Unraveling the Potential of SGK1 in Osteoporosis: From Molecular Mechanisms to Therapeutic Targets
Abstract
1. Introduction
2. The Structure and Activation Mechanism of SGK1
3. The Biological Function of SGK1
3.1. The Role of SGK1 in the Regulation of Ion Channels
3.2. SGK1 Is Involved in Cell Proliferation, Apoptosis, and Cell Survival
3.3. SGK1’s Role in Regulating Immunity and Inflammation
3.4. The Role of SGK1 in Cell Signaling and Regulation of Gene Expression
4. The Role of SGK1 in Different Types of Osteoporosis
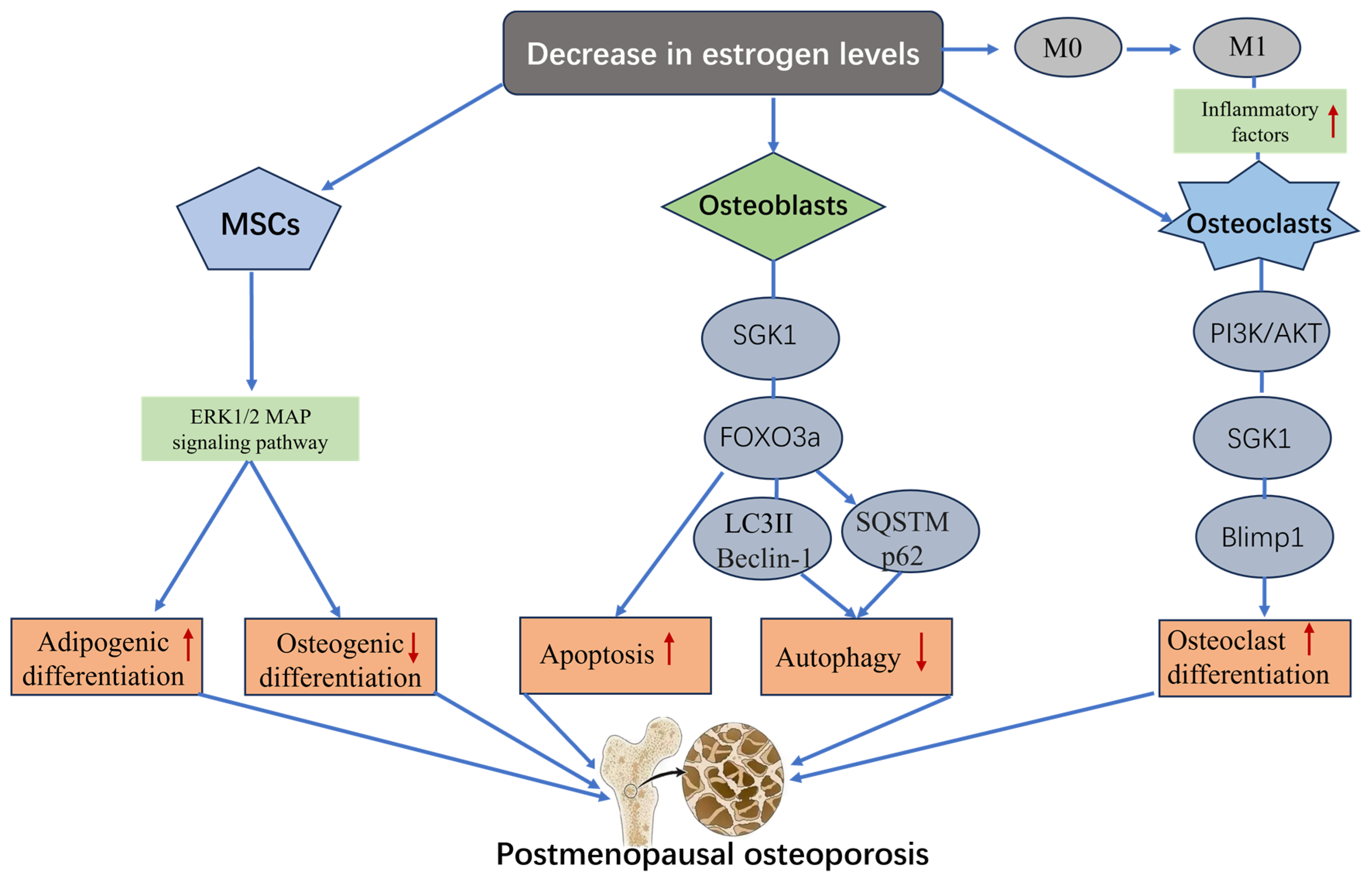
4.1. SGK1 and Postmenopausal Osteoporosis
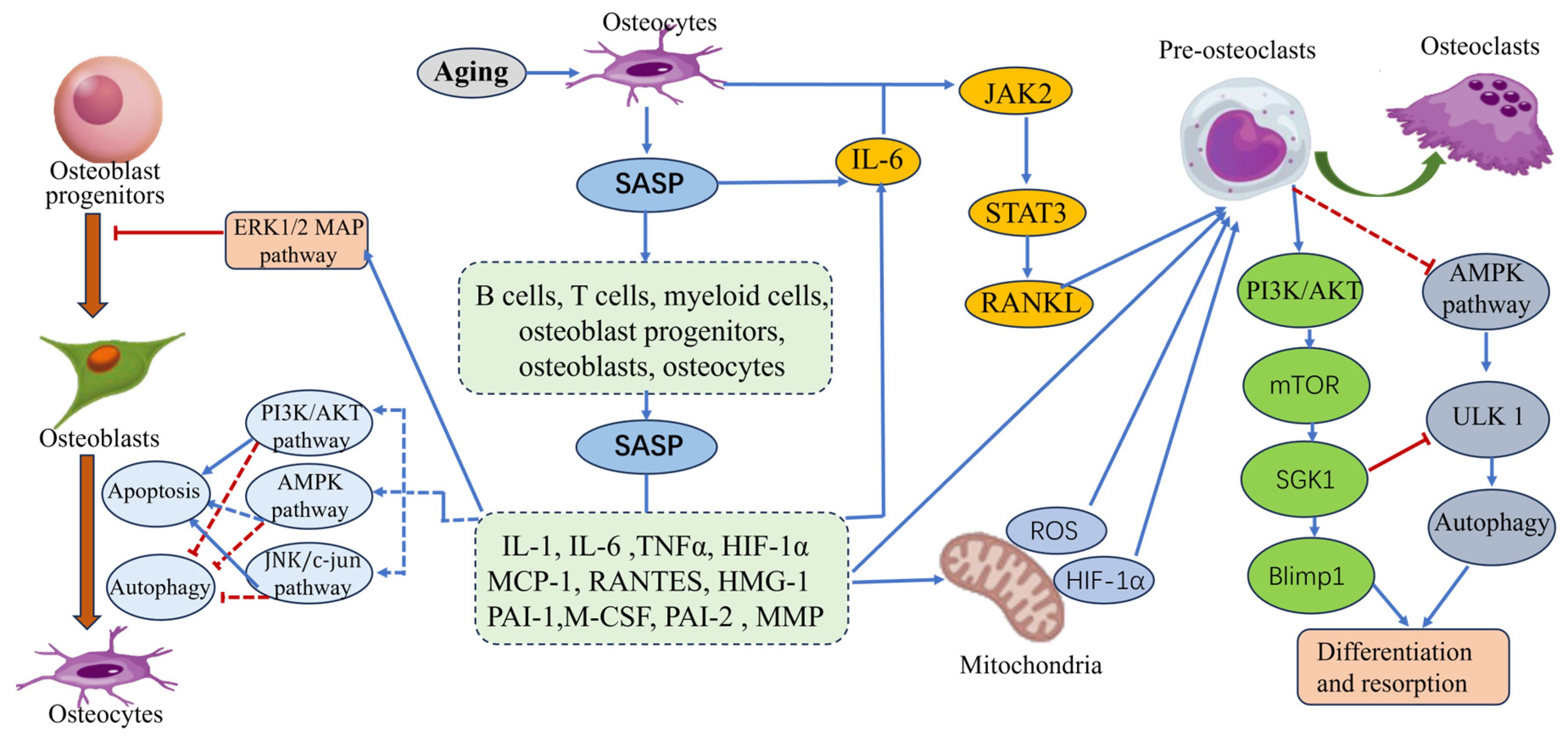
4.2. SGK1 and Senile Osteoporosis
4.2.1. Abnormal Activation of SGK1 by Inflammatory Factors
4.2.2. Regulation of the Autophagy Process by SGK1
4.3. SGK1 and Diabetic Osteoporosis
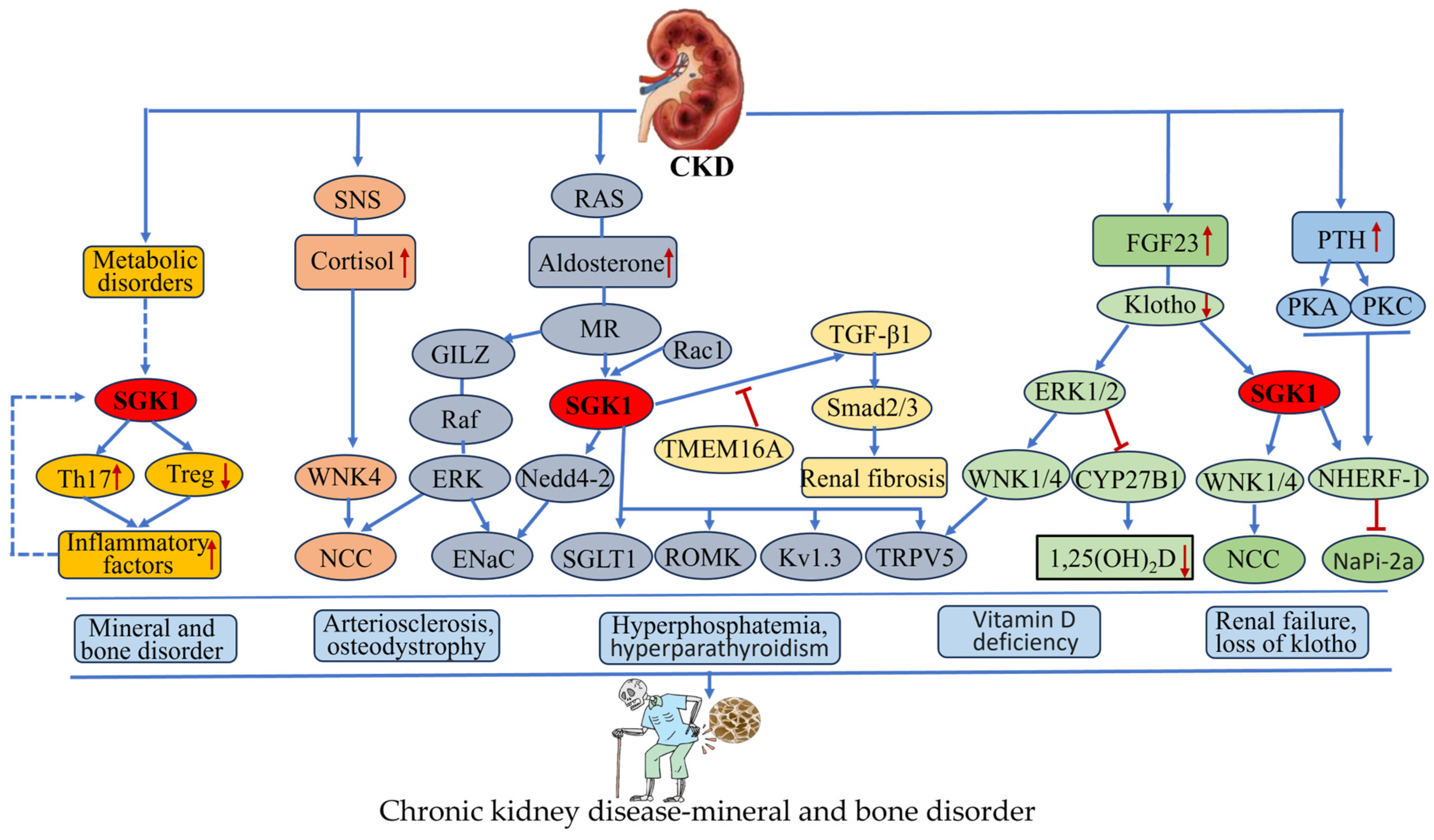
4.4. SGK1 and Chronic Kidney Disease—Mineral and Bone Disorder (CKD-MBD)
5. The Potential of SGK1 as a Therapeutic Target for Osteoporosis
5.1. GSK650394 and QGY-5-114-A
5.2. EMD638683
5.3. SI113 and 17a
5.4. ZINC00319000
5.5. Herbacetin (HBT)
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Yang, C.; Yang, F.; Yang, A.; Xiao, H.; Peng, B.; Chen, C.; Geng, B.; Xia, Y. Targeting the mTOR-Autophagy Axis: Unveiling Therapeutic Potentials in Osteoporosis. Biomolecules 2024, 14, 1452. [Google Scholar] [CrossRef]
- Gullborg, E.J.; Kim, J.H.; Ward, C.M.; Simcock, X.C. Optimizing Treatment Strategies for Distal Radius Fractures in Osteoporosis: A Comparative Review. Medicina 2024, 60, 1848. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.M.; Saniee, N.; Mousaviasl, S.; Radmanesh, E.; Doustimotlagh, A.H. The Role of Osteocalcin in Patients with Osteoporosis: A Systematic Review. Iran. J. Public Health 2024, 53, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global prevalence of osteoporosis among the world older adults: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 669. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.K.; Goya, L.; Ge, Y.; Maiyar, A.C.; Firestone, G.L. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell Biol. 1993, 13, 2031–2040. [Google Scholar] [CrossRef]
- Webster, M.K.; Goya, L.; Firestone, G.L. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J. Biol. Chem. 1993, 268, 11482–11485. [Google Scholar] [CrossRef]
- Firestone, G.L.; Giampaolo, J.R.; O’Keeffe, B.A. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol. Biochem. 2003, 13, 1–12. [Google Scholar] [CrossRef]
- Leong, M.L.; Maiyar, A.C.; Kim, B.; O’Keeffe, B.A.; Firestone, G.L. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J. Biol. Chem. 2003, 278, 5871–5882. [Google Scholar] [CrossRef]
- Tessier, M.; Woodgett, J.R. Serum and glucocorticoid-regulated protein kinases: Variations on a theme. J. Cell Biochem. 2006, 98, 1391–1407. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Ashraf, G.M.; Farhat, J.; Bilgrami, A.L.; Eapen, M.S.; Sohal, S.S.; Yadav, D.K.; Hassan, M.I. Mechanistic insights into the role of serum-glucocorticoid kinase 1 in diabetic nephropathy: A systematic review. Int. J. Biol. Macromol. 2021, 193, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Buse, P.; Tran, S.H.; Luther, E.; Phu, P.T.; Aponte, G.W.; Firestone, G.L. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J. Biol. Chem. 1999, 274, 7253–7263. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Chong, F.L.; Yuan, C.C.; Liu, Y.L.; Yang, H.M.; Wang, W.W.; Fang, Q.J.; Wu, W.; Wang, M.Z.; Tu, Y.; et al. Fucoidan Ameliorates Renal Injury-Related Calcium-Phosphorus Metabolic Disorder and Bone Abnormality in the CKD-MBD Model Rats by Targeting FGF23-Klotho Signaling Axis. Front. Pharmacol. 2020, 11, 586725. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Park, J.; Tran, H.; Hu, L.S.; Hemmings, B.A.; Greenberg, M.E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell Biol. 2001, 21, 952–965. [Google Scholar] [CrossRef]
- Mikosz, C.A.; Brickley, D.R.; Sharkey, M.S.; Moran, T.W.; Conzen, S.D. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J. Biol. Chem. 2001, 276, 16649–16654. [Google Scholar] [CrossRef]
- Chaudhary, M.; Sharma, V.; Bedi, O.; Kaur, A.; Singh, T.G. SGK-1 Signalling Pathway is a Key Factor in Cell Survival in Ischemic Injury. Curr. Drug Targets 2023, 24, 1117–1126. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, Y.S.; Hong, J.; Chaugule, S.; Chun, H.; van der Meulen, M.C.H.; Xu, R.; Greenblatt, M.B.; Shim, J.H. Biphasic regulation of osteoblast development via the ERK MAPK-mTOR pathway. Elife 2022, 11, e78069. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Song, C.; Mi, B.; Zhang, H.; Kang, H.; Liu, H.; Sun, Y.; Wang, J.; Lei, Z.; et al. Serum- and Glucocorticoid-inducible Kinase 1 is Essential for Osteoclastogenesis and Promotes Breast Cancer Bone Metastasis. Mol. Cancer Ther. 2020, 19, 650–660. [Google Scholar] [CrossRef]
- Lang, F.; Böhmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef]
- Basnet, R.; Gong, G.Q.; Li, C.; Wang, M.W. Serum and glucocorticoid inducible protein kinases (SGKs): A potential target for cancer intervention. Acta Pharm. Sin. 2018, 8, 767–771. [Google Scholar] [CrossRef]
- Waldegger, S.; Barth, P.; Forrest, J.N., Jr.; Greger, R.; Lang, F. Cloning of sgk serine-threonine protein kinase from shark rectal gland—A gene induced by hypertonicity and secretagogues. Pflug. Arch. 1998, 436, 575–580. [Google Scholar] [CrossRef]
- Hertweck, M.; Göbel, C.; Baumeister, R.C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Kobayashi, T.; Deak, M.; Morrice, N.; Cohen, P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem. J. 1999, 344 Pt 1, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Cohen, P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 1999, 339 Pt 2, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lauro, D.; Pastore, D.; Capuani, B.; Pacifici, F.; Palmirotta, R.; Abete, P.; Roselli, M.; Bellia, A.; Federici, M.; Di Daniele, N.; et al. Role of Serum and Glucocorticoid-Inducible Kinase (SGK)-1 in Senescence: A Novel Molecular Target Against Age-Related Diseases. Curr. Med. Chem. 2015, 22, 3765–3788. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Jang, H.; Park, Y.; Jang, J. Serum and glucocorticoid-regulated kinase 1: Structure, biological functions, and its inhibitors. Front. Pharmacol. 2022, 13, 1036844. [Google Scholar] [CrossRef]
- Geraghty, K.M.; Chen, S.; Harthill, J.E.; Ibrahim, A.F.; Toth, R.; Morrice, N.A.; Vandermoere, F.; Moorhead, G.B.; Hardie, D.G.; MacKintosh, C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem. J. 2007, 407, 231–241. [Google Scholar] [CrossRef]
- Zhou, R.; Snyder, P.M. Nedd4-2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J. Biol. Chem. 2005, 280, 4518–4523. [Google Scholar] [CrossRef]
- Gleason, C.E.; Oses-Prieto, J.A.; Li, K.H.; Saha, B.; Situ, G.; Burlingame, A.L.; Pearce, D. Phosphorylation at distinct subcellular locations underlies specificity in mTORC2-mediated activation of SGK1 and Akt. J. Cell Sci. 2019, 132, jcs224931. [Google Scholar] [CrossRef]
- Bell, L.M.; Leong, M.L.; Kim, B.; Wang, E.; Park, J.; Hemmings, B.A.; Firestone, G.L. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J. Biol. Chem. 2000, 275, 25262–25272. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Liu, Q.; Wang, S.; Sheng, C.; Chen, J.; Tan, J.; Wang, X.; Shao, L.; Zhou, L. Role of serum- and glucocorticoid-inducible kinase 1 in the regulation of hepatic gluconeogenesis. J. Mol. Endocrinol. 2023, 71. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C.; Zacharopoulou, N.; Voelkl, J.; Alesutan, I. Serum- and glucocorticoid-inducible kinase 1 and the response to cell stress. Cell Stress. 2018, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ikumawoyi, V.O.; Lynch, K.D.; Iverson, D.T.; Call, M.R.; Yue, G.E.; Prasad, B.; Clarke, J.D. Microcystin-LR activates serine/threonine kinases and alters the phosphoproteome in human HepaRG cells. Toxicon 2024, 249, 108072. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, Y.; Fu, Z.; Wu, S.; Lan, H.; Zhou, X.; Shen, W.; Lou, Y. The role of serum-glucocorticoid regulated kinase 1 in reproductive viability: Implications from prenatal programming and senescence. Mol. Biol. Rep. 2024, 51, 376. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.; Manis, A.D.; Nesterov, V.; Korbmacher, C. Regulation of distal tubule sodium transport: Mechanisms and roles in homeostasis and pathophysiology. Pflug. Arch. 2022, 474, 869–884. [Google Scholar] [CrossRef]
- Hanin, A.; Comi, M.; Sumida, T.S.; Hafler, D.A. Cholesterol promotes IFNG mRNA expression in CD4(+) effector/memory cells by SGK1 activation. Life Sci. Alliance 2024, 7, e202402890. [Google Scholar] [CrossRef]
- Sumida, T.S.; Lincoln, M.R.; He, L.; Park, Y.; Ota, M.; Oguchi, A.; Son, R.; Yi, A.; Stillwell, H.A.; Leissa, G.A.; et al. An autoimmune transcriptional circuit drives FOXP3(+) regulatory T cell dysfunction. Sci. Transl. Med. 2024, 16, eadp1720. [Google Scholar] [CrossRef]
- Valinsky, W.C.; Touyz, R.M.; Shrier, A. Aldosterone, SGK1, and ion channels in the kidney. Clin. Sci. 2018, 132, 173–183. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, F.; Luo, Y.; Wang, L.; Huang, S.; Jin, F. Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis. Int. J. Mol. Sci. 2016, 17, 1307. [Google Scholar] [CrossRef]
- Kamynina, E.; Staub, O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na(+) transport. Am. J. Physiol. Ren. Physiol. 2002, 283, F377–F387. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Shumilina, E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. Faseb J. 2013, 27, 3–12. [Google Scholar] [CrossRef]
- Verrey, F.; Fakitsas, P.; Adam, G.; Staub, O. Early transcriptional control of ENaC (de)ubiquitylation by aldosterone. Kidney Int. 2008, 73, 691–696. [Google Scholar] [CrossRef]
- Pearce, D. The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol. Metab. 2001, 12, 341–347. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, F.; Tian, X.; Zhang, Q.; Xu, C.; Ji, B.; Zhang, Y.A.; Du, L.; Han, J.; Li, L.; et al. Inhibition the ubiquitination of ENaC and Na,K-ATPase with erythropoietin promotes alveolar fluid clearance in sepsis-induced acute respiratory distress syndrome. Biomed. Pharmacother. 2024, 174, 116447. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Shabbir, W.; Takagi, E.; Duan, X.P.; Leite Dellova, D.C.A.; Demko, J.; Manis, A.; Loffing-Cueni, D.; Loffing, J.; Sørensen, M.V.; et al. Potassium Activates mTORC2-dependent SGK1 Phosphorylation to Stimulate Epithelial Sodium Channel: Role in Rapid Renal Responses to Dietary Potassium. J. Am. Soc. Nephrol. 2023, 34, 1019–1038. [Google Scholar] [CrossRef]
- Khachab, M.; Kanaan, A.; Awad, D.; Deeba, E.; Osman, S.; Nassar, C.F. Colectomy induces an aldosterone-mediated increase in jejunal glucose uptake in rats. Life Sci. 2017, 174, 43–49. [Google Scholar] [CrossRef]
- Dieter, M.; Palmada, M.; Rajamanickam, J.; Aydin, A.; Busjahn, A.; Boehmer, C.; Luft, F.C.; Lang, F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes. Res. 2004, 12, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Gedney, J.R.; Mattia, V.; Figueroa, M.; Barksdale, C.; Fannin, E.; Silverman, J.; Xiong, Y.; Mukherjee, R.; Jones, J.A.; Ruddy, J.M. Biomechanical dysregulation of SGK-1 dependent aortic pathologic markers in hypertension. Front. Cardiovasc. Med. 2024, 11, 1359734. [Google Scholar] [CrossRef]
- Li, P.; Hao, Y.; Pan, F.H.; Zhang, M.; Ma, J.Q.; Zhu, D.L. SGK1 inhibitor reverses hyperglycemia partly through decreasing glucose absorption. J. Mol. Endocrinol. 2016, 56, 301–309. [Google Scholar] [CrossRef]
- Schwab, M.; Lupescu, A.; Mota, M.; Mota, E.; Frey, A.; Simon, P.; Mertens, P.R.; Floege, J.; Luft, F.; Asante-Poku, S.; et al. Association of SGK1 gene polymorphisms with type 2 diabetes. Cell Physiol. Biochem. 2008, 21, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Ramos, C.; Velazquez-Garcia, S.; Vastola-Mascolo, A.; Hernández, G.; Faresse, N.; Alvarez de la Rosa, D. SGK1 activation exacerbates diet-induced obesity, metabolic syndrome and hypertension. J. Endocrinol. 2020, 244, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lv, W.; Li, Z.; Han, W. SGK1 protein expression is a prognostic factor of lung adenocarcinoma that regulates cell proliferation and survival. Int. J. Clin. Exp. Pathol. 2019, 12, 391–408. [Google Scholar]
- Maiyar, A.C.; Leong, M.L.; Firestone, G.L. Importin-alpha mediates the regulated nuclear targeting of serum- and glucocorticoid-inducible protein kinase (Sgk) by recognition of a nuclear localization signal in the kinase central domain. Mol. Biol. Cell 2003, 14, 1221–1239. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ö.; Yurt, K.; Pektaş, A.N.; Berk, Ş. Evaluation and normalization of a set of reliable reference genes for quantitative sgk-1 gene expression analysis in Caenorhabditis elegans-focused cancer research. Nucleosides Nucleotides Nucleic Acids 2024, 44, 91–110. [Google Scholar] [CrossRef]
- Hong, F.; Larrea, M.D.; Doughty, C.; Kwiatkowski, D.J.; Squillace, R.; Slingerland, J.M. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol. Cell 2008, 30, 701–711. [Google Scholar] [CrossRef]
- Lang, F.; Henke, G.; Embark, H.M.; Waldegger, S.; Palmada, M.; Böhmer, C.; Vallon, V. Regulation of channels by the serum and glucocorticoid-inducible kinase—Implications for transport, excitability and cell proliferation. Cell Physiol. Biochem. 2003, 13, 41–50. [Google Scholar] [CrossRef]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Meng, T.; Tian, P.; Chen, J.; Liu, A.; Zheng, Y.; Su, G. SGK1 is involved in doxorubicin-induced chronic cardiotoxicity and dysfunction through activation of the NFκB pathway. Int. Immunopharmacol. 2023, 125, 111151. [Google Scholar] [CrossRef]
- Tao, G.Z.; Lehwald, N.; Jang, K.Y.; Baek, J.; Xu, B.; Omary, M.B.; Sylvester, K.G. Wnt/β-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. J. Biol. Chem. 2013, 288, 17214–17224. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef]
- Dijkers, P.F.; Medema, R.H.; Lammers, J.W.; Koenderman, L.; Coffer, P.J. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 2000, 10, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Biggs, W.H., 3rd; Meisenhelder, J.; Hunter, T.; Cavenee, W.K.; Arden, K.C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 1999, 96, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Kanai, F.; Stehn, J.; Xu, J.; Sarbassova, D.; Frangioni, J.V.; Dalal, S.N.; DeCaprio, J.A.; Greenberg, M.E.; Yaffe, M.B. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 2002, 156, 817–828. [Google Scholar] [CrossRef]
- Tran, H.; Brunet, A.; Grenier, J.M.; Datta, S.R.; Fornace, A.J., Jr.; DiStefano, P.S.; Chiang, L.W.; Greenberg, M.E. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 2002, 296, 530–534. [Google Scholar] [CrossRef]
- Náray-Fejes-Tóth, A.; Canessa, C.; Cleaveland, E.S.; Aldrich, G.; Fejes-Tóth, G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. J. Biol. Chem. 1999, 274, 16973–16978. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Xue, H.; Jing, D.; Wang, Y.; Zhou, G.; Zhu, F. Role of Serum/Glucocorticoid-Regulated Kinase 1 (SGK1) in Immune and Inflammatory Diseases. Inflammation 2023, 46, 1612–1625. [Google Scholar] [CrossRef]
- Brescia, C.; Dattilo, V.; D’Antona, L.; Chiarella, E.; Tallerico, R.; Audia, S.; Rocca, V.; Iuliano, R.; Trapasso, F.; Perrotti, N.; et al. RANBP1, a member of the nuclear-cytoplasmic trafficking-regulator complex, is the terminal-striking point of the SGK1-dependent Th17(+) pathological differentiation. Front. Immunol. 2023, 14, 1213805. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Xiao, S.; Thalhamer, T.; Madi, A.; Han, T.; Kuchroo, V. SGK1 Governs the Reciprocal Development of Th17 and Regulatory T Cells. Cell Rep. 2018, 22, 653–665. [Google Scholar] [CrossRef]
- Martín-Fernández, B.; Rubio-Navarro, A.; Cortegano, I.; Ballesteros, S.; Alía, M.; Cannata-Ortiz, P.; Olivares-Álvaro, E.; Egido, J.; de Andrés, B.; Gaspar, M.L.; et al. Aldosterone Induces Renal Fibrosis and Inflammatory M1-Macrophage Subtype via Mineralocorticoid Receptor in Rats. PLoS ONE 2016, 11, e0145946. [Google Scholar] [CrossRef]
- Burgon, J.; Robertson, A.L.; Sadiku, P.; Wang, X.; Hooper-Greenhill, E.; Prince, L.R.; Walker, P.; Hoggett, E.E.; Ward, J.R.; Farrow, S.N.; et al. Serum and glucocorticoid-regulated kinase 1 regulates neutrophil clearance during inflammation resolution. J. Immunol. 2014, 192, 1796–1805. [Google Scholar] [CrossRef]
- Han, H.; Liu, C.; Li, M.; Wang, J.; Liu, Y.S.; Zhou, Y.; Li, Z.C.; Hu, R.; Li, Z.H.; Wang, R.M.; et al. Increased intracellular Cl(-) concentration mediates neutrophil extracellular traps formation in atherosclerotic cardiovascular diseases. Acta Pharmacol. Sin. 2022, 43, 2848–2861. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Amann, K.; Nasir, O.; Friedrich, B.; Sandulache, D.; Jahovic, N.; Risler, T.; Vallon, V.; Wulff, P.; Kuhl, D.; et al. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J. Mol. Med. 2006, 84, 737–746. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Hormones 2013, 12, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.A.; Xu, G.F.; Yan, L.J.; Zeng, W.W.; Ji, Q.Q.; Wu, J.D.; Tang, Q.Y. SGK1 inhibits cellular apoptosis and promotes proliferation via the MEK/ERK/p53 pathway in colitis. World J. Gastroenterol. 2015, 21, 6180–6193. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, B.; Wärntges, S.; Klingel, K.; Sauter, M.; Kandolf, R.; Risler, T.; Müller, G.A.; Witzgall, R.; Kriz, W.; Gröne, H.J.; et al. Up-regulation of the human serum and glucocorticoid-dependent kinase 1 in glomerulonephritis. Kidney Blood Press. Res. 2002, 25, 303–307. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Cen, Y.; Yang, J.; Su, L.; Wang, F.; Zhu, D.; Zhao, L.; Li, Y. Manganese induces neuronal apoptosis by activating mTOR signaling pathway in vitro and in vivo. Food Chem. Toxicol. 2024, 185, 114508. [Google Scholar] [CrossRef]
- You, H.; Jang, Y.; You-Ten, A.I.; Okada, H.; Liepa, J.; Wakeham, A.; Zaugg, K.; Mak, T.W. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc. Natl. Acad. Sci. USA 2004, 101, 14057–14062. [Google Scholar] [CrossRef]
- David, S.; Kalb, R.G. Serum/glucocorticoid-inducible kinase can phosphorylate the cyclic AMP response element binding protein, CREB. FEBS Lett. 2005, 579, 1534–1538. [Google Scholar] [CrossRef]
- Mason, J.A.; Davison-Versagli, C.A.; Leliaert, A.K.; Pape, D.J.; McCallister, C.; Zuo, J.; Durbin, S.M.; Buchheit, C.L.; Zhang, S.; Schafer, Z.T. Oncogenic Ras differentially regulates metabolism and anoikis in extracellular matrix-detached cells. Cell Death Differ. 2016, 23, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chae, J.S.; Kim, K.J.; Hwang, S.G.; Yoon, K.W.; Kim, E.K.; Yun, H.J.; Cho, J.H.; Kim, J.; Kim, B.W.; et al. Negative regulation of SEK1 signaling by serum- and glucocorticoid-inducible protein kinase 1. EMBO J. 2007, 26, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Kuntzsch, D.; Bergann, T.; Dames, P.; Fromm, A.; Fromm, M.; Davis, R.A.; Melzig, M.F.; Schulzke, J.D. The plant-derived glucocorticoid receptor agonist Endiandrin A acts as co-stimulator of colonic epithelial sodium channels (ENaC) via SGK-1 and MAPKs. PLoS ONE 2012, 7, e49426. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.J.; Su, C.C.; Ma, Y.L.; Lee, E.H. SGK1 phosphorylation of IkappaB Kinase alpha and p300 Up-regulates NF-kappaB activity and increases N-Methyl-D-aspartate receptor NR2A and NR2B expression. J. Biol. Chem. 2009, 284, 4073–4089. [Google Scholar] [CrossRef]
- Subarajan, P.; Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: A Review of Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2024, 53, 497–512. [Google Scholar] [CrossRef]
- Yu, X.H.; Xu, X.M.; Zhang, S.X. Low-dose dexamethasone promotes osteoblast viability by activating autophagy via the SGK1/FOXO3a signaling pathway. Cell Biol. Int. 2023, 47, 669–678. [Google Scholar] [CrossRef]
- Kong, Y.; Nie, Z.K.; Li, F.; Guo, H.M.; Yang, X.L.; Ding, S.F. MiR-320a was highly expressed in postmenopausal osteoporosis and acts as a negative regulator in MC3T3E1 cells by reducing MAP9 and inhibiting PI3K/AKT signaling pathway. Exp. Mol. Pathol. 2019, 110, 104282. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; Fernández, M.; Santibañez, J.F. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J. Cell Biochem. 2004, 92, 745–754. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, G.; Li, W. Weighted gene correlation network analysis reveals novel biomarkers associated with mesenchymal stromal cell differentiation in early phase. PeerJ 2020, 8, e8907. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhao, G.; Yang, K.; Tao, L. Obesity and lipid metabolism in the development of osteoporosis (Review). Int. J. Mol. Med. 2024, 54, 61. [Google Scholar] [CrossRef]
- Zha, L.; He, L.; Liang, Y.; Qin, H.; Yu, B.; Chang, L.; Xue, L. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 2018, 102, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Fu, Z.; Tian, Y.; Hu, M.; Wang, Q.; Zhou, Y.; Wang, N.; Zhang, Q.; Jin, F. Estrogen-sensitive activation of SGK1 induces M2 macrophages with anti-inflammatory properties and a Th2 response at the maternal-fetal interface. Reprod. Biol. Endocrinol. 2023, 21, 50. [Google Scholar] [CrossRef]
- Sweeney, E.E.; Fan, P.; Jordan, V.C. Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: An insight into the women’s health initiative study. Cancer Res. 2014, 74, 7060–7068. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, X.; Liu, S.; Wang, S.; Ni, X. Isoflavones suppress cyclic adenosine 3′,5′-monophosphate regulatory element-mediated transcription in osteoblastic cell line. J. Nutr. Biochem. 2011, 22, 865–873. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, J.; Li, J.; Su, T.; Deng, X.; Fu, Y.; Liang, X.; Cui, H. From death to birth: How osteocyte death promotes osteoclast formation. Front. Immunol. 2025, 16, 1551542. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Chen, Y.; Singla, R.K.; Cao, D.; Shen, B. Exploring cytokines dynamics: Uncovering therapeutic concepts for metabolic disorders in postmenopausal women- diabetes, metabolic bone diseases, and non-alcohol fatty liver disease. Ageing Res. Rev. 2024, 101, 102505. [Google Scholar] [CrossRef]
- Pan, B.; Chen, C.; Zhao, Y.; Cai, J.; Fu, S.; Liu, J. SIRT3: A Potential Target of Different Types of Osteoporosis. Cell Biochem. Biophys. 2024, 82, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Public health impact of osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1243–1251. [Google Scholar] [CrossRef]
- Koehne, T.; Vettorazzi, E.; Küsters, N.; Lüneburg, R.; Kahl-Nieke, B.; Püschel, K.; Amling, M.; Busse, B. Trends in trabecular architecture and bone mineral density distribution in 152 individuals aged 30–90 years. Bone 2014, 66, 31–38. [Google Scholar] [CrossRef]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, Q.; Yang, J.; Ni, B. Tumor Necrosis Factor Alpha Promotes Osteoclast Formation via PI3K/Akt Pathway-Mediated Blimp1 Expression Upregulation. J. Cell Biochem. 2017, 118, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Sapra, L. The Rising Era of “Immunoporosis”: Role of Immune System in the Pathophysiology of Osteoporosis. J. Inflamm. Res. 2022, 15, 1667–1698. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shen, S.; Zhang, S.; Huang, M.; Zhang, L.; Chen, X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front. Endocrinol. 2022, 13, 898634. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Z.; Ma, Y.; Jin, J.; Qi, F.; Li, S.; Liu, C.; Lyu, F.J.; Zheng, Q. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int. J. Biol. Sci. 2020, 16, 2675–2691. [Google Scholar] [CrossRef]
- Young, A.R.J.; Cassidy, L.D.; Narita, M. Autophagy and senescence, converging roles in pathophysiology as seen through mouse models. Adv. Cancer Res. 2021, 150, 113–145. [Google Scholar] [CrossRef]
- Chen, K.; Yang, Y.H.; Jiang, S.D.; Jiang, L.S. Decreased activity of osteocyte autophagy with aging may contribute to the bone loss in senile population. Histochem. Cell Biol. 2014, 142, 285–295. [Google Scholar] [CrossRef]
- Onal, M.; Piemontese, M.; Xiong, J.; Wang, Y.; Han, L.; Ye, S.; Komatsu, M.; Selig, M.; Weinstein, R.S.; Zhao, H.; et al. Suppression of autophagy in osteocytes mimics skeletal aging. J. Biol. Chem. 2013, 288, 17432–17440. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.; Guo, J.; Wang, Q.; Cao, J.; Wang, H.; Li, G.; Mao, J.; Zou, X.; Chen, D.; et al. Nano-Hydroxyapatite Modulates Osteoblast Differentiation Through Autophagy Induction via mTOR Signaling Pathway. J. Biomed. Nanotechnol. 2019, 15, 405–415. [Google Scholar] [CrossRef]
- Fu, L.; Wu, W.; Sun, X.; Zhang, P. Glucocorticoids Enhanced Osteoclast Autophagy Through the PI3K/Akt/mTOR Signaling Pathway. Calcif. Tissue Int. 2020, 107, 60–71. [Google Scholar] [CrossRef]
- Ma, J.; Du, D.; Liu, J.; Guo, L.; Li, Y.; Chen, A.; Ye, T. Hydrogen sulphide promotes osteoclastogenesis by inhibiting autophagy through the PI3K/AKT/mTOR pathway. J. Drug Target. 2020, 28, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V. Remission of type 2 diabetes mellitus: Emerging concepts and proposed diagnostic criteria. World J. Methodol. 2024, 14, 95210. [Google Scholar] [CrossRef] [PubMed]
- Leungsuwan, D.S.; Chandran, M. Bone Fragility in Diabetes and its Management: A Narrative Review. Drugs 2024, 84, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Ferrelli, F.; Pastore, D.; Capuani, B.; Lombardo, M.F.; Blot-Chabaud, M.; Coppola, A.; Basello, K.; Galli, A.; Donadel, G.; Romano, M.; et al. Serum glucocorticoid inducible kinase (SGK)-1 protects endothelial cells against oxidative stress and apoptosis induced by hyperglycaemia. Acta Diabetol. 2015, 52, 55–64. [Google Scholar] [CrossRef]
- Lang, F.; Klingel, K.; Wagner, C.A.; Stegen, C.; Warntges, S.; Friedrich, B.; Lanzendorfer, M.; Melzig, J.; Moschen, I.; Steuer, S.; et al. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2000, 97, 8157–8162. [Google Scholar] [CrossRef]
- Ullrich, S.; Berchtold, S.; Ranta, F.; Seebohm, G.; Henke, G.; Lupescu, A.; Mack, A.F.; Chao, C.M.; Su, J.; Nitschke, R.; et al. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 2005, 54, 1090–1099. [Google Scholar] [CrossRef]
- Liu, H.; Yu, J.; Xia, T.; Xiao, Y.; Zhang, Q.; Liu, B.; Guo, Y.; Deng, J.; Deng, Y.; Chen, S.; et al. Hepatic serum- and glucocorticoid-regulated protein kinase 1 (SGK1) regulates insulin sensitivity in mice via extracellular-signal-regulated kinase 1/2 (ERK1/2). Biochem. J. 2014, 464, 281–289. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Y.; Li, S.; Wu, L.; Fejes-Toth, G.; Naray-Fejes-Toth, A.; Soukas, A.A. Serum- and glucocorticoid-induced kinase drives hepatic insulin resistance by directly inhibiting AMP-activated protein kinase. Cell Rep. 2021, 37, 109785. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, Y.; Yuan, F.; Liu, Y.; Jiang, X.; Deng, J.; Fejes-Toth, G.; Naray-Fejes-Toth, A.; Chen, S.; Chen, Y.; et al. SGK1/FOXO3 Signaling in Hypothalamic POMC Neurons Mediates Glucocorticoid-Increased Adiposity. Diabetes 2018, 67, 569–580. [Google Scholar] [CrossRef]
- Di Pietro, N.; Panel, V.; Hayes, S.; Bagattin, A.; Meruvu, S.; Pandolfi, A.; Hugendubler, L.; Fejes-Tóth, G.; Naray-Fejes-Tóth, A.; Mueller, E. Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates adipocyte differentiation via forkhead box O1. Mol. Endocrinol. 2010, 24, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J.; Voit, T. Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: Impaired glucose transport into brain—A review. Eur. J. Pediatr. 2002, 161, 295–304. [Google Scholar] [CrossRef]
- Lang, F.; Görlach, A.; Vallon, V. Targeting SGK1 in diabetes. Expert. Opin. Ther. Targets 2009, 13, 1303–1311. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Tariq, F.; Ahmad, M.; Subhan, M.; Zaid Alvi, S.M.; Tariq, M.U.; Ullah, S.; Khalid, A.; Bibi, R.; Shafique Ur Rehman, M.; Abbas, A. The Management of Osteoporosis in Chronic Kidney Disease: A Review of Diagnostic and Therapeutic Approaches. Cureus 2024, 16, e73882. [Google Scholar] [CrossRef]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho signaling axis in the kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef]
- Fujita, T. Mechanism of salt-sensitive hypertension: Focus on adrenal and sympathetic nervous systems. J. Am. Soc. Nephrol. 2014, 25, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Liu, J.; Chen, X.S.; Cheng, L.M.; Liu, W.L.; Chen, X.F.; Li, Y.J.; Guan, Y.Y.; Zeng, X.; Du, Y.H. Blockade of TMEM16A protects against renal fibrosis by reducing intracellular Cl(-) concentration. Br. J. Pharmacol. 2022, 179, 3043–3060. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, C.; Niu, J.; Xiong, Y.; He, Z.; Xu, H.; Zhang, M.; Wang, H.; Xu, Q.; Wang, X.; et al. Inhibitory effects of Eplerenone on angiogenesis via modulating SGK1/TGF-β pathway in contralateral kidney of CKD pregnancy rats. Cell. Signal. 2024, 122, 111346. [Google Scholar] [CrossRef]
- Sierra-Ramos, C.; Velazquez-Garcia, S.; Keskus, A.G.; Vastola-Mascolo, A.; Rodríguez-Rodríguez, A.E.; Luis-Lima, S.; Hernández, G.; Navarro-González, J.F.; Porrini, E.; Konu, O.; et al. Increased SGK1 activity potentiates mineralocorticoid/NaCl-induced kidney injury. Am. J. Physiol. Ren. Physiol. 2021, 320, F628–F643. [Google Scholar] [CrossRef]
- Bhalla, V.; Soundararajan, R.; Pao, A.C.; Li, H.; Pearce, D. Disinhibitory pathways for control of sodium transport: Regulation of ENaC by SGK1 and GILZ. Am. J. Physiol. Ren. Physiol. 2006, 291, F714–F721. [Google Scholar] [CrossRef]
- Diakov, A.; Korbmacher, C. A novel pathway of epithelial sodium channel activation involves a serum- and glucocorticoid-inducible kinase consensus motif in the C terminus of the channel’s alpha-subunit. J. Biol. Chem. 2004, 279, 38134–38142. [Google Scholar] [CrossRef]
- Vallon, V.; Lang, F. New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure. Curr. Opin. Nephrol. Hypertens. 2005, 14, 59–66. [Google Scholar] [CrossRef]
- Boehmer, C.; Wilhelm, V.; Palmada, M.; Wallisch, S.; Henke, G.; Brinkmeier, H.; Cohen, P.; Pieske, B.; Lang, F. Serum and glucocorticoid inducible kinases in the regulation of the cardiac sodium channel SCN5A. Cardiovasc. Res. 2003, 57, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Palmada, M.; Embark, H.M.; Wyatt, A.W.; Böhmer, C.; Lang, F. Negative charge at the consensus sequence for the serum- and glucocorticoid-inducible kinase, SGK1, determines pH sensitivity of the renal outer medullary K+ channel, ROMK1. Biochem. Biophys. Res. Commun. 2003, 307, 967–972. [Google Scholar] [CrossRef]
- Henke, G.; Maier, G.; Wallisch, S.; Boehmer, C.; Lang, F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid inducible kinase SGK1. J. Cell Physiol. 2004, 199, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Yoshida, S.; Shibata, S.; Nagase, T.; Gotoda, T.; Ando, K.; Fujita, T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: Possible contribution of fat-derived factors. J. Am. Soc. Nephrol. 2006, 17, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Sugatani, T.; Agapova, O.; Fang, Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 2017, 100, 80–86. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Si, Z.; Yang, Y.; Li, S.; Xue, Y. Dapagliflozin reverses the imbalance of T helper 17 and T regulatory cells by inhibiting SGK1 in a mouse model of diabetic kidney disease. FEBS Open Bio 2021, 11, 1395–1405. [Google Scholar] [CrossRef]
- Lang, F.; Artunc, F.; Vallon, V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr. Opin. Nephrol. Hypertens. 2009, 18, 439–448. [Google Scholar] [CrossRef]
- Gulzar, M.; Noor, S.; Hasan, G.M.; Hassan, M.I. The role of serum and glucocorticoid-regulated kinase 1 in cellular signaling: Implications for drug development. Int. J. Biol. Macromol. 2024, 258, 128725. [Google Scholar] [CrossRef] [PubMed]
- Halland, N.; Schmidt, F.; Weiss, T.; Li, Z.; Czech, J.; Saas, J.; Ding-Pfennigdorff, D.; Dreyer, M.K.; Strübing, C.; Nazare, M. Rational Design of Highly Potent, Selective, and Bioavailable SGK1 Protein Kinase Inhibitors for the Treatment of Osteoarthritis. J. Med. Chem. 2022, 65, 1567–1584. [Google Scholar] [CrossRef]
- Halland, N.; Schmidt, F.; Weiss, T.; Saas, J.; Li, Z.; Czech, J.; Dreyer, M.; Hofmeister, A.; Mertsch, K.; Dietz, U.; et al. Discovery of N-[4-(1H-Pyrazolo [3,4-b]pyrazin-6-yl)-phenyl]-sulfonamides as Highly Active and Selective SGK1 Inhibitors. ACS Med. Chem. Lett. 2015, 6, 73–78. [Google Scholar] [CrossRef]
- Sherk, A.B.; Frigo, D.E.; Schnackenberg, C.G.; Bray, J.D.; Laping, N.J.; Trizna, W.; Hammond, M.; Patterson, J.R.; Thompson, S.K.; Kazmin, D.; et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008, 68, 7475–7483. [Google Scholar] [CrossRef] [PubMed]
- Mansley, M.K.; Wilson, S.M. Effects of nominally selective inhibitors of the kinases PI3K, SGK1 and PKB on the insulin-dependent control of epithelial Na+ absorption. Br. J. Pharmacol. 2010, 161, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Maestro, I.; Boya, P.; Martinez, A. Serum- and glucocorticoid-induced kinase 1, a new therapeutic target for autophagy modulation in chronic diseases. Expert. Opin. Ther. Targets 2020, 24, 231–243. [Google Scholar] [CrossRef]
- Fagerli, U.M.; Ullrich, K.; Stühmer, T.; Holien, T.; Köchert, K.; Holt, R.U.; Bruland, O.; Chatterjee, M.; Nogai, H.; Lenz, G.; et al. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a prominent target gene of the transcriptional response to cytokines in multiple myeloma and supports the growth of myeloma cells. Oncogene 2011, 30, 3198–3206. [Google Scholar] [CrossRef]
- Jin, L.Y.; Huo, S.C.; Guo, C.; Liu, H.Y.; Xu, S.; Li, X.F. GSK 650394 Inhibits Osteoclasts Differentiation and Prevents Bone Loss via Promoting the Activities of Antioxidant Enzymes In Vitro and In Vivo. Oxid. Med. Cell Longev. 2022, 2022, 3458560. [Google Scholar] [CrossRef]
- Liang, X.; Lan, C.; Zhou, J.; Fu, W.; Long, X.; An, Y.; Jiao, G.; Wang, K.; Li, Y.; Xu, J.; et al. Development of a new analog of SGK1 inhibitor and its evaluation as a therapeutic molecule of colorectal cancer. J. Cancer 2017, 8, 2256–2262. [Google Scholar] [CrossRef]
- Ackermann, T.F.; Boini, K.M.; Beier, N.; Scholz, W.; Fuchss, T.; Lang, F. EMD638683, a novel SGK inhibitor with antihypertensive potency. Cell Physiol. Biochem. 2011, 28, 137–146. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, H.; Peng, F.; Liu, Z.; Ding, K.; Song, J.; Li, L.; Chen, J.; Shao, Q.; Yan, S.; et al. Complement C3a activates osteoclasts by regulating the PI3K/PDK1/SGK3 pathway in patients with multiple myeloma. Cancer Biol. Med. 2021, 18, 721–733. [Google Scholar] [CrossRef]
- Poetsch, F.; Henze, L.A.; Estepa, M.; Moser, B.; Pieske, B.; Lang, F.; Eckardt, K.U.; Alesutan, I.; Voelkl, J. Role of SGK1 in the Osteogenic Transdifferentiation and Calcification of Vascular Smooth Muscle Cells Promoted by Hyperglycemic Conditions. Int. J. Mol. Sci. 2020, 21, 7207. [Google Scholar] [CrossRef]
- Towhid, S.T.; Liu, G.L.; Ackermann, T.F.; Beier, N.; Scholz, W.; Fuchß, T.; Toulany, M.; Rodemann, H.P.; Lang, F. Inhibition of colonic tumor growth by the selective SGK inhibitor EMD638683. Cell Physiol. Biochem. 2013, 32, 838–848. [Google Scholar] [CrossRef]
- Du, Y.N.; Tang, X.F.; Xu, L.; Chen, W.D.; Gao, P.J.; Han, W.Q. SGK1-FoxO1 Signaling Pathway Mediates Th17/Treg Imbalance and Target Organ Inflammation in Angiotensin II-Induced Hypertension. Front. Physiol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Ren, J.; Li, T.; Lv, S.; Li, C.; Liu, Z.; Yang, M. The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Stagno, M.J.; Yan, J.; Schleicher, S.; Yu, W.; Honisch, S.; Lang, F.; Fuchs, J.; Seitz, G. Serum and Glucocorticoid Inducible Kinase 1-Sensitive Survival, Proliferation and Migration of Rhabdomyosarcoma Cells. Cell Physiol. Biochem. 2017, 43, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, L.; Amato, R.; Talarico, C.; Ortuso, F.; Menniti, M.; Dattilo, V.; Iuliano, R.; Gigliotti, F.; Artese, A.; Costa, G.; et al. SI113, a specific inhibitor of the Sgk1 kinase activity that counteracts cancer cell proliferation. Cell Physiol. Biochem. 2015, 35, 2006–2018. [Google Scholar] [CrossRef]
- Talarico, C.; D’Antona, L.; Scumaci, D.; Barone, A.; Gigliotti, F.; Fiumara, C.V.; Dattilo, V.; Gallo, E.; Visca, P.; Ortuso, F.; et al. Preclinical model in HCC: The SGK1 kinase inhibitor SI113 blocks tumor progression in vitro and in vivo and synergizes with radiotherapy. Oncotarget 2015, 6, 37511–37525. [Google Scholar] [CrossRef]
- Conza, D.; Mirra, P.; Calì, G.; Tortora, T.; Insabato, L.; Fiory, F.; Schenone, S.; Amato, R.; Beguinot, F.; Perrotti, N.; et al. The SGK1 inhibitor SI113 induces autophagy, apoptosis, and endoplasmic reticulum stress in endometrial cancer cells. J. Cell Physiol. 2017, 232, 3735–3743. [Google Scholar] [CrossRef]
- Rango, E.; D’Antona, L.; Iovenitti, G.; Brai, A.; Mancini, A.; Zamperini, C.; Trivisani, C.I.; Marianelli, S.; Fallacara, A.L.; Molinari, A.; et al. Si113-prodrugs selectively activated by plasmin against hepatocellular and ovarian carcinoma. Eur. J. Med. Chem. 2021, 223, 113653. [Google Scholar] [CrossRef]
- Mohammad, T.; Siddiqui, S.; Shamsi, A.; Alajmi, M.F.; Hussain, A.; Islam, A.; Ahmad, F.; Hassan, M.I. Virtual Screening Approach to Identify High-Affinity Inhibitors of Serum and Glucocorticoid-Regulated Kinase 1 Among Bioactive Natural Products: Combined Molecular Docking and Simulation Studies. Molecules 2020, 25, 823. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhao, Z.; Zhong, R.; Tan, X. A comprehensive review of herbacetin: From chemistry to pharmacological activities. J. Ethnopharmacol. 2021, 279, 114356. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Vicente, B.; Proença, C.; Silva, V.L.M.; Silva, A.M.S.; Corvo, M.L.; Fernandes, E.; Freitas, M. Herbacetin Inhibits Human Fructose 1,6-Bisphosphatase Among a Panel of Chromone Derivatives and Pyrazoles, Demonstrating Positive Effects on Insulin-Resistant HepG2 Cells. Chem. Biol. Drug Des. 2024, 104, e70017. [Google Scholar] [CrossRef]
- Amiran, M.R.; Taghdir, M.; Abasi Joozdani, F. Molecular insights into the behavior of the allosteric and ATP-competitive inhibitors in interaction with AKT1 protein: A molecular dynamics study. Int. J. Biol. Macromol. 2023, 242, 124853. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, T.; Xu, F.; Luo, F.; Zhou, H.; Liu, F.; Xie, X.; Liu, Y.; Wang, J.; Guo, Z.; et al. Discovery of Flavonoids as Novel Inhibitors of ATP Citrate Lyase: Structure-Activity Relationship and Inhibition Profiles. Int. J. Mol. Sci. 2022, 23, 10747. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Yu, M.; Shang, Y.; Chang, Y.; Zhao, H.; Kang, Y.; Zhao, L.; Xu, L.; Zhao, X.; et al. Discovery of Herbacetin as a Novel SGK1 Inhibitor to Alleviate Myocardial Hypertrophy. Adv. Sci. 2022, 9, e2101485. [Google Scholar] [CrossRef]
- Embark, H.M.; Setiawan, I.; Poppendieck, S.; van de Graaf, S.F.; Boehmer, C.; Palmada, M.; Wieder, T.; Gerstberger, R.; Cohen, P.; Yun, C.C.; et al. Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol. Biochem. 2004, 14, 203–212. [Google Scholar] [CrossRef]
- Aspernig, H.; Heimbucher, T.; Qi, W.; Gangurde, D.; Curic, S.; Yan, Y.; Donner von Gromoff, E.; Baumeister, R.; Thien, A. Mitochondrial Perturbations Couple mTORC2 to Autophagy in C. elegans. Cell Rep. 2019, 29, 1399–1409.e5. [Google Scholar] [CrossRef]
- Willebrand, R.; Kleinewietfeld, M. The role of salt for immune cell function and disease. Immunology 2018, 154, 346–353. [Google Scholar] [CrossRef]



| Inhibitor | Structure | IC50 | Pathways Targeted | Disease | Ref. |
|---|---|---|---|---|---|
| GSK650394 (C25H22N2O2) |  | 62 nM | mTOR-FoxO3 pathway PI3K-Akt signaling | Cancer Osteoporosis Age-related diseases Diabetes Inflammation | [144,145,146,147,148] |
| QGY-5-114-A |  | 122.9 μM | ND | Colorectal cancer Metastatic bone tumor | [149] |
| EMD638683 (C18H18F2N2O4) | 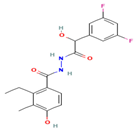 | 3 μM | NF-κB signaling pathway | Colorectal tumor Osteoporosis Hypertension Diabetes | [49,141,150,151,152,153,154,155,156] |
| SI113 (C23H24N6O) |  | 600 nM | Caspases-PARP signaling pathway | Ovarian cancer Hepatocellular carcinoma Endometrial cancer Osteoporosis | [157,158,159,160] |
| 17a (C23H21ClFN5O4S) |  | NA | ND | Hepatocellular carcinoma Glioblastoma | [142] |
| ZINC00319000 (C19H15O6) |  | NA | ND | Cancer Metastatic bone tumor | [161] |
| Herbacetin (C15H10O7) |  | 750 nM | mTOR-FoxO3 signaling pathway ROS production | Hepatocellular carcinoma Osteoporosis Cardiac hypertrophy Diabetes | [162,163,164,165,166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Chen, C.; Chen, R.; Yang, C.; Liu, Z.; Wen, L.; Xiao, H.; Geng, B.; Xia, Y. Unraveling the Potential of SGK1 in Osteoporosis: From Molecular Mechanisms to Therapeutic Targets. Biomolecules 2025, 15, 686. https://doi.org/10.3390/biom15050686
Yang F, Chen C, Chen R, Yang C, Liu Z, Wen L, Xiao H, Geng B, Xia Y. Unraveling the Potential of SGK1 in Osteoporosis: From Molecular Mechanisms to Therapeutic Targets. Biomolecules. 2025; 15(5):686. https://doi.org/10.3390/biom15050686
Chicago/Turabian StyleYang, Fei, Changshun Chen, Rongjin Chen, Chenghui Yang, Zirui Liu, Lei Wen, Hefang Xiao, Bin Geng, and Yayi Xia. 2025. "Unraveling the Potential of SGK1 in Osteoporosis: From Molecular Mechanisms to Therapeutic Targets" Biomolecules 15, no. 5: 686. https://doi.org/10.3390/biom15050686
APA StyleYang, F., Chen, C., Chen, R., Yang, C., Liu, Z., Wen, L., Xiao, H., Geng, B., & Xia, Y. (2025). Unraveling the Potential of SGK1 in Osteoporosis: From Molecular Mechanisms to Therapeutic Targets. Biomolecules, 15(5), 686. https://doi.org/10.3390/biom15050686






