Embryonic Origins of Cancer: Insights from Double Homeobox 4 Regulation
Abstract
1. Introduction
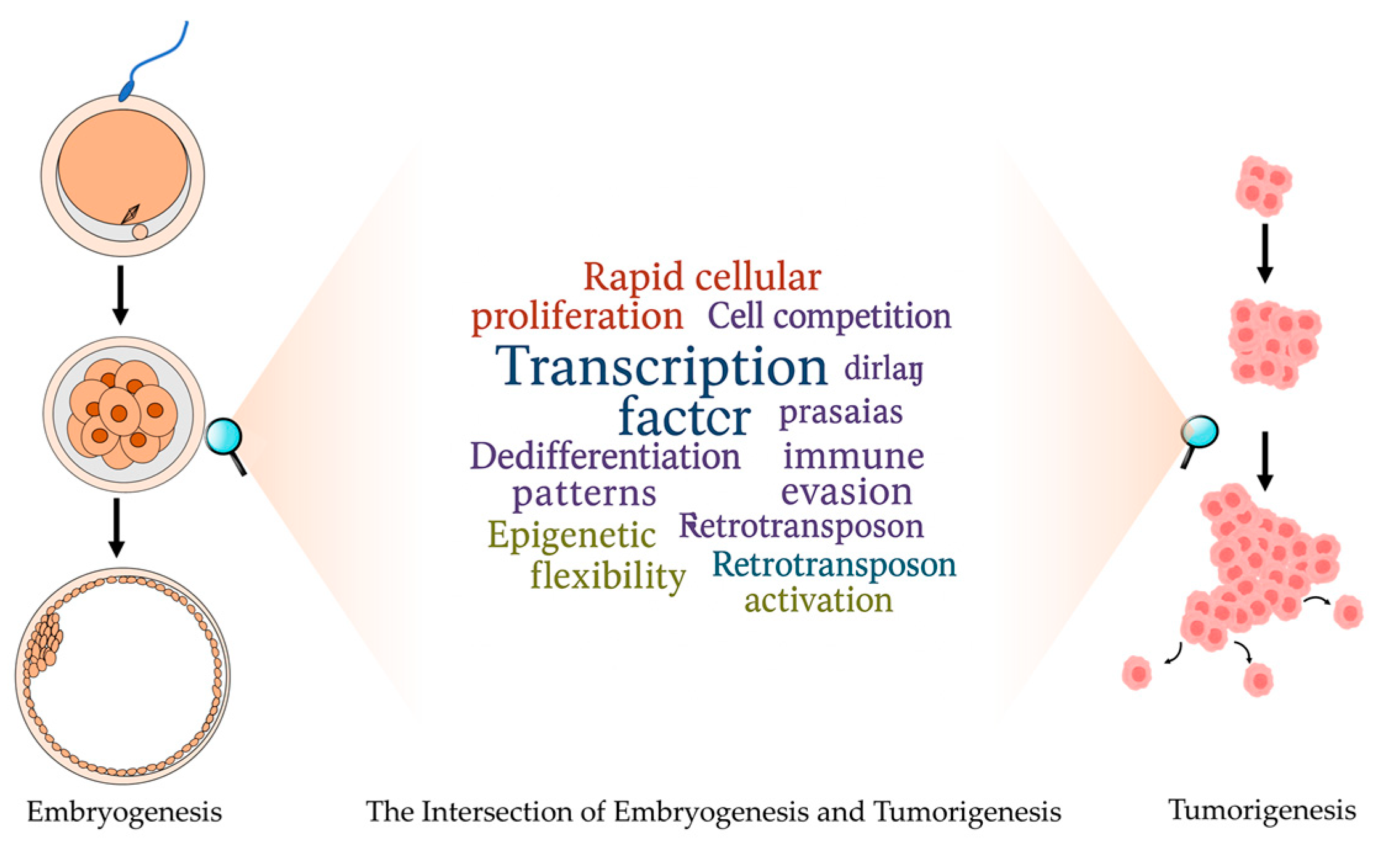
2. The Intersection of Embryogenesis and Tumorigenesis
3. The Role of DUX4 in Early Embryonic Development
4. The Role of DUX4 in Tumorigenesis
5. The Analogous Function of DUX4 Between Embryogenesis and Oncogenesis
6. Clinical Implications of DUX4-Induced Embryonic Features
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendrickson, P.G.; Doráis, J.A.; Grow, E.J.; Whiddon, J.L.; Lim, J.-W.; Wike, C.L.; Weaver, B.D.; Pflueger, C.; Emery, B.R.; Wilcox, A.L. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 2017, 49, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Toso, E.A.; Ener, E.T.; Gearhart, M.D.; Yin, L.; Lüttmann, F.F.; Magli, A.; Shi, K.; Kim, J.; Aihara, H. Antagonism among DUX family members evolved from an ancestral toxic single homeodomain protein. iScience 2023, 26, 107823. [Google Scholar] [CrossRef]
- Wu, J.; Huang, B.; Chen, H.; Yin, Q.; Liu, Y.; Xiang, Y.; Zhang, B.; Liu, B.; Wang, Q.; Xia, W. The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 2016, 534, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Ooga, M.; Fulka, H.; Hashimoto, S.; Suzuki, M.G.; Aoki, F. Analysis of chromatin structure in mouse preimplantation embryos by fluorescent recovery after photobleaching. Epigenetics 2016, 11, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Karpukhina, A.; Tiukacheva, E.; Dib, C.; Vassetzky, Y.S. Control of DUX4 expression in facioscapulohumeral muscular dystrophy and cancer. Trends Mol. Med. 2021, 27, 588–601. [Google Scholar] [CrossRef]
- Mocciaro, E.; Runfola, V.; Ghezzi, P.; Pannese, M.; Gabellini, D. DUX4 role in normal physiology and in FSHD muscular dystrophy. Cells 2021, 10, 3322. [Google Scholar] [CrossRef]
- Snider, L.; Geng, L.N.; Lemmers, R.J.; Kyba, M.; Ware, C.B.; Nelson, A.M.; Tawil, R.; Filippova, G.N.; van der Maarel, S.M.; Tapscott, S.J. Facioscapulohumeral dystrophy: Incomplete suppression of a retrotransposed gene. PLoS Genet. 2010, 6, e1001181. [Google Scholar] [CrossRef]
- Lang, D.; Marwitz, S.; Kugler, C.; Reinmuth, N.; Vollmer, E.; Zabel, P.; Reck, M.; Goldmann, T. Double homeobox protein DUX4 in the human lung: Expression under normal and pathological conditions. GJMR-C 2014, 2, 1–9. [Google Scholar]
- Lee, J.K.; Bosnakovski, D.; Toso, E.A.; Dinh, T.; Banerjee, S.; Bohl, T.E.; Shi, K.; Orellana, K.; Kyba, M.; Aihara, H. Crystal structure of the double homeodomain of DUX4 in complex with DNA. Cell Rep. 2018, 25, 2955–2962. [Google Scholar] [CrossRef]
- Choi, S.H.; Gearhart, M.D.; Cui, Z.; Bosnakovski, D.; Kim, M.; Schennum, N.; Kyba, M. DUX4 recruits p300/CBP through its C-terminus and induces global H3K27 acetylation changes. Nucleic Acids Res. 2016, 44, 5161–5173. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Wahl, G.M. Cancer as a disease of development gone awry. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 397–421. [Google Scholar] [CrossRef]
- Yu, X.; Xu, J. A ‘Goldmine’ for digging cancer-specific targets: The genes essential for embryo development but non-essential for adult life. J. Mol. Cell Biol. 2020, 12, 669–673. [Google Scholar] [CrossRef]
- Aiello, N.M.; Stanger, B.Z. Echoes of the embryo: Using the developmental biology toolkit to study cancer. Dis. Models Mech. 2016, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Sturmey, R.G. Parallels between embryo and cancer cell metabolism. Biochem. Soc. Trans. 2013, 41, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patel, S.; Mirza, S.; Rawal, R.M. Unravelling the link between embryogenesis and cancer metastasis. Gene 2018, 642, 447–452. [Google Scholar] [CrossRef]
- Nagahata, Y.; Kawamoto, H. Evolutionary reversion in tumorigenesis. Front. Oncol. 2023, 13, 1282417. [Google Scholar] [CrossRef] [PubMed]
- Triolo, V.A. Nineteenth century foundations of cancer research origins of experimental research. Cancer Res. 1964, 24, 4–27. [Google Scholar]
- Sell, S. On the stem cell origin of cancer. Am. J. Pathol. 2010, 176, 2584–2594. [Google Scholar] [CrossRef]
- Wahl, G.M.; Spike, B.T. Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. NPJ Breast Cancer 2017, 3, 14. [Google Scholar] [CrossRef]
- Triolo, V.A. Nineteenth century foundations of cancer research advances in tumor pathology, nomenclature, and theories of oncogenesis. Cancer Res. 1965, 25, 75–106. [Google Scholar]
- Pantel, K.; Brakenhoff, R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Penzo-Méndez, A.I.; Stanger, B.Z. Organ-size regulation in mammals. Cold Spring Harb. Perspect. Biol. 2015, 7, a019240. [Google Scholar] [CrossRef]
- Baker, N.E. Emerging mechanisms of cell competition. Nature Rev. Genet. 2020, 21, 683–697. [Google Scholar] [CrossRef]
- Johnston, L.A. Competitive interactions between cells: Death, growth, and geography. Science 2009, 324, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Penzo-Méndez, A.I.; Stanger, B.Z. Cell competition in vertebrate organ size regulation. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Mintz, B.; Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA 1975, 72, 3585–3589. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.C. Experimental production of testicular teratomas in mice. Proc. Natl. Acad. Sci. USA 1964, 52, 654–661. [Google Scholar] [CrossRef]
- Stevens, L.C. Experimental production of testicular teratomas in the mouse. Int. J. Androl. 1981, 4, 54–59. [Google Scholar] [CrossRef]
- Molyneux, G.; Geyer, F.C.; Magnay, F.-A.; McCarthy, A.; Kendrick, H.; Natrajan, R.; MacKay, A.; Grigoriadis, A.; Tutt, A.; Ashworth, A. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 2010, 7, 403–417. [Google Scholar] [CrossRef]
- Krah, N.M.; De La O, J.-P.; Swift, G.H.; Hoang, C.Q.; Willet, S.G.; Chen Pan, F.; Cash, G.M.; Bronner, M.P.; Wright, C.V.; MacDonald, R.J. The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. eLife 2015, 4, e07125. [Google Scholar] [CrossRef]
- Saha, S.K.; Parachoniak, C.A.; Ghanta, K.S.; Fitamant, J.; Ross, K.N.; Najem, M.S.; Gurumurthy, S.; Akbay, E.A.; Sia, D.; Cornella, H. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature 2014, 513, 110–114. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.D. An overview of epithelio-mesenchymal transformation. Cells Tissues Organs 1995, 154, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef]
- Li, J.; Stanger, B.Z. The tumor as organizer model. Science 2019, 363, 1038–1039. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Yin, Y.; Chughtai, M.I.; Tan, B.; Yaseen, A.; Rehman, Z.U. Understanding the immune system in fetal protection and maternal infections during pregnancy. J. Immunol. Res. 2022, 2022, 7567708. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Shiota, G. Immune evasion by cancer stem cells. Regen. Ther. 2021, 17, 20–33. [Google Scholar] [CrossRef]
- Gupta, I.; Hussein, O.; Sastry, K.S.; Bougarn, S.; Gopinath, N.; Chin-Smith, E.; Sinha, Y.; Korashy, H.M.; Maccalli, C. Deciphering the complexities of cancer cell immune evasion: Mechanisms and therapeutic implications. Adv. Cancer Biol.-Metastasis 2023, 8, 100107. [Google Scholar] [CrossRef]
- Kumar, D.; Cinghu, S.; Oldfield, A.J.; Yang, P.; Jothi, R. Decoding the function of bivalent chromatin in development and cancer. Genome Res. 2021, 31, 2170–2184. [Google Scholar] [CrossRef]
- Glancy, E.; Choy, N.; Eckersley-Maslin, M.A. Bivalent chromatin: A developmental balancing act tipped in cancer. Biochem. Soc. Trans. 2024, 52, 217–229. [Google Scholar] [CrossRef]
- Vasilyev, S.; Tolmacheva, E.; Lebedev, I. Epigenetic regulation and role of LINE-1 retrotransposon in embryogenesis. Russ. J. Genet. 2016, 52, 1219–1226. [Google Scholar] [CrossRef]
- Li, W. Retrotransposon Activity and DNA Methylation Control in Bovine Preimplantation Embryos. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2017. [Google Scholar]
- Zhang, R.; Yu, J. LINE-1 retrotransposition L1-FGGY promotes the occurrence and progression of lung squamous cell carcinoma by inhibiting the tumor-suppressor gene FGGY. Cancer Res. 2018, 78, 3096. [Google Scholar] [CrossRef]
- Mustafin, R.N. Influence of retroelements on oncogenes and tumor suppressors in carcinogenesis: A review. J. Mod. Oncol. 2021, 23, 666–673. [Google Scholar] [CrossRef]
- Smith, A.A.; Nip, Y.; Bennett, S.R.; Hamm, D.C.; Lemmers, R.J.; van der Vliet, P.J.; Setty, M.; van der Maarel, S.M.; Tapscott, S.J. DUX4 expression in cancer induces a metastable early embryonic totipotent program. Cell Rep. 2023, 42, 113114. [Google Scholar] [CrossRef]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic genome activation in vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Nykänen, S.; Vuoristo, S. DUX4, the rockstar of embryonic genome activation? Int. J. Dev. Biol. 2024, 68, 161–168. [Google Scholar] [CrossRef]
- Li, Y.; Wu, B.; Liu, H.; Gao, Y.; Yang, C.; Chen, X.; Zhang, J.; Chen, Y.; Gu, Y.; Li, J. Structural basis for multiple gene regulation by human DUX4. Biochem. Biophys. Res. Commun. 2018, 505, 1161–1167. [Google Scholar] [CrossRef]
- Whiddon, J.L.; Langford, A.T.; Wong, C.-J.; Zhong, J.W.; Tapscott, S.J. Conservation and innovation in the DUX4-family gene network. Nat. Genet. 2017, 49, 935–940. [Google Scholar] [CrossRef]
- Taubenschmid-Stowers, J.; Rostovskaya, M.; Santos, F.; Ljung, S.; Argelaguet, R.; Krueger, F.; Nichols, J.; Reik, W. 8C-like cells capture the human zygotic genome activation program in vitro. Cell Stem Cell 2022, 29, 449–459. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhou, D.; Zhang, T.; Chen, X.; Ren, J.; He, C.; Meng, F.; Zhou, Q.; Yang, Q. Telomeres cooperate in zygotic genome activation by affecting DUX4/Dux transcription. iScience 2023, 26, 106158. [Google Scholar] [CrossRef]
- Chew, G.-L.; Campbell, A.E.; De Neef, E.; Sutliff, N.A.; Shadle, S.C.; Tapscott, S.J.; Bradley, R.K. DUX4 suppresses MHC class I to promote cancer immune evasion and resistance to checkpoint blockade. Dev. Cell 2019, 50, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Full, F.; Walter, S.; Neugebauer, E.; Tan, J.; Drayman, N.; Franke, V.; Tay, S.; Landthaler, M.; Akalin, A.; Ensser, A. Herpesviruses mimic zygotic genome activation to promote viral replication. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Knopp, P.; Krom, Y.D.; Banerji, C.R.; Panamarova, M.; Moyle, L.A.; den Hamer, B.; van der Maarel, S.M.; Zammit, P.S. DUX4 induces a transcriptome more characteristic of a less-differentiated cell state and inhibits myogenesis. J. Cell Sci. 2016, 129, 3816–3831. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, R.A.; Wu, W.; Nanjo, S.; Olivas, V.; Lin, Y.K.; Ponce, R.K.; Oyama, R.; Kondo, T.; Bivona, T.G. CIC-DUX4 oncoprotein drives sarcoma metastasis and tumorigenesis via distinct regulatory programs. J. Clin. Investig. 2019, 129, 3401–3406. [Google Scholar] [CrossRef]
- Okimoto, R.A.; Wu, W.; Bivona, T.G. Molecular and functional dissection of the CIC-DUX4 fusion in undifferentiated round cell sarcoma. Proc. Clin. Cancer Res. 2018, 24, 66. [Google Scholar] [CrossRef]
- Watson, S.; Kendall, G.; Delattre, O.; Tirode, F.; Amatruda, J.F. Expression of a human CIC-DUX4 fusion is sufficient to induce malignant small round blue cell tumors in zebrafish: A new model of CIC-DUX4 sarcoma for translational research. Cancer Res. 2016, 76, 4190. [Google Scholar] [CrossRef]
- Ganassi, M.; Figeac, N.; Reynaud, M.; Ortuste Quiroga, H.P.; Zammit, P.S. Antagonism between DUX4 and DUX4c highlights a pathomechanism operating through β-catenin in facioscapulohumeral muscular dystrophy. Front. Cell Dev. Biol. 2022, 10, 802573. [Google Scholar] [CrossRef]
- Preussner, J.; Zhong, J.; Sreenivasan, K.; Günther, S.; Engleitner, T.; Künne, C.; Glatzel, M.; Rad, R.; Looso, M.; Braun, T. Oncogenic amplification of zygotic dux factors in regenerating p53-deficient muscle stem cells defines a molecular cancer subtype. Cell Stem Cell 2018, 23, 794–805. [Google Scholar] [CrossRef]
- Sharma, V.; Harafuji, N.; Belayew, A.; Chen, Y.-W. DUX4 differentially regulates transcriptomes of human rhabdomyosarcoma and mouse C2C12 cells. PLoS ONE 2013, 8, e64691. [Google Scholar] [CrossRef]
- Smith, S.C.; Buehler, D.; Choi, E.-Y.K.; McHugh, J.B.; Rubin, B.P.; Billings, S.D.; Balzer, B.; Thomas, D.G.; Lucas, D.R.; Goldblum, J.R. CIC-DUX sarcomas demonstrate frequent MYC amplification and ETS-family transcription factor expression. Mod. Pathol. 2015, 28, 57–68. [Google Scholar] [CrossRef]
- Vanderplanck, C.; Tassin, A.; Ansseau, E.; Charron, S.; Wauters, A.; Lancelot, C.; Vancutsem, K.; Laoudj-Chenivesse, D.; Belayew, A.; Coppée, F. Overexpression of the double homeodomain protein DUX4c interferes with myofibrillogenesis and induces clustering of myonuclei. Skelet. Muscle 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Chen, Y.W.; Sharma, V.; Narola, J.; Harafuji, N.; Shi, R. Over-expressing DUX4 induces pathways regulating myogenesis and apoptosis. FASEB J. 2011, 25, 359. [Google Scholar]
- Spens, A.E.; Sutliff, N.A.; Bennett, S.R.; Campbell, A.E.; Tapscott, S.J. Human DUX4 and mouse Dux interact with STAT1 and broadly inhibit interferon-stimulated gene induction. Elife 2023, 12, e82057. [Google Scholar] [CrossRef]
- Pineda, J.M.B.; Bradley, R.K. DUX4 is a common driver of immune evasion and immunotherapy failure in metastatic cancers. eLife 2024, 12, RP89017. [Google Scholar] [CrossRef] [PubMed]
- Falco, G.; Lee, S.-L.; Stanghellini, I.; Bassey, U.C.; Hamatani, T.; Ko, M.S. Zscan4: A novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev. Biol. 2007, 307, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Storm, M.P.; Kumpfmueller, B.; Bone, H.K.; Buchholz, M.; Sanchez Ripoll, Y.; Chaudhuri, J.B.; Niwa, H.; Tosh, D.; Welham, M.J. Zscan4 is regulated by PI3-kinase and DNA-damaging agents and directly interacts with the transcriptional repressors LSD1 and CtBP2 in mouse embryonic stem cells. PLoS ONE 2014, 9, e89821. [Google Scholar] [CrossRef]
- Zalzman, M.; Falco, G.; Sharova, L.V.; Nishiyama, A.; Thomas, M.; Lee, S.-L.; Stagg, C.A.; Hoang, H.G.; Yang, H.-T.; Indig, F.E. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 2010, 464, 858–863. [Google Scholar] [CrossRef]
- Meltzer, W.A.; Gupta, A.; Lin, P.N.; Brown, R.A.; Benyamien-Roufaeil, D.S.; Khatri, R.; Mahurkar, A.A.; Song, Y.; Taylor, R.J.; Zalzman, M. Reprogramming Chromosome Ends by Functional Histone Acetylation. Int. J. Mol. Sci. 2024, 25, 3898. [Google Scholar] [CrossRef]
- Jung, J.; Yu, B.; Diaz, A. Group 3 medulloblastomas exploit a transient mechanism of telomere repair mediated by zscan4 which is targetable for therapeutic benefit with brain-penetrant drugs. Neuro-Oncology 2024, 26, viii120. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.D.; Modzelewski, A.J.; Siomi, H. Retrotransposon renaissance in early embryos. Trends Genet. 2024, 40, 39–51. [Google Scholar] [CrossRef]
- Helman, E. Somatic Retrotransposition in the Cancer Genome. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2013. [Google Scholar]
- Criscione, S.W.; Zhang, Y.; Thompson, W.; Sedivy, J.M.; Neretti, N. Transcriptional landscape of repetitive elements in normal and cancer human cells. BMC Genom. 2014, 15, 583. [Google Scholar] [CrossRef]
- Bosnakovski, D.; da Silva, M.T.; Sunny, S.T.; Ener, E.T.; Toso, E.A.; Yuan, C.; Cui, Z.; Walters, M.A.; Jadhav, A.; Kyba, M. A novel P300 inhibitor reverses DUX4-mediated global histone H3 hyperacetylation, target gene expression, and cell death. Sci. Adv. 2019, 5, eaaw7781. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Jin, H.; Dang, Y.; Zhao, P.; Li, S.; Shi, Y.; Wang, S.; Zhang, K. p300/CBP-catalyzed acetylation safeguards minor zygotic genome activation via activating DUX. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dandapat, A.; Hartweck, L.M.; Bosnakovski, D.; Kyba, M. Expression of the human FSHD-linked DUX4 gene induces neurogenesis during differentiation of murine embryonic stem cells. Stem Cells Dev. 2013, 22, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Paino, F.; Mele, L.; Papaccio, G.; Regad, T.; Lombardi, A.; Papaccio, F.; Desiderio, V.; Tirino, V. HDAC2 depletion promotes osteosarcoma’s stemness both in vitro and in vivo: A study on a putative new target for CSCs directed therapy. J. Exp. Clin. Cancer Res. 2018, 37, 296. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Ener, E.T.; Cooper, M.S.; Gearhart, M.D.; Knights, K.A.; Xu, N.C.; Palumbo, C.A.; Toso, E.A.; Marsh, G.P.; Maple, H.J. Inactivation of the CIC-DUX4 oncogene through P300/CBP inhibition, a therapeutic approach for CIC-DUX4 sarcoma. Oncogenesis 2021, 10, 68. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sukumar, S. The Hox genes and their roles in oncogenesis. Nat. Rev. Cancer 2010, 10, 361–371. [Google Scholar] [CrossRef]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Wierstra, I.; Alves, J. FOXM1, a typical proliferation-associated transcription factor. Biol. Chem. 2007, 388, 1257–1274. [Google Scholar] [CrossRef]
| Shared Features of Embryonic and Cancer Cells | Role of DUX4 | Reference |
|---|---|---|
| Uncontrolled proliferation | Promotes proliferation and blocks differentiation | [62,63] |
| Immune evasion | Suppresses MHC I, aiding immune escape | [52,64,65] |
| Undergo early developmental processes | Triggers cleavage-stage transcription | [66,67,68,69,70] |
| Retrotransposon activation | Activates HERV-L | [1,45,71,72,73] |
| Chromatin remodeling | Recruits p300/CBP to drive gene activation | [10,51,74,75] |
| Pluripotency and stemness | Induces totipotent-like state and self-renewal programs | [1,74,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, B.; Ma, H.; Wang, L.; Guo, Z.; Wang, F.; Liu, D.; Zhang, D. Embryonic Origins of Cancer: Insights from Double Homeobox 4 Regulation. Biomolecules 2025, 15, 721. https://doi.org/10.3390/biom15050721
Fu B, Ma H, Wang L, Guo Z, Wang F, Liu D, Zhang D. Embryonic Origins of Cancer: Insights from Double Homeobox 4 Regulation. Biomolecules. 2025; 15(5):721. https://doi.org/10.3390/biom15050721
Chicago/Turabian StyleFu, Bo, Hong Ma, Liang Wang, Zhenhua Guo, Fang Wang, Di Liu, and Dongjie Zhang. 2025. "Embryonic Origins of Cancer: Insights from Double Homeobox 4 Regulation" Biomolecules 15, no. 5: 721. https://doi.org/10.3390/biom15050721
APA StyleFu, B., Ma, H., Wang, L., Guo, Z., Wang, F., Liu, D., & Zhang, D. (2025). Embryonic Origins of Cancer: Insights from Double Homeobox 4 Regulation. Biomolecules, 15(5), 721. https://doi.org/10.3390/biom15050721






