Bispecific Antibodies, Nanobodies and Extracellular Vesicles: Present and Future to Cancer Target Therapy
Abstract
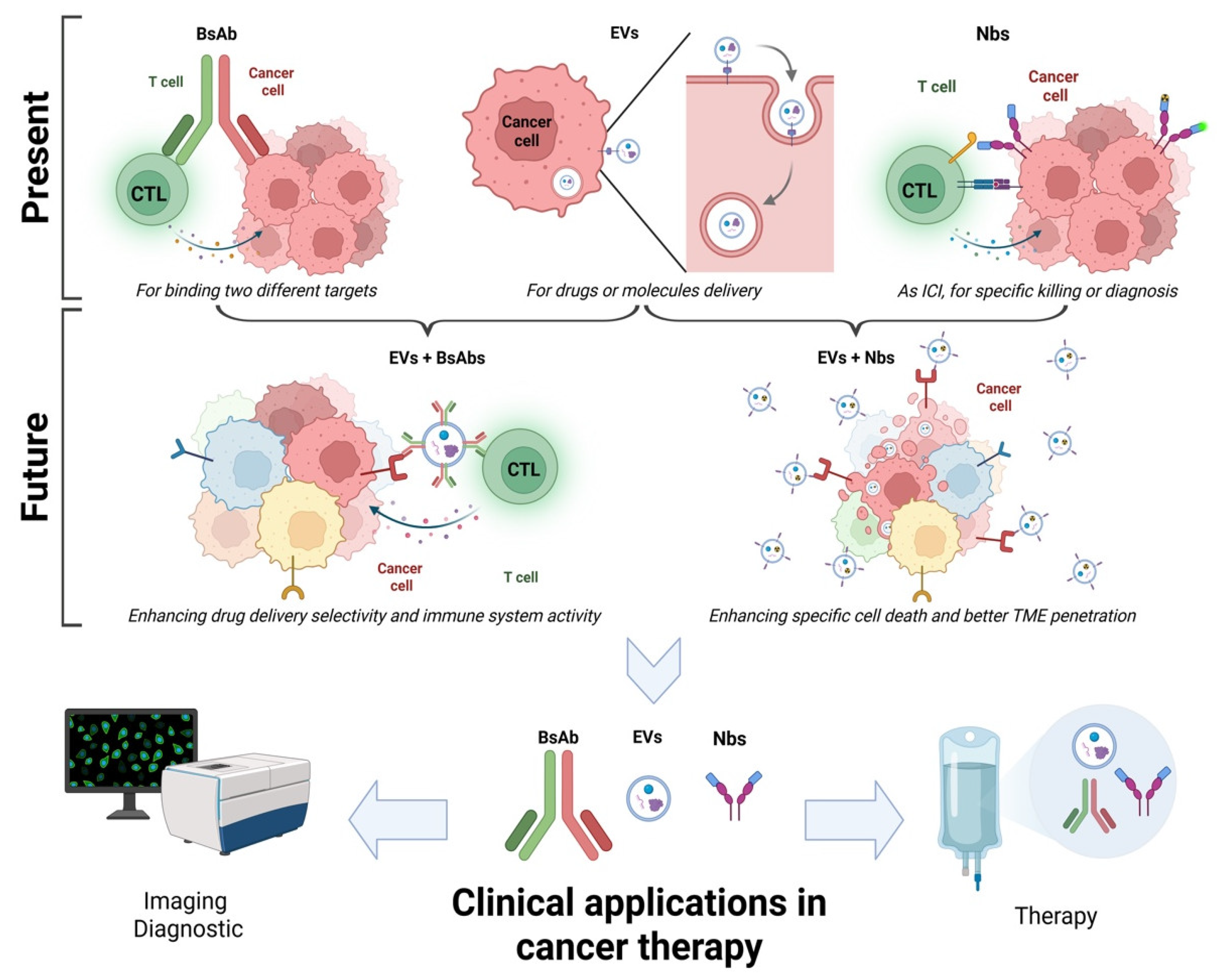
1. Introduction
2. Bispecific Antibodies (BsAbs) in Cancer Therapy
2.1. BsAb Classification by Their Mechanism of Action
2.1.1. BsAbs That Block Two Pathways
2.1.2. BsAbs Against Immune Checkpoints
2.1.3. BsAbs That Aim at the Activation of the Immune System
2.2. Advantages and Limitations
3. Nanobodies (Nbs) in Cancer Therapy
3.1. Current Applications in Cancer Therapy
3.1.1. Nbs for Imaging and Diagnosis
3.1.2. Nbs for Cancer Treatment
3.2. Limitations and Future Prospects
4. Extracellular Vesicles as a Drug Delivery Platform
4.1. Applications in Targeted Cancer Therapy
4.2. Challenges and Future Directions
5. Comparative Discussion
5.1. Comparing BsAbs, Nbs and EVs in Cancer Therapy
5.2. Potential Combinations
5.3. Regulatory and Translational Challenges
5.4. Clinical Considerations and Translational Barriers
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Xu, M.; Han, X.; Xiong, H.; Gao, Y.; Xu, B.; Zhu, G.; Li, J. Cancer Nanomedicine: Emerging Strategies and Therapeutic Potentials. Molecules 2023, 28, 5145. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Deivayanai, V.C.; Thamarai, P.; Karishma, S.; Saravanan, A.; Yaashikaa, P.R.; Vickram, A.S.; Hemavathy, R.V.; Kumar, R.R.; Rishikesavan, S.; Shruthi, S. A comprehensive review on advances in nanoparticle-mediated cancer therapeutics: Current research and future perspectives. Cancer Pathog. Ther. 2024; in press. [Google Scholar] [CrossRef]
- Bandini, S.; Ulivi, P.; Rossi, T. Extracellular Vesicles, Circulating Tumor Cells, and Immune Checkpoint Inhibitors: Hints and Promises. Cells 2024, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Yahya, E.B.; Mohamed Ibrahim Mohamed, M.; Rashid, S.; Iqbal, M.O.; Kontek, R.; Abdulsamad, M.A.; Allaq, A.A. Recent Advances in Molecular Mechanisms of Cancer Immunotherapy. Cancers 2023, 15, 2721. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef]
- Kreamer, K.M. Immune Checkpoint Blockade: A New Paradigm in Treating Advanced Cancer. J. Adv. Pract. Oncol. 2014, 5, 418–431. [Google Scholar] [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Kasana, A.; Verma, V. Next-Generation Therapeutic Antibodies for Cancer Treatment: Advancements, Applications, and Challenges. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.K.; Odongo, S.; Radwanska, M.; Magez, S. NANOBODIES(R): A Review of Diagnostic and Therapeutic Applications. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef]
- Alexander, E.; Leong, K.W. Discovery of nanobodies: A comprehensive review of their applications and potential over the past five years. J. Nanobiotechnol. 2024, 22, 661. [Google Scholar] [CrossRef]
- Ratnikova, N.M.; Kravchenko, Y.; Ivanova, A.; Zhuchkov, V.; Frolova, E.; Chumakov, S. A Novel Anti-CD47 Nanobody Tetramer for Cancer Therapy. Antibodies 2024, 13, 2. [Google Scholar] [CrossRef]
- Sergazy, S.; Seydahmetova, R.; Gulyayev, A.; Shulgau, Z.; Aljofan, M. The Role of Exosomes in Cancer Progression and Therapy. Biology 2025, 14, 27. [Google Scholar] [CrossRef]
- Jovcevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Johnson, V.; Vasu, S.; Kumar, U.S.; Kumar, M. Surface-Engineered Extracellular Vesicles in Cancer Immunotherapy. Cancers 2023, 15, 2838. [Google Scholar] [CrossRef] [PubMed]
- Cerne, K.; Kelhar, N.; Resnik, N.; Herzog, M.; Vodnik, L.; Veranic, P.; Kobal, B. Characteristics of Extracellular Vesicles from a High-Grade Serous Ovarian Cancer Cell Line Derived from a Platinum-Resistant Patient as a Potential Tool for Aiding the Prediction of Responses to Chemotherapy. Pharmaceuticals 2023, 16, 907. [Google Scholar] [CrossRef]
- Forder, A.; Hsing, C.Y.; Trejo Vazquez, J.; Garnis, C. Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis. Cells 2021, 10, 3429. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Rival, C.; Mandal, M.; Cramton, K.; Qiao, H.; Arish, M.; Sun, J.; McCann, J.V.; Dudley, A.C.; Solga, M.D.; Erdbrugger, U.; et al. B cells secrete functional antigen-specific IgG antibodies on extracellular vesicles. Sci. Rep. 2024, 14, 16970. [Google Scholar] [CrossRef]
- Gutknecht, M.F.; Holodick, N.E.; Rothstein, T.L. B cell extracellular vesicles contain monomeric IgM that binds antigen and enters target cells. iScience 2023, 26, 107526. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.D.; Longjohn, M.N.; Gormley, D.J.B.; Smith, R.H.; Dang-Lawson, M.; Matsuuchi, L.; Gold, M.R.; Christian, S.L. CD24 and IgM Stimulation of B Cells Triggers Transfer of Functional B Cell Receptor to B Cell Recipients Via Extracellular Vesicles. J. Immunol. 2021, 207, 3004–3015. [Google Scholar] [CrossRef]
- Barok, M.; Puhka, M.; Vereb, G.; Szollosi, J.; Isola, J.; Joensuu, H. Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer 2018, 18, 504. [Google Scholar] [CrossRef]
- Wang, S.; Chen, K.; Lei, Q.; Ma, P.; Yuan, A.Q.; Zhao, Y.; Jiang, Y.; Fang, H.; Xing, S.; Fang, Y.; et al. The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol. Med. 2021, 13, e14291. [Google Scholar] [CrossRef]
- Tavarozzi, R.; Manzato, E. The Role of Bispecific Antibodies in Non-Hodgkin’s Lymphoma: From Structure to Prospective Clinical Use. Antibodies 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Morita, A.; Hara, N.; Makimoto, G.; Ninomiya, K.; Fujii, M.; Rai, K.; Ohashi, K.; Hotta, K.; Tabata, M.; et al. Efficacy of amivantamab, a bi-specific antibody targeting EGFR and MET, in ALK-rearranged non-small-cell lung cancer cell lines. Lung Cancer 2025, 201, 108415. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Babitzki, G.; Klaman, I.; Krieter, O.; Lechner, K.; Bendell, J.; Vega Harring, S.; Heil, F. Predictive potential of angiopoietin-2 in a mCRC subpopulation treated with vanucizumab in the McCAVE trial. Front. Oncol. 2023, 13, 1157596. [Google Scholar] [CrossRef]
- Fu, S.; Corr, B.R.; Culm-Merdek, K.; Mockbee, C.; Youssoufian, H.; Stagg, R.; Naumann, R.W.; Wenham, R.M.; Rosengarten, R.D.; Benjamin, L.; et al. Phase Ib Study of Navicixizumab Plus Paclitaxel in Patients With Platinum-Resistant Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J. Clin. Oncol. 2022, 40, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Lumish, M.; Chui, M.H.; Zhou, Q.; Iasonos, A.; Sarasohn, D.; Cohen, S.; Friedman, C.; Grisham, R.; Konner, J.; Kyi, C.; et al. A phase 2 trial of zanidatamab in HER2-overexpressed advanced endometrial carcinoma and carcinosarcoma (ZW25-IST-2). Gynecol. Oncol. 2024, 182, 75–81. [Google Scholar] [CrossRef]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R.; Hamilton, E.; Chmielowski, B.; Ulahannan, S.V.; Connolly, R.M.; Santa-Maria, C.A.; Wang, J.; Bahadur, S.W.; et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: A phase 1 trial. Nat. Med. 2023, 29, 2814–2824. [Google Scholar] [CrossRef]
- Tebotelimab Is Safe and Effective Across Multiple Cancer Types. Cancer Discov. 2024, 14, OF4. [CrossRef]
- Ruan, D.Y.; Wei, X.L.; Liu, F.R.; Hu, X.C.; Zhang, J.; Ji, D.M.; Huang, D.Z.; Zhao, Y.Q.; Pan, H.M.; Liao, W.J.; et al. The first-in-class bispecific antibody IBI318 (LY3434172) targeting PD-1 and PD-L1 in patients with advanced tumors: A phase Ia/Ib study. J. Hematol. Oncol. 2024, 17, 118. [Google Scholar] [CrossRef]
- Zhang, P.F.; Zhang, W.H.; Liu, X.J.; He, D.; Yang, K.; Gou, H.F.; Hu, J.K. Chemotherapy combined with cadonilimab (AK104) as neoadjuvant treatment for locally advanced gastric/gastro-oesophageal junction adenocarcinoma: Study protocol for a single-arm, phase II clinical trial. BMJ Open 2024, 14, e081529. [Google Scholar] [CrossRef]
- Li, H.; Zhao, W.; Li, C.; Shen, H.; Li, M.; Wang, C.; Han, C.; Yi, C.; Wang, J.; Meng, X.; et al. The efficacy and safety of a novel PD-1/CTLA-4 bispecific antibody cadonilimab (AK104) in advanced non-small cell lung cancer: A multicenter retrospective observational study. Thorac. Cancer 2024, 15, 2327–2338. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Ren, S.; Zhang, Z.; Xiong, A.; Su, C.; Zhou, J.; Yu, X.; Hu, Y.; Zhang, X.; et al. A Phase 1b Study of Ivonescimab, a Programmed Cell Death Protein-1 and Vascular Endothelial Growth Factor Bispecific Antibody, as First- or Second-Line Therapy for Advanced or Metastatic Immunotherapy-Naive NSCLC. J. Thorac. Oncol. 2024, 19, 465–475. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Chen, J.; Zhuang, L.; Du, Y.; Yu, Q.; Zhuang, W.; Zhao, Y.; Zhou, M.; Zhang, W.; et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): An open-label, multicenter, phase II trial. EClinicalMedicine 2023, 62, 102106. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Moreau, A.; Melchiorri, D.; Camarero, J.; Josephson, F.; Olimpier, O.; Bergh, J.; Karres, D.; Tzogani, K.; Gisselbrecht, C.; et al. Blinatumomab for Acute Lymphoblastic Leukemia: The First Bispecific T-Cell Engager Antibody to Be Approved by the EMA for Minimal Residual Disease. Oncologist 2020, 25, e709–e715. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Varon, B.; Horowitz, N.A.; Khatib, H. Novel Bispecific T-Cell Engagers for the Treatment of Relapsed B Cell Non-Hodgkin Lymphomas: Current Knowledge and Treatment Considerations. Patient Prefer. Adherence 2024, 18, 2159–2167. [Google Scholar] [CrossRef]
- Phillips, T.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Crump, M.; Trneny, M.; Bartlett, N.L.; Zaucha, J.; Wrobel, T.; Offner, F.; et al. Glofitamab in Relapsed/Refractory Mantle Cell Lymphoma: Results From a Phase I/II Study. J. Clin. Oncol. 2025, 43, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Ivasko, S.M.; Anders, K.; Grunewald, L.; Launspach, M.; Klaus, A.; Schwiebert, S.; Ruf, P.; Lindhofer, H.; Lode, H.N.; Andersch, L.; et al. Combination of GD2-directed bispecific trifunctional antibody therapy with Pd-1 immune checkpoint blockade induces anti-neuroblastoma immunity in a syngeneic mouse model. Front. Immunol. 2022, 13, 1023206. [Google Scholar] [CrossRef]
- Ruf, P.; Bauer, H.W.; Schoberth, A.; Kellermann, C.; Lindhofer, H. First time intravesically administered trifunctional antibody catumaxomab in patients with recurrent non-muscle invasive bladder cancer indicates high tolerability and local immunological activity. Cancer Immunol. Immunother. 2021, 70, 2727–2735. [Google Scholar] [CrossRef]
- Hassel, J.C.; Stanhope, S.; Greenshields-Watson, A.; Machiraju, D.; Enk, A.; Holland, C.; Abdullah, S.E.; Benlahrech, A.; Orloff, M.; Nathan, P.; et al. Tebentafusp Induces a T-Cell-Driven Rash in Melanocyte-Bearing Skin as an Adverse Event Consistent with the Mechanism of Action. J. Investig. Dermatol. 2025, 145, 559–572.e559. [Google Scholar] [CrossRef]
- Vitek, L.; Goronflot, T.; Dutriaux, C.; Deleuze, A.; Le Corre, Y.; Duval-Modeste, A.B.; Fresnard, C.; Jeudy, G.; Lamoureux, A.; Gaudy-Marqueste, C.; et al. Efficacy and Tolerability of Tebentafusp in Metastatic Uveal Melanoma: A Real-life Retrospective Multicentre Study. Acta Derm. Venereol. 2024, 104, adv41297. [Google Scholar] [CrossRef]
- Kim, W.S.; Shortt, J.; Zinzani, P.L.; Mikhailova, N.; Radeski, D.; Ribrag, V.; Domingo Domenech, E.; Sawas, A.; Alexis, K.; Emig, M.; et al. A Phase II Study of Acimtamig (AFM13) in Patients with CD30-Positive, Relapsed, or Refractory Peripheral T-cell Lymphomas. Clin. Cancer Res. 2025, 31, 65–73. [Google Scholar] [CrossRef]
- Shang, J.; Hu, S.; Wang, X. Targeting natural killer cells: From basic biology to clinical application in hematologic malignancies. Exp. Hematol. Oncol. 2024, 13, 21. [Google Scholar] [CrossRef]
- Zhu, A.; Bai, Y.; Nan, Y.; Ju, D. Natural killer cell engagers: From bi-specific to tri-specific and tetra-specific engagers for enhanced cancer immunotherapy. Clin. Transl. Med. 2024, 14, e70046. [Google Scholar] [CrossRef]
- Khoshtinat Nikkhoi, S.; Yang, G.; Owji, H.; Grizotte-Lake, M.; Cohen, R.I.; Gil Gonzalez, L.; Massumi, M.; Hatefi, A. Bispecific immune cell engager enhances the anticancer activity of CD16+ NK cells and macrophages in vitro, and eliminates cancer metastasis in NK humanized NOG mice. J. Immunother. Cancer 2024, 12, e008295. [Google Scholar] [CrossRef]
- Nikkhoi, S.K.; Li, G.; Eleya, S.; Yang, G.; Vandavasi, V.G.; Hatefi, A. Bispecific killer cell engager with high affinity and specificity toward CD16a on NK cells for cancer immunotherapy. Front. Immunol. 2022, 13, 1039969. [Google Scholar] [CrossRef]
- Huang, S.; van Duijnhoven, S.M.J.; Sijts, A.; van Elsas, A. Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J. Cancer Res. Clin. Oncol. 2020, 146, 3111–3122. [Google Scholar] [CrossRef]
- Nie, S.; Wang, Z.; Moscoso-Castro, M.; D’Souza, P.; Lei, C.; Xu, J.; Gu, J. Biology drives the discovery of bispecific antibodies as innovative therapeutics. Antib. Ther. 2020, 3, 18–62. [Google Scholar] [CrossRef]
- Li, C.L.; Ma, X.Y.; Yi, P. Bispecific Antibodies, Immune Checkpoint Inhibitors, and Antibody-Drug Conjugates Directing Antitumor Immune Responses: Challenges and Prospects. Cell Biochem. Funct. 2024, 42, e70011. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Yu, L.; Sun, M.; Zhang, Q.; Zhou, Q.; Wang, Y. Harnessing the immune system by targeting immune checkpoints: Providing new hope for Oncotherapy. Front. Immunol. 2022, 13, 982026. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Qian, H.; Shi, Y.; Tao, Z. CD8(+) T cell-based cancer immunotherapy. J. Transl. Med. 2024, 22, 394. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, Q. Clinical Progresses and Challenges of Bispecific Antibodies for the Treatment of Solid Tumors. Mol. Diagn. Ther. 2024, 28, 669–702. [Google Scholar] [CrossRef]
- Herrera, M.; Pretelli, G.; Desai, J.; Garralda, E.; Siu, L.L.; Steiner, T.M.; Au, L. Bispecific antibodies: Advancing precision oncology. Trends Cancer 2024, 10, 893–919. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef]
- Kang, J.; Sun, T.; Zhang, Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front. Immunol. 2022, 13, 1020003. [Google Scholar] [CrossRef]
- Attia, M.S.; Kijanka, G.; Nguyen, N.T.; Zhang, J.; An, H. Advances and prospects of RNA delivery nanoplatforms for cancer therapy. Acta Pharm. Sin. B 2025, 15, 52–96. [Google Scholar] [CrossRef]
- Xue, F.; Yao, H.; Cui, L.; Huang, Y.; Shao, C.; Shen, N.; Hu, J.; Tang, Z.; Chen, X. An Fc Binding Peptide-Based Facile and Versatile Build Platform for Multispecific Antibodies. Nano Lett. 2023, 23, 4191–4200. [Google Scholar] [CrossRef]
- Ai, Z.; Wang, B.; Song, Y.; Cheng, P.; Liu, X.; Sun, P. Prodrug-based bispecific antibodies for cancer therapy: Advances and future directions. Front. Immunol. 2025, 16, 1523693. [Google Scholar] [CrossRef]
- Maali, A.; Gholizadeh, M.; Feghhi-Najafabadi, S.; Noei, A.; Seyed-Motahari, S.S.; Mansoori, S.; Sharifzadeh, Z. Nanobodies in cell-mediated immunotherapy: On the road to fight cancer. Front. Immunol. 2023, 14, 1012841. [Google Scholar] [CrossRef]
- Muyldermans, S. A guide to: Generation and design of nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Xie, A.; Liu, W.; He, Z.; Wan, Y.; Mao, W. Unveiling the new chapter in nanobody engineering: Advances in traditional construction and AI-driven optimization. J. Nanobiotechnol. 2025, 23, 87. [Google Scholar] [CrossRef]
- Hosseininejad-Chafi, M.; Eftekhari, Z.; Oghalaie, A.; Behdani, M.; Sotoudeh, N.; Kazemi-Lomedasht, F. Nanobodies as innovative immune checkpoint modulators: Advancing cancer immunotherapy. Med. Oncol. 2024, 42, 36. [Google Scholar] [CrossRef]
- Brytan, W.; Padrela, L. Structural modifications for the conversion of proteins and peptides into stable dried powder formulations: A review. J. Drug Deliv. Sci. Technol. 2023, 89, 104992. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; Aa, F.V.; et al. Phase I Trial of (131)I-GMIB-Anti-HER2-VHH1, a New Promising Candidate for HER2-Targeted Radionuclide Therapy in Breast Cancer Patients. J. Nucl. Med. 2021, 62, 1097–1105. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Liu, T.; Li, L.; Zhou, N.; Ma, X.; Meng, X.; Liu, J.; Zhu, H.; Jia, B.; et al. High in-vivo stability in preclinical and first-in-human experiments with [(18)F]AlF-RESCA-MIRC213: A (18)F-labeled nanobody as PET radiotracer for diagnosis of HER2-positive cancers. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 302–313. [Google Scholar] [CrossRef]
- Zhao, L.; Gong, J.; Liao, S.; Huang, W.; Zhao, J.; Xing, Y. Preclinical evaluation and preliminary clinical study of (68)Ga-NODAGA-NM-01 for PET imaging of PD-L1 expression. Cancer Imaging 2025, 25, 6. [Google Scholar] [CrossRef]
- Bai, Z.; Xie, X.; Li, C.; Wang, Y.; Wang, Y.; Li, H.; Gao, R.; Jia, B. Claudin18.2-Targeted SPECT/CT Imaging for Gastric Cancer: Preclinical Evaluation and Clinical Translation of the (99m)Tc-Labeled Nanobody (PHG102) Radiotracer. ACS Pharmacol. Transl. Sci. 2024, 7, 2465–2475. [Google Scholar] [CrossRef]
- Broos, K.; Lecocq, Q.; Xavier, C.; Bridoux, J.; Nguyen, T.T.; Corthals, J.; Schoonooghe, S.; Lion, E.; Raes, G.; Keyaerts, M.; et al. Evaluating a Single Domain Antibody Targeting Human PD-L1 as a Nuclear Imaging and Therapeutic Agent. Cancers 2019, 11, 872. [Google Scholar] [CrossRef]
- Sun, Y.; Hao, Z.; Gao, H.; Yang, G.; Pan, B.; Zhu, M.; Wan, Y.; Shi, J.; Huo, L.; Chen, H.; et al. [(99m)Tc]Tc-MY6349 Probe for Trop2-Targeted SPECT Imaging: From Preclinical to Pilot Clinical Study. J. Nucl. Med. 2025, 66, 543–551. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, R.; Shi, G.; Jiang, Y.; Li, Z.; Xu, X.; Ma, J.; Huang, J.; Fu, C.; Zhou, H.; et al. Cadherin 17 Nanobody-Mediated Near-Infrared-II Fluorescence Imaging-Guided Surgery and Immunotoxin Delivery for Colorectal Cancer. Biomater. Res. 2024, 28, 0041. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Liu, A.; Hou, X.; Lai, Z.; Li, J.; Yang, N.; Tan, J.; Mo, F.; Hu, Z.; Yang, X.; et al. Screening and antitumor effect of an anti-CTLA-4 nanobody. Oncol. Rep. 2018, 39, 511–518. [Google Scholar] [CrossRef]
- Blanchetot, C.; Verzijl, D.; Mujic-Delic, A.; Bosch, L.; Rem, L.; Leurs, R.; Verrips, C.T.; Saunders, M.; de Haard, H.; Smit, M.J. Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function. J. Biol. Chem. 2013, 288, 25173–25182. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, K.; Amjad Zanjani, F.S.; Hagh, A.G.; Azizi, M.D.; Rasouli, S.J.; Kashanian, S. Efficient growth inhibition of EGFR over-expressing tumor cells by an anti-EGFR nanobody. Mol. Biol. Rep. 2013, 40, 6737–6745. [Google Scholar] [CrossRef]
- Behdani, M.; Zeinali, S.; Khanahmad, H.; Karimipour, M.; Asadzadeh, N.; Azadmanesh, K.; Khabiri, A.; Schoonooghe, S.; Habibi Anbouhi, M.; Hassanzadeh-Ghassabeh, G.; et al. Generation and characterization of a functional Nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol. Immunol. 2012, 50, 35–41. [Google Scholar] [CrossRef]
- Verhaar, E.R.; Woodham, A.W.; Ploegh, H.L. Nanobodies in cancer. Semin. Immunol. 2021, 52, 101425. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.M.; Kim, S.J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Che, Z.; Wang, W.; Zhang, L.; Lin, Z. Therapeutic strategies targeting CD47-SIRPα signaling pathway in gastrointestinal cancers treatment. J. Pharm. Anal. 2025, 15, 101099. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.-A.; Han, H.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Goncalves, M.O.; Di Iorio, J.F.; Marin, G.V.; Meneghetti, P.; Negreiros, N.G.S.; Torrecilhas, A.C. Extracellular vesicles. Curr. Top. Membr. 2024, 94, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Islam, S.U.; Shahzad, R.; Khan, S.; Lee, Y.S. Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol. Ther. 2021, 223, 107806. [Google Scholar] [CrossRef] [PubMed]
- Radler, J.; Gupta, D.; Zickler, A.; Andaloussi, S.E. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol. Ther. 2023, 31, 1231–1250. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, L.; Razeghian-Jahromi, I.; Zarei-Behjani, Z.; Bagheri, Z.; Razmkhah, M. Composition, Biogenesis, and Role of Exosomes in Tumor Development. Stem Cells Int. 2022, 2022, 8392509. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Akbari, A.; Nazari-Khanamiri, F.; Ahmadi, M.; Shoaran, M.; Rezaie, J. Engineered Exosomes for Tumor-Targeted Drug Delivery: A Focus on Genetic and Chemical Functionalization. Pharmaceutics 2022, 15, 66. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, H.; He, Z.; Chen, A.; Cheng, P. Extracellular vesicles as an emerging drug delivery system for cancer treatment: Current strategies and recent advances. Biomed. Pharmacother. 2022, 153, 113480. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, D.; Pang, L.; Liu, S. Extracellular vesicles for cancer therapy: Potential, progress, and clinical challenges. Front. Bioeng. Biotechnol. 2024, 12, 1476737. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Hanachi, P.; Montaseri, A. Extracellular vesicles of immune cells; immunomodulatory impacts and therapeutic potentials. Clin. Immunol. 2023, 248, 109237. [Google Scholar] [CrossRef]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune cells-derived exosomes function as a double-edged sword: Role in disease progression and their therapeutic applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal 2022, 20, 69. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Babaker, M.A.; Aljoud, F.A.; Alkhilaiwi, F.; Algarni, A.; Ahmed, A.; Khan, M.I.; Saadeldin, I.M.; Alzahrani, F.A. The Therapeutic Potential of Milk Extracellular Vesicles on Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 6812. [Google Scholar] [CrossRef]
- Mecocci, S.; Trabalza-Marinucci, M.; Cappelli, K. Extracellular Vesicles from Animal Milk: Great Potentialities and Critical Issues. Animals 2022, 12, 3231. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef]
- Fan, X.; Fang, S.-B.; Fu, Q.-L. Extracellular Vesicle-Based Clinical Trials. In Extracellular Vesicles: From Bench to Bedside; Wang, Q., Zheng, L., Eds.; Springer Nature: Singapore, 2024; pp. 541–553. [Google Scholar]
- Van Delen, M.; Derdelinckx, J.; Wouters, K.; Nelissen, I.; Cools, N. A systematic review and meta-analysis of clinical trials assessing safety and efficacy of human extracellular vesicle-based therapy. J. Extracell. Vesicles 2024, 13, e12458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Gu, Y.; Zhu, Z.; Zhang, H.; Liu, W.; Xu, B.; Zhou, F.; Zhang, M.; Hua, K.; Wu, L.; et al. Exosome Mediated Cytosolic Cisplatin Delivery Through Clathrin-Independent Endocytosis and Enhanced Anti-cancer Effect via Avoiding Endosome Trapping in Cisplatin-Resistant Ovarian Cancer. Front. Med. 2022, 9, 810761. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Smaglo, B.G.; Mahadevan, K.K.; Kirtley, M.L.; McAndrews, K.M.; Mendt, M.; Yang, S.; Maldonado, A.S.; Sugimoto, H.; Salvatierra, M.E.; et al. KRAS (G12D) -Specific Targeting with Engineered Exosomes Reprograms the Immune Microenvironment to Enable Efficacy of Immune Checkpoint Therapy in PDAC Patients. medRxiv 2025. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Zhou, N.; Xu, P.; Wang, J.; Gao, Y.; Jin, X.; Liang, X.; Lv, J.; Zhang, Y.; et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat. Biomed. Eng. 2020, 4, 743–753. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Massaro, C.; Min, W.; Pegtel, D.M.; Baglio, S.R. Harnessing EV communication to restore antitumor immunity. Adv. Drug Deliv. Rev. 2021, 176, 113838. [Google Scholar] [CrossRef]
- Wang, X.; Yin, Z.; Liu, N.; Zhang, H.; Wang, Z.; Che, J.; Li, J.; Zheng, A. Research progress on nanobodies in the diagnosis and treatment of natural toxin poisoning. Nano Today 2025, 62, 102711. [Google Scholar] [CrossRef]
- Chen, J.; Tan, Q.; Yang, Z.; Jin, Y. Engineered extracellular vesicles: Potentials in cancer combination therapy. J. Nanobiotechnol. 2022, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, C.; Xiao, K. Engineered extracellular vesicles-like biomimetic nanoparticles as an emerging platform for targeted cancer therapy. J. Nanobiotechnol. 2023, 21, 287. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Zhang, Y. Reprogramming Exosomes for Immunotherapy. Methods Mol. Biol. 2020, 2097, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Mamand, D.R.; Mohammad, D.K.; Zheng, W.; Jawad Wiklander, R.; Sych, T.; Zickler, A.M.; Liang, X.; Sharma, H.; Lavado, A.; et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat. Biomed. Eng. 2024, 8, 1453–1468. [Google Scholar] [CrossRef]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, L.; Cao, Y.; Liu, Z.; Wang, Y.; Cheng, H.; Feng, Y.; Yao, S.; Yin, Y.; Wu, Z.; et al. Reprogramming tumor-associated macrophages by a dually targeted milk exosome system as a potent monotherapy for cancer. J. Control Release 2024, 366, 395–409. [Google Scholar] [CrossRef]
- Zhang, R.; Li, D.; Zhou, Z.; Hong, H.; Shi, J.; Wu, Z. Chemo-Enzymatic Functionalization of Bovine Milk Exosomes with an EGFR Nanobody for Target-specific Drug Delivery. Chembiochem 2024, 25, e202400512. [Google Scholar] [CrossRef]
- Broer, L.N.; Knapen, D.G.; de Groot, D.A.; Mol, P.G.M.; Kosterink, J.G.W.; de Vries, E.G.E.; Lub-de Hooge, M.N. Monoclonal antibody biosimilars for cancer treatment. iScience 2024, 27, 110115. [Google Scholar] [CrossRef]
- Kumari, S.; Raj, S.; Babu, M.A.; Bhatti, G.K.; Bhatti, J.S. Antibody-drug conjugates in cancer therapy: Innovations, challenges, and future directions. Arch. Pharm. Res. 2024, 47, 40–65. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Z.; Shao, L.; Kong, X.; Hou, X.; Tian, D.; Sun, Y.; Xiao, Y.; Yu, L. Nanobody-derived nanobiotechnology tool kits for diverse biomedical and biotechnology applications. Int. J. Nanomed. 2016, 11, 3287–3303. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gainkam, L.O.T.; Caveliers, V.; Vanhove, C.; Keyaerts, M.; De Baetselier, P.; Bossuyt, A.; Revets, H.; Lahoutte, T. SPECT imaging with 99m Tc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression. Mol. Imaging Biol. 2008, 10, 167–175. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.; Jin, D.; Liu, Y. Nanobody—A versatile tool for cancer diagnosis and therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1697. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Li, K.; Li, H.; Lv, G.; Lin, J.; Qiu, L. Immuno-PET imaging of 68 Ga-labeled nanobody Nb109 for dynamic monitoring the PD-L1 expression in cancers. Cancer Immunol. Immunother. 2021, 70, 1721–1733. [Google Scholar] [CrossRef]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical Translation of Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, e2301010. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.S.; Jiang, W. Engineering therapeutical extracellular vesicles for clinical translation. Trends Biotechnol. 2025, 43, 61–82. [Google Scholar] [CrossRef]
- Mizenko, R.R.; Brostoff, T.; Rojalin, T.; Koster, H.J.; Swindell, H.S.; Leiserowitz, G.S.; Wang, A.; Carney, R.P. Tetraspanins are unevenly distributed across single extracellular vesicles and bias sensitivity to multiplexed cancer biomarkers. J. Nanobiotechnol. 2021, 19, 250. [Google Scholar] [CrossRef]
- Han, C.; Kang, H.; Yi, J.; Kang, M.; Lee, H.; Kwon, Y.; Jung, J.; Lee, J.; Park, J. Single-vesicle imaging and co-localization analysis for tetraspanin profiling of individual extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12047. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, L.; Gong, M.; Su, G.; Zhu, S.; Zhang, W.; Wang, S.; Li, Z.; Chen, C.; Li, L. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano 2018, 12, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Görgens, A.; Bremer, M.; Ferrer-Tur, R.; Murke, F.; Tertel, T.; Horn, P.A.; Thalmann, S.; Welsh, J.A.; Probst, C.; Guerin, C. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J. Extracell. Vesicles 2019, 8, 1587567. [Google Scholar] [CrossRef]
- Hung, M.E.; Lenzini, S.B.; Stranford, D.M.; Leonard, J.N. Enrichment of extracellular vesicle subpopulations via affinity chromatography. Extracell. RNA Methods Protoc. 2018, 1740, 109–124. [Google Scholar]
| BsAbs Mechanism of Action | Name | Target 1 | Target 2 | Application | Phase | Reference |
|---|---|---|---|---|---|---|
| Blocking two pathways | Amivantamab | EGFR | MET | NSCLC with EGFR mutations | Approved | [33] |
| Vanucizumab | VEGF | Ang-2 | Colorectal and metastatic cancers | Phase I/II | [34] | |
| Navicixizumab | DLL4 | Ovarian cancer | Phase Ib | [35] | ||
| Zanidatamab | HER2 | HER2 | Solid tumors | Phase II | [36] | |
| Against immune checkpoints | Tebotelimab | PD-1 | LAG-3 | Multiple cancer types | Phase II/III | [37,38] |
| IBI318 | PD-L1 | NK/T-cell lymphoma | Phase I/II | [39] | ||
| Cadonilimab | CTLA-4 | Adenocarcinoma | Phase II | [40] | ||
| NSCLC resistant anti-PD-1/PD-L1 | Phase Ib/II | [41] | ||||
| Ivonescimab | VEGF | Advanced NSCLC | Phase I/II | [42,43] | ||
| Activate the immune system | Blinatumomab | CD3 | CD19 | R/R Ph −/+ B-ALL | Approved | [16,44,45] |
| NHL | ||||||
| Glofitamab | CD20 | R/R DLBCL | Approved | [46] | ||
| R/R MCL | Phase I | [47] | ||||
| Teclistamab | BCMA | Follicular NHL and MM | Approved | [48] | ||
| Catumaxomab | EpCAM | Intraperitoneal malignant ascites | Approved | [49,50] | ||
| Tebentafusp | HLA-A*02:01 | Uveal melanoma | Approved | [51,52] | ||
| Acimtamig | CD16a | CD30 | R/R PTCL | Phase II | [53,54,55] | |
| E5C1 | HER2 | Ovarian metastatic cancer | Preclinical | [56,57] |
| Advantages | Disadvantages |
|---|---|
| High specificity | Some immunogenic |
| One molecule, two targets | Off-target effects |
| Block surface receptors | High production costs |
| Recruit cells | Poor tumor infiltration and penetration |
| Better than mAbs | Complex and huge |
| Activate and potentiate the immune system | Difficult cross-biological barriers |
| Nbs Use | Name | Target | Application | Phase | Reference |
|---|---|---|---|---|---|
| For imaging and diagnosis | 131I-GMIB | HER2 | PET | Phase I | [76] |
| 18F-AlF-RESCA-MIRC213 | Preclinical | [77] | |||
| 68Ga-NODAGA-NM-01 | PD-L1 | Phase I | [78] | ||
| 99mTc-PHG102 | CLDN18.2 | SPECT | Preclinical | [79] | |
| 99mTc-MY6349 | Trop2 | Phase I | [80] | ||
| 99mTc-K2 | PD-L1 | Preclinical | [81] | ||
| E8-IR800CW | CDH17 | NIR | Preclinical | [82] | |
| For cancer treatment | KN035 | PD-L1 | Solid tumors | Phase I/II | [83] |
| Nb16 | CTLA-4 | Melanoma | Preclinical | [84] | |
| 12A4 | CXCL12 | Pre-B lymphoma | Preclinical | [85] | |
| OA-cb6 | EGFR | Epithelial cancers | Preclinical | [86] | |
| 3VGR19 | VEGFR | Angiogenesis in solid tumors | Preclinical | [87] |
| Advantages | Disadvantages |
|---|---|
| Low size | Short half-life time |
| High affinity, stability, solubility | Low biodisponibility |
| Low immunogenicity | Kidney accumulation |
| Low production costs | Some off-target effects |
| Well tumor infiltration and penetration | Immunogenicity |
| Unique physico-chemical properties | Treatment resistance |
| Easily modified | Difficulties crossing biological barriers |
| EVs’ Source | EV Engineering | Application | Phase | Reference |
|---|---|---|---|---|
| Fresh milk-derived exosomes | Yes, loaded with cisplatin | Resistant ovarian carcinoma | Preclinical | [114] |
| Ascite-derived exosomes | No | Colorectal cancer | Phase I | [115] |
| DC-derived exosomes | Yes, tumor antigen-loaded | NSCLC | Phase II | [116] |
| MVs from tumor cells | Yes, MTX packaging | Cholangiocarcinoma | Phase I | [118] |
| Plant exosomes | Yes, loaded with curcumin | Colon cancer | Phase I | [NCT01294072] |
| MSC-derived exosomes | Yes, KrasG12D siRNA added | Metastatic pancreatic cancer | Phase I | [117] |
| Grape-derived exosomes | No | Head and neck cancer | Phase I | [NCT01668849] |
| Advantages | Disadvantages |
|---|---|
| Biological origin, high biocompatible | Heterogeneity between EVs |
| Easily modified and functionalized | Source of EVs is relevant |
| Able to cross biological barriers | Production scaling-up |
| Different source available | Standardization methods for production |
| Natural delivery system | Loading is complex |
| High stability | Modifications may affect integrity |
| Low immunogenicity |
| BsAbs | Nbs | EVs | |
|---|---|---|---|
| Size | 150 kDa or 14 × 8 nm | 15 kDa or 4 × 2.5 nm | 50–500 nm |
| Origin | Usually, animals | Camelids and fishes | Plants and animal |
| Production complexity | Medium | Medium | High |
| Immunogenicity | High | Low | The lowest |
| Half-life | Medium | Low | High |
| Tumor penetration | Low | High | Medium |
| Production costs | High | Low | Medium |
| Stability | Medium | High | High |
| Off-target effects | Yes | Yes | Yes, but low |
| Cross biological barriers | Poor | Not easily | Easily |
| Activity | Intrinsic | Intrinsic | Not always |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizama-Muñoz, A.; Plaza-Diaz, J. Bispecific Antibodies, Nanobodies and Extracellular Vesicles: Present and Future to Cancer Target Therapy. Biomolecules 2025, 15, 639. https://doi.org/10.3390/biom15050639
Lizama-Muñoz A, Plaza-Diaz J. Bispecific Antibodies, Nanobodies and Extracellular Vesicles: Present and Future to Cancer Target Therapy. Biomolecules. 2025; 15(5):639. https://doi.org/10.3390/biom15050639
Chicago/Turabian StyleLizama-Muñoz, Asier, and Julio Plaza-Diaz. 2025. "Bispecific Antibodies, Nanobodies and Extracellular Vesicles: Present and Future to Cancer Target Therapy" Biomolecules 15, no. 5: 639. https://doi.org/10.3390/biom15050639
APA StyleLizama-Muñoz, A., & Plaza-Diaz, J. (2025). Bispecific Antibodies, Nanobodies and Extracellular Vesicles: Present and Future to Cancer Target Therapy. Biomolecules, 15(5), 639. https://doi.org/10.3390/biom15050639







