Biomarkers and Mental Disorders: A Relevance Analysis Using a Random Forest Algorithm
Abstract
1. Introduction
2. Material and Methods
2.1. Participants and Eligibility
2.2. Procedures and Instruments
2.3. Characteristics of the Volunteers Studied
2.4. Depression and Anxiety Score
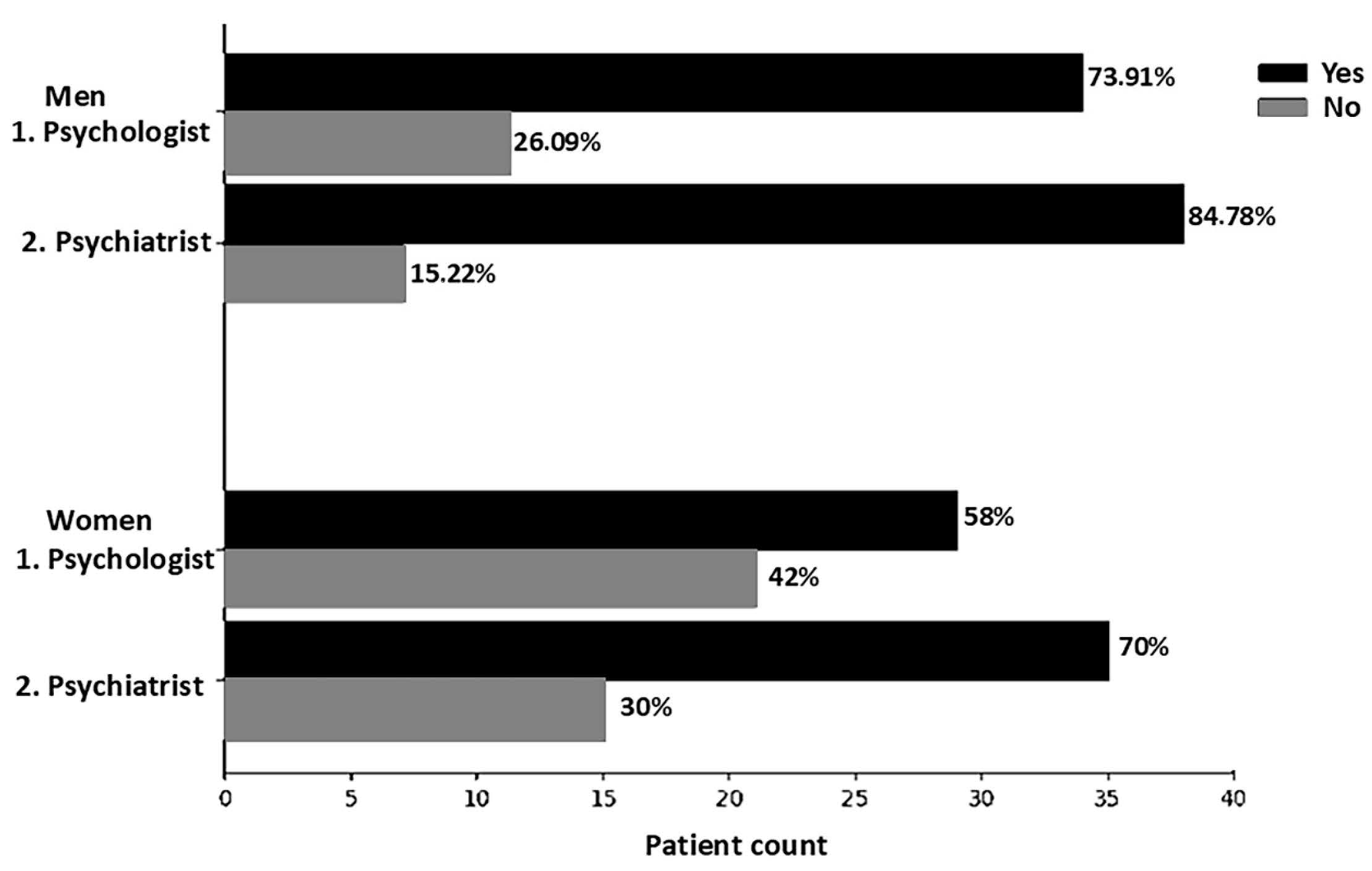
2.5. Alcohol and Tobacco Consumption Patterns
2.6. Blood Collection
2.7. Analysis of the Results
2.7.1. Random Forest and Cross-Validation
2.7.2. Importance Network Between Biomarkers
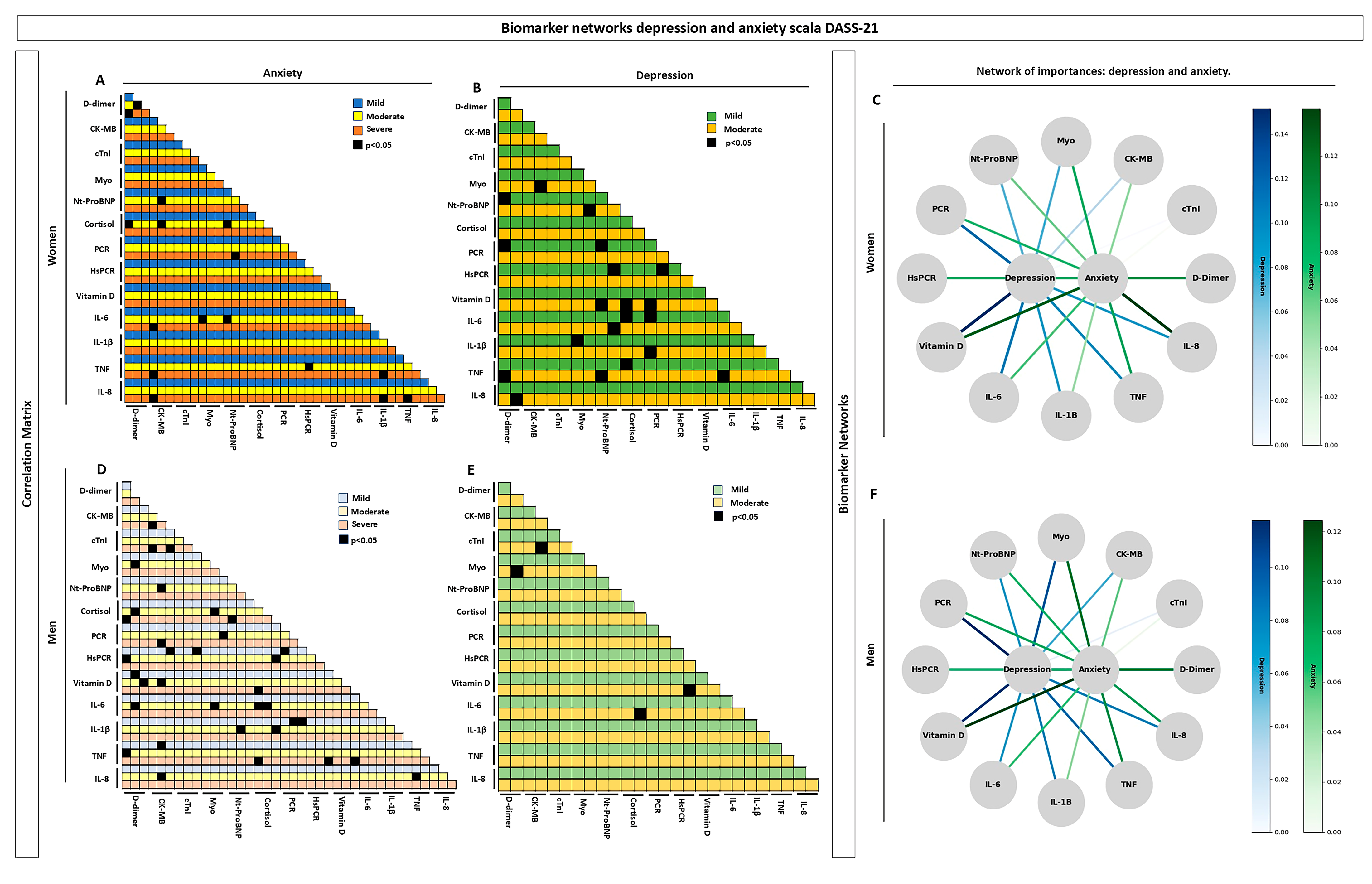
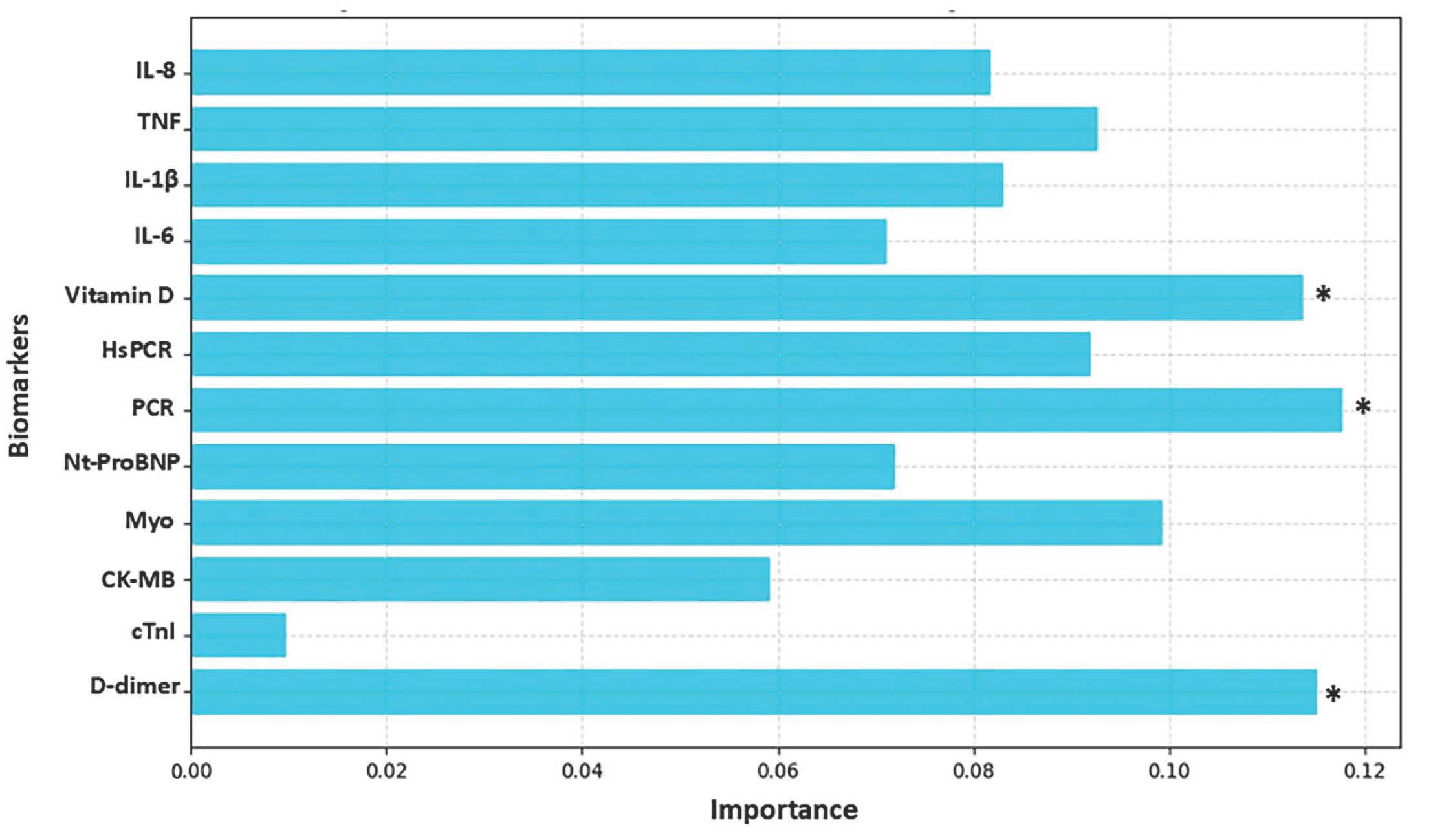
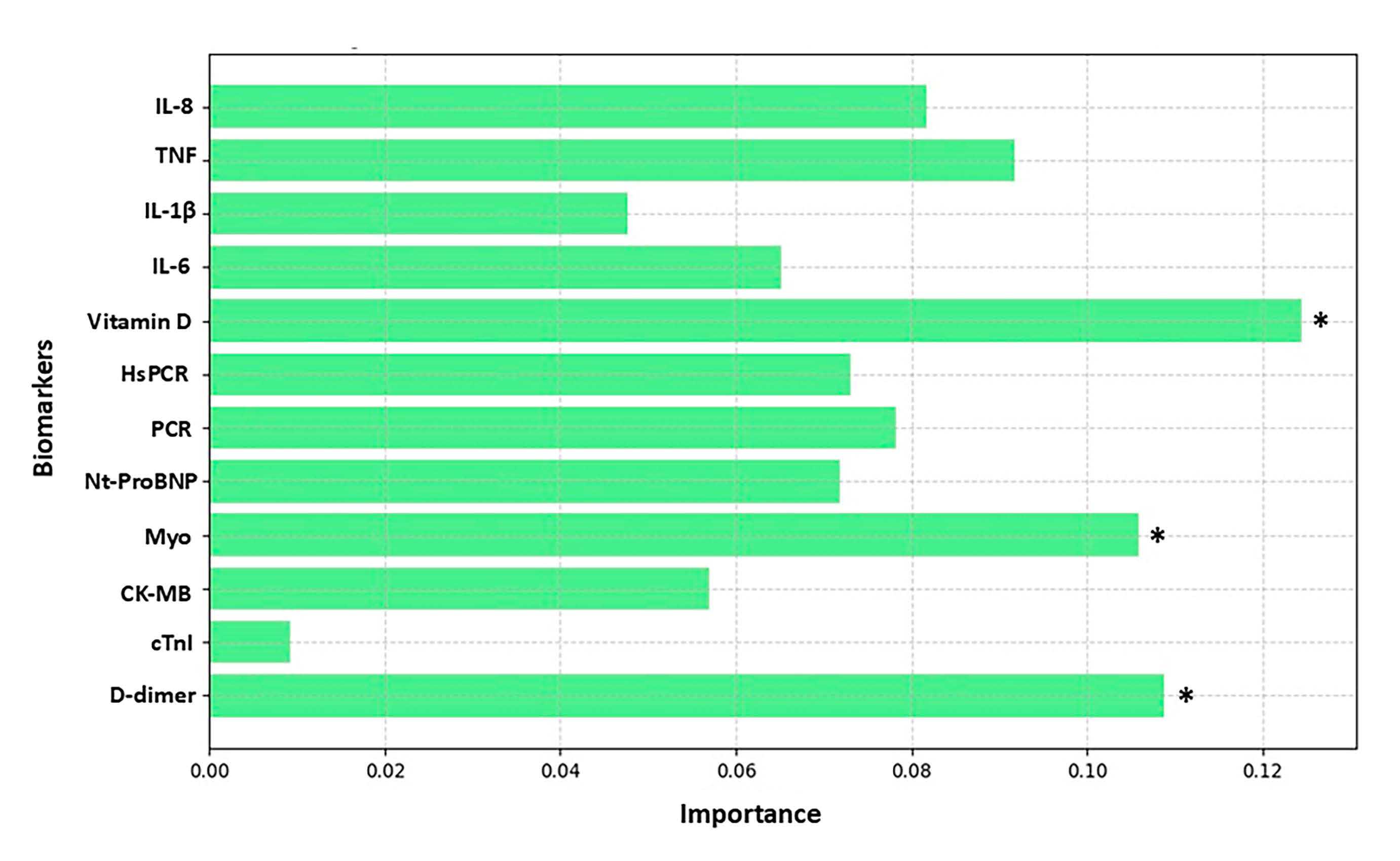
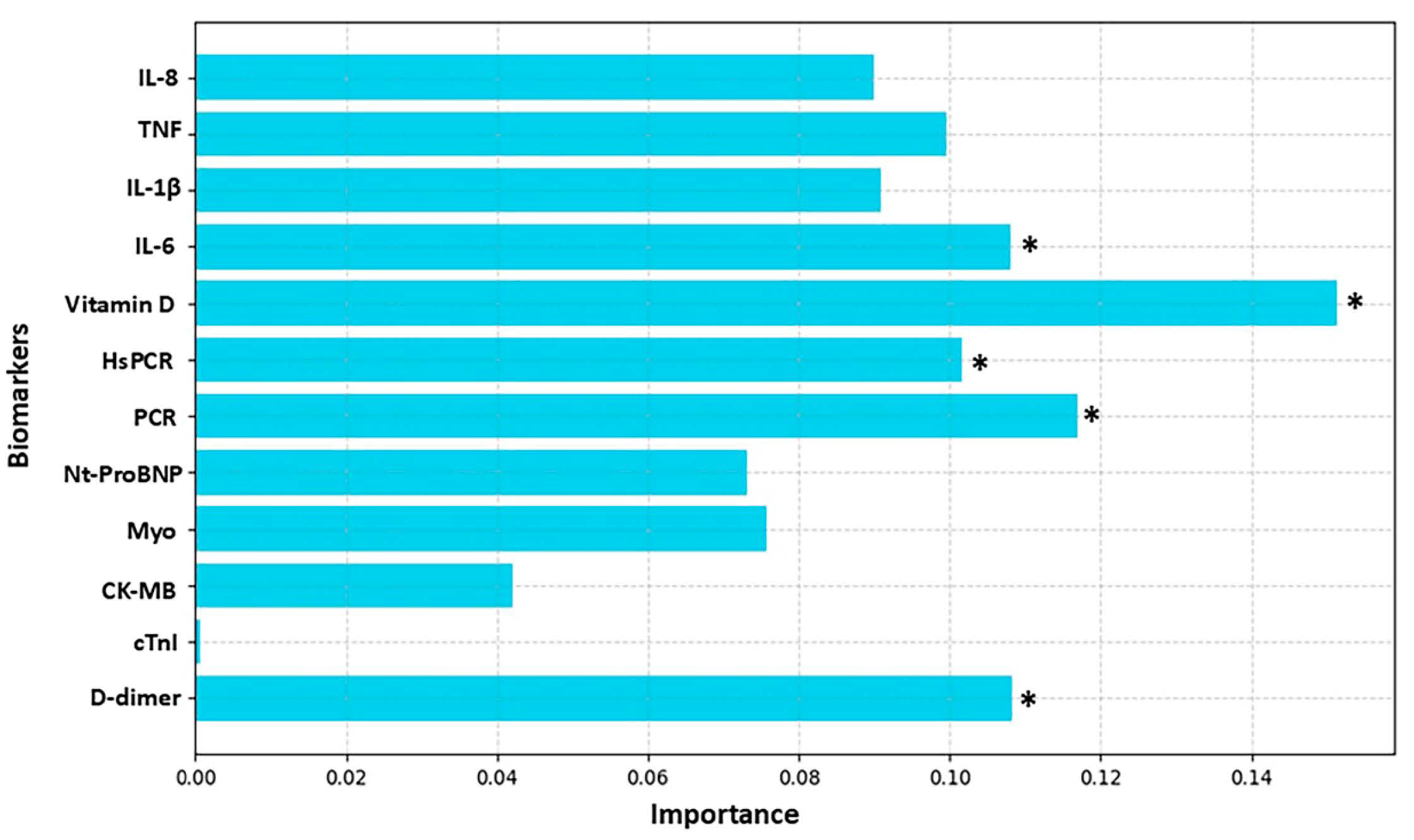
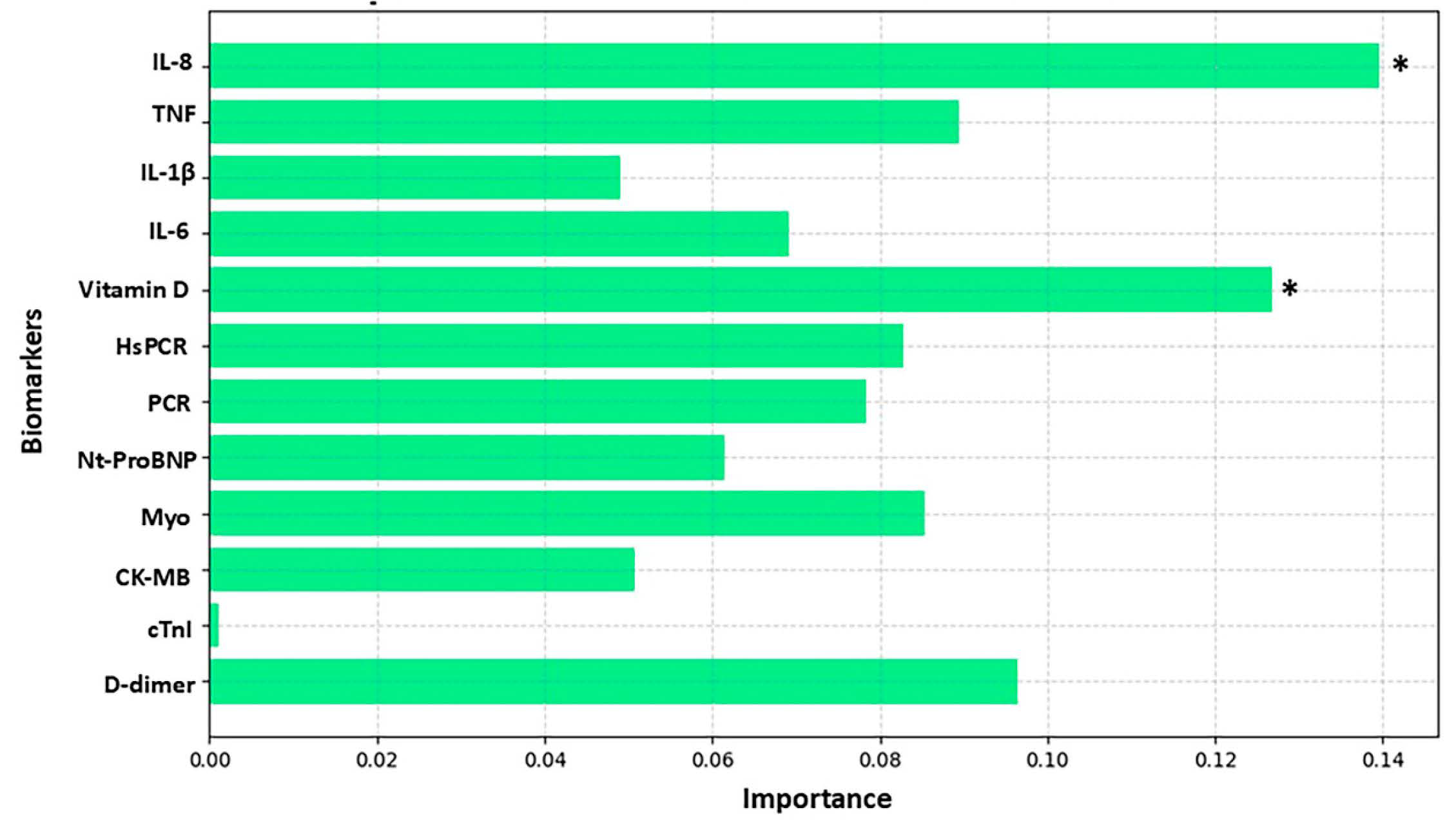
3. Results
Characteristics of the Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2022, 24. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 7, 137–150. [Google Scholar] [CrossRef]
- World Health Organization. Anxiety Disorders. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/anxiety-disorders (accessed on 16 September 2024).
- Vismari, L.; Alves, G.J.; Palermo-Neto, J. Depressão, antidepressivos e sistema imune: Um novo olhar sobre um velho problema. Arch. Clin. Psychiatry 2008, 35, 196–204. [Google Scholar] [CrossRef]
- Abelha, L. Depressão, uma questão de saúde pública. Cad. Saúde Colet. 2014, 22, 223. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef]
- Craske, M.G.; Stein, M.B.; Eley, T.C.; Milad, M.R.; Holmes, A.; Rapee, R.M.; Wittchen, H.-U. Anxiety disorders. Nat. Rev. Dis. Prim. 2017, 3, 17024. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Rev. Bras. de Psiquiatr. 2020, 42, 657–672. [Google Scholar] [CrossRef]
- Gonçalves, A.M.C.; Teixeira, M.T.B.; Gama, J.R.d.A.; Lopes, C.S.; e Silva, G.A.; Gamarra, C.J.; Duque, K.d.C.D.; Machado, M.L.S.M. Prevalência de depressão e fatores associados em mulheres atendidas pela Estratégia de Saúde da Família. J. Bras. de Psiquiatr. 2018, 67, 101–109. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, M.; Liu, Y.; Jiang, J.; Wang, Y. Recent advances in anxiety disorders: Focus on animal models and pathological mechanisms. Anim. Model. Exp. Med. 2023, 6, 559–572. [Google Scholar] [CrossRef]
- Martins, B.G.; da Silva, W.R.; Maroco, J.; Campos, J.A.D.B. Escala de Depressão, Ansiedade e Estresse: Propriedades psicométricas e prevalência das afetividades. J. Bras. de Psiquiatr. 2019, 68, 32–41. [Google Scholar] [CrossRef]
- Silveira, G.E.L.; Viana, L.G.; Sena, M.M.; Alencar, M.M.S.d.C.; Soares, P.R.A.L.; Aquino, P.d.S.; Ribeiro, S.G. Sintomas de ansiedade e depressão no ambiente acadêmico: Um estudo transversal. Acta Paul. de Enferm. 2022, 35. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Comorbid depression and anxiety: Integration of insights from attachment theory and cognitive neuroscience, and their implications for research and treatment. Front. Behav. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Müller, N.; Ackenheil, M. Psychoneuroimmunology and the cytokine action in the CNS: Implications for psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1998, 22, 1–33. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Y.; Xu, Y.; Xian, X.; Wang, R.; Cai, L.; Li, G.; Li, Y. Interleukin-6-white matter network differences explained the susceptibility to depression after stressful life events. J. Affect. Disord. 2022, 305, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Poletti, S.; Vai, B.; Mazza, M.G.; Lorenzi, C.; Brioschi, S.; Aggio, V.; Branchi, I.; Colombo, C.; Furlan, R.; et al. Higher baseline interleukin-1β and TNF-α hamper antidepressant response in major depressive disorder. Eur. Neuropsychopharmacol. 2021, 42, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J. Role of interleukin 8 in depression and other psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110173. [Google Scholar] [CrossRef]
- Teo, C.H.; Wong, A.C.H.; Sivakumaran, R.N.; Parhar, I.; Soga, T. Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7129. [Google Scholar] [CrossRef]
- Porto, C.M.; Silva, T.d.P.S.d.; Sougey, E.B. Contributions of vitamin D in the management of depressive symptoms and cardiovascular risk factors: Study protocol for a randomized, double-blind, placebo-controlled clinical trial. Trials 2019, 20, 583. [Google Scholar] [CrossRef]
- Aguiar, F.J.; Ferreira-Júnior, M.; Sales, M.M.; Cruz-Neto, L.M.; Fonseca, L.A.; Sumita, N.M.; Duarte, N.J.; Lichtenstein, A.; Duarte, A.J. Proteína C reativa: Aplicações clínicas e propostas para utilização racional. Front. Public Health 2013, 59, 85–92. [Google Scholar] [CrossRef]
- Lin, D.; Wang, L.; Yan, S.; Zhang, Q.; Zhang, J.H.; Shao, A. The Role of Oxidative Stress in Common Risk Factors and Mechanisms of Cardio-Cerebrovascular Ischemia and Depression. Oxidative Med. Cell. Longev. 2019, 2019, 2491927. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Błaziak, M.; Urban, S.; Wietrzyk, W.; Jura, M.; Iwanek, G.; Stańczykiewicz, B.; Kuliczkowski, W.; Zymliński, R.; Pondel, M.; Berka, P.; et al. An Artificial Intelligence Approach to Guiding the Management of Heart Failure Patients Using Predictive Models: A Systematic Review. Biomedicines 2022, 10, 2188. [Google Scholar] [CrossRef] [PubMed]
- Karanasiou, G.S.; Tripoliti, E.E.; Papadopoulos, T.G.; Kalatzis, F.G.; Goletsis, Y.; Naka, K.K.; Bechlioulis, A.; Errachid, A.; Fotiadis, D.I. Predicting adherence of patients with HF through machine learning techniques. Health Technol. Lett. 2016, 3, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Szymczak, S. A review on longitudinal data analysis with random forest. Brief. \Bioinform. 2023, 24, bbad002. [Google Scholar] [CrossRef]
- Thapelo, T.S.; Mpoeleng, D.; Hillhouse, G. Informed Random Forest to Model Associations of Epidemiological Priors, Government Policies, and Public Mobility. MDM Policy Pr. 2023, 8, 23814683231218716. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random survival forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Schonlau, M.; Zou, R.Y. The random forest algorithm for statistical learning. Stata J. Promot. Commun. Stat. Stata 2020, 20, 3–29. [Google Scholar] [CrossRef]
- Freiberger, N.; Lynch, T.; Read, G.L.; Blouin, A.M. (Men)tal health: Perceptions of depression in men and women. SSM Ment. Health 2023, 4. [Google Scholar] [CrossRef]
- Jalal, N.; Mehmood, A.; Choi, G.S.; Ashraf, I. A novel improved random forest for text classification using feature ranking and optimal number of trees. J. King Saud Univ. Comput. Inf. Sci. 2022, 34, 2733–2742. [Google Scholar] [CrossRef]
- Pashaei, E.; Ozen, M.; Aydin, N. Random Forest in Splice Site Prediction of Human Genome. In XIV Mediterranean Conference on Medical and Biological Engineering and Computing 2016. IFMBE Proceedings; Kyriacou, E., Christofides, S., Pattichis, C., Eds.; Springer: Cham, Switzerland, 2016; Volume 57. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Dadkhah, M.; Jafarzadehgharehziaaddin, M.; Molaei, S.; Akbari, M.; Gholizadeh, N.; Fathi, F. Major depressive disorder: Biomarkers and biosensors. Clin. Chim. Acta 2023, 547, 117437. [Google Scholar] [CrossRef]
- Bohannan, Z.S.; Coffman, F.; Mitrofanova, A. Random survival forest model identifies novel biomarkers of event-free survival in high-risk pediatric acute lymphoblastic leukemia. Comput. Struct. Biotechnol. J. 2022, 20, 583–597. [Google Scholar] [CrossRef]
- Morssinkhof, M.; van Wylick, D.; Priester-Vink, S.; van der Werf, Y.; Heijer, M.D.; Heuvel, O.v.D.; Broekman, B. Associations between sex hormones, sleep problems and depression: A systematic review. Neurosci. Biobehav. Rev. 2020, 118, 669–680. [Google Scholar] [CrossRef]
- Müller, S.; Chang, H.-C.; Köhler, H. Perturbation of the idiotypic network. Cell. Immunol. 1989, 119, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Radakovich, N.; Nagy, M.; Nazha, A. Machine learning in haematological malignancies. Lancet Haematol 2020, 7, e541–e550. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Eck, S.R.; Sanchez, E.O. Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacology 2018, 44, 129–139. [Google Scholar] [CrossRef]
- Seeman, M.V.; González-Rodríguez, A. Stratification by Sex and Hormone Level When Contrasting Men and Women in Schizophrenia Trials Will Improve Personalized Treatment. J. Pers. Med. 2021, 11, 929. [Google Scholar] [CrossRef]
- Lei, Y.; Li, S.; Liu, Z.; Wan, F.; Tian, T.; Li, S.; Zhao, D.; Zeng, J. A deep-learning framework for multi-level peptide–protein interaction prediction. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hamada, T.; Higashi, M.; Matsuo, K.; Maemura, K.; Kurahara, H.; Horinouchi, M.; Hiraki, T.; Sugimoto, T.; Akahane, T.; et al. Predicted Prognosis of Patients with Pancreatic Cancer by Machine Learning. Clin. Cancer Res. 2020, 26, 2411–2421. [Google Scholar] [CrossRef]
- Gal, O.; Auslander, N.; Fan, Y.; Meerzaman, D. Predicting Complete Remission of Acute Myeloid Leukemia: Machine Learning Applied to Gene Expression. Cancer Informatics 2019, 18. [Google Scholar] [CrossRef]
- Remes, O.; Mendes, J.F.; Templeton, P. Biological, Psychological, and Social Determinants of Depression: A Review of Recent Literature. Brain Sci 2021, 11. [Google Scholar] [CrossRef]
- Bøen, H.; Dalgard, O.S.; Bjertness, E. The importance of social support in the associations between psychological distress and somatic health problems and socio-economic factors among older adults living at home: A cross sectional study. BMC Geriatr. 2012, 12, 27. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Cuarenta, A. Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Belmaker, R.; Agam, G. Major Depressive Disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef]
- Weitz, J.I.; Fredenburgh, J.C.; Eikelboom, J.W. A Test in Context: D-Dimer. J. Am. Coll. Cardiology 2017, 70, 2411–2420. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr. Rev. 2009, 67, 481–492. [Google Scholar] [CrossRef]
- Musazadeh, V.; Keramati, M.; Ghalichi, F.; Kavyani, Z.; Ghoreishi, Z.; Alras, K.A.; Albadawi, N.; Salem, A.; Albadawi, M.I.; Salem, R.; et al. Vitamin D protects against depression: Evidence from an umbrella meta-analysis on interventional and observational meta-analyses. Pharmacol. Res. 2022, 187, 106605. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Schilliger, Z.; Perroud, N.; Piguet, C. Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder. Biomedicines 2021, 9, 1313. [Google Scholar] [CrossRef]
- Leonard, B. Psychopathology of depression. Drugs Today 2007, 43, 705–716. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Pan, Y.; Wang, H.; Chen, Y.; Lai, J.; Hu, S. Clinical Characteristics of Asymptomatic Thromboembolism in Psychiatric Inpatients: A Retrospective Study. Neuropsychiatr. Dis. Treat. 2024, ume 20, 515–522. [Google Scholar] [CrossRef]
- Parkin, L.; Balkwill, A.; Sweetland, S.; Reeves, G.K.; Green, J.; Beral, V.; the Million Women Study Collaborators; Abbiss, H.; Abbott, S.; Alison, R.; et al. Antidepressants, Depression, and Venous Thromboembolism Risk: Large Prospective Study of UK Women. J. Am. Hear. Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Keller, T.C.S.; Lechauve, C.; Keller, A.S.; Brooks, S.; Weiss, M.J.; Columbus, L.; Ackerman, H.; Cortese-Krott, M.M.; Isakson, B.E. The role of globins in cardiovascular physiology. Physiol. Rev. 2022, 102, 859–892. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.L.R.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Barbosa, N.M.M.V.; da Silva, P.R.; Coutinho, H.D.M.; de Menezes, I.R.A.; Felipe, C.F.B. Biochemical aspects of the inflammatory process: A narrative review. Biomed. Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef] [PubMed]
| Variables | Woman (N = 50) | Confidence Interval [CI 95%] (Women) | Range (Women) | Man (N = 46) | Confidence Interval [CI 95%] (Men) | Range (Men) | |
|---|---|---|---|---|---|---|---|
| Age (years) | 41.61 ± 12.68 | [19.00–69.00] | 50 | 38 ± 12.34 | [19.00–72.00] | 53 | |
| Weight (kg) | 73.16 ±17.82 | [45.00–115.00] | 70 | 78.23 ± 14.83 | [54.00–117.00] | 63 | |
| Height (kg) | 163.8 ± 0.066 | [152.00–190.00] | 0.38 | 172.82 ± 0.075 | [153.00–188.00] | 0.35 | |
| BMI (kg/m2) | 27.27 ± 6.507 | [17.5–43.82] | 26.67 | 26.16 ± 4.3 | [16.41–39.09] | 22.68 | |
| Obesity degree | Underweight | 4 (8%) | 2 (4.34%) | ||||
| Ideal | 17 (34%) | 15 (32.6%) | |||||
| Overweight | 16 (32%) | 21 (45.65%) | |||||
| Obesity degree I | 6 (12%) | 6 (13.04%) | |||||
| Obesity degree II | 4 (8%) | 1 (2.17%) | |||||
| Obesity degree III | 3 (6%) | 1 (2.17%) | |||||
| Smoking | Smoker | 36 (72%) | 25 (54.34%) | ||||
| Non-smoker | 11 (22%) | 19 (41.30%) | |||||
| Quit smoking more than 5 years ago | 3 (%) | 2 (4.34%) | |||||
| Alcoholism | Non-drinker or occasional drinker (up to once a week) | 40 (80%) | 34 (73.91%) | ||||
| Regular drinker (more than once a week) | 10 (20%) | 12 (26.08%) |
| Variables | Category | Woman (N = 50) | MD/SD (W) | CI 95% (W) | Man (N = 46) | MD/SD (M) | CI 95% (M) |
|---|---|---|---|---|---|---|---|
| Depression Score | No depression: 0–9 | 29 (58%) | 2 ± 2.86 | 0.79 [1.20–2.79] | 36 (78.26%) | 3 ± 2.64 | 0.76 [2.23–3.76] |
| Mild: 10–13 * | 7 (14%) | 12 ± 0.45 | 0.12 [11.87–12.12] | 2 (4.34%) | 10 ± 0 | - | |
| Moderate: 14–20 | 14 (28%) | 18 ± 2.3 | 0.63 [7.36–18.63] | 7 (15.21%) | 17 ± 1.88 | 0.54 [16.45–17.5] | |
| Severe: >21 | - | - | - | 1 (2.17%) | 21 ± 0 | - | |
| Anxiety Score | No Anxiety: 0–7 | 28 (60.86%) | 2 ± 2.3 | 0.63 [1.36–2.63] | 29 (63.04%) | 2 ± 2.4 | 0.69 [1.30–2.69] |
| Mild: 8–9 | 2 (4.34%) | 8.5 ± 0.5 | 0.13 [8.16–8.83] | 5 (10.86%) | 8 ± 0.43 | 0.12 [7.87–8.12] | |
| Moderate: 10–14 | 8 (17.39%) | 14 ± 1.08 | 0.29 [13.70–14.29] | 8 (17.39%) | 11 ± 1.81 | 0.52 [10.47–11.52] | |
| Severe: >21 | 12 (26.08%) | 16 ± 2.5 | 0.69 [15.30–16.69] | 4 (8.69%) | 16 ± 1.08 | 0.31 [15.68–16.31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodolpho, J.M.A.; Godoy, K.F.; Fragelli, B.D.L.; Bianchi, J.; Duarte, F.O.; Camillo, L.; Silva, G.B.; Andrade, P.H.M.; Prado, J.A.; Speglich, C.; et al. Biomarkers and Mental Disorders: A Relevance Analysis Using a Random Forest Algorithm. Biomolecules 2025, 15, 793. https://doi.org/10.3390/biom15060793
Rodolpho JMA, Godoy KF, Fragelli BDL, Bianchi J, Duarte FO, Camillo L, Silva GB, Andrade PHM, Prado JA, Speglich C, et al. Biomarkers and Mental Disorders: A Relevance Analysis Using a Random Forest Algorithm. Biomolecules. 2025; 15(6):793. https://doi.org/10.3390/biom15060793
Chicago/Turabian StyleRodolpho, Joice M. A., Krissia F. Godoy, Bruna D. L. Fragelli, Jaqueline Bianchi, Fernanda O. Duarte, Luciana Camillo, Gustavo B. Silva, Paulo H. M. Andrade, Juliana A. Prado, Carlos Speglich, and et al. 2025. "Biomarkers and Mental Disorders: A Relevance Analysis Using a Random Forest Algorithm" Biomolecules 15, no. 6: 793. https://doi.org/10.3390/biom15060793
APA StyleRodolpho, J. M. A., Godoy, K. F., Fragelli, B. D. L., Bianchi, J., Duarte, F. O., Camillo, L., Silva, G. B., Andrade, P. H. M., Prado, J. A., Speglich, C., & Anibal, F. F. (2025). Biomarkers and Mental Disorders: A Relevance Analysis Using a Random Forest Algorithm. Biomolecules, 15(6), 793. https://doi.org/10.3390/biom15060793






