J. Clin. Med. 2019, 8, 2027. [Google Scholar] [CrossRef]
- Wei, M.T.; Le, A.K.; Chang, M.S.; Hsu, H.; Nguyen, P.; Zhang, J.Q.; Wong, C.; Wong, C.; Cheung, R.; Nguyen, M.H. Antiviral therapy and the development of osteopenia/osteoporosis among Asians with chronic hepatitis B. J. Med. Virol. 2019, 91, 1288–1294. [Google Scholar] [CrossRef]
- Kim, W.; Chung, Y.; Kim, S.H.; Park, S.; Bae, J.H.; Kim, G.; Lee, S.J.; Kim, J.E.; Park, B.W.; Lim, S.K.; et al. Increased sclerostin levels after further ablation of remnant estrogen by aromatase inhibitors. Endocrinol. Metab. 2015, 30, 58–64. [Google Scholar] [CrossRef]
- Guañabens, N.; Ruiz-Gaspà, S.; Gifre, L.; Miquel, R.; Peris, P.; Monegal, A.; Dubrueil, M.; Arias, A.; Parés, A. Sclerostin Expression in Bile Ducts of Patients with Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J. Bone Miner. Res. 2016, 31, 1725–1733. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.; Li, Y.; Tang, M.; Wan, S.; Tian, H.; Chen, X. Decreased Sclerostin Secretion in Humans and Mice with Nonalcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 707505. [Google Scholar] [CrossRef]
- Suen, P.K.; Qin, L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: A general review. J. Orthop. Transl. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Chan, B.Y.; Fuller, E.S.; Russell, A.K.; Smith, S.M.; Smith, M.M.; Jackson, M.T.; Cake, M.A.; Read, R.A.; Bateman, J.F.; Sambrook, P.N.; et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthr. Cartil. 2011, 19, 874–885. [Google Scholar] [CrossRef]
- Thompson, M.L.; Jimenez-Andrade, J.M.; Mantyh, P.W. Sclerostin Immunoreactivity Increases in Cortical Bone Osteocytes and Decreases in Articular Cartilage Chondrocytes in Aging Mice. J. Histochem. Cytochem. 2016, 64, 179–189. [Google Scholar] [CrossRef]
- Zhou, S.; Ge, Y.; Li, Y.; Bao, Z.; Yao, C.; Teng, H.; Jiang, Q. Accelerated development of instability-induced osteoarthritis in transgenic mice overexpressing SOST. Int. J. Clin. Exp. Pathol. 2017, 10, 10830–10840. [Google Scholar] [PubMed]
- Bouaziz, W.; Funck-Brentano, T.; Lin, H.; Marty, C.; Ea, H.K.; Hay, E.; Cohen-Solal, M. Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res. Ther. 2015, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Roudier, M.; Li, X.; Niu, Q.T.; Pacheco, E.; Pretorius, J.K.; Graham, K.; Yoon, B.R.; Gong, J.; Warmington, K.; Ke, H.Z.; et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheumatol. 2013, 65, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Liang, Y.; Chen, P.; Zhang, Y.; Yin, X.; Zhang, M. In-depth proteomics approach reveals novel biomarkers of cardiac remodelling after myocardial infarction: An exploratory analysis. J. Cell. Mol. Med. 2020, 24, 10042–10051. [Google Scholar] [CrossRef]
- González-Salvatierra, S.; García-Fontana, C.; Lacal, J.; Andújar-Vera, F.; Martínez-Heredia, L.; Sanabria-de la Torre, R.; Ferrer-Millán, M.; Moratalla-Aranda, E.; Muñoz-Torres, M.; García-Fontana, B. Cardioprotective function of sclerostin by reducing calcium deposition, proliferation, and apoptosis in human vascular smooth muscle cells. Cardiovasc. Diabetol. 2023, 22, 301. [Google Scholar] [CrossRef]
- Yuan, J.; Pedrini, S.; Thota, R.; Doecke, J.; Chatterjee, P.; Sohrabi, H.R.; Teunissen, C.E.; Verberk, I.M.W.; Stoops, E.; Vanderstichele, H.; et al. Elevated plasma sclerostin is associated with high brain amyloid-β load in cognitively normal older adults. NPJ Aging 2023, 9, 17. [Google Scholar] [CrossRef]
- Amado, C.A.; García-Unzueta, M.; Agüero, J.; Martín-Audera, P.; Fueyo, P.; Lavín, B.A.; Guerra, A.R.; Muñoz, P.; Tello, S.; Berja, A.; et al. Associations of serum sclerostin levels with body composition, pulmonary function, and exacerbations in COPD patients. Pulmonology 2024, 30, 512–521. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Rosengren, B.E. Training and bone—From health to injury. Scand. J. Med. Sci. Sports 2012, 22, e15–e23. [Google Scholar] [CrossRef]
- Krahenbühl, T.; Guimarães, R.F.; Barros Filho, A.A.; Gonçalves, E.M. Bone Geometry and Physical Activity in Children and Adolescents: Systematic Review. Rev. Paul. Pediatr. 2018, 36, 230–237. [Google Scholar] [CrossRef]
- Winther, A.; Dennison, E.; Ahmed, L.A.; Furberg, A.S.; Grimnes, G.; Jorde, R.; Gjesdal, C.G.; Emaus, N. The Tromsø Study: Fit Futures: A study of Norwegian adolescents’ lifestyle and bone health. Arch. Osteoporos. 2014, 9, 185. [Google Scholar] [CrossRef]
- Akaike, A.; Suzuki, D.; Okuyama, S.; Kudo, Y.; Shimizu, H.; Takanashi, S.; Makino, K.; Yokoyama, J.; Nakaji, S. Associations between physical physique/fitness in children and bone development during puberty: A 4-year longitudinal study. Sci. Rep. 2022, 12, 13427. [Google Scholar] [CrossRef] [PubMed]
- Marin-Puyalto, J.; Mäestu, J.; Gomez-Cabello, A.; Lätt, E.; Remmel, L.; Purge, P.; Casajús, J.A.; Vicente-Rodríguez, G.; Jürimäe, J. Vigorous physical activity patterns affect bone growth during early puberty in boys. Osteoporos. Int. 2018, 29, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Boudenot, A.; Pallu, S.; Uzbekov, R.; Dolleans, E.; Toumi, H.; Lespessailles, E. Free-fall landing and interval running have different effects on trabecular bone mass and microarchitecture, serum osteocalcin, biomechanical properties, SOST expression and on osteocyte-related characteristics. Appl. Physiol. Nutr. Metab. 2021, 46, 1525–1534. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, L.; Wang, Z.; Zhao, X.; Zou, J. Endocrine Regulation of Extra-skeletal Organs by Bone-derived Secreted Protein and the effect of Mechanical Stimulation. Front. Cell Dev. Biol. 2021, 9, 778015. [Google Scholar] [CrossRef]
- Pickering, M.E.; Simon, M.; Sornay-Rendu, E.; Chikh, K.; Carlier, M.C.; Raby, A.L.; Szulc, P.; Confavreux, C.B. Serum Sclerostin Increases After Acute Physical Activity. Calcif. Tissue Int. 2017, 101, 170–173. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P.; Tillmann, V. Serum sclerostin and cytokine responses to prolonged sculling exercise in highly-trained male rowers. J. Sports Sci. 2021, 39, 591–597. [Google Scholar] [CrossRef]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.E.; Josse, A.R.; Klentrou, P. Response of Sclerostin and Bone Turnover Markers to High Intensity Interval Exercise in Young Women: Does Impact Matter? Biomed. Res. Int. 2018, 2018, 4864952. [Google Scholar] [CrossRef]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.E.; Josse, A.R.; Klentrou, P. Cytokine and Sclerostin Response to High-Intensity Interval Running versus Cycling. Med. Sci. Sports Exerc. 2019, 51, 2458–2464. [Google Scholar] [CrossRef]
- Falk, B.; Haddad, F.; Klentrou, P.; Ward, W.; Kish, K.; Mezil, Y.; Radom-Aizik, S. Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos. Int. 2016, 27, 1245–1249. [Google Scholar] [CrossRef]
- Hinton, P.S.; Nigh, P.; Thyfault, J. Serum sclerostin decreases following 12months of resistance- or jump-training in men with low bone mass. Bone 2017, 96, 85–90. [Google Scholar] [CrossRef]
- Spatz, J.M.; Fields, E.E.; Yu, E.W.; Divieti Pajevic, P.; Bouxsein, M.L.; Sibonga, J.D.; Zwart, S.R.; Smith, S.M. Serum sclerostin increases in healthy adult men during bed rest. J. Clin. Endocrinol. Metab. 2012, 97, E1736–E1740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Li, W.; Lei, L.; Zhang, L. Role of SOST in Response to Mechanical Stimulation in Bone and Extraosseous Organs. Biomolecules 2025, 15, 856. https://doi.org/10.3390/biom15060856
Chen M, Li W, Lei L, Zhang L. Role of SOST in Response to Mechanical Stimulation in Bone and Extraosseous Organs. Biomolecules. 2025; 15(6):856. https://doi.org/10.3390/biom15060856
Chicago/Turabian StyleChen, Minyou, Wenjing Li, Le Lei, and Lingli Zhang. 2025. "Role of SOST in Response to Mechanical Stimulation in Bone and Extraosseous Organs" Biomolecules 15, no. 6: 856. https://doi.org/10.3390/biom15060856
APA StyleChen, M., Li, W., Lei, L., & Zhang, L. (2025). Role of SOST in Response to Mechanical Stimulation in Bone and Extraosseous Organs. Biomolecules, 15(6), 856. https://doi.org/10.3390/biom15060856




