Combination of Periodontal Ligament Stem Cells and Metformin via Organic Cation Transporters for Periodontal Regeneration in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Sources and Treatment
2.2. Western Blot Analysis
2.3. Immunofluorescence Staining
2.4. Cell Proliferation Assay
2.5. Metformin Uptake Assay
2.6. OCT1 Knockdown via Lentivirus-Mediated shRNA Transduction
2.7. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.8. ALP Activity Assay and Alizarin Red S (ARS) Staining
2.9. In Vivo Periodontal Defect Model in Rats
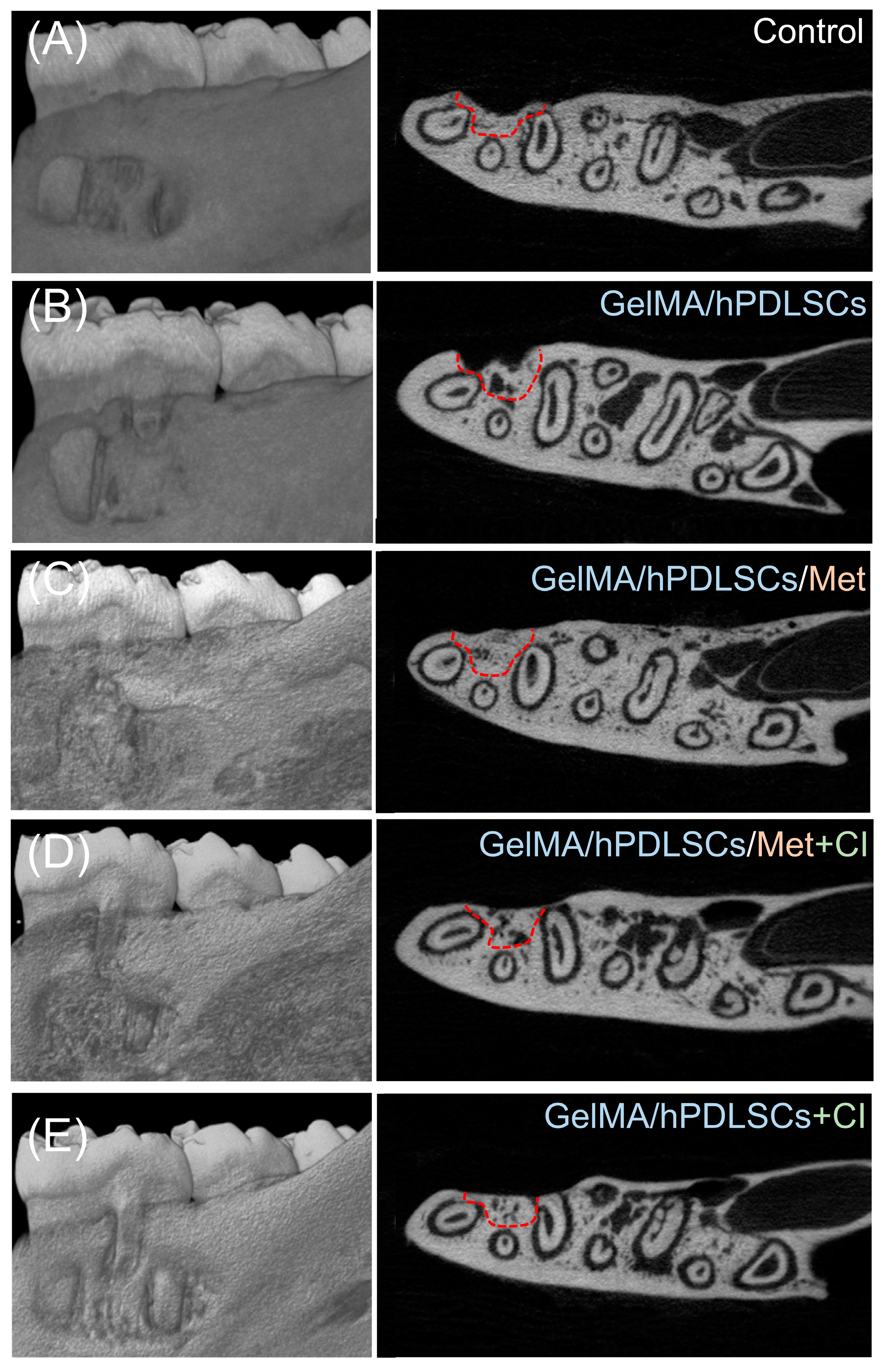
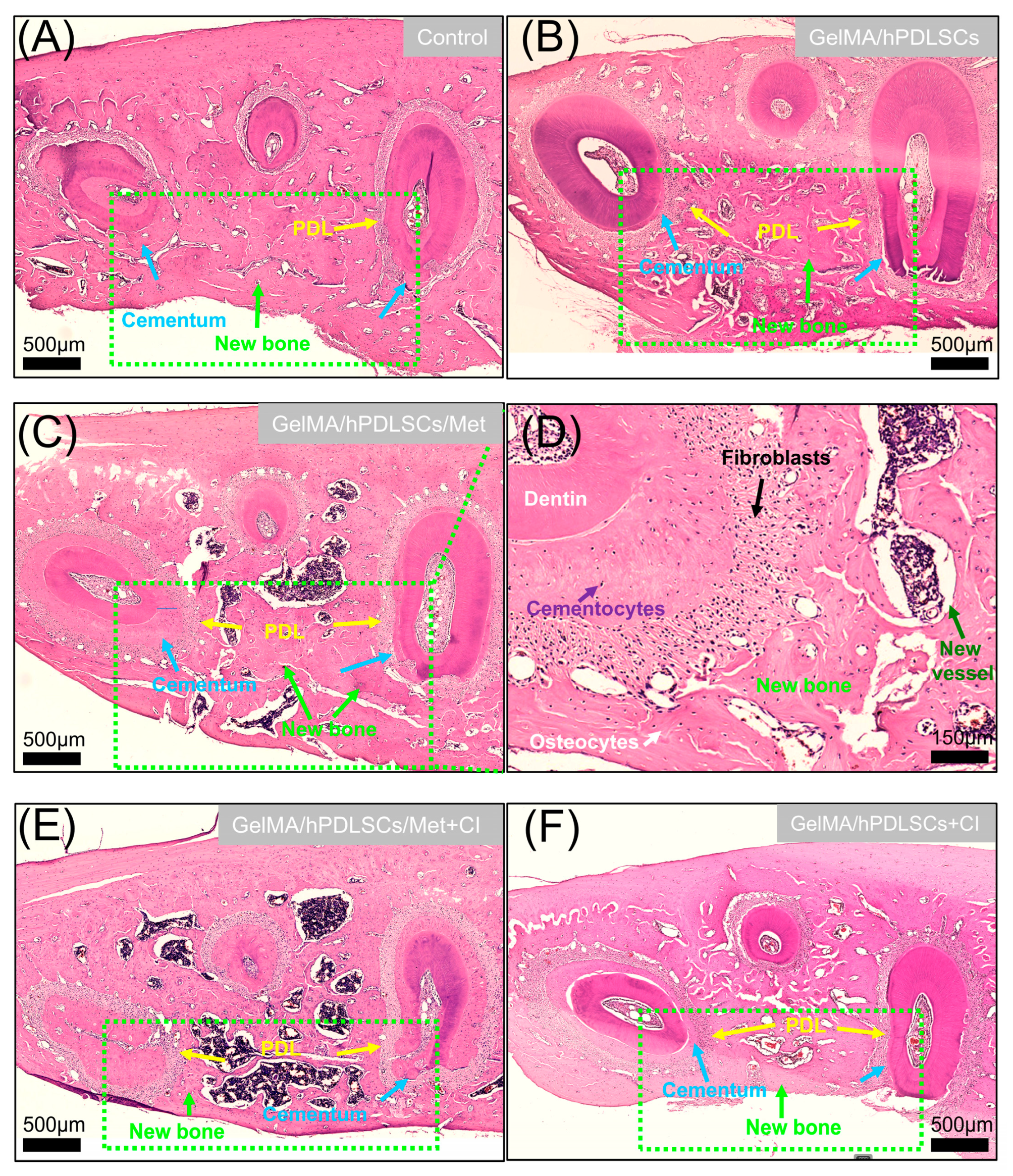
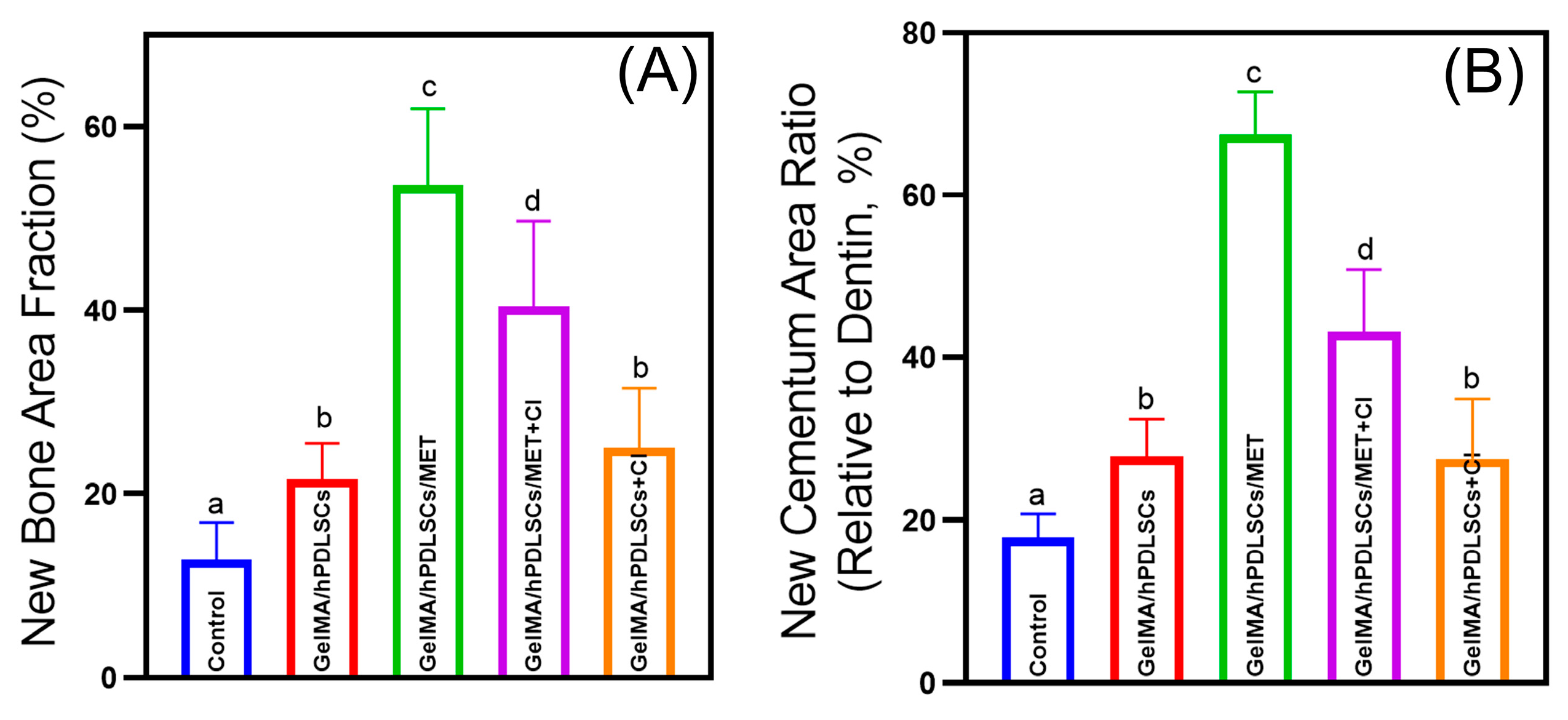
2.10. Micro-CT and Histomorphometric Analyses
2.11. Data Analysis
3. Results
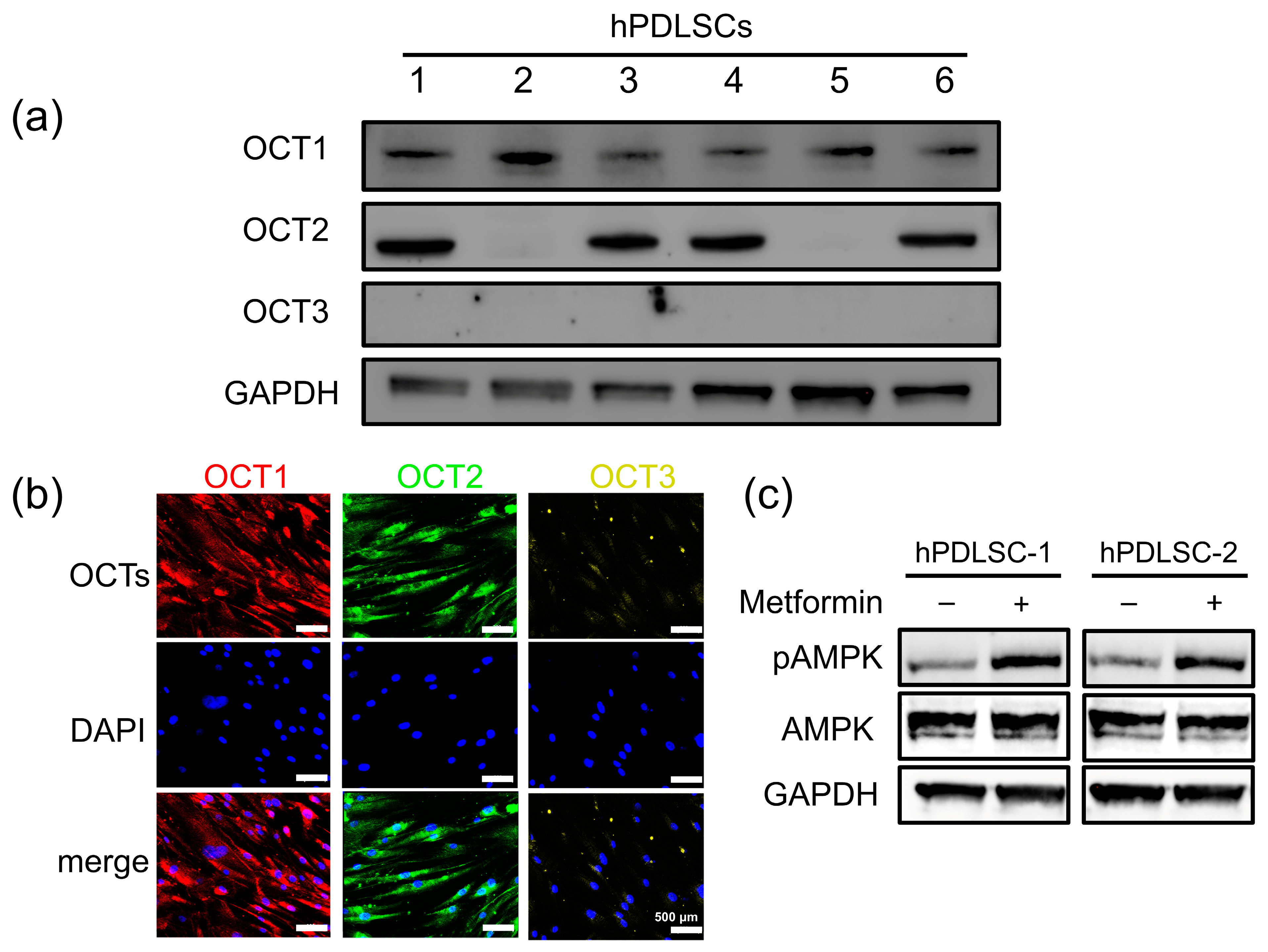
3.1. Differential Expression and Functional Activity of OCTs in hPDLSCs
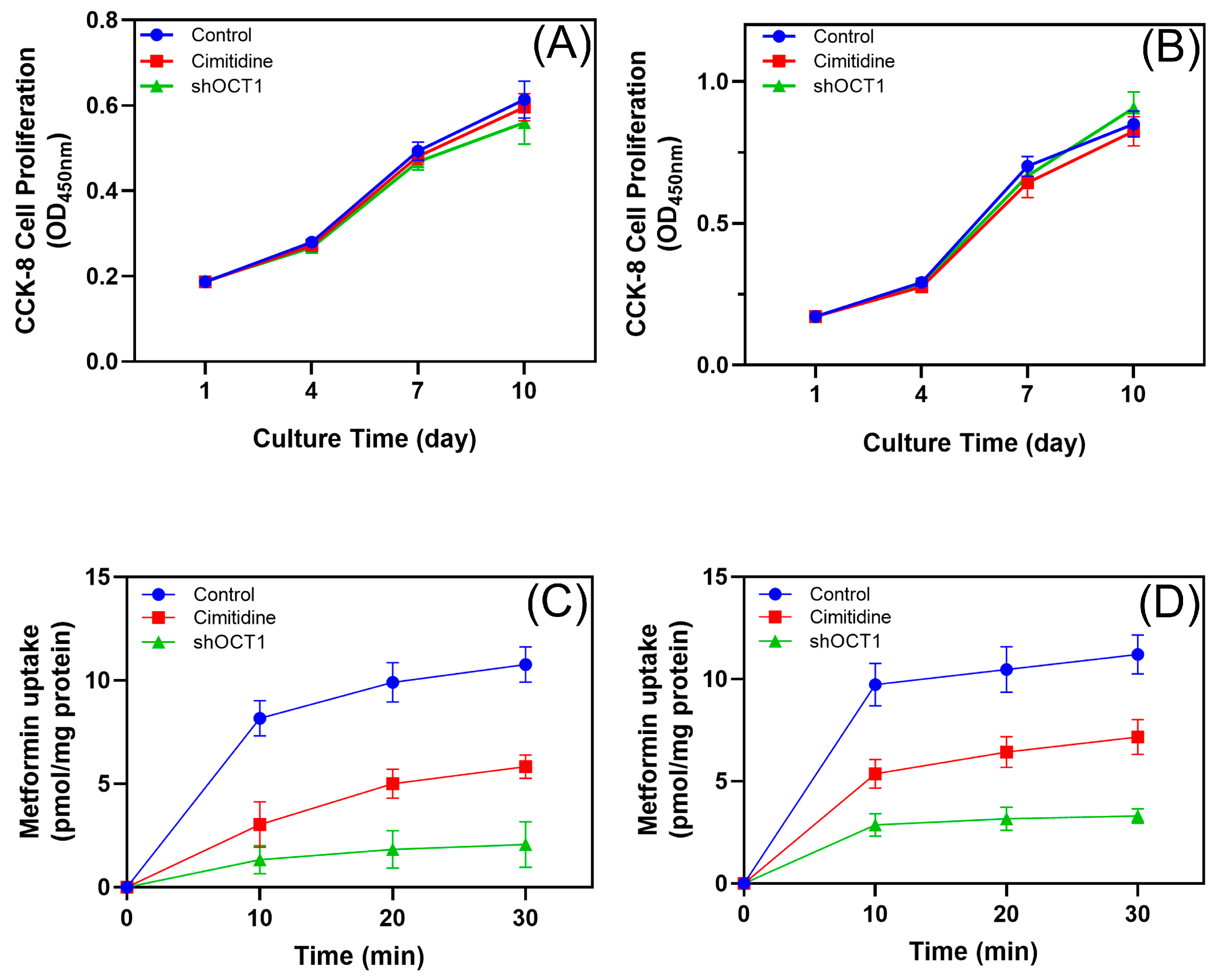
3.2. Role of OCTs in Metformin Uptake in hPDLSCs
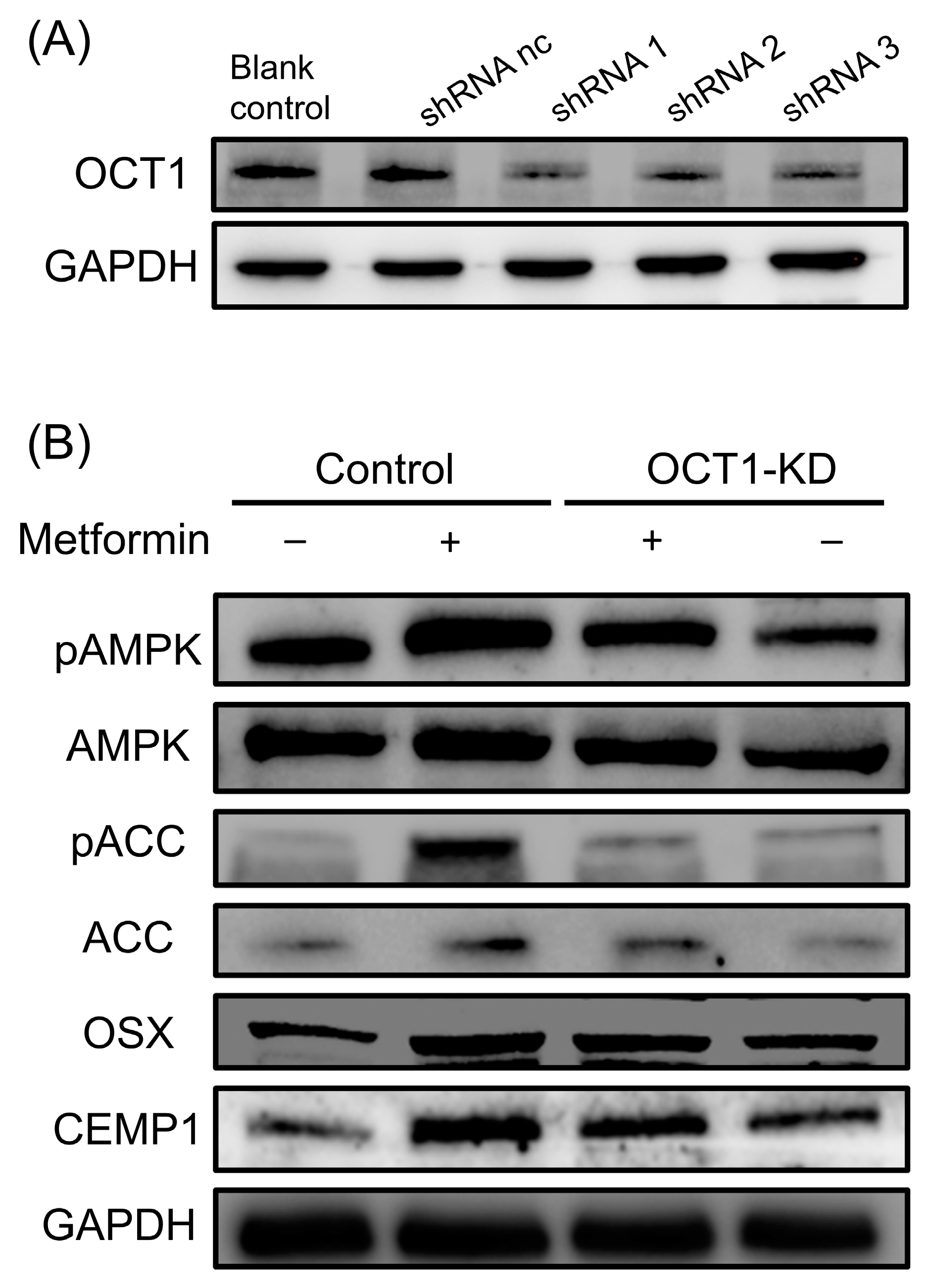
3.3. Role of OCT1 in Metformin-Induced AMPK/ACC Activation and Osteogenic/Cementogenic Differentiation
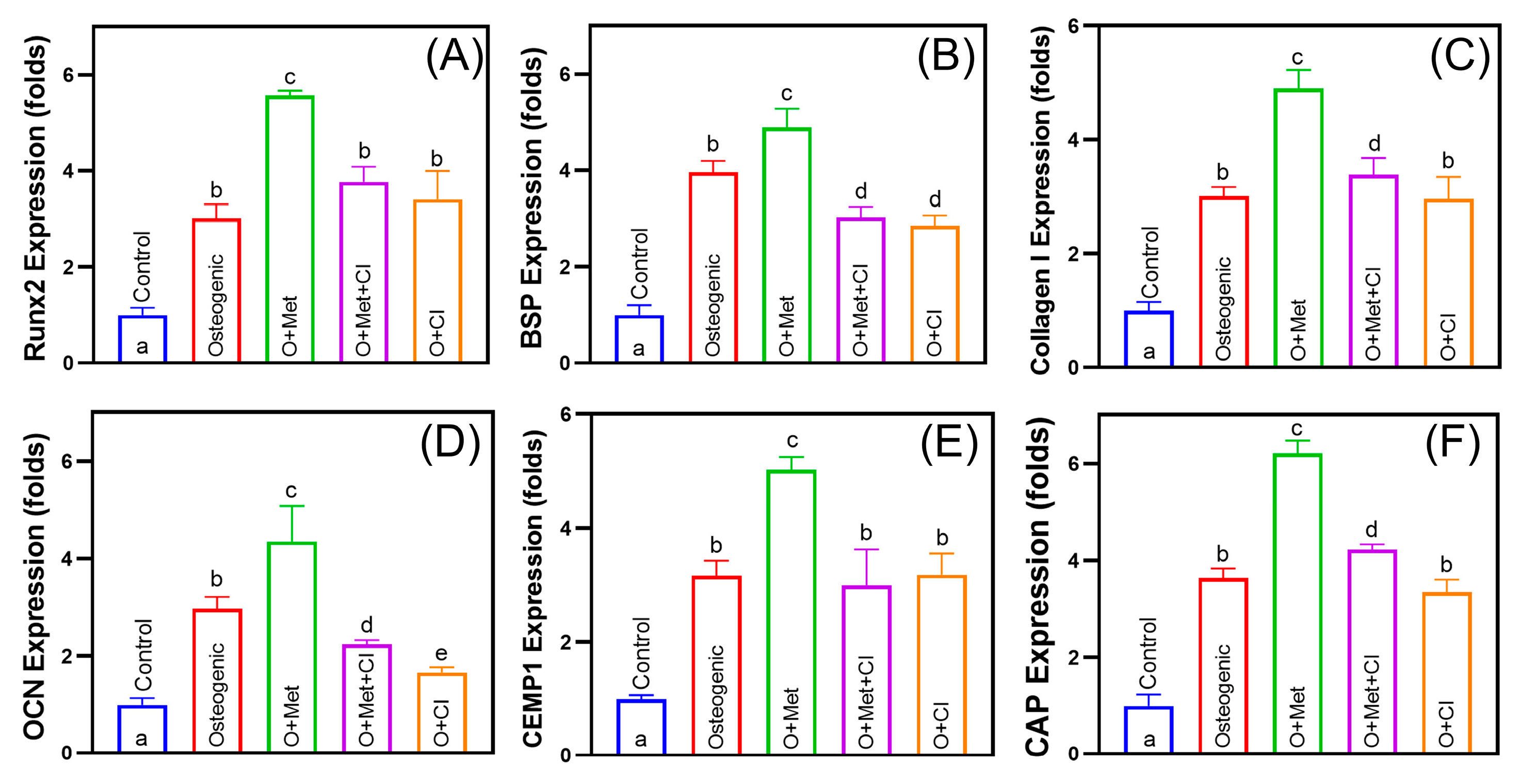
3.4. Cimetidine Inhibits Metformin-Induced Osteogenic and Cementogenic Differentiation
3.5. Cimetidine Attenuates Metformin-Induced Periodontal Regeneration In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OCTs | Organic cation transporters |
| hPDLSCs | Human periodontal ligament stem cells |
| HPLC | High-performance liquid chromatography |
| PDLSCs | Periodontal ligament stem cells |
| ARS | Alizarin Red S |
| hUC-MSCs | Human umbilical cord mesenchymal stromal cells |
| ACC1 | Acetyl-CoA carboxylase 1 |
| DDIs | Drug–drug interactions |
| AUC | Area under the curve |
References
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Therapeutic profile in the treatment of type 2 diabetes. Diabetes Obes. Metab. 2024, 26, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Kimura, T.; Obata, A.; Shimoda, M.; Kaku, K. Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One after Another in the Long History. Int. J. Mol. Sci. 2021, 22, 2596. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Pradeep, A.; Rao, N.S.; Naik, S.B.; Kumari, M. Efficacy of Varying Concentrations of Subgingivally Delivered Metformin in the Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2013, 84, 212–220. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, Z.; Xu, H.H.K.; Dai, Z.; Yu, K.; Xiao, L.; Schneider, A.; Weir, M.D.; Oates, T.W.; Bai, Y.; et al. Effects of Metformin Delivery via Biomaterials on Bone and Dental Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 15905. [Google Scholar] [CrossRef]
- Kassebaum, N.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Ruan, J.; Weir, M.D.; Ma, T.; Ren, K.; Schneider, A.; Oates, T.W.; Li, A.; Zhao, L.; et al. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J. Dent. 2020, 92, 103259. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Weir, M.D.; Schneider, A.; Ma, T.; Oates, T.W.; Xu, H.H.K.; Zhang, K.; Bai, Y. Periodontal ligament stem cell-based bioactive constructs for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1071472. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Z.; Qiao, Q.; Li, S.; Yu, W.; Guan, X.; Schneider, A.; Weir, M.D.; Xu, H.H.; Zhang, K.; et al. Injectable periodontal ligament stem cell-metformin-calcium phosphate scaffold for bone regeneration and vascularization in rats. Dent. Mater. 2023, 39, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Kawoosa, F.; Shah, Z.A.; Masoodi, S.R.; Amin, A.; Rasool, R.; Fazili, K.M.; Dar, A.H.; Lone, A.; Bashir, S.U. Role of human organic cation transporter-1 (OCT-1/SLC22A1) in modulating the response to metformin in patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Hanke, N.; Türk, D.; Selzer, D.; Ishiguro, N.; Ebner, T.; Wiebe, S.; Müller, F.; Stopfer, P.; Nock, V.; Lehr, T. A Comprehensive Whole-Body Physiologically Based Pharmacokinetic Drug–Drug–Gene Interaction Model of Metformin and Cimetidine in Healthy Adults and Renally Impaired Individuals. Clin. Pharmacokinet. 2020, 59, 1419–1431. [Google Scholar] [CrossRef]
- Mato, E.P.M.; Guewo-Fokeng, M.; Essop, M.F.; Owira, P.M.O. Genetic polymorphisms of organic cation transporters 1 (OCT1) and responses to metformin therapy in individuals with type 2 diabetes mellitus: A systematic review protocol. Syst. Rev. 2018, 7, 105. [Google Scholar] [CrossRef]
- García, M.A.; Contreras, D.; González, P.M. Metformin Transport in Native MDCK-Wt and MDCK-II Monolayers Unveils Functional Inter-Strains Differences Influencing Drug Permeability. Pharm. Res. 2020, 37, 1–14. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.; et al. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Nies, A.T.; Hofmann, U.; Resch, C.; Schaeffeler, E.; Rius, M.; Schwab, M. Proton Pump Inhibitors Inhibit Metformin Uptake by Organic Cation Transporters (OCTs). PLoS ONE 2011, 6, e22163. [Google Scholar] [CrossRef]
- Samodelov, S.L.; Kullak-Ublick, G.A.; Gai, Z.; Visentin, M. Organic Cation Transporters in Human Physiology, Pharmacology, and Toxicology. Int. J. Mol. Sci. 2020, 21, 7890. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K.-I. Gene Expression Levels and Immunolocalization of Organic Ion Transporters in the Human Kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Koepsell, H.; Winter, S.; Burk, O.; Klein, K.; Kerb, R.; Zanger, U.M.; Keppler, D.; Schwab, M.; Schaeffeler, E. Expression of Organic Cation Transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) Is Affected by Genetic Factors and Cholestasis in Human Liver. Hepatology 2009, 50, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Sogame, Y.; Kitamura, A.; Yabuki, M.; Komuro, S. A comparison of uptake of metformin and phenformin mediated by hOCT1 in human hepatocytes. Biopharm. Drug Dispos. 2009, 30, 476–484. [Google Scholar] [CrossRef]
- Kimura, N.; Okuda, M.; Inui, K.-I. Metformin Transport by Renal Basolateral Organic Cation Transporter hOCT2. Pharm. Res. 2005, 22, 255–259. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Schneider, A.; Gao, X.; Ren, K.; Weir, M.D.; Zhang, N.; Zhang, K.; Zhang, L.; Bai, Y.; et al. Human periodontal ligament stem cell seeding on calcium phosphate cement scaffold delivering metformin for bone tissue engineering. J. Dent. 2019, 91, 103220. [Google Scholar] [CrossRef]
- Wang, P.; Ma, T.; Guo, D.; Hu, K.; Shu, Y.; Xu, H.H.K.; Schneider, A. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 12, 437–446. [Google Scholar] [CrossRef]
- Di Pietro, M.; Velazquez, C.; Matzkin, M.E.; Frungieri, M.B.; Peña, M.G.; de Zúñiga, I.; Pascuali, N.; Irusta, G.; Bianchi, M.S.; Parborell, F.; et al. Metformin has a direct effect on ovarian cells that is dependent on organic cation transporters. Mol. Cell. Endocrinol. 2020, 499, 110591. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiao, Q.; Sun, Y.; Wang, J.; Li, X.; Zhang, L.; Yang, H.; Zhang, K.; Zhang, N.; Bai, Y. Human periodontal ligament stem cells and metformin to enhance periodontal regeneration in rats. J. Dent. 2025, 156, 105700. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Qiao, Q.; Zhang, L.; Xie, X.; Weir, M.D.; Schneider, A.; Xu, H.H.K.; Zhang, N.; Zhang, K.; et al. Human Periodontal Ligament Stem Cell and Umbilical Vein Endothelial Cell Co-Culture to Prevascularize Scaffolds for Angiogenic and Osteogenic Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 12363. [Google Scholar] [CrossRef]
- Meyer, M.J.; Tuerkova, A.; Römer, S.; Wenzel, C.; Seitz, T.; Gaedcke, J.; Oswald, S.; Brockmöller, J.; Zdrazil, B.; Tzvetkov, M.V. Differences in Metformin and Thiamine Uptake between Human and Mouse Organic Cation Transporter 1: Structural Determinants and Potential Consequences for Intrahepatic Concentrations. Drug Metab. Dispos. 2020, 48, 1380–1392. [Google Scholar] [CrossRef]
- Umehara, K.-I.; Iwatsubo, T.; Noguchi, K.; Usui, T.; Kamimura, H. Effect of cationic drugs on the transporting activity of human and rat OCT/Oct 1–3 in vitro and implications for drug–drug interactions. Xenobiotica 2008, 38, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, Q.; Yu, W.; Huang, J.; Yang, M.; Chen, W.; Han, W. Optimized protocol for high-titer lentivirus production and transduction of primary fibroblasts. J. Basic Microbiol. 2021, 61, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.D.; Xu, H.H. Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair. Acta Biomater. 2010, 6, 4118–4126. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Gao, X.; Ma, T.; Weir, M.D.; Zou, J.; Song, B.; Lin, Z.; Schneider, A.; Xu, H.H. Metformin Enhances the Differentiation of Dental Pulp Cells into Odontoblasts by Activating AMPK Signaling. J. Endod. 2018, 44, 576–584. [Google Scholar] [CrossRef]
- Furuta, K.; Sato, S.; Miyake, T.; Okamoto, E.; Ishine, J.; Ishihara, S.; Amano, Y.; Adachi, K.; Kinoshita, Y. Anti-tumor effects of cimetidine on hepatocellular carcinomas in diethylnitrosamine-treated rats. Oncol. Rep. 2008, 19, 361–368. [Google Scholar] [CrossRef][Green Version]
- Yu, M.; Luo, D.; Qiao, J.; Guo, J.; He, D.; Jin, S.; Tang, L.; Wang, Y.; Shi, X.; Mao, J.; et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact. Mater. 2022, 10, 93–106. [Google Scholar] [CrossRef]
- Daghrery, A.; Ferreira, J.A.; Araújo, I.J.d.S.; Clarkson, B.H.; Eckert, G.J.; Bhaduri, S.B.; Malda, J.; Bottino, M.C. A Highly Ordered, Nanostructured Fluorinated CaP-Coated Melt Electrowritten Scaffold for Periodontal Tissue Regeneration. Adv. Healthc. Mater. 2021, 10, 2101152. [Google Scholar] [CrossRef]
- Bak, E.J.; Park, H.G.; Kim, M.; Kim, S.W.; Kim, S.; Choi, S.; Cha, J.; Yoo, Y. The Effect of Metformin on Alveolar Bone in Ligature-Induced Periodontitis in Rats: A Pilot Study. J. Periodontol. 2010, 81, 412–419. [Google Scholar] [CrossRef]
- Ren, C.; Hao, X.; Wang, L.; Hu, Y.; Meng, L.; Zheng, S.; Ren, F.; Bu, W.; Wang, H.; Li, D.; et al. Metformin Carbon Dots for Promoting Periodontal Bone Regeneration via Activation of ERK/AMPK Pathway. Adv. Healthc. Mater. 2021, 10, 2100196. [Google Scholar] [CrossRef]
- Lozano, E.; Briz, O.; Macias, R.I.R.; Serrano, M.A.; Marin, J.J.G.; Herraez, E. Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition. J. Pers. Med. 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Al Jofi, F.E.; Ma, T.; Guo, D.; Schneider, M.P.; Shu, Y.; Xu, H.H.; Schneider, A. Functional organic cation transporters mediate osteogenic response to metformin in human umbilical cord mesenchymal stromal cells. Cytotherapy 2018, 20, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, X.; Ling-Ling, E.; Wang, D.-S.; Liu, H.-C. The transmembrane transport of metformin by osteoblasts from rat mandible. Arch. Oral Biol. 2009, 54, 951–962. [Google Scholar] [CrossRef]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Brown, C.; Cheatham, S.; Castro, R.A.; Leabman, M.K.; Urban, T.J.; Chen, L.; Yee, S.W.; Choi, J.H.; et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenetics Genom. 2009, 19, 497–504. [Google Scholar] [CrossRef]
- Burkewitz, K.; Zhang, Y.; Mair, W.B. AMPK at the Nexus of Energetics and Aging. Cell Metab. 2014, 20, 10–25. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Yan, H.; Jiao, G.; Wang, H.; Chi, H.; Zhou, H.; Chen, L.; Shan, Y.; Chen, Y. Resveratrol Protects Osteoblasts Against Dexamethasone-Induced Cytotoxicity Through Activation of AMP-Activated Protein Kinase. Drug Des. Dev. Ther. 2020, 14, 4451–4463. [Google Scholar] [CrossRef]
- Gao, B.; Cheng, X.; Wu, Y.; Jiang, B. Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells. Open Life Sci. 2024, 19, 20220884. [Google Scholar] [CrossRef]
- Metformin Drug Interactions. 2024. Available online: https://www.drugs.com/drug-interactions/metformin.html (accessed on 22 November 2024).
- Wang, Z.-J.; Yin, O.Q.; Tomlinson, B.; Chow, M.S. OCT2 polymorphisms and in-vivo renal functional consequence: Studies with metformin and cimetidine. Pharmacogenetics Genom. 2008, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Burt, H.; Neuhoff, S.; Almond, L.; Gaohua, L.; Harwood, M.D.; Jamei, M.; Rostami-Hodjegan, A.; Tucker, G.; Rowland-Yeo, K. Metformin and cimetidine: Physiologically based pharmacokinetic modelling to investigate transporter mediated drug–drug interactions. Eur. J. Pharm. Sci. 2016, 88, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ailabouni, A.S.; Singh, D.K.; Thakur, A.; Boone, E.C.; Gaedigk, A.; Paine, M.F.; Prasad, B. Quantitative Contributions of Hepatic and Renal Organic Cation Transporters to the Clinical Pharmacokinetic Cimetidine–Metformin Interaction. Clin. Pharmacol. Ther. 2025. [Google Scholar] [CrossRef]
- Oshima, R.; Yamada, M.; Kurogi, E.; Ogino, Y.; Serizawa, Y.; Tsuda, S.; Ma, X.; Egawa, T.; Hayashi, T. Evidence for organic cation transporter-mediated metformin transport and 5′-adenosine monophosphate-activated protein kinase activation in rat skeletal muscles. Metabolism 2015, 64, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Rodríguez-Hermosa, J.-I.; Sabater, M.; Pardo, G.; Ricart, W.; Fernández-Real, J.M. OCT1 Expression in Adipocytes Could Contribute to Increased Metformin Action in Obese Subjects. Diabetes 2011, 60, 168–176. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| OCN | GCAAAGGTGCAGCCTTTGTG | GGCTCCCAGCCATTGATACAG |
| RUNX2 | TCTGGCCTTCCACTCTCAGT | GACTGGCGGGGTGTAAGTAA |
| Collagen I | CTGACCTTCCTGCGCCTGATGTCC | GTCTGGGGCACCAACGTCCAAGGG |
| BSP | GAACCACTTCCCCACCTTTT | TCTGACCATCATAGCCATCG |
| CAP | CCTGGCTCACCTTCTACGAC | CCTCAAGCAAGGCAAATGTC |
| CEMP1 | GGGCACATCAAGCACTGACAG | CCCTTAGGAAGTGGCTGTCCAG |
| GAPDH | GCACCGTCAAGGCTGAGAAC | ATGGTGGTGAAGACGCCAGT |
| Group | Treatment Description | Cimetidine Administration |
|---|---|---|
| (1) Control | 0.9% NaCl | no |
| (2) GelMA/hPDLSCs | GelMA hydrogel with hPDLSCs (5 × 106/mL) | no |
| (3) GelMA/hPDLSCs/Met | GelMA hydrogel with hPDLSCs (5 × 106/mL) and metformin (100 μM) | no |
| (4) GelMA/hPDLSCs/Met + CI | GelMA hydrogel with hPDLSCs (5 × 106/mL) and metformin (100 μM) | 100 mg/kg/day in water |
| (5) GelMA/hPDLSCs + CI | GelMA hydrogel with hPDLSCs (5 × 106/mL) | 100 mg/kg/day in water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Q.; Zhao, Z.; Sun, Y.; Wang, J.; Li, X.; Zhang, L.; Yang, H.; Zhang, N.; Zhang, K.; Bai, Y. Combination of Periodontal Ligament Stem Cells and Metformin via Organic Cation Transporters for Periodontal Regeneration in Rats. Biomolecules 2025, 15, 663. https://doi.org/10.3390/biom15050663
Qiao Q, Zhao Z, Sun Y, Wang J, Li X, Zhang L, Yang H, Zhang N, Zhang K, Bai Y. Combination of Periodontal Ligament Stem Cells and Metformin via Organic Cation Transporters for Periodontal Regeneration in Rats. Biomolecules. 2025; 15(5):663. https://doi.org/10.3390/biom15050663
Chicago/Turabian StyleQiao, Qingchen, Zeqing Zhao, Yaxi Sun, Jing Wang, Xiaowei Li, Li Zhang, Hao Yang, Ning Zhang, Ke Zhang, and Yuxing Bai. 2025. "Combination of Periodontal Ligament Stem Cells and Metformin via Organic Cation Transporters for Periodontal Regeneration in Rats" Biomolecules 15, no. 5: 663. https://doi.org/10.3390/biom15050663
APA StyleQiao, Q., Zhao, Z., Sun, Y., Wang, J., Li, X., Zhang, L., Yang, H., Zhang, N., Zhang, K., & Bai, Y. (2025). Combination of Periodontal Ligament Stem Cells and Metformin via Organic Cation Transporters for Periodontal Regeneration in Rats. Biomolecules, 15(5), 663. https://doi.org/10.3390/biom15050663







