Abstract
Acute kidney injury (AKI) causes damage to the renal epithelium, initiating a reparative process intended to restore renal function. Although effective repair can result in the complete recovery of kidney function, this process is frequently incomplete. In instances where repair is unsuccessful, the kidney experiences maladaptive alterations that may progressively result in chronic kidney disease (CKD), a phenomenon referred to as failed repair. This condition is precipitated by hypotensive, septic, or toxic insults, which initiate a series of pathophysiological processes, including microcirculatory dysfunction, the activation of inflammatory responses, and the death of tubular epithelial cells. These events collectively compromise renal function and trigger a complex repair response. This review provides a comprehensive examination of the multifactorial mechanisms underlying the initiation and progression of AKI, the regenerative pathways facilitating structural recovery in severely damaged kidneys, and the critical transition from adaptive repair to maladaptive remodeling. Central to this transition are mechanisms such as epigenetic reprogramming, G2/M cell-cycle arrest, cellular senescence, mitochondrial dysfunction, metabolism reprogramming, and cell death, which collectively drive the progression of CKD. These mechanistic insights offer a robust foundation for the development of targeted therapeutic strategies aimed at enhancing adaptive renal repair.
1. Introduction
CKD affects approximately 10% of the global population and frequently progresses to kidney failure [1]. There are no available therapies to reverse its progression, and this leads to significant morbidity and mortality. A common pathological feature across all forms of CKD, regardless of the etiology, is the impairment of epithelial repair, which is increasingly recognized as a significant contributor to interstitial fibrosis [2]. Understanding the initial cellular events that trigger kidney fibrogenesis will enhance our comprehension of CKD pathophysiology and may reveal new and effective therapeutic targets. AKI is characterized by a rapid loss of renal function [3]. Recent clinical and experimental studies have demonstrated a significant association between AKI and CKD, previously regarded as separate conditions [4,5,6]. AKI can contribute to CKD onset and progression, while pre-existing CKD may increase susceptibility to AKI. This review aims to summarize the interrelationship between AKI and CKD and examine the potential mechanisms that contribute to AKI-CKD transition. Of note, epithelial–esenchymal transition (EMT) is also a key mechanism in AKI-CKD progression, but its detailed discussion is beyond the scope of this review.
2. Renal Tubular Regeneration: Balancing Adaptive Repair and Maladaptive Responses
The mechanisms underlying renal tubular regeneration following injury have been progressively elucidated through ongoing scientific exploration, particularly regarding whether it is facilitated by surviving mature cells or renal stem cell proliferation. In 2008, researchers demonstrated that surviving renal tubular epithelial cells are the primary source for the repair after AKI [7]. Researchers utilized DNA-labeling techniques to trace cellular processes and found that following acute kidney injury (AKI), proximal tubular epithelial cells (PTCs) in the mouse kidney undergo random dedifferentiation, proliferation, and subsequent redifferentiation into mature PTCs to facilitate tissue repair [8]. Subsequent investigations demonstrated that damaged PTCs initiate proliferation post-AKI, with the majority of the repair process being completed by day 14. However, approximately 20% of PTCs remain in a compromised and dedifferentiated state at this juncture [9]. The damaged proximal tubular cells (PTCs) demonstrate the expression of specific injury-associated genes, such as Havcr1 (Kidney Injury Molecule 1, KIM1), Krt8 (Keratin 8), Krt20 (Keratin 20), and Lcn2 (neutrophil gelatinase-associated lipocalin, NGAL) [10]. Previous research has shown that following acute kidney injury (AKI), the injured proximal tubular cells (PTCs) in a compromised state nearly vanish within two days. By the fourteenth day post-injury, a novel and distinct cell population emerges. These cells undergo differentiation and proliferation, constituting approximately 30% of the PTC population. Notably, around 8% of these cells persist even six weeks post-injury. This observation suggests that this process may play a crucial role in kidney repair and regeneration [11]. In the aforementioned study, the newly identified cell type demonstrated a unique gene expression profile absent in healthy or early-stage acute kidney injury (AKI) proximal tubular cells (PTCs) in mice. This gene set includes markers such as Vcam1, Dcdc2a, and Sema5a, which are considered indicative of the injury repair process. At later time points, PTCs exhibited the downregulation of terminal differentiation marker genes, suggesting an impairment in the differentiation process of PTCs. Consequently, researchers classified these cells as failed-repair proximal tubular cells (FR-PTCs) [11,12]. Subsequent research has corroborated that injury results in the persistent presence of FR-PTCs, marked by Vcam1+/Ccl2+ expression. These cells display a senescence-associated secretory phenotype, distinguished by the upregulation of proinflammatory and profibrotic gene expression, which contributes to the progression from acute kidney injury (AKI) to chronic kidney disease (CKD) [13]. In a murine model of acute kidney injury (AKI) induced by ischemia–reperfusion injury (IRI), single-nucleus RNA sequencing analysis demonstrated that failed-repair proximal tubular cells (FR-PTCs) are marked by a downregulation of genes associated with normal differentiation and proliferation, such as Hnf4a, alongside an upregulation of genes related to injury, including Havcr1, Krt20, Ccl2, and Vcam1. Among these, Vcam1 showed the most pronounced increase [14]. The majority of FR-PTCs were isolated from samples collected four weeks post-injury. Nevertheless, Vcam1-positive PTCs, which serve as injury markers, were still detectable six months following acute kidney injury (AKI). This persistence suggests their potential role in the transition from AKI to chronic kidney disease (CKD) [15].
Two studies published in 2015 and 2016 examined the role of SOX9, a transcription factor regulating cell proliferation and differentiation during kidney repair, following AKI [16,17]. The 2015 study examined the transcriptome of PTCs and identified that Sox9 expression was rapidly upregulated within 4 h post-injury, peaking at 24–48 h. Compared to sham-operated kidneys, SOX9 expression increased 20-fold. Immunofluorescence analysis demonstrated that in uninjured kidneys, SOX9-positive (SOX9+) proximal tubular cells (PTCs) were exceedingly rare, comprising only 0.05% of the proximal tubular cell population, while SOX9+ distal tubular cells were also limited in number. Following acute kidney injury (AKI), 10.6% of proximal tubular cells expressed SOX9. A majority of these SOX9+ cells coexpressed the dedifferentiation marker KIM-1, with 40% of them actively proliferating. Previous studies have demonstrated that following AKI, SOX9+ PTCs re-enter mitosis and significantly promote the extensive repair of the injured proximal tubules [16,17]. A recent study by Sanjeev Kumar et al. demonstrated the critical role of SOX9 expression after AKI in renal tissue repair [18]. The research revealed that SOX9+ cells continued to proliferate on the 10th day post-AKI. The expression level of SOX9 was found to be downregulated; however, partially recovered proximal tubular cells (PTCs) continued to express SOX9. This persistent expression of SOX9 led to renal interstitial fibrosis and tissue scarring. Importantly, the researchers identified that inhibiting SOX9 activity could hinder the progression of renal fibrosis. SOX9-positive cells were associated with the expression of fibrotic markers. Furthermore, the research team demonstrated that SOX9-positive PTCs secrete Wnt4, a key factor in sustaining Wnt-β-catenin activity within the profibrotic fibroblast microenvironment. Studies involving human kidney transplant biopsy specimens support these findings, revealing a positive correlation between SOX9 expression and the severity of renal interstitial fibrosis. Moreover, SOX9 expression was correlated with cadherin 6 (CDH6), Wnt2b, and the expression of profibrotic genes [18]. These findings underscore the critical importance of sustained SOX9 expression in the progression of renal fibrosis following acute kidney injury (AKI). They contribute to a deeper understanding of the mechanisms underlying kidney repair and identify novel molecular targets for future therapeutic interventions against chronic kidney disease (CKD). Notably, the expression of SOX9 in human transplant kidney biopsy specimens shows a positive correlation with the extent of fibrosis, highlighting its potential as a therapeutic target. Future research should concentrate on devising spatiotemporally specific regulatory strategies to reconcile the dual roles of SOX9 in early repair processes and late-stage fibrosis.
During acute kidney injury (AKI), injured proximal tubular cells (PTCs) can undergo processes of dedifferentiation, proliferation, and subsequent redifferentiation, collectively termed adaptive repair, to facilitate endogenous recovery. PTCs that successfully engage in adaptive repair contribute to renal recovery post-AKI. However, in instances of severe injury, PTCs may be unable to repair effectively due to disruptions in energy metabolism or DNA damage, resulting in what is termed “maladaptive repair”. PTCs undergoing maladaptive repair are not merely passive victims of AKI; rather, they exhibit distinct characteristics such as cell-cycle arrest, apoptosis, metabolic dysfunction, senescence, and sustained inflammatory responses. These features culminate in tubular atrophy and degeneration, interstitial fibrosis, and a decline in renal function, thereby advancing the transition from AKI to chronic kidney disease (CKD). While adaptive repair mechanisms can effectively restore tubular architecture following mild to moderate injury, severe or recurrent insults may lead to dysregulated repair processes.
3. Mechanisms of Maladaptive Repair
3.1. Epigenetic Reprogramming
Emerging evidence suggests that epigenetic regulation is pivotal in determining cell fate by orchestrating temporal and spatial regulation [19,20]. Chromatin accessibility, which refers to the degree of chromatin compaction, is a fundamental characteristic of chromatin states and is widely acknowledged as a hallmark of active cisregulatory elements [21,22]. Regions of accessible chromatin, characterized by reduced nucleosome presence and diminished chromatin compaction, serve as sites for the recruitment of transcription factors (TFs) through DNA-specific interactions. The binding of TFs, in turn, plays a crucial role in establishing and maintaining the openness of these regulatory regions. Based on the sequence characteristics of accessible chromatin regions, TF binding can be predicted, allowing for the mapping of dynamic TF regulatory networks. Consequently, analyzing chromatin dynamics and profiling TF regulatory networks following AKI may illuminate the epigenetic mechanisms underlying cellular responses to varying degrees of injury, thereby identifying key regulators involved in both adaptive and maladaptive kidney repair.
In related studies, researchers have systematically defined cell type–specific epigenetic signatures in healthy kidneys [23] as well as in kidneys from patients with autosomal dominant polycystic kidney disease and CKD [24,25]. These investigations revealed that FR-PTCs exhibit a significant enrichment of nuclear factor κB (NF-κB) transcription factor binding sites within accessible chromatin regions. This increased accessibility of NF-κB motifs in FR-PTCs suggests a central role for this proinflammatory transcription factor in driving inflammation, fibrosis, and the AKI-CKD.
Recent research by Muto et al. delineates the dynamic epigenetic landscape underlying the transition from AKI-CKD [14]. Utilizing single-nucleus multiomic profiling of approximately 280,000 nuclei throughout a mouse AKI time course, and corroborating findings in human AKI samples, the research demonstrates that fibroblast-like proximal tubular cells (FR-PTCs) exhibit enduring changes in chromatin accessibility, predominantly influenced by NF-κB transcription factor activity. NF-κB orchestrates proinflammatory and profibrotic gene expression programs through conserved cisregulatory elements (CREs), including chemokines such as CCL2 and CSF1, and anti-phagocytic signals like CD47, which collectively recruit immune cell subsets and maintain fibrotic microenvironments. Cross-species regulatory network analysis identified CREB5 as a bifunctional regulator, facilitating tubular proliferation during acute repair phases while contributing to maladaptive FR-PTC states in chronic phases. Knockdown of CREB5 resulted in suppressed proliferation via activation of the p53 pathway, underscoring its context-dependent roles. Additionally, immune cell profiling revealed macrophage heterogeneity, including CCR2+ M1 and CSF1R+ subsets. This study pioneers an integrative methodological approach by combining single-nucleus assay for transposase-accessible chromatin sequencing (snATAC-seq), single-nucleus RNA sequencing (snRNA-seq), and cleavage under targets and release using nuclease (CUT&RUN) to map temporal chromatin dynamics and validate conserved transcription factors.
3.2. Cellular Senescence and G2/M Cell-Cycle Arrest
Maladaptive repair of PTCs may occur due to the inability to complete normal mitosis, resulting in cells entering a state of cell-cycle arrest or senescence. Cellular senescence is an irreversible state of cell-cycle arrest induced by various injurious factors. These factors result in upregulating the tumor suppressor gene P53, which subsequently activates downstream P21CIP1 (P21) expression. This, subsequently, inhibits multiple cyclin expressions, resulting in cell-cycle arrest. Moreover, P16INK4A/pRb (P16), which inhibits cyclin-dependent kinases (CDKs), including CDK4/6, is upregulated, stabilizing cells in a cycle arrest state [26]. In contrast to quiescent and fully differentiated cells, senescent cells cannot re-enter the cell cycle in response to stimuli. Senescent cells exhibit specific changes in morphology, metabolism, gene expression, and chromatin remodeling and develop a proinflammatory phenotype [27,28]. In a mouse model of nephrotoxicity caused by repeated low-dose intraperitoneal cisplatin injection, PTCs underwent senescence, which contributed to the AKI-CKD progression. Pharmacological clearance of senescent PTCs reduced renal inflammation, mitigated renal interstitial fibrosis, and enhanced kidney function [29]. These findings suggest that the senescence of PTCs following injury is an essential mechanism in the AKI-CKD progression. In kidney samples from clinical patients with CKD, PTCs senescence persisted despite the alleviation of ureteral obstruction. Furthermore, the degree of cellular senescence correlated with the severity of fibrosis [30]. Renal scRNA-seq analysis revealed that senescent PTCs can facilitate AKI-CKD progression by activating mesenchymal fibroblasts, resulting in renal interstitial fibrosis [31].
Cell-cycle checkpoints involve cyclins, CDKs, and cyclin-dependent kinase inhibitors (CKIs), which inhibit cell division in response to DNA damage or cellular stress. These checkpoints safe-guard cellular energy by inhibiting unnecessary progression through the cell cycle during adverse conditions [32]. Previous studies have demonstrated that G2/M cell-cycle arrest significantly contributes to maladaptive repair after AKI and AKI-to-CKD progression. In various AKI models, including bilateral IRI, unilateral IRI, aristolochic acid-induced injury, and unilateral ureteral obstruction (UUO), injured PTCs exhibit G2/M phase arrest. This is accompanied by the synthesis of profibrotic growth factors, which contribute to the progression of renal fibrosis [33]. The pharmacological inhibition of cells in G2/M cell-cycle arrest diminishes renal interstitial fibrosis. Conversely, an increased proportion of cells arrested in the G2/M phase exacerbates renal interstitial fibrosis [34].
3.3. Mitochondrial Dysfunction and Metabolism Reprogramming
3.3.1. Mitochondrial Damage
Acute kidney injury (AKI) can lead to mitochondrial dysfunction, predominantly as a consequence of hypoxia interfering with the electron transport chain (ETC) within mitochondria. This interference results in the overproduction of reactive oxygen species (ROS), which are detrimental free radicals. The excessive ROS exacerbate damage to renal tubular cells, thereby contributing to the progression of injury [35]. Recent studies have demonstrated that mitochondrial dysfunction is a key factor driving AKI-to-CKD progression. This dysfunction hinders energy production, enhances oxidative stress, and initiates maladaptive repair mechanisms, leading to chronic kidney injury and fibrosis [35]. Mitochondrial homeostasis is maintained through mitochondrial dynamics, mitophagy, and mitochondrial biogenesis. Mitochondrial dynamics involve two distinct processes: fission, regulated by DRP1 (Dynamin-Related Protein 1), and fusion, regulated by MFN1 (Mitofusin 1), MFN2 (Mitofusin 2), and OPA1 (Optic Atrophy 1) [36,37]. Mitophagy refers to the selective degradation of damaged mitochondria via autophagy. This process is regulated by several pathways, including the PINK1–PARK2 pathway, the BNIP3 and NIX pathways, and the FUNDC1 pathway. Additionally, mitochondrial biogenesis, which is primarily governed by peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), fulfills the high energy demands of the cell and replenishes mitochondrial content during cellular proliferation [38,39]. Animal studies have demonstrated that regulating the genes associated with mitochondrial homeostasis influences renal inflammation, kidney fibrosis, and apoptosis [40]. Increased levels of PGC-1α in renal tubular cells enhance mitochondrial mass and ameliorate kidney injury [41]. Conversely, PGC-1α deficiency in a sepsis-induced AKI model results in severe renal dysfunction [42]. Studies have demonstrated that improving mitochondrial dynamics by inhibiting Drp1-dependent fission, preserving mitochondrial membrane potential, and regulating mitophagy can mitigate mitochondrial dysfunction. Accordingly, regulating mitochondrial dynamics may offer new therapeutic avenues for AKI. Several studies have demonstrated that autophagy acts as a protective mechanism by eliminating excessively produced collagen, reducing oxidative stress, and inhibiting endothelial-to-mesenchymal transition, thus preventing renal interstitial fibrosis after AKI [43]. However, some studies indicate that the prolonged activation of autophagy during renal repair can induce a profibrotic phenotype in tubular cells, including senescence and the secretion of proinflammatory and profibrotic cytokines. Therefore, the role and regulatory mechanisms of autophagy in maladaptive renal repair necessitate additional investigation.
3.3.2. Metabolism Reprogramming
PTCs constitute approximately 50% of the total cellular population in the kidney and exhibit a significant energetic demand necessary for maintaining acid–base homeostasis, as well as for the reabsorption and secretion of water, ions, and other compounds [44]. PTCs predominantly depend on mitochondrial fatty acid oxidation (FAO) to generate the requisite adenosine triphosphate (ATP). In contrast to cortical tubule segments (specifically, the S1 and S2 segments), medullary (S3) segments exhibit a greater reliance on glycolysis rather than oxidative phosphorylation (OXPHOS), attributed to the reduced partial pressure of oxygen (pO2) in the medulla compared to in the cortex [45]. FAO is instrumental in mediating the response of proximal tubular epithelial cells to injury, given that lipids provide an essential energy source for the regeneration of proliferative tubules. During the initial phase of ischemia, FAO is temporarily upregulated in murine proximal tubular cells, a phenomenon that aligns with successful cellular repair in vivo. This observation has been substantiated by analyses of human datasets [46]. Conversely, the impairment of FAO is a pathogenic characteristic of CKD. Patients with CKD exhibit a significant reduction in FAO and OXPHOS, which is evidenced by the accumulation of intracellular lipids and the diminished expression of key transcription factors associated with mitochondrial metabolism [47,48]. These transcription factors include estrogen-related receptor α and PGC1α, which are crucial for mitochondrial biogenesis and fatty acid metabolism; mitochondrial transcription factor A (TFAM), which is essential for mitochondrial DNA transcription and replication; as well as peroxisome proliferator-activated receptor α (PPARα) and Krüppel-like factor 15 (KLF15), which are involved in the regulation of fatty acid catabolism [49,50,51]. Several mechanisms govern the suppression of fatty acid oxidation (FAO) associated with chronic kidney disease (CKD) in proximal tubular cells (PTCs). Transforming growth factor-beta (TGFβ)-induced SMAD3 is pivotal in this process, as it inhibits the expression of genes responsible for fatty acid uptake and oxidation. This inhibition occurs through SMAD3’s binding to the promoter region of Ppargc1a and the subsequent suppression of histone 3 lysine 4 monomethylation (H3K4Me1). The impairment of FAO in PTCs promotes their transition to a secretory phenotype, resulting in the increased expression of inflammatory cytokines such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor (TNF), which in turn attract macrophages to the site of injury. Importantly, a study published in 2024 revealed that the conditional deletion of Cpt1a in proximal tubular epithelial cells (PTECs) in vivo led to enhanced macrophage infiltration, while exerting a minimal effect on kidney injury and fibrosis [52]. This observation suggests that peroxisomal FAO may compensate for the adverse effects associated with Cpt1a deletion and impaired mitochondrial FAO. Furthermore, this finding underscores the role of FAO in the pathogenesis of CKD by influencing both cellular energy metabolism and the accumulation of toxic lipids.
Lipid droplets are present in almost all damaged renal parenchymal cells. A 2015 large-scale genome-wide transcriptome analysis revealed that inflammation and metabolism are the most dysregulated pathways in diseased human kidneys [49]. The study found that in human and mice fibrotic kidneys, the levels of key enzymes and regulators associated with FAO in PTCs were diminished, accompanied by lipid accumulation. Mice exhibiting tubular-specific overexpression of the long-chain fatty acid transporter CD36 predominantly deposited fatty acid, stearic acid, palmitic acid, linoleic acid, and docosahexaenoic acid in the kidney [53]. Significant lipid accumulation in PTCs has been observed in angiotensin II-induced rats, high-fat-diet-fed mice, and cisplatin-induced nephrotoxicity models [54,55].
A previous study has demonstrated that prolonged fatty acid uptake (10-day palmitic acid treatment) stimulates inflammatory and profibrotic mechanisms in mouse renal PTCs [56]. Recent research demonstrated that injured PTCs exhibit a transient lipid accumulation and a significant increase in FAO-related gene expression within 6 h post-injury [12]. Subsequent analysis using the bulk RNA sequencing of cells treated with oleic acid for 6 h and collected on day 2 demonstrated the upregulation of genes involved in DNA replication, the cell cycle, and proliferation, signifying a repair state with high energy demands. Similarly, in vivo experiments revealed that PTCs expressing the repair marker Mki67 were most prevalent in the kidneys of IRI mice on the second day after injury. These findings indicate that lipid deposition within the first 6 h following kidney injury may serve as a vital energy source for damaged epithelial cells. The proliferation and expansion of PTCs facilitate tubular repair during this phase [12]. CD36 functions as a transporter for long-chain fatty acids, which subsequently accumulate within the cell and form lipid droplets, with PLIN2 acting as a surface protein. Fatty acyl-coenzyme A (CoA) is synthesized from lipids through ACSL-mediated lipolysis or lipophagy and is utilized in ACOX1-mediated peroxisomal β-oxidation or CPT-mediated mitochondrial β-oxidation. This metabolic pathway generates acetyl-CoA, a critical substrate for the tricarboxylic acid (TCA) cycle, which is essential for energy production. The transport of acyl-CoA into mitochondria via CPT1 and CPT2 is associated with reduced fatty acid oxidation (FAO), inefficient NADH production, decreased electron transport chain (ETC) activity, and insufficient ATP levels, ultimately leading to mitochondrial dysfunction. Furthermore, evidence from animal models indicates that the inhibition of genes involved in mitochondrial biogenesis, particularly PGC-1α and PPARα, results in mitochondrial DNA instability (Figure 1).
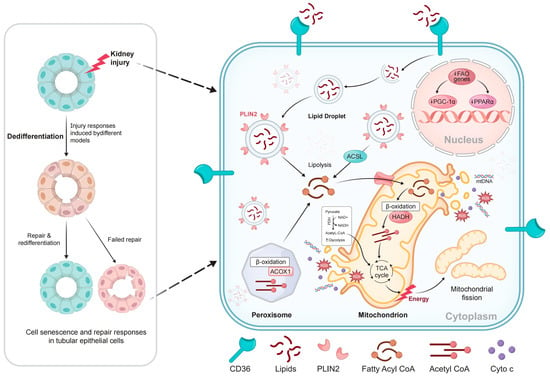
Figure 1.
Proposed model of activated lipid metabolism in injured proximal tubule cells: CD36 functions as a transporter for long-chain fatty acids. Within the cell, these fatty acids accumulate and form lipid droplets, with PLIN2 serving as a surface protein. Fatty acyl-coenzyme A (CoA) is produced from lipids through ACSL-mediated lipolysis or lipophagy and is utilized in ACOX1-mediated peroxisomal β-oxidation or CPT-mediated mitochondrial β-oxidation. This process generates acetyl-CoA, a key substrate for TCA, which produces energy. CPT1 and CPT2, which transport Acyl-CoA into mitochondria, contribute to decreased FAO, ineffective NADH production, reduced ETC activity, and inadequate ATP levels, ultimately leading to mitochondrial dysfunction. Furthermore, data from animal models indicate that the suppression of genes involved in mitochondrial biogenesis, particularly PGC-1α and PPARα, leads to mitochondrial DNA instability.
3.4. Programmed Cell Death
Mitochondrial damage can precipitate various forms of cell death, including apoptosis, necroptosis, pyroptosis, and ferroptosis, also promoting the release of the proinflammatory cytokines that facilitate immune cell infiltration and fibroblast activation. However, further research is necessary to elucidate the intricate relationships between metabolic processes, various forms of cell death, and fibrotic responses within the renal microenvironment.
3.4.1. Ferroptosis
Ferroptosis is an iron-dependent form of cell death driven by lipid peroxidation. Through the creation of a mouse UUO-induced renal fibrosis model, researchers observed that PTCs exhibited the typical features of ferroptosis concurrent with the development of fibrotic pathological phenotypes in the kidneys. The inhibition of ferroptosis via liproxstatin-1 diminished the expression of profibrotic factors and the activation of myofibroblasts in the UUO model, consequently alleviating renal interstitial fibrosis [57]. Subsequent research employing diverse models of kidney injury and repair, in conjunction with single-cell RNA sequencing (scRNA-seq) and murine genetic techniques, has elucidated that proximal tubular cells (PTCs) adopt a distinctive proinflammatory phenotype and undergo ferroptosis following injury [10]. Although these inflammatory PTCs manifest transiently and revert to normal after mild injury, their accumulation following severe kidney injury results in chronic inflammation and renal interstitial fibrosis. Furthermore, the transient inflammatory PTC state is marked by the significant downregulation of glutathione metabolism genes, making the cells more susceptible to ferroptosis. Mild injury-induced ferroptosis inhibits the redifferentiation of injured PTCs into normal PTCs, resulting in the accumulation and persistence of inflammatory PTCs. This promotes maladaptive repair and leads to renal interstitial fibrosis. The study demonstrated that the pharmacological inhibition of ferroptosis significantly alleviates inflammation and diminishes renal interstitial fibrosis after AKI, affirming that ferroptosis is a crucial mechanism regulating the repair and regeneration of PTCs following kidney injury [10].
3.4.2. Pyroptosis
Pyroptosis is a form of inflammatory cell death marked by cellular swelling and the secretion of extracellular vesicles from the plasma membrane. Gasdermin D (GSDMD), a substrate for all inflammatory caspases, is essential in executing inflammasome-induced cell death, thus facilitating pyroptosis [58]. Recent observations have identified pyroptosis as a significant process in various kidney diseases. The NLRP3 inflammasome, a pivotal component in the pyroptotic pathway, plays a critical role in modulating innate immune responses by regulating the maturation and secretion of proinflammatory cytokines, such as interleukin-1β (IL-1β) [59].
Researchers have detected the activation of Casp3 and the cleavage of the GSDME protein in a mouse UUO-induced renal fibrosis model, and knockout of GSDME or Casp3 inhibited tubular cell pyroptosis, thereby alleviating hydronephrosis, interstitial fibrosis, and inflammatory cell infiltration [60]. Meanwhile recent single-cell data analysis revealed that ferroptosis and pyroptosis in PTCs induce cell death and exacerbate cellular damage, leading to tubular repair failure and facilitating AKI-to-CKD progression [61]. The findings demonstrated the persistent enrichment of cell death-related genes (pyroptosis and ferroptosis) in injured PTCs, strongly associated with maladaptive repair in AKI-CKD progression. The pharmacological inhibition of pyroptosis and ferroptosis enhanced maladaptive renal repair and interstitial fibrosis after severe IRI, thereby mitigating AKI-to-CKD progression [61,62].
These studies highlight the critical roles of pyroptosis and ferroptosis in facilitating renal fibrosis and hindering post-injury repair. The findings enhance our understanding of the mechanisms involved in kidney injury and repair and provide potential therapeutic targets for preventing and treating AKI-CKD progression.
3.4.3. Necroptosis
Necroptosis, a regulated lytic form of cell death, is characterized by the specific formation of a necrosome comprising receptor-interacting protein kinase (RIPK) 1, RIPK3, and the mixed lineage kinase domain-like protein (MLKL) [63,64]. Our previous research demonstrated that the necroptosis of tubular cells, induced by cisplatin nephrotoxicity, initiates necroinflammation [65]. The inhibition of RIPK1 (via Nec-1) [66] and the deletion of RIPK3 or MLKL mitigates renal dysfunction following IRI [67,68,69,70]. Moreover, the deletion of caspase 8 leads to severe chronic inflammation; however, the simultaneous deletion of RIPK3 and caspase 8 inhibits this inflammatory response [71]. These findings highlight the role of necrosome components in modulating inflammatory reactions.
Studies demonstrate that RIPK3-MLKL necroptotic signaling activates the NLRP3 inflammasome when caspase 8 is suppressed. Caspase 8 activation in the kidney was effectively inhibited after IRI [72]. Mice lacking Ripk3 or MLKL demonstrated decreased NLRP3 inflammasome activation after a similar initial kidney injury as observed in WT mice (35 min ischemia), indicating that RIPK3 and MLKL are involved in inflammasome activation. The study clarified that RIPK3-MLKL-dependent necroptosis triggers NLRP3 inflammasome activation in an auto-amplification loop, which plays a significant role in AKI-to-CKD progression [73].
4. Signaling Pathways During the Renal Tubular Repair Process
Recent studies have demonstrated that the upregulation of Notch, Wnt, and Hedgehog (Hh) signaling pathways in renal tubular epithelial cells is a key event that drives their differentiation, a process critically linked to the pathogenesis of CKD [74,75,76]. The augmented signaling induces phenotypic alterations in proximal tubular cells (PTCs), disrupting normal cellular function and diminishing the regenerative capacity of the renal epithelium. This, in turn, contributes to maladaptive repair mechanisms and fibrosis. Moreover, the interaction among these signaling pathways collectively influences the processes of kidney injury and repair [77,78]. Understanding these intricate interactions provides valuable insights into the molecular underpinnings of CKD and highlights potential therapeutic targets.
4.1. Wnt
The Wnt pathway is a universally present and highly conserved signal transduction pathway in organisms, essential for numerous physiological processes, including embryonic development, cell proliferation, differentiation, migration, and maintenance of tissue homeostasis [79]. Wnt proteins are secreted glycoproteins that function by binding to the Frizzled family receptors and co-receptors of low-density-lipoprotein receptor-related protein 5/6 [80]; they recruit and activate the intracellular disheveled protein near the cell membrane, subsequently exerting physiological effects in a β-catenin-dependent or -independent manner [81]. After β-catenin translocation into the nucleus and its binding to T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, it activates downstream pathways, including TGF-β1/SMAD signaling, Snail1, and Twist1 [82].
Wnt expression at the transcriptional and translational levels significantly increased in the IRI [83] and was closely associated with severity [84]. Pretreatment with a Wnt agonist [85] and exogenous Wnt 1 h before ischemia may mitigate kidney injury [86], while tubular-specific knockout of β-catenin exacerbated ischemia-induced kidney injury and nephrotoxicity [87]. Wnt4 or β-catenin primarily protects PTCs from apoptosis and mitigates AKI kidney injury by enhancing cell proliferation and inhibiting the activation of p53 and Bax [88,89]. Additionally, Wnt is implicated in cellular communication in the kidney. The Wnt7 interaction between macrophages and renal tubular cells is essential in kidney injury [90]. The intercellular communication of Wnt between proximal renal tubules and activated perivascular fibroblasts/pericytes promotes interstitial fibrosis [91,92], and extrarenal myeloid-derived Wnt is essential for the activation of macrophages and fibroblasts contributing to renal fibrosis [93,94]. The function of Wnt/β-catenin in kidney injury repair is complex. In several chronic kidney injury conditions, including UUO [95] and nephropathy [96], the upregulation of the Wnt pathway has also been observed, and this upregulation appears to exacerbate chronic kidney injury. Transgenic mice exhibiting activated β-catenin have been demonstrated to spontaneously develop renal lesions and fibrosis [97]. In chronic kidney injury models, administering various antagonists (including secreted frizzled-related protein 4, Klotho, and the small molecule inhibitor ICG-001) to inhibit the Wnt/β-catenin signal has resulted in a reduction in renal fibrosis and the alleviation of proteinuria [98]. In CKDs, Wnt is typically considered harmful; however, some studies have identified that the interaction between tubular β-catenin and FoxO3 may confer a protective effect in CKD [99].
In addition to the previously mentioned pharmacological inhibitory effects, recent studies indicate that some endogenous small molecules, proteins, and RNAs exacerbate chronic kidney injury by upregulating the Wnt pathway, while pregnane X receptor, combined with melatonin and poricoic acid A, exhibits the opposite effect. Previous studies have demonstrated that the classical Wnt pathway, specifically the β-catenin-dependent pathway, is essential in the bidirectional role in kidney injury repair. It mitigates kidney injury during the acute phase; however, continuous activation can cause CKD progression (Table 1).

Table 1.
The regulation of the Wnt pathway in kidney injury.
4.2. Notch
The Notch pathway, similar to the Wnt pathway, is a highly conserved intercellular signal transduction pathway that is essential for various physiological processes, including cell proliferation, differentiation, and apoptosis [113]. The Notch signaling pathway is fundamentally constituted by Notch receptors, ligands, and intracellular effector molecules. In mammals, there are four distinct Notch receptors (Notch1–4), with ligands predominantly belonging to the Delta-like (Dll1, Dll3, Dll4) and Jagged (Jag1, Jag2) families. Upon ligand–receptor interaction, the Notch receptor undergoes enzymatic cleavage, resulting in the release of the Notch intracellular domain. This domain then translocates to the nucleus, where it associates with transcription factors to activate downstream target genes [114], thus regulating the physiological functions of cells.
In various kidney injury models, the ligands and receptors associated with Notch signal transduction are upregulated [115,116], and the degree of fibrosis is positively correlated with Notch levels. Furthermore, the expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function [117], while inhibiting the Notch pathway mitigates kidney injury [118]. Notch is extensively distributed. While its expression level is low in healthy organisms, the expression levels of various cells, including myeloid-derived cells, podocytes, epithelial cells, and endothelial cells, are significantly increased in chronic kidneys. Additionally, the reactivation of Notch signal transduction in the kidneys of animal models and patients with CKD has been demonstrated to result in epithelial damage and the progression of fibrosis. Inhibiting the Notch pathway can mitigate renal fibrosis progression [74]. Activated Notch inhibits the expression of the adaptor protein Disabled-2 in tubular epithelial cells, thereby protecting them from TGF-β-induced epithelial-to-mesenchymal transition and IRI [119]. The overexpression of active Notch1 in tubular epithelial cells aggravated renal fibrosis, while the inhibition of Notch resulted in the amelioration of renal fibrosis [120]. The intercellular signal crosstalk of Notch significantly contributes to kidney injury repair. Notch signal transduction induces renal fibrosis through endothelial-to-mesenchymal transition surrounding the renal tubules [121]. In Diabetic Nephropathy (DN), the Notch signal transduction of podocytes is related to the degree of glomerulosclerosis [117]. Furthermore, previous studies have found that the Notch pathway in bone marrow-derived cells is essential in chronic kidney injury. In DN, the Notch pathway in macrophages promotes macrophage polarization, exacerbating the inflammatory reaction, fibrosis, and necroptosis of the kidneys. Inhibiting the Notch pathway in macrophages alleviates the pathological changes in renal cells [122]. Moreover, in the UUO model, myeloid-specific targeting of Notch ameliorates renal fibrosis in mice by diminishing the infiltration and activation of bone marrow-derived macrophages [123]. Comprehensive studies have revealed that specifically blocking Notch in ferroptosis suppressor protein 1-positive cells derived from bone marrow can improve renal fibrosis in UUO mice [124]. While substantial research evidence suggests that the Notch pathway exacerbates renal fibrosis and CKD progression, it serves as a double-edged sword in AKI. The Notch pathway is involved in the repair process of gentamicin-induced AKI [118,125], while Notch 3 aggravates the IRI [126] (Table 2).

Table 2.
Notch pathway in the repair of renal injury.
4.3. Hedgehog
The Hedgehog signaling pathway primarily comprises ligands, receptors, and intracellular signaling molecules with transcription factors. The Hedgehog ligands comprise Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh). The primary transmembrane receptors comprise patched (Ptch) and smoothened (Smo). With no Hh signal, Ptch inhibits Smo activity. After the binding of the Hh protein to Ptch, the inhibitory effect of Ptch on Smo is alleviated. Activated Smo can transmit the signal to the Gli family proteins of mammalian transcription factors through intracellular signaling molecules. Gli proteins subsequently translocate to the nucleus to initiate the transcription of downstream target genes [134].
The Shh signal has been widely studied in kidney injury. Activating Shh in the early stage of AKI mitigates AKI induced by IR and sepsis, which may be linked to the fact that the early Shh pathway helps cell proliferation and reduces cell apoptosis [135,136]. During CKD, the Hedgehog signaling pathway is essential in renal fibrosis development, primarily through the secretion of Hh ligands by epithelial cells and the upregulation of Gli1 in myofibroblasts [137]. Shh expression in the tubular epithelial cells of mouse models of renal fibrosis induced by UUO and IRI is elevated, and the inhibition of the Shh signaling pathway has a protective effect in various kidney injury models, including hypertension [138], diabetes [139], UUO [140], and ciaplatin [140]. Cyclopmine is an effective SMO inhibitor, and its inhibition of the Hh signal significantly alleviates the progression of renal fibrosis in mouse models of IRI and obstructive nephropathy [141]. The genetic ablation of Gli1 reduces kidney injury in mouse models of renal fibrosis [142]. The roles of other Hh proteins in kidney injury repair are being gradually investigated. Ihh release from tumor necrosis factor-activated renal epithelia drives renal fibrosis [143,144]. The Hh signal plays an important role in intercellular communication. The Shh ligand shuttled by exosomes derived from renal tubules plays an essential role in renal fibrosis [145].
Notch, Wnt, and Hedgehog signaling pathways are essential for embryonic development, cellular proliferation, differentiation, and migration. Their expression levels are higher and essential in kidney injury repair. In the early stage of AKI, these characteristics promote cell proliferation and reduce cell death, partially alleviating kidney injury. However, continuous overactivation exacerbates epithelial–mesenchymal transition and renal fibrosis, thereby promoting the transformation of AKI-CKD (Table 3).

Table 3.
The regulation of the Hedgehog pathway in kidney injury.
5. Emerging Therapeutic Strategies and Future Directions
5.1. Exosomes in Kidney Injury and Repair
Exosomes (Exos), which are a heterogeneous group of double-membrane-bound vesicles, play a crucial role in mediating intercellular communication by shuttling molecular cargo from donor cells to recipient cells. Exos released from different cells exert distinct effects and can be selectively taken up by neighboring or distant cells, thereby reprogramming the recipient cells based on their bioactive compounds [146]. Researchers found that FRC-Exos protect the kidneys from sepsis-induced injury [147]. Exosomes from multiple sources, particularly those derived from mesenchymal stem cells, have demonstrated a reparative role in various types of AKI by promoting the proliferation and repair of renal tubules, regulating autophagy, inhibiting inflammatory responses and oxidative stress, suppressing fibrosis progression, and facilitating angiogenesis [148]. However, paracrine exosomes in the kidneys are predominantly detrimental, especially in the cellular communication between RTECs and inflammatory cells [149,150]. Injury-induced exosomes micro374b-5p derived from RTECs promote the activation of M1 macrophages [150], while exosomes derived from macrophages cause glomerular endothelial cell dysfunction [151], trigger pyroptosis [152], and participate in the apoptotic injury and inflammatory responses that promote AKI. In the advanced stage of AKI, exosomes significantly contribute to the mechanism of renal fibrosis and repair. The primary source of exosomes for mitigating the AKI-CKD progression derived from renal tubular cells activates fibroblasts and facilitates kidney fibrosis [153].
Current research in the field of extracellular vesicles is focused on enhancing the targeting efficiency of Exos to specific tissue types. Exosomal transport represents a novel mechanism for intercellular communication during injury [154]. Notably, FRC-Exos demonstrate a capacity to specifically target damaged renal tissue, as evidenced by fluorescence observed in PTCs following coculture with FRC-Exos. Additionally, the incorporation of a ligand derived from an LTH-targeting peptide segment, which selectively binds to KIM-1 expressed on injured kidney cells, significantly improves the targeting efficacy of Exos toward kidney tubular cells [154]. These findings suggest that Exos are promising drug-delivery vehicles with great targeted therapeutic potential for AKI-CKD.
5.2. Metabolic Interventions
The activation of PPARα has emerged as a promising strategy to restore FAO and counteract lipotoxicity in PTCs. PPARα agonists such as fenofibrate have been shown to upregulate carnitine palmitoyl transferase 1a (CPT1a), thereby improving mitochondrial function and reducing lipid-induced damage [155]. Preclinical studies in various models of kidney injury indicate that fenofibrate not only mitigates renal lipid accumulation but also enhances overall renal function. Moreover, ongoing research into novel, more selective PPARα modulators promises to refine this therapeutic approach by enhancing efficacy while minimizing off-target effects.
Aberrant glycolytic reprogramming is a critical driver of maladaptive repair and fibrosis in the kidney. Small-molecule inhibitors of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), such as 3PO, have been shown to reduce glycolytic flux [156]. By doing so, these inhibitors can reverse the histone acetylation changes that drive fibrotic processes. Preclinical studies indicate that modulating glycolysis may restore metabolic homeostasis in injured renal cells, suggesting that the further optimization of PFKFB3 inhibitors could yield effective antifibrotic therapies.
5.3. Clearance of Senescent Cells
The accumulation of senescent cells, characterized by the expression of p16INK4a, contributes to chronic inflammation and the progression of renal interstitial fibrosis. Senolytic agents, such as the combination of dasatinib and quercetin, have demonstrated the capacity to selectively eliminate these p16INK4a-positive cells, thereby attenuating fibrosis and preserving renal function [29]. This approach is supported by emerging clinical data in age-related diseases and holds significant promise for translation into renal therapies.
By targeting core mechanisms, developing innovative technologies, and advancing precision medicine, there is hope for breaking the vicious cycle of AKI-CKD progression in the future. The key challenge lies in balancing efficacy and safety while achieving personalized interventions through the integration of multiomic data. Interdisciplinary collaborations will accelerate the clinical translation of therapeutic strategies. Aging contributes to immunosenescence and may elucidate the increased susceptibility to AKI-CKD progression. Considering the aging population and the escalating burden of age-associated progressive CKD, developing innovative therapies for this widespread disease should be prioritized.
The most prominent current limitation in AKI-CKD transition research lies in the substantial disconnect between basic research and clinical practice. While mechanistic studies have made significant progress in identifying molecular pathways, translational research remains limited in both quantity and depth. Moving forward, extensive clinical studies will be essential to effectively bridge this gap and transform theoretical knowledge into practical therapeutic applications (Table 4).

Table 4.
Emerging therapeutic strategies targeting AKI-CKD transition.
6. Conclusions
AKI frequently results in considerable morbidity and mortality due to the intricate processes involved in renal damage and subsequent recovery. Post-AKI, renal tubular cells may undergo either adaptive or maladaptive repair pathways. Maladaptive repair is characterized by phenomena such as cellular senescence, cell-cycle arrest, mitochondrial dysfunction, metabolic reprogramming, programmed cell death, and epigenetic reprogramming. Key signaling pathways, including Notch, Wnt, and Hedgehog, play significant roles in these processes. Additionally, exosomes demonstrate a dual role in the context of AKI. A thorough understanding of these mechanisms is crucial for the development of therapeutic strategies aimed at preventing the progression of AKI to CKD. Future research should prioritize the detailed elucidation of these pathways and the identification of therapeutic targets to improve patient outcomes.
Author Contributions
All authors contributed crucially to the manuscript. Conceptualization, D.X. and X.Z. Writing—original draft preparation, D.X. and J.P. Writing—review and editing, Z.P. and Y.L. Supervision, D.X. and X.Z. Project administration, Z.P. and Y.L. Funding acquisition, Z.P. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81772046 to Peng; No. 82241039 to Peng); the Program of Excellent Doctoral (Postdoctoral) of Zhongnan Hospital of Wuhan University (Grant No. ZNYB2020008 to Prof. Li); and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund, Project CXPY2020012 to Prof. Li.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AKI | Acute kidney injury |
| CKD | Chronic kidney disease |
| PTCs | Proximal tubular epithelial cells |
| GSDMD | Gasdermin D |
| IL-1β | Interleukin-1β |
| RIPK | Receptor-interacting protein kinase |
| MLKL | Mixed lineage kinase domain-like protein |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| Notch1–4 | Notch receptors |
| TCA | Tricarboxylic acid cycle |
| Dll | Delta-like |
| Jag | Jagged |
| Hh | Hedgehog |
| Shh | Sonic Hedgehog |
| Ihh | Indian Hedgehog |
| Dhh | Desert Hedgehog |
| Ptch/Smo | The primary transmembrane receptors comprise patched and smoothened |
| Havcr1/KIM1 | Kidney Injury Molecule 1 |
| Krt8 | Keratin 8 |
| Krt20 | Keratin 20 |
| Lcn2/NGAL | Neutrophil gelatinase-associated lipocalin |
| SOX9+ | SOX9-positive |
| CDH6 | Cadherin 6 |
| NF-κB | Nuclear factor κB |
| CREs | Conserved cisregulatory elements |
| P21 | P21CIP1 |
| P16 | P16INK4A/pRb |
| CDKS | Cyclin-dependent kinases |
| CKIs | Cyclin-dependent kinase inhibitors |
| ETC | Electron transport chain |
| DRP1 | Dynamin-Related Protein 1 |
| MFN1 | Mitofusin 1 |
| MFN2 | Mitofusin 2 |
| OPA1 | Optic Atrophy 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1α |
| FAO | Fatty acid oxidation |
| ATP | Adenosine triphosphate |
| TFAM | Mitochondrial transcription factor A |
| PPARα | Proliferator-activated receptor α (PPARα) |
| KLF15 | Krüppel-like factor 15 |
| TGFβ | Transforming growth factor-beta |
| H2K4Me1 | Histone 3 lysine 4 monomethylation |
| OXPHOS | Oxidative phosphorylation |
References
- Hocaoglu, H.; Wang, L.; Yang, M.; Yue, S.; Sieber, M. Heritable shifts in redox metabolites during mitochondrial quiescence reprogramme progeny metabolism. Nat. Metab. 2021, 3, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Khamissi, F.Z.; Ning, L.; Kefaloyianni, E.; Dun, H.; Arthanarisami, A.; Keller, A.; Atkinson, J.J.; Li, W.; Wong, B.; Dietmann, S.; et al. Identification of kidney injury–released circulating osteopontin as causal agent of respiratory failure. Sci. Adv. 2022, 8, eabm5900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Griffin, K.A.; Lan, R.; Geng, H.; Saikumar, P.; Bidani, A.K. Acute kidney injury: A springboard for progression in chronic kidney disease. Am. J. Physiol. Physiol. 2010, 298, F1078–F1094. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef]
- Balzer, M.S.; Rohacs, T.; Susztak, K. How Many Cell Types Are in the Kidney and What Do They Do? Annu. Rev. Physiol. 2022, 84, 507–531. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefaloyianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Investig. 2019, 129, 5501–5517. [Google Scholar] [CrossRef]
- Ide, S.; Kobayashi, Y.; Ide, K.; Strausser, S.A.; Abe, K.; Herbek, S.; O’Brien, L.L.; Crowley, S.D.; Barisoni, L.; Tata, A.; et al. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. eLife 2021, 10, e68603. [Google Scholar] [CrossRef]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dixon, E.E.; Wu, H.; Humphreys, B.D. Comprehensive single-cell transcriptional profiling defines shared and unique epithelial injury responses during kidney fibrosis. Cell Metab. 2022, 34, 1977–1998.e9. [Google Scholar] [CrossRef] [PubMed]
- Ledru, N.; Wilson, P.C.; Muto, Y.; Yoshimura, Y.; Wu, H.; Li, D.; Asthana, A.; Tullius, S.G.; Waikar, S.S.; Orlando, G.; et al. Predicting proximal tubule failed repair drivers through regularized regression analysis of single cell multiomic sequencing. Nat. Commun. 2024, 15, 1291. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Dixon, E.E.; Yoshimura, Y.; Ledru, N.; Kirita, Y.; Wu, H.; Humphreys, B.D. Epigenetic reprogramming driving successful and failed repair in acute kidney injury. Sci. Adv. 2024, 10, eado2849. [Google Scholar] [CrossRef]
- Gerhardt, L.M.; Koppitch, K.; van Gestel, J.; Guo, J.; Cho, S.; Wu, H.; Kirita, Y.; Humphreys, B.D.; McMahon, A.P. Lineage Tracing and Single-Nucleus Multiomics Reveal Novel Features of Adaptive and Maladaptive Repair after Acute Kidney Injury. J. Am. Soc. Nephrol. 2023, 34, 554–571. [Google Scholar] [CrossRef]
- Kang, H.M.; Huang, S.; Reidy, K.; Han, S.H.; Chinga, F.; Susztak, K. Sox9-Positive Progenitor Cells Play a Key Role in Renal Tubule Epithelial Regeneration in Mice. Cell Rep. 2016, 14, 861–871. [Google Scholar] [CrossRef]
- Kumar, S.; Liu, J.; Pang, P.; Krautzberger, A.M.; Reginensi, A.; Akiyama, H.; Schedl, A.; Humphreys, B.D.; McMahon, A.P. Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Rep. 2015, 12, 1325–1338. [Google Scholar] [CrossRef]
- Aggarwal, S.; Wang, Z.; Pacheco, D.R.F.; Rinaldi, A.; Rajewski, A.; Callemeyn, J.; Van Loon, E.; Lamarthée, B.; Covarrubias, A.E.; Hou, J.; et al. SOX9 switch links regeneration to fibrosis at the single-cell level in mammalian kidneys. Science 2024, 383, eadd6371. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct cell reprogramming: Approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef]
- Moris, N.; Pina, C.; Arias, A.M. Transition states and cell fate decisions in epigenetic landscapes. Nat. Rev. Genet. 2016, 17, 693–703. [Google Scholar] [CrossRef]
- Klemm, S.L.; Shipony, Z.; Greenleaf, W.J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019, 20, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shu, X.; Zhu, P.; Pei, D. Chromatin accessibility dynamics during cell fate reprogramming. Embo Rep. 2021, 22, e51644. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Wilson, P.C.; Ledru, N.; Wu, H.; Dimke, H.; Waikar, S.S.; Humphreys, B.D. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat. Commun. 2021, 12, 2190. [Google Scholar] [CrossRef]
- Muto, Y.; Dixon, E.E.; Yoshimura, Y.; Wu, H.; Omachi, K.; Ledru, N.; Wilson, P.C.; King, A.J.; Olson, N.E.; Gunawan, M.G.; et al. Defining cellular complexity in human autosomal dominant polycystic kidney disease by multimodal single cell analysis. Nat. Commun. 2022, 13, 6497. [Google Scholar] [CrossRef]
- Wilson, P.C.; Muto, Y.; Wu, H.; Karihaloo, A.; Waikar, S.S.; Humphreys, B.D. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat. Commun. 2022, 13, 5253. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Verzola, D.; Gandolfo, M.T.; Gaetani, G.; Ferraris, A.; Mangerini, R.; Ferrario, F.; Villaggio, B.; Gianiorio, F.; Tosetti, F.; Weiss, U.; et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Physiol. 2008, 295, F1563–F1573. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.-R.; He, Y.-N.; Cai, G.-Y.; Zhang, J.-G.; Lin, L.-R.; Zhan, J.; Zhang, J.-H.; Xiao, H.-S. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl. Res. 2012, 159, 454–463. [Google Scholar] [CrossRef]
- Yamashita, N.; Nakai, K.; Nakata, T.; Nakamura, I.; Kirita, Y.; Matoba, S.; Humphreys, B.D.; Tamagaki, K.; Kusaba, T. Cumulative DNA damage by repeated low-dose cisplatin injection promotes the transition of acute to chronic kidney injury in mice. Sci. Rep. 2021, 11, 20920. [Google Scholar] [CrossRef]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The senescence-associated secretome as an indicator of age and medical risk. J. Clin. Investig. 2020, 5, e133668. [Google Scholar] [CrossRef]
- O’sullivan, E.D.; Mylonas, K.J.; Bell, R.; Carvalho, C.; Baird, D.P.; Cairns, C.; Gallagher, K.M.; Campbell, R.; Docherty, M.; Laird, A.; et al. Single-cell analysis of senescent epithelia reveals targetable mechanisms promoting fibrosis. J. Clin. Investig. 2022, 7, e133668. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fogo, A.B. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 2010, 21, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Cosentino, C.C.; Skrypnyk, N.I.; Brilli, L.L.; Chiba, T.; Novitskaya, T.; Woods, C.; West, J.; Korotchenko, V.N.; McDermott, L.; Day, B.W.; et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 2013, 24, 943–953. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Gall, J.M.; Wang, Z.; Bonegio, R.G.; Havasi, A.; Liesa, M.; Vemula, P.; Borkan, S.C. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J. Am. Soc. Nephrol. 2015, 26, 1092–1102. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, Y.; Quirós, P.M.; Wei, Q.; López-Otín, C.; Dong, Z. OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am. J. Physiol. Physiol. 2014, 306, F1318–F1326. [Google Scholar] [CrossRef]
- Jesinkey, S.R.; Funk, J.A.; Stallons, L.J.; Wills, L.P.; Megyesi, J.K.; Beeson, C.C.; Schnellmann, R.G. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2014, 25, 1157–1162. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Tang, C.; Han, H.; Liu, Z.; Liu, Y.; Yin, L.; Cai, J.; He, L.; Liu, Y.; Chen, G.; Zhang, Z.; et al. Activation of BNIP3-mediated mitophagy protects against renal ischemia–reperfusion injury. Cell Death Dis. 2019, 10, 677. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.Z.; Chen, L.; Chou, C.-L.; Jung, H.J.; Lee, J.W.; Knepper, M.A. Representation and relative abundance of cell-type selective markers in whole-kidney RNA-Seq data. Kidney Int. 2019, 95, 787–796. [Google Scholar] [CrossRef]
- Wang, G.; Heijs, B.; Kostidis, S.; Mahfouz, A.; Rietjens, R.G.J.; Bijkerk, R.; Koudijs, A.; van der Pluijm, L.A.K.; Berg, C.W.V.D.; Dumas, S.J.; et al. Analyzing cell-type-specific dynamics of metabolism in kidney repair. Nat. Metab. 2022, 4, 1109–1118. [Google Scholar] [CrossRef]
- Gaudry, S.; Grolleau, F.; Barbar, S.; Martin-Lefevre, L.; Pons, B.; Boulet, É.; Boyer, A.; Chevrel, G.; Montini, F.; Bohe, J.; et al. Continuous renal replacement therapy versus intermittent hemodialysis as first modality for renal replacement therapy in severe acute kidney injury: A secondary analysis of AKIKI and IDEAL-ICU studies. Crit. Care 2022, 26, 93. [Google Scholar] [CrossRef]
- Miguel, V.; Tituaña, J.; Herrero, J.I.; Herrero, L.; Serra, D.; Cuevas, P.; Barbas, C.; Puyol, D.R.; Márquez-Expósito, L.; Ruiz-Ortega, M.; et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Investig. 2021, 131, e140695. [Google Scholar] [CrossRef]
- Afshinnia, F.; Rajendiran, T.M.; Soni, T.; Byun, J.; Wernisch, S.; Sas, K.M.; Hawkins, J.; Bellovich, K.; Gipson, D.; Michailidis, G.; et al. Impaired β-Oxidation and Altered Complex Lipid Fatty Acid Partitioning with Advancing CKD. J. Am. Soc. Nephrol. 2018, 29, 295–306. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.-A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Dhillon, P.; Park, J.; del Pozo, C.H.; Li, L.; Doke, T.; Huang, S.; Zhao, J.; Kang, H.M.; Shrestra, R.; Balzer, M.S.; et al. The Nuclear Receptor ESRRA Protects from Kidney Disease by Coupling Metabolism and Differentiation. Cell Metab. 2021, 33, 379–394.e8. [Google Scholar] [CrossRef]
- Piret, S.E.; Attallah, A.A.; Gu, X.; Guo, Y.; Gujarati, N.A.; Henein, J.; Zollman, A.; Hato, T.; Ma’ayan, A.; Revelo, M.P.; et al. Loss of proximal tubular transcription factor Krüppel-like factor 15 exacerbates kidney injury through loss of fatty acid oxidation. Kidney Int. 2021, 100, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, S.; Ivanova, A.; Osaki, Y.; Funk, S.; Yang, H.; Viquez, O.; Delgado, R.; Lu, D.; Mignemi, M.P.; Tonello, J.; et al. Tubular CPT1A deletion minimally affects aging and chronic kidney injury. JCI Insight 2024, 9, e171961. [Google Scholar] [CrossRef] [PubMed]
- Portilla, D.; Li, S.; Nagothu, K.; Megyesi, J.; Kaissling, B.; Schnackenberg, L.; Safirstein, R.; Beger, R. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 2006, 69, 2194–2204. [Google Scholar] [CrossRef]
- De Vries, A.P.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; DD’Agati, V.; Lamb, H.J.; Barlovic, D.P.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef]
- Mori, Y.; Ajay, A.K.; Chang, J.-H.; Mou, S.; Zhao, H.; Kishi, S.; Li, J.; Brooks, C.R.; Xiao, S.; Woo, H.-M.; et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 2021, 33, 1042–1061.e7. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Ru, F.; Gan, Y.; Li, B.; Xia, W.; Dai, G.; He, Y.; Chen, Z. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021, 12, 843. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Devant, P.; Kagan, J.C. Molecular mechanisms of gasdermin D pore-forming activity. Nat. Immunol. 2023, 24, 1064–1075. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Huang, Z.-X.; Chen, H.; Lan, R.; Wang, Z.; Lai, K.; Chen, H.; Chen, Z.; Zou, Z.; et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 2021, 28, 2333–2350. [Google Scholar] [CrossRef]
- Balzer, M.S.; Doke, T.; Yang, Y.-W.; Aldridge, D.L.; Hu, H.; Mai, H.; Mukhi, D.; Ma, Z.; Shrestha, R.; Palmer, M.B.; et al. Single-cell analysis highlights differences in druggable pathways underlying adaptive or fibrotic kidney regeneration. Nat. Commun. 2022, 13, 4018. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, X.; Zhu, Z.; Luo, Z.; Hao, Y.; Yang, X.; Feng, J.; Zhang, Z.; Hu, J.; Jian, Y.; et al. STING contributes to lipopolysaccharide-induced tubular cell inflammation and pyroptosis by activating endoplasmic reticulum stress in acute kidney injury. Cell Death Dis. 2024, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhong, C.-Q.; Zhang, D.-W. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat. Immunol. 2011, 12, 1143–1149. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Ren, J.; Huang, D.; He, W.-T.; Song, Y.; Yang, C.; Li, W.; Zheng, X.; Chen, P.; et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014, 24, 105–121. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Linkermann, A.; Bräsen, J.H.; Himmerkus, N.; Liu, S.; Huber, T.B.; Kunzendorf, U.; Krautwald, S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012, 81, 751–761. [Google Scholar] [CrossRef]
- Von Mässenhausen, A.; Tonnus, W.; Himmerkus, N.; Parmentier, S.; Saleh, D.; Rodriguez, D.; Ousingsawat, J.; Ang, R.L.; Weinberg, J.M.; Sanz, A.B.; et al. Phenytoin inhibits necroptosis. Cell Death Dis. 2018, 9, 359. [Google Scholar] [CrossRef]
- Linkermann, A.; Bräsen, J.H.; Darding, M.; Jin, M.K.; Sanz, A.B.; Heller, J.-O.; De Zen, F.; Weinlich, R.; Ortiz, A.; Walczak, H.; et al. Two independent pathways of regulated necrosis mediate ischemia–reperfusion injury. Proc. Natl. Acad. Sci. USA 2013, 110, 12024–12029. [Google Scholar] [CrossRef]
- Liu, W.; Chen, B.; Wang, Y.; Meng, C.; Huang, H.; Huang, X.-R.; Qin, J.; Mulay, S.R.; Anders, H.-J.; Qiu, A.; et al. RGMb protects against acute kidney injury by inhibiting tubular cell necroptosis via an MLKL-dependent mechanism. Proc. Natl. Acad. Sci. USA 2018, 115, E1475–E1484. [Google Scholar] [CrossRef]
- Oberst, A.; Dillon, C.P.; Weinlich, R.; McCormick, L.L.; Fitzgerald, P.; Pop, C.; Hakem, R.; Salvesen, G.S.; Green, D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011, 471, 363–367. [Google Scholar] [CrossRef]
- Kang, T.-B.; Yang, S.-H.; Toth, B.; Kovalenko, A.; Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 2013, 38, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fang, Y.; Wu, J.; Chen, H.; Zou, Z.; Zhang, X.; Shao, J.; Xu, Y. RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis. 2018, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Bielesz, B.; Sirin, Y.; Si, H.; Niranjan, T.; Gruenwald, A.; Ahn, S.; Kato, H.; Pullman, J.; Gessler, M.; Haase, V.H.; et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Investig. 2010, 120, 4040–4054. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT-β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Fabian, S.L.; Penchev, R.R.; St-Jacques, B.; Rao, A.N.; Sipilä, P.; West, K.A.; McMahon, A.P.; Humphreys, B.D. Hedgehog-Gli pathway activation during kidney fibrosis. Am. J. Pathol. 2012, 180, 1441–1453. [Google Scholar] [CrossRef]
- Borggrefe, T.; Lauth, M.; Zwijsen, A.; Huylebroeck, D.; Oswald, F.; Giaimo, B.D. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia pathways. Biochim. Biophys. Acta Bioenerg. 2016, 1863, 303–313. [Google Scholar] [CrossRef]
- D’Cruz, R.; Kim, Y.; Mulder, J.; Ibeh, N.; Jiang, N.; Tian, Y.; Rosenblum, N.D. Hedgehog signalling in Foxd1+ embryonic kidney stromal progenitors controls nephron formation via Cxcl12 and Wnt5a. J. Pathol. 2023, 261, 385–400. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Nusse, R. Wnt signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011163. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Tanaka, H.; Okado, T.; Shimamura, H.; Inoshita, S.; Kuwahara, M.; Sasaki, S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J. Am. Soc. Nephrol. 2003, 14, 1223–1233. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, D.; Tan, R.J.; Fu, H.; Zhou, L.; Hou, F.F.; Liu, Y. Sustained Activation of Wnt/β-Catenin Signaling Drives AKI to CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1727–1740. [Google Scholar] [CrossRef]
- Kuncewitch, M.; Yang, W.-L.; Corbo, L.; Khader, A.; Nicastro, J.; Coppa, G.F.; Wang, P. WNT Agonist Decreases Tissue Damage and Improves Renal Function After Ischemia-Reperfusion. Shock 2015, 43, 268–275. [Google Scholar] [CrossRef]
- Hong, X.; Zhou, Y.; Wang, D.; Lyu, F.; Guan, T.; Liu, Y.; Xiao, L. Exogenous Wnt1 Prevents Acute Kidney Injury and Its Subsequent Progression to Chronic Kidney Disease. Front. Physiol. 2021, 12, 745816. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Lin, L.; Zhou, L.; Igarashi, P.; Liu, Y. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int. 2012, 82, 537–547. [Google Scholar] [CrossRef]
- Wang, Z.; Havasi, A.; Gall, J.M.; Mao, H.; Schwartz, J.H.; Borkan, S.C. β-catenin promotes survival of renal epithelial cells by inhibiting Bax. J. Am. Soc. Nephrol. 2009, 20, 1919–1928. [Google Scholar] [CrossRef]
- Ming, W.-H.; Wen, L.; Hu, W.-J.; Qiao, R.-F.; Zhou, Y.; Su, B.-W.; Bao, Y.-N.; Gao, P.; Luan, Z.-L. The crosstalk of Wnt/β-catenin signaling and p53 in acute kidney injury and chronic kidney disease. Kidney Res. Clin. Pract. 2024, 43, 724–738. [Google Scholar] [CrossRef]
- Lin, S.L.; Li, B.; Rao, S.; Yeo, E.J.; Hudson, T.E.; Nowlin, B.T.; Pei, H.; Chen, L.; Zheng, J.J.; Carroll, T.J.; et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 4194–4199. [Google Scholar] [CrossRef]
- Maarouf, O.H.; Aravamudhan, A.; Rangarajan, D.; Kusaba, T.; Zhang, V.; Welborn, J.; Gauvin, D.; Hou, X.; Kramann, R.; Humphreys, B.D. Paracrine Wnt1 Drives Interstitial Fibrosis without Inflammation by Tubulointerstitial Cross-Talk. J. Am. Soc. Nephrol. 2016, 27, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, Y.; Muranaka, Y.; Sun, D.; Goto, T.; Zhou, H.; Sakakima, M.; Fukasawa, H.; Yonemura, K.; Yamamoto, T.; Hishida, A. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch. 2005, 446, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, J.; Huang, W.; Ren, Q.; Feng, J.; Liao, J.; Fu, H.; Zhou, L.; Liu, Y. Myeloid-derived Wnts play an indispensible role in macrophage and fibroblast activation and kidney fibrosis. Int. J. Biol. Sci. 2024, 20, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Mhaidly, R.; Croizer, H.; Kieffer, Y.; Leclere, R.; Vincent-Salomon, A.; Robley, C.; Anglicheau, D.; Rabant, M.; Sannier, A.; et al. WNT-dependent interaction between inflammatory fibroblasts and FOLR2+ macrophages promotes fibrosis in chronic kidney disease. Nat. Commun. 2024, 15, 743. [Google Scholar] [CrossRef]
- Surendran, K.; Schiavi, S.; Hruska, K.A. Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 2005, 16, 2373–2384. [Google Scholar] [CrossRef]
- He, W.; Kang, Y.S.; Dai, C.; Liu, Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011, 22, 90–103. [Google Scholar] [CrossRef]
- DiRocco, D.P.; Kobayashi, A.; Taketo, M.M.; McMahon, A.P.; Humphreys, B.D. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. JASN 2013, 24, 1399–1412. [Google Scholar] [CrossRef]
- Zhou, D.; Tan, R.J.; Fu, H.; Liu, Y. Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Mod. Pathol. 2016, 96, 156–167. [Google Scholar] [CrossRef]
- Nlandu-Khodo, S.; Osaki, Y.; Scarfe, L.; Yang, H.; Phillips-Mignemi, M.; Tonello, J.; Saito-Diaz, K.; Neelisetty, S.; Ivanova, A.; Huffstater, T.; et al. Tubular β-catenin and FoxO3 interactions protect in chronic kidney disease. J. Clin. Investig. 2020, 5, e135454. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, Y.; Wang, H.; Luo, X.; Zeng, T.; Huang, H.; Xiao, L.; Jia, N.; Li, A. Fibroblast growth factor 21 alleviates unilateral ureteral obstruction-induced renal fibrosis by inhibiting Wnt/β-catenin signaling pathway. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119620. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Z.; Huang, X.; Shu, S.; Hu, X.; Zheng, M.; Tang, C.; Liu, Y.; Chen, G.; Sun, L.; et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 2020, 97, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Yiu, W.H.; Li, Y.; Lok, S.W.; Chan, K.W.; Chan, L.Y.; Leung, J.C.; Lai, K.N.; Tsu, J.H.; Chao, J.; Huang, X.-R.; et al. Protective role of kallistatin in renal fibrosis via modulation of Wnt/β-catenin signaling. Clin. Sci. 2021, 135, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Gao, M.; Zeng, X. TMEM16A inhibits renal tubulointerstitial fibrosis via Wnt/β-catenin signaling during hypertension nephropathy. Cell. Signal. 2024, 117, 111088. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.-H.; Luan, Z.-L.; Yao, Y.; Liu, H.-C.; Hu, S.-Y.; Du, C.-X.; Zhang, C.; Zhao, Y.-H.; Huang, Y.-Z.; Sun, X.-W.; et al. Pregnane X receptor activation alleviates renal fibrosis in mice via interacting with p53 and inhibiting the Wnt7a/β-catenin signaling. Acta Pharmacol. Sin. 2023, 44, 2075–2090. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Cao, G.; Zhao, H.; Chen, L.; Yang, T.; Wang, M.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and β-catenin pathway in AKI-to-CKD continuum. Ther. Adv. Chronic Dis. 2019, 10, 2040622319869116. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Yan, J.; Wang, X.; Xu, M.; Wang, M.; Fan, B.; Liu, J.; Lin, N.; Wang, X.; et al. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat. Commun. 2022, 13, 1600. [Google Scholar] [CrossRef]
- Xie, H.; Miao, N.; Xu, D.; Zhou, Z.; Ni, J.; Yin, F.; Wang, Y.; Cheng, Q.; Chen, P.; Li, J.; et al. FoxM1 promotes Wnt/β-catenin pathway activation and renal fibrosis via transcriptionally regulating multi-Wnts expressions. J. Cell. Mol. Med. 2021, 25, 1958–1971. [Google Scholar] [CrossRef]
- Gong, W.; Luo, C.; Peng, F.; Xiao, J.; Zeng, Y.; Yin, B.; Chen, X.; Li, S.; He, X.; Liu, Y.; et al. Brahma-related gene-1 promotes tubular senescence and renal fibrosis through Wnt/β-catenin/autophagy axis. Clin. Sci. 2021, 135, 1873–1895. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, B.; Huang, W.; Ma, M.; Zhang, F.; Jiang, L.; Qian, C.; Wan, X.; Cao, C. IKKα aggravates renal fibrogenesis by positively regulating the Wnt/β-catenin pathway. Immunology 2023, 168, 120–134. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, H.; Hong, Y.; Ma, M.; Wan, X.; Cao, C. Indoleamine-2,3-Dioxygenase Activates Wnt/β-Catenin Inducing Kidney Fibrosis after Acute Kidney Injury. Gerontology 2021, 67, 611–619. [Google Scholar] [CrossRef]
- Kuang, Q.; Wu, S.; Xue, N.; Wang, X.; Ding, X.; Fang, Y. Selective Wnt/β-Catenin Pathway Activation Concomitant with Sustained Overexpression of miR-21 is Responsible for Aristolochic Acid-Induced AKI-to-CKD Transition. Front. Pharmacol. 2021, 12, 667282. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Cao, R.; Li, Q.; Yin, L. The Long Noncoding RNA-H19 Mediates the Progression of Fibrosis from Acute Kidney Injury to Chronic Kidney Disease by Regulating the miR-196a/Wnt/β-Catenin Signaling. Nephron 2022, 146, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Sprinzak, D.; Blacklow, S.C. Biophysics of Notch Signaling. Annu. Rev. Biophys. 2021, 50, 157–189. [Google Scholar] [CrossRef]
- Kramer, J.; Schwanbeck, R.; Pagel, H.; Cakiroglu, F.; Rohwedel, J.; Just, U. Inhibition of Notch Signaling Ameliorates Acute Kidney Failure and Downregulates Platelet-Derived Growth Factor Receptor β in the Mouse Model. Cells Tissues Organs 2016, 201, 109–117. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Liao, G.; Liu, D.; Zhou, S.; Zhang, J.; Liao, J.; Guo, Z.; Li, Y.; Yang, S.; et al. The role of regulatory T cells in the pathogenesis of acute kidney injury. J. Cell. Mol. Med. 2023, 27, 3202–3212. [Google Scholar] [CrossRef]
- Murea, M.; Park, J.-K.; Sharma, S.; Kato, H.; Gruenwald, A.; Niranjan, T.; Si, H.; Thomas, D.B.; Pullman, J.M.; Melamed, M.L.; et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010, 78, 514–522. [Google Scholar] [CrossRef]
- Elkhoely, A. Liraglutide ameliorates gentamicin-induced acute kidney injury in rats via PGC-1α- mediated mitochondrial biogenesis: Involvement of PKA/CREB and Notch/Hes-1 signaling pathways. Int. Immunopharmacol. 2023, 114, 109578. [Google Scholar] [CrossRef]
- Schütte-Nütgen, K.; Edeling, M.; Mendl, G.; Krahn, M.P.; Edemir, B.; Weide, T.; Kremerskothen, J.; Michgehl, U.; Pavenstädt, H. Getting a Notch closer to renal dysfunction: Activated Notch suppresses expression of the adaptor protein Disabled-2 in tubular epithelial cells. FASEB J. 2019, 33, 821–832. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Diao, Z.; Han, X.; Li, D.; Ruan, X.; Liu, W. C1q/tumor necrosis factor-related protein-3 improves renal fibrosis via inhibiting notch signaling pathways. J. Cell. Physiol. 2019, 234, 22352–22364. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, X.; Tan, T.K.; Zhao, H.; Zhang, Y.; Liu, L.; Zhang, J.; Wang, L.; Cao, Q.; Wang, Y.; et al. Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial–mesenchymal transition. Nephrol. Dial. Transplant. 2017, 32, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, X.; Zhu, Y.; Yu, S.; Liu, T.; Zhang, X.; Chen, D.; Du, S.; Chen, T.; Chen, S.; et al. Excessive Activation of Notch Signaling in Macrophages Promote Kidney Inflammation, Fibrosis, and Necroptosis. Front. Immunol. 2022, 13, 835879. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Ma, P.; An, D.; Zhao, J.; Liang, S.; Ye, Y.; Lu, Y.; Zhang, P.; Liu, X.; et al. Myeloid-specific targeting of Notch ameliorates murine renal fibrosis via reduced infiltration and activation of bone marrow-derived macrophage. Protein Cell 2019, 10, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, M.; Huang, F.; Cheng, O.H.; Xiao, X.; Lee, T.H.; Truong, L.; Cheng, J. Notch Blockade Specifically in Bone Marrow-Derived FSP-1-Positive Cells Ameliorates Renal Fibrosis. Cells 2023, 12, 214. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Zhang, T.; Zuo, W. Notch-mediated Sox9 + cell activation contributes to kidney repair after partial nephrectomy. Life Sci. 2018, 193, 104–109. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Conway, E.M.; Harris, R.C. Survivin mediates renal proximal tubule recovery from AKI. J. Am. Soc. Nephrol. JASN 2013, 24, 2023–2033. [Google Scholar] [CrossRef]
- Shimizu, S.; Yoshioka, K.; Aki, S.; Takuwa, Y. Class II phosphatidylinositol 3-kinase-C2α is essential for Notch signaling by regulating the endocytosis of γ-secretase in endothelial cells. Sci. Rep. 2021, 11, 5199. [Google Scholar] [CrossRef]
- Soni, H.; Matthews, A.T.; Pallikkuth, S.; Gangaraju, R.; Adebiyi, A. γ-secretase inhibitor DAPT mitigates cisplatin-induced acute kidney injury by suppressing Notch1 signaling. J. Cell. Mol. Med. 2019, 23, 260–270. [Google Scholar] [CrossRef]
- Sörensen-Zender, I.; Rong, S.; Susnik, N.; Zender, S.; Pennekamp, P.; Melk, A.; Haller, H.; Schmitt, R. Renal tubular Notch signaling triggers a prosenescent state after acute kidney injury. Am. J. Physiol. Physiol. 2014, 306, F907–F915. [Google Scholar] [CrossRef]
- Lavoz, C.; Poveda, J.; Marquez-Exposito, L.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Ortiz, A.; Egido, J.; Mezzano, S.; Ruiz-Ortega, M. Gremlin activates the Notch pathway linked to renal inflammation. Clin. Sci. 2018, 132, 1097–1115. [Google Scholar] [CrossRef]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, Q.; Veeraragoo, P.; Yu, H.; Xiao, Z. Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the γ-secretase inhibitor DAPT has a nephroprotective effect. Ren. Fail. 2011, 33, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-S.; Fei, M.; Yang, Y.-X.; Cai, W.-W. MiR-201-5p alleviates lipopolysaccharide-induced renal cell dysfunction by targeting NOTCH3. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5592–5603. [Google Scholar]
- Zhang, Y.; Beachy, P.A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Chen, F.; Song, H.; Zhou, X. Overexpression of CISD2 alleviates septic acute kidney injury via activating Sonic Hedgehog signaling pathway. Cell. Mol. Biol. 2024, 70, 238–242. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, Y.; Jiang, D.-S.; Chen, Y. Hydroxytyrosol inhibits apoptosis in ischemia/reperfusion-induced acute kidney injury via activating Sonic Hedgehog signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12380–12388. [Google Scholar]
- Edeling, M.; Ragi, G.; Huang, S.; Pavenstädt, H.; Susztak, K. Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016, 12, 426–439. [Google Scholar] [CrossRef]
- Guan, Y.; Quan, D.; Chen, K.; Kang, L.; Yang, D.; Wu, H.; Yan, M.; Wu, S.; Lv, L.; Zhang, G. Kaempferol inhibits renal fibrosis by suppression of the sonic hedgehog signaling pathway. Phytomedicine 2023, 108, 154246. [Google Scholar] [CrossRef]
- Zhao, X.-P.; Chang, S.-Y.; Pang, Y.; Liao, M.-C.; Peng, J.; Ingelfinger, J.R.; Chan, J.S.D.; Zhang, S.-L. Hedgehog interacting protein activates sodium–glucose cotransporter 2 expression and promotes renal tubular epithelial cell senescence in a mouse model of type 1 diabetes. Diabetologia 2023, 66, 223–240. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Fu, R.; He, Y.; Xiao, L.; Peng, F.; Yuan, C. Polysaccharides extracted from Balanophora polyandra Griff (BPP) ameliorate renal Fibrosis and EMT via inhibiting the Hedgehog pathway. J. Cell. Mol. Med. 2021, 25, 2828–2840. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Zhou, L.; Tan, R.J.; Xiao, L.; Liang, M.; Hou, F.F.; Liu, Y. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J. Am. Soc. Nephrol. 2014, 25, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhou, D.; Hao, S.; Zhou, L.; He, W.; Nie, J.; Hou, F.F.; Liu, Y. Sonic hedgehog signaling mediates epithelial–mesenchymal communication and promotes renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 801–813. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, E.D.; Mylonas, K.J.; Xin, C.; Baird, D.P.; Carvalho, C.; Docherty, M.-H.; Campbell, R.; Matchett, K.P.; Waddell, S.H.; Walker, A.D.; et al. Indian Hedgehog release from TNF-activated renal epithelia drives local and remote organ fibrosis. Sci. Transl. Med. 2023, 15, eabn0736. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J. Indian Hedgehog links kidney injury to fibrosis. Nat. Rev. Nephrol. 2023, 19, 478. [Google Scholar] [CrossRef]
- Liu, X.; Miao, J.; Wang, C.; Zhou, S.; Chen, S.; Ren, Q.; Hong, X.; Wang, Y.; Hou, F.F.; Zhou, L.; et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 2020, 97, 1181–1195. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Li, Y.; Hu, C.; Zhai, P.; Zhang, J.; Jiang, J.; Suo, J.; Hu, B.; Wang, J.; Weng, X.; Zhou, X.; et al. Fibroblastic reticular cell-derived exosomes are a promising therapeutic approach for septic acute kidney injury. Kidney Int. 2024, 105, 508–523. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, J.; Tan, Y.; Li, Y.; Peng, Z. Exosomes Highlight Future Directions in the Treatment of Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 15568. [Google Scholar] [CrossRef]
- Ding, C.; Zheng, J.; Wang, B.; Li, Y.; Xiang, H.; Dou, M.; Qiao, Y.; Tian, P.; Ding, X.; Xue, W. Exosomal MicroRNA-374b-5p From Tubular Epithelial Cells Promoted M1 Macrophages Activation and Worsened Renal Ischemia/Reperfusion Injury. Front. Cell Dev. Biol. 2020, 8, 587693. [Google Scholar] [CrossRef]
- Wang, W.; Sai, W.-L.; Yang, B. The role of macrophage polarization and interaction with renal tubular epithelial cells in ischemia-reperfusion induced acute kidney injury. Sheng Li Xue Bao [Acta Physiol. Sin.] 2022, 74, 28–38. [Google Scholar]
- Xiang, H.; Xu, Z.; Zhang, C.; Xiong, J. Macrophage-derived exosomes mediate glomerular endothelial cell dysfunction in sepsis-associated acute kidney injury. Cell Biosci. 2023, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.X.; Mao, Y.; Cao, Q.; Chen, Y.; Zhou, L.B.; Li, S.; Chen, H.; Chen, J.H.; Zhou, G.P.; Jin, R. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J. Cell. Mol. Med. 2021, 25, 4786–4799. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Peng, R.; Mao, L.; Fang, F.; Xu, B.; Chen, M. Injured tubular epithelial cells activate fibroblasts to promote kidney fibrosis through miR-150-containing exosomes. Exp. Cell Res. 2020, 392, 112007. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.; Pourhanifeh, M.H.; Fadaei, S.; Velayati, A.A.; Mirzaei, H.; Hamblin, M.R. Non-coding RNAs and Exosomes: Their Role in the Pathogenesis of Sepsis. Mol. Ther.-Nucleic Acids 2020, 21, 51–74. [Google Scholar] [CrossRef]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.-C.; Staels, B. Sorting out the roles of PPAR in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Jiang, S.; Fu, D.; Lu, X.; Lu, M.; Li, Y.; Luo, D.; Wu, K.; Xu, Y.; et al. The glycolytic enzyme PFKFB3 drives kidney fibrosis through promoting histone lactylation-mediated NF-κB family activation. Kidney Int. 2024, 106, 226–240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).