Orphan Nuclear Receptors TR2 and TR4 in Erythropoiesis: From Mechanisms to Therapies
Abstract
1. Introduction
2. Structures of TR2/TR4

3. TR2/TR4 in Erythropoiesis
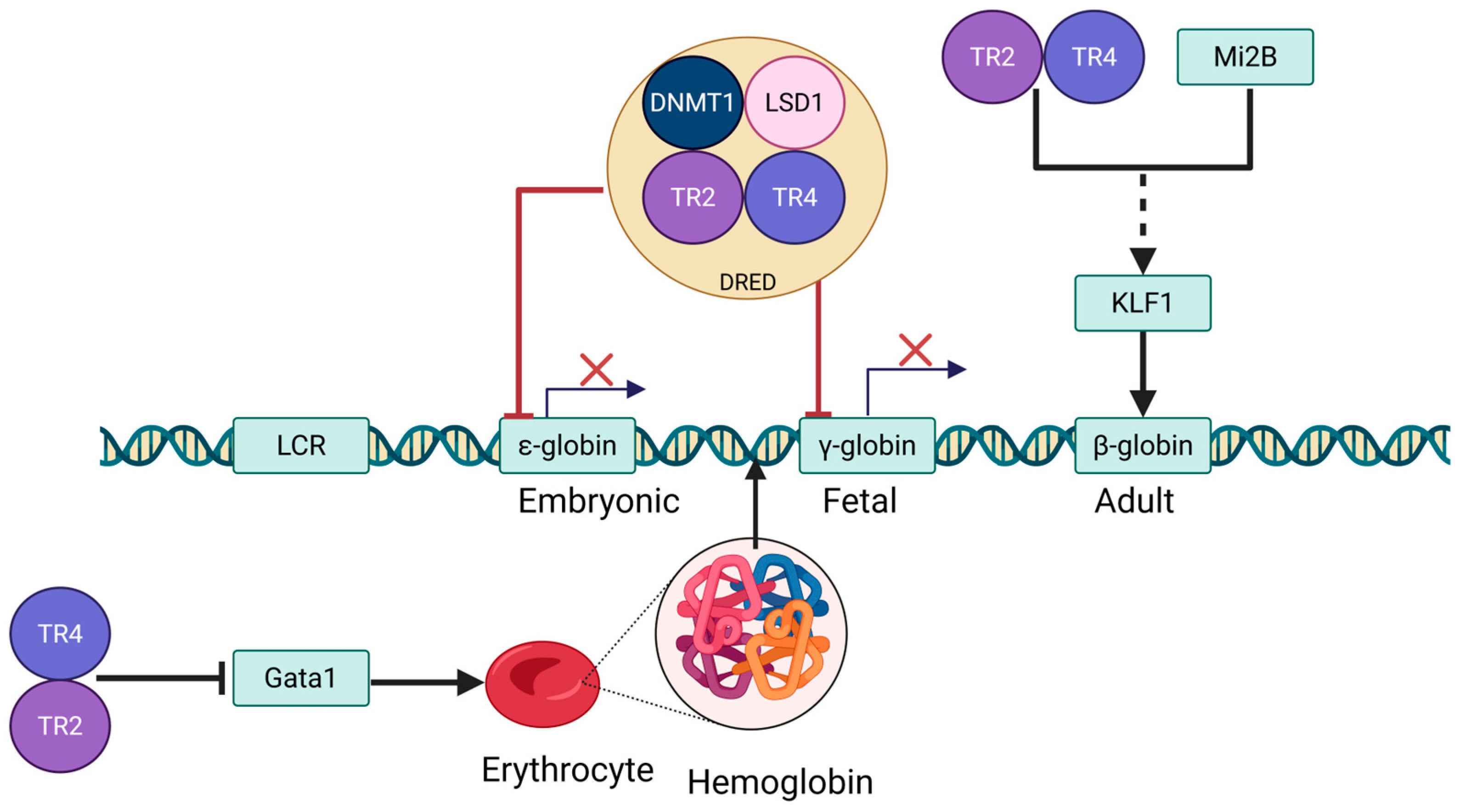
3.1. Erythrocyte Differentiation and Globin Switching
3.2. Role of TR2/TR4 in Erythropoiesis
4. TR2/TR4 in Hemoglobin Expression
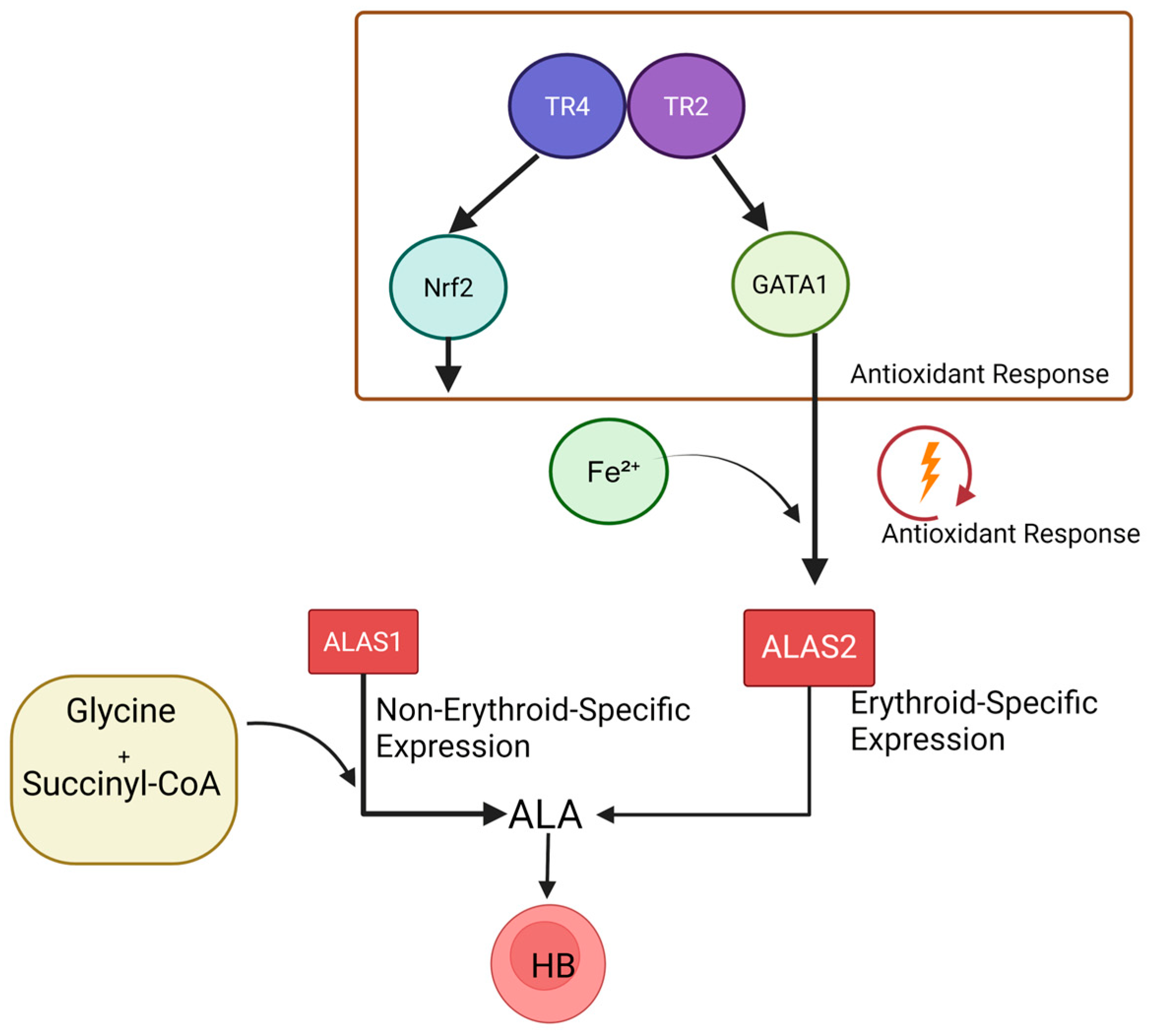
4.1. Coordinated Regulation of TR2/TR4 and GATA
4.2. Coordinated Regulation of TR2/TR4 and KLF1
4.3. TR2/TR4 in the Regulation of Heme Biosynthesis
5. TR2/TR4 in Erythrocyte Disorders
5.1. TR2/TR4 in SCD
5.2. TR2/TR4 in β-Thalassemia
5.3. TR2/TR4 in HPFH
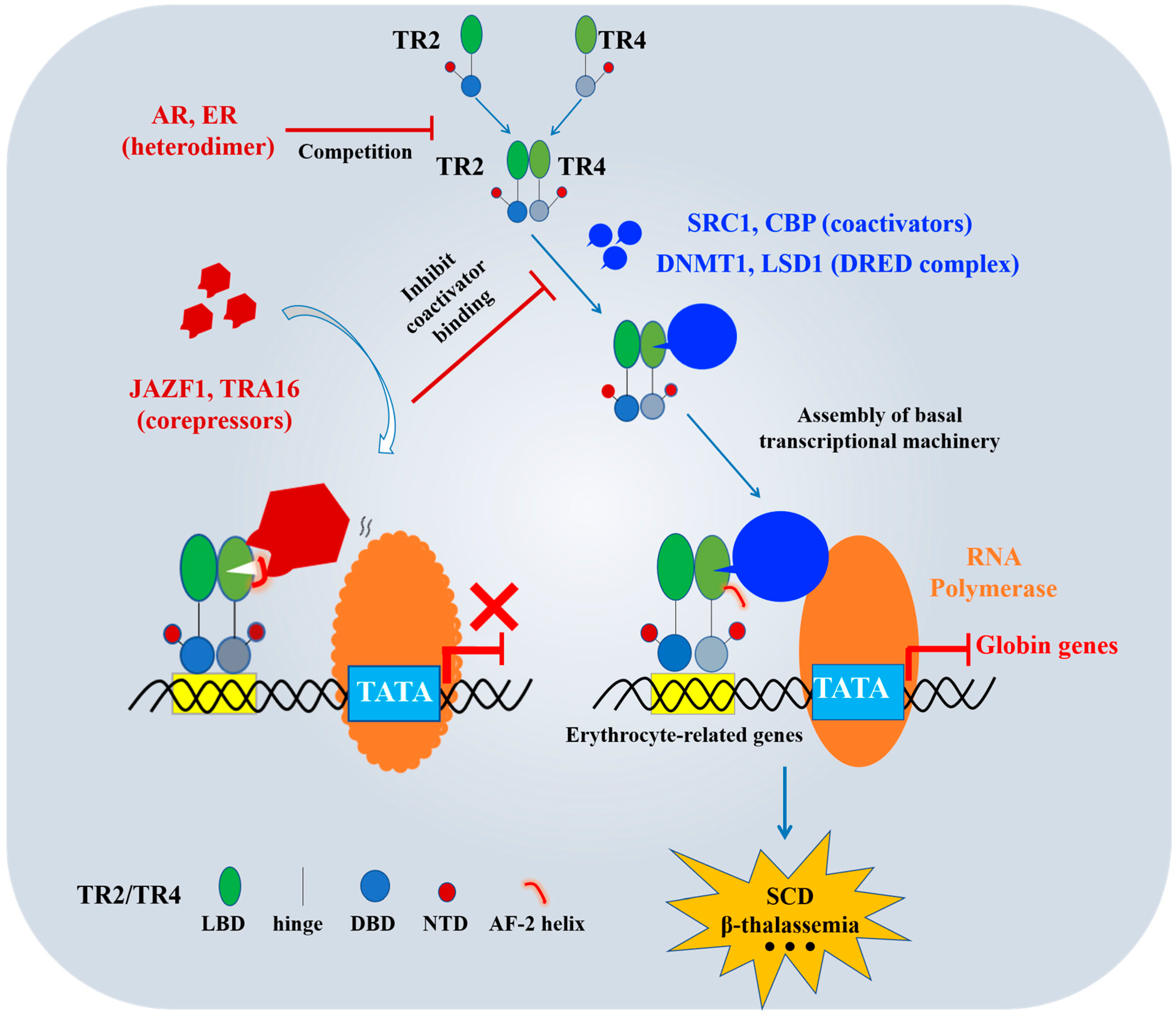
6. TR2/TR4-Targeted Coregulators
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Zhang, B.; Wang, Y.; Jiang, R.; Liu, J.; Wei, Y.; Gao, X.; Zhu, Y.; Wang, X.; Sun, M.; et al. An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption. Nature 2023, 622, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Caulier, A.L.; Sankaran, V.G. Molecular and cellular mechanisms that regulate human erythropoiesis. Blood 2022, 139, 2450–2459. [Google Scholar] [CrossRef]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; D’Alessandro, A.; Nikolaidis, M.G. Erythrocyte metabolism. Acta Physiol 2024, 240, e14081. [Google Scholar] [CrossRef]
- Nevezhin, E.V.; Vlasova, N.V.; Pyatnitskiy, I.A.; Lysenko, E.P.; Malakhov, M.V. On the mechanism of erythrocyte hemolysis induced by photooxidized psoralen. Biochemistry 2015, 80, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Peschle, C.; Migliaccio, A.R.; Migliaccio, G.; Petrini, M.; Calandrini, M.; Russo, G.; Mastroberardino, G.; Presta, M.; Gianni, A.M.; Comi, P.; et al. Embryonic—Fetal Hb switch in humans: Studies on erythroid bursts generated by embryonic progenitors from yolk sac and liver. Proc. Natl. Acad. Sci. USA 1984, 81, 2416–2420. [Google Scholar] [CrossRef]
- Huang, P.; Peslak, S.A.; Ren, R.; Khandros, E.; Qin, K.; Keller, C.A.; Giardine, B.; Bell, H.W.; Lan, X.; Sharma, M.; et al. HIC2 controls developmental hemoglobin switching by repressing BCL11A transcription. Nat. Genet. 2022, 54, 1417–1426. [Google Scholar] [CrossRef]
- Zaninetti, C.; Rivera, J.; Vater, L.; Ohlenforst, S.; Leinøe, E.; Böckelmann, D.; Freson, K.; Thiele, T.; Makhloufi, H.; Rath, M.; et al. Aggregates of nonmuscular myosin IIA in erythrocytes associate with GATA1- and GFI1B-related thrombocytopenia. J. Thromb. Haemost. JTH 2024, 22, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jearawiriyapaisarn, N.; Lee, M.P.; Hosoya, T.; Wu, Q.; Myers, G.; Lim, K.C.; Kurita, R.; Nakamura, Y.; Vojtek, A.B.; et al. BAP1 regulation of the key adaptor protein NCoR1 is critical for γ-globin gene repression. Genes. Dev. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Roscoe, B.P.; Liu, P.; Yao, Q.; Lazzarotto, C.R.; Clement, K.; Cole, M.A.; Luk, K.; Baricordi, C.; et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 2019, 25, 776–783. [Google Scholar] [CrossRef]
- Tanabe, O.; Shen, Y.; Liu, Q.; Campbell, A.D.; Kuroha, T.; Yamamoto, M.; Engel, J.D. The TR2 and TR4 orphan nuclear receptors repress Gata1 transcription. Genes. Dev. 2007, 21, 2832–2844. [Google Scholar] [CrossRef]
- Tanabe, O.; Katsuoka, F.; Campbell, A.D.; Song, W.; Yamamoto, M.; Tanimoto, K.; Engel, J.D. An embryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer. EMBO J. 2002, 21, 3434–3442. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Lehmann, J.M.; Willson, T.M. Orphan nuclear receptors: Shifting endocrinology into reverse. Science 1999, 284, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.P.; Tanabe, O.; Shi, L.; Jearawiriyapaisarn, N.; Lucas, D.; Engel, J.D. The orphan nuclear receptor TR4 regulates erythroid cell proliferation and maturation. Blood 2017, 130, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Yang, D.R.; Yang, G.; Lin, C.Y.; Chang, H.C.; Li, G.; Chang, C. TR2 and TR4 Orphan Nuclear Receptors: An Overview. Curr. Top. Dev. Biol. 2017, 125, 357–373. [Google Scholar]
- Lee, H.J.; Lee, Y.F.; Chang, C. TR4 orphan receptor represses the human steroid 21-hydroxylase gene expression through the monomeric AGGTCA motif. Biochem. Biophys. Res. Commun. 2001, 285, 1361–1368. [Google Scholar] [CrossRef][Green Version]
- Cui, S.; Kolodziej, K.E.; Obara, N.; Amaral-Psarris, A.; Demmers, J.; Shi, L.; Engel, J.D.; Grosveld, F.; Strouboulis, J.; Tanabe, O. Nuclear receptors TR2 and TR4 recruit multiple epigenetic transcriptional corepressors that associate specifically with the embryonic β-type globin promoters in differentiated adult erythroid cells. Mol. Cell Biol. 2011, 31, 3298–3311. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Boelens, J.J.; Cancio, M.; Hankins, J.S.; Bhad, P.; Azizy, M.; Lewandowski, A.; Zhao, X.; Chitnis, S.; Peddinti, R.; et al. CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease. New Engl. J. Med. 2023, 389, 820–832. [Google Scholar] [CrossRef]
- Wang, Y.; Myers, G.; Yu, L.; Deng, K.; Balbin-Cuesta, G.; Singh, S.A.; Guan, Y.; Khoriaty, R.; Engel, J.D. TR4 and BCL11A repress γ-globin transcription via independent mechanisms. Blood 2024, 144, 2762–2772. [Google Scholar] [CrossRef]
- Xia, L.; Shen, D.; Wang, H.; Ren, L.; Chen, Y.; Li, G. Identification of Small-Molecule Regulators of Testicular Receptor 4 via a Drug Repurposing Screening. ACS Omega 2020, 5, 30625–30632. [Google Scholar] [CrossRef]
- Xia, L.; Shen, D.; Zhang, Y.; Lu, J.; Wang, M.; Wang, H.; Chen, Y.; Xue, D.; Xie, D.; Li, G. Targeting the TR4 nuclear receptor with antagonist bexarotene can suppress the proopiomelanocortin signalling in AtT-20 cells. J. Cell Mol. Med. 2021, 25, 2404–2417. [Google Scholar] [CrossRef]
- Papageorgiou, D.N.; Karkoulia, E.; Amaral-Psarris, A.; Burda, P.; Kolodziej, K.; Demmers, J.; Bungert, J.; Stopka, T.; Strouboulis, J. Distinct and overlapping DNMT1 interactions with multiple transcription factors in erythroid cells: Evidence for co-repressor functions. Biochim. Biophys. Acta 2016, 1859, 1515–1526. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Fu, G.Q.; Zhao, J.; Cheng, S.Z.; Li, Y.; Yi, L.N.; Li, Z.; Zhang, L.; Zhang, Z.B.; Dai, J.; et al. Di(2-ethylhexyl)phthalate induces reproductive toxicity via JAZF1/TR4 pathway and oxidative stress in pubertal male rats. Toxicol. Ind. Health 2019, 35, 228–238. [Google Scholar] [CrossRef]
- Chang, C.; Kokontis, J. Identification of a new member of the steroid receptor super-family by cloning and sequence analysis. Biochem. Biophys. Res. Commun. 1988, 155, 971–977. [Google Scholar] [CrossRef]
- Chang, C.; Da Silva, S.L.; Ideta, R.; Lee, Y.; Yeh, S.; Burbach, J.P. Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc. Natl. Acad. Sci. USA 1994, 91, 6040–6044. [Google Scholar] [CrossRef] [PubMed]
- Giguère, V.; Hollenberg, S.M.; Rosenfeld, M.G.; Evans, R.M. Functional domains of the human glucocorticoid receptor. Cell 1986, 46, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V.; Ong, E.S.; Segui, P.; Evans, R.M. Identification of a receptor for the morphogen retinoic acid. Nature 1987, 330, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Giguère, V.; Yang, N.; Segui, P.; Evans, R.M. Identification of a new class of steroid hormone receptors. Nature 1988, 331, 91–94. [Google Scholar] [CrossRef]
- Sar, P. Nuclear receptor: Structure and function. Progress. Mol. Biol. Transl. Sci. 2023, 196, 209–227. [Google Scholar]
- Liu, Y.; Ma, L.; Li, M.; Tian, Z.; Yang, M.; Wu, X.; Wang, X.; Shang, G.; Xie, M.; Chen, Y.; et al. Structures of human TR4LBD-JAZF1 and TR4DBD-DNA complexes reveal the molecular basis of transcriptional regulation. Nucleic Acids Res. 2023, 51, 1443–1457. [Google Scholar] [CrossRef]
- Zhou, X.E.; Suino-Powell, K.M.; Xu, Y.; Chan, C.W.; Tanabe, O.; Kruse, S.W.; Reynolds, R.; Engel, J.D.; Xu, H.E. The orphan nuclear receptor TR4 is a vitamin A-activated nuclear receptor. J. Biol. Chem. 2011, 286, 2877–2885. [Google Scholar] [CrossRef]
- Nandakumar, S.K.; Ulirsch, J.C.; Sankaran, V.G. Advances in understanding erythropoiesis: Evolving perspectives. Br. J. Haematol. 2016, 173, 206–218. [Google Scholar] [CrossRef]
- Kasbekar, D.P. Fly clock, my clock, and lamin B receptor. J. Genet. 2024, 103, 1. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 53, D1670–D1676. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; Tsukamoto, S.; Suzuki, M.; Yamamoto-Mukai, H.; Yamamoto, M.; Philipsen, S.; Ohneda, K. Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood 2008, 111, 4375–4385. [Google Scholar] [CrossRef] [PubMed]
- Abdulhay, N.J.; Fiorini, C.; Verboon, J.M.; Ludwig, L.S.; Ulirsch, J.C.; Zieger, B.; Lareau, C.A.; Mi, X.; Roy, A.; Obeng, E.A.; et al. Impaired human hematopoiesis due to a cryptic intronic GATA1 splicing mutation. J. Exp. Med. 2019, 216, 1050–1060. [Google Scholar] [CrossRef]
- Wagenblast, E.; Azkanaz, M.; Smith, S.A.; Shakib, L.; McLeod, J.L.; Krivdova, G.; Araújo, J.; Shultz, L.D.; Gan, O.I.; Dick, J.E.; et al. Functional profiling of single CRISPR/Cas9-edited human long-term hematopoietic stem cells. Nat. Commun. 2019, 10, 4730. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, Z.; Olivey, H.E.; Gurbuxani, S.; Crispino, J.D.; Svensson, E.C. FOG-1-mediated recruitment of NuRD is required for cell lineage re-enforcement during haematopoiesis. EMBO J. 2010, 29, 457–468. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; He, S.; Yu, D. The crucial role of NRF2 in erythropoiesis and anemia: Mechanisms and therapeutic opportunities. Arch. Biochem. Biophys. 2024, 754, 109948. [Google Scholar] [CrossRef]
- Wang, X.; Thein, S.L. Suzuki ing from fetal to adult hemoglobin. Nat. Genet. 2018, 50, 478–480. [Google Scholar] [CrossRef]
- Qiu, C.; Hanson, E.; Olivier, E.; Inada, M.; Kaufman, D.S.; Gupta, S.; Bouhassira, E.E. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp. Hematol. 2005, 33, 1450–1458. [Google Scholar] [CrossRef]
- Traxler, E.A.; Yao, Y.; Wang, Y.D.; Woodard, K.J.; Kurita, R.; Nakamura, Y.; Hughes, J.R.; Hardison, R.C.; Blobel, G.A.; Li, C.; et al. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016, 22, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.; Sun, Y.; Wang, Y.; Benmhammed, H.; Cui, S. Roles of Nuclear Orphan Receptors TR2 and TR4 during Hematopoiesis. Genes (Basel) 2024, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Iarovaia, O.V.; Kovina, A.P.; Petrova, N.V.; Razin, S.V.; Ioudinkova, E.S.; Vassetzky, Y.S.; Ulianov, S.V. Genetic and Epigenetic Mechanisms of β-Globin Gene Switching. Biochemistry. Biokhimiia 2018, 83, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Qiu, H.Y.; Sun, S.; Fu, Z.C.; Wang, G.Q.; Qian, X.; Wang, L.; Zhai, X.; Wei, J.; Wang, Y.; et al. Base editing of the HBG promoter induces potent fetal hemoglobin expression with no detectable off-target mutations in human HSCs. Cell Stem Cell 2023, 30, 1624–1639.e1628. [Google Scholar] [CrossRef]
- Shi, L.; Sierant, M.C.; Gurdziel, K.; Zhu, F.; Cui, S.; Kolodziej, K.E.; Strouboulis, J.; Guan, Y.; Tanabe, O.; Lim, K.C.; et al. Biased, non-equivalent gene-proximal and -distal binding motifs of orphan nuclear receptor TR4 in primary human erythroid cells. PLoS Genet. 2014, 10, e1004339. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Z.; Jiang, H.; Wang, B.; Du, K.; Huang, C.; Wang, C.; Yang, T.; Wang, Y.; Liu, Y.; et al. Angelica sinensis polysaccharides promote extramedullary stress erythropoiesis via ameliorating splenic glycolysis and EPO/STAT5 signaling-regulated macrophages. J. Mol. Histol. 2024, 55, 661–673. [Google Scholar] [CrossRef]
- Liu, X.F.; Li, X.Y.; Zheng, P.S.; Yang, W.T. DAX1 promotes cervical cancer cell growth and tumorigenicity through activation of Wnt/β-catenin pathway via GSK3β. Cell Death Dis. 2018, 9, 339. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, S.; Zhang, S.; Li, Y.Y.; Wang, H.; Chen, Y.; Lu, H. Vitexin protects against high glucose-induced endothelial cell apoptosis and oxidative stress via Wnt/β-catenin and Nrf2 signalling pathway. Arch. Physiol. Biochem. 2024, 130, 275–284. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Lu, M.; Wu, Q.; Yuan, Q.; Hu, C.; Miao, J.; Zhang, Y.; Li, H.; Hou, F.F.; et al. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 2019, 95, 830–845. [Google Scholar] [CrossRef]
- Sharma, N.; Sistla, R.; Andugulapati, S.B. Yohimbine ameliorates liver inflammation and fibrosis by regulating oxidative stress and Wnt/β-catenin pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 123, 155182. [Google Scholar] [CrossRef]
- Tanabe, O.; McPhee, D.; Kobayashi, S.; Shen, Y.; Brandt, W.; Jiang, X.; Campbell, A.D.; Chen, Y.T.; Chang, C.; Yamamoto, M.; et al. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 2007, 26, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Tanabe, O.; Sierant, M.; Shi, L.; Campbell, A.; Lim, K.C.; Engel, J.D. Compound loss of function of nuclear receptors Tr2 and Tr4 leads to induction of murine embryonic β-type globin genes. Blood 2015, 125, 1477–1487. [Google Scholar] [CrossRef]
- Cui, S.; Lim, K.C.; Shi, L.; Lee, M.; Jearawiriyapaisarn, N.; Myers, G.; Campbell, A.; Harro, D.; Iwase, S.; Trievel, R.C.; et al. The LSD1 inhibitor RN-1 induces fetal hemoglobin synthesis and reduces disease pathology in sickle cell mice. Blood 2015, 126, 386–396. [Google Scholar] [CrossRef]
- Schote, A.B.; Turner, J.D.; Schiltz, J.; Muller, C.P. Nuclear receptors in human immune cells: Expression and correlations. Mol. Immunol. 2007, 44, 1436–1445. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, W.; Benda, C.; Huang, Z.; Ahmed, T.; Liu, P.; Guo, X.; Ibañez, D.P.; Luo, Z.; Zhang, M.; et al. NCoR/SMRT co-repressors cooperate with c-MYC to create an epigenetic barrier to somatic cell reprogramming. Nat. Cell Biol. 2018, 20, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Liebhaber, S.A.; Russell, J.E. Expression and developmental control of the human alpha-globin gene cluster. Ann. N. Y. Acad. Sci. 1998, 850, 54–63. [Google Scholar] [CrossRef]
- Okumura-Nakanishi, S.; Saito, M.; Niwa, H.; Ishikawa, F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J. Biol. Chem. 2005, 280, 5307–5317. [Google Scholar] [CrossRef]
- Park, S.W.; Hu, X.; Gupta, P.; Lin, Y.P.; Ha, S.G.; Wei, L.N. SUMOylation of Tr2 orphan receptor involves Pml and fine-tunes Oct4 expression in stem cells. Nat. Struct. Mol. Biol. 2007, 14, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Oh, S.P.; Okano, M.; Jüttermann, R.; Goss, K.A.; Jaenisch, R.; Li, E. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development 1996, 122, 3195–3205. [Google Scholar] [CrossRef]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef]
- Holshouser, S.; Cafiero, R.; Robinson, M.; Kirkpatrick, J.; Casero, R.A., Jr.; Hyacinth, H.I.; Woster, P.M. Epigenetic Reexpression of Hemoglobin F Using Reversible LSD1 Inhibitors: Potential Therapies for Sickle Cell Disease. ACS Omega 2020, 5, 14750–14758. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, S.; Nagai, M.; Kitagawa, T. Structural origin of cooperativity in human hemoglobin: A view from different roles of α and β subunits in the α(2)β(2) tetramer. Biophys. Rev. 2022, 14, 483–498. [Google Scholar] [CrossRef]
- Barbarani, G.; Łabedz, A.; Ronchi, A.E. β-Hemoglobinopathies: The Test Bench for Genome Editing-Based Therapeutic Strategies. Front. Genome Ed. 2020, 2, 571239. [Google Scholar] [CrossRef] [PubMed]
- Mairbäurl, H.; Weber, R.E. Oxygen transport by hemoglobin. Compr. Physiol. 2012, 2, 1463–1489. [Google Scholar] [CrossRef]
- Gottlieb, E.R.; Ziegler, J.; Morley, K.; Rush, B.; Celi, L.A. Assessment of Racial and Ethnic Differences in Oxygen Supplementation Among Patients in the Intensive Care Unit. JAMA Intern. Med. 2022, 182, 849–858. [Google Scholar] [CrossRef]
- Arbiol-Roca, A.; Imperiali, C.E.; Dot-Bach, D.; Valero-Politi, J.; Dastis-Arias, M. Stability of pH, Blood Gas Partial Pressure, Hemoglobin Oxygen Saturation Fraction, and Lactate Concentration. Ann. Lab. Med. 2020, 40, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, S. Heme-based oxygen gasoreceptors. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E178–E181. [Google Scholar] [CrossRef]
- Vávra, J.; Sergunin, A.; Jeřábek, P.; Shimizu, T.; Martínková, M. Signal transduction mechanisms in heme-based globin-coupled oxygen sensors with a focus on a histidine kinase (AfGcHK) and a diguanylate cyclase (YddV or EcDosC). Biol. Chem. 2022, 403, 1031–1042. [Google Scholar] [CrossRef]
- Hovan, A.; Berta, M.; Sedláková, D.; Miskovsky, P.; Bánó, G.; Sedlák, E. Heme is responsible for enhanced singlet oxygen deactivation in cytochrome c. Phys. Chem. Chem. Phys. PCCP 2021, 23, 15557–15563. [Google Scholar] [CrossRef]
- Lin, D.L.; Wu, S.Q.; Chang, C. The genomic structure and chromosomal location of the human TR2 orphan receptor, a member of the steroid receptor superfamily. Endocrine 1998, 8, 123–134. [Google Scholar] [CrossRef]
- Campbell, A.D.; Cui, S.; Shi, L.; Urbonya, R.; Mathias, A.; Bradley, K.; Bonsu, K.O.; Douglas, R.R.; Halford, B.; Schmidt, L.; et al. Forced TR2/TR4 expression in sickle cell disease mice confers enhanced fetal hemoglobin synthesis and alleviated disease phenotypes. Proc. Natl. Acad. Sci. USA 2011, 108, 18808–18813. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamamoto, M.; Engel, J.D. Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies. Mol. Cell Biol. 2014, 34, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hu, T.; Ho, M.H.; Wang, Y.; Yu, M.; Patel, N.; Pi, W.; Choi, J.H.; Xu, H.; Ganapathy, V.; et al. Hydroxyurea differentially modulates activator and repressors of γ-globin gene in erythroblasts of responsive and non-responsive patients with sickle cell disease in correlation with Index of Hydroxyurea Responsiveness. Haematologica 2017, 102, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Crossley, M.; Tsang, A.P.; Bieker, J.J.; Orkin, S.H. Regulation of the erythroid Kruppel-like factor (EKLF) gene promoter by the erythroid transcription factor GATA-1. J. Biol. Chem. 1994, 269, 15440–15444. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, K.; Sun, C.W.; Pawlik, K.M.; Townes, T.M. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat. Genet. 2010, 42, 742–744. [Google Scholar] [CrossRef]
- Yue, F.; Zhou, Z.; Wang, L.; Wang, M.; Song, L. A conserved zinc finger transcription factor GATA involving in the hemocyte production of scallop Chlamys farreri. Fish Shellfish Immunol. 2014, 39, 125–135. [Google Scholar] [CrossRef]
- Abunimye, D.A.; Okafor, I.M.; Okorowo, H.; Obeagu, E.I. The role of GATA family transcriptional factors in haematological malignancies: A review. Medicine 2024, 103, e37487. [Google Scholar] [CrossRef]
- Bagu, E.T.; Santos, M.M. Friend of GATA suppresses the GATA-induced transcription of hepcidin in hepatocytes through a GATA-regulatory element in the HAMP promoter. J. Mol. Endocrinol. 2011, 47, 299–313. [Google Scholar] [CrossRef]
- Romano, O.; Petiti, L.; Felix, T.; Meneghini, V.; Portafax, M.; Antoniani, C.; Amendola, M.; Bicciato, S.; Peano, C.; Miccio, A. GATA Factor-Mediated Gene Regulation in Human Erythropoiesis. iScience 2020, 23, 101018. [Google Scholar] [CrossRef]
- Ikonomi, P.; Noguchi, C.T.; Miller, W.; Kassahun, H.; Hardison, R.; Schechter, A.N. Levels of GATA-1/GATA-2 transcription factors modulate expression of embryonic and fetal hemoglobins. Gene 2000, 261, 277–287. [Google Scholar] [CrossRef]
- Doerfler, P.A.; Feng, R.; Li, Y.; Palmer, L.E.; Porter, S.N.; Bell, H.W.; Crossley, M.; Pruett-Miller, S.M.; Cheng, Y.; Weiss, M.J. Activation of γ-globin gene expression by GATA1 and NF-Y in hereditary persistence of fetal hemoglobin. Nat. Genet. 2021, 53, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, Y.W.; Kang, J.; Yun, W.J.; Kim, A. Erythroid specific activator GATA-1-dependent interactions between CTCF sites around the β-globin locus. Biochim. Et Biophys. Acta. Gene Regul. Mech. 2017, 1860, 416–426. [Google Scholar] [CrossRef]
- Martyn, G.E.; Wienert, B.; Kurita, R.; Nakamura, Y.; Quinlan, K.G.R.; Crossley, M. A natural regulatory mutation in the proximal promoter elevates fetal globin expression by creating a de novo GATA1 site. Blood 2019, 133, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Funnell, A.P.; Mak, K.S.; Twine, N.A.; Pelka, G.J.; Norton, L.J.; Radziewic, T.; Power, M.; Wilkins, M.R.; Bell-Anderson, K.S.; Fraser, S.T.; et al. Generation of mice deficient in both KLF3/BKLF and KLF8 reveals a genetic interaction and a role for these factors in embryonic globin gene silencing. Mol. Cell Biol. 2013, 33, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.; Desai, M.; Gnanapragasam, M.N.; Wang, S.Z.; Zu Zhu, S.; Williams, D.C., Jr.; Ginder, G.D. Mi2β-mediated silencing of the fetal γ-globin gene in adult erythroid cells. Blood 2013, 121, 3493–3501. [Google Scholar] [CrossRef]
- Hunter, G.A.; Ferreira, G.C. Molecular enzymology of 5-aminolevulinate synthase, the gatekeeper of heme biosynthesis. Biochim. Biophys. Acta 2011, 1814, 1467–1473. [Google Scholar] [CrossRef]
- Stojanovski, B.M.; Hunter, G.A.; Na, I.; Uversky, V.N.; Jiang, R.H.Y.; Ferreira, G.C. 5-Aminolevulinate synthase catalysis: The catcher in heme biosynthesis. Mol. Genet. Metab. 2019, 128, 178–189. [Google Scholar] [CrossRef]
- Kubota, Y.; Nomura, K.; Katoh, Y.; Yamashita, R.; Kaneko, K.; Furuyama, K. Novel Mechanisms for Heme-dependent Degradation of ALAS1 Protein as a Component of Negative Feedback Regulation of Heme Biosynthesis. J. Biol. Chem. 2016, 291, 20516–20529. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, D.; Zhu, Q.; Xiang, Y.; He, Y.; Zhang, H. ALAS2 overexpression alleviates oxidative stress-induced ferroptosis in aortic aneurysms via GATA1 activation. J. Thorac. Dis. 2024, 16, 2510–2527. [Google Scholar] [CrossRef]
- Peoc’h, K.; Nicolas, G.; Schmitt, C.; Mirmiran, A.; Daher, R.; Lefebvre, T.; Gouya, L.; Karim, Z.; Puy, H. Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias. Mol. Genet. Metab. 2019, 128, 190–197. [Google Scholar] [CrossRef]
- Doshi, B.S.; Abramowsky, C.; Briones, M.; Bunting, S.T. Concomitant a novel ALAS2 mutation and GATA1 mutation in a newborn: A case report and review of the literature. Am. J. Blood Res. 2014, 4, 41–45. [Google Scholar]
- Hu, S.T.; Li, X.Y.; Chen, H.; Xu, L.H.; Fang, J.P. Knockdown of ALAS2 Affects Erythroid Differentiation by Down-regulating Mitophagy Receptor BNIP3L. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 1710–1717. [Google Scholar]
- Tang, J.; Tang, Q.X.; Liu, S. METTL3-modified lncRNA-SNHG8 binds to PTBP1 to regulate ALAS2 expression to increase oxidative stress and promote myocardial infarction. Mol. Cell Biochem. 2023, 478, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Massaiu, I.; Campodonico, J.; Mapelli, M.; Salvioni, E.; Valerio, V.; Moschetta, D.; Myasoedova, V.A.; Cappellini, M.D.; Pompilio, G.; Poggio, P.; et al. Dysregulation of Iron Metabolism-Linked Genes at Myocardial Tissue and Cell Levels in Dilated Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 2887. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, Y.; Luo, J.; He, X.; Ye, M.; Li, G.; Zhang, Y.; Bai, J.; Zhang, D.; Chang, C. Targeting TR4 nuclear receptor with antagonist bexarotene increases docetaxel sensitivity to better suppress the metastatic castration-resistant prostate cancer progression. Oncogene 2020, 39, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- To-Figueras, J.; Erwin, A.L.; Aguilera, P.; Millet, O.; Desnick, R.J. Congenital erythropoietic porphyria. Liver Int. Off. J. Int. Assoc. Study Liver 2024, 44, 1842–1855. [Google Scholar] [CrossRef]
- Demosthenous, C.; Vlachaki, E.; Apostolou, C.; Eleftheriou, P.; Kotsiafti, A.; Vetsiou, E.; Mandala, E.; Perifanis, V.; Sarafidis, P. Beta-thalassemia: Renal complications and mechanisms: A narrative review. Hematology 2019, 24, 426–438. [Google Scholar] [CrossRef]
- Uhelski, M.L.; Simone, D.A. Sensitization of nociceptors and dorsal horn neurons contributes to pain in sickle cell disease. Neurosci. Lett. 2019, 705, 20–26. [Google Scholar] [CrossRef]
- Zheng, G.; Orkin, S.H. Transcriptional Repressor BCL11A in Erythroid Cells. Adv. Exp. Med. Biol. 2024, 1459, 199–215. [Google Scholar]
- Esrick, E.B.; Lehmann, L.E.; Biffi, A.; Achebe, M.; Brendel, C.; Ciuculescu, M.F.; Daley, H.; MacKinnon, B.; Morris, E.; Federico, A.; et al. Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. New Engl. J. Med. 2021, 384, 205–215. [Google Scholar] [CrossRef]
- Han, Y.; Tan, X.; Jin, T.; Zhao, S.; Hu, L.; Zhang, W.; Kurita, R.; Nakamura, Y.; Liu, J.; Li, D.; et al. CRISPR/Cas9-based multiplex genome editing of BCL11A and HBG efficiently induces fetal hemoglobin expression. Eur. J. Pharmacol. 2022, 918, 174788. [Google Scholar] [CrossRef] [PubMed]
- Melamed, D.; Nov, Y.; Malik, A.; Yakass, M.B.; Bolotin, E.; Shemer, R.; Hiadzi, E.K.; Skorecki, K.L.; Livnat, A. De novo mutation rates at the single-mutation resolution in a human HBB gene region associated with adaptation and genetic disease. Genome Res. 2022, 32, 488–498. [Google Scholar] [CrossRef]
- Enakaya, N.A.; Jefferson, A.; Chew-Martinez, D.; Matthews, J.S. Design, Synthesis, and Evaluation of Allosteric Effectors for Hemoglobin. Acc. Chem. Res. 2023, 56, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef]
- Susan, P.; Kevin, K.; Sylvia, S.; Frans, A.K.; Aidan, D.F.; Abdullah, K.; Mehdi, N.; Gershwin, B.; Aliya, U.; Lanetta, B.; et al. PB-04 Induces HbF Expression in a Ph1b Trial in Sickle Cell Disease. J. Sick. Cell Dis. 2024, 1, S1. [Google Scholar]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. New Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef]
- Rankine-Mullings, A.E.; Nevitt, S.J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst. Rev. 2022, 9, Cd002202. [Google Scholar] [PubMed]
- Sales, R.R.; Nogueira, B.L.; Tosatti, J.A.G.; Gomes, K.B.; Luizon, M.R. Do Genetic Polymorphisms Affect Fetal Hemoglobin (HbF) Levels in Patients With Sickle Cell Anemia Treated With Hydroxyurea? A Systematic Review and Pathway Analysis. Front. Pharmacol. 2021, 12, 779497. [Google Scholar] [CrossRef]
- Kargutkar, N.; Sawant-Mulay, M.; Hariharan, P.; Chandrakala, S.; Nadkarni, A. Role of microRNA in hydroxyurea mediated HbF induction in sickle cell anaemia patients. Sci. Rep. 2023, 13, 369. [Google Scholar] [CrossRef]
- López Rubio, M.; Argüello Marina, M. The Current Role of Hydroxyurea in the Treatment of Sickle Cell Anemia. J. Clin. Med. 2024, 13, 6404. [Google Scholar] [CrossRef] [PubMed]
- Veith, R.; Galanello, R.; Papayannopoulou, T.; Stamatoyannopoulos, G. Stimulation of F-cell production in patients with sickle-cell anemia treated with cytarabine or hydroxyurea. N. Engl. J. Med. 1985, 313, 1571–1575. [Google Scholar] [CrossRef]
- Jaing, T.H.; Chang, T.Y.; Chen, S.H.; Lin, C.W.; Wen, Y.C.; Chiu, C.C. Molecular genetics of β-thalassemia: A narrative review. Medicine 2021, 100, e27522. [Google Scholar] [CrossRef]
- Lu, H.Y.; Orkin, S.H.; Sankaran, V.G. Fetal Hemoglobin Regulation in Beta-Thalassemia. Hematol. Oncol. Clin. North. Am. 2023, 37, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, L.; Jin, T.; Han, Y.; Liu, J.; Zhang, W.; Biao, Y.; An, B.; Huang, S. Correlations between Multiple SNPs and HbF Levels in β-Thalassemia Carriers. Clin. Lab. 2023, 69, 1875–1881. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. New Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Movahedi Motlagh, F.; Soleimanpour-Lichaei, H.R.; Shamsara, M.; Etemadzadeh, A.; Modarressi, M.H. CRISPR/Cas9 Ablated BCL11A Unveils the Genes with Possible Role of Globin Switching. Adv. Pharm. Bull. 2023, 13, 799–805. [Google Scholar] [CrossRef]
- Sii-Felice, K.; Negre, O.; Brendel, C.; Tubsuwan, A.; Morel-À-l’Huissier, E.; Filardo, C.; Payen, E. Innovative Therapies for Hemoglobin Disorders. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 625–647. [Google Scholar] [CrossRef]
- Krivega, I.; Byrnes, C.; de Vasconcellos, J.F.; Lee, Y.T.; Kaushal, M.; Dean, A.; Miller, J.L. Inhibition of G9a methyltransferase stimulates fetal hemoglobin production by facilitating LCR/γ-globin looping. Blood 2015, 126, 665–672. [Google Scholar] [CrossRef]
- Sharma, D.C.; Singhal, S.; Woike, P.; Rai, S.; Yadav, M.; Gaur, R. Hereditary persistence of fetal hemoglobin. Asian J. Transfus. Sci. 2020, 14, 185–186. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, X.; Zhang, D.; Lan, Z.; Cai, X.; Bian, C.; Zhang, J. Steroid receptor coactivator-1: The central intermediator linking multiple signals and functions in the brain and spinal cord. Genes. Dis. 2022, 9, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.A.; Samuels, H.H. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol. Cell Biol. 2000, 20, 5048–5063. [Google Scholar] [CrossRef]
- Heery, D.M.; Hoare, S.; Hussain, S.; Parker, M.G.; Sheppard, H. Core LXXLL motif sequences in CREB-binding protein, SRC1, and RIP140 define affinity and selectivity for steroid and retinoid receptors. J. Biol. Chem. 2001, 276, 6695–6702. [Google Scholar] [CrossRef]
- Wang, Y.; Lonard, D.M.; Yu, Y.; Chow, D.C.; Palzkill, T.G.; Wang, J.; Qi, R.; Matzuk, A.J.; Song, X.; Madoux, F.; et al. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014, 74, 1506–1517. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, K.; Xu, J.; Chen, X.; Sheng, C.; Zhang, D.; Yang, Y.; Sun, L.; Zhao, H.; Wang, X.; et al. Acetyltransferases CBP/p300 Control Transcriptional Switch of β-Catenin and Stat1 Promoting Osteoblast Differentiation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2023, 38, 1885–1899. [Google Scholar] [CrossRef]
- Janknecht, R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol. Histopathol. 2002, 17, 657–668. [Google Scholar]
- Kim, E.; Liu, N.C.; Yu, I.C.; Lin, H.Y.; Lee, Y.F.; Sparks, J.D.; Chen, L.M.; Chang, C. Metformin inhibits nuclear receptor TR4-mediated hepatic stearoyl-CoA desaturase 1 gene expression with altered insulin sensitivity. Diabetes 2011, 60, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Carrero, P.; Okamoto, K.; Coumailleau, P.; O’Brien, S.; Tanaka, H.; Poellinger, L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol. Cell Biol. 2000, 20, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Fujino, S.; Nakanishi, G.; Kim, Y.S.; Jetten, A.M. TIP27: A novel repressor of the nuclear orphan receptor TAK1/TR4. Nucleic Acids Res. 2004, 32, 4194–4204. [Google Scholar] [CrossRef]
- Lin, S.J.; Zhang, Y.; Liu, N.C.; Yang, D.R.; Li, G.; Chang, C. Minireview: Pathophysiological roles of the TR4 nuclear receptor: Lessons learned from mice lacking TR4. Mol. Endocrinol. 2014, 28, 805–821. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Dong, T.; Kim, E.; Lin, W.J.; Chang, C. Identification of a novel testicular orphan receptor-4 (TR4)-associated protein as repressor for the selective suppression of TR4-mediated transactivation. J. Biol. Chem. 2003, 278, 7709–7717. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zheng, Q.; Zhang, J.; Dong, B.; Zhu, S.; Huang, X.; Wang, Y.; Zhao, B.; Li, S.; Xiong, H.; et al. Testicular orphan nuclear receptor 4-associated protein 16 promotes non-small cell lung carcinoma by activating estrogen receptor β and blocking testicular orphan nuclear receptor 2. Oncol. Rep. 2013, 29, 297–305. [Google Scholar] [CrossRef]
- Lee, Y.F.; Shyr, C.R.; Thin, T.H.; Lin, W.J.; Chang, C. Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: A unique signaling pathway in the steroid receptor superfamily. Proc. Natl. Acad. Sci. USA 1999, 96, 14724–14729. [Google Scholar] [CrossRef]
- Shyr, C.R.; Hu, Y.C.; Kim, E.; Chang, C. Modulation of estrogen receptor-mediated transactivation by orphan receptor TR4 in MCF-7 cells. J. Biol. Chem. 2002, 277, 14622–14628. [Google Scholar] [CrossRef] [PubMed]
- Beinsteiner, B.; Markov, G.V.; Bourguet, M.; McEwen, A.G.; Erb, S.; Patel, A.K.M.; El Khaloufi El Khaddar, F.Z.; Lecroisey, C.; Holzer, G.; Essabri, K.; et al. A novel nuclear receptor subfamily enlightens the origin of heterodimerization. BMC Biol. 2022, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Hara, H. Allogeneic hematopoietic stem cell transplantation. Ther. Apher. Dial. 2003, 7, 285–291. [Google Scholar] [CrossRef]
- Stefánsson, B.V.; Haraldsson, B.; Nilsson, U. Dosing of erythropoiesis-stimulating agents can be reduced by a new administration regimen. Nephron Extra 2011, 1, 45–54. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Fu, X.; Ren, M. The Progress and Evolving Trends in Nucleic-Acid-Based Therapies. Biomolecules 2025, 15, 376. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, H.; Ren, M.; Yu, Q.; Xu, Q.; Fu, X. Orphan Nuclear Receptors TR2 and TR4 in Erythropoiesis: From Mechanisms to Therapies. Biomolecules 2025, 15, 798. https://doi.org/10.3390/biom15060798
Liu Y, Yang H, Ren M, Yu Q, Xu Q, Fu X. Orphan Nuclear Receptors TR2 and TR4 in Erythropoiesis: From Mechanisms to Therapies. Biomolecules. 2025; 15(6):798. https://doi.org/10.3390/biom15060798
Chicago/Turabian StyleLiu, Yunlong, Helian Yang, Mengtian Ren, Qing Yu, Qingyang Xu, and Xiuping Fu. 2025. "Orphan Nuclear Receptors TR2 and TR4 in Erythropoiesis: From Mechanisms to Therapies" Biomolecules 15, no. 6: 798. https://doi.org/10.3390/biom15060798
APA StyleLiu, Y., Yang, H., Ren, M., Yu, Q., Xu, Q., & Fu, X. (2025). Orphan Nuclear Receptors TR2 and TR4 in Erythropoiesis: From Mechanisms to Therapies. Biomolecules, 15(6), 798. https://doi.org/10.3390/biom15060798





