Role of Tumor Necrosis Factor in Tuberculosis
Abstract
1. Introduction
2. Tumor Necrosis Factor and Its Receptors
Tumor Necrosis Factor and Its Receptors in Tuberculosis
3. Levels of TNF and Its Membrane and Soluble Receptors in Tuberculosis
3.1. Levels of TNF and Its Soluble Receptors in Tuberculosis
3.2. Expression of Type 1 and Type 2 TNF Membrane Receptors in Tuberculosis
4. Stimulation of TNF Production by Mycobacterial Components
5. Protective Effects of TNF During Tuberculosis Infection
5.1. Role of TNF in Apoptosis of M. tuberculosis-Infected Macrophages
5.2. Role of TNF in the Immunometabolism of Macrophages Infected by M. tuberculosis
5.3. Role of TNF in the Maturation of Dendritic Cells
5.4. TNF Stimulates the Production of Chemokines and Adhesion Molecules
5.5. Role of TNF in Granuloma Formation and Maintenance
6. Anti-TNF Therapy in Autoimmune Diseases and Tuberculosis
6.1. Negative Effects of Anti-TNF Therapy on Antituberculous Immunity
6.2. Anti-TNF Therapy in Autoimmune Disease and Tuberculosis Reactivation
7. The Role of the Membrane Form of TNF in the Immune Response to Tuberculosis
8. Role of TNF of Myeloid and T Cell Origin in the Immune Response in Tuberculosis
9. Effect of Drug-Resistant M. tuberculosis Strains on TNF-Mediated Immunity
10. Dual Role of TNF in Protection and Pathogenesis
11. TNF Anti-Inflammatory Activity
12. Autoantibodies to TNF in Tuberculosis
13. Tuberculosis Cases in Patients with TNF Deficiency
14. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2024. 2024. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024/ (accessed on 19 February 2025).
- Menzies, N.A.; Swartwood, N.; Testa, C.; Malyuta, Y.; Hill, A.N.; Marks, S.M.; Cohen, T.; Salomon, J.A. Time Since Infection and Risks of Future Disease for Individuals with Mycobacterium tuberculosis Infection in the United States. Epidemiology 2021, 32, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16077. [CrossRef] [PubMed]
- Pai, M. Tuberculosis: The story after the Primer. Nat. Rev. Dis. Primers 2020, 6, 29. [Google Scholar] [CrossRef]
- Mootoo, A.; Stylianou, E.; Arias, M.A.; Reljic, R. TNF-α in tuberculosis: A cytokine with a split personality. Inflamm. Allergy Drug Targets 2009, 8, 53–62. [Google Scholar] [CrossRef]
- Dorhoi, A.; Kaufmann, S.H.E. Tumor necrosis factor alpha in mycobacterial infection. Semin. Immunol. 2014, 26, 203–209. [Google Scholar] [CrossRef]
- Yuk, J.-M.; Kim, J.K.; Kim, I.S.; Jo, E.-K. TNF in Human Tuberculosis: A Double-Edged Sword. Immune Netw. 2024, 24, e4. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Pennica, D.; Nedwin, G.E.; Hayflick, J.S.; Seeburg, P.H.; Derynck, R.; Palladino, M.A.; Kohr, W.J.; Aggarwal, B.B.; Goeddel, D.V. Human tumour necrosis factor: Precursor structure, expression and homology to lymphotoxin. Nature 1984, 312, 724–729. [Google Scholar] [CrossRef]
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S.D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: Ramifications for the complex physiology of TNF. Cell 1988, 53, 45–53. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Gooz, M. ADAM-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 1997, 45, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.; Davis, M. TNF receptor 2 pathway: Drug target for autoimmune diseases. Nat. Rev. Drug Discov. 2010, 9, 482–493. [Google Scholar] [CrossRef]
- Dopp, J.M.; Sarafian, T.A.; Spinella, F.M.; Kahn, M.A.; Shau, H.; de Vellis, J. Expression of the p75 TNF receptor is linked to TNF-induced NFκB translocation and oxyradical neutralization in glial cells. Neurochem. Res. 2002, 27, 1535–1542. [Google Scholar] [CrossRef]
- Kuno, R.; Yoshida, Y.; Nitta, A.; Nabeshima, T.; Wang, J.; Sonobe, Y.; Kawanokuchi, J.; Takeuchi, H.; Mizuno, T.; Suzumura, A. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res. 2006, 1116, 12–18. [Google Scholar] [CrossRef]
- Ware, C.F.; Crowe, P.D.; Vanarsdale, T.L.; Andrews, J.L.; Grayson, M.H.; Jerzy, R.; Smith, C.A.; Goodwin, R.G. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J. Immunol. 1991, 147, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Alshevskaya, A.A.; Kireev, F.D.; Laushkina, Z.A.; Lopatnikova, J.A.; Gladkikh, V.S.; Sennikova, J.A.; Karaulov, A.V.; Sennikov, S.V. Enhanced expression of TNF-α type-1 receptors by immune cells in active pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2018, 22, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Fallahi-Sichani, M.; Schaller, M.A.; Kirschner, D.E.; Kunkel, S.L.; Linderman, J.J. Identification of key processes that control tumor necrosis factor availability in a tuberculosis granuloma. PLoS Comput. Biol. 2010, 6, e1000778. [Google Scholar] [CrossRef]
- Irwin, M.R.; Mak, S.; Mann, D.L.; Qu, R.; Penninger, J.M.; Yan, A.; Dawood, F.; Wen, W.H.; Shou, Z.; Liu, P. Tissue expression and immunolocalization of tumor necrosis factor-α in postinfarction dysfunctional myocardium. Circulation 1999, 99, 1492–1498. [Google Scholar] [CrossRef]
- Grell, M.; Becke, F.M.; Wajant, H.; Männel, D.N.; Scheurich, P. Tumor necrosis factor (TNF) receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur. J. Immunol. 1998, 28, 257–263. [Google Scholar] [CrossRef]
- Pan, S.; An, P.; Zhang, R.; He, X.; Yin, G.; Min, W. Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: Role in endothelial cell migration and angiogenesis. Mol. Cell. Biol. 2002, 22, 7512–7523. [Google Scholar] [CrossRef] [PubMed]
- Beldi, G.; Khosravi, M.; Abdelgawad, M.E.; Salomon, B.L.; Uzan, G.; Haouas, H.; Naserian, S. TNFα/TNFR2 signaling pathway: An active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res. Ther. 2020, 11, 281. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Hsu, H.; Shu, H.B.; Pan, M.G.; Goeddel, D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996, 84, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-J.; Chio, I.I.C.; Lin, W.-J.; Duncan, G.; Chau, H.; Katz, D.; Huang, H.-L.; Pike, K.A.; Hao, Z.; Su, Y.-W.; et al. Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 12429–12434. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Lazo, P.S. Signal transduction by tumor necrosis factor receptors. Cell. Signal. 2012, 24, 1297–1305. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Grell, M.; Douni, E.; Wajant, H.; Löhden, M.; Clauss, M.; Maxeiner, B.; Georgopoulos, S.; Lesslauer, W.; Kollias, G.; Pfizenmaier, K.; et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 1995, 83, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Marra, L.E.; Zhang, Z.X.; Joe, B.; Campbell, J.; Levy, G.A.; Penninger, J.; Zhang, L. IL-10 induces regulatory T cell apoptosis by up-regulation of the membrane form of TNF-α. J. Immunol. 2004, 172, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, B.; Li, X.; Zhao, X.; Wan, L.; Lin, G.; Yu, M.; Wang, J.; Jiang, X.; Feng, W.; et al. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J. Immunol. 2014, 192, 1320–1331. [Google Scholar] [CrossRef]
- Grell, M.; Zimmermann, G.; Gottfried, E.; Chen, C.M.; Grünwald, U.; Huang, D.C.; Lee, Y.H.W.; Dürkop, H.; Engelmann, H.; Scheurich, P.; et al. Induction of cell death by tumour necrosis factor (TNF) receptor 2,CD40 and CD30: A role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 1999, 18, 3034–3043. [Google Scholar] [CrossRef] [PubMed]
- Fotin-Mleczek, M.; Henkler, F.; Samel, D.; Reichwein, M.; Hausser, A.; Parmryd, I.; Scheurich, P.; Schmid, J.A.; Wajant, H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J. Cell Sci. 2002, 115, 2757–2770. [Google Scholar] [CrossRef]
- Faustman, D.L.; Davis, M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front. Immunol. 2013, 4, 478. [Google Scholar] [CrossRef]
- Vassalli, P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 1992, 10, 411–452. [Google Scholar] [CrossRef]
- Laskov, R.; Lancz, G.; Ruddle, N.H.; McGrath, K.M.; Specter, S.; Klein, T.; Djeu, J.Y.; Friedman, H. Production of tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) by murine pre-B and B cell lymphomas. J. Immunol. 1990, 144, 3424–3430. [Google Scholar] [CrossRef]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 1990, 346, 274–276. [Google Scholar] [CrossRef]
- Dubravec, D.B.; Spriggs, D.R.; Mannick, J.A.; Rodrick, M.L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 1990, 87, 6758–6761. [Google Scholar] [CrossRef]
- Sennikov, S.V.; Krysov, S.V.; Silkov, A.N.; Injelevskaya, T.V.; Kozlov, V.A. Production of IL-10, TNF-alpha; IFN-gamma, TGF-beta1 by different populations of erythroid cells derived from human embryonal liver. Cytokine 2002, 17, 221–225. [Google Scholar] [CrossRef]
- Köck, A.; Schwarz, T.; Kirnbauer, R.; Urbanski, A.; Perry, P.; Ansel, J.C.; Luger, T.A. Human keratinocytes are a source for tumor necrosis factor alpha: Evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J. Exp. Med. 1990, 172, 1609–1614. [Google Scholar] [CrossRef]
- Sawada, M.; Kondo, N.; Suzumura, A.; Marunouchi, T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989, 491, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.J.; Libby, P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J. Immunol. 1989, 142, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Keshav, S.; Lawson, L.; Chung, L.P.; Stein, M.; Perry, V.H.; Gordon, S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J. Exp. Med. 1990, 171, 327–332. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Baud, L.; Oudinet, J.P.; Bens, M.; Noe, L.; Peraldi, M.N.; Rondeau, E.; Etienne, J.; Ardaillou, R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989, 35, 1111–1118. [Google Scholar] [CrossRef]
- Sugarman, B.J.; Aggarwal, B.B.; Hass, P.E.; Figari, I.S.; Palladino, M.A.; Shepard, H.M. Recombinant human tumor necrosis factor-alpha: Effects on proliferation of normal and transformed cells in vitro Science. Science, 1985; 230, 943–945. [Google Scholar]
- Selmaj, K.W.; Farooq, M.; Norton, W.T.; Raine, C.S.; Brosnan, C.F. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J. Immunol. 1990, 144, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Branch, D.R.; Turner, A.R.; Guilbert, L.J. Synergistic stimulation of macrophage proliferation by the monokines tumor necrosis factor-alpha and colony-stimulating factor 1. Blood 1989, 73, 307–311. [Google Scholar] [CrossRef]
- Kehrl, J.H.; Miller, A.; Fauci, A.S. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J. Exp. Med. 1987, 166, 786–791. [Google Scholar] [CrossRef]
- Shalaby, M.R.; Espevik, T.; Rice, G.C.; Ammann, A.J.; Figari, I.S.; E Ranges, G.; A Palladino, M. The involvement of human tumor necrosis factors-alpha and -beta in the mixed lymphocyte reaction. J. Immunol. 1988, 141, 499–503. [Google Scholar] [CrossRef]
- Scheurich, P.; Thoma, B.; Ucer, U.; Pfizenmaier, K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: Induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J. Immunol. 1987, 138, 1786–1790. [Google Scholar] [CrossRef]
- Yokota, S.; Geppert, T.D.; Lipsky, P.E. Enhancement of antigen- and mitogen-induced human T lymphocyte proliferation by tumor necrosis factor-alpha. J. Immunol. 1988, 140, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.R.; Gifford, G.E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J. Immunol. 1980, 125, 1671–1677. [Google Scholar] [CrossRef]
- Schuger, L.; Varani, J.; Marks, R.M.; Kunkel, S.L.; Johnson, K.J.; Ward, P.A. Cytotoxicity of tumor necrosis factor-alpha for human umbilical vein endothelial cells. Lab. Investig. 1989, 61, 62–68. [Google Scholar]
- Sato, N.; Goto, T.; Haranaka, K.; Satomi, N.; Nariuchi, H.; Mano-Hirano, Y.; Sawasaki, Y. Actions of tumor necrosis factor on cultured vascular endothelial cells: Morphologic modulation, growth inhibition, and cytotoxicity. J. Natl. Cancer Inst. 1986, 76, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Selmaj, K.W.; Raine, C.S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol. 1988, 23, 339–346. [Google Scholar] [CrossRef]
- Taverne, J.; Rayner, D.C.; van der Meide, P.H.; Lydyard, P.M.; Bidey, S.P.; Cooke, A. Cytotoxicity of tumor necrosis factor for thyroid epithelial cells and its regulation by interferon-γ. Eur. J. Immunol. 1987, 17, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Palombella, V.J.; Vilcek, J. Mitogenic and cytotoxic actions of tumor necrosis factor in BALB/c 3T3 cells: Role of phospholipase activation. J. Biol. Chem. 1989, 264, 18128–18136. [Google Scholar] [CrossRef]
- Tracey, K.J.; Beutler, B.; Lowry, S.F.; Merryweather, J.; Wolpe, S.; Milsark, I.W.; Hariri, R.J.; Fahey, T.J.; Zentella, A.; Albert, J.D.; et al. Shock and tissue injury induced by recombinant human cachectin. Science 1986, 234, 470–474. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Bowersox, O.; Tribble, H.; Lee, S.H.; Shepard, H.M.; Liggitt, D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am. J. Pathol. 1987, 128, 410–425. [Google Scholar] [PubMed]
- Tracey, K.J.; Wei, H.; Manogue, K.R.; Fong, Y.; Hesse, D.G.; Nguyen, H.T.; Kuo, G.C.; Beutler, B.; Cotran, R.S.; Cerami, A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J. Exp. Med. 1988, 167, 1211–1227. [Google Scholar] [CrossRef]
- Remick, D.G.; Kunkel, S.L. Pathophysiologic alterations induced by tumor necrosis factor. Int. Rev. Exp. Pathol. 1993, 34, 7–25. [Google Scholar] [CrossRef]
- Feldmann, M.; Brennan, F.M.; Elliott, M.J.; Williams, R.O.; Maini, R.N. TNF α is an effective therapeutic target for rheumatoid arthritis. Ann. N. Y. Acad. Sci. 1995, 766, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Cerami, A. The biology of cachectin/TNF—A primary mediator of the host response. Annu. Rev. Immunol. 1989, 7, 625–655. [Google Scholar] [CrossRef] [PubMed]
- Kollias, G.; Douni, E.; Kassiotis, G.; Kontoyiannis, D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol. Rev. 1999, 169, 175–194. [Google Scholar] [CrossRef]
- Apostolaki, M.; Armaka, M.; Victoratos, P.; Kollias, G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr. Dir. Autoimmun. 2010, 11, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Probert, L.; Akassoglou, K.; Pasparakis, M.; Kontogeorgos, G.; Kollias, G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 1995, 92, 11294–11298. [Google Scholar] [CrossRef]
- Akassoglou, K.; Probert, L.; Kontogeorgos, G.; Kollias, G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 1997, 158, 438–445. [Google Scholar] [CrossRef]
- Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002, 2, 364–371. [Google Scholar] [CrossRef]
- Palazzi, C.; D’Angelo, S.; Gilio, M.; Leccese, P.; Padula, A.; Olivieri, I. Pharmacological therapy of spondyloarthritis. Expert. Opin. Pharmacother. 2015, 16, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, K.A.; Targan, S.R. Tumor necrosis factor: Biology and therapeutic inhibitors. Gastroenterology 2000, 119, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Kruithof, E.; van den Bosch, F.; van den Bossche, N.; Herssens, A.; Mielants, H.; de Keyser, F.; Veys, E.M. Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: A new perspective on the role of host defence in the pathogenesis of the disease? Ann. Rheum. Dis. 2003, 62, 829–834. [Google Scholar] [CrossRef]
- Mohan, A.K.; Coté, T.R.; Siegel, J.N.; Braun, M.M. Infectious complications of biologic treatments of rheumatoid arthritis. Curr. Opin. Rheumatol. 2003, 15, 179–184. [Google Scholar] [CrossRef]
- Giroir, B.P.; Johnson, J.H.; Brown, T.; Allen, G.L.; Beutler, B. The tissue distribution of tumor necrosis factor biosynthesis during endotoxemia. J. Clin. Investig. 1992, 90, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, J.D.; Riminton, D.S.; Cyster, J.G.; Körner, H. Tumor necrosis factor: A master-regulator of leukocyte movement. Immunol. Today 2000, 21, 110–113. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Tumanov, A.V.; Liepinsh, D.J.; Kruglov, A.A.; Marakusha, B.I.; Shakhov, A.N.; Murakami, T.; Drutskaya, L.N.; Förster, I.; Clausen, B.E.; et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: Protective and deleterious effects. Immunity 2005, 22, 93–104. [Google Scholar] [CrossRef]
- Engelmann, H.; Aderka, D.; Rubinstein, M.; Rotman, D.; Wallach, D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J. Biol. Chem. 1989, 264, 11974–11980. [Google Scholar] [CrossRef]
- Engelmann, H.; Novick, D.; Wallach, D. Two tumor necrosis factor-binding proteins purified from human urine: Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J. Biol. Chem. 1990, 265, 1531–1536. [Google Scholar] [CrossRef]
- Seckinger, P.; Isaaz, S.; Dayer, J.M. Purification and biologic characterization of a specific tumor necrosis factor α inhibitor. J. Biol. Chem. 1989, 264, 11966–11973. [Google Scholar] [CrossRef]
- Olsson, I.; Lantz, M.; Nilsson, E.; Peetre, C.; Thysell, H.; Grubb, A.; Adolf, G. Isolation and characterization of a tumor necrosis factor binding protein from urine. Eur. J. Haematol. 1989, 42, 270–275. [Google Scholar] [CrossRef]
- Nophar, Y.; Kemper, O.; Brakebusch, C.; Englemann, H.; Zwang, R.; Aderka, D.; Holtmann, H.; Wallach, D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990, 9, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Peschon, J.J.; Slack, J.L.; Reddy, P.; Stocking, K.L.; Sunnarborg, S.W.; Lee, D.C.; Russell, W.E.; Castner, B.J.; Johnson, R.S.; Fitzner, J.N.; et al. An essential role for ectodomain shedding in mammalian development. Science 1998, 282, 1281–1284. [Google Scholar] [CrossRef]
- Seitz, C.; Muller, P.; Krieg, R.C.; Mannel, D.N.; Hehlgans, T. A novel p75TNF receptor isoform mediating NFκB activation. J. Biol. Chem. 2001, 276, 19390–19395. [Google Scholar] [CrossRef] [PubMed]
- Lainez, B.; Fernandez-Real, J.M.; Romero, X.; Esplugues, E.; Cañete, J.D.; Ricart, W.; Engel, P. Identification and characterization of a novel spliced variant that encodes human soluble tumor necrosis factor receptor 2. Int. Immunol. 2004, 16, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; Straczkowski, M.; Lainez, B.; Chacón, M.R.; Kowalska, I.; López-Bermejo, A.; García-España, A.; Nikolajuk, A.; Kinalska, I.; Ricart, W. An alternative spliced variant of circulating soluble tumor necrosis factor-α receptor-2 is paradoxically associated with insulin action. Eur. J. Endocrinol. 2006, 154, 723–730. [Google Scholar] [CrossRef][Green Version]
- Fernández-Real, J.M.; Botas-Cervero, P.; Lainez, B.; Ricart, W.; Delgado, E. An alternatively spliced soluble TNF-α receptor is associated with metabolic disorders: A replication study. Clin. Immunol. 2006, 121, 236–241. [Google Scholar] [CrossRef]
- Esteve, E.; Botas, P.; Delgado, E.; López-Bermejo, A.; Lainez, B.; Engel, P.; Ricart, W.; Fernández-Real, J.M. Soluble TNF-alpha receptor 2 produced by alternative splicing is paradoxically associated with markers of liver injury. Clin. Immunol. 2007, 123, 89–94. [Google Scholar] [CrossRef]
- Sennikov, S.V.; Silkov, A.N.; Kozlov, V.A. The role of alternative splicing of cytokine genes in formation of the polymorphic structure of the cytokine network. Med. Immunol. 2001, 3, 389–400. [Google Scholar]
- Gregory, A.P.; Dendrou, C.A.; Attfield, K.E.; Haghikia, A.; Xifara, D.K.; Butter, F.; Poschmann, G.; Kaur, G.; Lambert, L.; Leach, O.A.; et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 2012, 488, 508–511. [Google Scholar] [CrossRef]
- Rittore, C.; Sanchez, E.; Soler, S.; Barat-Houari, M.; Albers, M.; Obici, I.; McDermott, M.F.; Touitou, I.; Grandemange, S. Identification of a new exon 2-skipped TNFR1 transcript: Regulation by three functional polymorphisms of the TNFR-associated periodic syndrome (TRAPS) gene. Ann. Rheum. Dis. 2014, 73, 290–297. [Google Scholar] [CrossRef]
- Aderka, D.; Engelmann, H.; Shemer-Avni, Y.; Hornik, V.; Galil, A.; Sarov, B.; Wallach, D. Variation in serum levels of the soluble TNF receptors among healthy individuals. Lymphokine Cytokine Res. 1992, 11, 157–159. [Google Scholar] [PubMed]
- Aderka, D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor. Rev. 1996, 7, 231–240. [Google Scholar] [CrossRef]
- Piguet, P.F.; Grau, G.E.; Hauser, C.; Vassalli, P. Tumor necrosis factor is a critical mediator in hapten induced irritant and contact hypersensitivity reactions. J. Exp. Med. 1991, 173, 673–679. [Google Scholar] [CrossRef]
- Collart, M.A.; Belin, D.; Vassalli, J.D.; de Kossodo, S.; Vassalli, P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J. Exp. Med. 1986, 164, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Nedwin, G.E.; Svedersky, L.P.; Bringman, T.S.; Palladino, M.A., Jr.; Goeddel, D.V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J. Immunol. 1985, 135, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rivera, G.L.; Madera-Sandoval, R.L.; León-Pedroza, J.I.; Ferat-Osorio, E.; Salazar-Rios, E.; Hernández-Aceves, J.A.; Guadarrama-Aranda, U.; López-Macías, C.; Wong-Baeza, I.; Arriaga-Pizano, L.A. Increased TNF-α production in response to IL-6 in patients with systemic inflammation without infection. Clin. Exp. Immunol. 2022, 209, 225–235. [Google Scholar] [CrossRef]
- Jovanovic, D.V.; Di Battista, J.A.; Martel-Pelletier, J.; Jolicoeur, F.C.; He, Y.; Zhang, M.; Mineau, F.; Pelletier, J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 1998, 160, 3513–3521. [Google Scholar] [CrossRef]
- Philip, R.; Epstein, L.B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, γ-interferon and interleukin-1. Nature 1986, 323, 86–89. [Google Scholar] [CrossRef]
- Kindler, V.; Sappino, A.P.; Grau, G.E.; Piguet, P.F.; Vassalli, P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989, 56, 731–740. [Google Scholar] [CrossRef]
- Chatenoud, L.; Ferran, C.; Reuter, A.; Legendre, C.; Gevaert, Y.; Kreis, H.; Franchimont, P.; Bach, J.F. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma. N. Engl. J. Med. 1989, 320, 1420–1421. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, D.; Schandene, L.; Goldman, M.; Crusiaux, A.; Vereerstraeten, P.; De Pauw, L.; Wybran, J.; Kinnaert, P.; Dupont, E.; Toussaint, C. Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation 1989, 47, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Yocum, D.E.; Esparza, L.; Dubry, S.; Benjamin, J.B.; Volz, R.; Scuderi, P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell. Immunol. 1989, 122, 131–145. [Google Scholar] [CrossRef]
- Hofman, F.M.; Hinton, D.R.; Johnson, K.; Merrill, J.E. Tumor necrosis factor identified in multiple sclerosis brain. J. Exp. Med. 1989, 170, 607–612. [Google Scholar] [CrossRef]
- Maury, C.P.; Teppo, A.M. Tumor necrosis factor in the serum of patients with systemic lupus erythematosus. Arthritis Rheum. 1989, 32, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Maury, C.P.; Teppo, A.M. Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. J. Intern. Med. 1989, 225, 333–336. [Google Scholar] [CrossRef]
- Watts, A.D.; Hunt, N.H.; Wanigasekara, Y.; Bloomfield, G.; Wallach, D.; Roufogalis, B.D.; Chaudhri, G. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in ‘reverse signalling’. EMBO J. 1999, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Eissner, G.; Kolch, W.; Scheurich, P. Ligands working as receptors: Reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor. Rev. 2004, 15, 353–366. [Google Scholar] [CrossRef]
- Deveci, F.; Akbulut, H.H.; Turgut, T.; Muz, M.H. Changes in serum cytokine levels in active tuberculosis with treatment. Mediat. Inflamm. 2005, 2005, 256–262. [Google Scholar] [CrossRef]
- Sampath, P.; Rajamanickam, A.; Thiruvengadam, K.; Natarajan, A.P.; Hissar, S.; Dhanapal, M.; Thangavelu, B.; Jayabal, L.; Ramesh, P.M.; Ranganathan, U.D.; et al. Cytokine upsurge among drug-resistant tuberculosis endorse the signatures of hyper inflammation and disease severity. Sci. Rep. 2023, 13, 785. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Mahmoudi, H. Evaluation of TNF-α cytokine production in patients with tuberculosis compared to healthy people. GMS Hyg. Infect. Control 2018, 13, Doc09. [Google Scholar] [CrossRef]
- Olobo, J.O.; Geletu, M.; Demissie, A.; Eguale, T.; Hiwot, K.; Aderaye, G.; Britton, S. Circulating TNF-α, TGF-β, and IL-10 in Tuberculosis Patients and Healthy Contacts. Scand. J. Immunol. 2001, 53, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, I.V.; Churilov, L.P.; Robertovn, I.; Nikolaev, A.V.; Starshinova, A.A.; Yablonsky, P.K. Vitamin D, Cathelicidin, Prolactin, Autoantibodies, and Cytokines in Different Forms of Pulmonary Tuberculosis versus Sarcoidosis. Isr. Med. Assoc. J. 2017, 19, 499–505. [Google Scholar]
- Kireev, F.D.; Lopatnikova, J.A.; Laushkina, Z.A.; Sennikov, S.V. Autoantibodies to Tumor Necrosis Factor in Patients with Active Pulmonary Tuberculosis. Front. Biosci. Landmark Ed. 2022, 27, 133. [Google Scholar] [CrossRef]
- Vankayalapati, R.; Wizel, B.; Weis, S.E.; Klucar, P.; Shams, H.; Samten, B.; Barnes, P.F. Serum cytokine concentrations do not parallel Mycobacterium tuberculosis-induced cytokine production in patients with tuberculosis. Clin. Infect. Dis. 2003, 36, 24–28. [Google Scholar] [CrossRef]
- Júnior, D.R.A.; Santos, S.A.; de Castro, I.; Andrade, D.R. Correlation between serum tumor necrosis factor alpha levels and clinical severity of tuberculosis. Braz. J. Infect. Dis. 2008, 12, 226–233. [Google Scholar] [CrossRef]
- Shepelkova, G.S.; Evstifeev, V.V.; Berezovskiy, Y.S.; Ergeshova, A.E.; Tarasov, R.V.; Bagirov, M.A.; Yeremeev, V.V. Characteristics of Pulmonary Inflammation in Patients with Different Forms of Active Tuberculosis. Int. J. Mol. Sci. 2024, 25, 11795. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Reed, M.B.; Freeman, S.; Mathema, B.; Kreiswirth, B.; Barry, C.E.; Kaplan, G. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 2004, 72, 5511–5514. [Google Scholar] [CrossRef]
- Theus, S.A.; Cave, M.D.; Eisenach, K.D. Intracellular macrophage growth rates and cytokine profiles of Mycobacterium tuberculosis strains with different transmission dynamics. J. Infect. Dis. 2005, 191, 453–460. [Google Scholar] [CrossRef]
- Krishnan, N.; Malaga, W.; Constant, P.; Caws, M.; Chau, T.T.H.; Salmons, J.; Lan, N.T.N.; Bang, N.D.; Daffé, M.; Young, D.B.; et al. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS ONE 2011, 6, e23870. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, N.; López, B.; Geffner, L.; García, C.S.Y.; Schierloh, P.; Barrera, L.; de la Barrera, S.; Sakai, S.; Kawamura, I.; Mitsuyama, M.; et al. Two genetically-related multidrug-resistant Mycobacterium tuberculosis strains induce divergent outcomes of infection in two human macrophage models. Infect. Genet. Evol. 2013, 16, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Nau, G.J.; Richmond, J.F.; Schlesinger, A.; Jennings, E.G.; Lander, E.S.; Young, R.A. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 2002, 99, 1503–1508. [Google Scholar] [CrossRef]
- Dao, D.N.; Sweeney, K.; Hsu, T.; Gurcha, S.S.; Nascimento, I.P.; Roshevsky, D.; Besra, G.S.; Chan, J.; Porcelli, S.A.; Jacobs, W.R., Jr. Mycolic acid modification by the mmaA4 gene of M. tuberculosis modulates IL-12 production. PLoS Pathog. 2008, 4, e1000081. [Google Scholar] [CrossRef]
- Henao, J.; Sánchez, D.; Muñoz, C.H.; Mejía, N.; Arias, M.A.; García, L.F.; Barrera, L.F. Human splenic macrophages as a model for in vitro infection with Mycobacterium tuberculosis. Tuberculosis 2007, 87, 509–517. [Google Scholar] [CrossRef]
- Silver, R.F.; Li, Q.; Ellner, J.J. Expression of virulence of Mycobacterium tuberculosis within human monocytes: Virulence correlates with intracellular growth and induction of tumor necrosis factor alpha but not with evasion of lymphocyte-dependent monocyte effector functions. Infect. Immun. 1998, 66, 1190–1199. [Google Scholar] [CrossRef]
- Tsao, T.C.; Li, L.; Hsieh, M.; Liao, S.; Chang, K.S. Soluble TNF-alpha receptor and IL-1 receptor antagonist elevation in BAL in active pulmonary TB. Eur. Respir. J. 1999, 14, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Keeton, R.; Toit, J.P.D.; Hsu, N.J.; Dube, F.; Jacobs, M. Immune control of Mycobacterium tuberculosis is dependent on both soluble TNFRp55 and soluble TNFRp75. Immunology 2021, 164, 524–540. [Google Scholar] [CrossRef]
- Roca, F.J.; Whitworth, L.J.; Prag, H.A.; Murphy, M.P.; Ramakrishnan, L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science 2022, 376, eabh2841. [Google Scholar] [CrossRef]
- Apt, A.; Kramnik, I. Man and mouse TB: Contradictions and solutions. Tuberculosis 2009, 89, 195–198. [Google Scholar] [CrossRef]
- Young, D. Animal models of tuberculosis. Eur. J. Immunol. 2009, 39, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A. Systems approach to understand the immune response in tuberculosis: An iterative process between mouse models and human disease. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 173–177. [Google Scholar] [CrossRef][Green Version]
- Ramakrishnan, L. The zebrafish guide to tuberculosis immunity and treatment. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.H. Protection and pathology in TB: Learning from the zebrafish model. Semin. Immunopathol. 2016, 38, 261–273. [Google Scholar] [CrossRef]
- Rook, G.A.; Taverne, J.; Leveton, C.; Steele, J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology 1987, 62, 229–234. [Google Scholar] [PubMed]
- Valone, S.E.; Rich, E.A.; Wallis, R.S.; Ellner, J.J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect. Immun. 1988, 56, 3313–3315. [Google Scholar] [CrossRef]
- Henderson, R.A.; Watkins, S.C.; Flynn, J.L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 1997, 159, 635–643. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Barber, D.L.; Hieny, S.; Feng, C.G.; Caspar, P.; Oland, S.; Gordon, S.; Sher, A. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 2011, 35, 1023–1034. [Google Scholar] [CrossRef]
- Faldt, J.; Dahlgren, C.; Ridell, M. Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS 2002, 110, 593–600. [Google Scholar] [CrossRef]
- Harari, A.; Rozot, V.; Enders, F.B.; Perreau, M.; Stalder, J.M.; Nicod, L.P.; Cavassini, M.; Calandra, T.; Blanchet, C.L.; Jaton, K.; et al. Dominant TNF-α+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat. Med. 2011, 17, 372–376. [Google Scholar] [CrossRef]
- Arora, P.; Foster, E.L.; Porcelli, S.A. CD1d and natural killer T cells in immunity to Mycobacterium tuberculosis. In The New Paradigm of Immunity to Tuberculosis; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; Volume 783, pp. 199–223. [Google Scholar]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.J.; Yu, Y.Y.; et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Peyrat, M.A.; Constant, P.; Davodeau, F.; David-Ameline, J.; Poquet, Y.; Vié, H.; Fournié, J.J.; Bonneville, M. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J. Immunol. 1995, 154, 5986–5994. [Google Scholar] [CrossRef]
- Barnes, P.F.; Chatterjee, D.; Abrams, J.S.; Lu, S.; Wang, E.; Yamamura, M.; Brennan, P.J.; Modlin, R.L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J. Immunol. 1992, 149, 541–547. [Google Scholar] [CrossRef]
- Aung, H.; Toossi, Z.; Wisnieski, J.J.; Wallis, R.S.; Culp, L.A.; Phillips, N.B.; Phillips, M.; Averill, L.E.; Daniel, T.M.; Ellner, J.J. Induction of monocyte expression of tumor necrosis factor alpha by the 30-kD alpha antigen of Mycobacterium tuberculosis and synergism with fibronectin. J. Clin. Investig. 1996, 98, 1261–1268. [Google Scholar] [CrossRef]
- Jung, S.B.; Yang, C.S.; Lee, J.S.; Shin, A.R.; Jung, S.S.; Son, J.W.; Harding, C.V.; Kim, H.J.; Park, J.K.; Paik, T.H.; et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect. Immun. 2006, 74, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, K.P.; Ganguli, G.; Naik, S.K.; Sonawane, A. Mycobacterium tuberculosis EsxL induces TNF-α secretion through activation of TLR2 dependent MAPK and NF-κB pathways. Mol. Immunol. 2021, 130, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Reiling, N.; Hölscher, C.; Fehrenbach, A.; Kröger, S.; Kirschning, C.J.; Goyert, S.; Ehlers, S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 2002, 169, 3480–3484. [Google Scholar] [CrossRef]
- Ruiz, A.; Guzmán-Beltrán, S.; Carreto-Binaghi, L.E.; Gonzalez, Y.; Juárez, E. DNA from virulent M. tuberculosis induces TNF-α production and autophagy in M1 polarized macrophages. Microb. Pathog. 2019, 132, 166–177. [Google Scholar] [CrossRef]
- Scanga, C.A.; Bafica, A.; Feng, C.G.; Cheever, A.W.; Hieny, S.; Sher, A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 2004, 72, 2400–2404. [Google Scholar] [CrossRef]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 206, 2879–2888. [Google Scholar] [CrossRef]
- Juarez, E.; Nuñez, C.; Sada, E.; Ellner, J.J.; Schwander, S.K.; Torres, M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir. Res. 2010, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, S.J.; Park, Y.M.; Jung, I.D.; Ryu, S.W.; Kim, D.J.; Park, J.H.; Park, J.H. Critical role of TRIF and MyD88 in Mycobacterium tuberculosis Hsp70-mediated activation of dendritic cells. Cytokine 2015, 71, 139–144. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Yonekawa, A.; Saijo, S.; Hoshino, Y.; Miyake, Y.; Ishikawa, E.; Suzukawa, M.; Inoue, H.; Tanaka, M.; Yoneyama, M.; Oh-Hora, M.; et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 2014, 41, 402–413. [Google Scholar] [CrossRef]
- Gandotra, S.; Jang, S.; Murray, P.J.; Salgame, P.; Ehrt, S. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect. Immun. 2007, 75, 5127–5134. [Google Scholar] [CrossRef]
- Divangahi, M.; Mostowy, S.; Coulombe, F.; Kozak, R.; Guillot, L.; Veyrier, F.; Kobayashi, K.S.; Flavell, R.A.; Gros, P.; Behr, M.A. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J. Immunol. 2008, 181, 7157–7165. [Google Scholar] [CrossRef] [PubMed]
- Toossi, Z. The inflammatory response in Mycobacterium tuberculosis infection. In Inflammation; Springer: Dordrecht, The Netherlands, 2000; Volume 48, pp. 513–519. [Google Scholar]
- Rajaram, M.V.; Ni, B.; Morris, J.D.; Brooks, M.N.; Carlson, T.K.; Bakthavachalu, B.; Schoenberg, D.R.; Torrelles, J.B.; Schlesinger, L.S. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc. Natl. Acad. Sci. USA 2011, 108, 17408–17413. [Google Scholar] [CrossRef]
- Fenton, M.J.; Vermeulen, M.W.; Kim, S.; Burdick, M.; Strieter, R.M.; Kornfeld, H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect. Immun. 1997, 65, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Hviid, L.; Kharazmi, A.; Kemp, M. Interferon-γ production by human T cells and natural killer cells in vitro in response to antigens from the two intracellular pathogens Mycobacterium tuberculosis and Leishmania major. Scand. J. Immunol. 1997, 46, 495–499. [Google Scholar] [CrossRef]
- Khan, T.A.; Mazhar, H.; Saleha, S.; Tipu, H.N.; Muhammad, N.; Abbas, M.N. Interferon-Gamma Improves Macrophages Function against M. tuberculosis in Multidrug-Resistant Tuberculosis Patients. Chemother. Res. Pract. 2016, 2016, 7295390. [Google Scholar] [CrossRef]
- Tailleux, L.; Pham-Thi, N.; Bergeron-Lafaurie, A.; Herrmann, J.L.; Charles, P.; Schwartz, O.; Scheinmann, P.; Lagrange, P.H.; de Blic, J.; Tazi, A.; et al. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005, 2, e381. [Google Scholar] [CrossRef]
- Lugo-Villarino, G.; Troegeler, A.; Balboa, L.; Lastrucci, C.; Duval, C.; Mercier, I.; Bénard, A.; Capilla, F.; Al Saati, T.; Poincloux, R.; et al. The C-Type Lectin Receptor DC-SIGN Has an Anti-Inflammatory Role in Human M(IL-4) Macrophages in Response to Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Nisa, A.; Kipper, F.C.; Panigrahy, D.; Tiwari, S.; Kupz, A.; Subbian, S. Different modalities of host cell death and their impact on Mycobacterium tuberculosis infection. Am. J. Physiol. Cell Physiol. 2022, 323, C1444–C1474. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Lyu, M.; Lai, H.; Niu, L.; Zhao, Z.; Liu, T.; Lei, S.; Ying, B. Host-pathogen dialogues in different cell death modes during Mycobacterium tuberculosis infection. Interdiscip. Med. 2024, 2, e20230044. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Y.; Yu, J.; Liu, Y.; Xia, J.; Kong, X.; Jin, X.; Li, J.; Lin, S.; Ruan, Y.; et al. Advancing roles and therapeutic potentials of pyroptosis in host immune defenses against tuberculosis. Biomolecules 2024, 14, 1255. [Google Scholar] [CrossRef]
- Wang, J.; Cao, H.; Xie, Y.; Xu, Z.; Li, Y.; Luo, H. Mycobacterium tuberculosis infection induces a novel type of cell death: Ferroptosis. Biomed. Pharmacother. 2024, 177, 117030. [Google Scholar] [CrossRef]
- Amaral, E.P.; Namasivayam, S.; Queiroz, A.T.; Fukutani, E.; Hilligan, K.L.; Aberman, K.; Fisher, L.; Bomfim, C.C.; Kauffman, K.; Buchanan, J.; et al. BACH1 promotes tissue necrosis and Mycobacterium tuberculosis susceptibility. Nat. Microbiol. 2024, 9, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Martin, C.J.; Nunes-Alves, C.; Divangahi, M.; Remold, H.G. Lipids, apoptosis, and cross-presentation: Links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect. 2011, 13, 749–756. [Google Scholar] [CrossRef]
- Schaible, U.E.; Winau, F.; Sieling, P.A.; Fischer, K.; Collins, H.L.; Hagens, K.; Modlin, R.L.; Brinkmann, V.; Kaufmann, S.H. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 2003, 9, 1039–1046. [Google Scholar] [CrossRef]
- Jiao, X.; Lo-Man, R.; Guermonprez, P.; Fiette, L.; Dériaud, E.; Burgaud, S.; Gicquel, B.; Winter, N.; Leclerc, C. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 2002, 168, 1294–1301. [Google Scholar] [CrossRef]
- den Haan, J.M.; Bevan, M.J. Antigen presentation to CD8+ T cells: Cross-priming in infectious diseases. Curr. Opin. Immunol. 2001, 13, 437–441. [Google Scholar] [CrossRef]
- Behar, S.M. Antigen-specific CD8(+) T cells and protective immunity to tuberculosis. In The New Paradigm of Immunity to Tuberculosis; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; Volume 783, pp. 141–163. [Google Scholar]
- Cohen, S.B.; Crawley, J.B.; Kahan, M.C.; Feldmann, M.; Foxwell, B.M. Interleukin-10 rescues T cells from apoptotic cell death: Association with an upregulation of Bcl-2. Immunology 1997, 92, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bingisser, R.; Stey, C.; Weller, M.; Groscurth, P.; Russi, E.; Frei, K. Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am. J. Respir. Cell. Mol. Biol. 1996, 15, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Balcewicz-Sablinska, M.K.; Keane, J.; Kornfeld, H.; Remold, H.G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 1998, 161, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Olivier, M.; Gros, P.; Barrera, L.F.; García, L.F. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J. Immunol. 1999, 162, 6122–6131. [Google Scholar] [CrossRef]
- Keane, J.; Remold, H.G.; Kornfeld, H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 2000, 164, 2016–2020. [Google Scholar] [CrossRef]
- Stutz, M.D.; Allison, C.C.; Ojaimi, S.; Preston, S.P.; Doerflinger, M.; Arandjelovic, P.; Whitehead, L.; Bader, S.M.; Batey, D.; Asselin-Labat, M.L.; et al. Macrophage and neutrophil death programs differentially confer resistance to tuberculosis. Immunity 2021, 54, 1758–1771.e7. [Google Scholar] [CrossRef]
- Tukiman, M.H.; Norazmi, M.N. Immunometabolism of Immune Cells in Mucosal Environment Drives Effector Responses against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2022, 23, 8531. [Google Scholar] [CrossRef]
- Marrocco, A.; Ortiz, L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. Immunol. 2022, 13, 936167. [Google Scholar] [CrossRef]
- Mahla, R.S.; Kumar, A.; Tutill, H.J.; Krishnaji, S.T.; Sathyamoorthy, B.; Noursadeghi, M.; Breuer, J.; Pandey, A.K.; Kumar, H. NIX-mediated mitophagy regulate metabolic reprogramming in phagocytic cells during mycobacterial infection. Tuberculosis 2021, 126, 102046. [Google Scholar] [CrossRef]
- Maoldomhnaigh, C.Ó.; Cox, D.J.; Phelan, J.J.; Mitermite, M.; Murphy, D.M.; Leisching, G.; Thong, L.; O’Leary, S.M.; Gogan, K.M.; McQuaid, K.; et al. Lactate Alters Metabolism in Human Macrophages and Improves Their Ability to Kill Mycobacterium tuberculosis. Front. Immunol. 2021, 12, 663695. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G. Antigen presentation in the lung. Am. J. Respir. Crit. Care Med. 2000, 162, S151–S156. [Google Scholar] [CrossRef] [PubMed]
- Stenger, S.; Hanson, D.A.; Teitelbaum, R.; Dewan, P.; Niazi, K.R.; Froelich, C.J.; Ganz, T.; Thoma-Uszynski, S.; Melián, A.; Bogdan, C.; et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998, 282, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Schwarz, F.; Divaris, N.; Kay, C.; Carsons, S.E. Mechanisms of tumor necrosis factor-granulocyte-macrophage colony-stimulating factor-induced dendritic cell development. Blood 1993, 82, 3019–3028. [Google Scholar] [CrossRef]
- Iwamoto, S.; Iwai, S.; Tsujiyama, K.; Kurahashi, C.; Takeshita, K.; Naoe, M.; Masunaga, A.; Ogawa, Y.; Oguchi, K.; Miyazaki, A. TNF-α drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J. Immunol. 2007, 179, 1449–1457. [Google Scholar] [CrossRef]
- Buettner, M.; Meinken, C.; Bastian, M.; Bhat, R.; Stössel, E.; Faller, G.; Cianciolo, G.; Ficker, J.; Wagner, M.; Röllinghoff, M.; et al. Inverse correlation of maturity and antibacterial activity in human dendritic cells. J. Immunol. 2005, 174, 4203–4209. [Google Scholar] [CrossRef]
- Cheadle, E.J.; Selby, P.J.; Jackson, A.M. Mycobacterium bovis bacillus Calmette-Guérin-infected dendritic cells potently activate autologous T cells via a B7 and interleukin-12-dependent mechanism. Immunology 2003, 108, 79–88. [Google Scholar] [CrossRef]
- Hanekom, W.A.; Mendillo, M.; Manca, C.; Haslett, P.A.; Siddiqui, M.R.; Barry, C.; Kaplan, G. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 2003, 188, 257–266. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Xu, H.; Lin, S.; Zhou, Z.; Li, D.; Zhang, X.; Yu, M.; Zhao, R.; Wang, Y.; Qian, J.; Li, X.; et al. New genetic and epigenetic insights into the chemokine system: The latest discoveries aiding progression toward precision medicine. Cell. Mol. Immunol. 2023, 20, 739–776. [Google Scholar] [CrossRef] [PubMed]
- Algood, H.M.; Lin, P.L.; Flynn, J.L. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 2005, 41, S189–S193. [Google Scholar] [CrossRef] [PubMed]
- Slight, S.R.; Khader, S.A. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor. Rev. 2013, 24, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Khader, S.A. Chemokines in tuberculosis: The good, the bad and the ugly. Semin. Immunol. 2014, 26, 552–558. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016, 4, 10-1128. [Google Scholar] [CrossRef]
- Ho, A.W.; Wong, C.K.; Lam, C.W. Tumor necrosis factor-α up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology 2008, 213, 533–544. [Google Scholar] [CrossRef]
- Thompson, W.L.; van Eldik, L.J. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes. Brain Res. 2009, 1287, 47–57. [Google Scholar] [CrossRef]
- Mercer, P.F.; Williams, A.E.; Scotton, C.J.; José, R.J.; Sulikowski, M.; Moffatt, J.D.; Murray, L.A.; Chambers, R.C. Proteinase-activated receptor-1; CCL2, and CCL7 regulate acute neutrophilic lung inflammation. Am. J. Respir. Cell. Mol. Biol. 2014, 50, 144–157. [Google Scholar] [CrossRef]
- Zhang, F.; Mears, J.R.; Shakib, L.; Beynor, J.I.; Shanaj, S.; Korsunsky, I.; Nathan, A.; Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium; Donlin, L.T.; Raychaudhuri, S. IFN-γ and TNF-α drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 lungs and inflammatory diseases with tissue inflammation. Genome Med. 2021, 13, 64. [Google Scholar] [CrossRef]
- Montecucco, F.; Steffens, S.; Burger, F.; Da Costa, A.; Bianchi, G.; Bertolotto, M.; Mach, F.; Dallegri, F.; Ottonello, L. Tumor necrosis factor-alpha (TNF-α) induces integrin CD11b/CD18 (Mac-1) up-regulation and migration to the CC chemokine CCL3 (MIP-1α) on human neutrophils through defined signalling pathways. Cell. Signal 2008, 20, 557–568. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Phillips, K.L.; Chiverton, N.; Haddock, G.; Bunning, R.A.; Cross, A.K.; Shapiro, I.M.; Le Maitre, C.L.; Risbud, M.V. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum 2013, 65, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kochumon, S.; Chandy, B.; Shenouda, S.; Koshy, M.; Hasan, A.; Arefanian, H.; Al-Mulla, F.; Sindhu, S. TNF-α Drives the CCL4 Expression in Human Monocytic Cells: Involvement of the SAPK/JNK and NF-κB Signaling Pathways. Cell. Physiol. Biochem. 2019, 52, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Kochumon, S.; Shenouda, S.; Wilson, A.; Al-Mulla, F.; Ahmad, R. The Cooperative Induction of CCL4 in Human Monocytic Cells by TNF-α and Palmitate Requires MyD88 and Involves MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4658. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Hong, J.H.; Seo, Y.S. Tumour necrosis factor-alpha and interferon-gamma synergistically activate the RANTES promoter through nuclear factor κB and interferon regulatory factor 1 (IRF-1) transcription factors. Biochem. J. 2000, 350, 131–138. [Google Scholar] [CrossRef]
- Homma, T.; Matsukura, S.; Hirose, T.; Ohnishi, T.; Kimura, T.; Kurokawa, M.; Ieki, K.; Odaka, M.; Suzuki, S.; Watanabe, S.; et al. Cooperative activation of CCL5 expression by TLR3 and tumor necrosis factor-α or interferon-γ through nuclear factor-κB or STAT-1 in airway epithelial cells. Int. Arch. Allergy Immunol. 2010, 152 (Suppl. 1), 9–17. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoelscher, G.L.; Ingram, J.A.; Bethea, S.; Norton, H.J.; Hanley, E.N. Production and expression of RANTES (CCL5) by human disc cells and modulation by IL-1-β and TNF-α in 3D culture. Exp. Mol. Pathol. 2014, 96, 133–138. [Google Scholar] [CrossRef]
- Hoeck, J.; Woisetschläger, M. STAT6 mediates eotaxin-1 expression in IL-4 or TNF-α-induced fibroblasts. J. Immunol. 2001, 166, 4507–4515. [Google Scholar] [CrossRef]
- Yoshifuku, K.; Matsune, S.; Ohori, J.; Sagara, Y.; Fukuiwa, T.; Kurono, Y. IL-4 and TNF-alpha increased the secretion of eotaxin from cultured fibroblasts of nasal polyps with eosinophil infiltration. Rhinology 2007, 45, 235–241. [Google Scholar]
- Nakamura, K.; Okada, M.; Yoneda, M.; Takamoto, S.; Nakade, Y.; Tamori, K.; Aso, K.; Makino, I. Macrophage inflammatory protein-2 induced by TNF-α plays a pivotal role in concanavalin A-induced liver injury in mice. J. Hepatol. 2001, 35, 217–224. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Bhattacharyya, A.; Bai, J.; Mifflin, R.C.; Ernst, P.B.; Mitra, S.; Crowe, S.E. Tumor necrosis factor (TNF)-alpha-induced IL-8 expression in gastric epithelial cells: Role of reactive oxygen species and AP endonuclease-1/redox factor (Ref)-1. Cytokine 2009, 46, 359–369. [Google Scholar] [CrossRef]
- Patel, A.B.; Tsilioni, I.; Weng, Z.; Theoharides, T.C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 2018, 27, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-α Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-κB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10519. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Shigeishi, H.; Taki, M.; Nishi, H.; Higashikawa, K.; Takechi, M.; Kamata, N. Regulation of CXCL9/10/11 in oral keratinocytes and fibroblasts. J. Dent. Res. 2008, 87, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Asaoka, T.; Eguchi, H.; Yokota, Y.; Kubo, M.; Kinoshita, M.; Urakawa, S.; Iwagami, Y.; Tomimaru, Y.; Akita, H.; et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020, 111, 323–333. [Google Scholar] [CrossRef]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.Y.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef]
- Hardaker, E.L.; Bacon, A.M.; Carlson, K.; Roshak, A.K.; Foley, J.J.; Schmidt, D.B.; Buckley, P.T.; Comegys, M.; Panettieri, R.A.; Sarau, H.M.; et al. Regulation of TNF-alpha- and IFN-gamma-induced CXCL10 expression: Participation of the airway smooth muscle in the pulmonary inflammatory response in chronic obstructive pulmonary disease. FASEB J. 2004, 18, 191–193. [Google Scholar] [CrossRef]
- Ohta, K.; Ishida, Y.; Fukui, A.; Nishi, H.; Naruse, T.; Takechi, M.; Kamata, N. Itraconazole inhibits TNF-α-induced CXCL10 expression in oral fibroblasts. Oral. Dis. 2015, 21, 106–112. [Google Scholar] [CrossRef]
- Qiu, Z.X.; Sha, Z.S.; Che, X.M.; Wang, M.Y. Correlation analysis of ADAMTS-4, VCAM-1, and TAK1 expression in cartilage tissue from spine tuberculosis. Genet. Mol. Res. 2017, 16, gmr16038961. [Google Scholar] [CrossRef]
- Hamzaoui, A.; Hamzaoui, K.; Kahan, A.; Chabbou, A. Levels of soluble VCAM-1, soluble ICAM-1, and soluble E-selectin in patients with tuberculous pleuritis. Mediat. Inflamm. 1996, 5, 276–279. [Google Scholar] [CrossRef]
- Feng, C.G.; Britton, W.J.; Palendira, U.; Groat, N.L.; Briscoe, H.; Bean, A.G. Up-regulation of VCAM-1 and differential expansion of β integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J. Immunol. 2000, 164, 4853–4860. [Google Scholar] [CrossRef]
- Clay, H.; Davis, J.M.; Beery, D.; Huttenlocher, A.; Lyons, S.E.; Ramakrishnan, L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2007, 2, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Clay, H.; Volkman, H.E.; Ramakrishnan, L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 2008, 29, 283–294. [Google Scholar] [CrossRef]
- Fenhalls, G.; Wong, A.; Bezuidenhout, J.; van Helden, P.; Bardin, P.; Lukey, P.T. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect. Immun. 2000, 68, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.L.; Flynn, J.L.; Reinhart, T.A. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect. Immun. 2003, 71, 7023–7034. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Bean, A.G.; Demangel, C.; France, M.P.; Briscoe, H.; Britton, W.J. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 2002, 168, 4620–4627. [Google Scholar] [CrossRef]
- Algood, H.M.; Lin, P.L.; Yankura, D.; Jones, A.; Chan, J.; Flynn, J.L. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J. Immunol. 2004, 172, 6846–6857. [Google Scholar] [CrossRef]
- Lee, J.; Remold, H.G.; Ieong, M.H.; Kornfeld, H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J. Immunol. 2006, 176, 4267–4274. [Google Scholar] [CrossRef]
- O’Sullivan, M.P.; O’Leary, S.; Kelly, D.M.; Keane, J. A caspase-independent pathway mediates macrophage cell death in response to Mycobacterium tuberculosis infection. Infect. Immun. 2007, 75, 1984–1993. [Google Scholar] [CrossRef]
- Bean, A.G.; Roach, D.R.; Briscoe, H.; France, M.P.; Korner, H.; Sedgwick, J.D.; Britton, W.J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 1999, 162, 3504–3511. [Google Scholar] [CrossRef]
- Wallis, R.S. Tumour necrosis factor antagonists: Structure, function, and tuberculosis risks. Lancet Infect. Dis. 2008, 8, 601–611. [Google Scholar] [CrossRef]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef]
- Martínez, N.O.; Noiseux, R.C.; Martín, C.J.A.; Lara, G.V. Reactivation tuberculosis in a patient with anti-TNF-alpha treatment. Am. J. Gastroenterol. 2001, 96, 1665–1666. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Keane, J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin. Exp. Immunol. 2010, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Champsi, J.; Young, L.S.; Bermudez, L.E. Production of TNF-alpha; IL-6; TGF-beta, and expression of receptors for TNF-alpha and IL-6, during murine Mycobacterium avium infection. Immunology 1995, 84, 549–554. [Google Scholar]
- Senaldi, G.; Yin, S.; Shaklee, C.L.; Piguet, P.F.; Mak, T.W.; Ulich, T.R. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J. Immunol. 1996, 157, 5022–5026. [Google Scholar] [CrossRef] [PubMed]
- Agnholt, J.; Dahlerup, J.F.; Kaltoft, K. The effect of etanercept and infliximab on the production of tumour necrosis factor α, interferon-γ and GM-CSF in in vivo activated intestinal T lymphocyte cultures. Cytokine 2003, 23, 76–85. [Google Scholar] [CrossRef]
- Netea, M.G.; Radstake, T.; Joosten, L.A.; van der Meer, J.W.; Barrera, P.; Kullberg, B.J. Salmonella septicemia in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Association with decreased interferon-γ production and Toll-like receptor 4 expression. Arthritis Rheum. 2003, 48, 1853–1857. [Google Scholar] [CrossRef]
- Popa, C.; Barrera, P.; Joosten, L.A.; van Riel, P.L.; Kullberg, B.J.; van der Meer, J.W.; Netea, M.G. Cytokine production from stimulated whole blood cultures in rheumatoid arthritis patients treated with various TNF blocking agents. Eur. Cytokine Netw. 2009, 20, 88–93. [Google Scholar] [CrossRef]
- Lügering, A.; Schmidt, M.; Lügering, N.; Pauels, H.G.; Domschke, W.; Kucharzik, T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology 2001, 121, 1145–1157. [Google Scholar] [CrossRef]
- van den Brande, J.M.; Braat, H.; van den Brink, G.R.; Versteeg, H.H.; Bauer, C.A.; Hoedemaeker, I.; van Montfrans, C.; Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology 2003, 124, 1774–1785. [Google Scholar] [CrossRef]
- van den Brande, J.; Hommes, D.W.; Peppelenbosch, M.P. Infliximab induced T lymphocyte apoptosis in Crohn’s disease. J. Rheumatol. Suppl. 2005, 74, 26–30. [Google Scholar]
- Bruns, H.; Meinken, C.; Schauenberg, P.; Härter, G.; Kern, P.; Modlin, R.L.; Antoni, C.; Stenger, S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J. Clin. Investig. 2009, 119, 1167–1177. [Google Scholar] [CrossRef]
- Nadkarni, S.; Mauri, C.; Ehrenstein, M.R. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J. Exp. Med. 2007, 204, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef]
- Scott-Browne, J.P.; Shafiani, S.; Tucker-Heard, G.; Ishida-Tsubota, K.; Fontenot, J.D.; Rudensky, A.Y.; Bevan, M.J.; Urdahl, K.B. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 2007, 204, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Scallon, B.; Cai, A.; Solowski, N.; Rosenberg, A.; Song, X.Y.; Shealy, D.; Wagner, C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J. Pharmacol. Exp. Ther. 2002, 301, 418–426. [Google Scholar] [CrossRef]
- Tubach, F.; Salmon, D.; Ravaud, P.; Allanore, Y.; Goupille, P.; Bréban, M.; Pallot-Prades, B.; Pouplin, S.; Sacchi, A.; Chichemanian, R.M.; et al. Research Axed on Tolerance of Biotherapies Group. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009, 60, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Hove, T.T.; van Montfrans, C.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut 2002, 50, 206–211. [Google Scholar] [CrossRef]
- Mitoma, H.; Horiuchi, T.; Hatta, N.; Tsukamoto, H.; Harashima, S.; Kikuchi, Y.; Otsuka, J.; Okamura, S.; Fujita, S.; Harada, M. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-α. Gastroenterology 2005, 128, 376–392. [Google Scholar] [CrossRef]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Tamimoto, Y.; Kimoto, Y.; Uchino, A.; To, K.; Harashima, S.; Hatta, N.; Harada, M. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor α-expressing cells: Comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008, 58, 1248–1257. [Google Scholar] [CrossRef]
- Agnholt, J.; Kaltoft, K. Infliximab downregulates interferon-γ production in activated gut T-lymphocytes from patients with Crohn’s disease. Cytokine 2001, 15, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Saliu, O.Y.; Sofer, C.; Stein, D.S.; Schwander, S.K.; Wallis, R.S. Tumor-necrosis-factor blockers: Differential effects on mycobacterial immunity. J. Infect. Dis. 2006, 194, 486–492. [Google Scholar] [CrossRef]
- Hamdi, H.; Mariette, X.; Godot, V.; Weldingh, K.; Hamid, A.M.; Prejean, M.V.; Baron, G.; Lemann, M.; Puechal, X.; Breban, M.; et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res. Ther. 2006, 8, R114. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.S.; Cohen, J.; Fei, J.; Zaba, L.C.; Cardinale, I.; Toyoko, K.; Ott, J.; Krueger, J.G. Insights into gene modulation by therapeutic TNF and IFNγ antibodies: TNF regulates IFNγ production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J. Investig. Dermatol. 2008, 128, 655–666. [Google Scholar] [CrossRef]
- Dieli, F.; Troye-Blomberg, M.; Ivanyi, J.; Fournié, J.J.; Bonneville, M.; Peyrat, M.A.; Sireci, G.; Salerno, A. Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 2000, 30, 1512–1519. [Google Scholar] [CrossRef]
- Giardina, A.R.; Accardo-Palumbo, A.; Ciccia, F.; Ferrante, A.; Principato, A.; Impastato, R.; Triolo, G. Blocking TNF in vitro with infliximab determines the inhibition of expansion and interferon gamma production of Vγ9/Vδ2 T lymphocytes from patients with active rheumatoid arthritis. A role in the susceptibility to tuberculosis? Reumatismo 2009, 61, 21–26. [Google Scholar] [CrossRef][Green Version]
- Siebert, S.; Tsoukas, A.; Robertson, J.; McInnes, I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol. Rev. 2015, 67, 280–309. [Google Scholar] [CrossRef]
- Steed, P.M.; Tansey, M.G.; Zalevsky, J.; Zhukovsky, E.A.; Desjarlais, J.R.; Szymkowski, D.E.; Abbott, C.; Carmichael, D.; Chan, C.; Cherry, L.; et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science 2003, 301, 1895–1898. [Google Scholar] [CrossRef]
- Olleros, M.L.; Guler, R.; Vesin, D.; Parapanov, R.; Marchal, G.; Martinez-Soria, E.; Corazza, N.; Pache, J.C.; Mueller, C.; Garcia, I. Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-Guerin and Mycobacterium tuberculosis infections. Am. J. Pathol. 2005, 166, 1109–1120. [Google Scholar] [CrossRef]
- Goletti, D.; Petrone, L.; Ippolito, G.; Niccoli, L.; Nannini, C.; Cantini, F. Preventive therapy for tuberculosis in rheumatological patients undergoing therapy with biological drugs. Expert. Rev. Anti Infect. Ther. 2018, 16, 501–512. [Google Scholar] [CrossRef]
- Winthrop, K.L. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat. Clin. Pract. Rheumatol. 2006, 2, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ha, R.; Keynan, Y.; Rueda, Z.V. Increased susceptibility to pneumonia due to tumour necrosis factor inhibition and prospective immune system rescue via immunotherapy. Front. Cell. Infect. Microbiol. 2022, 12, 980868. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Hope, J.C.; Keane, J. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis. J. Infect. Dis. 2008, 198, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Olleros, M.L.; Guler, R.; Corazza, N.; Vesin, D.; Eugster, H.P.; Marchal, G.; Chavarot, P.; Mueller, C.; Garcia, I. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette-Guérin infection in the absence of secreted TNF and lymphotoxin-α. J. Immunol. 2002, 168, 3394–3401. [Google Scholar] [CrossRef]
- Fremond, C.; Allie, N.; Dambuza, I.; Grivennikov, S.I.; Yeremeev, V.; Quesniaux, V.F.; Jacobs, M.; Ryffel, B. Membrane TNF confers protection to acute mycobacterial infection. Respir. Res. 2005, 6, 136. [Google Scholar] [CrossRef]
- Saunders, B.M.; Tran, S.; Ruuls, S.; Sedgwick, J.D.; Briscoe, H.; Britton, W.J. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J. Immunol. 2005, 174, 4852–4859. [Google Scholar] [CrossRef]
- Denis, M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: Killing effector mechanism depends on the generation of reactive nitrogen intermediates. J. Leukoc. Biol. 1991, 49, 380–387. [Google Scholar] [CrossRef]
- Bekker, L.G.; Freeman, S.; Murray, P.J.; Ryffel, B.; Kaplan, G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J. Immunol. 2001, 166, 6728–6734. [Google Scholar] [CrossRef]
- MacMicking, J.D.; North, R.J.; LaCourse, R.; Mudgett, J.S.; Shah, S.K.; Nathan, C.F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 1997, 94, 5243–5248. [Google Scholar] [CrossRef]
- Cooper, A.M.; Pearl, J.E.; Brooks, J.V.; Ehlers, S.; Orme, I.M. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 2000, 68, 6879–6882. [Google Scholar] [CrossRef]
- Garcia, I.; Guler, R.; Vesin, D.; Olleros, M.L.; Vassalli, P.; Chvatchko, Y.; Jacobs, M.; Ryffel, B. Lethal Mycobacterium bovis Bacillus Calmette Guérin infection in nitric oxide synthase 2-deficient mice: Cell-mediated immunity requires nitric oxide synthase 2. Lab. Investig. 2000, 80, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc. Natl. Acad. Sci. USA 1989, 86, 9030–9033. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Magram, J.; Ferrante, J.; Orme, I.M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 1997, 186, 39–45. [Google Scholar] [CrossRef]
- Balboa, L.; Barrios-Payan, J.; González-Domínguez, E.; Lastrucci, C.; Lugo-Villarino, G.; Mata-Espinoza, D.; Schierloh, P.; Kviatcovsky, D.; Neyrolles, O.; Maridonneau-Parini, I.; et al. Diverging biological roles among human monocyte subsets in the context of tuberculosis infection. Clin. Sci. 2015, 129, 319–330. [Google Scholar] [CrossRef]
- Hadadi, E.; Zhang, B.; Baidžajevas, K.; Yusof, N.; Puan, K.J.; Ong, S.M.; Yeap, W.H.; Rotzschke, O.; Kiss-Toth, E.; Wilson, H.; et al. Differential IL-1β secretion by monocyte subsets is regulated by Hsp27 through modulating mRNA stability. Sci. Rep. 2016, 6, 39035. [Google Scholar] [CrossRef]
- Beham, A.W.; Puellmann, K.; Laird, R.; Fuchs, T.; Streich, R.; Breysach, C.; Raddatz, D.; Oniga, S.; Peccerella, T.; Findeisen, P.; et al. A TNF-regulated recombinatorial macrophage immune receptor implicated in granuloma formation in tuberculosis. PLoS Pathog. 2011, 7, e1002375. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cruz, A.; Vesin, D.; Ramon-Luing, L.; Zuñiga, J.; Quesniaux, V.F.J.; Ryffel, B.; Lascurain, R.; Garcia, I.L. Chávez-Galán. CD3+ Macrophages Deliver Proinflammatory Cytokines by a CD3- and Transmembrane TNF-Dependent Pathway and Are Increased at the BCG-Infection Site. Front. Immunol. 2019, 10, 2550. [Google Scholar] [CrossRef]
- Moody, D.B.; Guy, M.R.; Grant, E.; Cheng, T.Y.; Brenner, M.B.; Besra, G.S.; Porcelli, S.A. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 2000, 192, 965–976. [Google Scholar] [CrossRef]
- Moody, D.B.; Ulrichs, T.; Mühlecker, W.; Young, D.C.; Gurcha, S.S.; Grant, E.; Rosat, J.P.; Brenner, M.B.; Costello, C.E.; Besra, G.S.; et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 2000, 404, 884–888. [Google Scholar] [CrossRef]
- Gras, S.; Van Rhijn, I.; Shahine, A.; Cheng, T.Y.; Bhati, M.; Tan, L.L.; Halim, H.; Tuttle, K.D.; Gapin, L.; Le Nours, J.; et al. T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat. Commun. 2016, 7, 13257. [Google Scholar] [CrossRef]
- Chavez-Galan, L.; Vesin, D.; Uysal, H.; Blaser, G.; Benkhoucha, M.; Ryffel, B.; Quesniaux, V.F.J.; Garcia, I. Transmembrane Tumor Necrosis Factor Controls Myeloid-Derived Suppressor Cell Activity via TNF Receptor 2 and Protects from Excessive Inflammation during BCG-Induced Pleurisy. Front. Immunol. 2017, 8, 999. [Google Scholar] [CrossRef] [PubMed]
- Uysal, H.; Chavez-Galan, L.; Vesin, D.; Blaser, G.; Benkhoucha, M.; Ryffel, B.; Quesniaux, V.F.J.; Garcia, I. Transmembrane TNF and Partially TNFR1 Regulate TNFR2 Expression and Control Inflammation in Mycobacterial-Induced Pleurisy. Int. J. Mol. Sci. 2018, 19, 1959. [Google Scholar] [CrossRef] [PubMed]
- Allie, N.; Grivennikov, S.I.; Keeton, R.; Hsu, N.J.; Bourigault, M.L.; Court, N.; Fremond, C.; Yeremeev, V.; Shebzukhov, Y.; Ryffel, B.; et al. Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection. Sci. Rep. 2013, 3, 1809. [Google Scholar] [CrossRef] [PubMed]
- Segueni, N.; Benmerzoug, S.; Rose, S.; Gauthier, A.; Bourigault, M.L.; Reverchon, F.; Philippeau, A.; Erard, F.; Le Bert, M.; Bouscayrol, H.; et al. Innate myeloid cell TNFR1 mediates first line defence against primary Mycobacterium tuberculosis infection. Sci. Rep. 2016, 6, 22454. [Google Scholar] [CrossRef]
- Zganiacz, A.; Santosuosso, M.; Wang, J.; Yang, T.; Chen, L.; Anzulovic, M.; Alexander, S.; Gicquel, B.; Wan, Y.; Bramson, J.; et al. TNF-α is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J. Clin. Investig. 2004, 113, 401–413. [Google Scholar] [CrossRef]
- Yokobori, N.; García, C.A.S.Y.; Geffner, L.; Schierloh, P.; López, B.; Ritacco, V.; Barrera, L.; de la Barrera, S.; del Carmen Saisiain, M. Differential induction of macrophage cell death by antigens of a clustered and a non-clustered multidrug-resistant Mycobacterium tuberculosis strain from Haarlem family. FEMS Immunol. Med. Microbiol. 2012, 66, 363–371. [Google Scholar] [CrossRef]
- Geffner, L.; Basile, J.I.; Yokobori, N.; Kviatcovsky, D.; García, C.S.Y.; Ritacco, V.; López, B.; del Carmen Sasiain, M.; de la Barrera, S. Mycobacterium tuberculosis multidrug resistant strain M induces an altered activation of cytotoxic CD8+ T cells. PLoS ONE 2014, 9, e97837. [Google Scholar] [CrossRef]
- Kviatcovsky, D.; Rivadeneyra, L.; Balboa, L.; Yokobori, N.; López, B.; Ritacco, V.; Schattner, M.; del Carmen Sasiain, M.; de la Barrera, S. Mycobacterium tuberculosis Multidrug-Resistant Strain M Induces Low IL-8 and Inhibits TNF-α Secretion by Bronchial Epithelial Cells Altering Neutrophil Effector Functions. Mediat. Inflamm. 2017, 2017, 2810606. [Google Scholar] [CrossRef]
- Rook, G.A. The role of activated macrophages in protection and immunopathology in tuberculosis. Res. Microbiol. 1990, 141, 253–256. [Google Scholar] [CrossRef]
- Byrd, T.F. Tumor necrosis factor alpha (TNFalpha) promotes growth of virulent Mycobacterium tuberculosis in human monocytes iron-mediated growth suppression is correlated with decreased release of TNFalpha from iron-treated infected monocytes. J. Clin. Investig. 1997, 99, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Kanost, A.R.; Teixeira, L.; Johnson, J.; Hejal, R.; Aung, H.; Wilkinson, R.J.; Hirsch, C.S.; Toossi, Z. Role of cellular activation and tumor necrosis factor-α in the early expression of Mycobacterium tuberculosis 85B mRNA in human alveolar macrophages. J. Infect. Dis. 2004, 190, 341–351. [Google Scholar] [CrossRef][Green Version]
- Linge, I.; Kondratieva, E.; Apt, A. Prolonged B-Lymphocyte-Mediated Immune and Inflammatory Responses to Tuberculosis Infection in the Lungs of TB-Resistant Mice. Int. J. Mol. Sci. 2023, 24, 1140. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.D.; Zhu, G.; Tsai, M.C.; Mohan, V.P.; Marino, S.; Kirschner, D.E.; Huang, L.; Flynn, J.; Chan, J. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect. Immun. 2008, 76, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.; Glassman, I.; Campbell, G.; Yeganyan, S.; Nguyen, J.; Shin, A.; Venketaraman, V. Host Cell Death and Modulation of Immune Response against Mycobacterium tuberculosis Infection. Int. J. Mol. Sci. 2024, 25, 6255. [Google Scholar] [CrossRef]
- Xu, W.; Snell, L.M.; Guo, M.; Boukhaled, G.; Macleod, B.L.; Li, M.; Tullius, M.V.; Guidos, C.J.; Tsao, M.S.; Divangahi, M.; et al. Early innate and adaptive immune perturbations determine long-term severity of chronic virus and Mycobacterium tuberculosis coinfection. Immunity 2021, 54, 526–541.e1-e7. [Google Scholar] [CrossRef]
- Brenner, D.A.; O’Hara, M.; Angel, P.; Chojkier, M.; Karin, M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-α. Nature 1989, 337, 661–663. [Google Scholar] [CrossRef]
- Solis-Herruzo, J.A.; Brenner, D.A.; Chojkier, M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J. Biol. Chem. 1988, 263, 5841–5845. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Raju, R.M.; Sharma, K.; Zhang, Y.J.; Eugenin, E.A.; Prideaux, B.; Daudelin, I.B.; Chen, P.Y.; Booty, M.G.; Kim, J.H.; et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat. Med. 2016, 22, 531–538. [Google Scholar] [CrossRef]
- Roca, F.J.; Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef]
- Roca, F.J.; Whitworth, L.J.; Redmond, S.; Jones, A.A.; Ramakrishnan, L. TNF Induces Pathogenic Programmed Macrophage Necrosis in Tuberculosis through a Mitochondrial-Lysosomal-Endoplasmic Reticulum Circuit. Cell 2019, 178, 1344–1361.e11. [Google Scholar] [CrossRef] [PubMed]
- Łabuzek, K.; Liber, S.; Gabryel, B.; Okopień, B. Metformin has adenosine-monophosphate activated protein kinase (AMPK)-independent effects on LPS-stimulated rat primary microglial cultures. Pharmacol. Rep. 2010, 62, 827–848. [Google Scholar] [CrossRef]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef] [PubMed]
- Tsenova, L.; Singhal, A. Effects of host-directed therapies on the pathology of tuberculosis. J. Pathol. 2020, 250, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.K.; Pinn, M.L.; Karakousis, P.C. Metformin Adjunctive Therapy Does Not Improve the Sterilizing Activity of the First-Line Antitubercular Regimen in Mice. Antimicrob. Agents Chemother. 2017, 61, e00652-17. [Google Scholar] [CrossRef]
- Giai, C.; Gonzalez, C.; Ledo, C.; Garofalo, A.; Di Genaro, M.S.; Sordelli, D.O.; Gomez, M.I. Shedding of tumor necrosis factor receptor 1 induced by protein A decreases tumor necrosis factor alpha availability and inflammation during systemic Staphylococcus aureus infection. Infect. Immun. 2013, 81, 4200–4207. [Google Scholar] [CrossRef]
- Rodrigues, M.F.; Alves, C.C.; Figueiredo, B.B.; Rezende, A.B.; Wohlres-Viana, S.; Silva, V.L.; Machado, M.A.; Teixeira, H.C. Tumour necrosis factor receptors and apoptosis of alveolar macrophages during early infection with attenuated and virulent Mycobacterium bovis. Immunology 2013, 139, 503–512. [Google Scholar] [CrossRef]
- Keeton, R.; Allie, N.; Dambuza, I.; Abel, B.; Hsu, N.J.; Sebesho, B.; Randall, P.; Burger, P.; Fick, E.; Quesniaux, V.F.; et al. Soluble TNFRp75 regulates host protective immunity against Mycobacterium tuberculosis. J. Clin. Investig. 2014, 124, 1537–1551. [Google Scholar] [CrossRef]
- Dambuza, I.M.; Keeton, R.; Hsu, N.J.; Allie, N.; Quesniaux, V.F.; Ryffel, B.; Jacobs, M. Persistent p55TNFR expression impairs T cell responses during chronic tuberculosis and promotes reactivation. Sci. Rep. 2016, 6, 39499. [Google Scholar] [CrossRef]
- Ruiz, A.; Palacios, Y.; Garcia, I.; Chavez-Galan, L. Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Int. J. Mol. Sci. 2021, 22, 5461. [Google Scholar] [CrossRef]
- Garcia, I.; Olleros, M.L.; Quesniaux, V.F.; Jacobs, M.; Allie, N.; Nedospasov, S.A.; Szymkowski, D.E.; Ryffel, B. Roles of soluble and membrane TNF and related ligands in mycobacterial infections: Effects of selective and non-selective TNF inhibitors during infection. Adv. Exp. Med. Biol. 2011, 691, 187–201. [Google Scholar] [CrossRef]
- Téllez-Navarrete, N.A.; Ramon-Luing, L.A.; Muñoz-Torrico, M.; Preciado-García, M.; Medina-Quero, K.; Hernandez-Pando, R.; Chavez-Galan, L. Anti-tuberculosis chemotherapy alters TNFR2 expression on CD4+ lymphocytes in both drug-sensitive and -resistant tubercu losis: However, only drug-resistant tuberculosis maintains a pro-inflammatory profile after a long time. Mol. Med. 2021, 27, 76. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, H.-M.; Kim, T.S.; Kim, J.K.; Sohn, K.M.; Jo, E.-K. Mycobacterium fortuitum induces A20 expression that impairs macrophage inflammatory responses. Pathog. Dis. 2016, 74, ftw015. [Google Scholar] [CrossRef]
- Cheng, A.; Holland, S.M. Anti-cytokine autoantibodies: Mechanistic insights and disease associations. Nat. Rev. Immunol. 2024, 24, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, L.; Amurrio, C.; Martín, G.; García-Cebrian, F.; Bicandi, J.; Lardelli, P.; Suarez, M.D.; Cisterna, R. Detection of anti-interferon-gamma autoantibodies in subjects infected by Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 1998, 2, 62–68. [Google Scholar] [PubMed]
- Yi, F.; Zhang, X. High-throughput autoantibody analysis in malignant pleural effusion and tuberculosis pleural effusion. Medicine 2019, 98, e17253. [Google Scholar] [CrossRef] [PubMed]
- van Loghem, E. Allotypic markers. Monogr. Allergy 1986, 19, 40–51. [Google Scholar] [PubMed]
- De La Fuente, J.; Gortázar, C.; Juste, R. Complement component 3: A new paradigm in tuberculosis vaccine. Expert Rev. Vaccines 2016, 15, 275–277. [Google Scholar] [CrossRef]
- Krupa, A.; Kato, H.; Matthay, M.A.; Kurdowska, A.K. Proinflammatory activity of anti-IL-8 autoantibody:IL-8 complexes in alveolar edema fluid from patients with acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L1105–L1113. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Madden, K.B.; Morris, S.C.; Holmes, J.M.; Boiani, N.; Katona, I.M.; Maliszewski, C.R. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 1993, 151, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Courtney, L.P.; Phelps, J.L.; Karavodin, L.M. An anti-IL-2 antibody increases serum half-life and improves anti-tumor efficacy of human recombinant interleukin-2. Immunopharmacology 1994, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.A.; Neehus, A.L.; Ogishi, M.; Meynier, V.; Krebs, A.; Lazarov, T.; Lee, A.M.; Arango-Franco, C.A.; Yang, R.; Orrego, J.; et al. Tuberculosis in otherwise healthy adults with inherited TNF deficiency. Nature 2024, 633, 417–425. [Google Scholar] [CrossRef]
- Rigato, O.; Ujvari, S.; Castelo, A.; Salomão, R. Tumor necrosis factor alpha (TNF-α) and sepsis: Evidence for a role in host defense. Infection 1996, 24, 314–318. [Google Scholar] [CrossRef] [PubMed]
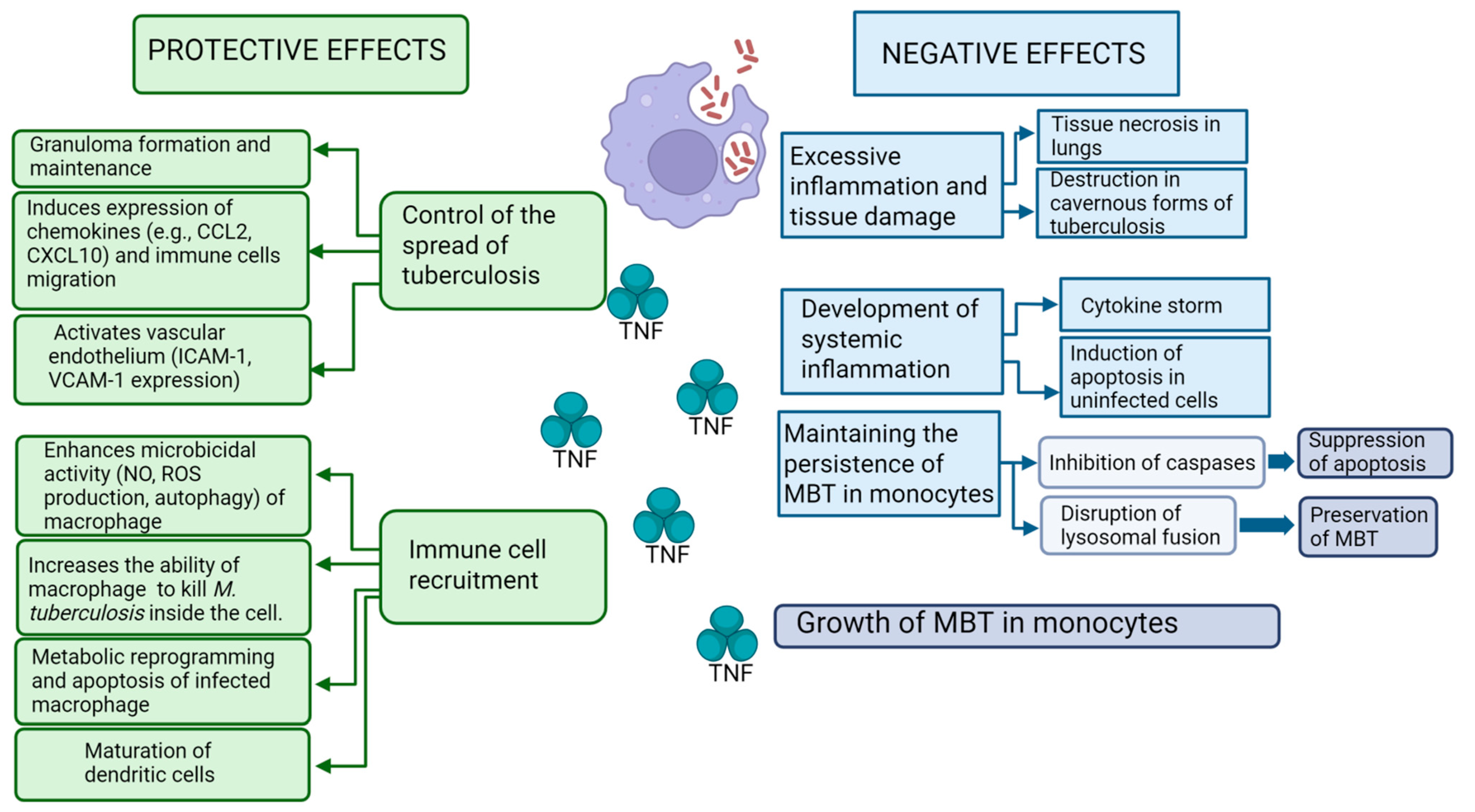
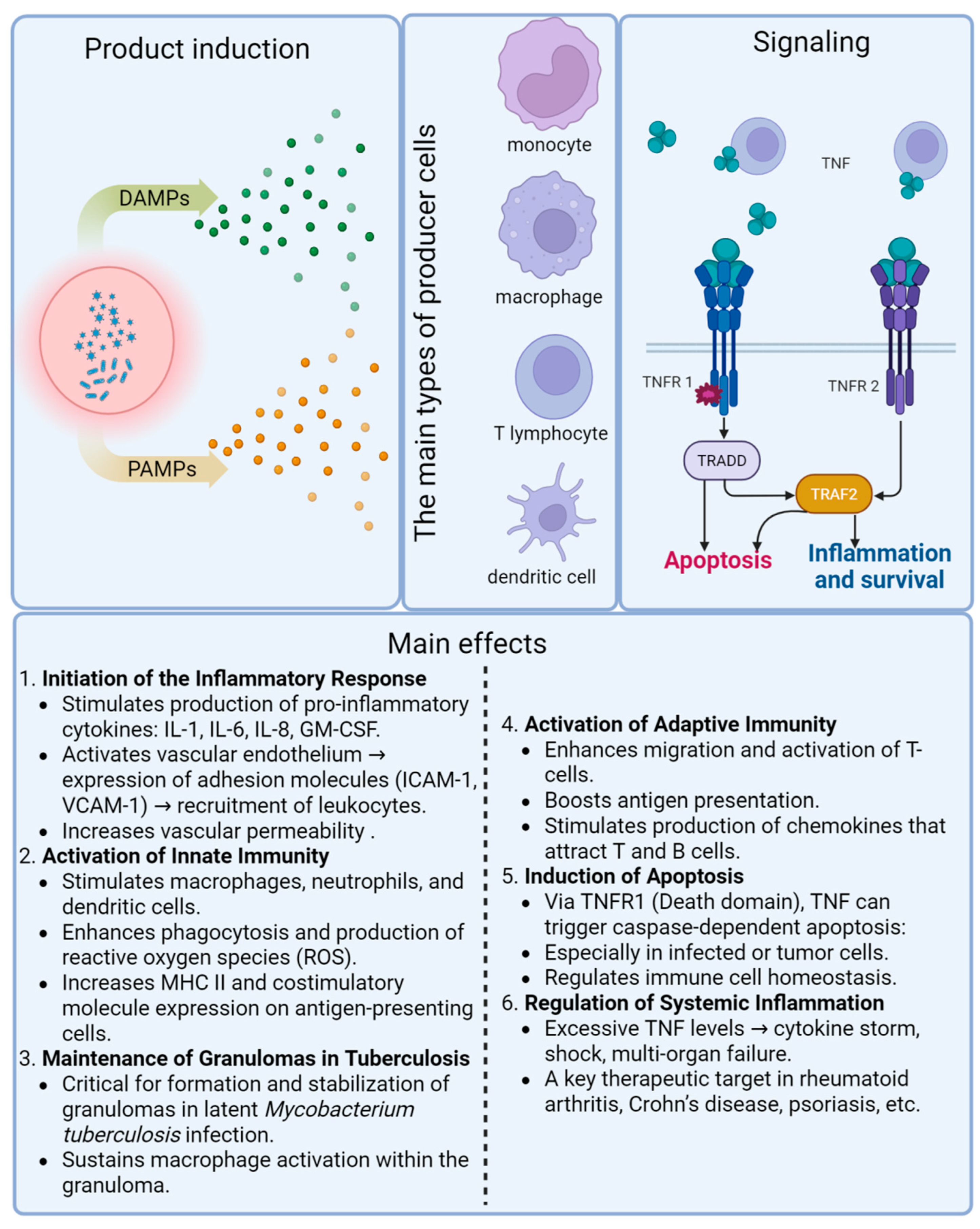
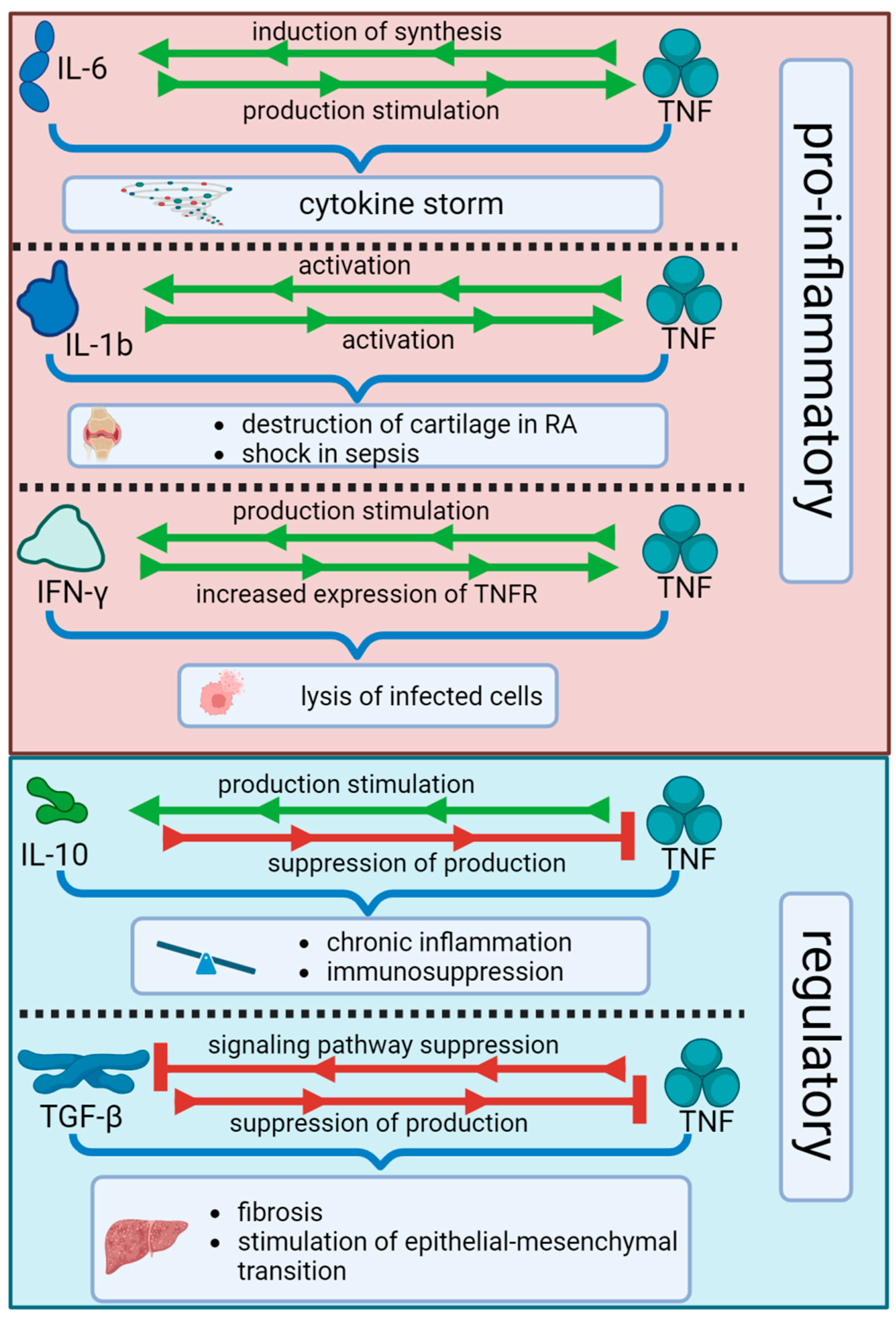
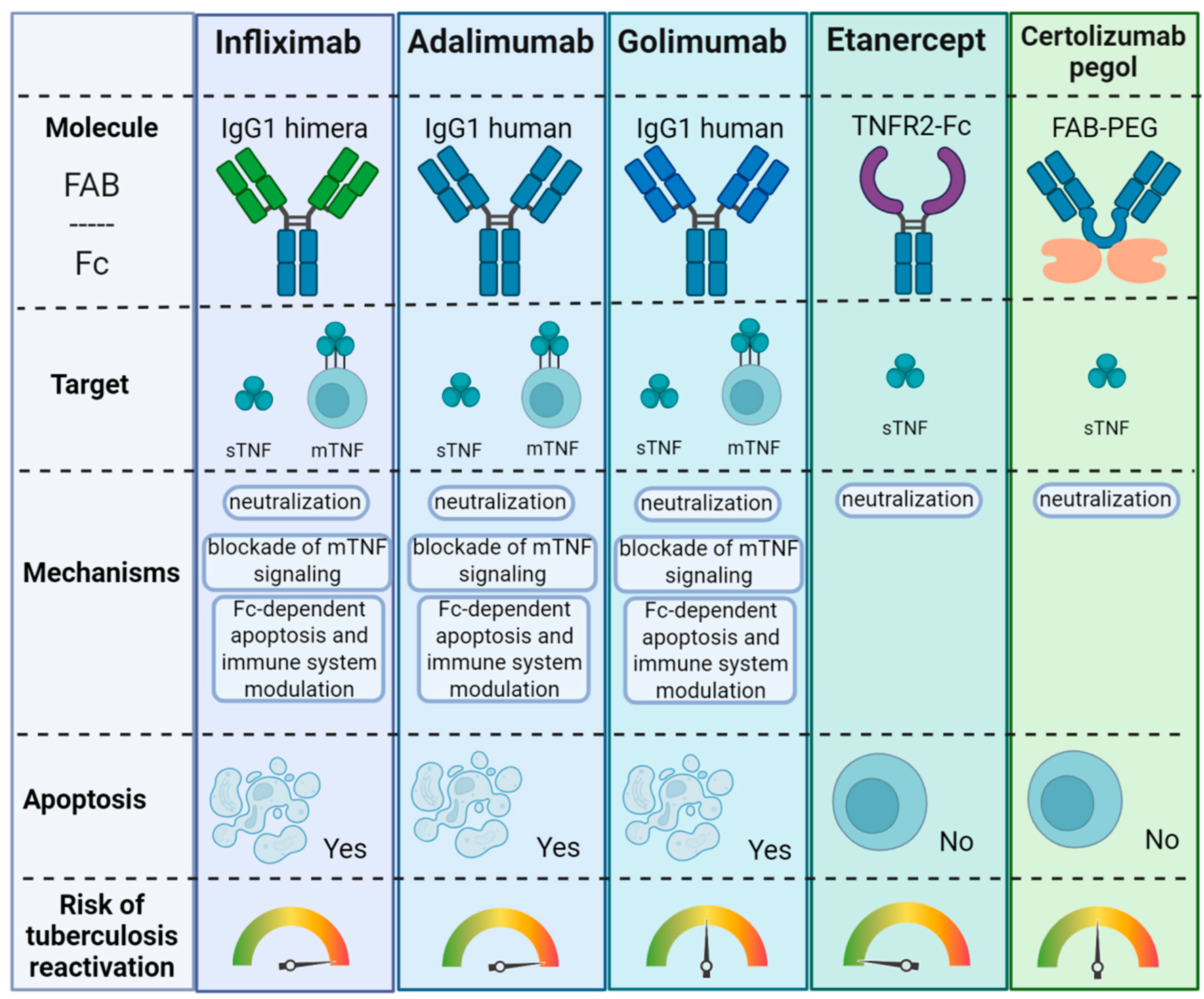
| Function | Cellular Targets | Mechanism | Biological Outcome |
|---|---|---|---|
| Initiation of Inflammatory Response | Endothelium, macrophages, dendritic cells | Induction of IL-1, IL-6, IL-8, GM-CSF, increased permeability and adhesion molecule expression | Recruitment of immune cells to the infection site |
| Activation of Innate Immunity | Macrophages, neutrophils, DCs | Enhances ROS production, phagocytosis, MHC-II, and co-stimulatory molecule expression | Efficient pathogen clearance |
| Maintenance of Granulomas | Macrophages, T cells | Sustains macrophage activation and cell recruitment within granulomas | Latent TB control, containment of M. tuberculosis |
| Adaptive Immunity Stimulation | T lymphocytes, APCs | Enhances migration, antigen presentation, chemokine secretion | Amplified Th1 response and bacterial killing |
| Apoptosis of Infected Cells | Macrophages, epithelial cells, infected APCs | TNFR1 signaling activates caspases via the death domain | Elimination of intracellular pathogen niches |
| Regulation of Systemic Inflammation | Systemic immune network | High TNF levels cause cytokine storms, and affect distant organs | Potential immunopathology: tissue necrosis, shock, multi-organ damage |
| Interaction with Other Cytokines | IFN-γ, IL-1β, IL-10, TGF-β pathways | Modulates expression and effects of synergistic (IFN-γ) and regulatory (IL-10, TGF-β) cytokines | Balanced immune response; dysregulation can lead to immunopathology or tolerance |
| Stimulation of MTB Growth (Pathological) | Monocytes | Inhibition of apoptosis, lysosome fusion; metabolic shift to glycolysis | Persistence and intracellular survival of M. tuberculosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kireev, F.D.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sennikov, S.V. Role of Tumor Necrosis Factor in Tuberculosis. Biomolecules 2025, 15, 709. https://doi.org/10.3390/biom15050709
Kireev FD, Lopatnikova JA, Alshevskaya AA, Sennikov SV. Role of Tumor Necrosis Factor in Tuberculosis. Biomolecules. 2025; 15(5):709. https://doi.org/10.3390/biom15050709
Chicago/Turabian StyleKireev, Fedor D., Julia A. Lopatnikova, Alina A. Alshevskaya, and Sergey V. Sennikov. 2025. "Role of Tumor Necrosis Factor in Tuberculosis" Biomolecules 15, no. 5: 709. https://doi.org/10.3390/biom15050709
APA StyleKireev, F. D., Lopatnikova, J. A., Alshevskaya, A. A., & Sennikov, S. V. (2025). Role of Tumor Necrosis Factor in Tuberculosis. Biomolecules, 15(5), 709. https://doi.org/10.3390/biom15050709






