Melatonin Interplay in Physiology and Disease—The Fountain of Eternal Youth Revisited
Abstract
1. Introduction
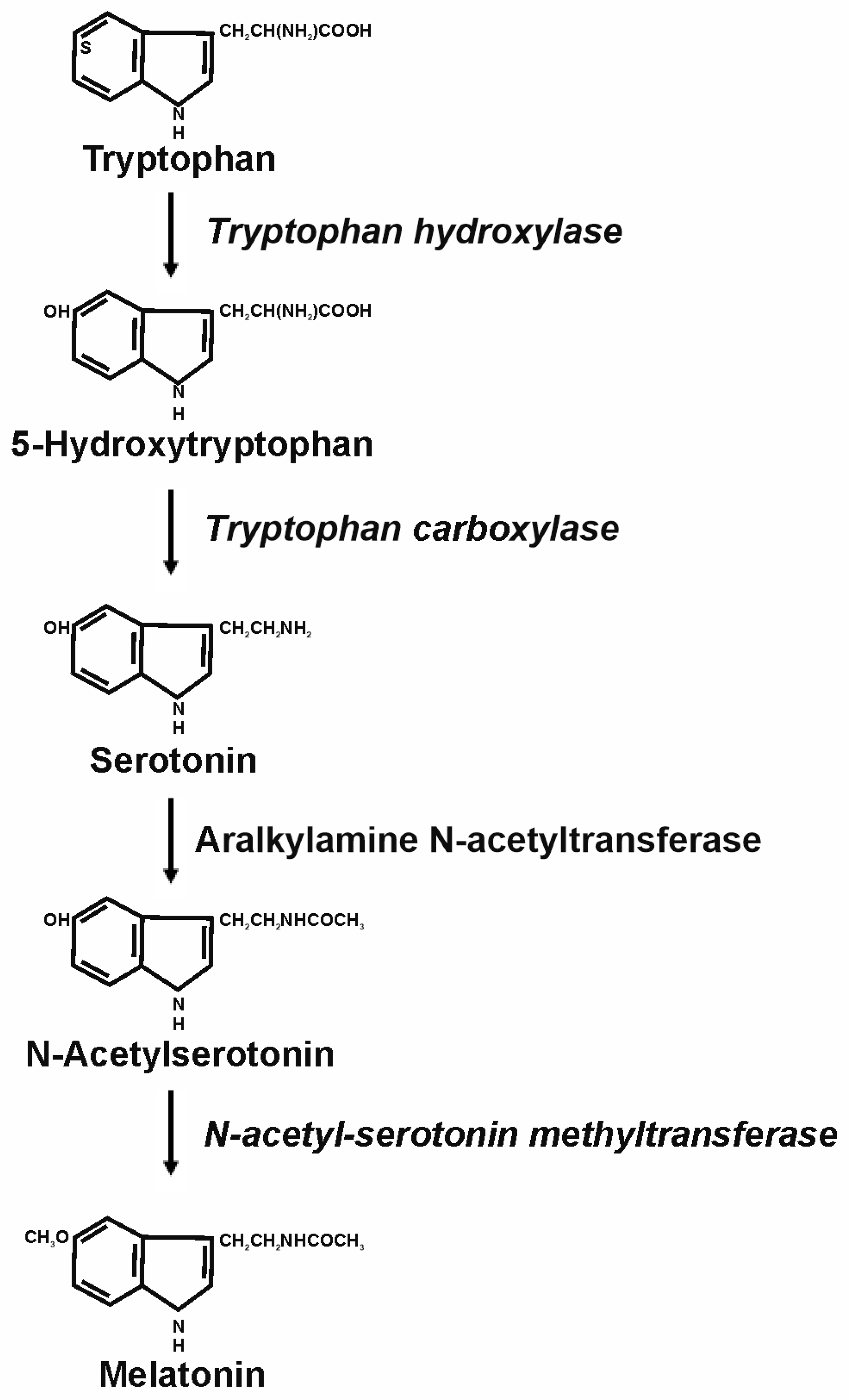
2. Melatonin and Its Physiological Functions
3. Antioxidant Effects of Melatonin
4. Melatonin and Neurological Diseases
5. Melatonin and Cancer
6. Melatonin and Immune-Related Diseases
7. Melatonin, Aging, and Frailty
8. Melatonin and Healthy Aging: Impairment of Sleep–Wake Cycle and Disease
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Beta-amyloid |
| AD | Alzheimer’s disease |
| ADAMTS | Disintegrin and metalloprotease with thrombospondin motifs |
| AKT | Protein kinase B |
| ALS | Amyotrophic lateral sclerosis |
| CAFs | Cancer-associated fibroblasts |
| Ca2+ | Calcium |
| Cas | Caspase |
| CAT | Catalase |
| Con A | Concanavalin A |
| COX-2 | Cycloxigenase-2 |
| CXCL | Chemotactic cytokine |
| Cyt C | Cytochrome C |
| EGFR-TKI | EGFR-tyrosine kinase inhibitor |
| ERK | Extracellular signal-regulated kinase |
| FoxOs | Forkhead box O |
| FOSL1 | Fos-related antigen 1 |
| GP | Glutathione peroxidase |
| GSH | Glutathione |
| HO-1 | Hemoxygenase 1 |
| HIF-1α | Hypoxia-induced factor 1 alpha |
| HPDG | 15-Hydroxyprostaglandin dehydrogenase |
| aMT6s | 6-hydroxymelatonin sulfate |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IGF-1 | Insulin-like growth factor 1 |
| IGFBP3 | Insulin-like growth factor binding protein-3 |
| lncRNAs | Long non-coding RNAs |
| LPO | Lipid hydroperoxide |
| mTOR | Mammalian target of rapamycin |
| MAPK | Mitogen-activated protein kinases |
| MCP1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MLL | Mixed lineage leukemia |
| MMP-2 | Matrix metalloproteinase 2 |
| MT1 | Melatonin receptor type 1 |
| MT2 | Melatonin receptor type 2 |
| miRNAs | Micro-ribonucleic acids |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| NETosis | Program for formation of neutrophil extracellular traps |
| NKAP | NFKB-activating protein |
| NF-κB | Nuclear factor kappa B |
| NGFR | Nerve growth factor receptor |
| NK | Natural killer |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NQO1 | NADPH: quinone oxidoreductase |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| PD | Parkinson’s disease |
| PI3K | Phosphatidylinositol 3-kinase |
| RANKL | Tumor-secreted receptor activator of NF-κB ligand |
| ROR | Retinoic acid-related orphan receptor |
| ROS | Reactive oxygen species |
| SIRT | Sirtuin |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor Alpha |
| VEGF | Vascular endothelial growth factor |
References
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martínez-Reig, M.; Mas-Bargues, C.; Inglés, M. Oxidative Stress and Exceptional Human Longevity: Systematic Review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rzheshevsky, A. V Fatal “Triad”: Lipotoxicity, Oxidative Stress, and Phenoptosis. Biochemistry 2013, 78, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.A.; Simões, J.; Costa, R.; Pimenta, L.; Gonçalves, N.J.; Albuquerque, C.; Cunha, C.; Zdravevski, E.; Lameski, P.; Garcia, N.M.; et al. Thought on Food: A Systematic Review of Current Approaches and Challenges for Food Intake Detection. Sensors 2022, 22, 6443. [Google Scholar] [CrossRef]
- Giannone, F.; Ebrahimi, C.; Endrass, T.; Hansson, A.C.; Schlagenhauf, F.; Sommer, W.H. Bad Habits-Good Goals? Meta-Analysis and Translation of the Habit Construct to Alcoholism. Transl. Psychiatry 2024, 14, 298. [Google Scholar] [CrossRef]
- Dhand, R.; Sohal, H. Good Sleep, Bad Sleep! The Role of Daytime Naps in Healthy Adults. Curr. Opin. Pulm. Med. 2006, 12, 379–382. [Google Scholar] [CrossRef]
- Waldhauser, F.; Kovács, J.; Reiter, E. Age-Related Changes in Melatonin Levels in Humans and Its Potential Consequences for Sleep Disorders. Exp. Gerontol. 1998, 33, 759–772. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Vendemiale, G.; La Viola, M.; De Cata, A.; Carughi, S.; Greco, A.; Balzanelli, M.; Tarquini, R. Circadian Variations of Cortisol, Melatonin and Lymphocyte Subpopulations in Geriatric Age. Int. J. Immunopathol. Pharmacol. 2010, 23, 289–296. [Google Scholar] [CrossRef]
- Magri, F.; Sarra, S.; Cinchetti, W.; Guazzoni, V.; Fioravanti, M.; Cravello, L.; Ferrari, E. Qualitative and Quantitative Changes of Melatonin Levels in Physiological and Pathological Aging and in Centenarians. J. Pineal Res. 2004, 36, 256–261. [Google Scholar] [CrossRef]
- Berisha, G.; Sedliak, M.; Zeman, M.; Hamar, D.; Cvečka, J.; Tirpáková, V.; Vajda, M.; Oreská, Ľ.; Černáčková, A.; Čupka, M.; et al. Can Lifelong Endurance Exercise Improve Ageing through Beneficial Effects on Circadian Timing Function, Muscular Performance and Health Status in Men? Protocol for a Comparative Cross-Sectional Study. Eur. J. Transl. Myol. 2023, 33, 10–4081. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Gubin, G.D.; Gapon, L.I.; Weinert, D. Daily Melatonin Administration Attenuates Age-Dependent Disturbances of Cardiovascular Rhythms. Curr. Aging Sci. 2016, 9, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Nachvak, S.M.; Fazelian, S.; Moradi, F.; Persad, E.; Heshmati, J. Effect of Melatonin Supplementation on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis. Pharmacol. Res. 2020, 161, 105210. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninejad, P.; Sheikhhossein, F.; Djafari, F.; Tijani, A.J.; Mohammadpour, S.; Shab-Bidar, S. Effects of Melatonin Supplementation on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200030. [Google Scholar] [CrossRef]
- EU Clinical Trials Register EU Clinical Trials Register: Clinical Trials for Melatonin. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=Melatonin (accessed on 6 April 2025).
- Gonzalez, A. Antioxidants and Neuron-Astrocyte Interplay in Brain Physiology: Melatonin, a Neighbor to Rely On. Neurochem. Res. 2021, 46, 34–50. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of Melatonin and 5-Methoxyindole-3-Acetic Acid from Bovine Pineal Glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef]
- Arendt, J.; Aulinas, A. Physiology of the Pineal Gland and Melatonin; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Hsing, A.W.; Meyer, T.E.; Niwa, S.; Quraishi, S.M.; Chu, L.W. Measuring Serum Melatonin in Epidemiologic Studies. Cancer Epidemiol. Biomark. Prev. 2010, 19, 932–937. [Google Scholar] [CrossRef]
- Felder-Schmittbuhl, M.P.; Hicks, D.; Ribelayga, C.P.; Tosini, G. Melatonin in the Mammalian Retina: Synthesis, Mechanisms of Action and Neuroprotection. J. Pineal Res. 2024, 76, e12951. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Maitra, S.K. Gut Melatonin in Vertebrates: Chronobiology and Physiology. Front. Endocrinol. 2015, 6, 112. [Google Scholar] [CrossRef]
- Cardenas-Padilla, A.J.; Jimenez-Trejo, F.; Cerbon, M.; Medrano, A. The Role of Melatonin on Caprine (Capra Hircus) Sperm Freezability: A Review. Antioxidants 2024, 13, 1466. [Google Scholar] [CrossRef] [PubMed]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P.M. Gene Expression of the Key Enzymes of Melatonin Synthesis in Extrapineal Tissues of the Rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on Its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef]
- Gómez-Corvera, A.; Cerrillo, I.; Molinero, P.; Naranjo, M.C.; Lardone, P.J.; Sanchez-Hidalgo, M.; Carrascosa-Salmoral, M.P.; Medrano-Campillo, P.; Guerrero, J.M.; Rubio, A. Evidence of Immune System Melatonin Production by Two Pineal Melatonin Deficient Mice, C57BL/6 and Swiss Strains. J. Pineal Res. 2009, 47, 15–22. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of Highly Elevated Levels of Melatonin in Bone Marrow: Its Origin and Significance. Biochim. Biophys. Acta 1999, 1472, 206–214. [Google Scholar] [CrossRef]
- Kvetnoy, I.M. Extrapineal Melatonin: Location and Role within Diffuse Neuroendocrine System. Histochem. J. 1999, 31, 1–12. [Google Scholar] [CrossRef]
- Bubenik, G.A. Localization, Physiological Significance and Possible Clinical Implication of Gastrointestinal Melatonin. Biol. Signals Recept. 2001, 10, 350–366. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal Melatonin: Localization, Function, and Clinical Relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Kozioł, K.; Broda, D.; Romerowicz-Misielak, M.; Nowak, S.; Koziorowski, M. Melatonin Concentration in Peripheral Blood and Melatonin Receptors (MT1 and MT2) in the Testis and Epididymis of Male Roe Deer during Active Spermatogenesis. Theriogenology 2020, 149, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Grasselli, F. Role of Melatonin in Ovarian Function. Animals 2024, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for Melatonin Synthesis in Mouse and Human Bone Marrow Cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ait Abdellah, S.; Raverot, V.; Gal, C.; Guinobert, I.; Bardot, V.; Blondeau, C.; Claustrat, B. Bioavailability of Melatonin after Administration of an Oral Prolonged-Release Tablet and an Immediate-Release Sublingual Spray in Healthy Male Volunteers. Drugs R. D. 2023, 23, 257–265. [Google Scholar] [CrossRef]
- Aldhous, M.; Franey, C.; Wright, J.; Arendt, J. Plasma Concentrations of Melatonin in Man Following Oral Absorption of Different Preparations. Br. J. Clin. Pharmacol. 1985, 19, 517–521. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.K.; Mielke, L.V.; Jønsson, B.; Jenstrup, M.T.; Gögenur, I.; Rosenberg, J. Pharmacokinetics of Repeated Melatonin Drug Administrations Prior to and After Surgery. Clin. Drug Investig. 2016, 36, 1045–1050. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical Pharmacokinetics of Melatonin: A Systematic Review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Binks, H.; E Vincent, G.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef]
- Pereira, G.A.; Gomes Domingos, A.L.; Aguiar, A.S. de Relationship between Food Consumption and Improvements in Circulating Melatonin in Humans: An Integrative Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 670–678. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Manwiwattanakul, G.; Loypimai, P.; Kerr, W.L. Melatonin and Its Derivative Contents in Tropical Fruits and Fruit Tablets. J. Food Compos. Anal. 2021, 103, 104109. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Calvo, J.R.; Maldonado-Aibar, M.D.; Millan-Linares, M.D.C.; Montserrat-de la Paz, S. Mediterranean Diet and Melatonin: A Systematic Review. Antioxidants 2023, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-Y.; Lam, K.-L.; Li, X.; Kong, A.P.-S.; Cheung, P.C.-K. Circadian Disruption-Induced Metabolic Syndrome in Mice Is Ameliorated by Oat β-Glucan Mediated by Gut Microbiota. Carbohydr. Polym. 2021, 267, 118216. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. Melatonin Receptors in Humans: Biological Role and Clinical Relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Tamura, H.; Reiter, R.J. Melatonin as a Naturally Occurring Co-Substrate of Quinone Reductase-2, the Putative MT3 Melatonin Membrane Receptor: Hypothesis and Significance. J. Pineal Res. 2007, 43, 317–320. [Google Scholar] [CrossRef]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Garcia-Sanchez, L.; Ruy, D.C.; Sanchez-Correa, B.; Fernandez-Bermejo, M.; Tarazona, R.; Salido, G.M.; Gonzalez, A. Melatonin Induces Calcium Mobilization and Influences Cell Proliferation Independently of MT1/MT2 Receptor Activation in Rat Pancreatic Stellate Cells. Cell Biol. Toxicol. 2015, 31, 95–110. [Google Scholar] [CrossRef]
- Xie, X.; Ding, D.; Bai, D.; Zhu, Y.; Sun, W.; Sun, Y.; Zhang, D. Melatonin Biosynthesis Pathways in Nature and Its Production in Engineered Microorganisms. Synth. Syst. Biotechnol. 2022, 7, 544–553. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and Its Metabolites vs Oxidative Stress: From Individual Actions to Collective Protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin Metabolism in the Central Nervous System. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocrinol. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The Regulations and Role of Circadian Clock and Melatonin in Uterine Receptivity and Pregnancy-An Immunological Perspective. Am. J. Reprod. Immunol. 2017, 78, e12715. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.-M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and Its Ubiquitous Anticancer Effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef]
- Ma, Q.; Reiter, R.J.; Chen, Y. Role of Melatonin in Controlling Angiogenesis under Physiological and Pathological Conditions. Angiogenesis 2020, 23, 91–104. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Bartolini, D.; Arcangeletti, M.; Ciffolilli, S.; Murdolo, G.; Piroddi, M.; Papa, S.; Reiter, R.J.; Galli, F. Melatonin Regulates Mesenchymal Stem Cell Differentiation: A Review. J. Pineal Res. 2014, 56, 382–397. [Google Scholar] [CrossRef]
- Nadri, P.; Ansari-Mahyari, S.; Jafarpour, F.; Mahdavi, A.H.; Tanhaei Vash, N.; Lachinani, L.; Dormiani, K.; Nasr-Esfahani, M.H. Melatonin Accelerates the Developmental Competence and Telomere Elongation in Ovine SCNT Embryos. PLoS ONE 2022, 17, e0267598. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.M.; Reiter, R.J. Melatonin-Immune System Relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-Antioxidant Response Element Pathway: A Review of Its Regulation by Melatonin and the Proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Melatonin and Aging. Subcell. Biochem. 2023, 103, 291–307. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Chen, G.-H. Treatment of Circadian Rhythm Sleep-Wake Disorders. Curr. Neuropharmacol. 2022, 20, 1022–1034. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; Blood, M.L.; Stevenson, J.; Keith, L.D. Melatonin Administration to Blind People: Phase Advances and Entrainment. J. Biol. Rhythm. 1991, 6, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Popović, B.; Velimirović, M.; Stojković, T.; Brajović, G.; De Luka, S.R.; Milovanović, I.; Stefanović, S.; Nikolić, D.; Ristić-Djurović, J.L.; Petronijević, N.D.; et al. The Influence of Ageing on the Extrapineal Melatonin Synthetic Pathway. Exp. Gerontol. 2018, 110, 151–157. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, Human Aging, and Age-Related Diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in Aging and Disease -Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis. 2012, 3, 194–225. [Google Scholar]
- InpharmD Does Supplemental Melatonin Suppress Endogenous Melatonin Production? Available online: https://inpharmd.com/does-supplemental-melatonin-suppress-endogenous-melatonin-production (accessed on 6 April 2025).
- Zhdanova, I.V.; Wurtman, R.J.; Balcioglu, A.; Kartashov, A.I.; Lynch, H.J. Endogenous Melatonin Levels and the Fate of Exogenous Melatonin: Age Effects. J. Gerontol. A. Biol. Sci. Med. Sci. 1998, 53, B293–B298. [Google Scholar] [CrossRef] [PubMed]
- Givler, D.; Givler, A.; Luther, P.M.; Wenger, D.M.; Ahmadzadeh, S.; Shekoohi, S.; Edinoff, A.N.; Dorius, B.K.; Jean Baptiste, C.; Cornett, E.M.; et al. Chronic Administration of Melatonin: Physiological and Clinical Considerations. Neurol. Int. 2023, 15, 518–533. [Google Scholar] [CrossRef]
- Markus, R.P.; Sousa, K.S.; da Silveira Cruz-Machado, S.; Fernandes, P.A.; Ferreira, Z.S. Possible Role of Pineal and Extra-Pineal Melatonin in Surveillance, Immunity, and First-Line Defense. Int. J. Mol. Sci. 2021, 22, 12143. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Mohammadzadeh, A.; Adib, A.; Darband, S.G.; Sadighparvar, S.; Mihanfar, A.; Majidinia, M.; Yousefi, B. Melatonin-Mediated Regulation of Autophagy: Making Sense of Double-Edged Sword in Cancer. J. Cell. Physiol. 2019, 234, 17011–17022. [Google Scholar] [CrossRef]
- Mahmood, D. Pleiotropic Effects of Melatonin. Drug Res. 2019, 69, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Herrera, T.; Benítez, V.; Arribas, S.M.; López de Pablo, A.L.; Esteban, R.M.; Martín-Cabrejas, M.A. Estimation of Scavenging Capacity of Melatonin and Other Antioxidants: Contribution and Evaluation in Germinated Seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and Physical Properties and Potential Mechanisms: Melatonin as a Broad Spectrum Antioxidant and Free Radical Scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Qi, W.; Manchester, L.C.; Karbownik, M.; Calvo, J.R. Pharmacology and Physiology of Melatonin in the Reduction of Oxidative Stress in Vivo. Biol. Signals Recept. 2000, 9, 160–171. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging in Vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ryer-Powder, J.E.; Forman, H.J. Adhering Lung Macrophages Produce Superoxide Demonstrated with Desferal-Mn(IV). Free Radic. Biol. Med. 1989, 6, 513–518. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Clea Ruy, D.; Garcia-Sanchez, L.; Jimenez-Blasco, D.; Fernandez-Bermejo, M.; Bolaños, J.P.; Salido, G.M.; Gonzalez, A. Melatonin Induces the Expression of Nrf2-Regulated Antioxidant Enzymes via PKC and Ca2+ Influx Activation in Mouse Pancreatic Acinar Cells. Free Radic. Biol. Med. 2015, 87, 226–236. [Google Scholar] [CrossRef]
- Estaras, M.; Peña, F.J.; Tapia, J.A.; Fernandez-Bermejo, M.; Mateos, J.M.; Vara, D.; Roncero, V.; Blanco, G.; Lopez, D.; Salido, G.M.; et al. Melatonin Modulates Proliferation of Pancreatic Stellate Cells through Caspase-3 Activation and Changes in Cyclin A and D Expression. J. Physiol. Biochem. 2020, 76, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Estaras, M.; Gonzalez-portillo, M.R.; Fernandez-bermejo, M.; Mateos, J.M.; Vara, D.; Blanco-fernandez, G.; Lopez-guerra, D.; Roncero, V.; Salido, G.M.; González, A. Melatonin Induces Apoptosis and Modulates Cyclin Expression and Mapk Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia. Int. J. Mol. Sci. 2021, 22, 5555. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Estaras, M.; Martinez-Morcillo, S.; Martinez, R.; García, A.; Estévez, M.; Santofimia-Castaño, P.; Tapia, J.A.; Moreno, N.; Pérez-López, M.; et al. Melatonin Modulates Red-Ox State and Decreases Viability of Rat Pancreatic Stellate Cells. Sci. Rep. 2020, 10, 6352. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Yi, W.; Li, Y.; Fan, C.; Xin, Z.; Jiang, S.; Di, S.; Qu, Y.; Reiter, R.J.; et al. A Review of Melatonin as a Suitable Antioxidant against Myocardial Ischemia-Reperfusion Injury and Clinical Heart Diseases. J. Pineal Res. 2014, 57, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Edres, H.A.; Taha, N.M.; Lebda, M.A.; Elfeky, M.S. The Potential Neuroprotective Effect of Allicin and Melatonin in Acrylamide-Induced Brain Damage in Rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 58768–58780. [Google Scholar] [CrossRef]
- Ates, G.; Tamer, S.; Ozkok, E.; Yorulmaz, H.; Gundogan, G.I.; Aksu, A.; Balkis, N. Utility of Melatonin on Brain Injury, Synaptic Transmission, and Energy Metabolism in Rats with Sepsis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025, 398, 1509–1519. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Xu, M.-M.; Sun, Y.; Ding, Z.-X.; Wei, Y.-Y.; Zhang, D.-W.; Wang, Y.-G.; Shen, J.-L.; Wu, H.-M.; Fei, G.-H. Melatonin Attenuates LPS-Induced Pyroptosis in Acute Lung Injury by Inhibiting NLRP3-GSDMD Pathway via Activating Nrf2/HO-1 Signaling Axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Chowdhury, O.; Ghosh, S.; Das, A.; Liu, H.; Shang, P.; Stepicheva, N.A.; Hose, S.; Sinha, D.; Chattopadhyay, S. Sustained Systemic Inflammation Increases Autophagy and Induces EMT/Fibrotic Changes in Mouse Liver Cells: Protection by Melatonin. Cell. Signal. 2023, 101, 110521. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; García-Gil, F.A.; López-Pingarrón, L.; Alatorre-Jiménez, M.A.; Ramírez, J.M.; Tan, D.-X.; García, J.J.; Reiter, R.J. Melatonin Role Preventing Steatohepatitis and Improving Liver Transplantation Results. Cell. Mol. Life Sci. 2016, 73, 2911–2927. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, L.; Tao, J.; Li, L. Protective Role of Melatonin in Early-Stage and End-Stage Liver Cirrhosis. J. Cell. Mol. Med. 2019, 23, 7151–7162. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Hu, H.; Zhang, W.; Zhang, Y.; Ge, Y.; Ma, T.; Wu, J.; Li, L.; Sun, M.; et al. Melatonin Attenuates Sepsis-Induced Acute Kidney Injury by Promoting Mitophagy through SIRT3-Mediated TFAM Deacetylation. Autophagy 2024, 20, 151–165. [Google Scholar] [CrossRef]
- Promsan, S.; Lungkaphin, A. The Roles of Melatonin on Kidney Injury in Obese and Diabetic Conditions. Biofactors 2020, 46, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Hsu, H.-C.; Chang, Y.-C.; Yu, C.-Y.; Liu, C.-T.; Sung, W.-W. Melatonin Exhibits Partial Protective Effects against Gemcitabine- and Cisplatin-Induced Kidney and Reproductive Injuries in Mice. Aging 2023, 15, 14372–14383. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, T.; Wu, H.; Yang, N.; Xu, S. Melatonin Attenuates Bisphenol A-Induced Colon Injury by Dual Targeting Mitochondrial Dynamics and Nrf2 Antioxidant System via Activation of SIRT1/PGC-1α Signaling Pathway. Free Radic. Biol. Med. 2023, 195, 13–22. [Google Scholar] [CrossRef]
- Zielińska, M.; Jarmuż, A.; Sałaga, M.; Kordek, R.; Laudon, M.; Storr, M.; Fichna, J. Melatonin, but Not Melatonin Receptor Agonists Neu-P11 and Neu-P67, Attenuates TNBS-Induced Colitis in Mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 511–519. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Jena, G.B.; Tikoo, K.B.; Kumar, V. Melatonin Modulated Autophagy and Nrf2 Signaling Pathways in Mice with Colitis-Associated Colon Carcinogenesis. Mol. Carcinog. 2016, 55, 255–267. [Google Scholar] [CrossRef]
- Aykutoglu, G.; Tartik, M.; Darendelioglu, E.; Ayna, A.; Baydas, G. Melatonin and Vitamin E Alleviate Homocysteine-Induced Oxidative Injury and Apoptosis in Endothelial Cells. Mol. Biol. Rep. 2020, 47, 5285–5293. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Foglio, E.; Rossini, C.; Castrezzati, S.; Lonati, C.; Rezzani, R. Vascular Endothelial Cells and Dysfunctions: Role of Melatonin. Front. Biosci. (Elite Ed.) 2013, 5, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kim, M.; Jeong, S.; Kim, S.; Moon, H.; Kim, H.; Lee, M.Y.; Kim, J.; Kim, H.-S.; Choi, M.; et al. Melatonin Alleviates Myocardial Dysfunction through Inhibition of Endothelial-to-Mesenchymal Transition via the NF-ΚB Pathway. J. Pineal Res. 2024, 76, e12958. [Google Scholar] [CrossRef]
- Su, C.-M.; Tsai, C.-H.; Chen, H.-T.; Wu, Y.-S.; Chang, J.-W.; Yang, S.-F.; Tang, C.-H. Melatonin Improves Muscle Injury and Differentiation by Increasing Pax7 Expression. Int. J. Biol. Sci. 2023, 19, 1049–1062. [Google Scholar] [CrossRef]
- Zhu, G.-Z.; Zhao, K.; Li, H.-Z.; Wu, D.-Z.; Chen, Y.-B.; Han, D.; Gao, J.-W.; Chen, X.-Y.; Yu, Y.-P.; Huang, Z.-W.; et al. Melatonin Ameliorates Age-Related Sarcopenia by Inhibiting Fibrogenic Conversion of Satellite Cell. Mol. Med. 2024, 30, 238. [Google Scholar] [CrossRef] [PubMed]
- Leonardo-Mendonça, R.C.; Ocaña-Wilhelmi, J.; de Haro, T.; de Teresa-Galván, C.; Guerra-Hernández, E.; Rusanova, I.; Fernández-Ortiz, M.; Sayed, R.K.A.; Escames, G.; Acuña-Castroviejo, D. The Benefit of a Supplement with the Antioxidant Melatonin on Redox Status and Muscle Damage in Resistance-Trained Athletes. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2017, 42, 700–707. [Google Scholar] [CrossRef]
- Gu, C.; Zhou, Q.; Hu, X.; Ge, X.; Hou, M.; Wang, W.; Liu, H.; Shi, Q.; Xu, Y.; Zhu, X.; et al. Melatonin Rescues the Mitochondrial Function of Bone Marrow-Derived Mesenchymal Stem Cells and Improves the Repair of Osteoporotic Bone Defect in Ovariectomized Rats. J. Pineal Res. 2024, 76, e12924. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, L.; Li, Y.; Yan, G.; Feng, C.; Liu, T.; Gong, R.; Yuan, Y.; Wang, N.; Idiiatullina, E.; et al. Melatonin Protects Bone Marrow Mesenchymal Stem Cells against Iron Overload-Induced Aberrant Differentiation and Senescence. J. Pineal Res. 2017, 63, e12422. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L.J.; Kraig, E.; Orr, M.E. Biological Aging Processes Underlying Cognitive Decline and Neurodegenerative Disease. J. Clin. Invest. 2022, 132, e158453. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Olcese, J.M.; Cao, C.; Mori, T.; Mamcarz, M.B.; Maxwell, A.; Runfeldt, M.J.; Wang, L.; Zhang, C.; Lin, X.; Zhang, G.; et al. Protection against Cognitive Deficits and Markers of Neurodegeneration by Long-Term Oral Administration of Melatonin in a Transgenic Model of Alzheimer Disease. J. Pineal Res. 2009, 47, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, C.; Wang, J.; Huang, X.; Yu, H.; Li, S.; Li, S.; Zhang, Z.; Liu, J.; Yang, X.; et al. Melatonin Ameliorates Cognitive Deficits through Improving Mitophagy in a Mouse Model of Alzheimer’s Disease. J. Pineal Res. 2021, 71, e12774. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, M.; Cheng, L.; Ding, L.; Lv, Q.; Huang, Z.; Zhou, J.; Chen, J.; Wang, P.; Zhang, S.; et al. Melatonin Modulates TLR4/MyD88/NF-ΚB Signaling Pathway to Ameliorate Cognitive Impairment in Sleep-Deprived Rats. Front. Pharmacol. 2024, 15, 1430599. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lan, G.; Li, R.; Mei, Y.; Shui, X.; Gu, X.; Wang, L.; Zhang, T.; Gan, C.-L.; Xia, Y.; et al. Melatonin Ameliorates Tau-Related Pathology via the MiR-504-3p and CDK5 Axis in Alzheimer’s Disease. Transl. Neurodegener. 2022, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Zhang, Y.-C.; Chatterjie, N.; Grundke-Iqbal, I.; Iqbal, K.; Wang, J.-Z. Effect of Melatonin and Melatonylvalpromide on Beta-Amyloid and Neurofilaments in N2a Cells. Neurochem. Res. 2008, 33, 1138–1144. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wang, X.; Xia, Y.; Huang, J.; Wang, T.; Lin, Z.; Xiong, N. Melatonin Ameliorates Parkinson’s Disease via Regulating Microglia Polarization in a RORα-Dependent Pathway. NPJ Park. Dis. 2022, 8, 90. [Google Scholar] [CrossRef]
- Hadoush, H.; Lababneh, T.; Banihani, S.A.; Al-Jarrah, M.; Jamous, M. Melatonin and Dopamine Serum Level Associations with Motor, Cognitive, and Sleep Dysfunctions in Patients with Parkinson’s Disease: A Cross-Sectional Research Study. NeuroRehabilitation 2020, 46, 539–549. [Google Scholar] [CrossRef]
- Patki, G.; Lau, Y.-S. Melatonin Protects against Neurobehavioral and Mitochondrial Deficits in a Chronic Mouse Model of Parkinson’s Disease. Pharmacol. Biochem. Behav. 2011, 99, 704–711. [Google Scholar] [CrossRef]
- Lv, Q.-K.; Tao, K.-X.; Yao, X.-Y.; Pang, M.-Z.; Cao, B.-E.; Liu, C.-F.; Wang, F. Melatonin MT1 Receptors Regulate the Sirt1/Nrf2/Ho-1/Gpx4 Pathway to Prevent α-Synuclein-Induced Ferroptosis in Parkinson’s Disease. J. Pineal Res. 2024, 76, e12948. [Google Scholar] [CrossRef]
- Jiménez-Delgado, A.; Ortiz, G.G.; Delgado-Lara, D.L.; González-Usigli, H.A.; González-Ortiz, L.J.; Cid-Hernández, M.; Cruz-Serrano, J.A.; Pacheco-Moisés, F.P. Effect of Melatonin Administration on Mitochondrial Activity and Oxidative Stress Markers in Patients with Parkinson’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 5577541. [Google Scholar] [CrossRef]
- Asemi-Rad, A.; Moafi, M.; Aliaghaei, A.; Abbaszadeh, H.-A.; Abdollahifar, M.-A.; Ebrahimi, M.-J.; Heidari, M.H.; Sadeghi, Y. The Effect of Dopaminergic Neuron Transplantation and Melatonin Co-Administration on Oxidative Stress-Induced Cell Death in Parkinson’s Disease. Metab. Brain Dis. 2022, 37, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic Lateral Sclerosis: A Neurodegenerative Disorder Poised for Successful Therapeutic Translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tang, C.; Wei, C.; Zhu, Y.; Xu, R. Melatonin Induces Autophagy in Amyotrophic Lateral Sclerosis Mice via Upregulation of SIRT1. Mol. Neurobiol. 2022, 59, 4747–4760. [Google Scholar] [CrossRef]
- Zhang, Y.; Cook, A.; Kim, J.; Baranov, S.V.; Jiang, J.; Smith, K.; Cormier, K.; Bennett, E.; Browser, R.P.; Day, A.L.; et al. Melatonin Inhibits the Caspase-1/Cytochrome c/Caspase-3 Cell Death Pathway, Inhibits MT1 Receptor Loss and Delays Disease Progression in a Mouse Model of Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2013, 55, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, J.H.; Bartels, C.; Pölking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Hüther, G.; et al. Reduced Oxidative Damage in ALS by High-Dose Enteral Melatonin Treatment. J. Pineal Res. 2006, 41, 313–323. [Google Scholar] [CrossRef]
- Bald, E.M.; Nance, C.S.; Schultz, J.L. Melatonin May Slow Disease Progression in Amyotrophic Lateral Sclerosis: Findings from the Pooled Resource Open-Access ALS Clinic Trials Database. Muscle Nerve 2021, 63, 572–576. [Google Scholar] [CrossRef]
- National Cancer Institute Age and Cancer Risk. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 6 April 2025).
- Del Castillo-Vaquero, A.; Salido, G.M.; Gonzalez, A. Melatonin Induces Calcium Release from CCK-8- and Thapsigargin-Sensitive Cytosolic Stores in Pancreatic AR42J Cells. J. Pineal Res. 2010, 49, 256–263. [Google Scholar] [CrossRef]
- Gonzalez, A.; del Castillo-Vaquero, A.; Miro-Moran, A.; Tapia, J.A.; Salido, G.M. Melatonin Reduces Pancreatic Tumor Cell Viability by Altering Mitochondrial Physiology. J. Pineal Res. 2011, 50, 250–260. [Google Scholar] [CrossRef]
- Leja-Szpak, A.; Jaworek, J.; Pierzchalski, P.; Reiter, R.J. Melatonin Induces Pro-Apoptotic Signaling Pathway in Human Pancreatic Carcinoma Cells (PANC-1). J. Pineal Res. 2010, 49, 248–255. [Google Scholar] [CrossRef]
- Li, M.; Hao, B.; Zhang, M.; Reiter, R.J.; Lin, S.; Zheng, T.; Chen, X.; Ren, Y.; Yue, L.; Abay, B.; et al. Melatonin Enhances Radiofrequency-Induced NK Antitumor Immunity, Causing Cancer Metabolism Reprogramming and Inhibition of Multiple Pulmonary Tumor Development. Signal Transduct. Target. Ther. 2021, 6, 330. [Google Scholar] [CrossRef]
- Chen, X.; Hao, B.; Li, D.; Reiter, R.J.; Bai, Y.; Abay, B.; Chen, G.; Lin, S.; Zheng, T.; Ren, Y.; et al. Melatonin Inhibits Lung Cancer Development by Reversing the Warburg Effect via Stimulating the SIRT3/PDH Axis. J. Pineal Res. 2021, 71, e12755. [Google Scholar] [CrossRef] [PubMed]
- Laothong, U.; Pinlaor, P.; Boonsiri, P.; Pairojkul, C.; Priprem, A.; Johns, N.P.; Charoensuk, L.; Intuyod, K.; Pinlaor, S. Melatonin Inhibits Cholangiocarcinoma and Reduces Liver Injury in Opisthorchis Viverrini-Infected and N-Nitrosodimethylamine-Treated Hamsters. J. Pineal Res. 2013, 55, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Byun, J.-K.; Kim, N.-Y.; Jin, J.; Woo, H.; Choi, Y.-K.; Park, K.-G. Melatonin Inhibits Glycolysis in Hepatocellular Carcinoma Cells by Downregulating Mitochondrial Respiration and MTORC1 Activity. BMB Rep. 2022, 55, 459–464. [Google Scholar] [CrossRef]
- Ordoñez, R.; Carbajo-Pescador, S.; Prieto-Dominguez, N.; García-Palomo, A.; González-Gallego, J.; Mauriz, J.L. Inhibition of Matrix Metalloproteinase-9 and Nuclear Factor Kappa B Contribute to Melatonin Prevention of Motility and Invasiveness in HepG2 Liver Cancer Cells. J. Pineal Res. 2014, 56, 20–30. [Google Scholar] [CrossRef]
- Ji, G.; Zhou, W.; Li, X.; Du, J.; Li, X.; Hao, H. Melatonin Inhibits Proliferation and Viability and Promotes Apoptosis in Colorectal Cancer Cells via Upregulation of the MicroRNA-34a/449a Cluster. Mol. Med. Rep. 2021, 23, 187. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, D.; Yang, X.; Xue, X.; Zuo, L.; Zhou, Q.; Hu, R.; Wang, Y. Melatonin Inhibits Colon Cancer RKO Cell Migration by Downregulating Rho-associated Protein Kinase Expression via the P38/MAPK Signaling Pathway. Mol. Med. Rep. 2017, 16, 9383–9392. [Google Scholar] [CrossRef] [PubMed]
- Radajewska, A.; Moreira, H.; Bęben, D.; Siwiela, O.; Szyjka, A.; Gębczak, K.; Nowak, P.; Frąszczak, J.; Emhemmed, F.; Muller, C.D.; et al. Combination of Irinotecan and Melatonin with the Natural Compounds Wogonin and Celastrol for Colon Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 9544. [Google Scholar] [CrossRef]
- Lee, J.H.; Yun, C.W.; Han, Y.-S.; Kim, S.; Jeong, D.; Kwon, H.Y.; Kim, H.; Baek, M.-J.; Lee, S.H. Melatonin and 5-Fluorouracil Co-Suppress Colon Cancer Stem Cells by Regulating Cellular Prion Protein-Oct4 Axis. J. Pineal Res. 2018, 65, e12519. [Google Scholar] [CrossRef]
- Tanriover, G.; Dilmac, S.; Aytac, G.; Farooqi, A.A.; Sindel, M. Effects of Melatonin and Doxorubicin on Primary Tumor And Metastasis in Breast Cancer Model. Anticancer. Agents Med. Chem. 2022, 22, 1970–1983. [Google Scholar] [CrossRef]
- Odeh, L.H.; Talib, W.H.; Basheti, I.A. Synergistic Effect of Thymoquinone and Melatonin against Breast Cancer Implanted in Mice. J. Cancer Res. Ther. 2018, 14, S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Karadas, A.K.; Dilmac, S.; Aytac, G.; Tanriover, G. Melatonin Decreases Metastasis, Primary Tumor Growth and Angiogenesis in a Mice Model of Breast Cancer. Hum. Exp. Toxicol. 2021, 40, 1545–1557. [Google Scholar] [CrossRef]
- Hsieh, T.-Y.; Sung, W.-W.; Chang, Y.-C.; Yu, C.-Y.; Lu, L.-Y.; Dong, C.; Lee, T.-H.; Chen, S.-L. Melatonin Induces Cell Cycle Arrest and Suppresses Tumor Invasion in Urinary Bladder Urothelial Carcinoma. Aging 2023, 15, 3107–3119. [Google Scholar] [CrossRef]
- Wu, J.; Tan, Z.; Li, H.; Lin, M.; Jiang, Y.; Liang, L.; Ma, Q.; Gou, J.; Ning, L.; Li, X.; et al. Melatonin Reduces Proliferation and Promotes Apoptosis of Bladder Cancer Cells by Suppressing O-GlcNAcylation of Cyclin-Dependent-like Kinase 5. J. Pineal Res. 2021, 71, e12765. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chuang, F.-C.; Chang, C.-L.; Huang, C.-R.; Chen, H.-H.; Yip, H.-K.; Chen, Y.-T. Melatonin-Assisted Cisplatin Suppresses Urinary Bladder Cancer Cell Proliferation and Growth through Inhibiting PrP(C)-Regulated Cell Stress and Cell Proliferation Signaling. Int. J. Mol. Sci. 2023, 24, 3353. [Google Scholar] [CrossRef]
- Martins Longaretti, L.; Luciano, J.A.; Strapazzon, G.; Pereira, M.; Damiani, A.P.; Rohr, P.; Rigo, F.K.; de Oliveira, C.A.; Steiner, B.T.; Vilela, T.C.; et al. Anti-Genotoxic and Anti-Mutagenic Effects of Melatonin Supplementation in a Mouse Model of Melanoma. Drug Chem. Toxicol. 2022, 45, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.; Negrín, G.; Estévez, F.; Loro, J.; Reiter, R.J.; Quintana, J. Melatonin Decreases Cell Proliferation and Induces Melanogenesis in Human Melanoma SK-MEL-1 Cells. J. Pineal Res. 2010, 49, 45–54. [Google Scholar] [CrossRef]
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and Pro-Apoptotic Activity of Melatonin Analogues on Melanoma and Breast Cancer Cells. Oncotarget 2017, 8, 68338–68353. [Google Scholar] [CrossRef]
- Bilska, B.; Schedel, F.; Piotrowska, A.; Stefan, J.; Zmijewski, M.; Pyza, E.; Reiter, R.J.; Steinbrink, K.; Slominski, A.T.; Tulic, M.K.; et al. Mitochondrial Function Is Controlled by Melatonin and Its Metabolites in Vitro in Human Melanoma Cells. J. Pineal Res. 2021, 70, e12728. [Google Scholar] [CrossRef]
- Hao, J.; Fan, W.; Li, Y.; Tang, R.; Tian, C.; Yang, Q.; Zhu, T.; Diao, C.; Hu, S.; Chen, M.; et al. Melatonin Synergizes BRAF-Targeting Agent Vemurafenib in Melanoma Treatment by Inhibiting INOS/HTERT Signaling and Cancer-Stem Cell Traits. J. Exp. Clin. Cancer Res. 2019, 38, 48. [Google Scholar] [CrossRef]
- Fernandez-Gil, B.I.; Otamendi-Lopez, A.; Bechtle, A.; Vazquez-Ramos, C.A.; Qosja, N.; Suarez-Meade, P.; Sarabia-Estrada, R.; Jentoft, M.E.; Guerrero-Cázares, H.; Escames, G.; et al. Melatonin Treatment Triggers Metabolic and Intracellular PH Imbalance in Glioblastoma. Cells 2022, 11, 3467. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Agomelatine or Ramelteon as Treatment Adjuncts in Glioblastoma and Other M1- or M2-Expressing Cancers. Contemp. Oncol. 2015, 19, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Doğanlar, O.; Doğanlar, Z.B.; Delen, E.; Doğan, A. The Role of Melatonin in Angio-MiR-Associated Inhibition of Tumorigenesis and Invasion in Human Glioblastoma Tumour Spheroids. Tissue Cell 2021, 73, 101617. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wang, F.; Ling, E.-A.; Liu, S.; Wang, L.; Yang, Y.; Yao, L.; Chen, X.; Wang, F.; et al. Melatonin Antagonizes Hypoxia-Mediated Glioblastoma Cell Migration and Invasion via Inhibition of HIF-1α. J. Pineal Res. 2013, 55, 121–130. [Google Scholar] [CrossRef]
- Bostanci, A.; Doganlar, O. Melatonin Enhances Temozolomide-Induced Apoptosis in Glioblastoma and Neuroblastoma Cells. Exp. Oncol. 2024, 46, 87–100. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Wanggou, S.; Lin, D.; Su, J.; Li, X.; Tao, E. A Natural Compound Melatonin Enhances the Effects of Nimotuzumab via Inhibiting EGFR in Glioblastoma. Cancer Lett. 2024, 592, 216920. [Google Scholar] [CrossRef]
- Song, J.; Ma, S.-J.; Luo, J.-H.; Zhang, H.; Wang, R.-X.; Liu, H.; Li, L.; Zhang, Z.-G.; Zhou, R.-X. Melatonin Induces the Apoptosis and Inhibits the Proliferation of Human Gastric Cancer Cells via Blockade of the AKT/MDM2 Pathway. Oncol. Rep. 2018, 39, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, C.; Zhong, X.; Wu, J.; Li, G. Melatonin Induces AGS Gastric Cancer Cell Apoptosis via Regulating PERK/EIF2α and HSF1/NF-ΚB Signaling Pathway. Ann. Clin. Lab. Sci. 2022, 52, 40–47. [Google Scholar]
- Huang, Y.; Yuan, K.; Tang, M.; Yue, J.; Bao, L.; Wu, S.; Zhang, Y.; Li, Y.; Wang, Y.; Ou, X.; et al. Melatonin Inhibiting the Survival of Human Gastric Cancer Cells under ER Stress Involving Autophagy and Ras-Raf-MAPK Signalling. J. Cell. Mol. Med. 2021, 25, 1480–1492. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Y.; Zhang, H.; He, W.; Zhou, Q.; Gui, S.; Wang, Y. Melatonin Inhibits Cell Growth and Migration, but Promotes Apoptosis in Gastric Cancer Cell Line, SGC7901. Biotech. Histochem. 2013, 88, 281–289. [Google Scholar] [CrossRef]
- Cheng, L.; Li, S.; He, K.; Kang, Y.; Li, T.; Li, C.; Zhang, Y.; Zhang, W.; Huang, Y. Melatonin Regulates Cancer Migration and Stemness and Enhances the Anti-Tumour Effect of Cisplatin. J. Cell. Mol. Med. 2023, 27, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, H.; Dan, Z.; Shu, C.; Zhu, R.; Yang, Q.; Wang, Y.; Zhu, H. Melatonin Potentiates Sensitivity to 5-Fluorouracil in Gastric Cancer Cells by Upregulating Autophagy and Downregulating Myosin Light-Chain Kinase. J. Cancer 2023, 14, 2608–2618. [Google Scholar] [CrossRef]
- Hao, L.; Dong, Y.; Zhang, J.-J.; He, H.-G.; Chen, J.-G.; Zhang, S.-Q.; Zhang, Q.-J.; Wu, W.; Han, C.-H.; Shi, Z.-D. Melatonin Decreases Androgen-Sensitive Prostate Cancer Growth by Suppressing SENP1 Expression. Transl. Androl. Urol. 2022, 11, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Nyamsambuu, A.; Khan, M.A.; Zhou, X.; Chen, H.-C. Molecular Mechanism of Inhibitory Effects of Melatonin on Prostate Cancer Cell Proliferation, Migration and Invasion. PLoS ONE 2022, 17, e0261341. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Hevia, D.; Quiros-Gonzalez, I.; Rodriguez-Garcia, A.; Gonzalez-Menendez, P.; Cepas, V.; Gonzalez-Pola, I.; Sainz, R.M. IGFBP3 and MAPK/ERK Signaling Mediates Melatonin-Induced Antitumor Activity in Prostate Cancer. J. Pineal Res. 2017, 62, e12373. [Google Scholar] [CrossRef]
- Hevia, D.; Gonzalez-Menendez, P.; Fernandez-Fernandez, M.; Cueto, S.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Mayo, J.C.; Sainz, R.M. Melatonin Decreases Glucose Metabolism in Prostate Cancer Cells: A (13)C Stable Isotope-Resolved Metabolomic Study. Int. J. Mol. Sci. 2017, 18, 1620. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Schmit, T.L.; Reagan-Shaw, S.R.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N. Melatonin, a Novel Sirt1 Inhibitor, Imparts Antiproliferative Effects against Prostate Cancer in Vitro in Culture and in Vivo in TRAMP Model. J. Pineal Res. 2011, 50, 140–149. [Google Scholar] [CrossRef]
- Zharinov, G.M.; Bogomolov, O.A.; Chepurnaya, I.V.; Neklasova, N.Y.; Anisimov, V.N. Melatonin Increases Overall Survival of Prostate Cancer Patients with Poor Prognosis after Combined Hormone Radiation Treatment. Oncotarget 2020, 11, 3723–3729. [Google Scholar] [CrossRef]
- Wen, Y.-C.; Lin, Y.-W.; Chu, C.-Y.; Yang, Y.-C.; Yang, S.-F.; Liu, Y.-F.; Hsiao, M.; Lee, W.-J.; Chien, M.-H. Melatonin-Triggered Post-Transcriptional and Post-Translational Modifications of ADAMTS1 Coordinately Retard Tumorigenesis and Metastasis of Renal Cell Carcinoma. J. Pineal Res. 2020, 69, e12668. [Google Scholar] [CrossRef]
- Xue, K.-H.; Jiang, Y.-F.; Bai, J.-Y.; Zhang, D.-Z.; Chen, Y.-H.; Ma, J.-B.; Zhu, Z.-J.; Wang, X.; Guo, P. Melatonin Suppresses Akt/MTOR/S6K Activity, Induces Cell Apoptosis, and Synergistically Inhibits Cell Growth with Sunitinib in Renal Carcinoma Cells via Reversing Warburg Effect. Redox Rep. 2023, 28, 2251234. [Google Scholar] [CrossRef]
- Baş, E.; Nazıroğlu, M. Treatment with Melatonin and Selenium Attenuates Docetaxel-Induced Apoptosis and Oxidative Injury in Kidney and Testes of Mice. Andrologia 2019, 51, e13320. [Google Scholar] [CrossRef]
- Elbanan, M.E.; Amer, M.E.; El-Missiry, M.A.; Othman, A.I.; Shabana, S.M. Melatonin Protected against Kidney Impairment Induced by 5-Fluorouracil in Mice. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli Pirzaman, A.; Mansoori, R.; Hosseini, S.M.; Abolhosseini, A.; Khosravi, S.; Moghadamnia, A.A.; Kazemi, S. The Effect of Melatonin on Capecitabine-Induced Hepatic and Renal Toxicity in Rats. Hum. Exp. Toxicol. 2024, 43, 9603271231223506. [Google Scholar] [CrossRef]
- Franchi, A. Epidemiology and Classification of Bone Tumors. Clin. Cases Miner. Bone Metab. 2012, 9, 92–95. [Google Scholar]
- Liu, P.-I.; Chang, A.-C.; Lai, J.-L.; Lin, T.-H.; Tsai, C.-H.; Chen, P.-C.; Jiang, Y.-J.; Lin, L.-W.; Huang, W.-C.; Yang, S.-F.; et al. Melatonin Interrupts Osteoclast Functioning and Suppresses Tumor-Secreted RANKL Expression: Implications for Bone Metastases. Oncogene 2021, 40, 1503–1515. [Google Scholar] [CrossRef]
- Okamoto, K. Role of RANKL in Cancer Development and Metastasis. J. Bone Miner. Metab. 2021, 39, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Saravanan, S.; Raghunandhakumar, S.; Anuradha, D. Melatonin Regulates Tumor Angiogenesis via MiR-424-5p/VEGFA Signaling Pathway in Osteosarcoma. Life Sci. 2020, 256, 118011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, J.; Li, B.; Du, J. Anticancer Effects of Melatonin via Regulating LncRNA JPX-Wnt/β-Catenin Signalling Pathway in Human Osteosarcoma Cells. J. Cell. Mol. Med. 2021, 25, 9543–9556. [Google Scholar] [CrossRef]
- Wang, X.; Su, P.; Kang, Y.; Xu, C.; Qiu, J.; Wu, J.; Sheng, P.; Huang, D.; Zhang, Z. Combination of Melatonin and Zoledronic Acid Suppressed the Giant Cell Tumor of Bone in Vitro and in Vivo. Front. Cell Dev. Biol. 2021, 9, 690502. [Google Scholar] [CrossRef]
- Hosseini, F.; Shanehbandi, D.; Soleimanpour, J.; Yousefi, B.; Alemi, F. Melatonin Increases the Sensitivity of Osteosarcoma Cells to Chemotherapy Drug Cisplatin. Drug Res. 2022, 72, 312–318. [Google Scholar] [CrossRef]
- Roth, J.A.; Kim, B.G.; Lin, W.L.; Cho, M.I. Melatonin Promotes Osteoblast Differentiation and Bone Formation. J. Biol. Chem. 1999, 274, 22041–22047. [Google Scholar] [CrossRef] [PubMed]
- Büyükavci, M.; Ozdemir, O.; Buck, S.; Stout, M.; Ravindranath, Y.; Savaşan, S. Melatonin Cytotoxicity in Human Leukemia Cells: Relation with Its pro-Oxidant Effect. Fundam. Clin. Pharmacol. 2006, 20, 73–79. [Google Scholar] [CrossRef]
- Perdomo, J.; Cabrera, J.; Estévez, F.; Loro, J.; Reiter, R.J.; Quintana, J. Melatonin Induces Apoptosis through a Caspase-Dependent but Reactive Oxygen Species-Independent Mechanism in Human Leukemia Molt-3 Cells. J. Pineal Res. 2013, 55, 195–206. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Sun, X.; Huang, L.-B.; Liu, X.-J.; Qin, G.; Wang, L.-N.; Zhang, X.-L.; Ke, Z.-Y.; Luo, J.-S.; Liang, C.; et al. Melatonin Inhibits MLL-Rearranged Leukemia via RBFOX3/HTERT and NF-ΚB/COX-2 Signaling Pathways. Cancer Lett. 2019, 443, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Puente-Moncada, N.; Turos-Cabal, M.; Sánchez-Sánchez, A.M.; Antolín, I.; Herrera, F.; Rodriguez-Blanco, J.; Duarte-Olivenza, C.; Rodriguez, C.; Martín, V. Role of Glucose Metabolism in the Differential Antileukemic Effect of Melatonin on Wild-type and FLT3-ITD Mutant Cells. Oncol. Rep. 2020, 44, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lomovsky, A.I.; Baburina, Y.L.; Fadeev, R.S.; Lomovskaya, Y.V.; Kobyakova, M.I.; Krestinin, R.R.; Sotnikova, L.D.; Krestinina, O. V Melatonin Can Enhance the Effect of Drugs Used in the Treatment of Leukemia. Biochemistry 2023, 88, 73–85. [Google Scholar] [CrossRef]
- Koh, W.; Jeong, S.-J.; Lee, H.-J.; Ryu, H.-G.; Lee, E.-O.; Ahn, K.S.; Bae, H.; Kim, S.-H. Melatonin Promotes Puromycin-Induced Apoptosis with Activation of Caspase-3 and 5′-Adenosine Monophosphate-Activated Kinase-Alpha in Human Leukemia HL-60 Cells. J. Pineal Res. 2011, 50, 367–373. [Google Scholar] [CrossRef]
- Landolt, L.; Spagnoli, G.C.; Hertig, A.; Brocheriou, I.; Marti, H.-P. Fibrosis and Cancer: Shared Features and Mechanisms Suggest Common Targeted Therapeutic Approaches. Nephrol. Dial. Transplant. 2022, 37, 1024–1032. [Google Scholar] [CrossRef]
- Estaras, M.; Martinez, R.; Garcia, A.; Ortiz-Placin, C.; Iovanna, J.L.; Santofimia-Castaño, P.; Gonzalez, A. Melatonin Modulates Metabolic Adaptation of Pancreatic Stellate Cells Subjected to Hypoxia. Biochem. Pharmacol. 2022, 202, 115118. [Google Scholar] [CrossRef]
- Estaras, M.; Moreno, N.; Santofimia-Castaño, P.; Martinez-Morcillo, S.; Roncero, V.; Blanco, G.; Lopez, D.; Fernandez-Bermejo, M.; Mateos, J.M.; Iovanna, J.L.; et al. Melatonin Induces Reactive Oxygen Species Generation and Changes in Glutathione Levels and Reduces Viability in Human Pancreatic Stellate Cells. J. Physiol. Biochem. 2019, 75, 185–197. [Google Scholar] [CrossRef]
- Estaras, M.; Gonzalez-Portillo, M.R.; Martinez, R.; Garcia, A.; Estevez, M.; Fernandez-Bermejo, M.; Mateos, J.M.; Vara, D.; Blanco-Fernández, G.; Lopez-Guerra, D.; et al. Melatonin Modulates the Antioxidant Defenses and the Expression of Proinflammatory Mediators in Pancreatic Stellate Cells Subjected to Hypoxia. Antioxidants 2021, 10, 577. [Google Scholar] [CrossRef]
- Liu, D.; Shi, K.; Fu, M.; Chen, F. Melatonin Indirectly Decreases Gastric Cancer Cell Proliferation and Invasion via Effects on Cancer-Associated Fibroblasts. Life Sci. 2021, 277, 119497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Q.; Hua, C.; Ci, X. Melatonin Alleviates Particulate Matter-Induced Liver Fibrosis by Inhibiting ROS-Mediated Mitophagy and Inflammation via Nrf2 Activation. Ecotoxicol. Environ. Saf. 2023, 268, 115717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, J.; Su, W.; Shan, H.; Zhang, B.; Wang, Y.; Shabanova, A.; Shan, H.; Liang, H. Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway. Int. J. Mol. Sci. 2018, 19, 1118. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahab, D.A.; El-Missiry, M.A.; Shabana, S.; Othman, A.I.; Amer, M.E. Melatonin Protects the Heart and Pancreas by Improving Glucose Homeostasis, Oxidative Stress, Inflammation and Apoptosis in T2DM-Induced Rats. Heliyon 2021, 7, e06474. [Google Scholar] [CrossRef]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Abd El-Aziz, M.K.; Siddiq Abduh, M.; Bin-Ammar, A.; Kamel, E.M.; Mahmoud, A.M. The Melatonin Receptor Agonist Agomelatine Protects against Acute Pancreatitis Induced by Cadmium by Attenuating Inflammation and Oxidative Stress and Modulating Nrf2/HO-1 Pathway. Int. Immunopharmacol. 2023, 124, 110833. [Google Scholar] [CrossRef]
- Cuesta, S.; Kireev, R.; García, C.; Forman, K.; Escames, G.; Vara, E.; Tresguerres, J.A.F. Beneficial Effect of Melatonin Treatment on Inflammation, Apoptosis and Oxidative Stress on Pancreas of a Senescence Accelerated Mice Model. Mech. Ageing Dev. 2011, 132, 573–582. [Google Scholar] [CrossRef]
- Popov, S.S.; Shulgin, K.K.; Popova, T.N.; Pashkov, A.N.; Agarkov, A.A.; de Carvalho, M.A.A.P. Effects of Melatonin-Aided Therapy on the Glutathione Antioxidant System Activity and Liver Protection. J. Biochem. Mol. Toxicol. 2015, 29, 449–457. [Google Scholar] [CrossRef]
- Pashkov, A.N.; Popov, S.S.; Semenikhina, A.V.; Rakhmanova, T.I. Glutathione System and Activity of NADPH-Generating Enzymes in the Liver of Intact Rats and Animals with Toxic Hepatitis Receiving Melatonin. Bull. Exp. Biol. Med. 2005, 139, 565–568. [Google Scholar] [CrossRef]
- Tuñón, M.J.; San-Miguel, B.; Crespo, I.; Laliena, A.; Vallejo, D.; Álvarez, M.; Prieto, J.; González-Gallego, J. Melatonin Treatment Reduces Endoplasmic Reticulum Stress and Modulates the Unfolded Protein Response in Rabbits with Lethal Fulminant Hepatitis of Viral Origin. J. Pineal Res. 2013, 55, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.; Kireev, R.; Forman, K.; García, C.; Escames, G.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A.F. Melatonin Improves Inflammation Processes in Liver of Senescence-Accelerated Prone Male Mice (SAMP8). Exp. Gerontol. 2010, 45, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, J.; Zhao, X.; Yang, W. Melatonin Ameliorates Lung Cell Inflammation and Apoptosis Caused by Klebsiella Pneumoniae via AMP-Activated Protein Kinase. Inflammopharmacology 2022, 30, 2345–2357. [Google Scholar] [CrossRef]
- Huo, C.; Tang, Y.; Li, X.; Han, D.; Gu, Q.; Su, R.; Liu, Y.; Reiter, R.J.; Liu, G.; Hu, Y.; et al. Melatonin Alleviates Lung Injury in H1N1-Infected Mice by Mast Cell Inactivation and Cytokine Storm Suppression. PLoS Pathog. 2023, 19, e1011406. [Google Scholar] [CrossRef] [PubMed]
- Ates, G.; Tamer, S.; Yorulmaz, H.; Mutlu, S.; Olgac, V.; Aksu, A.; Caglar, N.B.; Özkök, E. Melatonin Pretreatment Modulates Anti-Inflammatory, Antioxidant, YKL-40, and Matrix Metalloproteinases in Endotoxemic Rat Lung Tissue. Exp. Biol. Med. 2022, 247, 1080–1089. [Google Scholar] [CrossRef]
- Wang, M.-L.; Wei, C.-H.; Wang, W.-D.; Wang, J.-S.; Zhang, J.; Wang, J.-J. Melatonin Attenuates Lung Ischaemia-Reperfusion Injury via Inhibition of Oxidative Stress and Inflammation. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 761–767. [Google Scholar] [CrossRef]
- Ning, L.; Rui, X.; Guorui, L.; Tinglv, F.; Donghang, L.; Chenzhen, X.; Xiaojing, W.; Qing, G. A Novel Mechanism for the Protection against Acute Lung Injury by Melatonin: Mitochondrial Quality Control of Lung Epithelial Cells Is Preserved through SIRT3-Dependent Deacetylation of SOD2. Cell. Mol. Life Sci. 2022, 79, 610. [Google Scholar] [CrossRef]
- Li, Y.; Ma, B.; Wang, Z.; Chen, Y.; Dong, Y. The Effect Mechanism of N6-Adenosine Methylation (M6A) in Melatonin Regulated LPS-Induced Colon Inflammation. Int. J. Biol. Sci. 2024, 20, 2491–2506. [Google Scholar] [CrossRef]
- Gao, T.; Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin-Mediated MT2 Attenuates Colitis Induced by Dextran Sodium Sulfate via PI3K/AKT/Nrf2/SIRT1/RORα/NF-ΚB Signaling Pathways. Int. Immunopharmacol. 2021, 96, 107779. [Google Scholar] [CrossRef]
- Sadeghi, H.; Bagheri, H.; Shekarchi, B.; Javadi, A.; Najafi, M. Mitigation of Radiation-Induced Gastrointestinal System Injury by Melatonin: A Histopathological Study. Curr. Drug Res. Rev. 2020, 12, 72–79. [Google Scholar] [CrossRef]
- Paulis, G. Inflammatory Mechanisms and Oxidative Stress in Prostatitis: The Possible Role of Antioxidant Therapy. Res. Rep. Urol. 2018, 10, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lao, Y.; Li, R.; You, C.; Qing, L.; Xiao, X.; Liu, S.; Wang, W.; Zhao, Y.; Dong, Z. Network Pharmacological Analysis and Experimental Study of Melatonin in Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 397, 8691–8706. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.-G.; Du, H.-X.; Zhan, C.-S.; Liu, Y.; Zhang, M.; Chen, X.-G.; Wen, L.-P.; Zhang, L.; Liang, C.-Z. Melatonin Attenuates Prostatic Inflammation and Pelvic Pain via Sirt1-Dependent Inhibition of the NLRP3 Inflammasome in an EAP Mouse Model. Prostate 2021, 81, 1179–1190. [Google Scholar] [CrossRef]
- Tamarindo, G.H.; Gobbo, M.G.; Taboga, S.R.; Almeida, E.A.; Góes, R.M. Melatonin Ameliorates Degenerative Alterations Caused by Age in the Rat Prostate and Mitigates High-Fat Diet Damages. Cell Biol. Int. 2021, 45, 92–106. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, L.; Wu, X.; Liu, Y.; Wan, Y.; Hu, J.; Zhang, X.; Wu, Q. Melatonin Ameliorates Renal Dysfunction in Glyphosate- and Hard Water-Treated Mice. Ecotoxicol. Environ. Saf. 2022, 241, 113803. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Dos Santos, M.; Veronese, F.V.; Rezzani, R. NLRP3 Inflammasome Modulation by Melatonin Supplementation in Chronic Pristane-Induced Lupus Nephritis. Int. J. Mol. Sci. 2019, 20, 3466. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lu, K.-C.; Lin, G.-J.; Hsieh, H.-Y.; Chu, P.; Lin, S.-H.; Sytwu, H.-K. Melatonin Enhances Endogenous Heme Oxygenase-1 and Represses Immune Responses to Ameliorate Experimental Murine Membranous Nephropathy. J. Pineal Res. 2012, 52, 460–469. [Google Scholar] [CrossRef]
- Sener, G.; Tuğtepe, H.; Velioğlu-Oğünç, A.; Cetinel, S.; Gedik, N.; Yeğen, B.C. Melatonin Prevents Neutrophil-Mediated Oxidative Injury in Escherichia Coli-Induced Pyelonephritis in Rats. J. Pineal Res. 2006, 41, 220–227. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; et al. Natural Ingredients to Improve Immunity. Pharmaceuticals 2023, 16, 528. [Google Scholar] [CrossRef]
- Regodón, S.; Martín-Palomino, P.; Fernández-Montesinos, R.; Herrera, J.L.; Carrascosa-Salmoral, M.P.; Píriz, S.; Vadillo, S.; Guerrero, J.M.; Pozo, D. The Use of Melatonin as a Vaccine Agent. Vaccine 2005, 23, 5321–5327. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.R.; Porchia, B.F.M.M.; Pagni, R.L.; Souza, P.d.C.; Pegoraro, R.; Rodrigues, K.B.; Barros, T.B.; Aps, L.R.d.M.M.; de Araújo, E.F.; Calich, V.L.G.; et al. The Combined Use of Melatonin and an Indoleamine 2,3-Dioxygenase-1 Inhibitor Enhances Vaccine-Induced Protective Cellular Immunity to HPV16-Associated Tumors. Front. Immunol. 2018, 9, 1914. [Google Scholar] [CrossRef]
- Negrette, B.; Bonilla, E.; Valero, N.; Pons, H.; Garcia Tamayo, J.; Chacín-Bonilla, L.; Medina-Leendertz, S.; Añez, F. Melatonin Treatment Enhances the Efficiency of Mice Immunization with Venezuelan Equine Encephalomyelitis Virus TC-83. Neurochem. Res. 2001, 26, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Izabelle, C.; Poder, S.L.; Real, F.; Zhu, A.; Tu, L.; Ghigna, M.R.; Klonjkowski, B.; Bomsel, M.; Jockers, R.; et al. Therapeutic Potential of Melatonin and Melatonergic Drugs on K18-HACE2 Mice Infected with SARS-CoV-2. J. Pineal Res. 2022, 72, e12772. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, X.; Wei, M.; Yu, S.; Ding, Y.; Cheng, B. Melatonin Regulates the Immune Response and Improves Sjögren’s Syndrome-like Symptoms in NOD/Ltj Mice. Biochem. Pharmacol. 2022, 201, 115073. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. Role and Therapeutic Potential of Melatonin in Various Type of Cancers. Onco. Targets. Ther. 2021, 14, 2019–2052. [Google Scholar] [CrossRef]
- Hekmatirad, S.; Moloudizargari, M.; Fallah, M.; Rahimi, A.; Poortahmasebi, V.; Asghari, M.H. Cancer-Associated Immune Cells and Their Modulation by Melatonin. Immunopharmacol. Immunotoxicol. 2023, 45, 788–801. [Google Scholar] [CrossRef]
- Giannoulia-Karantana, A.; Vlachou, A.; Polychronopoulou, S.; Papassotiriou, I.; Chrousos, G.P. Melatonin and Immunomodulation: Connections and Potential Clinical Applications. Neuroimmunomodulation 2006, 13, 133–144. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Lee, K.-Y.; Wu, S.-M.; Kuo, D.-Y.; Shueng, P.-W.; Lin, C.-W. Melatonin Downregulates PD-L1 Expression and Modulates Tumor Immunity in KRAS-Mutant Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 5649. [Google Scholar] [CrossRef]
- Chan, Y.-T.; Tan, H.-Y.; Lu, Y.; Zhang, C.; Cheng, C.-S.; Wu, J.; Wang, N.; Feng, Y. Pancreatic Melatonin Enhances Anti-Tumor Immunity in Pancreatic Adenocarcinoma through Regulating Tumor-Associated Neutrophils Infiltration and NETosis. Acta Pharm. Sin. B 2023, 13, 1554–1567. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, Y.; Tang, H.; Wang, H.; Jiang, E.; Shao, Z.; Liu, K.; Zhou, X.; Shang, Z. Melatonin Inhibits EMT and PD-L1 Expression through the ERK1/2/FOSL1 Pathway and Regulates Anti-Tumor Immunity in HNSCC. Cancer Sci. 2022, 113, 2232–2245. [Google Scholar] [CrossRef]
- Wang, K.; Cai, R.; Fei, S.; Chen, X.; Feng, S.; Zhang, L.; Liu, H.; Zhang, Z.; Song, J.; Zhou, R. Melatonin Enhances Anti-Tumor Immunity by Targeting Macrophages PD-L1 via Exosomes Derived from Gastric Cancer Cells. Mol. Cell. Endocrinol. 2023, 568–569, 111917. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-C.; Wu, M.-D.; Zhang, X.-X.; Liu, Y.-F.; Wang, C.-L. Identification of Prognostic Melatonin-Related LncRNA Signature in Tumor Immune Microenvironment and Drug Resistance for Breast Cancer. Asian J. Surg. 2023, 46, 3529–3541. [Google Scholar] [CrossRef]
- Dai, Z.; Lin, B.; Cao, Y.; Wang, L.; Liao, K.; Guo, L.; Zhang, J. Melatonin Reverses EGFR-TKI Therapeutic Resistance by Modulating Crosstalk between Circadian-Related Gene Signature and Immune Infiltration Patterns in Patients with COVID-19 and Lung Adenocarcinoma. Comput. Biol. Med. 2024, 180, 108937. [Google Scholar] [CrossRef] [PubMed]
- Haddad, C.F.; Haddad, J.M.; Veiga, E.C.d.A.; Sorpreso, I.C.E.; Simões, R.S.; Baracat, E.C.; Soares Júnior, J.M. Melatonin and Organ Transplantation: What Is the Relationship? Rev. Assoc. Med. Bras. 2020, 66, 353–358. [Google Scholar] [CrossRef]
- Sapmaz, T.; Sevgin, K.; Topkaraoglu, S.; Tekayev, M.; Aktas, S.; Coskun, G.; Polat, S.; Sapmaz, E.; Irkorucu, O. Comparison of Melatonin, Oxytetracycline, and N-Acetylcysteine Pre-Treatments in Autologous Intraperitoneal Ovarian Transplantation in Rats. Biochem. Biophys. Res. Commun. 2022, 606, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hemadi, M.; Abolhassani, F.; Akbari, M.; Sobhani, A.; Pasbakhsh, P.; Ahrlund-Richter, L.; Modaresi, M.H.; Salehnia, M. Melatonin Promotes the Cumulus-Oocyte Complexes Quality of Vitrified-Thawed Murine Ovaries; with Increased Mean Number of Follicles Survival and Ovary Size Following Heterotopic Transplantation. Eur. J. Pharmacol. 2009, 618, 84–90. [Google Scholar] [CrossRef]
- Aierken, A.; Li, B.; Liu, P.; Cheng, X.; Kou, Z.; Tan, N.; Zhang, M.; Yu, S.; Shen, Q.; Du, X.; et al. Melatonin Treatment Improves Human Umbilical Cord Mesenchymal Stem Cell Therapy in a Mouse Model of Type II Diabetes Mellitus via the PI3K/AKT Signaling Pathway. Stem Cell Res. Ther. 2022, 13, 164. [Google Scholar] [CrossRef]
- Li, Z.; Nickkholgh, A.; Yi, X.; Bruns, H.; Gross, M.-L.; Hoffmann, K.; Mohr, E.; Zorn, M.; Büchler, M.W.; Schemmer, P. Melatonin Protects Kidney Grafts from Ischemia/Reperfusion Injury through Inhibition of NF-KB and Apoptosis after Experimental Kidney Transplantation. J. Pineal Res. 2009, 46, 365–372. [Google Scholar] [CrossRef]
- Coskun, A.; Yegen, C.; Arbak, S.; Attaallah, W.; Gunal, O.; Elmas, M.A.; Ucal, Y.; Can, O.; Baş, B.; Yildirim, Z.; et al. Melatonin in Preservation Solutions Prevents Ischemic Injury in Rat Kidneys. PLoS ONE 2022, 17, e0273921. [Google Scholar] [CrossRef]
- Erdoğan, M.M.; Erdemli, M.E.; Özhan, O.; Erdemli, Z.; Gözükara Bağ, H.G.; Vardı, N. Effect of Melatonin on Increasing the Effectiveness of Liver Preservation Solution. Turk. J. Gastroenterol. 2023, 34, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Ferrigno, A.; Bertone, R.; Rizzo, V.; Richelmi, P.; Bertè, F.; Reiter, R.J.; Freitas, I. Exogenous Melatonin Enhances Bile Flow and ATP Levels after Cold Storage and Reperfusion in Rat Liver: Implications for Liver Transplantation. J. Pineal Res. 2005, 38, 223–230. [Google Scholar] [CrossRef]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Roldan, M.; Diez, E.R.; Pueyo, E. Melatonin to Rescue the Aged Heart: Antiarrhythmic and Antioxidant Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 8876792. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.-H.; Li, C.-J.; Lin, L.-T. Melatonin Supplementation Attenuates Cuproptosis and Ferroptosis in Aging Cumulus and Granulosa Cells: Potential for Improving IVF Outcomes in Advanced Maternal Age. Reprod. Biol. Endocrinol. 2024, 22, 138. [Google Scholar] [CrossRef]
- Sayed, R.K.A.; Mokhtar, D.M.; Fernández-Ortiz, M.; Fernández-Martínez, J.; Aranda-Martínez, P.; Escames, G.; Acuña-Castroviejo, D. Lack of Retinoid Acid Receptor-Related Orphan Receptor Alpha Accelerates and Melatonin Supplementation Prevents Testicular Aging. Aging 2020, 12, 12648–12668. [Google Scholar] [CrossRef]
- Akbulut, K.G.; Aktas, S.H.; Akbulut, H. The Role of Melatonin, Sirtuin2 and FoXO1 Transcription Factor in the Aging Process of Colon in Male Rats. Biogerontology 2015, 16, 99–108. [Google Scholar] [CrossRef]
- Eşrefoğlu, M.; Iraz, M.; Ateş, B.; Gül, M. Not Only Melatonin but Also Caffeic Acid Phenethyl Ester Protects Kidneys against Aging-Related Oxidative Damage in Sprague Dawley Rats. Ultrastruct. Pathol. 2012, 36, 244–251. [Google Scholar] [CrossRef]
- Bocheva, G.; Bakalov, D.; Iliev, P.; Tafradjiiska-Hadjiolova, R. The Vital Role of Melatonin and Its Metabolites in the Neuroprotection and Retardation of Brain Aging. Int. J. Mol. Sci. 2024, 25, 5122. [Google Scholar] [CrossRef]
- Cristòfol, R.; Porquet, D.; Corpas, R.; Coto-Montes, A.; Serret, J.; Camins, A.; Pallàs, M.; Sanfeliu, C. Neurons from Senescence-Accelerated SAMP8 Mice Are Protected against Frailty by the Sirtuin 1 Promoting Agents Melatonin and Resveratrol. J. Pineal Res. 2012, 52, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular Mechanisms and Diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.-M.; Jang, S.K.; Kim, G.-H.; Park, J.-Y.; Joo, S.-S. Pharmacological Advantages of Melatonin in Immunosenescence by Improving Activity of T Lymphocytes. J. Biomed. Res. 2016, 30, 314–321. [Google Scholar] [CrossRef]
- Paredes, S.D.; Barriga, C.; Rodríguez, A.B. Melatonin and Tryptophan as Therapeutic Agents against the Impairment of the Sleep-Wake Cycle and Immunosenescence Due to Aging in Streptopelia Risoria. Neuro Endocrinol. Lett. 2007, 28, 757–760. [Google Scholar]
- Paredes, S.D.; Terrón, M.P.; Marchena, A.M.; Barriga, C.; Pariente, J.A.; Reiter, R.J.; Rodríguez, A.B. Tryptophan Modulates Cell Viability, Phagocytosis and Oxidative Metabolism in Old Ringdoves. Basic Clin. Pharmacol. Toxicol. 2007, 101, 56–62. [Google Scholar] [CrossRef]
- Rai, S.; Haldar, C.; Singh, R. Modulation of Immunity in Young-Adult and Aged Squirrel, Funambulus Pennanti by Melatonin and p-Chlorophenylalanine. Immun. Ageing 2009, 6, 5. [Google Scholar] [CrossRef]
- Baeza, I.; Alvarado, C.; Alvarez, P.; Salazar, V.; Castillo, C.; Ariznavarreta, C.; Fdez-Tresguerres, J.A.; De la Fuente, M. Improvement of Leucocyte Functions in Ovariectomised Aged Rats after Treatment with Growth Hormone, Melatonin, Oestrogens or Phyto-Oestrogens. J. Reprod. Immunol. 2009, 80, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.J.; Michan, S. Biology of Healthy Aging and Longevity. Rev. Investig. Clin. 2016, 68, 7–16. [Google Scholar]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef]
- Karadas, O.; Ozpinar, N.; Bilgic, E.; Ozcelik, F.; Karadas, S. The Physiological and Lifespan Alterations in Caenorhabditis Elegans Exposed to Different Dosages of Melatonin. Pak. J. Pharm. Sci. 2019, 32, 625–630. [Google Scholar]
- Ferrari, E.; Cravello, L.; Falvo, F.; Barili, L.; Solerte, S.B.; Fioravanti, M.; Magri, F. Neuroendocrine Features in Extreme Longevity. Exp. Gerontol. 2008, 43, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Harrison, S.L.; Stone, K.L.; Holton, K.F.; Barrett-Connor, E.; Ancoli-Israel, S.; Yaffe, K.; Ensrud, K.; Cawthon, P.M.; Redline, S.; et al. Association of Urinary Melatonin Levels and Aging-Related Outcomes in Older Men. Sleep Med. 2016, 23, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lingas, E.C. A Narrative Review of the Carcinogenic Effect of Night Shift and the Potential Protective Role of Melatonin. Cureus 2023, 15, e43326. [Google Scholar] [CrossRef] [PubMed]
- Zeitzer, J.M.; Duffy, J.F.; Lockley, S.W.; Dijk, D.-J.; Czeisler, C.A. Plasma Melatonin Rhythms in Young and Older Humans during Sleep, Sleep Deprivation, and Wake. Sleep 2007, 30, 1437–1443. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin and Healthy Aging. Vitam. Horm. 2021, 115, 67–88. [Google Scholar] [CrossRef]
- Marjot, T.; Ray, D.W.; Williams, F.R.; Tomlinson, J.W.; Armstrong, M.J. Sleep and Liver Disease: A Bidirectional Relationship. Lancet Gastroenterol. Hepatol. 2021, 6, 850–863. [Google Scholar] [CrossRef]
- Wang, C.; Holtzman, D.M. Bidirectional Relationship between Sleep and Alzheimer’s Disease: Role of Amyloid, Tau, and Other Factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef]
- Orr, W.C.; Fass, R.; Sundaram, S.S.; Scheimann, A.O. The Effect of Sleep on Gastrointestinal Functioning in Common Digestive Diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 616–624. [Google Scholar] [CrossRef]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between Circadian Clocks and Thyroid Function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Romigi, A.; Albanese, M.; Liguori, C.; Placidi, F.; Marciani, M.G.; Massa, R. Sleep-Wake Cycle and Daytime Sleepiness in the Myotonic Dystrophies. J. Neurodegener. Dis. 2013, 2013, 692026. [Google Scholar] [CrossRef]
- Hemmer, A.; Mareschal, J.; Dibner, C.; Pralong, J.A.; Dorribo, V.; Perrig, S.; Genton, L.; Pichard, C.; Collet, T.-H. The Effects of Shift Work on Cardio-Metabolic Diseases and Eating Patterns. Nutrients 2021, 13, 4178. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Yoo, S.-H.; Chen, Z.J. Manipulating the Circadian and Sleep Cycles to Protect against Metabolic Disease. Front. Endocrinol. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Jockers, R. Melatonin Controversies, an Update. J. Pineal Res. 2021, 70, e12702. [Google Scholar] [CrossRef] [PubMed]

| Effect | Fruit/Vegetable |
|---|---|
| Body clock synchronization | Cherries |
| Sleep | Bananas |
| Reproduction | Pineapples |
| Anti-inflammatory | Grapes |
| Antitumor | Mangoes |
| Cell differentiation | Nuts |
| Telomerase activity | Oats |
| Angiogenesis regulator | Tomatoes |
| Immune system activation | Mushrooms |
| Antioxidant | |
| Anti-aging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Placín, C.; Salido, G.M.; González, A. Melatonin Interplay in Physiology and Disease—The Fountain of Eternal Youth Revisited. Biomolecules 2025, 15, 682. https://doi.org/10.3390/biom15050682
Ortiz-Placín C, Salido GM, González A. Melatonin Interplay in Physiology and Disease—The Fountain of Eternal Youth Revisited. Biomolecules. 2025; 15(5):682. https://doi.org/10.3390/biom15050682
Chicago/Turabian StyleOrtiz-Placín, Cándido, Ginés María Salido, and Antonio González. 2025. "Melatonin Interplay in Physiology and Disease—The Fountain of Eternal Youth Revisited" Biomolecules 15, no. 5: 682. https://doi.org/10.3390/biom15050682
APA StyleOrtiz-Placín, C., Salido, G. M., & González, A. (2025). Melatonin Interplay in Physiology and Disease—The Fountain of Eternal Youth Revisited. Biomolecules, 15(5), 682. https://doi.org/10.3390/biom15050682






