Baicalin Relieves Glaesserella parasuis-Triggered Immunosuppression Through Polarization via MIF/CD74 Signaling Pathway in Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacteria and Drugs
2.3. Design of the Experiment
2.4. RT-PCR
2.5. Determination of Baicalin on CD3 Expression by Immunofluorescence
2.6. Detection of CD74 Expression by Immunohistochemistry
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
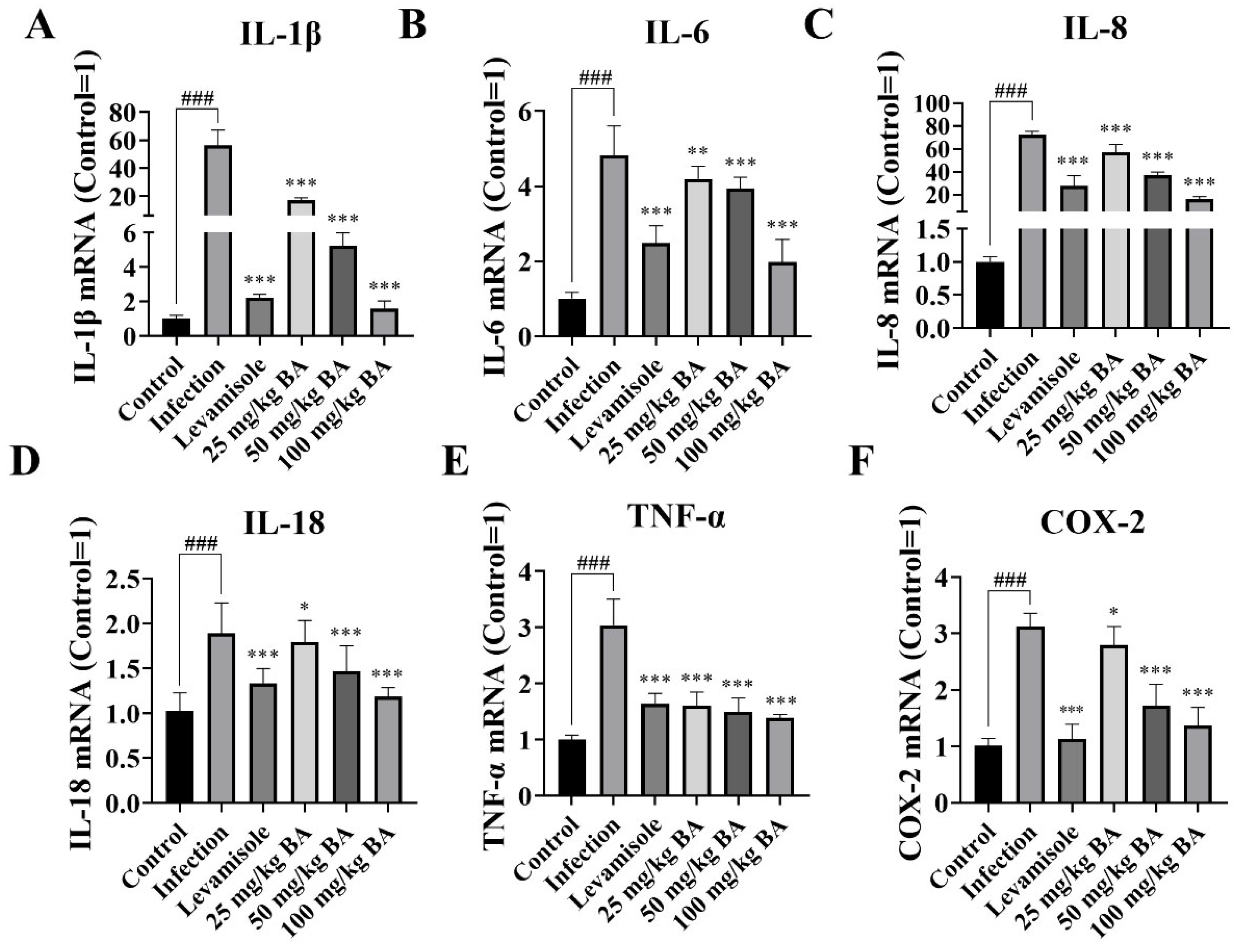
3.1. Baicalin Inhibited IL-1β, IL-6, IL-8, IL-18, TNF-α, and COX-2 Expression in Piglet Spleen Triggered by G. parasuis
3.2. Baicalin Improved MIF/CD74 Axis Levels in Piglet Spleen Elicited by G. parasuis
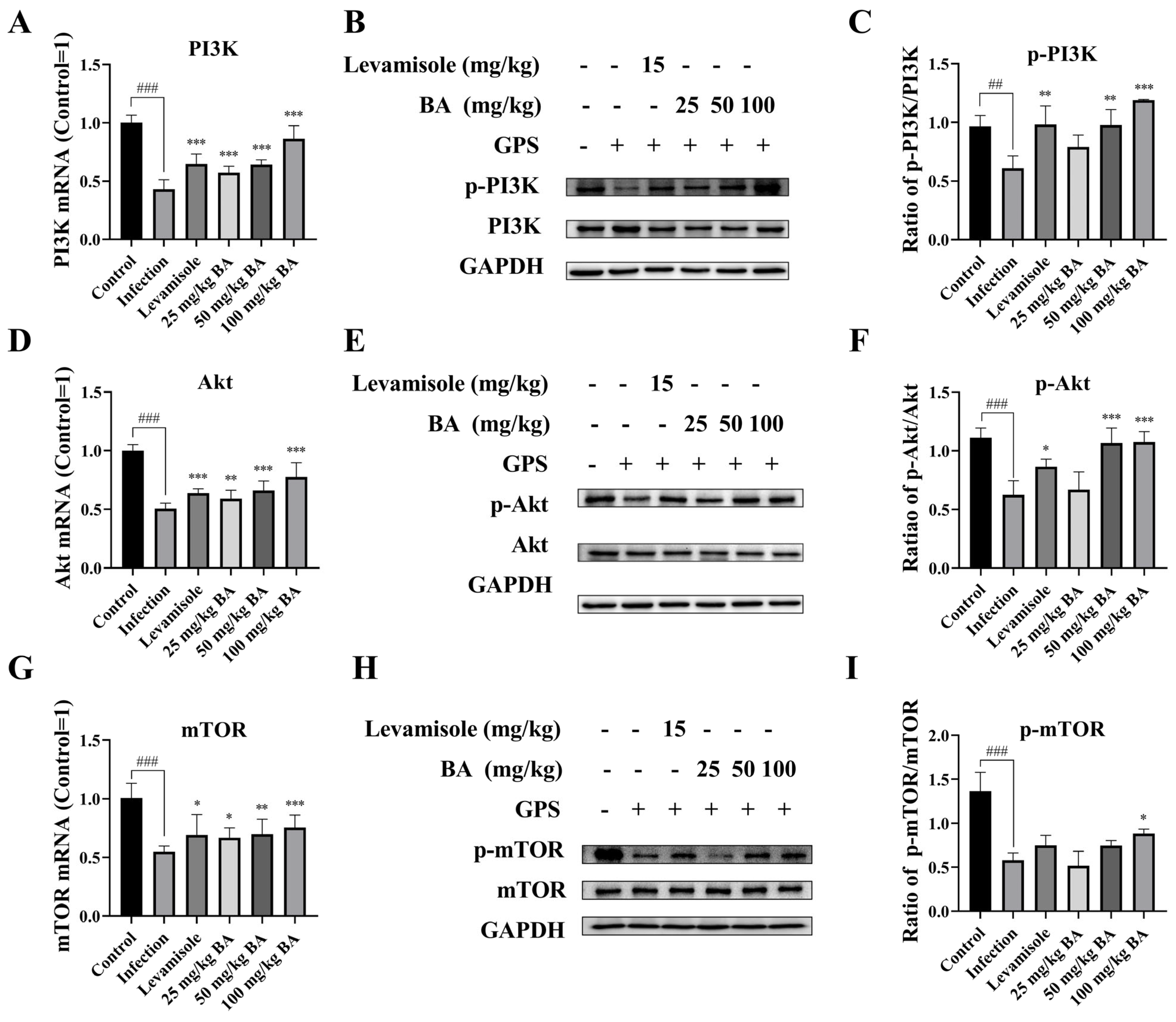
3.3. Baicalin Promoted PI3K/Akt/mTOR Pathway Activation in G. parasuis Triggered Piglet Spleen
3.4. Baicalin Regulated the RAF/MEK/ERK Signaling Activation in G. parasuis Triggered Piglet Spleen
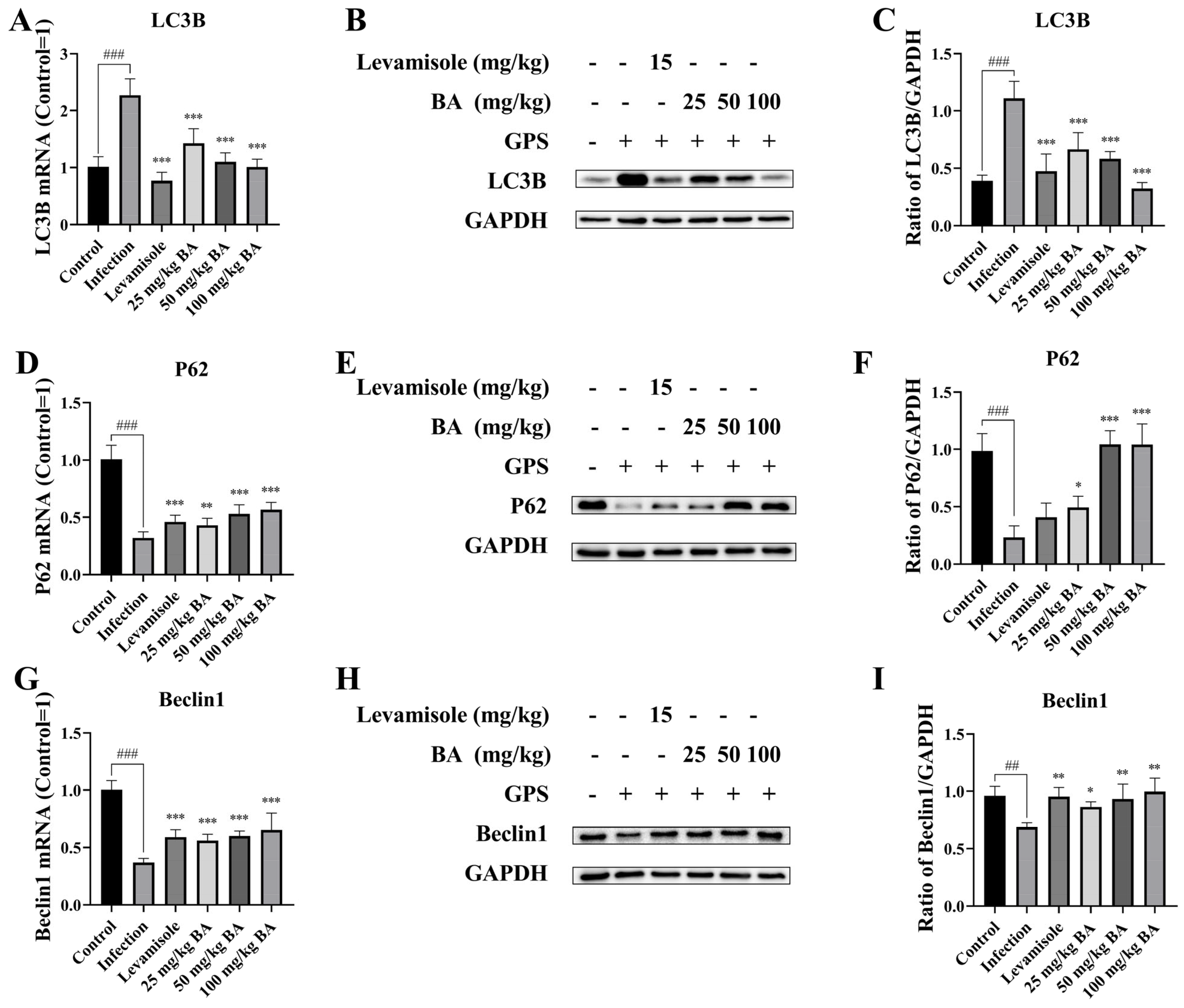
3.5. Baicalin Modified Autophagy-Related Proteins Beclin-1, P62, and LC3B Expression in G. parasuis-Triggered Piglet Spleens
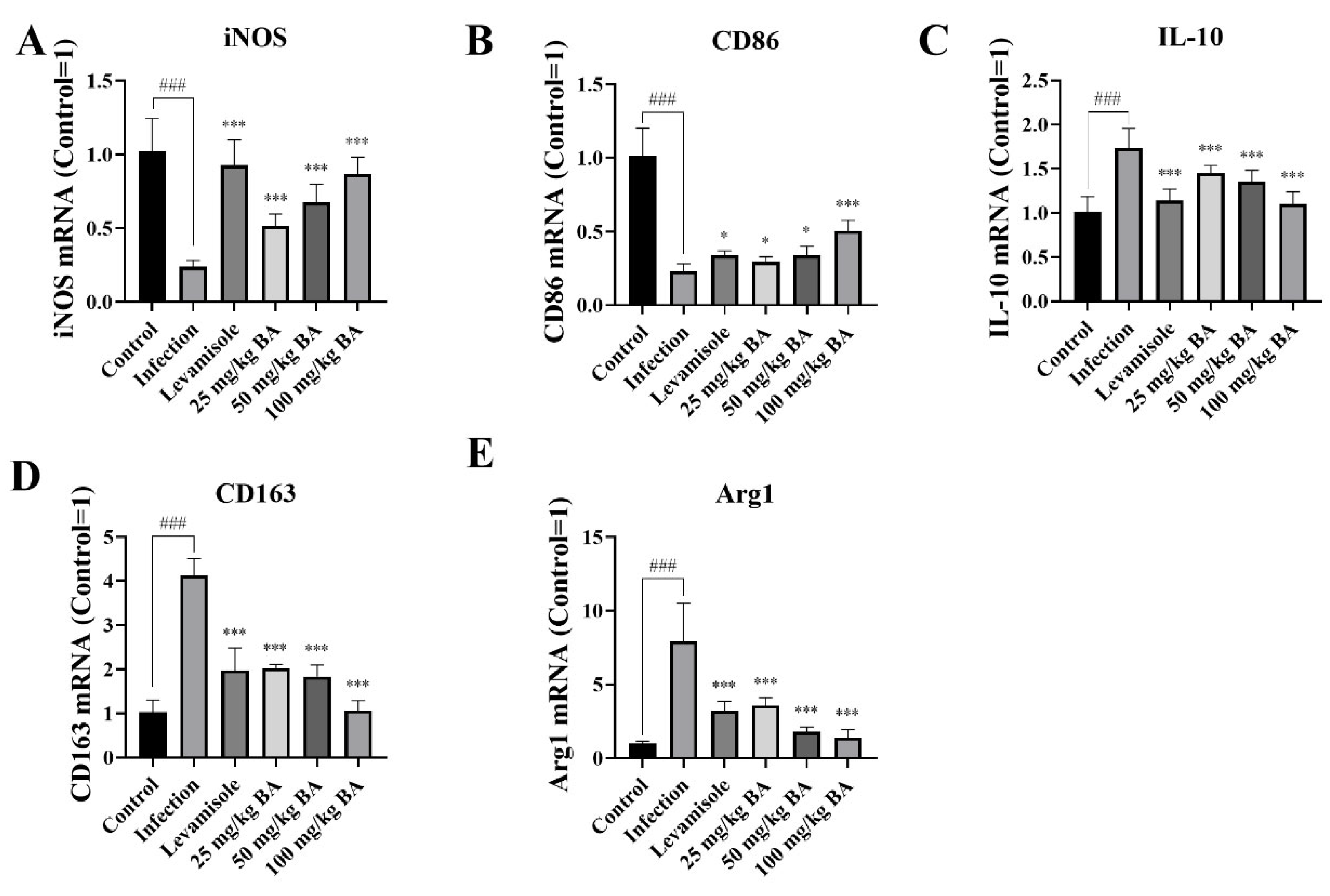
3.6. Baicalin and Levamisole Promoted M2 Polarization to M1 Polarization in G. parasuis-Triggered Piglet Spleens
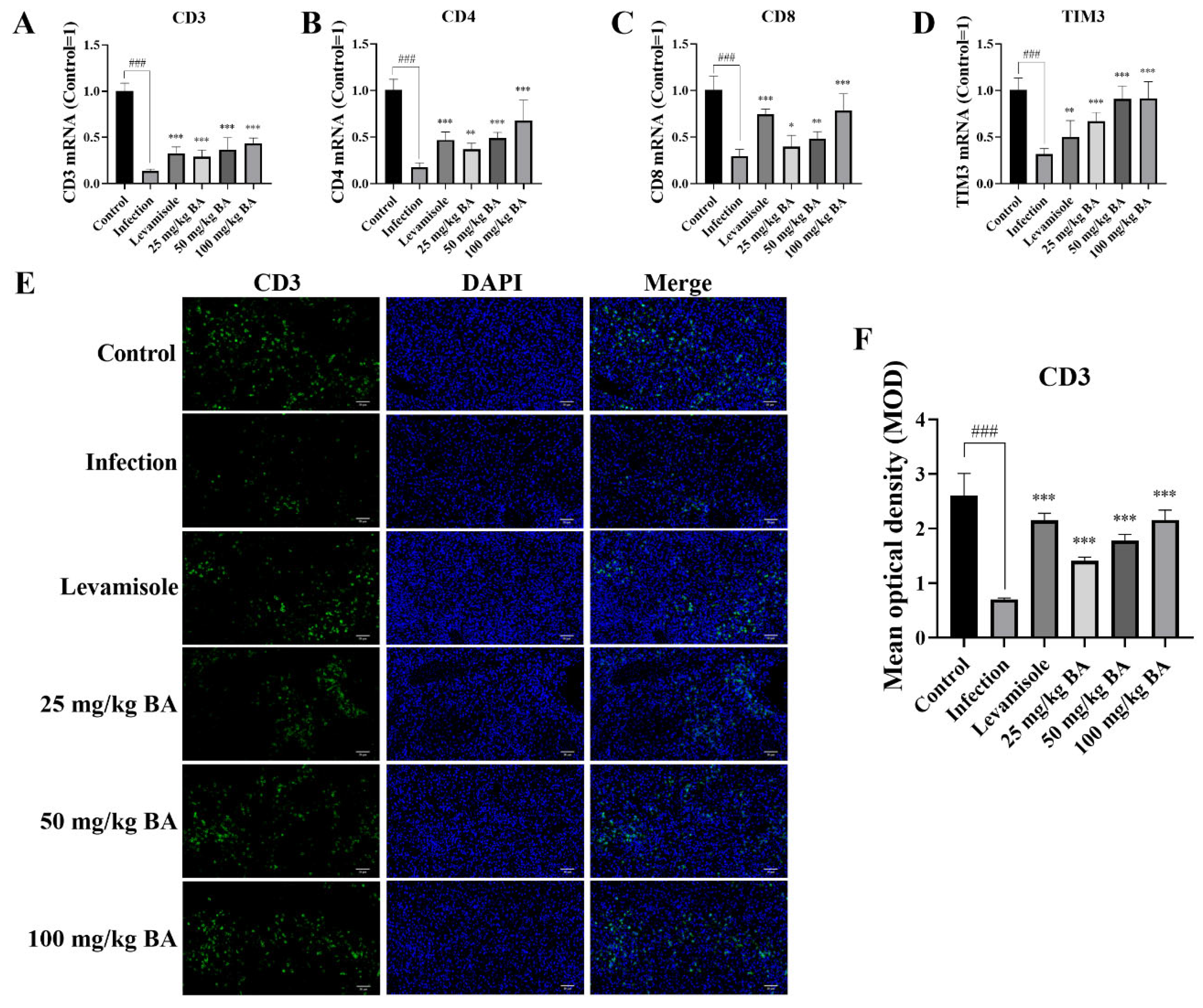
3.7. Baicalin Enhanced CD3, CD4, CD8, and TIM3 Levels in G. parasuis-Triggered Piglet Spleens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Zhu, N.; Liu, J.; Wen, S.; Xu, Y.; Xu, X.; Cai, X. The role of cytolethal distending toxin in Glaesserella parasuis JS0135 strain infection: Cytotoxicity, phagocytic resistance and pathogenicity. Vet. Microbiol. 2024, 295, 110168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, D.; Yao, X.; Luo, Y.; Yang, Z.; Ren, M.; Zhang, G.; Yu, Y.; Lu, A.; Wang, Y. Pan-genome wide association study of Glaesserella parasuis highlights genes associated with virulence and biofilm formation. Front. Microbiol. 2023, 14, 1160433. [Google Scholar] [CrossRef]
- Wu, C.F.; Hsu, C.Y.; Chou, C.C.; Wang, C.M.; Huang, S.W.; Kuo, H.C. Serotypes, virulence factors and multilocus sequence typing of Glaesserella parasuis from diseased pigs in Taiwan. PeerJ 2023, 11, e15823. [Google Scholar] [CrossRef]
- Macedo, N.; Gottschalk, M.; Strutzberg-Minder, K.; Van, C.N.; Zhang, L.; Zou, G.; Zhou, R.; Marostica, T.; Clavijo, M.J.; Tucker, A.; et al. Molecular characterization of Glaesserella parasuis strains isolated from North America, Europe and Asia by serotyping PCR and LS-PCR. Vet. Res. 2021, 52, 68. [Google Scholar] [CrossRef]
- Mao, W.; Wang, Z.; Wen, S.; Lin, Y.; Gu, J.; Sun, J.; Wang, H.; Cao, Q.; Xu, Y.; Xu, X.; et al. LRRC8A promotes Glaesserella parasuis cytolethal distending toxin-induced p53-dependent apoptosis in NPTr cells. Virulence 2023, 14, 2287339. [Google Scholar] [CrossRef]
- Liu, F.; Gao, Y.; Jiao, J.; Zhang, Y.; Li, J.; Ding, L.; Zhang, L.; Chen, Z.; Song, X.; Yang, G.; et al. Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation. Cells 2023, 12, 751. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, F.; Peng, J.; Zhao, D.; Xu, L.; Li, H.; Ma, S.; Peng, X.; Sheng, X.; Sun, Y.; et al. Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance. Cell Rep. Med. 2024, 5, 101686. [Google Scholar] [CrossRef]
- Silva, G.F.R.; Moreno, L.Z.; Matajira, C.E.C.; Silva, A.P.S.; Araújo, K.M.; Gomes, V.T.M.; Barbosa, M.R.F.; Sato, M.I.Z.; Moreno, A.M. Serotyping and Antimicrobial Susceptibility Profiling of Glaesserella parasuis Isolated from Diseased Swine in Brazil. Pathogens. 2022, 11, 1443. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Dong, Q.; Fu, Y.; Sun, Y.; Luo, R.; Tian, X.; Guo, L.; Liu, W.; Qiu, Y.; et al. PD-1/PD-L1 axis induced host immunosuppression via PI3K/Akt/mTOR signalling pathway in piglets infected by Glaesserella parasuis. BMC Vet. Res. 2024, 20, 141. [Google Scholar] [CrossRef]
- Tanese, K.; Ogata, D. The role of macrophage migration inhibitory factor family and CD74 in the pathogenesis of melanoma. Exp. Dermatol. 2024, 33, e15122. [Google Scholar] [CrossRef]
- Hong, W.C.; Lee, D.E.; Kang, H.W.; Kim, M.J.; Kim, M.; Kim, J.H.; Fang, S.; Kim, H.J.; Park, J.S. CD74 Promotes a Pro-Inflammatory Tumor Microenvironment by Inducing S100A8 and S100A9 Secretion in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 12993. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, E.; Rodrigo Riestra, M.; Marziali, F.; Mena Osuna, R.; Denizeau, J.; Maurin, M.; Saez, J.J.; Jouve, M.; Bonté, P.E.; Richer, W.; et al. CD74 supports accumulation and function of regulatory T cells in tumors. Nat. Commun. 2024, 15, 3749. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, G.; Cajander, S.; Bäckman, A.; Källman, J.; Söderquist, B.; Strålin, K. Expression of HLA-DRA and CD74 mRNA in whole blood during the course of complicated and uncomplicated Staphylococcus aureus bacteremia. Microbiol. Immunol. 2017, 61, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Tang, J.; Zhao, L.; Ruze, A.; Shan, X.F.; Gao, X.M. The role of CD74 in cardiovascular disease. Front. Cardiovasc. Med. 2022, 9, 1049143. [Google Scholar] [CrossRef]
- Merk, M.; Mitchell, R.A.; Endres, S.; Bucala, R. D-dopachrome tautomerase (D-DT or MIF-2): Doubling the MIF cytokine family. Cytokine 2012, 59, 10–17. [Google Scholar] [CrossRef]
- Bach, J.P.; Rinn, B.; Meyer, B.; Dodel, R.; Bacher, M. Role of MIF in inflammation and tumorigenesis. Oncology 2008, 75, 127–133. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Rendon, B.E.; Lamont, G.; Kim, E.J.; Al Rayyan, N.; Richie, J.; Albeituni, S.; Waigel, S.; Wise, A.; Mitchell, R.A. MIF Is Necessary for Late-Stage Melanoma Patient MDSC Immune Suppression and Differentiation. Cancer Immunol. Res. 2016, 4, 101–112. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Deji, Z.; Li, C.; Chen, C.; Ding, W.; Du, P.; Wang, X. Infliximab modifies CD74-mediated lymphatic abnormalities and adipose tissue alterations in creeping fat of Crohn’s disease. Inflamm. Res. 2024, 73, 1157–1172. [Google Scholar] [CrossRef]
- Trifone, C.; Salido, J.; Ruiz, M.J.; Leng, L.; Quiroga, M.F.; Salomón, H.; Bucala, R.; Ghiglione, Y.; Turk, G. Interaction Between Macrophage Migration Inhibitory Factor and CD74 in Human Immunodeficiency Virus Type I Infected Primary Monocyte-Derived Macrophages Triggers the Production of Proinflammatory Mediators and Enhances Infection of Unactivated CD4+ T Cells. Front. Immunol. 2018, 9, 1494. [Google Scholar] [CrossRef]
- Figueiredo, C.R.; Azevedo, R.A.; Mousdell, S.; Resende-Lara, P.T.; Ireland, L.; Santos, A.; Girola, N.; Cunha, R.; Schmid, M.C.; Polonelli, L.; et al. Blockade of MIF-CD74 Signalling on Macrophages and Dendritic Cells Restores the Antitumour Immune Response Against Metastatic Melanoma. Front. Immunol. 2018, 9, 1132. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Ghazanfar, S.; Abdel-Hamid, M.; Abdel-Latif, H.M.R.; Zhang, Z.; Naiel, M.A.E. Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: An overview. Vet. Res. Commun. 2023, 47, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.H.; Rassouli, A.; Mirzaei, S.; Hashemi, F. The potential immunomodulatory effect of levamisole in humans and farm animals. J. Adv. Vet. Anim. Res. 2023, 10, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, M.; Xu, M.; Ji, X.; Zong, X.; Zhang, Z.; Shang, W.; Zhang, L.; Fang, P. Baicalin suppresses macrophage JNK-mediated adipose tissue inflammation to mitigate insulin resistance in obesity. J. Ethnopharmacol. 2024, 332, 118355. [Google Scholar] [CrossRef]
- Zhang, H.J.; Luo, J.Z.; Lan, C.L.; Teng, X.; Ge, B.; Liu, J.Q.; Xie, H.X.; Yang, K.J.; Qin, C.J.; Zhou, X.; et al. Baicalin protects against hepatocyte injury caused by aflatoxin B1 via the TP53-related ferroptosis Pathway. Ecotoxicol. Environ. Saf. 2024, 281, 116661. [Google Scholar] [CrossRef]
- Guo, L.; Yue, M.; Ma, C.; Wang, Y.; Hou, J.; Li, H. Baicalin reduces inflammation to inhibit lung cancer via targeting SOCS1/NF-κB/STAT3 axis. Heliyon 2024, 10, e29361. [Google Scholar] [CrossRef]
- Chen, X.; Ishfaq, M.; Wang, J. Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury via inhibiting STIM1-regulated ceramide accumulation in DF-1 cells. Poult. Sci. 2023, 102, 102687. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Jia, Q.; Zheng, L.; Tang, Q.; Sai, L.; Zhang, W.; Du, Z.; Peng, C.; Bo, C.; et al. Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting TLR4/NF-?B pathway in rats. Physiol. Res. 2023, 72, 221–233. [Google Scholar] [CrossRef]
- Zong, B.; Xiao, Y.; Wang, P.; Liu, W.; Ren, M.; Li, C.; Fu, S.; Zhang, Y.; Qiu, Y. Baicalin Weakens the Virulence of Porcine Extraintestinal Pathogenic Escherichia coli by Inhibiting the LuxS/AI-2 Quorum-Sensing System. Biomolecules 2024, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wang, N.; Wen, D.; Guo, P.; Liu, Y.; Fu, S.; Ye, C.; Wu, Z.; Qiu, Y. Baicalin attenuates lipopolysaccharide-induced intestinal inflammatory injury via suppressing PARP1-mediated NF-κB and NLRP3 signalling pathway. Toxicon 2024, 239, 107612. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Tian, X.; Peng, C.; Zhang, D.; Zhou, L.; Yuan, Y.; He, J.; Guo, L.; Qiu, Y.; Ye, C.; et al. Baicalin inhibited PANX-1/P2Y6 signaling pathway activation in porcine aortic vascular endothelial cells infected by Glaesserella parasuis. Heliyon 2024, 10, e23632. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yang, Y.; Yang, L.; Qian, Y.; Liang, M.; Chen, H.; Zhang, J.; Qiu, Y.; Guo, L.; Fu, S. Quercetin Protects Blood-Brain Barrier Integrity via the PI3K/Akt/Erk Signaling Pathway in a Mouse Model of Meningitis Induced by Glaesserella parasuis. Biomolecules 2024, 14, 696. [Google Scholar] [CrossRef]
- Fu, S.; Li, J.; You, J.; Liu, S.; Dong, Q.; Fu, Y.; Luo, R.; Sun, Y.; Tian, X.; Liu, W.; et al. Baicalin attenuates PD-1/PD-L1 axis-induced immunosuppression in piglets challenged with Glaesserella parasuis by inhibiting the PI3K/Akt/mTOR and RAS/MEK/ERK signalling pathways. Vet. Res. 2024, 55, 95. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, J.H.; Lim, H.K.; Huang, L.; Choi, W.; Kopalli, S.R.; Lee, S.; Lee, B.H.; Lee, J.H.; Ju, Y.; et al. Millingtonia hortensis L. f. ethanol extract exerts in vivo and in vitro anti-inflammatory activities through inhibition of Syk kinase in NF-κB pathway. J. Ethnopharmacol. 2024, 332, 118386. [Google Scholar] [CrossRef]
- Yang, K.; Shan, X.; Songru, Y.; Fu, M.; Zhao, P.; Guo, W.; Xu, M.; Chen, H.; Lu, R.; Zhang, C. Network pharmacology integrated with experimental validation to elucidate the mechanisms of action of the Guizhi-Gancao Decoction in the treatment of phenylephrine-induced cardiac hypertrophy. Pharm. Biol. 2024, 62, 456–471. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Z.; Chen, J. Role of BACH1 in multiple myeloma. Hematology 2024, 29, 2352687. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Liu, S.; Dong, Q.; Fu, Y.; Luo, R.; Sun, Y.; Guo, L.; Lu, Q.; Ye, C.; et al. Anemoside B4 attenuates necrotic enteritis of laying hens induced by Clostridium perfringens via inhibiting NF-κB and PI3K/Akt/mTOR signalling pathways. Heliyon 2024, 10, e33161. [Google Scholar] [CrossRef]
- Campbell, I.L. Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res. Brain Res. Rev. 2005, 48, 166–177. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.; Kapurniotu, A.; Fingerle-Rowson, G.; Roger, T.; Leng, L.; Thiele, M.; Calandra, T.; Bucala, R.; Bernhagen, J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell. Signal. 2006, 18, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.S.; Zhihua, S.; Xuelian, T.; Min, X.; Rongjing, H.; Mei, L. Rosmarinic acid activates the Ras/Raf/MEK/ERK signaling pathway to regulate CD8+ T cells and autophagy to clear Chlamydia trachomatis in reproductive tract-infected mice. Mol. Immunol. 2024, 171, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Wang, Y.; Chen, L.; Peng, L.; Bin, Y.; Ding, P.; Zhang, R.; Tong, F.; Dong, X. Blocking the MIF-CD74 axis augments radiotherapy efficacy for brain metastasis in NSCLC via synergistically promoting microglia M1 polarization. J. Exp. Clin. Cancer Res. CR 2024, 43, 128. [Google Scholar] [CrossRef]
- Petrovic, M.; Majchrzak, O.B.; Marecar, R.; Laingoniaina, A.C.; Walker, P.R.; Borchard, G.; Jordan, O.; Tankov, S. Combining antimiR-25 and cGAMP Nanocomplexes Enhances Immune Responses via M2 Macrophage Reprogramming. Int. J. Mol. Sci. 2024, 25, 12787. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Y.; Liao, P.; Wang, J.; Li, Z.; Tan, J.; Zha, X.; Chen, S.; Li, Y.; Zhong, L. Different expression patterns of VISTA concurrent with PD-1, Tim-3, and TIGIT on T cell subsets in peripheral blood and bone marrow from patients with multiple myeloma. Front. Oncol. 2022, 12, 1014904. [Google Scholar] [CrossRef]
- Gong, X.; Cui, Q.; Zhang, W.; Shi, Y.; Zhang, P.; Zhang, C.; Hu, G.; Sahin, O.; Wang, L.; Shen, Z.; et al. Genomic insight into the diversity of Glaesserella parasuis isolates from 19 countries. mSphere 2024, 9, e0023124. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Zhao, Q.; Du, S.; Huang, X.; Wu, R.; Yan, Q.; Han, X.; Wen, Y.; Cao, S.J. HbpA from Glaesserella parasuis induces an inflammatory response in 3D4/21 cells by activating the MAPK and NF-κB signalling pathways and protects mice against G. parasuis when used as an immunogen. Vet. Res. 2024, 55, 93. [Google Scholar] [CrossRef]
- Ghoochani, A.; Schwarz, M.A.; Yakubov, E.; Engelhorn, T.; Doerfler, A.; Buchfelder, M.; Bucala, R.; Savaskan, N.E.; Eyüpoglu, I.Y. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene 2016, 35, 6246–6261. [Google Scholar] [CrossRef]
- Meza-Romero, R.; Benedek, G.; Leng, L.; Bucala, R.; Vandenbark, A.A. Predicted structure of MIF/CD74 and RTL1000/CD74 complexes. Metab. Brain Dis. 2016, 31, 249–255. [Google Scholar] [CrossRef]
- Calandra, T.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF): A Glucocorticoid Counter-Regulator within the Immune System. Crit. Rev. Immunol. 2017, 37, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.; Grieb, G.; Hristov, M.; Pallua, N.; Weber, C.; Bernhagen, J.; Steffens, G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J. Cell. Mol. Med. 2011, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, A.; Yano, H.; Pan, C.; Fujiwara, Y.; Anami, T.; Ibe, Y.; Motoshima, T.; Yatsuda, J.; Esumi, S.; Miura, Y.; et al. Potential protumor function of CD74 in clear cell renal cell carcinoma. Hum. Cell 2024, 37, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xu, J.; Zeng, F.; Wang, B.; Yang, Y.; Xu, J.; Sun, X.; Ren, T.; Tang, X. Integrative analysis of Ewing’s sarcoma reveals that the MIF-CD74 axis is a target for immunotherapy. Cell Commun. Signal. CCS 2025, 23, 23. [Google Scholar] [CrossRef]
- Pellegrino, B.; David, K.; Rabani, S.; Lampert, B.; Tran, T.; Doherty, E.; Piecychna, M.; Meza-Romero, R.; Leng, L.; Hershkovitz, D.; et al. CD74 promotes the formation of an immunosuppressive tumor microenvironment in triple-negative breast cancer in mice by inducing the expansion of tolerogenic dendritic cells and regulatory B cells. PLoS Biol. 2024, 22, e3002905. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Vishnupriya, S.; Priya Dharshini, L.C.; Sakthivel, K.M.; Rasmi, R.R. Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci. 2020, 260, 118308. [Google Scholar] [CrossRef]
- Hwang, H.J.; Ha, H.; Lee, B.S.; Kim, B.H.; Song, H.K.; Kim, Y.K. LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat. Commun. 2022, 13, 1436. [Google Scholar] [CrossRef]
- Jeong, S.J.; Zhang, X.; Rodriguez-Velez, A.; Evans, T.D.; Razani, B. p62/SQSTM1 and Selective Autophagy in Cardiometabolic Diseases. Antioxid. Redox Signal. 2019, 31, 458–471. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, F.; Wang, Y.; Liu, J.; Ming, X.; Hou, J.; Lv, B.; Fang, S.; Yu, B. Macrophage migration inhibitory factor promotes cardiac stem cell proliferation and endothelial differentiation through the activation of the PI3K/Akt/mTOR and AMPK pathways. Int. J. Mol. Med. 2016, 37, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Tan, H.L.; Huang, Q.; Ong, C.N.; Shen, H.M. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 2009, 5, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Deng, X.; Jiang, Q.; Li, G.; Zhang, J.; Zhang, N.; Xin, S.; Xu, K. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomed. Pharmacother. 2020, 125, 109895. [Google Scholar] [CrossRef]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2022, 85, 123–154. [Google Scholar] [CrossRef]
- Umbarkar, P.; Tousif, S.; Singh, A.P.; Anderson, J.C.; Zhang, Q.; Tallquist, M.D.; Woodgett, J.; Lal, H. Fibroblast GSK-3α Promotes Fibrosis via RAF-MEK-ERK Pathway in the Injured Heart. Circ. Res. 2022, 131, 620–636. [Google Scholar] [CrossRef]
- Jin, H.; Huang, X.; Pan, Q.; Ma, N.; Xie, X.; Wei, Y.; Yu, F.; Wen, W.; Zhang, B.; Zhang, P.; et al. The EIF3H-HAX1 axis increases RAF-MEK-ERK signaling activity to promote colorectal cancer progression. Nat. Commun. 2024, 15, 2551. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Qi, M.; Yu, F.; Ni, X.; Hong, H.; Xu, H.; Xu, S. Glyphosate induces autophagy in hepatic L8824 cell line through NO-mediated activation of RAS/RAF/MEK/ERK signaling pathway and energy metabolism disorders. Fish Shellfish Immunol. 2023, 137, 108772. [Google Scholar] [CrossRef]
- Li, X.; Ma, A.; Liu, K. Geniposide alleviates lipopolysaccharide-caused apoptosis of murine kidney podocytes by activating Ras/Raf/MEK/ERK-mediated cell autophagy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1524–1532. [Google Scholar] [CrossRef]
- Xie, J.; Wang, S.; Li, Z.; Ao, C.; Wang, J.; Wang, L.; Peng, X.; Zeng, K. 5-aminolevulinic acid photodynamic therapy reduces HPV viral load via autophagy and apoptosis by modulating Ras/Raf/MEK/ERK and PI3K/AKT pathways in HeLa cells. J. Photochem. Photobiol. B Biol. 2019, 194, 46–55. [Google Scholar] [CrossRef]
- Bardi, G.T.; Smith, M.A.; Hood, J.L. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 2018, 105, 63–72. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo, L.; Aran, G.; Téllez, É.; Amézaga, N.; Armengol, C.; López, D.; Prats, C.; Sarrias, M.R. CD5L Promotes M2 Macrophage Polarization through Autophagy-Mediated Upregulation of ID3. Front. Immunol. 2018, 9, 480. [Google Scholar] [CrossRef]
- Fang, X.; Lan, X.; Zhu, M.; He, M.; Sun, M.; Cao, Y.; Zhu, D.; Guo, D.; Luo, H. Puerarin Induces Macrophage M2 Polarization to Exert Antinonalcoholic Steatohepatitis Pharmacological Activity via the Activation of Autophagy. J. Agric. Food Chem. 2024, 72, 7187–7202. [Google Scholar] [CrossRef]
- Geng, P.; Zhu, H.; Zhou, W.; Su, C.; Chen, M.; Huang, C.; Xia, C.; Huang, H.; Cao, Y.; Shi, X. Baicalin Inhibits Influenza A Virus Infection via Promotion of M1 Macrophage Polarization. Front. Pharmacol. 2020, 11, 01298. [Google Scholar] [CrossRef]
- van Elsas, M.J.; Middelburg, J.; Labrie, C.; Roelands, J.; Schaap, G.; Sluijter, M.; Tonea, R.; Ovcinnikovs, V.; Lloyd, K.; Schuurman, J.; et al. Immunotherapy-activated T cells recruit and skew late-stage activated M1-like macrophages that are critical for therapeutic efficacy. Cancer Cell 2024, 42, 1032–1050.e10. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, C.; Wang, S.; Shi, D.; Wei, C.; Song, J.; Lin, X.; Dou, R.; Bai, J.; Xiang, Z.; et al. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun. Signal. CCS 2020, 18, 51. [Google Scholar] [CrossRef]
- Hong, S.M.; Lee, A.Y.; Kim, B.J.; Lee, J.E.; Seon, S.Y.; Ha, Y.J.; Ng, J.T.; Yoon, G.; Lim, S.B.; Morgan, M.J.; et al. NAMPT-Driven M2 Polarization of Tumor-Associated Macrophages Leads to an Immunosuppressive Microenvironment in Colorectal Cancer. Adv. Sci. 2024, 11, e2303177. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Y.; Li, Q.; Yao, J.; Yuan, X.; Zhang, Y.; Yin, X.; Saito, Y.; Fan, H.; Li, P.; et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 2022, 40, 36–52.e9. [Google Scholar] [CrossRef]
- Yeung, M.Y.; Grimmig, T.; Sayegh, M.H. Costimulation Blockade in Transplantation. Adv. Exp. Med. Biol. 2019, 1189, 267–312. [Google Scholar]
- Yuan, Y.; Hua, L.; Zhou, J.; Liu, D.; Ouyang, F.; Chen, X.; Long, S.; Huang, Y.; Liu, X.; Zheng, J.; et al. The effect of artesunate to reverse CLP-induced sepsis immunosuppression mice with secondary infection is tightly related to reducing the apoptosis of T cells via decreasing the inhibiting receptors and activating MAPK/ERK pathway. Int. Immunopharmacol. 2023, 124 Pt A, 110917. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Liu, Q.; Cheng, P.; Zhao, T.; Yang, T.; Zhao, Y.; Sha, W.; Zhao, Y.; Qu, H. Targeting regulatory T cells for cardiovascular diseases. Front. Immunol. 2023, 14, 1126761. [Google Scholar] [CrossRef] [PubMed]
- Finisguerra, V.; Dvorakova, T.; Formenti, M.; Van Meerbeeck, P.; Mignion, L.; Gallez, B.; Van den Eynde, B.J. Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression. J. Immunother. Cancer 2023, 11, e005719. [Google Scholar] [CrossRef] [PubMed]
- Naoi, Y.; Morinaga, T.; Nagasaki, J.; Ariyasu, R.; Ueda, Y.; Yamashita, K.; Zhou, W.; Kawashima, S.; Kawase, K.; Honobe-Tabuchi, A.; et al. CD106 in Tumor-Specific Exhausted CD8+ T Cells Mediates Immunosuppression by Inhibiting TCR Signaling. Cancer Res. 2024, 84, 2109–2122. [Google Scholar] [CrossRef] [PubMed]


| Gene | Nucleotide Sequence (5′-3′) | Tm (°C) | Length (bp) | |
|---|---|---|---|---|
| IL-1β | Forward | TCTGCATGAGCTTTGTGCAAG | 59.7 | 155 |
| Reverse | ACAGGGCAGACTCGAATTCAAC | 60.9 | ||
| IL-6 | Forward | CTTCTGGTGATGGCTACTG | 52.7 | 134 |
| Reverse | TTGCCGAGGATGTACTTAA | 50.0 | ||
| IL-8 | Forward | ACAGCAGTAACAACAACAAG | 50.2 | 117 |
| Reverse | GACCAGCACAGGAATGAG | 53.2 | ||
| IL-10 | Forward | CGTGGAGGAGGTGAAGAGTG | 55.4 | 178 |
| Reverse | TTAGTAGAGTCGTCATCCTGGAAG | 55.6 | ||
| IL-18 | Forward | AGTAACCATATCTGTGCAGTGT | 54.0 | 155 |
| Reverse | TCTTATCACCATGTCCAGGAAC | 53.0 | ||
| TNF-α | Forward | CGCTCTTCTGCCTACTGCACTTC | 60.7 | 164 |
| Reverse | CTGTCCCTCGGCTTTGACATT | 57.8 | ||
| CD74 | Forward | CTGGGAGTGTACCCGAAGC | 58.6 | 104 |
| Reverse | CGCAGCCAGTTCTCAAAGAG | 56.6 | ||
| MIF | Forward | GTGCGCCCTTTGCAGTCT | 59.5 | 146 |
| Reverse | CCGCGTTCATGTCGTAGTAGTT | 57.1 | ||
| COX-2 | Forward | TGTGAAAGGGAGGAAAGA | 49.7 | 133 |
| Reverse | CTGATGGGTGAAGTGCTG | 53.5 | ||
| TIM-3 | Forward | TCAAGCCTCATCACTTTGG | 53.7 | 145 |
| Reverse | TGACGGAGCAGTAACACTC | 50.8 | ||
| PI3K | Forward | TTGCTACAATCAATCGCCAGGAGAC | 59.3 | 147 |
| Reverse | CTTCCCGTTGTTGCCATCGTTTG | 59.7 | ||
| Akt | Forward | GGACGGGCACATCAAGATCACTG | 60.8 | 126 |
| Reverse | TAGTCGTTGTCCTCCAGCACCTC | 61.2 | ||
| mTOR | Forward | AGTACCTCCAGGACACCATGAACC | 60.9 | 108 |
| Reverse | CAGACCTCACAGCCACAGAAAGC | 61.0 | ||
| GAPDH | Forward | GGCACAGTCAAGGCGGAGAAC | 61.9 | 105 |
| Reverse | AGCACCAGCATCACCCCATTTG | 61.0 | ||
| LC3B | Forward | AGCCTTCTTCCTGTTAGTG | 51.3 | 135 |
| Reverse | TTCATTCCGAAAGTCTCC | 48.4 | ||
| P62 | Forward | CCCCAATGTGATCTGTGATG | 53.4 | 126 |
| Reverse | TTGCTGTGCTCCTTGTGAAT | 54.5 | ||
| Beclin-1 | Forward | TGAGGATACCCAAGCAAG | 51.2 | 146 |
| Reverse | ATGTGGAGAAAGGCAAGA | 50.4 | ||
| Inos | Forward | TGGAAGCGGTAACAAAGGA | 53.7 | 482 |
| Reverse | CACGAGGTCAGGAGGGATT | 56.8 | ||
| ARG1 | Forward | GGACCTGTGCTTTGCTGAT | 55.6 | 122 |
| Reverse | TTCCGTTCTTCTTGATTTCTG | 49.7 | ||
| RAF | Forward | CAACACTGATGCTGCTGGTAA | 55.4 | 113 |
| Reverse | CAGATGGCGACTTGGAATG | 53.8 | ||
| MEK | Forward | CGTGAATGAGCCACCTCCC | 60.7 | 149 |
| Reverse | CCACCTCGGACCGTTTGAT | 60.2 | ||
| ERK | Forward | GCTCTTGAAGACGCAGCAC | 58.6 | 108 |
| Reverse | CAGCAGGTTGGAAGGTTTG | 58.5 | ||
| CD86 | Forward | TGTGGGATGGTGTCCTTTG | 55.0 | 106 |
| Reverse | GTTCACTCGCCTTCCTGTT | 55.2 | ||
| CD3 | Forward | GTTTGCTGATGGTGGTGTA | 52.4 | 144 |
| Reverse | TGGGCTCATAGTCTGGATT | 52.5 | ||
| CD4 | Forward | AGCCTCAGTTACCGAGTT | 52.7 | 138 |
| Reverse | ATCCTCTTGTCTTCCACTTC | 51.1 | ||
| CD8 | Forward | GTTACATCTCTGGTTACAAGG | 49.9 | 139 |
| Reverse | AAGAAGACGGACATGAAGTT | 50.7 | ||
| CD163 | Forward | TGCTGTAGTCGCTGTTCT | 55.9 | 117 |
| Reverse | ACTTTCACCTCCACTCTTC | 54.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Dong, Q.; Fu, Y.; Luo, R.; Li, J.; Sun, Y.; Liu, S.; Qiu, Y.; Guo, L.; Hu, J. Baicalin Relieves Glaesserella parasuis-Triggered Immunosuppression Through Polarization via MIF/CD74 Signaling Pathway in Piglets. Biomolecules 2025, 15, 640. https://doi.org/10.3390/biom15050640
Fu S, Dong Q, Fu Y, Luo R, Li J, Sun Y, Liu S, Qiu Y, Guo L, Hu J. Baicalin Relieves Glaesserella parasuis-Triggered Immunosuppression Through Polarization via MIF/CD74 Signaling Pathway in Piglets. Biomolecules. 2025; 15(5):640. https://doi.org/10.3390/biom15050640
Chicago/Turabian StyleFu, Shulin, Qiaoli Dong, Yunjian Fu, Ronghui Luo, Jingyang Li, Yamin Sun, Siyu Liu, Yinsheng Qiu, Ling Guo, and Jin Hu. 2025. "Baicalin Relieves Glaesserella parasuis-Triggered Immunosuppression Through Polarization via MIF/CD74 Signaling Pathway in Piglets" Biomolecules 15, no. 5: 640. https://doi.org/10.3390/biom15050640
APA StyleFu, S., Dong, Q., Fu, Y., Luo, R., Li, J., Sun, Y., Liu, S., Qiu, Y., Guo, L., & Hu, J. (2025). Baicalin Relieves Glaesserella parasuis-Triggered Immunosuppression Through Polarization via MIF/CD74 Signaling Pathway in Piglets. Biomolecules, 15(5), 640. https://doi.org/10.3390/biom15050640






