Functional Thyroid Organoids—Powerful Stem Cell-Derived Models in Basic and Translational Research
Abstract
1. Introduction
2. Organoids: An Advanced Tool to Dissect Thyroid Development and Model Diseases
2.1. Existing Models of Thyroid Organoids
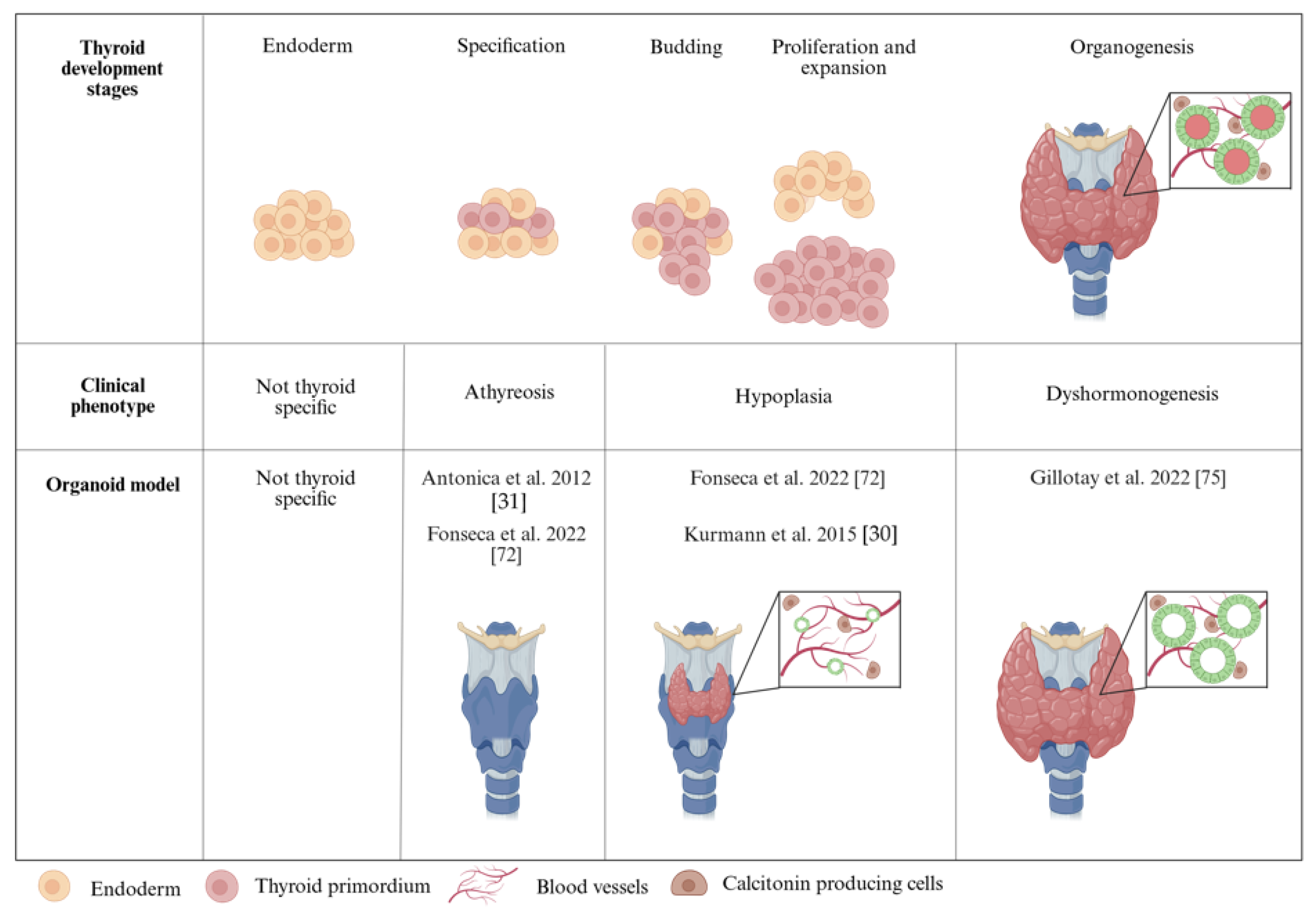
2.2. From Endoderm to Thyroid Specification
2.3. Thyroid Progenitor Expansion and Differentiation
2.4. Thyroid Hormone Synthesis
2.5. In Vivo Transplantation and Rescue of Hypothyroidism
3. Modeling Thyroid Health and Diseases Using Organoids
3.1. Modeling Congenital Hypothyroidism with Organoids
3.2. Organoid Modeling of Thyroid Autoimmune Diseases
3.3. Using Thyroid Organoids to Study Thyroid Disruption by EDCs
4. Challenges and Limitations in Using Thyroid Organoids for Therapeutic Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 131I | 131 iodine |
| 3D | Three-dimensional |
| AA | Activin A |
| AFE | Anterior Foregut Endoderm |
| AFU | Angiofollicular unit |
| ASCs | Adult Stem Cells |
| BKF | Benzo[k]fluoranthene |
| BMP | Bone Morphogenic Protein |
| C cells | Calcitonin-producing cells |
| cAMP | cyclic Adenosine Monophosphate |
| CH | Congenital Hypothyroidism |
| CHTD | Congenital Hypothyroidism with Thyroid Dysgenesis |
| DE | Definitive Endoderm |
| Dox | Doxycycline |
| DUOX | Dual oxidase |
| EBs | Embryonic Bodies |
| ECDs | Endocrine-Disrupting Chemicals |
| EGF | Epidermal Growth Factor |
| ESCs | Embryonic Stem Cells |
| FBS | Fetal Bovine Serum |
| FGF | Fibroblast Growth Factor |
| FNA | Fine Needle Aspiration |
| FSK | Forskolin |
| GMP | Good Manufacturing Practices |
| GW | Gestational Week |
| hFTOs | human Fetal Thyroid Organoids |
| HLA | Human Leukocyte Antigen |
| iPSCs | induced Pluripotent Stem Cells |
| KC | Kidney Capsule |
| KO | Knock-out |
| LIF | Leukemia Inhibitory Factor |
| mESCs | mouse Embryonic Stem Cells |
| NA | Not Applicable |
| NSG | NOD SCID gamma |
| NOD-SCID | Non-obese Diabetic/Severe Combined Immunodeficiency |
| OoC | Organ-On-a-Chip |
| PSC | Pluripotent Stem Cell |
| RA | Retinoic Acid |
| RIA | Radioiodine Ablation |
| scRNA-seq | single cell RNA sequencing |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TD | Thyroid Dysgenesis |
| TFCs | Thyroid Follicular Cells |
| TG | Thyroglobulin |
| TGF | Transforming Growth Factor |
| TPO | Thyroid Peroxidase |
| TSH | Thyroid Stimulating Hormone |
| TSHR | Thyroid Stimulating Hormone Receptor |
| WNT | wingless-type MMTV integrate site family |
References
- Nilsson, M.; Fagman, H. Development of the Thyroid Gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; Di Lauro, R. Thyroid Development and Its Disorders: Genetics and Molecular Mechanisms. Endocr. Rev. 2004, 25, 722–746. [Google Scholar] [CrossRef]
- Fagman, H.; Nilsson, M. Morphogenesis of the Thyroid Gland. Mol. Cell. Endocrinol. 2010, 323, 35–54. [Google Scholar] [CrossRef]
- Marotta, P.; Amendola, E.; Scarfò, M.; De Luca, P.; Zoppoli, P.; Amoresano, A.; De Felice, M.; Di Lauro, R. The Paired Box Transcription Factor Pax8 Is Essential for Function and Survival of Adult Thyroid Cells. Mol. Cell. Endocrinol. 2014, 396, 26–36. [Google Scholar] [CrossRef]
- Fagman, H.; Nilsson, M. Morphogenetics of Early Thyroid Development. J. Mol. Endocrinol. 2011, 46, R33–R42. [Google Scholar] [CrossRef]
- Haerlingen, B.; Opitz, R.; Vandernoot, I.; Trubiroha, A.; Gillotay, P.; Giusti, N.; Costagliola, S. Small-Molecule Screening in Zebrafish Embryos Identifies Signaling Pathways Regulating Early Thyroid Development. Thyroid 2019, 29, 1683–1703. [Google Scholar] [CrossRef]
- Haerlingen, B.; Opitz, R.; Vandernoot, I.; Molinaro, A.; Shankar, M.P.; Gillotay, P.; Trubiroha, A.; Costagliola, S. Mesodermal FGF and BMP Govern the Sequential Stages of Zebrafish Thyroid Specification. Development 2023, 150, dev201023. [Google Scholar] [CrossRef]
- Colin, I.M.; Denef, J.F.; Lengelé, B.; Many, M.C.; Gérard, A.C. Recent Insights into the Cell Biology of Thyroid Angiofollicular Units. Endocr. Rev. 2013, 34, 209–238. [Google Scholar] [CrossRef]
- Nilsson, M.; Fagman, H. Mechanisms of Thyroid Development and Dysgenesis: An Analysis Based on Developmental Stages and Concurrent Embryonic Anatomy. Curr. Top. Dev. Biol. 2013, 106, 123–170. [Google Scholar] [CrossRef]
- Wendl, T.; Lun, K.; Mione, M.; Favor, J.; Brand, M.; Wilson, S.W.; Rohr, K.B. Pax2.1 Is Required for the Development of Thyroid Follicles in Zebrafish. Development 2002, 129, 3751–3760. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human Organoids in Basic Research and Clinical Applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.W. A Brief History of Organoids. Am. J. Physiol. Cell Physiol. 2020, 319, 151–165. [Google Scholar] [CrossRef]
- Nitsch, L.; Tacchetti, C.; Tramontano, D. Suspension Culture Reveals a Morphogenetic Property of a Thyroid Epithelial Cell Line. Exp. Cell Res. 1984, 152, 22–30. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, G.E.; Stelzer, H.K.E. The Third Dimension Bridges the gap between Cell Culture and Live Tissue. Nat. Rev. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Fayet, G.; Michel-Béchet, M.; Lissitzky, S. Thyrotrophin-Induced Aggregation and Reorganization into Follicles of Isolated Porcine-Thyroid Cells in Culture. Eur. J. Biochem. 1971, 24, 100–111. [Google Scholar] [CrossRef]
- Ambesi-Impiombato, F.S.; Parkst, L.A.M.; Coontf, H.G. Culture of Hormone-Dependent Functional Epithelial Cells from Rat Thyroids (Thyroglobulin/Iodide Transport/Hormones/Differentiation/Aging). Cell Biol. 1980, 77, 3455–3459. [Google Scholar] [CrossRef]
- van der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- van der Vaart, J.; Bosmans, L.; Sijbesma, S.F.; Knoops, K.; van de Wetering, W.J.; Otten, H.G.; Begthel, H.; Borel Rinkes, I.H.M.; Korving, J.; Lentjes, E.G.W.M.; et al. Adult Mouse and Human Organoids Derived from Thyroid Follicular Cells and Modeling of Graves’ Hyperthyroidism. Proc. Natl. Acad. Sci. USA 2021, 118, e2117017118. [Google Scholar] [CrossRef]
- Massalha, H.; Trinh, M.K.; Armingol, E.; Tuck, L.; Predeus, A.; Mazin, P.; Sancho-Serra, C.; Oszlanczi, A.; Wood, Y.; Parks, C.; et al. A Developmental Cell Atlas of the Human Thyroid Gland. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ogundipe, V.M.L.; Groen, A.H.; Hosper, N.; Nagle, P.W.K.; Hess, J.; Faber, H.; Jellema, A.L.; Baanstra, M.; Links, T.P.; Unger, K.; et al. Generation and Differentiation of Adult Tissue-Derived Human Thyroid Organoids. Stem Cell Rep. 2021, 16, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Onishi, N.; Takami, H.; Seishima, R.; Inoue, H.; Hirata, Y.; Kameyama, K.; Tsuchihashi, K.; Sugihara, E.; Uchino, S.; et al. Development of a Functional Thyroid Model Based on an Organoid Culture System. Biochem. Biophys. Res. Commun. 2018, 497, 783–789. [Google Scholar] [CrossRef]
- Calà, G.; Sina, B.; De Coppi, P.; Giobbe, G.G.; Gerli, M.F.M. Primary Human Organoids Models: Current Progress and Key Milestones. Front. Bioeng. Biotechnol. 2023, 11, 1058970. [Google Scholar] [CrossRef]
- Deisenroth, C.; Soldatow, V.Y.; Ford, J.; Stewart, W.; Brinkman, C.; Lecluyse, E.L.; MacMillan, D.K.; Thomas, R.S. Development of an in Vitro Human Thyroid Microtissue Model for Chemical Screening. Toxicol. Sci. 2020, 174, 63–78. [Google Scholar] [CrossRef]
- Lan, L.; Cui, D.; Nowka, K.; Derwahl, M. Stem Cells Derived from Goiters in Adults Form Spheres in Response to Intense Growth Stimulation and Require Thyrotropin for Differentiation into Thyrocytes. J. Clin. Endocrinol. Metab. 2007, 92, 3681–3688. [Google Scholar] [CrossRef]
- Romitti, M.; Eski, S.E.; Fonseca, B.F.; Gillotay, P.; Singh, S.P.; Costagliola, S. Single-Cell Trajectory Inference Guided Enhancement of Thyroid Maturation In Vitro Using TGF-Beta Inhibition. Front. Endocrinol. 2021, 12, 657195. [Google Scholar] [CrossRef]
- Romitti, M.; Tourneur, A.; de Faria da Fonseca, B.; Doumont, G.; Gillotay, P.; Liao, X.H.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; et al. Transplantable Human Thyroid Organoids Generated from Embryonic Stem Cells to Rescue Hypothyroidism. Nat. Commun. 2022, 13, 7057. [Google Scholar] [CrossRef]
- Longmire, T.A.; Ikonomou, L.; Hawkins, F.; Christodoulou, C.; Cao, Y.; Jean, J.C.; Kwok, L.W.; Mou, H.; Rajagopal, J.; Shen, S.S.; et al. Efficient Derivation of Purified Lung and Thyroid Progenitors from Embryonic Stem Cells. Cell Stem Cell 2012, 10, 398–411. [Google Scholar] [CrossRef]
- Kurmann, A.A.; Serra, M.; Hawkins, F.; Rankin, S.A.; Mori, M.; Astapova, I.; Ullas, S.; Lin, S.; Bilodeau, M.; Rossant, J.; et al. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 527–542. [Google Scholar] [CrossRef]
- Antonica, F.; Kasprzyk, D.F.; Opitz, R.; Iacovino, M.; Liao, X.H.; Dumitrescu, A.M.; Refetoff, S.; Peremans, K.; Manto, M.; Kyba, M.; et al. Generation of Functional Thyroid from Embryonic Stem Cells. Nature 2012, 491, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Latif, R.; Davies, T.F. Thyroid Follicle Formation and Thyroglobulin Expression in Multipotent Endodermal Stem Cells. Thyroid 2013, 23, 385–391. [Google Scholar] [CrossRef]
- Ma, R.; Morshed, S.A.; Latif, R.; Davies, T.F. Thyroid Cell Differentiation from Murine Induced Pluripotent Stem Cells. Front. Endocrinol. 2015, 6, 56. [Google Scholar] [CrossRef]
- Ma, R.; Latif, R.; Davies, T.F. Human Embryonic Stem Cells Form Functional Thyroid Follicles. Thyroid 2015, 25, 455–461. [Google Scholar] [CrossRef]
- Dame, K.; Cincotta, S.; Lang, A.H.; Sanghrajka, R.M.; Zhang, L.; Choi, J.; Kwok, L.; Wilson, T.; Kańduła, M.M.; Monti, S.; et al. Thyroid Progenitors Are Robustly Derived from Embryonic Stem Cells through Transient, Developmental Stage-Specific Overexpression of Nkx2-1. Stem Cell Rep. 2017, 8, 216–225. [Google Scholar] [CrossRef]
- Undeutsch, H.J.; Posabella, A.; Alber, A.B.; Bawa, P.S.; Villacorta-Martin, C.; Wang, F.; Ikonomou, L.; Kotton, D.N.; Hollenberg, A.N. Derivation of Transplantable Human Thyroid Follicular Epithelial Cells from Induced Pluripotent Stem Cells. Stem Cell Rep. 2024, 19, 1690–1705. [Google Scholar] [CrossRef]
- Ma, R.; Shi, R.; Morshed, S.A.; Latif, R.; Davies, T.F. Derivation and 97% Purification of Human Thyroid Cells from Dermal Fibroblasts. Front. Endocrinol. 2020, 11, 446. [Google Scholar] [CrossRef]
- Solnica-Krezel, L.; Sepich, D.S. Gastrulation: Making and Shaping Germ Layers. Annu. Rev. Cell Dev. Biol. 2012, 28, 687–717. [Google Scholar] [CrossRef]
- Keller, G.M. In Vitro Differentiation of Embryonic Stem Cells. Curr. Opin. Cell Biol. 1995, 7, 862–869. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient Differentiation of Human Embryonic Stem Cells to Definitive Endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef]
- Lowe, L.A.; Yamada, S.; Kuehn, M.R. Genetic Dissection of Nodal Function in Patterning the Mouse Embryo. Development 2001, 128, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Ashe, H.L.; Briscoe, J. The Interpretation of Morphogen Gradients. Development 2006, 133, 385–394. [Google Scholar] [CrossRef]
- Lin, R.Y.; Kubo, A.; Keller, G.M.; Davies, T.F. Committing Embryonic Stem Cells to Differentiate into Thyrocyte-like Cells in Vitro. Endocrinology 2003, 144, 2644–2649. [Google Scholar] [CrossRef]
- Arufe, M.C.; Lu, M.; Lin, R.Y. Differentiation of Murine Embryonic Stem Cells to Thyrocytes Requires Insulin and Insulin-like Growth Factor-1. Biochem. Biophys. Res. Commun. 2009, 381, 264–270. [Google Scholar] [CrossRef]
- Ma, R.; Latif, R.; Davies, T.F. Thyrotropin-Independent Induction of Thyroid Endoderm from Embryonic Stem Cells by Activin A. Endocrinology 2009, 150, 1970–1975. [Google Scholar] [CrossRef][Green Version]
- Wulansari, N.; Sulistio, Y.A.; Darsono, W.H.W.; Kim, C.-H.; Lee, S.-H. LIF Maintains Mouse Embryonic Stem Cells Pluripotency by Modulating TET1 and JMJD2 Activity in a JAK2-Dependent Manner. Stem Cells 2021, 39, 750–760. [Google Scholar] [CrossRef]
- Tiso, N.; Filippi, A.; Pauls, S.; Bortolussi, M.; Argenton, F. BMP Signalling Regulates Anteroposterior Endoderm Patterning in Zebrafish. Mech. Dev. 2002, 118, 29–37. [Google Scholar] [CrossRef]
- Wills, A.; Dickinson, K.; Khokha, M.; Baker, J.C. Bmp Signaling Is Necessary and Sufficient for Ventrolateral Endoderm Specification in Xenopus. Dev. Dyn. 2008, 237, 2177–2186. [Google Scholar] [CrossRef]
- Green, M.D.; Chen, A.; Nostro, M.-C.; d’Souza, S.L.; Schaniel, C.; Lemischka, I.R.; Gouon-Evans, V.; Keller, G.; Snoeck, H.-W. Generation of Anterior Foregut Endoderm from Human Embryonic and Induced Pluripotent Stem Cells. Nat. Biotechnol. 2011, 29, 267–272. [Google Scholar] [CrossRef]
- Hao, J.; Daleo, M.A.; Murphy, C.K.; Yu, P.B.; Ho, J.N.; Hu, J.; Peterson, R.T.; Hatzopoulos, A.K.; Hong, C.C. Dorsomorphin, a Selective Small Molecule Inhibitor of BMP Signaling, Promotes Cardiomyogenesis in Embryonic Stem Cells. PLoS ONE 2008, 3, e2904. [Google Scholar] [CrossRef]
- Wendl, T.; Adzic, D.; Schoenebeck, J.J.; Scholpp, S.; Brand, M.; Yelon, D.; Rohr, K.B. Early Developmental Specification of the Thyroid Gland Depends on Han-Expressing Surrounding Tissue and on FGF Signals. Development 2007, 134, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Vandernoot, I.; Haerlingen, B.; Gillotay, P.; Trubiroha, A.; Janssens, V.; Opitz, R.; Costagliola, S. Enhanced Canonical Wnt Signaling During Early Zebrafish Development Perturbs the Interaction of Cardiac Mesoderm and Pharyngeal Endoderm and Causes Thyroid Specification Defects. Thyroid 2020, 31, 420–438. [Google Scholar] [CrossRef]
- Liang, S.; Johansson, E.; Barila, G.; Altschuler, D.L.; Fagman, H.; Nilsson, M. A Branching Morphogenesis Program Governs Embryonic Growth of the Thyroid Gland. Development 2018, 145, dev146829. [Google Scholar] [CrossRef]
- Liang, J.; Qian, J.; Yang, L.; Chen, X.; Wang, X.; Lin, X.; Wang, X.; Zhao, B. Modeling Human Thyroid Development by Fetal Tissue-Derived Organoid Culture. Adv. Sci. 2022, 9, 2105568. [Google Scholar] [CrossRef]
- Carvalho, D.J.; Kip, A.M.; Romitti, M.; Nazzari, M.; Tegel, A.; Stich, M.; Krause, C.; Caiment, F.; Costagliola, S.; Moroni, L.; et al. Thyroid-on-a-Chip: An Organoid Platform for In Vitro Assessment of Endocrine Disruption. Adv. Healthc. Mater. 2023, 12, 2201555. [Google Scholar] [CrossRef]
- Pellegata, A.F.; Tedeschi, A.M.; De Coppi, P. Whole Organ Tissue Vascularization: Engineering the Tree to Develop the Fruits. Front. Bioeng. Biotechnol. 2018, 6, 56. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Wang, Y.; Yao, W.; Tang, Y.; Tian, X.; Song, X.; Zhou, J. Advances in Thyroid Organoids Research and Applications. Endocr. Res. 2024, 49, 86–91. [Google Scholar] [CrossRef]
- Prete, A.; Matrone, A.; Plebani, R. State of the Art in 3D Culture Models Applied to Thyroid Cancer. Medicina 2024, 60, 520. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef]
- Rastogi, M.V.; LaFranchi, S.H. Congenital Hypothyroidism. Orphanet J. Rare Dis. 2010, 5, 17. [Google Scholar] [CrossRef]
- Koop, P.; Braverman, L.; Cooper, D. Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Stoupa, A.; Carré, A.; Polak, M.; Szinnai, G. Thyroid Disorders. In Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 495–527. [Google Scholar]
- Olivieri, A.; Corbetta, C.; Weber, G.; Vigone, M.C.; Fazzini, C.; Medda, E. Congenital Hypothyroidism Due to Defects of Thyroid Development and Mild Increase of TSH at Screening: Data from the Italian National Registry of Infants with Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2013, 98, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Deladoey, J. Congenital Hypothyroidism due to Thyroid Dysgenesis: From Epidemiology to Molecular Mechanisms. In A New Look at Hypothyroidism; InTech: Seoul, Republic of Korea, 2012. [Google Scholar]
- Polak, M.; Sura-Trueba, S.; Chauty, A.; Szinnai, G.; Carré, A.; Castanet, M. Molecular Mechanisms of Thyroid Dysgenesis. Horm. Res. Paediatr. 2004, 62, 14–21. [Google Scholar] [CrossRef]
- Didier-Mathon, H.; Stoupa, A.; Kariyawasam, D.; Yde, S.; Cochant-Priollet, B.; Groussin, L.; Sébag, F.; Cagnard, N.; Nitschke, P.; Luton, D.; et al. Borealin/CDCA8 Deficiency Alters Thyroid Development and Results in Papillary Tumor-like Structures. Front. Endocrinol. 2023, 14, 1286747. [Google Scholar] [CrossRef]
- Gordon, J. Hox Genes in the Pharyngeal Region: How Hoxa3 Controls Early Embryonic Development of the Pharyngeal Organs. Int. J. Dev. Biol. 2018, 62, 775–783. [Google Scholar] [CrossRef]
- Nicholas, A.K.; Serra, E.G.; Cangul, H.; Alyaarubi, S.; Ullah, I.; Schoenmakers, E.; Deeb, A.; Habeb, A.M.; Almaghamsi, M.; Peters, C.; et al. Comprehensive Screening of Eight Known Causative Genes in Congenital Hypothyroidism with Gland-in-Situ. J. Clin. Endocrinol. Metab. 2016, 101, 4521–4531. [Google Scholar] [CrossRef]
- Scoville, D.W.; Kang, H.S.; Jetten, A.M. Transcription Factor GLIS3: Critical Roles in Thyroid Hormone Biosynthesis, Hypothyroidism, Pancreatic Beta Cells and Diabetes. Pharmacol. Ther. 2020, 215, 107632. [Google Scholar] [CrossRef]
- Boros, E.; Vilain, C.; Driessens, N.; Heinrichs, C.; Van Vliet, G.; Brachet, C. Hypothyroidism due to Biallelic Variants in IYD: Description of 4 Families and a Novel Variant. Eur. J. Endocrinol. 2024, 191, K5–K9. [Google Scholar] [CrossRef]
- Szinnai, G. Genetics of Normal and Abnormal Thyroid Development in Humans. Best. Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 133–150. [Google Scholar] [CrossRef]
- Fonseca, B.F.; Barbée, C.; Romitti, M.; Eski, S.E.; Gillotay, P.; Monteyne, D.; Morga, D.P.; Refetoff, S.; Singh, S.P.; Costagliola, S. Foxe1 Orchestrates Thyroid and Lung Cell Lineage Divergence in Mouse Stem Cell-Derived Organoids. bioRxiv 2022. [Google Scholar] [CrossRef]
- De Felice, M.; Ovitt, C.; Biffali, E.; Rodriguez-Mallon, A.; Arra, C.; Anastassiadis, K.; Macchia, P.E.; Mattei, M.-G.; Mariano, A.; Schöler, H.; et al. A Mouse Model for Hereditary Thyroid Dysgenesis and Cleft Palate. Nat. Genet. 1998, 19, 395–398. [Google Scholar] [CrossRef]
- Clifton-Bligh, R.J.; Wentworth, J.M.; Heinz, P.; Crisp, M.S.; John, R.; Lazarus, J.H.; Ludgate, M.; Chatterjee, V.K. Mutation of the Gene Encoding Human TTF-2 Associated with Thyroid Agenesis, Cleft Palate and Choanal Atresia. Nat. Genet. 1998, 19, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Gillotay, P.; Romitti, M.; Dassy, B.; Haerlingen, B.; Parakkal Shankar, M.; Faria Fonseca, B.; Ziros, G.P.; Pal Singh, S.; Sykiotis, P.G.; Costagliola, S. Nrf2 Promotes Thyroid Development and Hormone Synthesis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Willemsen, M.A.A.P.; Breedveld, G.J.; Wouda, S.; Otten, B.J.; Yntema, J.L.; Lammens, M.; de Vries, B.B.A. Brain-Thyroid-Lung Syndrome: A Patient with a Severe Multi-System Disorder due to a de Novo Mutation in the Thyroid Transcription Factor 1 Gene. Eur. J. Pediatr. 2005, 164, 28–30. [Google Scholar] [CrossRef]
- Srivastava, P.; Kilian, K.A. Micro-Engineered Models of Development Using Induced Pluripotent Stem Cells. Front. Bioeng. Biotechnol. 2019, 7, 357. [Google Scholar] [CrossRef]
- Turhan, A.G.; Hwang, J.W.; Chaker, D.; Tasteyre, A.; Latsis, T.; Griscelli, F.; Desterke, C.; Bennaceur-Griscelli, A. IPSC-Derived Organoids as Therapeutic Models in Regenerative Medicine and Oncology. Front. Med. 2021, 8, 728543. [Google Scholar] [CrossRef]
- Ye, L.; Swingen, C.; Zhang, J. Induced Pluripotent Stem Cells and Their Potential for Basic and Clinical Sciences. Curr. Cardiol. Rev. 2013, 9, 63–72. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J. The Epidemiology of Thyroid Disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Primers 2022, 8, 30. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef]
- Moroni, L.; Barbaro, F.; Caiment, F.; Coleman, O.; Costagliola, S.; Di Conza, G.; Elviri, L.; Giselbrecht, S.; Krause, C.; Mota, C.; et al. Screened: A Multistage Model of Thyroid Gland Function for Screening Endocrine-Disrupting Chemicals in a Biologically Sex-Specific Manner. Int. J. Mol. Sci. 2020, 21, 3648. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by Stealth—Interference of Endocrine Disrupting Chemicals on Hormonal Crosstalk with Thyroid Axis Function in Humans and Other Animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef] [PubMed]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The Impact of Endocrine-Disrupting Chemical Exposure in the Mammalian Hypothalamic-Pituitary Axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef] [PubMed]
- Nazzari, M.; Romitti, M.; Hauser, D.; Carvalho, D.J.; Giselbrecht, S.; Moroni, L.; Costagliola, S.; Caiment, F. Investigation of the Effects of Phthalates on in Vitro Thyroid Models with RNA-Seq and ATAC-Seq. Front. Endocrinol. 2023, 14, 1200211. [Google Scholar] [CrossRef]
- Nazzari, M.; Romitti, M.; Kip, A.M.; Kamps, R.; Costagliola, S.; van de Beucken, T.; Moroni, L.; Caiment, F. Impact of Benzo[a]Pyrene, PCB153 and Sex Hormones on Human ESC-Derived Thyroid Follicles Using Single Cell Transcriptomics. Environ. Int. 2024, 188, 108748. [Google Scholar] [CrossRef]
- Dew, R.; Okosieme, O.; Dayan, C.; Eligar, V.; Khan, I.; Razvi, S.; Pearce, S.; Wilkes, S. Clinical, Behavioural and Pharmacogenomic Factors Influencing the Response to Levothyroxine Therapy in Patients with Primary Hypothyroidism—Protocol for a Systematic Review. Syst. Rev. 2017, 6, 60. [Google Scholar] [CrossRef]
- Kraut, E.; Farahani, P. A Systematic Review of Clinical Practice Guidelines’ Recommendations on Levothyroxine Therapy Alone versus Combination Therapy (LT4 plus LT3) for Hypothyroidism. Clin. Investig. Med. 2015, 38, E305–E313. [Google Scholar] [CrossRef]
- Watt, T.; Cramon, P.; Hegedüs, L.; Bjorner, J.B.; Bonnema, S.J.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Groenvold, M. The Thyroid-Related Quality of Life Measure ThyPRO Has Good Responsiveness and Ability to Detect Relevant Treatment Effects. J. Clin. Endocrinol. Metab. 2014, 99, 3708–3717. [Google Scholar] [CrossRef]
- Biondi, B.; Wartofsky, L. Treatment with Thyroid Hormone. Endocr. Rev. 2014, 35, 433–512. [Google Scholar] [CrossRef]
- Saravanan, P.; Chau, W.-F.; Roberts, N.; Vedhara, K.; Greenwood, R.; Dayan, C.M. Psychological Well-being in Patients on ‘Adequate’ Doses of l-thyroxine: Results of a Large, Controlled Community-based Questionnaire Study. Clin. Endocrinol. 2002, 57, 577–585. [Google Scholar] [CrossRef]
- Romitti, M.; Costagliola, S. Progress Toward and Challenges Remaining for Thyroid Tissue Regeneration. Endocrinology 2023, 164, bqad136. [Google Scholar] [CrossRef]
- Polanco, A.; Kuang, B.; Yoon, S. Bioprocess Technologies That Preserve the Quality of IPSCs. Trends Biotechnol. 2020, 38, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Posabella, A.; Alber, A.B.; Undeutsch, H.J.; Droeser, R.A.; Hollenberg, A.N.; Ikonomou, L.; Kotton, D.N. Derivation of Thyroid Follicular Cells From Pluripotent Stem Cells: Insights From Development and Implications for Regenerative Medicine. Front. Endocrinol. 2021, 12, 666565. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Girault, N.; Plinet, M.; Bouckenheimer, J.; Sansac, C.; Combe, M.; Mianné, J.; Bourguignon, C.; Fieldes, M.; Ahmed, E.; et al. Recurrent Genetic Abnormalities in Human Pluripotent Stem Cells: Definition and Routine Detection in Culture Supernatant by Targeted Droplet Digital PCR. Stem Cell Rep. 2020, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Choi, J.; Park, N.; Kang, J.; Kim, M.; Kim, Y.; Ju, J.H. Development of Immunocompatible Pluripotent Stem Cells via CRISPR-Based Human Leukocyte Antigen Engineering. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The Current Status and Biomedical Applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef]
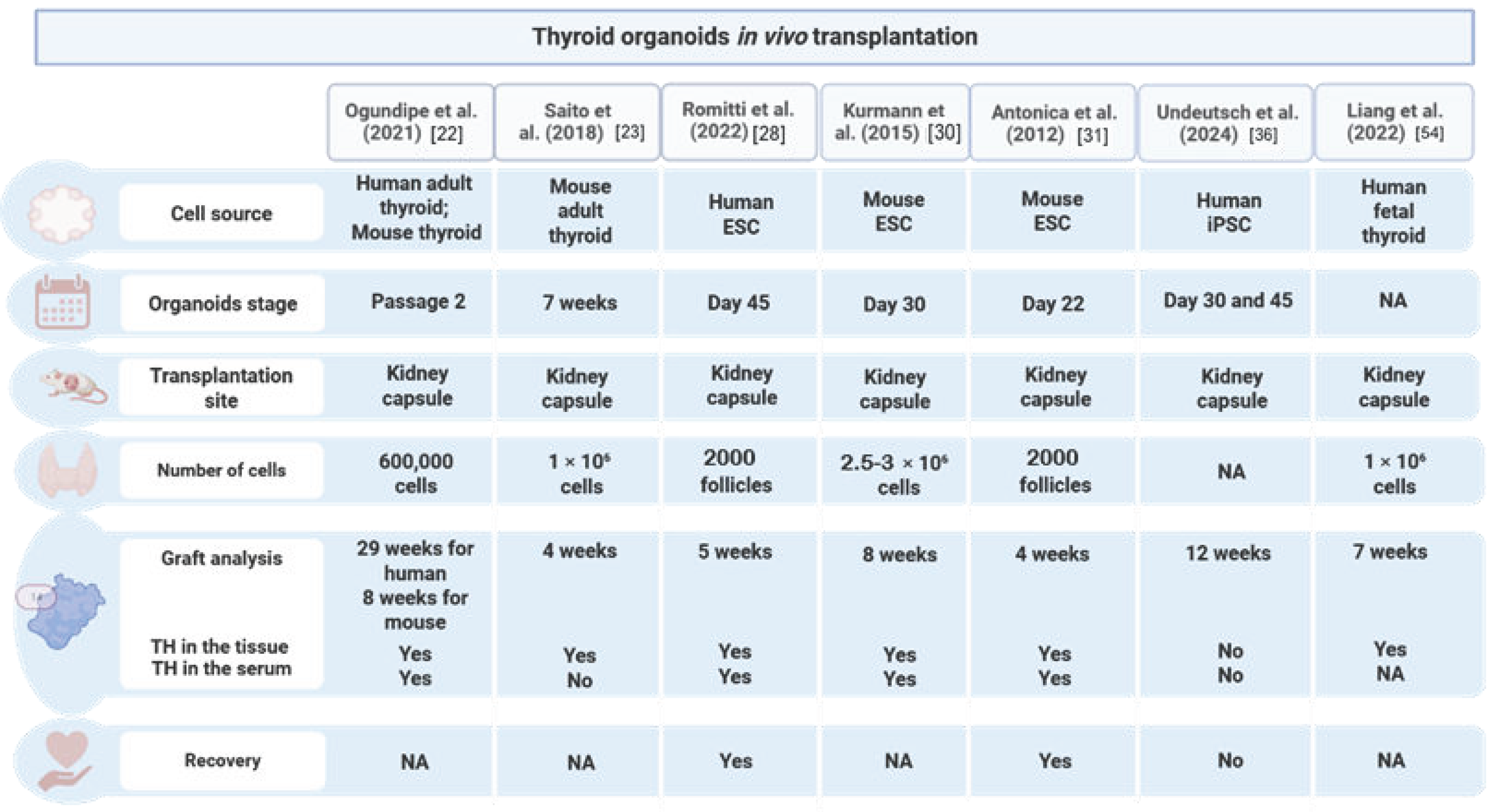
| Study | Cell Source | Type of Differentiation | Specification Efficiency | Total Efficiency | Maturation In Vitro | In Vitro Functionality | Transplantation | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T4 Detection | Tg-I Detection | T4 + Tissue | Systematic Recovery | |||||||
| Romitti et al., 2022 | human-ESCs | Forward programing | ~12% | ~25% | Full | Yes | Yes | Yes | Yes | [28] |
| Longmire et al., 2012 | mouse-ESCs | Directed differentiation * | 16% | ~60% | Partial | NA | NA | NA | NA | [29] |
| Kurmann et al., 2015 | mouse-ESCs | Directed differentiation * | 4.90% | 50% | Partial | No | NA | Yes | Yes | [30] |
| Antonica et al., 2012 | mouse-ESCs | Forward programing | NA | 60.5 ± 8.1% | Full | No | Yes | Yes | Yes | [31] |
| Ma et al., 2013 | mouse-ESCs | Forward programing | NA | NA | Partial | NA | NA | NA | NA | [32] |
| Ma et al., 2015a | mouse-iPSCs | Forward programing | NA | NA | Partial | NA | NA | NA | NA | [33] |
| Ma et al., 2015b | human-ESCs | Forward programing | NA | NA | Partial | NA | NA | NA | NA | [34] |
| Dame et al., 2017 | mouse-ESCs | Forward programing ** | ~5% | NA | Full | Yes | NA | No | No | [35] |
| Undeutsch et al., 2024 | human-iPSCs | Directed differentiation * | 35% | 73.90% | Partial | No | NA | No | No | [36] |
| Ma et al., 2020 | human-iPSCs | Forward programing | 34.29% | 58.35% | Full | Yes | NA | NA | NA | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shankar, M.P.; Boggian, A.; Aparicio-Quiñonez, D.; Djerbib, S.; Rios-Morris, E.; Costagliola, S.; Romitti, M. Functional Thyroid Organoids—Powerful Stem Cell-Derived Models in Basic and Translational Research. Biomolecules 2025, 15, 747. https://doi.org/10.3390/biom15050747
Shankar MP, Boggian A, Aparicio-Quiñonez D, Djerbib S, Rios-Morris E, Costagliola S, Romitti M. Functional Thyroid Organoids—Powerful Stem Cell-Derived Models in Basic and Translational Research. Biomolecules. 2025; 15(5):747. https://doi.org/10.3390/biom15050747
Chicago/Turabian StyleShankar, Meghna Parakkal, Alessandra Boggian, Daniela Aparicio-Quiñonez, Sami Djerbib, Eduardo Rios-Morris, Sabine Costagliola, and Mírian Romitti. 2025. "Functional Thyroid Organoids—Powerful Stem Cell-Derived Models in Basic and Translational Research" Biomolecules 15, no. 5: 747. https://doi.org/10.3390/biom15050747
APA StyleShankar, M. P., Boggian, A., Aparicio-Quiñonez, D., Djerbib, S., Rios-Morris, E., Costagliola, S., & Romitti, M. (2025). Functional Thyroid Organoids—Powerful Stem Cell-Derived Models in Basic and Translational Research. Biomolecules, 15(5), 747. https://doi.org/10.3390/biom15050747






