The SlymiR394-ZDS Module Enhances the Antioxidant Capacity of Tomato by Promoting Lycopene Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. RNA Extraction and Real-Time Quantitative PCR (qRT-PCR) Analysis
2.3. Determination of Red–Green Ratio of Tomato Fruits
2.4. Extraction and Content Determination of Lycopene
2.5. Measurement of Antioxidant Enzyme Activities
2.6. Measurement of Free Radical Scavenging Capacity of DPPH and ABTS
2.7. Plasmid Construction
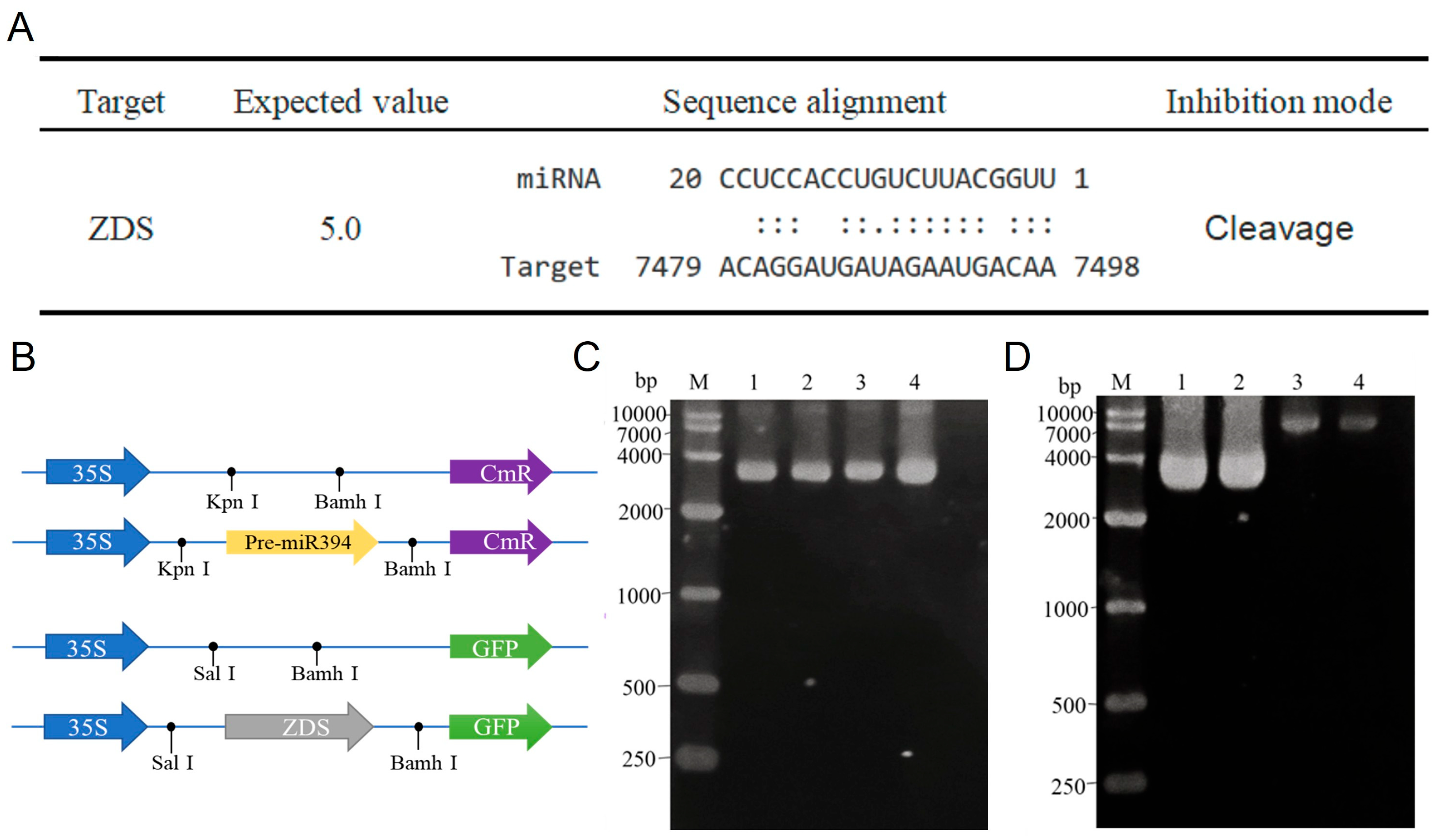
2.8. Interaction Prediction and Characterization of miR394 and ZDS
2.9. Data Processing
3. Results
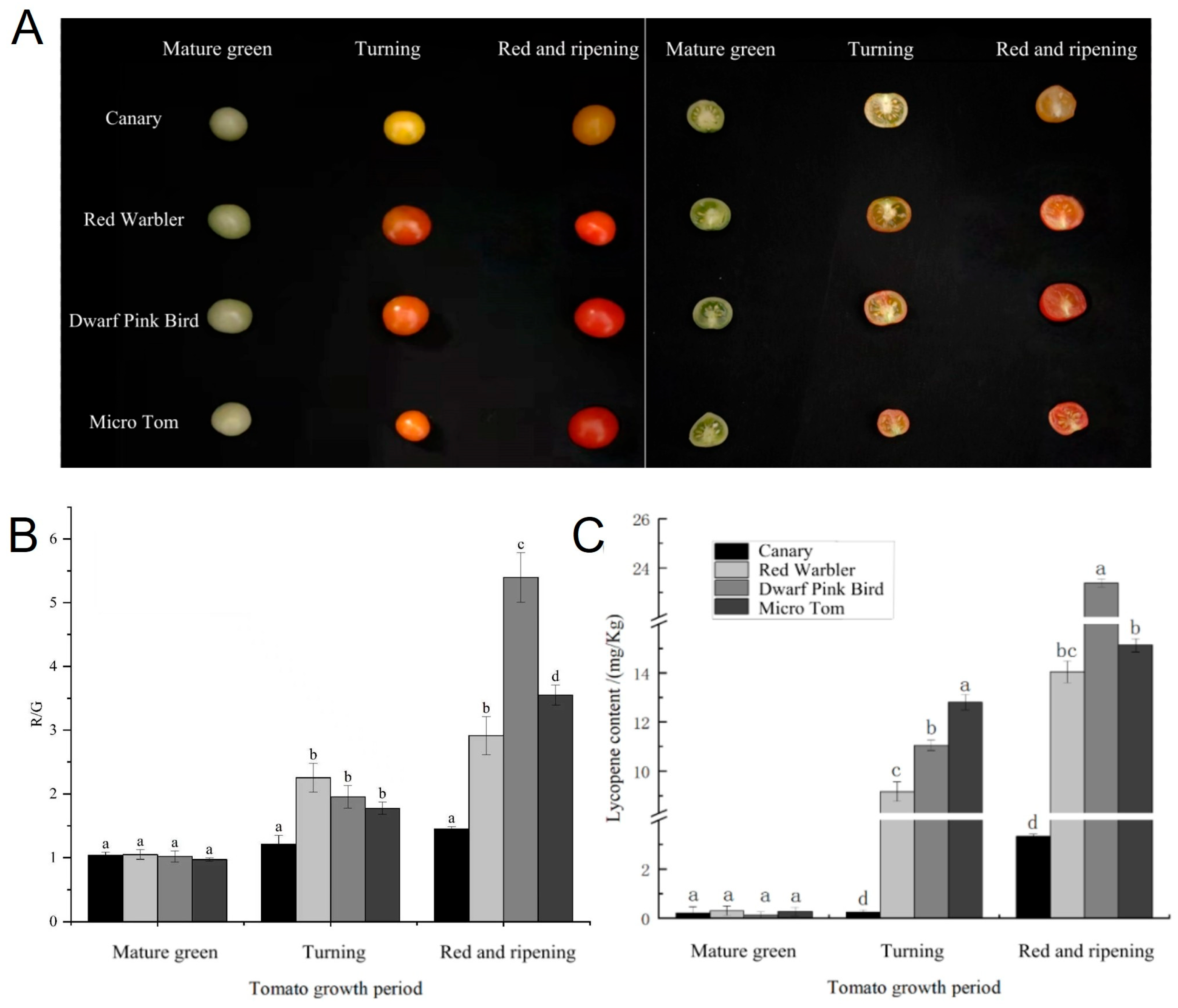
3.1. Lycopene Content in Tomato Cultivars with Distinct Fruit Colors
3.2. Differences in Antioxidant Capacity of Tomatoes with Different Fruit Colors
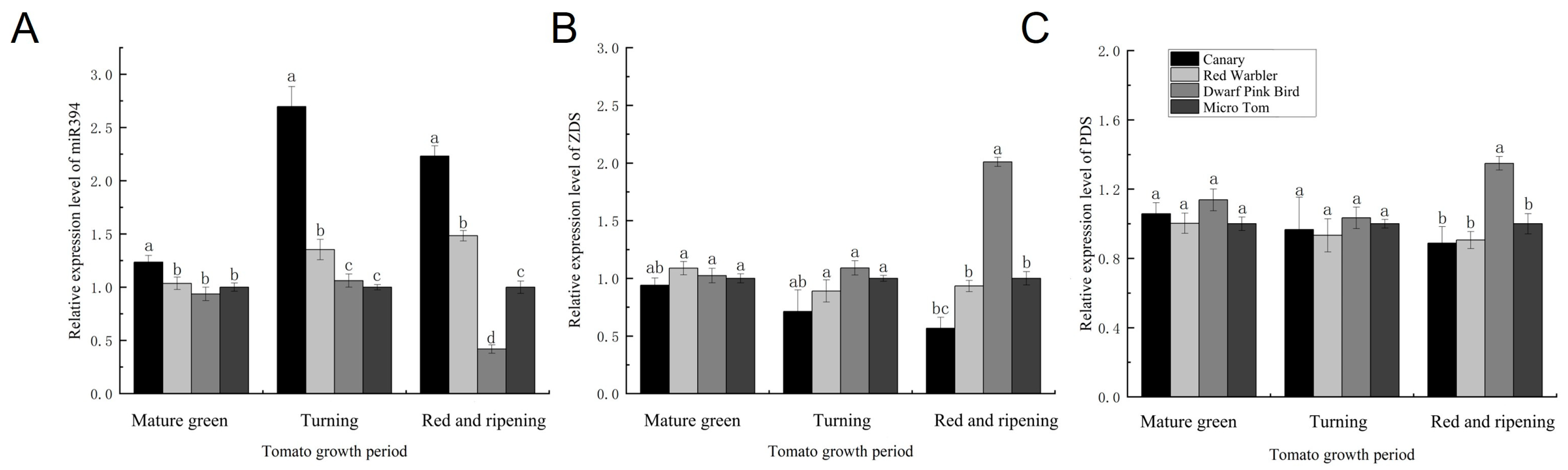
3.3. Expression Levels of miR394 and Lycopene Metabolic Pathway-Related Enzyme Genes in Different Tomato Varieties
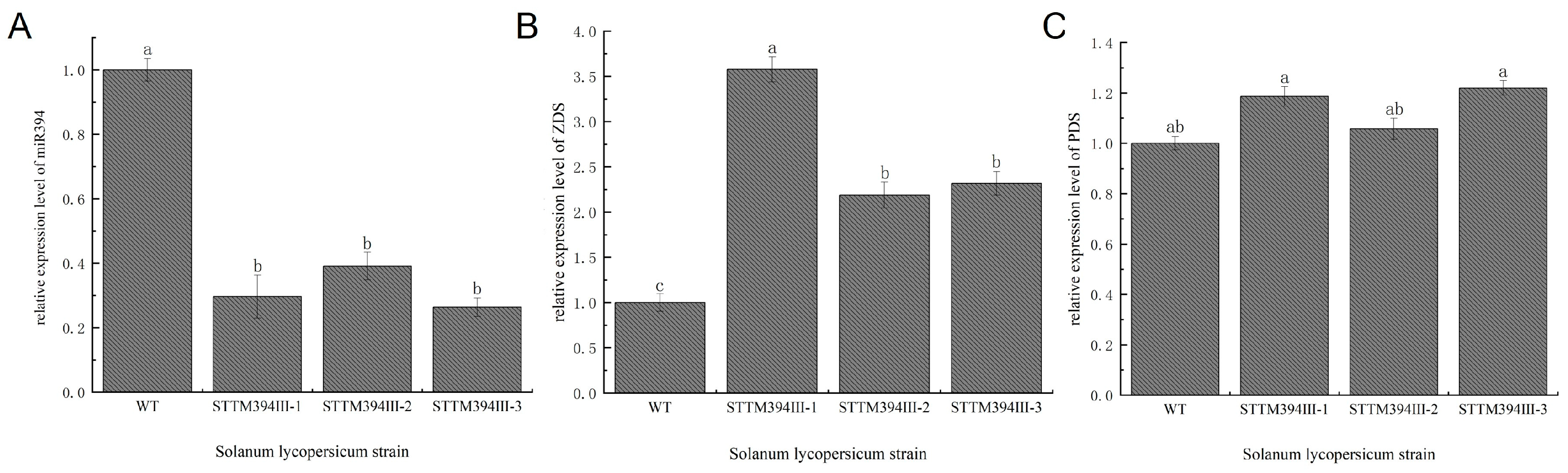
3.4. Obtaining of STTM394 Transgenic Tomato Plants
3.5. Determination of Lycopene Content in Transgenic Tomato Fruits
3.6. Determination of Antioxidant Capacity of Transgenic Tomato Fruits
3.7. Transgenic Tomato Fruit miR394 Regulates the Expression of Lycopene Biosynthetic Pathway-Related Enzyme Genes
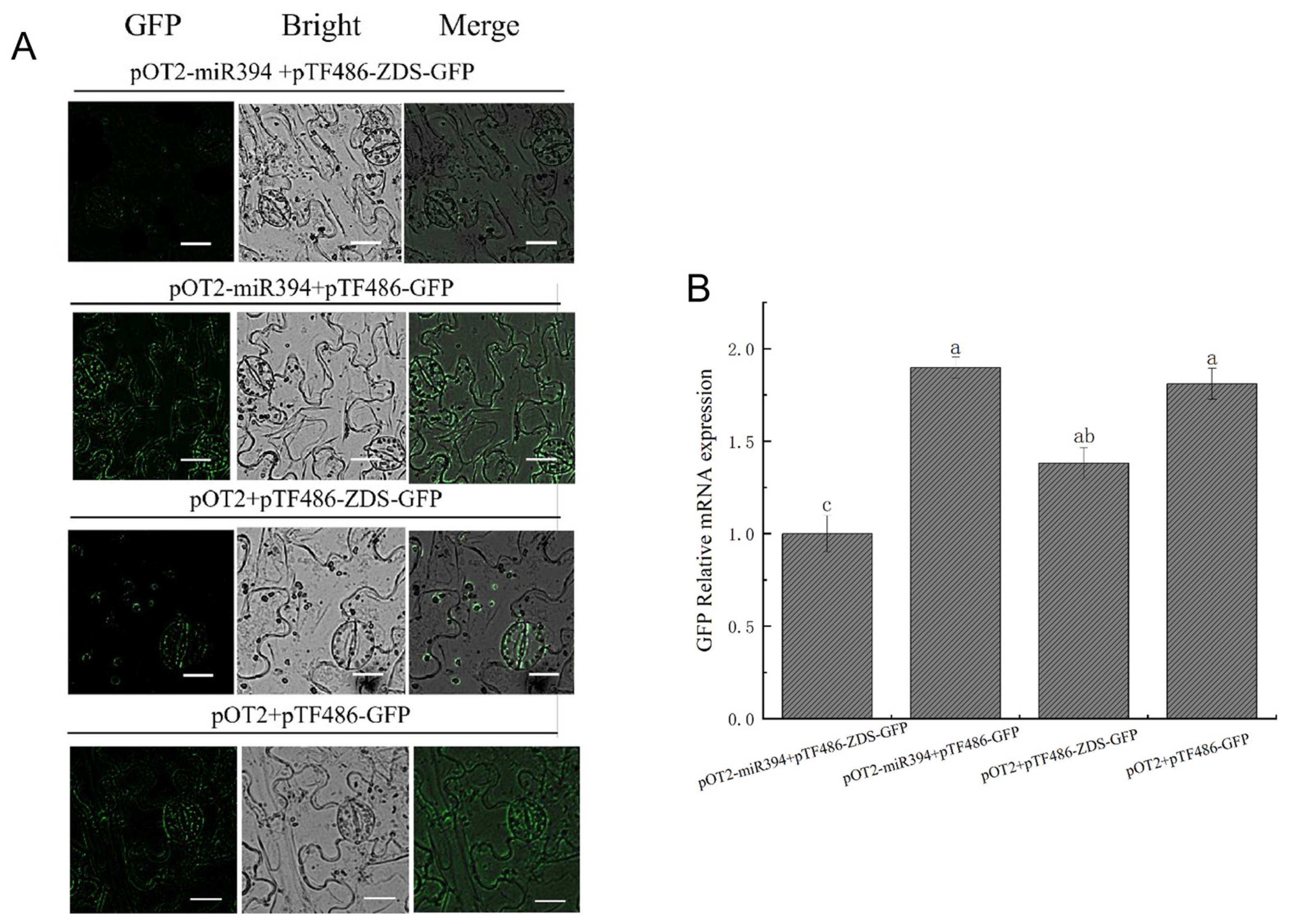
3.8. Analysis of SlymiR394 and ZDS Interactions
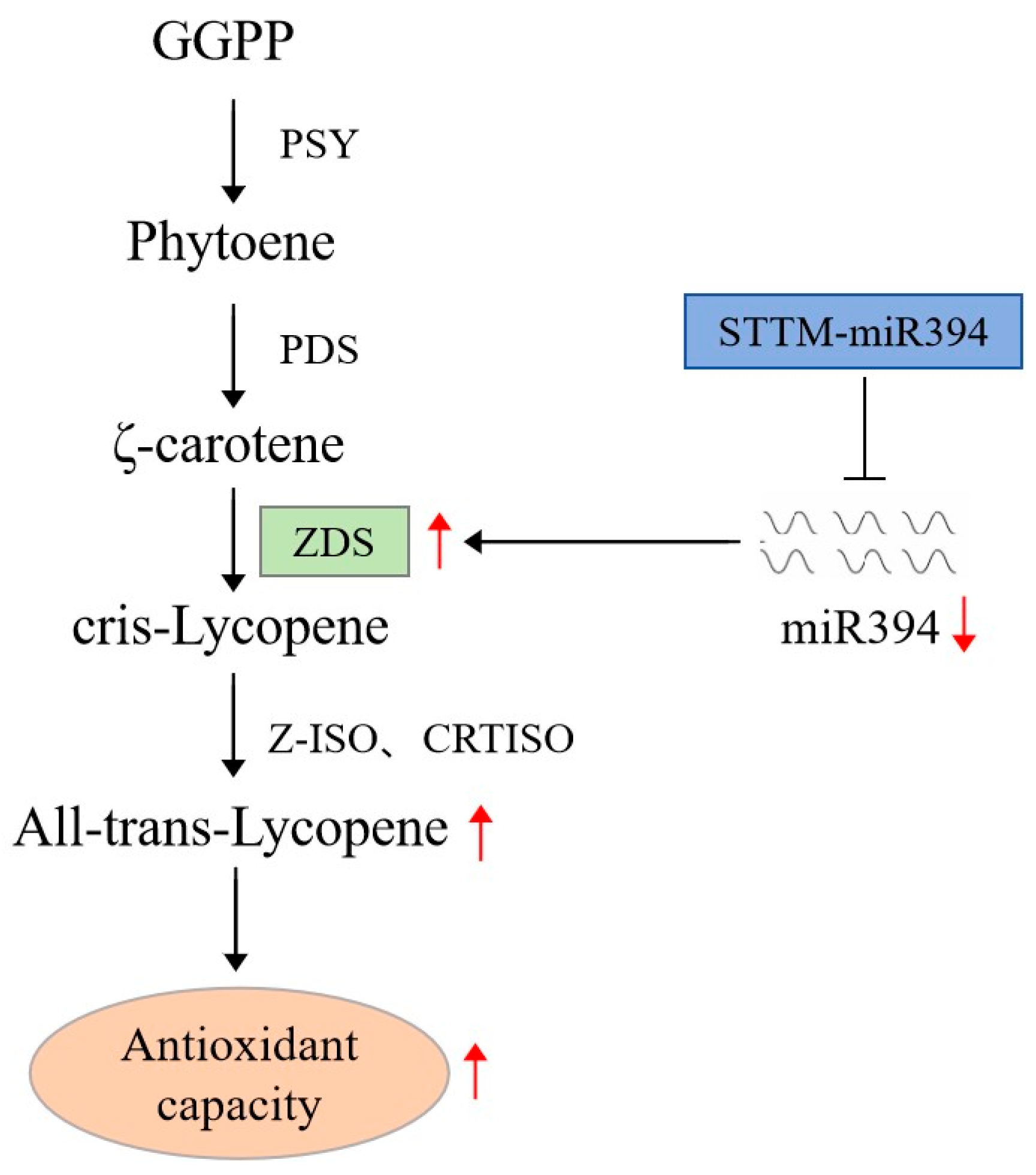
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W., Jr. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar] [CrossRef]
- Heber, D.; Lu, Q.-Y. Overview of Mechanisms of Action of Lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Sawardekar, S.B.; Patel, T.C.; Uchil, D. Comparative evaluation of antiplatelet effect of lycopene with aspirin and the effect of their combination on platelet aggregation: An in vitro study. Indian J. Pharmacol. 2016, 48, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Mills, L.M.; Moir, S.; Masson, L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2017, 76, 122–129. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8, e20. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R.V. Mechanisms of Action of Non-Enzymatic Antioxidants to Control Oxidative Stress During in Vitro Follicle Growth, Oocyte Maturation, and Embryo Development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between Ros and Autophagy in Cancer Cells, from Tumor Initiation to Cancer Therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef]
- Fraser, P.D.; Truesdale, M.R.; Bird, C.R.; Schuch, W.; Bramley, P.M. Carotenoid biosynthesis during tomato fruit development (evidence for tissue-specific gene expression). Plant Physiol. 1994, 105, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Pecker, I.; Chamovitz, D.; Linden, H.; Sandmann, G.; Hirschberg, J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc. Natl. Acad. Sci. USA 1992, 89, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Corona, V.; Aracri, B.; Kosturkova, G.; Bartley, G.E.; Pitto, L.; Giorgetti, L.; Scolnik, P.A.; Giuliano, G. Regulation of a carotenoid biosynthesis gene promoter during plant development. Plant J. 1996, 9, 505–512. [Google Scholar] [CrossRef]
- Bramley, P. The regulation and genetic manipulation of carotenoid biosynthesis in tomato fruit. Pure Appl. Chem. 1997, 69, 2159–2162. [Google Scholar] [CrossRef]
- Burns, J.; Fraser, P.D.; Bramley, P.M. Identification and quantification of carotenoids, tocopherols and chlorophylls in commonly consumed fruits and vegetables. Phytochemistry 2003, 62, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. Micrornas and Their Regulatory Roles in Plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Zhu, A.; Wu, X.; Ye, J.; Yu, K.; Guo, W.; Deng, X. Discovery and Comparative Profiling of Micrornas in a Sweet Orange Red-Flesh Mutant and Its Wild Type. BMC Genom. 2010, 11, 246. [Google Scholar] [CrossRef]
- Mekontso, F.N.; Wu, S.; Fu, R.; Li, W.; Meng, L.; Wang, Q.; Li, J.; Song, H.; Xu, X. Knockdown of Sly-Mir160a Using Short Tandem Target Mimic (Sttm) Enhanced Expression of Auxin Signaling Genes and Delayed Postharvest Ripening of Tomato Fruit. Postharvest Biol. Technol. 2023, 198, 112271. [Google Scholar] [CrossRef]
- Kaur, S.; Spillane, C. Reduction in Carotenoid Levels in the Marine Diatom Phaeodactylum tricornutum by Artificial Micrornas Targeted against the Endogenous Phytoene Synthase Gene. Mar. Biotechnol. 2015, 17, 1–7. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, X.; Ye, J.; Su, D.; Wang, L.; Liao, Y.; Zhang, W.; Wang, Q.; Chen, Q.; Xu, F. Multiomics Analysis Provides New Insights into the Regulatory Mechanism of Carotenoid Biosynthesis in Yellow Peach Peel. Mol. Hortic. 2023, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Knauer, S.; Holt, A.L.; Rubio-Somoza, I.; Tucker, E.J.; Hinze, A.; Pisch, M.; Javelle, M.; Timmermans, M.C.; Tucker, M.R.; Laux, T. A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev. Cell 2013, 24, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Song, J.B.; Shu, X.X.; Shen, Q.; Li, B.W.; Song, J.; Yang, Z.M. Altered fruit and seed development of transgenic rapeseed (Brassica napus) over-expressing microRNA394. PLoS ONE 2015, 10, e0125427. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Lin, L.B.; Xue, H.W. Rice miR394 suppresses leaf inclination through targeting an F-box gene, LEAF INCLINATION 4. J. Integr. Plant Biol. 2019, 61, 406–416. [Google Scholar] [CrossRef]
- Song, J.B.; Gao, S.; Wang, Y.; Li, B.W.; Zhang, Y.L.; Yang, Z.M. miR394 and its target gene LCR are involved in cold stress response in Arabidopsis. Plant Gene 2016, 5, 56–64. [Google Scholar] [CrossRef]
- Pan, Y.; Niu, M.; Liang, J.; Lin, E.; Tong, Z.; Zhang, J. Identification of heat-responsive miRNAs to reveal the miRNA-mediated regulatory network of heat stress response in Betula luminifera. Trees 2017, 31, 1635–1652. [Google Scholar] [CrossRef]
- Song, J.B.; Gao, S.; Sun, D.; Li, H.; Shu, X.X.; Yang, Z.M. miR394 and LCR are involved in Arabidopsis salt and drought stress responses in an abscisic acid-dependent manner. BMC Plant Biol. 2013, 13, 210. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.-J.; Shin, Y. Estimation of Daily Intake of Lycopene, Antioxidant Contents and Activities from Tomatoes, Watermelons, and Their Processed Products in Korea. Appl. Biol. Chem. 2020, 63, 50. [Google Scholar] [CrossRef]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Akpolat, M.Z.; Francis, D.M.; Chen, Y.J.; Rodriguez-Saona, L.E. High-Throughput Phenotyping Approach for Screening Major Carotenoids of Tomato by Handheld Raman Spectroscopy Using Chemometric Methods. Sensors 2020, 20, 3723. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat Exposure Alters the Expression of Sod, Pod, Apx and Cat Isozymes and Mitigates Low Cadmium Toxicity in Seedlings of Sensitive and Tolerant Rice Cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tang, G.; Yan, J.; Gu, Y.; Qiao, M.; Fan, R.; Mao, Y.; Tang, X. Construction of Short Tandem Target Mimic (Sttm) to Block the Functions of Plant and Animal Micrornas. Methods 2012, 58, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Jiang, C.; Fan, Z.; Li, Z.; Niu, D.; Li, Y.; Zheng, M.; Wang, Q.; Jin, H.; Guo, J. Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing miR472 and activating CNLs-mediated basal immunity in Arabidopsis. Mol. Plant Pathol. 2020, 21, 854–870. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.-C.; Logendra, L.; Janes, H. Correlation of Lycopene Measured by Hplc with the L*, a*, B* Color Readings of a Hydroponic Tomato and the Relationship of Maturity with Color and Lycopene Content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Kulawik, A.; Cielecka-Piontek, J.; Zalewski, P. The Importance of Antioxidant Activity for the Health-Promoting Effect of Lycopene. Nutrients 2023, 15, 3821. [Google Scholar] [CrossRef]
- Sankari, M.; Rao, P.R.; Hemachandran, H.; Pullela, P.K.; Doss, C.G.P.; Tayubi, I.A.; Subramanian, B.; Gothandam, K.M.; Singh, P.; Ramamoorthy, S. Prospects and progress in the production of valuable carotenoids: Insights from metabolic engineering, synthetic biology, and computational approaches. J. Biotechnol. 2018, 266, 89–101. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, Z.; Chen, F.; Zheng, L.; Pan, Y.; Xing, Q.; Tsao, R.; Li, H. Synergistic Antioxidant Effects of Petunidin and Lycopene in H9c2 Cells Submitted to Hydrogen Peroxide: Role of Akt/Nrf2 Pathway. J. Food Sci. 2020, 85, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Yu, B.; Gruber, M.Y.; Khachatourians, G.G.; Hegedus, D.D.; Hannoufa, A. Enhanced Seed Carotenoid Levels and Branching in Transgenic Brassica napus Expressing the Arabidopsis Mir156b Gene. J. Agric. Food Chem. 2010, 58, 9572–9578. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, J.; Muhammed Sadiq, P.A.; Raju, S.; Mukherjee, A. In Silico Analysis of Carotenoid Biosynthesis Pathway in Cassava (Manihot esculenta Crantz). J. Genet. 2022, 101, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Lu, Q.; Li, M.; Dai, X.; Shan, M.; Liu, Z.; Bai, M.-Y.; Xiang, F. Mir394 Modulates Brassinosteroid Signaling to Regulate Hypocotyl Elongation in Arabidopsis. Plant J. 2024, 119, 645–657. [Google Scholar] [CrossRef]
- Miskevish, F.; Lodeyro, A.; Ponso, M.A.; Bouzo, C.; Meeley, R.; Timmermans, M.C.; Dotto, M. Maize Mutants in Mir394-Regulated Genes Show Improved Drought Tolerance. Physiol. Plant. 2025, 177, e70155. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.; Chen, X. The ‘How’ and ‘Where’ of Plant microRNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef]
- Chen, X. A microRNA as a Translational Repressor of Apetala2 in Arabidopsis Flower Development. Science 2004, 303, 2022–2025. [Google Scholar] [CrossRef]
- Gandikota, M.; Birkenbihl, R.P.; Höhmann, S.; Cardon, G.H.; Saedler, H.; Huijser, P. The miRNA156/157 Recognition Element in the 3’ Utr of the Arabidopsis SBP Box Gene SPL3 Prevents Early Flowering by Translational Inhibition in Seedlings. Plant J. 2007, 49, 683–693. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Zhuang, X.; Yu, Y.; Liu, X.; Cui, X.; Ji, L.; Pan, Z.; Cao, X.; Mo, B.; et al. microRNAs Inhibit the Translation of Target mRNAs on the EndopLsamic Reticulum in Arabidopsis. Cell 2013, 153, 562–574. [Google Scholar] [CrossRef]




| Primer Name | Sequence (5′-3′) |
|---|---|
| miR394-RT | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACGGAGGT |
| miR394-F | CGCGTTGGCATTCTGTCC |
| miR394-R | GTGCAGGGTCCGAGGT |
| U6-F | CATCCGATAAAATTGGAACGA |
| U6-R | TTTGTGCGTGTCATCCTTGCG |
| Actin-F | GAAATAGCATAAGATGGCAGACG |
| Actin-R | ATACCCACCATCACACCAGTAT |
| PDS-F | CATGCCACGACCAGAAGATTG |
| PDS-R | CACCAGCAATAACAATCTCCAATG |
| ZDS-F | TTCATCCATCAACAGGGTACAT |
| ZDS-R | GCCACAAACCATTCCAAACTC |
| pTF486-ZDS-GFP-F | ACGCGTCGACGTCGGCCATAGCGGCCGCGGAAGTATTTGAATCAAGTGGCTCCT |
| pTF486-ZDS-GFP-R | CGGGATCCCGAATTCTTCCTTGTGCGATGC |
| pOT2-miR394-F | CGGGGTACCAGTAGTGAAGATTATTGAGAGAC |
| pOT2-miR394-R | CGCGGATCCTCATTATCATCAGTATCAACACA |
| STTM-miR394-F | GCCATTTAAATATGGTCTAAAGAAGAAGAATTTGGCATTCTctaGTCCACCTCCGAATTCGGTACGCTGAAATCACCAG |
| STTM-miR394-R | GCCATTTAAATTAGACCATAACAACAACAACGGAGGTGGACAtagGAATGCCAAAAGCTTGGGCTGTCCTCTCCAAATG |
| STTM-New-Common-PF | CGCACAACCCCACTATCCTT |
| STTM-New-Common-PR | GGGCCTGCAACCTTATCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Zhao, L.; Shi, J.; Chen, X.; Yang, Z.; Wu, Y. The SlymiR394-ZDS Module Enhances the Antioxidant Capacity of Tomato by Promoting Lycopene Synthesis. Biomolecules 2025, 15, 857. https://doi.org/10.3390/biom15060857
Zhao Q, Zhao L, Shi J, Chen X, Yang Z, Wu Y. The SlymiR394-ZDS Module Enhances the Antioxidant Capacity of Tomato by Promoting Lycopene Synthesis. Biomolecules. 2025; 15(6):857. https://doi.org/10.3390/biom15060857
Chicago/Turabian StyleZhao, Qiuyu, Li Zhao, Jiuzhi Shi, Xiaoxiao Chen, Zhenchao Yang, and Yongjun Wu. 2025. "The SlymiR394-ZDS Module Enhances the Antioxidant Capacity of Tomato by Promoting Lycopene Synthesis" Biomolecules 15, no. 6: 857. https://doi.org/10.3390/biom15060857
APA StyleZhao, Q., Zhao, L., Shi, J., Chen, X., Yang, Z., & Wu, Y. (2025). The SlymiR394-ZDS Module Enhances the Antioxidant Capacity of Tomato by Promoting Lycopene Synthesis. Biomolecules, 15(6), 857. https://doi.org/10.3390/biom15060857






