Exosome-Mediated Chemoresistance in Cancers: Mechanisms, Therapeutic Implications, and Future Directions
Abstract
1. Introduction
2. Dynamic Interplay of Active Molecules Exchanged in Tumor Microenvironment and Chemoresistance
2.1. Intrinsic Mechanisms of Chemoresistance in Tumor Cells
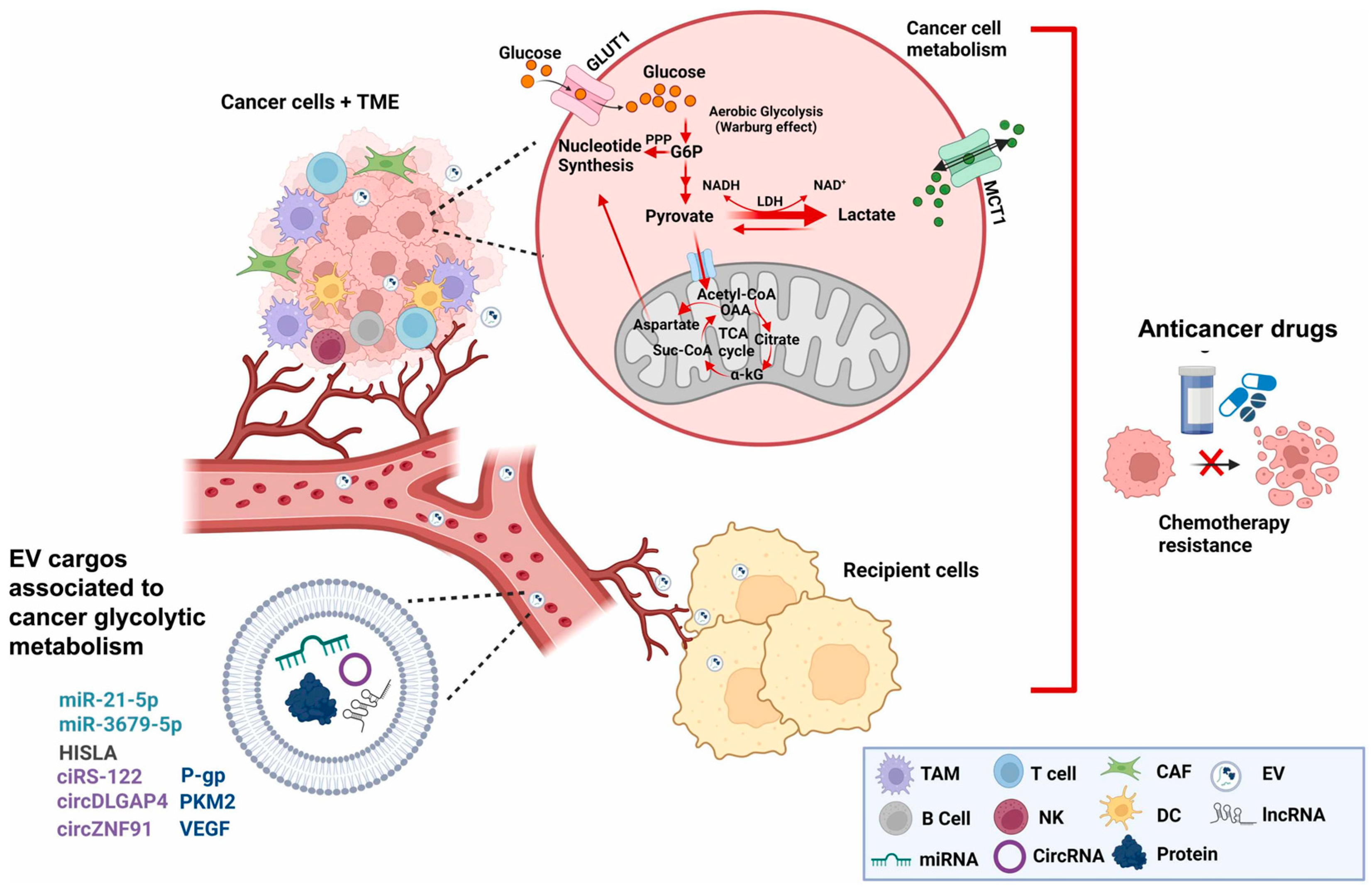
2.2. Exosomal Crosstalk and Metabolic Reprogramming in Cancer Chemoresistance
| Donor Cell | Recipient Cell | Resistance | Exosomal Components | Molecular Mechanisms | Ref. |
|---|---|---|---|---|---|
| Colorectal cancer (R) | Colorectal cancer (S) | Doxorubicin (DOX) | circ_0006174 | circ_0006174/miR-1205/CCND2 axis | [98] |
| 5-fluorouracil | hsa-circ-0004771 | hsa-circ-0004771/miR-653/ZEB2 pathway | [99] | ||
| 5-FU | circ_0000338 | circ_0000338/miR-217, miR-485-3p | [100] | ||
| Recipient T cells | FOLFOX (CRC) | miR-208b | miR-208b/PDCD4 axis | [101] | |
| Colorectal cancer (S) | Colorectal cancer (R) | Oxaliplatin | circular RNA FBXW7 | FBXW7/miR-18b-5p axis | [102] |
| Ovarian cancer (OE) | - | Cisplatin | miR-497 | PI3K/AKT/mTOR pathway | [103] |
| Ovarian cancer (R) (KD) | Macrophages | Cisplatin (OVa) | circ_C20orf11 | circ_C20orf11/miR-527/YWHAZ axis | [104] |
| High-grade serous ovarian cancer (OE) | High-grade serous ovarian cancer (R) | Cisplatin | LncRNA PLADE | LncRNA PLADE/HNRNPD/R-loop | [105] |
| Hepatocellular Carcinoma (R) | Hepatocellular carcinoma (S) | Lenvatinib | Circ-PAK1 | Circ-PAK1/14-3-3ξ/YAP/Hippo | [106] |
| Sorafenib | Circ-SORE | Circ-SORE/YBX1/PRP19 | [107] | ||
| Sorafenib | miR-494-3p | GOLPH3/miR-494-3p/PTEN axis | [108] | ||
| Bone marrow mesenchymal stem cell | Acute myeloid leukemia | Cytarabine | miR-10a | miR-10a/RPRD1A/wnt(β)-catenin pathway | [109] |
| Cytosine arabinoside | FTO | FTO/LncRNAGLCC1/IGF2BP1/c-Myc axis | [110] | ||
| Glioblastoma (R) | Glioblastoma (S) | Temozolomide | circCABIN1 | circCABIN1/miR-637/OLFML3/ErbB | [111] |
| Temozolomide | Cx43 | Bcl-2, Bax and cleaved caspase-3 | [112] | ||
| M2 tumor-associated macrophages | Non-small cell lung cancer | Osimertinib | MSTRG.292666.16 | miR-6836-5p/MAPK8IP3 axis | [113] |
| Non-small cell lung cancer (KD) (OE) | - | EGFR-TKIs | circRNA_102481 | circRNA_102481/miR-30a-5p/ROR1 axis | [114] |
| Non-small cell lung cancer (R) | Non-small cell lung cancer (S) | Gemcitabine | miRNA-222-3p | SOCS3/Stat3 signaling pathway | [115] |
| Cisplatin | miR-4443 | METTL3/FSP1pathway | [116] | ||
| Anlotinib | miR-136-5p | PPP2R2A/AkT pathway | [117] | ||
| Non-small cell lung cancer (KD) | - | Cisplatin | circ_0008928 | miR-488/HK2 Axis | [118] |
| Non-small cell lung cancer (OE) | - | Everolimus | miR-7-5p | MNK/eIF4E axis | [119] |
| Non-small cell lung cancer (S) | Non-small cell lung cancer (R) | Gefitinib | miR-7 | YAP | [120] |
| EGFR+ non-small cell lung cancer (R) | EGFR+ non-small cell lung cancer (S) | Osimertinib | miR-210-3p | - | [121] |
| Small cell lung cancer (KD) (OE) | - | Multidrug | miR-92b-3p | PTEN/AKT pathway | [122] |
| Tumor associated macrophage (hypoxic) | Epithelial ovarian cancer | Cisplatin | miR-223 | miR-223/PTEN/PI3K/AKT pathway | [123] |
| Omental stromal cells | Ovarian cancer | Paclitaxel | miR-21 | miR-21/APAF1 axis | [124] |
| Esophageal cancer (R) | Esophageal cancer (S) | Cisplatin | Circ_0000337 | miR-377-3p/JAK2 axis | [125] |
| Esophageal squamous cell carcinoma (R) | Paclitaxel | PD-L1 | PD-L1/STAT3/miR-21/PTEN/Akt axis | [126] | |
| Lung adenocarcinoma (S) | Lung adenocarcinoma (R) | Docetaxel | LOC85009 | USP5/USF1/ATG5 axis | [127] |
| Nasopharyngeal carcinoma (R) | Nasopharyngeal carcinoma (S) | Cisplatin | miR-106a-5p | miR-106a-5p/ARNT2/AKT axis | [128] |
| Taxol | DDX53 | - | [129] | ||
| Nasopharyngeal carcinoma | NPC-side population cells | Cisplatin | circPARD3 | miR-579-3p/SIRT1/SSRP1 axis | [130] |
| Gastrointestinal stromal tumor (R) | Gastrointestinal stromal tumor (S) | imatinib | - | USP32-Rab35 axis | [131] |
| Prostate cancer (R) | Prostate cancer (S) | Docetaxel | lincROR | lincROR/MYH9/β-catenin/HIF1a | [132] |
| Breast cancer (R) | Breast cancer (S) | Tamoxifen | miR-9-5p | ADIPOQ | [133] |
| DOX and PTX | miR-378a-3p, miR-378d | EZH2/STAT3 axis, DKK3, NUMB | [134] | ||
| Breast cancer stem cell, Breast cancer (R) | Breast cancer (S) | DOX and PTX | miR-155 | FOXO-3a | [64] |
| Triple-negative breast cancer (R) | Tumor associated macrophage | Doxorubicin | miR-770 | miR-770/STMN1 axis | [135] |
| Triple-negative breast cancer (KD) | - | Pirarubicin | CircEGFR | circEGFR/miR-1299/EGFR pathway | [136] |
| Senescent neutrophils | Breast cancer | Docetaxel | piRNA-17560 | FTO/ZEB1 signaling | [137] |
| Breast cancer stem cell (R) | Breast cancer (S) | Paclitaxelb | ANXA6 | YAP1 | [138] |
| Myeloid-derived suppressor cells | Prostate cancer | Castration | circMID1 | S100A9/circMID1/miR-506-3p/MID1 | [139] |
| Gastric cancer (R) | Gastric cancer (S) | Cisplatin | RPS3 | PI3K/Akt/cofilin-1 signaling axis | [140] |
| Doxorubicin | microRNA-501-5p | BLID | [141] | ||
| Gastric cancer (S) | Gastric cancer (R) | 5-FU, cisplatin | miR-107 | HMGA2/mTOR/P-gp pathway | [142] |
| Gastric cancer (KD) | - | Oxaliplatin | miR-374a-5p | Neurod1 | [143] |
| M2 tumor-associated macrophages | Hemangioma stem cells | Propranolol | miR-27a-3p | miR-27a-3p/DKK2 axis | [144] |
| Lung cancer | Cisplatin | miR-3679-5p | miR-3679-5R/NEDD4L/c-Myc axis | [145] | |
| Liver cancer (R) | Liver cancer (S) | Cisplatin | circRNA-G004213 | miR-513b-5p/PRPF39 | [146] |
| Mesenchymal stem cells | Gastric cancer | Cisplatin/vincristine | miR-301b-3p | miR-301b-3p/TXNIP | [147] |
| Breast cancer | Doxorubicin | miR-21-5p | miR-21-5p/S100A6 | [148] | |
| Renal cell carcinoma (R) | Renal cell carcinoma (S) | Sunitinib | LncRNA IGFL2-AS1 | IGFL2-AS1/hnRNPC/TP53INP2 axis | [149] |
| Advanced renal cell carcinoma (R) | Renal cell carcinoma (S) | Sunitinib | lncARSR | lncARSR/miR-34, miR-449/AXL, c-MET | [150] |
| Cancer associated fibroblasts | Myeloid-derived suppressor cells | DDP (ESCC) | miR-21/Non-exo IL-6 | IL-6/exo-miR-21-STAT3 axis | [151] |
| Epithelial ovarian cancer (R) | Epithelial ovarian cancer (S) | Cisplatin | miR-6836 | miR-6836/DLG2/Yap1/TEAD1 axis | [152] |
| Resistant pancreatic cancer stem cell | Pancreatic cancer | Gemcitabine | miR-210 | miR-210/mTOR pathway | [153] |
| Chronic myeloid leukemia (R) | Chronic myeloid leukemia (S) | Imatinib | IFITM3, CD146, CD36 | - | [154] |
| Oral squamous cell carcinoma (R) | Oral squamous cell carcinoma (S) | 5-FU | lncRNA APCDD1L-AS1 | miR-1224-5p/NSD2 axis | [155] |
| Neuroblastoma (R) | Neuroblastoma (S) | Doxorubicin | circDLGAP4 | circDLGAP4/miR-143/HK2 axis | [92] |
| Gliomas (R) | Gliomas (S) | Temozolomide | MIF | TIMP3/PI3K/AKT axis | [156] |
| circWDR62 | Circ-WDR162/miR-370-3p/MGMT | [157] | |||
| Circ_0072083 | miR-1252-5p/ALKBH5/NANOG axis | [158] | |||
| Glioblastoma stem cell | Normal astrocytes (transform to TAA) | Temozolomide | ALKBH7 | ALKBH7/APNG regulation network | [159] |
| Pancreatic cancer (hypoxic) | Pancreatic cancer (normoxic) | Gemcitabine | circZNF91(hypoxic) | miR-23b-3p/SIRT1/HIF-1α axis | [90] |
| Osteosarcoma (R) | Osteosarcoma (S) | Cisplatin | circ_103801 | - | [160] |
| M2 macrophage | Pancreatic cancer | Gemcitabine | miR-222-3p | mTOR/AKT/PI3K pathway and TSC1 | [161] |
| Cancer associated fibroblasts | Colorectal cancer | Oxaliplatin | lncRNA FAL1 | lnc-FAL1/Beclin1 and TRIM3 | [162] |
| multidrug | LINC00355 | LINC00355/miR-34b-5p/CRKL axis | [163] | ||
| Malignant lymphoma | Anti-pyrimidine | miR-4717-5p | miR-4717-5p/ENT2 axis | [164] | |
| Monocytic myeloid-derived suppressor cell | Cisplatin (ESCC) | IL-6 and Exo-miR21 | IL-6, exo-miR21-STAT3 signaling | [151] | |
| Vulvar squamous cell carcinoma | Cisplatin | lncRNA UCA1 | lncRNA UCA1/miR-103a/WEE1 axis | [165] | |
| Non-small cell lung cancer | Cisplatin | miRNA-130a | PUM2-Dependent Packaging | [166] | |
| Cisplatin | microRNA-20a | microRNA-20a/PTEN/PI3K-AKT pathway | [167] | ||
| Colon cancer | Methotrexate | miR-24-3p | miR-24-3p/CDX2/HEPH axis | [168] | |
| Bladder cancer | Cisplatin | LINC00355 | LINC00355/miR-34b-5p/ABCB1 axis | [169] | |
| PTX and DOX | miR-148b-3p | miR-148b-3p/PTEN/Wnt/β-catenin pathway | [170] | ||
| Gastric cancer | Oxaliplatin | DACT3-AS1 (down) | miR-181a-5p/SIRT1 axis | [171] | |
| Ovarian cancer | - | miR-296-3p | PTEN/AKT and SOCS6/STAT3 pathways | [172] | |
| Oral squamous cell carcinoma | Cisplatin | miR-876-3p | miR-876-3p/GATA1/IGFBP33 | [173] | |
| Pancreatic ductal adenocarcinoma | Gemcitabine | miR-3173-5p | miR-3173-5p/ACSL4 pathway | [174] | |
| Platinum | circBIRC6 | circBIRC6/XRCC4/SUMO1 | [175] | ||
| Esophageal squamous cell carcinoma | Cisplatin | RIG-I | RIG-I/IFN-β signaling | [176] | |
| Cancer associated fibroblasts (hypoxic) | Pancreatic cancer | Gemcitabine | miR-21 | HIF-1α/miR-21 and miR-21/RAS/AKT/ERK axis | [177] |
| Breast cancer | Paclitaxel | lncRNA H19 | lncRNA H19/miR-497/DNMT1 | [178] |
3. Drug Efflux and Chemoresistance
3.1. Mechanisms of Drug Efflux in Chemoresistance
3.2. Exosome-Mediated Drug Expulsion
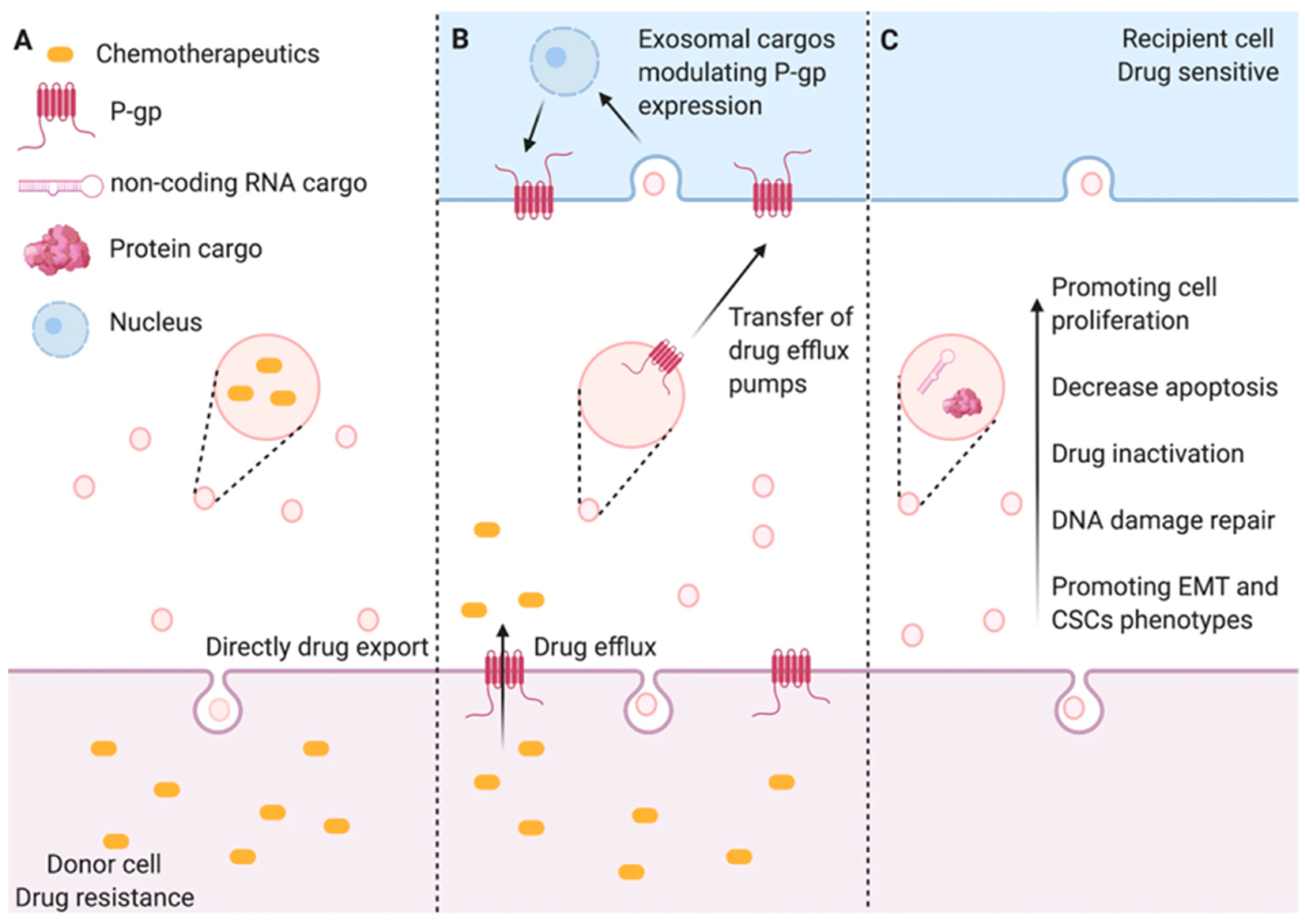
3.3. Distinction Between Efflux Transporters and Exosome-Mediated Efflux
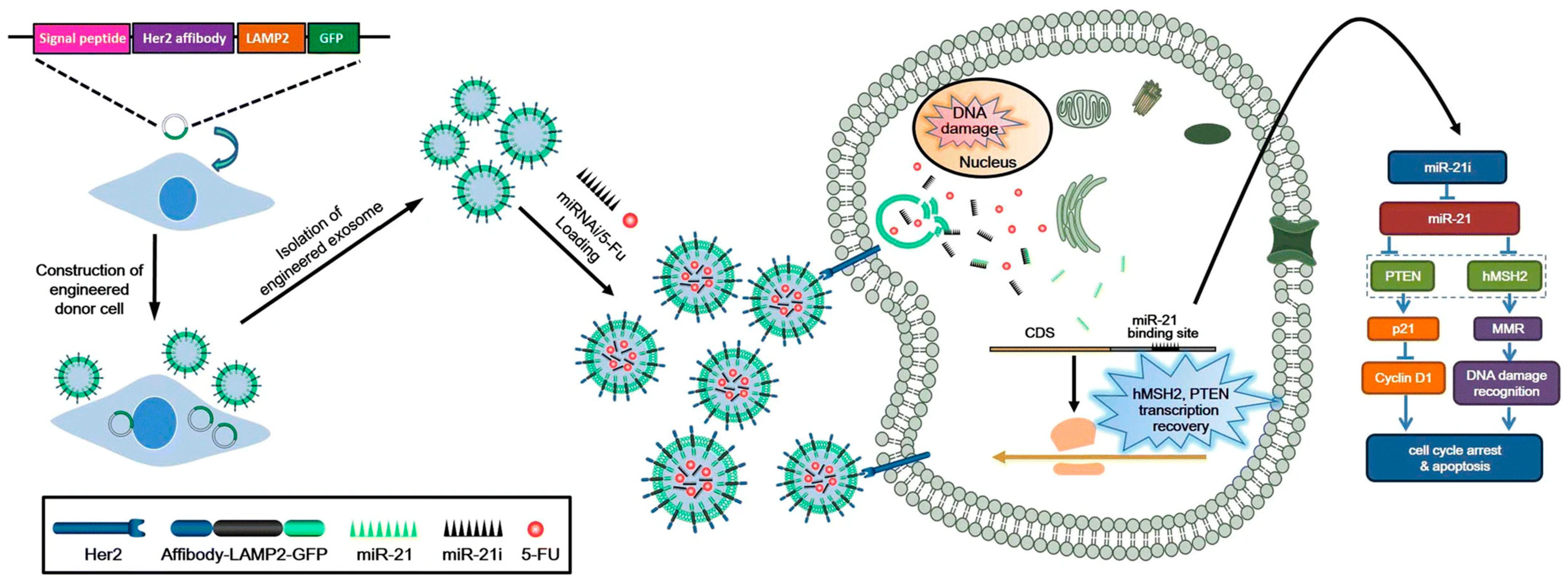
3.4. Therapeutic Potential of Engineered Exosomes
| Cell Type | Resistant Type | Exosomal Content | Mechanism | Ref |
|---|---|---|---|---|
| Human breast adenocarcinoma | Doxorubicin | P-gp | Two transfer modalities including P-gp containing microparticles and tunneling nanotubes | [199] |
| Human osteosarcoma | Doxorubicin | MDR-1/P-gp | Acquisition and dissemination of drug-resistant traits | [220] |
| Human brain endothelial cell | Doxycycline | Pgp-EGFP | A non-genetic way of intercellular transfer of P-gp occurs in non-cancer cells | [221] |
| Breast cancer | Docetaxel | P-gp | Mediate docetaxel resistance transfer in MCF-7 cell | [222] |
| Gastric cancer | Vincristine | CLIC1 | Induce the development of resistance to vincristine and related to upregulated P-gp and Bcl-2 | [223] |
| Hormone-refractory prostate cancer | Docetaxel | MDR-1/P-gp | Influence cellular proliferation, invasion, and response to docetaxel | [224] |
| Breast cancer | Palbociclib | TK1 and CDK9 mRNA expression in plasma-derived exosomes is associated with resistance to palbociclib | [225,226] | |
| Human breast adenocarcinoma and acute lymphoblastic leukemia cell | Doxorubicin | MRP1 | MPs shed from cells with a P-gp dominant resistance profile to re-template a pre-existing MRP1 dominant profile in recipient cells | [200] |
| MDR leukemia and breast cancer | Multidrug | P-gp and MRP1 | Change recipient cells’ transcriptional environment to reflect donor MDR phenotype | [227] |
| Human breast cancer cell | Adriamycin | UCH-L1 and P-gp | Transferring the chemoresistance phenotype in a time-dependent manner | [228] |
| Colorectal Cancer | 5-fluorouracil (5-FU) | p-STAT3 transferred by exosomes from 5-FU-resistant cells could induce chemotherapy resistance in recipient cells by reducing caspase cascade activation | [229,230] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MVBs | multivesicular bodies |
| ILVs | intraluminal vesicles |
| CAFs | cancer-associated fibroblasts |
| HNSCC | head and neck squamous cell carcinoma |
| ncRNAs | non-coding RNAs |
| ABC | ATP-binding cassette |
| BCRP | breast cancer resistance protein |
| SR-BI | scavenger receptor class B type I |
| AMOs | anti-miRNA oligonucleotides |
| OSCC | oral squamous cell carcinoma |
| MET | mesenchymal–epithelial transition |
| 5-FU | 5-fluorouracil |
| EMT | epithelial–mesenchymal transition |
| HCC | hepatocellular carcinoma |
| miRNA | microRNA |
| siRNA | small interfering RNA |
| oncomiRs | oncogenic miRNAs |
| TMZ | temozolomide |
References
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune Surveillance Properties of Human NK Cell-Derived Exosomes. J. Immunol. 2012, 189, 2833–2842. [Google Scholar] [CrossRef]
- Thery, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes: Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef]
- Raposo, G.; Tenza, D.; Mecheri, S.; Peronet, R.; Bonnerot, C.; Desaymard, C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell 1997, 8, 2631–2645. [Google Scholar] [CrossRef]
- van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef]
- Janiszewski, M.; do Carmo, A.O.; Pedro, M.A.; Silva, E.; Knobel, E.; Laurindo, F.R.M. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit. Care Med. 2004, 32, 818–825. [Google Scholar] [CrossRef]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Bu, N.; Yu, Y.-C.; Hua, W.; Xin, X.-Y. Exvivo experiments of human ovarian cancer ascites-derived exosomes presented by dendritic cells derived from umbilical cord blood for immunotherapy treatment. Clin. Med. Oncol. 2008, 2, 461–467. [Google Scholar] [CrossRef]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.A.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.T.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Greenwood, D.L.V.; Cappai, R.; Scheerlinck, J.P.Y.; Hill, A.F. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet. Immunol. Immunopathol. 2008, 124, 385–393. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Duan, X.B.; Geng, X.R.; Xie, J.X.; Gao, H. Antigen-specific activities of CD8+T cells in the nasal mucosa of patients with nasal allergy. Asian Pac. J. Allergy Immunol. 2012, 30, 107–113. [Google Scholar] [PubMed]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef]
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. Faseb J. 2009, 23, 1541–1557. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.E.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Sainte-Marie, J.; Philippot, J.R.; Bienvenue, A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: Evidence precluding a role for “aminophospholipid translocase”. J. Cell. Physiol. 1989, 140, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.X.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Williams, C.; Rodriguez-Barrueco, R.; Silva, J.M.; Zhang, W.J.; Hearn, S.; Elemento, O.; Paknejad, N.; Manova-Todorova, K.; Welte, K.; Bromberg, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar]
- Pan, W.; Miao, Q.; Yin, W.; Li, X.; Ye, W.; Zhang, D.; Deng, L.; Zhang, J.; Chen, M. The role and clinical applications of exosomes in cancer drug resistance. Cancer Drug Resist. 2024, 7, 43. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar]
- Douillard, J.Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V.; et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef]
- Wilting, R.H.; Dannenberg, J.H. Epigenetic mechanisms in tumorigenesis, tumor cell heterogeneity and drug resistance. Drug Resist. Updates 2012, 15, 21–38. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Kohan, H.G.; Asimakopoulos, A.G.; Sudha, T.; Sell, S.; Kannan, K.; Boroujerdi, M.; Davis, P.J.; Mousa, S.A. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget 2016, 7, 50365–50379. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Belisario, D.C.; Rebelo, R.; Assaraf, Y.G.; Giovannetti, E.; Kopecka, J.; Vasconcelos, M.H. The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug Resist. Updates 2022, 62, 100833. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, W.; Zhuo, H.; Zhu, D.; Rong, D.; Sun, J.; Song, J. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/ nuclear factor -kappa B (NF-κB) pathway. Bioengineered 2022, 13, 4786–4797. [Google Scholar] [CrossRef]
- Lyu, T.; Wang, Y.; Li, D.; Yang, H.; Qin, B.; Zhang, W.; Li, Z.; Cheng, C.; Zhang, B.; Guo, R.; et al. Exosomes from BM-MSCs promote acute myeloid leukemia cell proliferation, invasion and chemoresistance via upregulation of S100A4. Exp. Hematol. Oncol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Chen, L.; Yuan, X.; Luo, Q.; Liu, Y.; Xie, G.; Ma, Y.; Shen, L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. CR 2017, 36, 53. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, e9066–e9075. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, K.; Li, M.; Lin, X.; Mei, Y.; Huang, X.; Yang, H. Chemoresistance Transmission via Exosome-Transferred MMP14 in Pancreatic Cancer. Front. Oncol. 2022, 12, 844648. [Google Scholar] [CrossRef]
- Alharbi, M.; Lai, A.; Sharma, S.; Kalita-de Croft, P.; Godbole, N.; Campos, A.; Guanzon, D.; Salas-Burgos, A.; Carrion, F.; Zuñiga, F.A.; et al. Extracellular Vesicle Transmission of Chemoresistance to Ovarian Cancer Cells Is Associated with Hypoxia-Induced Expression of Glycolytic Pathway Proteins, and Prediction of Epithelial Ovarian Cancer Disease Recurrence. Cancers 2021, 13, 3388. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Wang, B. Exosomal transfer of miR-25-3p promotes the proliferation and temozolomide resistance of glioblastoma cells by targeting FBXW7. Int. J. Oncol. 2021, 59, 64. [Google Scholar] [CrossRef]
- Mao, L.; Li, J.; Chen, W.X.; Cai, Y.Q.; Yu, D.D.; Zhong, S.L.; Zhao, J.H.; Zhou, J.W.; Tang, J.H. Exosomes decrease sensitivity of breast cancer cells to adriamycin by delivering microRNAs. Tumour Biol. 2016, 37, 5247–5256. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, J.; Guo, D.; Zhang, S.; Huang, G.; Chen, Y.; Xu, C.; Chen, W.; Zhang, Q.; Zhao, C.; et al. MIAT shuttled by tumor-secreted exosomes promotes paclitaxel resistance in esophageal cancer cells by activating the TAF1/SREBF1 axis. J. Biochem. Mol. Toxicol. 2023, 37, e23380. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, J.; Qian, H.; Yan, Y.; Xu, W. Exosomal circRNA: Emerging insights into cancer progression and clinical application potential. J. Hematol. Oncol. 2023, 16, 67. [Google Scholar] [CrossRef]
- Lin, Z.; Ji, Y.; Zhou, J.; Li, G.; Wu, Y.; Liu, W.; Li, Z.; Liu, T. Exosomal circRNAs in cancer: Implications for therapy resistance and biomarkers. Cancer Lett. 2023, 566, 216245. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Ahmad, A.; Banerjee, S.; Azmi, A.S.; Kong, D.; Sarkar, F.H. Pancreatic cancer: Understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, H.; Bai, M.; Ning, T.; Deng, T.; Liu, R.; Fan, Q.; Zhu, K.; Li, J.; et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol. Oncol. 2020, 14, 539–555. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, L.; Lu, X.; Chen, Z.; Duerksen-Hughes, P.J.; Hu, H.; Zhu, X.; Yang, J. Cisplatin treatment leads to changes in nuclear protein and microRNA expression. Mutat. Res. 2012, 746, 66–77. [Google Scholar] [CrossRef]
- Guo, X.; Gao, C.; Yang, D.H.; Li, S. Exosomal circular RNAs: A chief culprit in cancer chemotherapy resistance. Drug Resist. Updates 2023, 67, 100937. [Google Scholar] [CrossRef]
- Qin, X.; Guo, H.; Wang, X.; Zhu, X.; Yan, M.; Wang, X.; Xu, Q.; Shi, J.; Lu, E.; Chen, W.; et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019, 20, 12. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Peixoto, A.; Neves, M.; Gaiteiro, C.; Reis, C.A.; Assaraf, Y.G.; Santos, L.L. Mechanisms of cisplatin resistance and targeting of cancer stem cells: Adding glycosylation to the equation. Drug Resist. Updates 2016, 24, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.; Phillips, L.M.; Shahar, T.; Hossain, A.; Gumin, J.; Kim, H.; Bean, A.J.; Calin, G.A.; Fueyo, J.; Walters, E.T.; et al. Exosomes from Glioma-Associated Mesenchymal Stem Cells Increase the Tumorigenicity of Glioma Stem-like Cells via Transfer of miR-1587. Cancer Res. 2017, 77, 5808–5819. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Mu, L.; Huang, K.; Li, X.; Tao, D.; Wu, Y.; Qin, J. Fibroblast-Derived Exosomes Contribute to Chemoresistance through Priming Cancer Stem Cells in Colorectal Cancer. PLoS ONE 2015, 10, e0125625. [Google Scholar] [CrossRef]
- Jie, Z.; Qianqian, S.; Mengna, W.; Wenjie, Z. The Emerging Roles of Exosomes in the Chemoresistance of Hepatocellular Carcinoma. Curr. Med. Chem. 2021, 28, 93–109. [Google Scholar]
- Santos, J.C.; Lima, N.d.S.; Sarian, L.O.; Matheu, A.; Ribeiro, M.L.; Derchain, S.F.M. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci. Rep. 2018, 8, 829. [Google Scholar] [CrossRef]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.T.; Lee, W.T.; Abedini, M.R.; Wang, P.W.; Shieh, D.B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef]
- Safaei, R.; Larson, B.J.; Cheng, T.C.; Gibson, M.A.; Otani, S.; Naerdemann, W.; Howell, S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005, 4, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiao, D.; Chen, L.; Xu, M.; Chen, S.; Huang, L.; Wang, F.; Chen, Z.; Cai, J.; Fu, L. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Mol. Cancer 2019, 18, 182. [Google Scholar] [CrossRef]
- Wang, Z.; He, J.; Bach, D.H.; Huang, Y.H.; Li, Z.; Liu, H.; Lin, P.; Yang, J. Induction of m(6)A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. J. Exp. Clin. Cancer Res. CR 2022, 41, 4. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.P.; et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Luo, M.; To, K.K.W.; Zhang, J.; Su, C.; Zhang, H.; An, S.; Wang, F.; Chen, D.; Fu, L. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol. Cancer 2021, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Polónia, B.; Xavier, C.P.R.; Kopecka, J.; Riganti, C.; Vasconcelos, M.H. The role of Extracellular Vesicles in glycolytic and lipid metabolic reprogramming of cancer cells: Consequences for drug resistance. Cytokine Growth Factor Rev. 2023, 73, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Comandatore, A.; Franczak, M.A.; Smolenski, R.T.; Peters, G.J.; Morelli, L.; Giovannetti, E. Role of exosomes in transferring chemoresistance through modulation of cancer glycolytic cell metabolism. Cytokine Growth Factor Rev. 2023, 73, 163–172. [Google Scholar] [CrossRef]
- Zeng, A.; Wei, Z.; Yan, W.; Yin, J.; Huang, X.; Zhou, X.; Li, R.; Shen, F.; Wu, W.; Wang, X.; et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018, 436, 10–21. [Google Scholar] [CrossRef]
- Hui, B.; Zhou, C.; Xu, Y.; Wang, R.; Dong, Y.; Zhou, Y.; Ding, J.; Zhang, X.; Xu, J.; Gu, Y. Exosomes secreted by Fusobacterium nucleatum-infected colon cancer cells transmit resistance to oxaliplatin and 5-FU by delivering hsa_circ_0004085. J. Nanobiotechnol. 2024, 22, 62. [Google Scholar] [CrossRef]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef]
- Sun, C.; Huang, X.; Li, J.; Fu, Z.; Hua, Y.; Zeng, T.; He, Y.; Duan, N.; Yang, F.; Liang, Y.; et al. Exosome-Transmitted tRF-16-K8J7K1B Promotes Tamoxifen Resistance by Reducing Drug-Induced Cell Apoptosis in Breast Cancer. Cancers 2023, 15, 899. [Google Scholar] [CrossRef]
- Meng, C.; Yang, Y.; Feng, W.; Ma, P.; Bai, R. Exosomal miR-331-3p derived from chemoresistant osteosarcoma cells induces chemoresistance through autophagy. J. Orthop. Surg. Res. 2023, 18, 892. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.; Li, C.; Fang, S.R.; Liu, W.F.; Qian, Y.Y.; Ma, J.Y.; Chang, L.G.; Chen, F.F.; Yang, Z.H.; et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 2022, 526, 142–154. [Google Scholar] [CrossRef]
- Balaji, S.; Kim, U.; Muthukkaruppan, V.; Vanniarajan, A. Emerging role of tumor microenvironment derived exosomes in therapeutic resistance and metastasis through epithelial-to-mesenchymal transition. Life Sci. 2021, 280, 119750. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Pan, T.; Lv, C.; Cao, L.; Li, L.; Zhou, X.; Li, G.; Li, H.; Vicencio, J.M.; Xu, Y.; et al. Exosomal transfer leads to chemoresistance through oxidative phosphorylation-mediated stemness phenotype in colorectal cancer. Theranostics 2023, 13, 5057–5074. [Google Scholar] [CrossRef]
- Ma, Y.; Yuwen, D.; Chen, J.; Zheng, B.; Gao, J.; Fan, M.; Xue, W.; Wang, Y.; Li, W.; Shu, Y.; et al. Exosomal Transfer Of Cisplatin-Induced miR-425-3p Confers Cisplatin Resistance In NSCLC Through Activating Autophagy. Int. J. Nanomed. 2019, 14, 8121–8132. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zang, W.; Qiu, Y.; Liao, L.; Zheng, X. Deubiquitinase OTUB2 exacerbates the progression of colorectal cancer by promoting PKM2 activity and glycolysis. Oncogene 2022, 41, 46–56. [Google Scholar] [CrossRef]
- Jing, Y.Y.; Cai, F.F.; Zhang, L.; Han, J.; Yang, L.; Tang, F.; Li, Y.B.; Chang, J.F.; Sun, F.; Yang, X.M.; et al. Epigenetic regulation of the Warburg effect by H2B monoubiquitination. Cell Death Differ. 2020, 27, 1660–1676. [Google Scholar] [CrossRef]
- Petanidis, S.; Domvri, K.; Porpodis, K.; Anestakis, D.; Freitag, L.; Hohenforst-Schmidt, W.; Tsavlis, D.; Zarogoulidis, K. Inhibition of kras-derived exosomes downregulates immunosuppressive BACH2/GATA-3 expression via RIP-3 dependent necroptosis and miR-146/miR-210 modulation. Biomed. Pharmacother. 2020, 122, 109461. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, Y.; Chen, Q.; Zhu, S.; Niu, Y.; Ye, Z.; Hu, P.; Chen, D.; Xu, P.; Chen, J.; et al. Hypoxic exosomal HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene 2021, 40, 5505–5517. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, C.; Xu, F.; Zhang, A.; Jin, M.; Zhang, K.; Liu, L.; Hua, Q.; Zhao, J.; Liu, J.; et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics 2021, 11, 2860–2875. [Google Scholar] [CrossRef]
- Tan, W.Q.; Yuan, L.; Wu, X.Y.; He, C.G.; Zhu, S.C.; Ye, M. Exosome-delivered circular RNA DLGAP4 induces chemoresistance via miR-143-HK2 axis in neuroblastoma. Cancer Biomark. Sect. A Dis. Markers 2022, 34, 375–384. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, B.; Liu, X.; Lin, L.; Wang, L.; Hong, Z.; Chen, J. Exosomal miR-21-5p derived from cisplatin-resistant SKOV3 ovarian cancer cells promotes glycolysis and inhibits chemosensitivity of its progenitor SKOV3 cells by targeting PDHA1. Cell Biol. Int. 2021, 45, 2140–2149. [Google Scholar] [CrossRef]
- Nguyen Cao, T.G.; Truong Hoang, Q.; Kang, J.H.; Kang, S.J.; Ravichandran, V.; Rhee, W.J.; Lee, M.; Ko, Y.T.; Shim, M.S. Bioreducible exosomes encapsulating glycolysis inhibitors potentiate mitochondria-targeted sonodynamic cancer therapy via cancer-targeted drug release and cellular energy depletion. Biomaterials 2023, 301, 122242. [Google Scholar] [CrossRef]
- Wu, S.; Yun, J.; Tang, W.; Familiari, G.; Relucenti, M.; Wu, J.; Li, X.; Chen, H.; Chen, R. Therapeutic m6A Eraser ALKBH5 mRNA-Loaded Exosome–Liposome Hybrid Nanoparticles Inhibit Progression of Colorectal Cancer in Preclinical Tumor Models. ACS Nano 2023, 17, 11838–11854. [Google Scholar] [CrossRef]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K.; et al. MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Yu, J.; Zhang, J.F.; Wang, C. Suppressing the secretion of exosomal miR-19b by gw4869 could regulate oxaliplatin sensitivity in colorectal cancer. Neoplasma 2019, 66, 39–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Lu, Y. Exosomal transfer of circ_0006174 contributes to the chemoresistance of doxorubicin in colorectal cancer by depending on the miR-1205/CCND2 axis. J. Physiol. Biochem. 2022, 78, 39–50. [Google Scholar] [CrossRef]
- Qiao, X.X.; Shi, H.B.; Xiao, L. Serum exosomal hsa-circ-0004771 modulates the resistance of colorectal cancer to 5-fluorouracil via regulating miR-653/ZEB2 signaling pathway. Cancer Cell Int. 2023, 23, 243. [Google Scholar] [CrossRef]
- Zhao, K.; Cheng, X.; Ye, Z.; Li, Y.; Peng, W.; Wu, Y.; Xing, C. Exosome-Mediated Transfer of circ_0000338 Enhances 5-Fluorouracil Resistance in Colorectal Cancer through Regulating MicroRNA 217 (miR-217) and miR-485-3p. Mol. Cell. Biol. 2021, 41, e00517-20. [Google Scholar] [CrossRef]
- Ning, T.; Li, J.; He, Y.; Zhang, H.; Wang, X.; Deng, T.; Liu, R.; Li, H.; Bai, M.; Fan, Q.; et al. Exosomal miR-208b related with oxaliplatin resistance promotes Treg expansion in colorectal cancer. Mol. Ther. 2021, 29, 2723–2736. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, A.; Peng, F.; Tan, X.; Wang, J.; Gong, X. Exosomal transfer of circular RNA FBXW7 ameliorates the chemoresistance to oxaliplatin in colorectal cancer by sponging miR-18b-5p. Neoplasma 2021, 68, 108–118. [Google Scholar] [CrossRef]
- Li, L.; He, D.; Guo, Q.; Zhang, Z.; Ru, D.; Wang, L.; Gong, K.; Liu, F.; Duan, Y.; Li, H. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J. Nanobiotechnol. 2022, 20, 50. [Google Scholar] [CrossRef]
- Yin, J.; Huang, H.Y.; Long, Y.; Ma, Y.; Kamalibaike, M.; Dawuti, R.; Li, L. circ_C20orf11 enhances DDP resistance by inhibiting miR-527/YWHAZ through the promotion of extracellular vesicle-mediated macrophage M2 polarization in ovarian cancer. Cancer Biol. Ther. 2021, 22, 440–454. [Google Scholar] [CrossRef]
- Liu, H.; Deng, S.; Yao, X.; Liu, Y.; Qian, L.; Wang, Y.; Zhang, T.; Shan, G.; Chen, L.; Zhou, Y. Ascites exosomal lncRNA PLADE enhances platinum sensitivity by inducing R-loops in ovarian cancer. Oncogene 2024, 43, 714–728. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, Y.; Shi, X.; Liu, H.; Zheng, Z.; Han, G.; Rong, D.; Zhang, C.; Tang, W.; Wang, X. CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3ζ. J. Exp. Clin. Cancer Res. CR 2022, 41, 281. [Google Scholar] [CrossRef]
- Xu, J.; Ji, L.; Liang, Y.; Wan, Z.; Zheng, W.; Song, X.; Gorshkov, K.; Sun, Q.; Lin, H.; Zheng, X.; et al. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct. Target. Ther. 2020, 5, 298. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, Z.; Qi, Y.; Peng, H.; Ma, W.; Wang, R.; Li, W. Golgi phosphoprotein 3 promotes angiogenesis and sorafenib resistance in hepatocellular carcinoma via upregulating exosomal miR-494-3p. Cancer Cell Int. 2022, 22, 35. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Li, X.; Ren, J.; Chen, L.; Chen, J.; Cao, Y. Exosomes from bone marrow mesenchymal stem cells decrease chemosensitivity of acute myeloid leukemia cells via delivering miR-10a. Biochem. Biophys. Res. Commun. 2022, 622, 149–156. [Google Scholar] [CrossRef]
- Kou, R.; Li, T.; Fu, C.; Jiang, D.; Wang, Y.; Meng, J.; Zhong, R.; Liang, C.; Dong, M. Exosome-shuttled FTO from BM-MSCs contributes to cancer malignancy and chemoresistance in acute myeloid leukemia by inducing m6A-demethylation: A nano-based investigation. Environ. Res. 2024, 244, 117783. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Q.; Gao, G.; Cao, Z.; Guan, Z.; Jia, B.; Wang, W.; Zhang, K.; Zhang, W.; Wang, S.; et al. Exosome-transmitted circCABIN1 promotes temozolomide resistance in glioblastoma via sustaining ErbB downstream signaling. J. Nanobiotechnol. 2023, 21, 45. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhang, L.L.; Bi, Q.C.; Gan, L.J.; Wei, M.J.; Hong, T.; Tan, R.J.; Lan, X.M.; Liu, L.H.; Han, X.J.; et al. Exosomal connexin 43 regulates the resistance of glioma cells to temozolomide. Oncol. Rep. 2021, 45, 44. [Google Scholar] [CrossRef]
- Wan, X.; Xie, B.; Sun, H.; Gu, W.; Wang, C.; Deng, Q.; Zhou, S. Exosomes derived from M2 type tumor-associated macrophages promote osimertinib resistance in non-small cell lung cancer through MSTRG.292666.16-miR-6836-5p-MAPK8IP3 axis. Cancer Cell Int. 2022, 22, 83. [Google Scholar] [CrossRef]
- Yang, B.; Teng, F.; Chang, L.; Wang, J.; Liu, D.L.; Cui, Y.S.; Li, G.H. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging 2021, 13, 13264–13286. [Google Scholar] [CrossRef]
- Wei, F.; Ma, C.; Zhou, T.; Dong, X.; Luo, Q.; Geng, L.; Ding, L.; Zhang, Y.; Zhang, L.; Li, N.; et al. Correction to: Exosomes derived from gemcitabine resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol. Cancer 2021, 20, 35. [Google Scholar] [CrossRef]
- Song, Z.; Jia, G.; Ma, P.; Cang, S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021, 276, 119399. [Google Scholar] [CrossRef]
- Gu, G.; Hu, C.; Hui, K.; Zhang, H.; Chen, T.; Zhang, X.; Jiang, X. Exosomal miR-136-5p Derived from Anlotinib-Resistant NSCLC Cells Confers Anlotinib Resistance in Non-Small Cell Lung Cancer Through Targeting PPP2R2A. Int. J. Nanomed. 2021, 16, 6329–6343. [Google Scholar] [CrossRef]
- Shi, Q.; Ji, T.; Ma, Z.; Tan, Q.; Liang, J. Serum Exosomes-Based Biomarker circ_0008928 Regulates Cisplatin Sensitivity, Tumor Progression, and Glycolysis Metabolism by miR-488/HK2 Axis in Cisplatin-Resistant Nonsmall Cell Lung Carcinoma. Cancer Biother. Radiopharm. 2023, 38, 558–571. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Ning, Y.; Zheng, H.; Zhan, Y.; Wang, H.; Yang, Y.; Luo, J.; Wen, Q.; Zang, H.; et al. Exosome-mediated miR-7-5p delivery enhances the anticancer effect of Everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer. Cell Death Dis. 2022, 13, 129. [Google Scholar] [CrossRef]
- Chen, R.; Qian, Z.; Xu, X.; Zhang, C.; Niu, Y.; Wang, Z.; Sun, J.; Zhang, X.; Yu, Y. Exosomes-transmitted miR-7 reverses gefitinib resistance by targeting YAP in non-small-cell lung cancer. Pharmacol. Res. 2021, 165, 105442. [Google Scholar] [CrossRef]
- Hisakane, K.; Seike, M.; Sugano, T.; Yoshikawa, A.; Matsuda, K.; Takano, N.; Takahashi, S.; Noro, R.; Gemma, A. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial-mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thoracic. Cancer 2021, 12, 1690–1698. [Google Scholar] [CrossRef]
- Li, M.; Shan, W.; Hua, Y.; Chao, F.; Cui, Y.; Lv, L.; Dou, X.; Bian, X.; Zou, J.; Li, H.; et al. Exosomal miR-92b-3p Promotes Chemoresistance of Small Cell Lung Cancer Through the PTEN/AKT Pathway. Front. Cell Dev. Biol. 2021, 9, 661602. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, H.; Yin, X.; Yang, M.; Wei, H.; Chen, Q.; Feng, F.; Liu, Y.; Xu, W.; Li, Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp. Clin. Cancer Res. CR 2019, 38, 81. [Google Scholar] [CrossRef]
- Au Yeung, C.L.; Co, N.N.; Tsuruga, T.; Yeung, T.L.; Kwan, S.Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.K.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7, 11150. [Google Scholar] [CrossRef]
- Zang, R.; Qiu, X.; Song, Y.; Wang, Y. Exosomes Mediated Transfer of Circ_0000337 Contributes to Cisplatin (CDDP) Resistance of Esophageal Cancer by Regulating JAK2 via miR-377-3p. Front. Cell Dev. Biol. 2021, 9, 673237. [Google Scholar] [CrossRef]
- Wang, H.; Qi, Y.; Lan, Z.; Liu, Q.; Xu, J.; Zhu, M.; Yang, T.; Shi, R.; Gao, S.; Liang, G. Exosomal PD-L1 confers chemoresistance and promotes tumorigenic properties in esophageal cancer cells via upregulating STAT3/miR-21. Gene Ther. 2023, 30, 88–100. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, H.; Chen, S.; Xie, Y.; Shi, L.; Xia, S.; Jiang, M.; Li, J.; Chen, D. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist. Updates 2023, 67, 100915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, C.; Chao, H.; Zhang, Y.; Li, Y.; Hou, J.; Huang, L. Exosomal transfer of miR-106a-5p contributes to cisplatin resistance and tumorigenesis in nasopharyngeal carcinoma. J. Cell. Mol. Med. 2021, 25, 9183–9198. [Google Scholar] [CrossRef]
- Yuan, F.; Zhou, Z.F. Exosomes derived from Taxol-resistant nasopharyngeal carcinoma (NPC) cells transferred DDX53 to NPC cells and promoted cancer resistance to Taxol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 127–138. [Google Scholar]
- Ai, J.; Tan, G.; Li, W.; Liu, H.; Li, T.; Zhang, G.; Zhou, Z.; Gan, Y. Exosomes loaded with circPARD3 promotes EBV-miR-BART4-induced stemness and cisplatin resistance in nasopharyngeal carcinoma side population cells through the miR-579-3p/SIRT1/SSRP1 axis. Cell Biol. Toxicol. 2023, 39, 537–556. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z.; Cui, Z.; Liu, Z.; Bian, Y.; Sun, H.; Wang, N.; He, Z.; Li, B.; Li, F.; et al. Deubiquitylation of Rab35 by USP32 promotes the transmission of imatinib resistance by enhancing exosome secretion in gastrointestinal stromal tumours. Oncogene 2023, 42, 894–910. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Y.; Liu, R.; Guo, S. Exosomal lincROR Promotes Docetaxel Resistance in Prostate Cancer through a β-catenin/HIF1α Positive Feedback Loop. Mol. Cancer Res. 2023, 21, 472–482. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Tang, W.; Huang, Q.; Mei, Y.; Yang, H. Correction to: Exosomes from tamoxifen-resistant breast cancer cells transmit drug resistance partly by delivering miR-9-5p. Cancer Cell Int. 2021, 21, 673. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, S.; Shi, Z.; Cao, L.; Liu, J.; Pan, T.; Zhou, D.; Zhang, J. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J. Exp. Clin. Cancer Res. CR 2021, 40, 120. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Sang, Y.; Song, X.; Zhang, H.; Liu, Y.; Jiang, L.; Yang, Q. MiR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1. Cell Death Dis. 2018, 9, 14. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.; Fan, Z.; Zhang, Y.; Ji, J.; Wei, D.; Zhang, F.; Sun, B.; Huang, P.; Ren, L. CircEGFR reduces the sensitivity of pirarubicin and regulates the malignant progression of triple-negative breast cancer via the miR-1299/EGFR axis. Int. J. Biol. Macromol. 2023, 244, 125295. [Google Scholar] [CrossRef]
- Ou, B.; Liu, Y.; Gao, Z.; Xu, J.; Yan, Y.; Li, Y.; Zhang, J. Senescent neutrophils-derived exosomal piRNA-17560 promotes chemoresistance and EMT of breast cancer via FTO-mediated m6A demethylation. Cell Death Dis. 2022, 13, 905. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, A.; Zhou, C. Breast Cancer Stem Cell-Derived ANXA6-Containing Exosomes Sustain Paclitaxel Resistance and Cancer Aggressiveness in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 718721. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Q.; Tang, Z.; Zhang, N.; Huang, Y.; Li, Z.; Dai, Y.; Yu, Q.; Zhu, J. Exosomes derived from myeloid-derived suppressor cells facilitate castration-resistant prostate cancer progression via S100A9/circMID1/miR-506-3p/MID1. J. Transl. Med. 2022, 20, 346. [Google Scholar] [CrossRef]
- Sun, M.Y.; Xu, B.; Wu, Q.X.; Chen, W.L.; Cai, S.; Zhang, H.; Tang, Q.F. Cisplatin-Resistant Gastric Cancer Cells Promote the Chemoresistance of Cisplatin-Sensitive Cells via the Exosomal RPS3-Mediated PI3K-Akt-Cofilin-1 Signaling Axis. Front. Cell Dev. Biol. 2021, 9, 618899. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Xu, Y.; Hou, S.; Huang, J.; Wang, B.; Zhao, J.; Xia, S.; Fan, S.; Yu, X.; et al. Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer. Cancer Lett. 2019, 459, 122–134. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Guo, L.; Liu, C.; Wang, P.; Ren, W. Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway. BMC Cancer 2021, 21, 1290. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, X.; Gu, H.; Ma, J.; Wen, X.; Zhou, J.; Qian, H.; Xu, W.; Qian, J.; Lin, J. miR-374a-5p: A New Target for Diagnosis and Drug Resistance Therapy in Gastric Cancer. Mol. Ther. Nucleic Acids 2019, 18, 320–331. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Guo, S.; Zhang, L.; Fan, X.; Zheng, J. Exosomal miR-27a-3p derived from tumor-associated macrophage suppresses propranolol sensitivity in infantile hemangioma. Cell. Immunol. 2021, 370, 104442. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Pan, H.; Wang, Y.; Shi, M.; Yu, H.; Wang, C.; Pan, X.; Chen, Z. Exosomes Derived From Macrophages Enhance Aerobic Glycolysis and Chemoresistance in Lung Cancer by Stabilizing c-Myc via the Inhibition of NEDD4L. Front. Cell Dev. Biol. 2020, 8, 620603. [Google Scholar] [CrossRef]
- Qin, L.; Zhan, Z.; Wei, C.; Li, X.; Zhang, T.; Li, J. Hsa-circRNA-G004213 promotes cisplatin sensitivity by regulating miR-513b-5p/PRPF39 in liver cancer. Mol. Med. Rep. 2021, 23, 421. [Google Scholar] [CrossRef]
- Zhu, T.; Hu, Z.; Wang, Z.; Ding, H.; Li, R.; Wang, J.; Wang, G. microRNA-301b-3p from mesenchymal stem cells-derived extracellular vesicles inhibits TXNIP to promote multidrug resistance of gastric cancer cells. Cell Biol. Toxicol. 2023, 39, 1923–1937. [Google Scholar] [CrossRef]
- Luo, T.; Liu, Q.; Tan, A.; Duan, L.; Jia, Y.; Nong, L.; Tang, J.; Zhou, W.; Xie, W.; Lu, Y.; et al. Mesenchymal Stem Cell-Secreted Exosome Promotes Chemoresistance in Breast Cancer via Enhancing miR-21-5p-Mediated S100A6 Expression. Mol. Ther. Oncolytics 2020, 19, 283–293. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Shu, G.; Cen, J.; Lu, J.; Zhou, M.; Huang, K.; Dong, J.; Li, J.; Lin, H.; et al. Extracellular Vesicle-Mediated Transfer of LncRNA IGFL2-AS1 Confers Sunitinib Resistance in Renal Cell Carcinoma. Cancer Res. 2023, 83, 103–116. [Google Scholar] [CrossRef]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.F.; et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell 2016, 29, 653–668. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, L.; Qin, G.; Qiao, Y.; Ren, F.; Shen, C.; Wang, S.; Liu, S.; Lian, J.; Wang, D.; et al. Cancer-associated fibroblasts induce monocytic myeloid-derived suppressor cell generation via IL-6/exosomal miR-21-activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma. Cancer Lett. 2021, 518, 35–48. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, Z.; Wang, J.; Ma, L.; Liu, Y.; Sun, L.; Song, Y. Extracellular vesicles carrying miR-6836 derived from resistant tumor cells transfer cisplatin resistance of epithelial ovarian cancer via DLG2-YAP1 signaling pathway. Int. J. Biol. Sci. 2023, 19, 3099–3114. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell. Oncol. 2020, 43, 123–136. [Google Scholar] [CrossRef]
- Hrdinova, T.; Toman, O.; Dresler, J.; Klimentova, J.; Salovska, B.; Pajer, P.; Bartos, O.; Polivkova, V.; Linhartova, J.; Machova Polakova, K.; et al. Exosomes released by imatinib-resistant K562 cells contain specific membrane markers, IFITM3, CD146 and CD36 and increase the survival of imatinib-sensitive cells in the presence of imatinib. Int. J. Oncol. 2021, 58, 238–250. [Google Scholar] [CrossRef]
- Li, S.; Shi, Z.; Fu, S.; Li, Q.; Li, B.; Sang, L.; Wu, D. Exosomal-mediated transfer of APCDD1L-AS1 induces 5-fluorouracil resistance in oral squamous cell carcinoma via miR-1224-5p/nuclear receptor binding SET domain protein 2 (NSD2) axis. Bioengineered 2021, 12, 7188–7204. [Google Scholar] [CrossRef]
- Wei, Q.T.; Liu, B.Y.; Ji, H.Y.; Lan, Y.F.; Tang, W.H.; Zhou, J.; Zhong, X.Y.; Lian, C.L.; Huang, Q.Z.; Wang, C.Y.; et al. Exosome-mediated transfer of MIF confers temozolomide resistance by regulating TIMP3/PI3K/AKT axis in gliomas. Mol. Ther. Oncolytics 2021, 22, 114–128. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Lin, X.; Zeng, Z.; Hu, J.; Hao, L.; Xu, J.; Wang, X.; Wang, H.; Li, Q. Exosomal circWDR62 promotes temozolomide resistance and malignant progression through regulation of the miR-370-3p/MGMT axis in glioma. Cell Death Dis. 2022, 13, 596. [Google Scholar] [CrossRef]
- Ding, C.; Yi, X.; Chen, X.; Wu, Z.; You, H.; Chen, X.; Zhang, G.; Sun, Y.; Bu, X.; Wu, X.; et al. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. J. Exp. Clin. Cancer Res. CR 2021, 40, 164. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Wu, A.; Huang, S.; Xu, Z.; Zhang, X.; Li, Z.; Li, H.; Dong, J. Transformed astrocytes confer temozolomide resistance on glioblastoma via delivering ALKBH7 to enhance APNG expression after educating by glioblastoma stem cells-derived exosomes. CNS Neurosci. Ther. 2024, 30, e14599. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.; Mi, C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol. Int. 2021, 45, 858–868. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Xiong, J.; Gou, S.; Cui, J.; Peng, T. miR-222-3p-containing macrophage-derived extracellular vesicles confer gemcitabine resistance via TSC1-mediated mTOR/AKT/PI3K pathway in pancreatic cancer. Cell Biol. Toxicol. 2023, 39, 1203–1214. [Google Scholar] [CrossRef]
- Zhu, S.; Mao, J.; Zhang, X.; Wang, P.; Zhou, Y.; Tong, J.; Peng, H.; Yang, B.; Fu, Q. CAF-derived exosomal lncRNA FAL1 promotes chemoresistance to oxaliplatin by regulating autophagy in colorectal cancer. Dig. Liver Dis. 2023, 56, 330–342. [Google Scholar] [CrossRef]
- Hu, J.H.; Tang, H.N.; Wang, Y.H. Cancer-associated fibroblast exosome LINC00355 promotes epithelial-mesenchymal transition and chemoresistance in colorectal cancer through the miR-34b-5p/CRKL axis. Cancer Gene Ther. 2024, 31, 259–272. [Google Scholar] [CrossRef]
- Kunou, S.; Shimada, K.; Takai, M.; Sakamoto, A.; Aoki, T.; Hikita, T.; Kagaya, Y.; Iwamoto, E.; Sanada, M.; Shimada, S.; et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma. Oncogene 2021, 40, 3989–4003. [Google Scholar] [CrossRef]
- Gao, Q.; Fang, X.; Chen, Y.; Li, Z.; Wang, M. Exosomal lncRNA UCA1 from cancer-associated fibroblasts enhances chemoresistance in vulvar squamous cell carcinoma cells. J. Obstet. Gynaecol. Res. 2021, 47, 73–87. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, P.; Li, H.X. CAFs-Derived Exosomal miRNA-130a Confers Cisplatin Resistance of NSCLC Cells Through PUM2-Dependent Packaging. Int. J. Nanomed. 2021, 16, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, W.; Huang, Y.; Zhuo, L.; Wang, S.; Chen, S.; Zhang, B.; Ke, B. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e989. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shi, Y.; Liu, J.B.; Wang, H.M.; Wang, P.Y.; Wu, Z.J.; Li, L.; Gu, L.P.; Cao, P.S.; Wang, G.R.; et al. Cancer-associated fibroblast-derived exosomal microRNA-24-3p enhances colon cancer cell resistance to MTX by down-regulating CDX2/HEPH axis. J. Cell. Mol. Med. 2021, 25, 3699–3713. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Wu, Z.; Zhang, L.; Liang, C.; Chen, X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim. Biophys. Sin. 2021, 53, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Zhou, X.; Gu, J.; Zhou, D.; Cheng, W.; Wu, H.; Wang, Y.; Tang, T.; Wang, X. Correction to: Downregulated exosomal microRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β-catenin pathway and upregulating PTEN. Cell Oncol. 2021, 44, 959. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, B.; Wang, L.; Liu, L.; Zhao, W.; Liu, C.; Ding, J.; Zhao, S.; Xu, B.; Yu, H.; et al. Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist. Updates 2023, 68, 100936. [Google Scholar] [CrossRef]
- Sun, L.; Ke, M.; Yin, M.; Zeng, Y.; Ji, Y.; Hu, Y.; Fu, S.; Zhang, C. Extracellular vesicle-encapsulated microRNA-296-3p from cancer-associated fibroblasts promotes ovarian cancer development through regulation of the PTEN/AKT and SOCS6/STAT3 pathways. Cancer Sci. 2024, 115, 155–169. [Google Scholar] [CrossRef]
- Kang, S.H.; Oh, S.Y.; Lee, K.Y.; Lee, H.J.; Kim, M.S.; Kwon, T.G.; Kim, J.W.; Lee, S.T.; Choi, S.Y.; Hong, S.H. Differential effect of cancer-associated fibroblast-derived extracellular vesicles on cisplatin resistance in oral squamous cell carcinoma via miR-876-3p. Theranostics 2024, 14, 460–479. [Google Scholar] [CrossRef]
- Qi, R.; Bai, Y.; Li, K.; Liu, N.; Xu, Y.; Dal, E.; Wang, Y.; Lin, R.; Wang, H.; Liu, Z.; et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist. Updates 2023, 68, 100960. [Google Scholar] [CrossRef]
- Zheng, S.; Tian, Q.; Yuan, Y.; Sun, S.; Li, T.; Xia, R.; He, R.; Luo, Y.; Lin, Q.; Fu, Z.; et al. Extracellular vesicle-packaged circBIRC6 from cancer-associated fibroblasts induce platinum resistance via SUMOylation modulation in pancreatic cancer. J. Exp. Clin. Cancer Res. 2023, 42, 324. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, S.; Hu, X.; Gao, F. Tumor-associated fibroblasts derived exosomes induce the proliferation and cisplatin resistance in esophageal squamous cell carcinoma cells through RIG-I/IFN-β signaling. Bioengineered 2022, 13, 12462–12474. [Google Scholar] [CrossRef]
- Deng, K.; Zou, F.; Xu, J.; Xu, D.; Luo, Z. Cancer-associated fibroblasts promote stemness maintenance and gemcitabine resistance via HIF-1α/miR-21 axis under hypoxic conditions in pancreatic cancer. Mol. Carcinog. 2024, 63, 524–537. [Google Scholar] [CrossRef]
- Tao, S.; Wang, J.; Li, F.; Shi, B.; Ren, Q.; Zhuang, Y.; Qian, X. Extracellular vesicles released by hypoxia-induced tumor-associated fibroblasts impart chemoresistance to breast cancer cells via long noncoding RNA H19 delivery. Faseb J. 2024, 38, e23165. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Giddings, E.L.; Champagne, D.P.; Wu, M.H.; Laffin, J.M.; Thornton, T.M.; Valenca-Pereira, F.; Culp-Hill, R.; Fortner, K.A.; Romero, N.; East, J.; et al. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat. Commun. 2021, 12, 2804. [Google Scholar] [CrossRef]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M.H. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, H.; Gu, J.; Xu, D.; Wang, M.; Qiao, J.; Yang, X.; Zhang, J.; Yao, M.; Gu, J.; et al. ABCA8-mediated efflux of taurocholic acid contributes to gemcitabine insensitivity in human pancreatic cancer via the S1PR2-ERK pathway. Cell Death Discov. 2021, 7, 6. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Z.; Li, J.; Liu, H.; Zhang, H.; Zhang, J.; Huang, W.; He, Y.; Zhu, S.; Huo, M.; et al. CHD4 promotes acquired chemoresistance and tumor progression by activating the MEK/ERK axis. Drug Resist. Updates 2023, 66, 100913. [Google Scholar] [CrossRef]
- Khalaf, B.H.; Suleiman, A.A.; Suwaid, M.A. Exploring the Regulatory Roles of miR-21, miR-15, and miR-let-7 in ABC Transporter-Mediated Chemoresistance: Implications for Breast Cancer Etiology and Treatment. Mol. Biotechnol. 2023, 67, 149–159. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Luh, F.; Ho, Y.-S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef]
- Meng, W.; Hao, Y.; He, C.; Li, L.; Zhu, G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer 2019, 18, 57. [Google Scholar] [CrossRef]
- Shedden, K.; Xie, X.T.; Chandaroy, P.; Chang, Y.T.; Rosania, G.R. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003, 63, 4331–4337. [Google Scholar]
- Peak, T.; Panigrahi, G.; Praharaj, P.; Chavez, J.; Chyr, J.; Singh, R.; Vander Griend, D.; Bitting, R.; Hemal, A.; Deep, G. PD65-01 do exosomes contribute to the development of enzalutamide-resistant prostate cancer? J. Urol. 2018, 199, e1224. [Google Scholar] [CrossRef]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef]
- Li, R.; Dong, C.; Jiang, K.; Sun, R.; Zhou, Y.; Yin, Z.; Lv, J.; Zhang, J.; Wang, Q.; Wang, L. Rab27B enhances drug resistance in hepatocellular carcinoma by promoting exosome-mediated drug efflux. Carcinogenesis 2020, 41, 1583–1591. [Google Scholar] [CrossRef]
- Wang, J.; Yeung, B.Z.; Wientjes, M.G.; Cui, M.; Peer, C.J.; Lu, Z.; Figg, W.D.; Woo, S.; Au, J.L. A Quantitative Pharmacology Model of Exosome-Mediated Drug Efflux and Perturbation-Induced Synergy. Pharmaceutics 2021, 13, 997. [Google Scholar] [CrossRef]
- Koch, R.; Aung, T.; Vogel, D.; Chapuy, B.; Wenzel, D.; Becker, S.; Sinzig, U.; Venkataramani, V.; von Mach, T.; Jacob, R.; et al. Nuclear Trapping through Inhibition of Exosomal Export by Indomethacin Increases Cytostatic Efficacy of Doxorubicin and Pixantrone. Clin. Cancer Res. 2016, 22, 395–404. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Mol. Aspects Med. 2023, 93, 101205. [Google Scholar] [CrossRef]
- Chapuy, B.; Koch, R.; Radunski, U.; Corsham, S.; Cheong, N.; Inagaki, N.; Ban, N.; Wenzel, D.; Reinhardt, D.; Zapf, A.; et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia 2008, 22, 1576–1586. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Levchenko, A.; Mehta, B.M.; Niu, X.; Kang, G.; Villafania, L.; Way, D.; Polycarpe, D.; Sadelain, M.; Larson, S.M. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1933–1938. [Google Scholar] [CrossRef]
- Bebawy, M.; Combes, V.; Lee, E.; Jaiswal, R.; Gong, J.; Bonhoure, A.; Grau, G.E. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009, 23, 1643–1649. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Hua, Y.; Sun, L.; Cheng, K.; Jia, Z.; Han, Y.; Dong, J.; Cui, Y.; Yang, Z. Exosomes play an important role in the process of psoralen reverse multidrug resistance of breast cancer. J. Exp. Clin. Cancer Res. 2016, 35, 186. [Google Scholar] [CrossRef]
- Pasquier, J.; Galas, L.; Boulangé-Lecomte, C.; Rioult, D.; Bultelle, F.; Magal, P.; Webb, G.; Le Foll, F. Different modalities of intercellular membrane exchanges mediate cell-to-cell p-glycoprotein transfers in MCF-7 breast cancer cells. J. Biol. Chem. 2012, 287, 7374–7387. [Google Scholar] [CrossRef]
- Lu, J.F.; Luk, F.; Gong, J.; Jaiswal, R.; Grau, G.E.; Bebawy, M. Microparticles mediate MRP1 intercellular transfer and the re-templating of intrinsic resistance pathways. Pharmacol. Res. 2013, 76, 77–83. [Google Scholar] [CrossRef]
- Kong, J.N.; He, Q.; Wang, G.; Dasgupta, S.; Dinkins, M.B.; Zhu, G.; Kim, A.; Spassieva, S.; Bieberich, E. Guggulsterone and bexarotene induce secretion of exosome-associated breast cancer resistance protein and reduce doxorubicin resistance in MDA-MB-231 cells. Int. J. Cancer 2015, 137, 1610–1620. [Google Scholar] [CrossRef]
- Dong, X.; Bai, X.; Ni, J.; Zhang, H.; Duan, W.; Graham, P.; Li, Y. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020, 11, 987. [Google Scholar] [CrossRef]
- Wang, J.; Yeung, B.Z.; Cui, M.; Peer, C.J.; Lu, Z.; Figg, W.D.; Guillaume Wientjes, M.; Woo, S.; Au, J.L. Exosome is a mechanism of intercellular drug transfer: Application of quantitative pharmacology. J. Control. Release 2017, 268, 147–158. [Google Scholar] [CrossRef]
- Debbi, L.; Guo, S.; Safina, D.; Levenberg, S. Boosting extracellular vesicle secretion. Biotechnol. Adv. 2022, 59, 107983. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.A.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.A.; Súarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Khoo, X.H.; Paterson, I.C.; Goh, B.H.; Lee, W.L. Cisplatin-Resistance in Oral Squamous Cell Carcinoma: Regulation by Tumor Cell-Derived Extracellular Vesicles. Cancers 2019, 11, 1166. [Google Scholar] [CrossRef]
- Hekmatirad, S.; Moloudizargari, M.; Moghadamnia, A.A.; Kazemi, S.; Mohammadnia-Afrouzi, M.; Baeeri, M.; Moradkhani, F.; Asghari, M.H. Inhibition of Exosome Release Sensitizes U937 Cells to PEGylated Liposomal Doxorubicin. Front. Immunol. 2021, 12, 692654. [Google Scholar] [CrossRef]
- Yang, L.; Peng, X.; Li, Y.; Zhang, X.; Ma, Y.; Wu, C.; Fan, Q.; Wei, S.; Li, H.; Liu, J. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol Cancer 2019, 18, 78. [Google Scholar] [CrossRef]
- Qian, L.; Yang, X.; Li, S.; Zhao, H.; Gao, Y.; Zhao, S.; Lv, X.; Zhang, X.; Li, L.; Zhai, L.; et al. Reduced O-GlcNAcylation of SNAP-23 promotes cisplatin resistance by inducing exosome secretion in ovarian cancer. Cell Death Discov. 2021, 7, 112. [Google Scholar] [CrossRef]
- Kirave, P.; Gondaliya, P.; Kulkarni, B.; Rawal, R.; Kalia, K. Exosome mediated miR-155 delivery confers cisplatin chemoresistance in oral cancer cells via epithelial-mesenchymal transition. Oncotarget 2020, 11, 1157–1171. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Ohno, S.-i.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Rezaei, R.; Baghaei, K.; Amani, D.; Piccin, A.; Hashemi, S.M.; Asadzadeh Aghdaei, H.; Zali, M.R. Exosome-mediated delivery of functionally active miRNA-375-3p mimic regulate epithelial mesenchymal transition (EMT) of colon cancer cells. Life Sci. 2021, 269, 119035. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Shi, Y.; Zhao, L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J. Nanobiotechnol. 2018, 16, 103. [Google Scholar] [CrossRef]
- Zhang, Q.; Haiyang, Z.; Tao, N.; Dongying, L.; Ting, D.; Rui, L.; Ming, B.; Kegan, Z.; Jialu, L.; Qian, F.; et al. Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer. Int. J. Nanomed. 2020, 15, 2323–2335. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, H.; Liu, R.; Deng, T.; Ning, T.; Bai, M.; Yang, Y.; Zhu, K.; Wang, J.; Duan, J.; et al. iRGD-modified exosomes effectively deliver CPT1A siRNA to colon cancer cells, reversing oxaliplatin resistance by regulating fatty acid oxidation. Mol. Oncol. 2021, 15, 3430–3446. [Google Scholar] [CrossRef]
- Si, J.; Li, W.; Li, X.; Cao, L.; Chen, Z.; Jiang, Z. Heparanase confers temozolomide resistance by regulation of exosome secretion and circular RNA composition in glioma. Cancer Sci. 2021, 112, 3491–3506. [Google Scholar] [CrossRef]
- Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Baldini, N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int. J. Oncol. 2016, 49, 189–196. [Google Scholar] [CrossRef]
- Noack, A.; Noack, S.; Buettner, M.; Naim, H.Y.; Löscher, W. Intercellular transfer of P-glycoprotein in human blood-brain barrier endothelial cells is increased by histone deacetylase inhibitors. Sci. Rep. 2016, 6, 29253. [Google Scholar] [CrossRef]
- Lv, M.M.; Zhu, X.Y.; Chen, W.X.; Zhong, S.L.; Hu, Q.; Ma, T.F.; Zhang, J.; Chen, L.; Tang, J.H.; Zhao, J.H. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014, 35, 10773–10779. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.; Li, X.; Liu, J.L.; Tian, L.; Chen, J.Q. Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer. Mol. Cell. Biochem. 2019, 462, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Rani, S.; O’Brien, K.; O’Neill, A.; Prencipe, M.; Sheikh, R.; Webb, G.; McDermott, R.; Watson, W.; Crown, J.; et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 2012, 7, e50999. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Bertolini, I.; Crucitta, S.; Fontanelli, L.; Rofi, E.; De Angelis, C.; Diodati, L.; Cavallero, D.; Gianfilippo, G.; Salvadori, B.; et al. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res. Treat. 2019, 178, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, D.; Padula, M.P.; Lu, J.F.; Jaiswal, R.; Djordjevic, S.P.; Bebawy, M. The Role of CD44 and ERM Proteins in Expression and Functionality of P-glycoprotein in Breast Cancer Cells. Molecules 2016, 21, 290. [Google Scholar] [CrossRef]
- Jaiswal, R.; Gong, J.; Sambasivam, S.; Combes, V.; Mathys, J.M.; Davey, R.; Grau, G.E.; Bebawy, M. Microparticle-associated nucleic acids mediate trait dominance in cancer. Faseb J. 2012, 26, 420–429. [Google Scholar] [CrossRef]
- Ning, K.; Wang, T.; Sun, X.; Zhang, P.; Chen, Y.; Jin, J.; Hua, D. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J. Surg. Oncol. 2017, 115, 932–940. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, R.-X.; Chan, K.-W.; Hu, J.; Zhang, J.; Wei, L.; Tan, H.; Yang, X.; Liu, H. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 320. [Google Scholar] [CrossRef]
- Goler-Baron, V.; Assaraf, Y.G. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS ONE 2011, 6, e16007. [Google Scholar] [CrossRef]
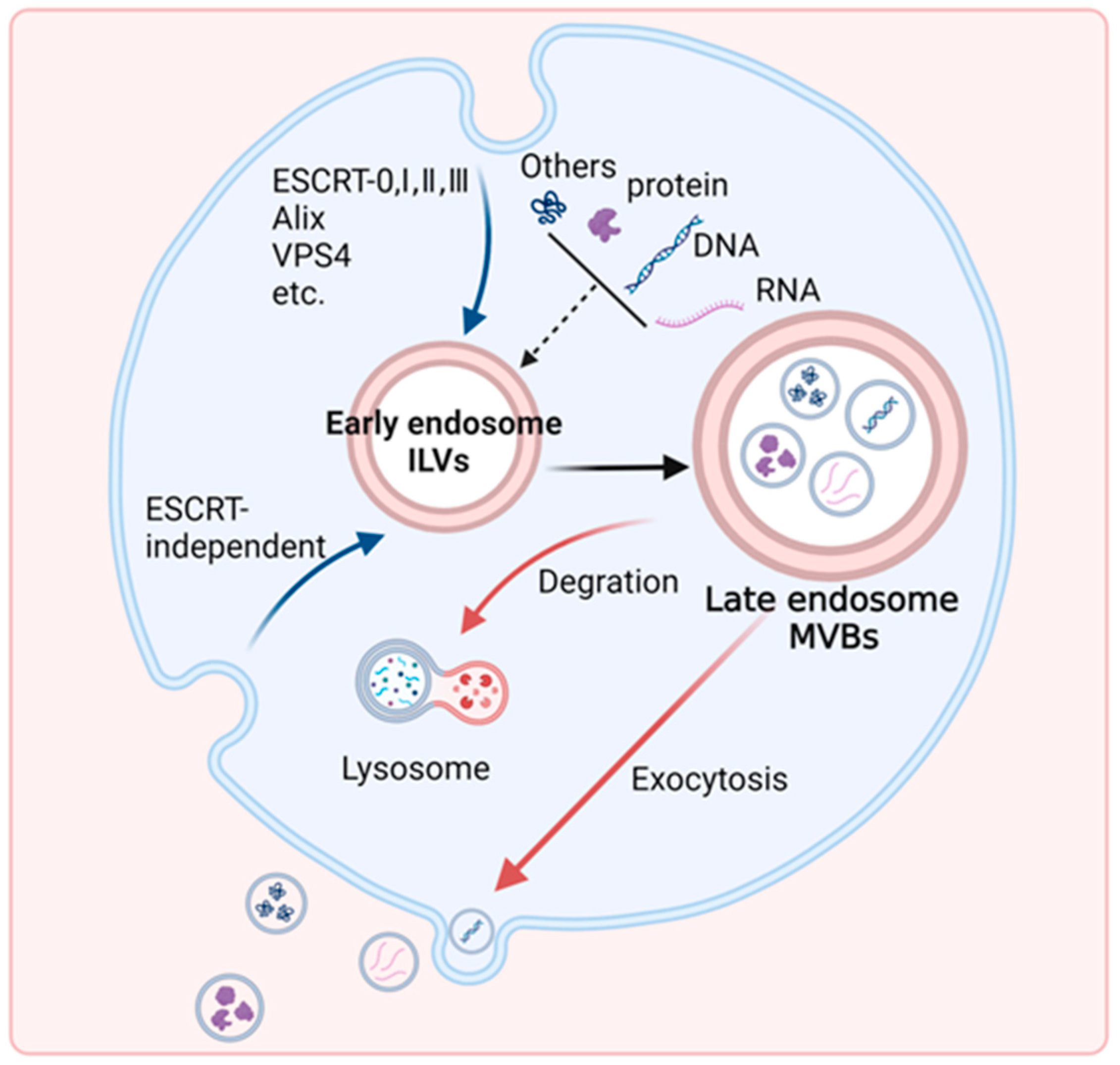
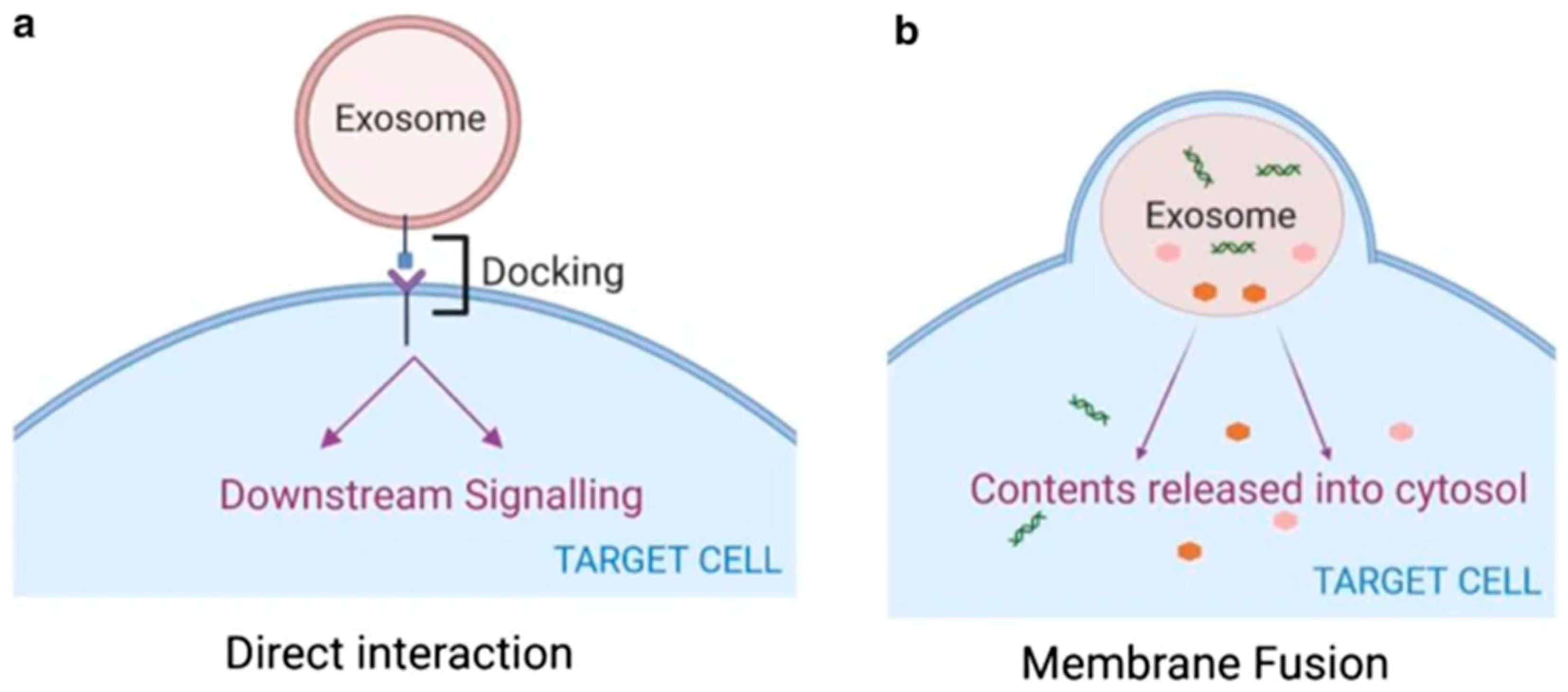
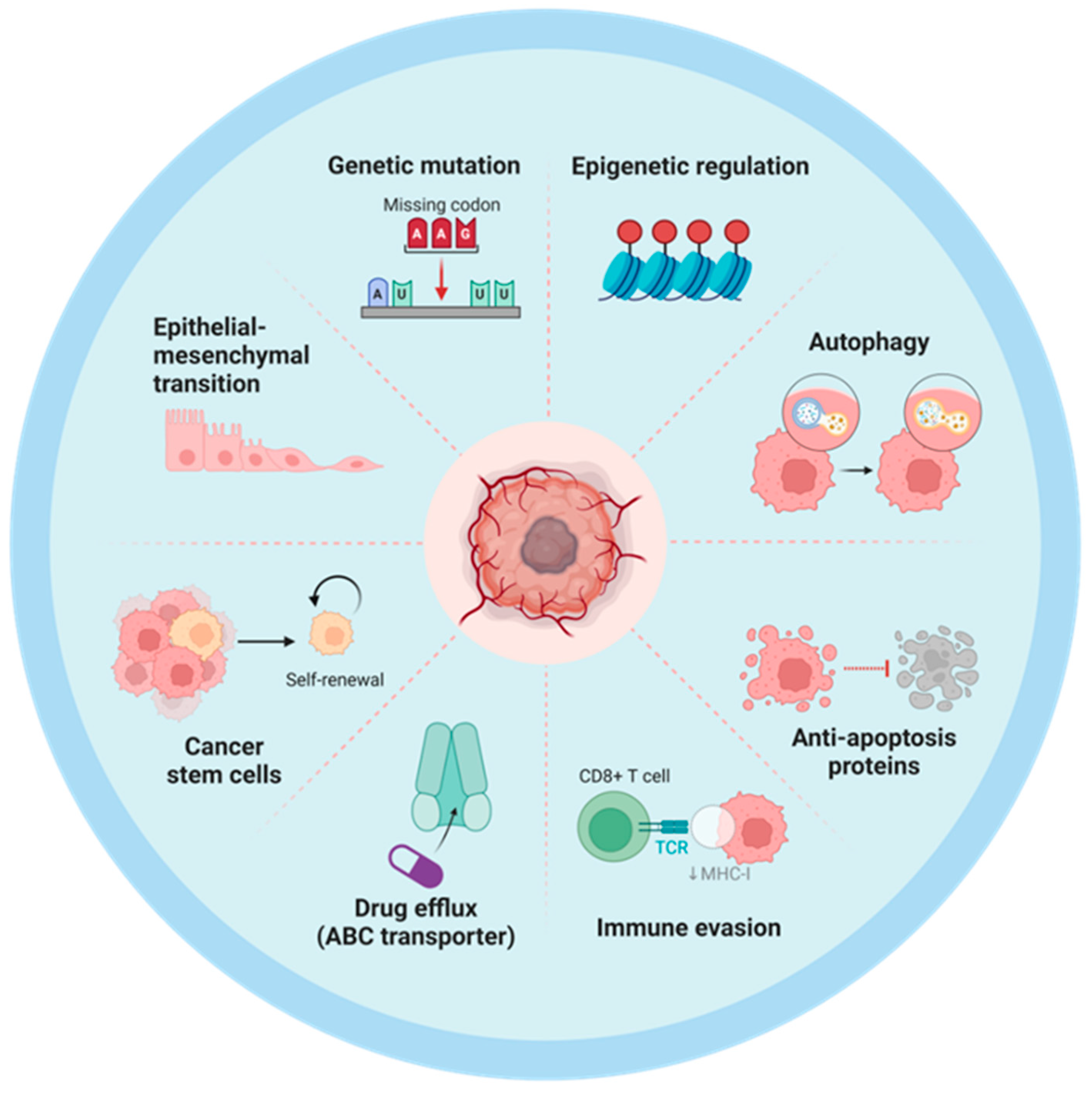
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liu, J.; Li, S.; Zhang, Y.; Ren, H. Exosome-Mediated Chemoresistance in Cancers: Mechanisms, Therapeutic Implications, and Future Directions. Biomolecules 2025, 15, 685. https://doi.org/10.3390/biom15050685
Liu G, Liu J, Li S, Zhang Y, Ren H. Exosome-Mediated Chemoresistance in Cancers: Mechanisms, Therapeutic Implications, and Future Directions. Biomolecules. 2025; 15(5):685. https://doi.org/10.3390/biom15050685
Chicago/Turabian StyleLiu, Gengqi, Jingang Liu, Silu Li, Yumiao Zhang, and He Ren. 2025. "Exosome-Mediated Chemoresistance in Cancers: Mechanisms, Therapeutic Implications, and Future Directions" Biomolecules 15, no. 5: 685. https://doi.org/10.3390/biom15050685
APA StyleLiu, G., Liu, J., Li, S., Zhang, Y., & Ren, H. (2025). Exosome-Mediated Chemoresistance in Cancers: Mechanisms, Therapeutic Implications, and Future Directions. Biomolecules, 15(5), 685. https://doi.org/10.3390/biom15050685





