Increased [18F]DPA-714 Uptake in the Skeletal Muscle of SOD1G93A Mice: A New Potential of Translocator Protein 18 kDa Imaging in Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiochemistry
2.2. Animal Models
2.3. Experimental Micro-PET Scanning and Image Analysis
2.4. Ex Vivo Studies
2.5. Autoradiography
2.6. Statistical Analysis
3. Results
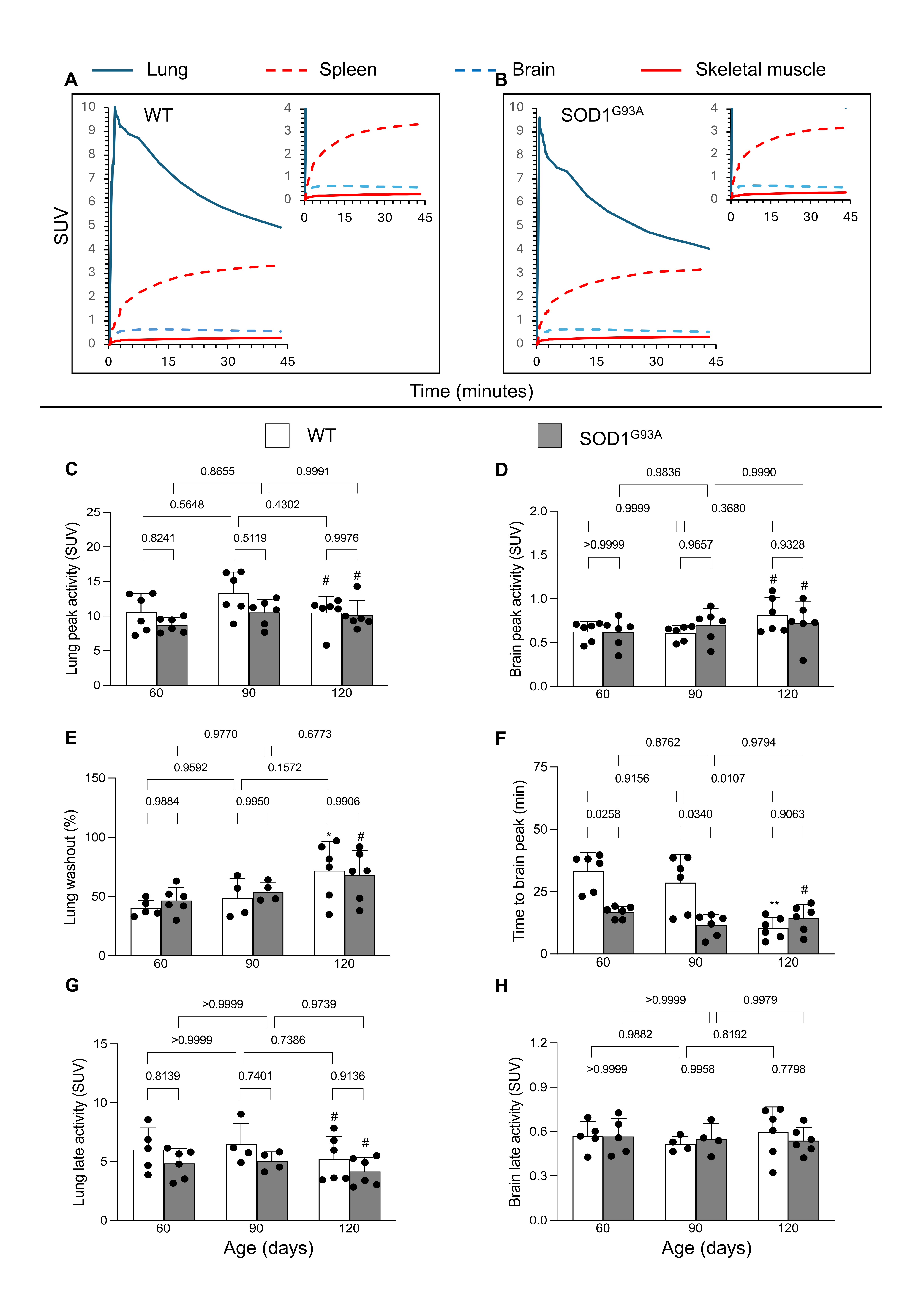
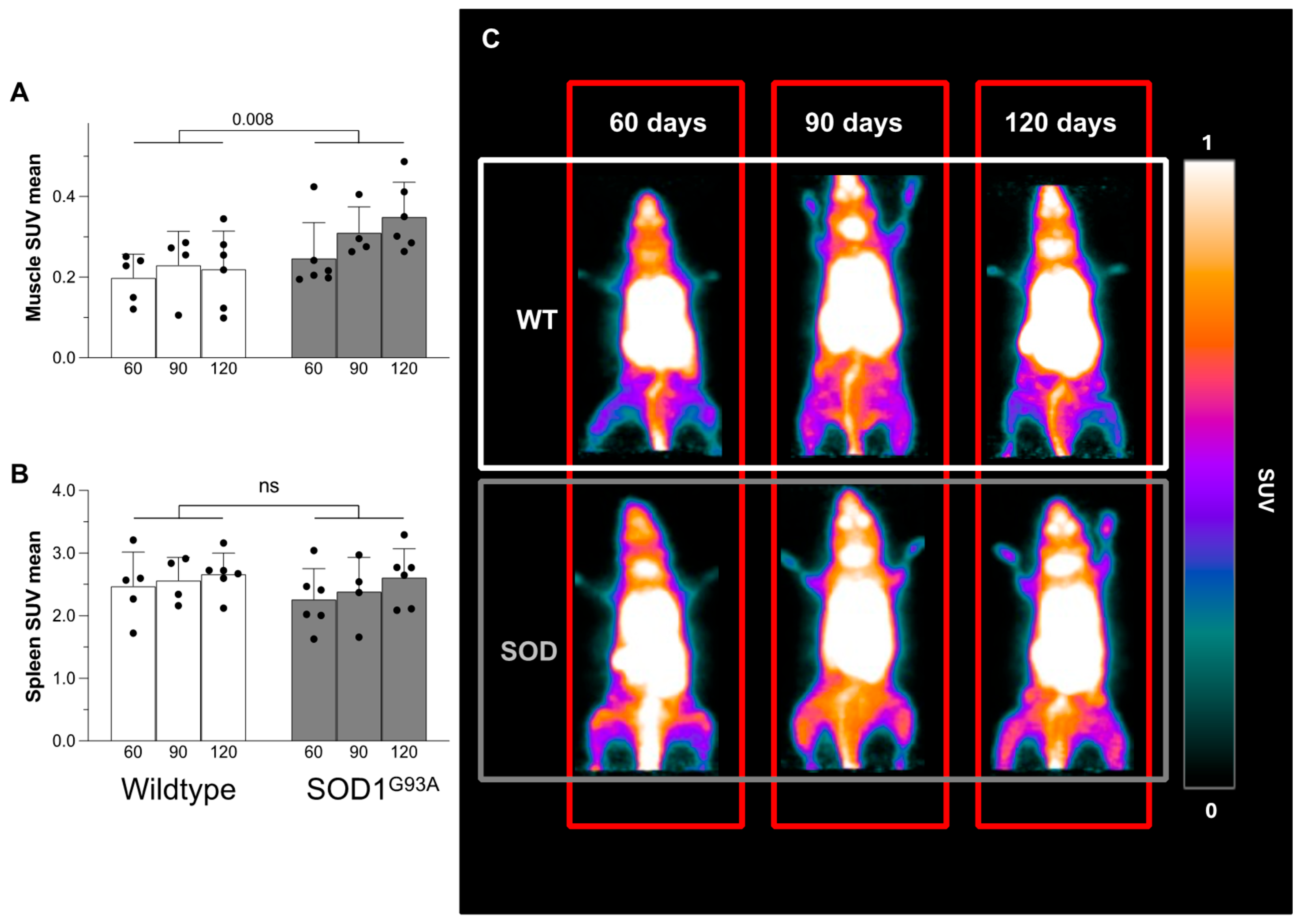
3.1. [18F]DPA-714 Uptake
3.2. Overexpression of TSPO in Skeletal Muscle of Transgenic Mice
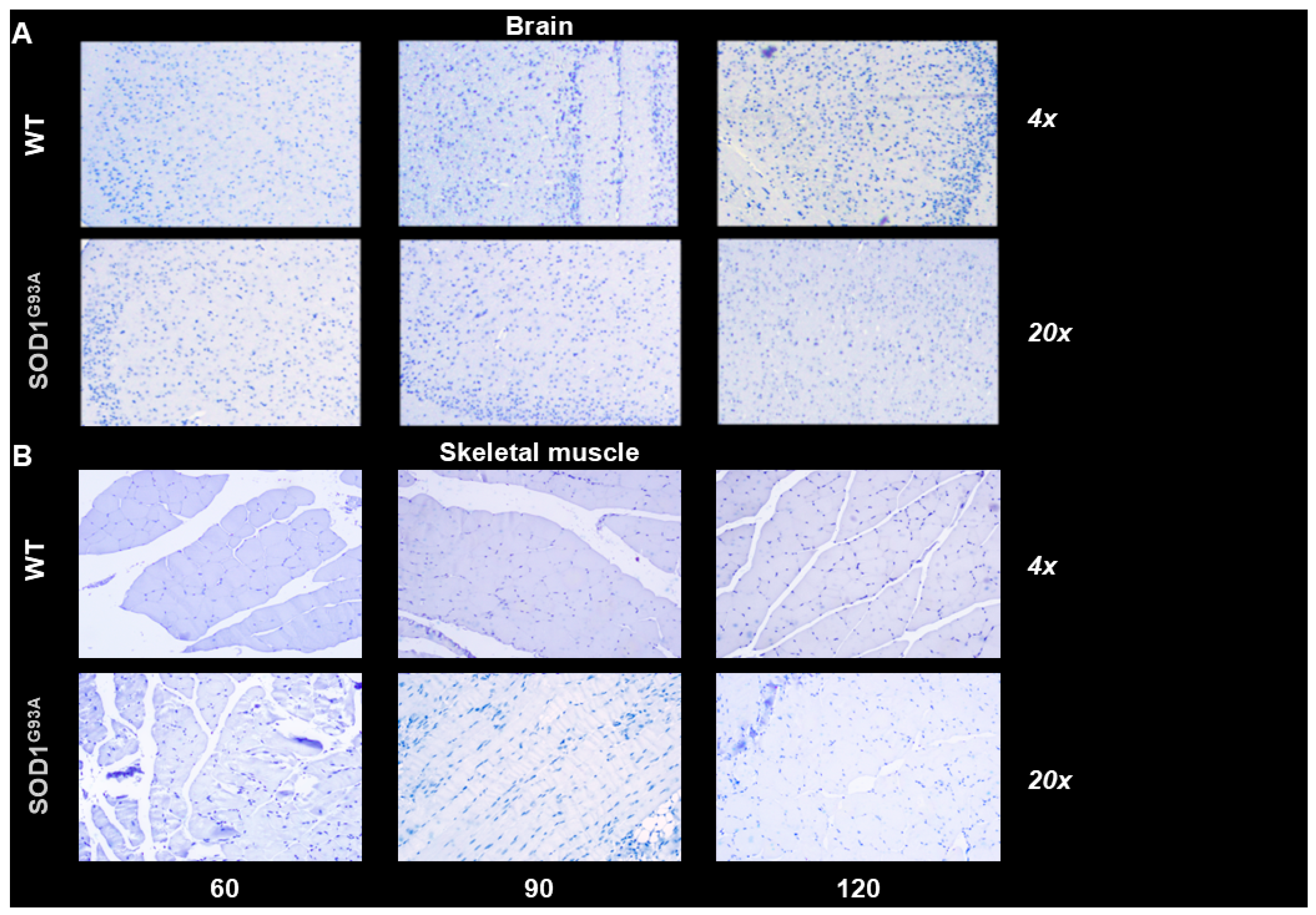
3.3. Histology and Immunohistochemistry of Skeletal Muscle
4. Discussion
PET Evaluation of TSPO Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Shefner, J.M.; Musaro, A.; Ngo, S.T.; Lunetta, C.; Steyn, F.J.; Robitaille, R.; De Carvalho, M.; Rutkove, S.; Ludolph, A.C.; Dupuis, L. Skeletal muscle in amyotrophic lateral sclerosis. Brain 2023, 146, 4425–4436. [Google Scholar] [CrossRef] [PubMed]
- Pikatza-Menoio, O.; Elicegui, A.; Bengoetxea, X.; Naldaiz-Gastesi, N.; López de Munain, A.; Gerenu, G.; Gil-Bea, F.J.; Alonso-Martín, S. The Skeletal Muscle Emerges as a New Disease Target in Amyotrophic Lateral Sclerosis. J. Pers. Med. 2021, 11, 671. [Google Scholar] [CrossRef]
- Loeffler, J.P.; Picchiarelli, G.; Dupuis, L.; Gonzalez De Aguilar, J.L. The Role of Skeletal Muscle in Amyotrophic Lateral Sclerosis. Brain Pathol. 2016, 26, 227–236. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870. [Google Scholar] [CrossRef]
- Van Weehaeghe, D.; Babu, S.; De Vocht, J.; Zürcher, N.R.; Chew, S.; Tseng, C.J.; Loggia, M.L.; Koole, M.; Rezaei, A.; Schramm, G.; et al. Moving Toward Multicenter Therapeutic Trials in Amyotrophic Lateral Sclerosis: Feasibility of Data Pooling Using Different Translocator Protein PET Radioligands. J. Nucl. Med. 2020, 61, 1621–1627. [Google Scholar] [CrossRef]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef]
- Gargiulo, S.; Anzilotti, S.; Coda, A.R.; Gramanzini, M.; Greco, A.; Panico, M.; Vinciguerra, A.; Zannetti, A.; Vicidomini, C.; Dollé, F.; et al. Imaging of brain TSPO expression in a mouse model of amyotrophic lateral sclerosis with 18F-DPA-714 and micro-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Su, P.; Piccini, P. Imaging of microglia in patients with neurodegenerative disorders. Front. Pharmacol. 2012, 3, 22967. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Robello, M.; Costa, B.; Da Pozzo, E.; Baglini, E.; Salerno, S.; Da Settimo, F.; Martini, C.; Taliani, S. An update into the medicinal chemistry of translocator protein (TSPO) ligands. Eur. J. Med. Chem. 2021, 209, 112924. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Fan, J.; Papadopoulos, V. Translocator protein (Tspo) gene promoter-driven green fluorescent protein synthesis in transgenic mice: An in vivo model to study Tspo transcription. Cell Tissue Res. 2012, 350, 261–275. [Google Scholar] [CrossRef]
- Fan, J.; Rone, M.B.; Papadopoulos, V. Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J. Biol. Chem. 2009, 284, 30484–30497. [Google Scholar] [CrossRef]
- Magrì, A.; Lipari, C.L.R.; Risiglione, P.; Zimbone, S.; Guarino, F.; Caccamo, A.; Messina, A. ERK1/2-dependent TSPO overactivation associates with the loss of mitophagy and mitochondrial respiration in ALS. Cell Death Dis. 2023, 14, 122. [Google Scholar] [CrossRef]
- Guilarte, T.R.; Rodichkin, A.N.; McGlothan, J.L.; Acanda De La Rocha, A.M.; Azzam, D.J. Imaging neuroinflammation with TSPO: A new perspective on the cellular sources and subcellular localization. Pharmacol. Ther. 2022, 234, 108048. [Google Scholar] [CrossRef]
- Frison, M.; Faccenda, D.; Abeti, R.; Rigon, M.; Strobbe, D.; England-Rendon, B.S.; Cash, D.; Barnes, K.; Sadeghian, M.; Sajic, M.; et al. The translocator protein (TSPO) is prodromal to mitophagy loss in neurotoxicity. Mol. Psychiatry 2021, 26, 2721–2739. [Google Scholar] [CrossRef]
- Harberts, E.; Datta, D.; Chen, S.; Wohler, J.E.; Oh, U.; Jacobson, S. Translocator protein 18 kDa (TSPO) expression in multiple sclerosis patients. J. Neuroimmune Pharmacol. 2013, 8, 51–57. [Google Scholar] [CrossRef][Green Version]
- Batoko, H.; Veljanovski, V.; Jurkiewicz, P. Enigmatic Translocator protein (TSPO) and cellular stress regulation. Trends Biochem. Sci. 2015, 40, 497–503. [Google Scholar] [CrossRef]
- Jaipuria, G.; Leonov, A.; Giller, K.; Vasa, S.K.; Jaremko, Ł.; Jaremko, M.; Linser, R.; Becker, S.; Zweckstetter, M. Cholesterol-mediated allosteric regulation of the mitochondrial translocator protein structure. Nat. Commun. 2017, 8, 14893. [Google Scholar] [CrossRef]
- Pan, J.H.; Kang, Y.Q.; Li, Q.; Xing, W.; Chen, Y.H.; Yan, Y.; Luo, D.X.; Qiu, Y.; Yuan, Y.F.; Zeng, W.A.; et al. TSPO is a novel biomarker for prognosis that regulates cell proliferation through influencing mitochondrial functions in HCC. Heliyon 2023, 9, e22590. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Middleton, R.J.; Banati, R.; Liu, G.J. The Translocator Protein (TSPO) in Mitochondrial Bioenergetics and Immune Processes. Cells 2020, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Cossu, V.; Bonifacino, T.; Bauckneht, M.; Torazza, C.; Bruno, S.; Castellani, P.; Ravera, S.; Milanese, M.; Venturi, C.; et al. Mechanisms underlying the predictive power of high skeletal muscle uptake of FDG in amyotrophic lateral sclerosis. EJNMMI Res. 2020, 10, 76. [Google Scholar] [CrossRef]
- Cybulska, K.A.; Bloemers, V.; Perk, L.R.; Laverman, P. Optimised GMP-compliant production of [18F]DPA-714 on the Trasis AllinOne module. EJNMMI Radiopharm. Chem. 2021, 6, 20. [Google Scholar] [CrossRef]
- CPMP/ICH/381/95—ICH Harmonised Tripartite Guideline—Validation of Analytical Procedures: Text and Methodology Q2(R1). 2014. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 10 January 2024).
- European Pharmacopoeia 9.5, 2.2.46 Chromatographic Separation Techniques (07/2016:20246). 2016.
- Saba, W.; Goutal, S.; Auvity, S.; Kuhnast, B.; Coulon, C.; Kouyoumdjian, V.; Buvat, I.; Leroy, C.; Tournier, N. Imaging the neuroimmune response to alcohol exposure in adolescent baboons: A TSPO PET study using 18F-DPA-714. Addict. Biol. 2018, 23, 1000–1009. [Google Scholar] [CrossRef]
- Vicidomini, C.; Panico, M.; Greco, A.; Gargiulo, S.; Coda, A.R.; Zannetti, A.; Gramanzini, M.; Roviello, G.N.; Quarantelli, M.; Alfano, B.; et al. In vivo imaging and characterization of [18F]DPA-714, a potential new TSPO ligand, in mouse brain and peripheral tissues using small-animal PET. Nucl. Med. Biol. 2015, 42, 309–316. [Google Scholar] [CrossRef]
- Martín, A.; Boisgard, R.; Thézé, B.; Van Camp, N.; Kuhnast, B.; Damont, A.; Kassiou, M.; Dollé, F.; Tavitian, B. Evaluation of the PBR/TSPO radioligand [18F]DPA-714 in a rat model of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 230–241. [Google Scholar] [CrossRef]
- Bauckneht, M.; Cossu, V.; Castellani, P.; Piccioli, P.; Orengo, A.M.; Emionite, L.; Di Giulio, F.; Donegani, M.I.; Miceli, A.; Raffa, S.; et al. FDG uptake tracks the oxidative damage in diabetic skeletal muscle: An experimental study. Mol. Metab. 2020, 31, 98–108. [Google Scholar] [CrossRef]
- Van Weehaeghe, D.; Van Schoor, E.; De Vocht, J.; Koole, M.; Attili, B.; Celen, S.; Declercq, L.; Thal, D.R.; Van Damme, P.; Bormans, G.; et al. TSPO Versus P2X7 as a Target for Neuroinflammation: An In Vitro and In Vivo Study. J. Nucl. Med. 2020, 61, 604–607. [Google Scholar] [CrossRef]
- Hegedus, J.; Putman, C.T.; Tyreman, N.; Gordon, T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Physiol. 2008, 586, 3337–3351. [Google Scholar] [CrossRef]
- Kovacs, G.G. Cellular reactions of the central nervous system. Handb. Clin. Neurol. 2017, 145, 13–23. [Google Scholar] [CrossRef]
- Romer, S.H.; Metzger, S.; Peraza, K.; Wright, M.C.; Jobe, D.S.; Song, L.S.; Rich, M.M.; Foy, B.D.; Talmadge, R.J.; Voss, A.A. A mouse model of Huntington’s disease shows altered ultrastructure of transverse tubules in skeletal muscle fibers. J. Gen. Physiol. 2021, 153, e202012637. [Google Scholar] [CrossRef] [PubMed]
- Corcia, P.; Tauber, C.; Vercoullie, J.; Arlicot, N.; Prunier, C.; Praline, J.; Nicolas, G.; Venel, Y.; Hommet, C.; Baulieu, J.L.; et al. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e52941. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Phatnani, H.; Kuligowski, M.; Tapia, J.C.; Carrasco, M.A.; Zhang, M.; Maniatis, T.; Carroll, M.C. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20960–20965. [Google Scholar] [CrossRef]
- Al-Sarraj, S.; King, A.; Cleveland, M.; Pradat, P.F.; Corse, A.; Rothstein, J.D.; Leigh, P.N.; Abila, B.; Bates, S.; Wurthner, J.; et al. Mitochondrial abnormalities and low grade inflammation are present in the skeletal muscle of a minority of patients with amyotrophic lateral sclerosis; an observational myopathology study. Acta Neuropathol. Commun. 2014, 2, 165. [Google Scholar] [CrossRef]
- Gatliff, J.; East, D.A.; Singh, A.; Alvarez, M.S.; Frison, M.; Matic, I.; Ferraina, C.; Sampson, N.; Turkheimer, F.; Campanella, M. A role for TSPO in mitochondrial Ca2+ homeostasis and redox stress signaling. Cell Death Dis. 2017, 8, e2896. [Google Scholar] [CrossRef]
- Carayon, P.; Portier, M.; Dussossoy, D.; Bord, A.; Petitprêtre, G.; Canat, X.; Le Fur, G.; Casellas, P. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood 1996, 87, 3170–3178. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Papadopoulos, V. The mitochondrial translocator protein (TSPO, 18 kDa): A key multifunctional molecule in liver diseases. Biochimie 2023, 224, 91–103. [Google Scholar] [CrossRef]
- James, M.L.; Fulton, R.R.; Vercoullie, J.; Henderson, D.J.; Garreau, L.; Chalon, S.; Dolle, F.; Costa, B.; Guilloteau, D.; Kassiou, M. DPA-714, a new translocator protein-specific ligand: Synthesis, radiofluorination, and pharmacologic characterization. J. Nucl. Med. 2008, 49, 814–822. [Google Scholar] [CrossRef]
- Thuillier, R.; Hauet, T. Role of translocator protein in renal ischemia reperfusion, renal preservation and acute kidney injury. Curr. Mol. Med. 2012, 12, 413–425. [Google Scholar]
- Guillemain, G.; Khemtemourian, L.; Brehat, J.; Morin, D.; Movassat, J.; Tourrel-Cuzin, C.; Lacapere, J.J. TSPO in pancreatic beta cells and its possible involvement in type 2 diabetes. Biochimie 2024, 224, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sheng, M.; Lv, J.; Cao, Y.; Chen, D.; Jia, L.; Sun, Y.; Ren, Y.; Li, L.; Weng, Y.; et al. Single-cell analysis reveals the immune heterogeneity and interactions in lungs undergoing hepatic ischemia-reperfusion. Int. Immunopharmacol. 2023, 124, 111043. [Google Scholar] [CrossRef] [PubMed]
- Wongso, H.; Kurniawan, A.; Setiadi, Y.; Kusumaningrum, C.E.; Widyasari, E.M.; Wibawa, T.H.A.; Mahendra, I.; Febrian, M.B.; Sriyani, M.E.; Halimah, I.; et al. Translocator Protein 18 kDa (TSPO): A Promising Molecular Target for Image-Guided Surgery of Solid Cancers. Adv. Pharm. Bull. 2024, 14, 86–104. [Google Scholar] [CrossRef]
- Gundersen, K.; Bruusgaard, J.C. Nuclear domains during muscle atrophy: Nuclei lost or paradigm lost? J. Physiol. 2008, 586, 2675–2681. [Google Scholar] [CrossRef]
- Schmalbruch, H.; Lewis, D.M. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 2000, 23, 617–626. [Google Scholar] [CrossRef]
- Zhou, B.; Zheng, Y.; Li, X.; Dong, H.; Yu, J.; Zou, Y.; Zhu, M.; Yu, Y.; Fang, X.; Zhou, M.; et al. FUS Mutation Causes Disordered Lipid Metabolism in Skeletal Muscle Associated with ALS. Mol. Neurobiol. 2022, 59, 7265–7277. [Google Scholar] [CrossRef]
- Badu-Mensah, A.; Guo, X.; McAleer, C.W.; Rumsey, J.W.; Hickman, J.J. Functional skeletal muscle model derived from SOD1-mutant ALS patient iPSCs recapitulates hallmarks of disease progression. Sci. Rep. 2020, 10, 14302. [Google Scholar] [CrossRef]
- Steyn, F.J.; Li, R.; Kirk, S.E.; Tefera, T.W.; Xie, T.Y.; Tracey, T.J.; Kelk, D.; Wimberger, E.; Garton, F.C.; Roberts, L.; et al. Altered skeletal muscle glucose-fatty acid flux in amyotrophic lateral sclerosis. Brain Commun. 2020, 2, fcaa154. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, C.; Riondato, M.; Dighero, E.; Democrito, A.; Losacco, S.; Emionite, L.; Nobbio, L.; Di Patrizi, I.; Camera, M.; Ghersi, C.; et al. Increased [18F]DPA-714 Uptake in the Skeletal Muscle of SOD1G93A Mice: A New Potential of Translocator Protein 18 kDa Imaging in Amyotrophic Lateral Sclerosis. Biomolecules 2025, 15, 799. https://doi.org/10.3390/biom15060799
Marini C, Riondato M, Dighero E, Democrito A, Losacco S, Emionite L, Nobbio L, Di Patrizi I, Camera M, Ghersi C, et al. Increased [18F]DPA-714 Uptake in the Skeletal Muscle of SOD1G93A Mice: A New Potential of Translocator Protein 18 kDa Imaging in Amyotrophic Lateral Sclerosis. Biomolecules. 2025; 15(6):799. https://doi.org/10.3390/biom15060799
Chicago/Turabian StyleMarini, Cecilia, Mattia Riondato, Edoardo Dighero, Alessia Democrito, Serena Losacco, Laura Emionite, Lucilla Nobbio, Irene Di Patrizi, Mattia Camera, Chiara Ghersi, and et al. 2025. "Increased [18F]DPA-714 Uptake in the Skeletal Muscle of SOD1G93A Mice: A New Potential of Translocator Protein 18 kDa Imaging in Amyotrophic Lateral Sclerosis" Biomolecules 15, no. 6: 799. https://doi.org/10.3390/biom15060799
APA StyleMarini, C., Riondato, M., Dighero, E., Democrito, A., Losacco, S., Emionite, L., Nobbio, L., Di Patrizi, I., Camera, M., Ghersi, C., Ghelardoni, M., Lanfranchi, F., Vitale, F., Carta, S., Chiesa, S., Torazza, C., Milanese, M., Bauckneht, M., Hamedani, M., ... Sambuceti, G. (2025). Increased [18F]DPA-714 Uptake in the Skeletal Muscle of SOD1G93A Mice: A New Potential of Translocator Protein 18 kDa Imaging in Amyotrophic Lateral Sclerosis. Biomolecules, 15(6), 799. https://doi.org/10.3390/biom15060799










